Abstract
The rodent primary somatosensory cortex is spontaneously active in the form of locally synchronous membrane depolarizations (UP states) separated by quiescent hyperpolarized periods (DOWN states) both under anesthesia and during quiet wakefulness. In vivo whole-cell recordings and tetrode unit recordings were combined with voltage-sensitive dye imaging to analyze the relationship of the activity of individual pyramidal neurons in layer 2/3 to the ensemble spatiotemporal dynamics of the spontaneous depolarizations. These were either brief and localized to an area of a barrel column or occurred as propagating waves dependent on local glutamatergic synaptic transmission in layer 2/3. Spontaneous activity inhibited the sensory responses evoked by whisker deflection, accounting almost entirely for the large trial-to-trial variability of sensory-evoked postsynaptic potentials and action potentials. Subthreshold sensory synaptic responses evoked while a cortical area was spontaneously depolarized were smaller, briefer and spatially more confined. Surprisingly, whisker deflections evoked fewer action potentials during the spontaneous depolarizations despite neurons being closer to threshold. The ongoing spontaneous activity thus regulates the amplitude and the time-dependent spread of the sensory response in layer 2/3 barrel cortex.
The neocortex is spontaneously active. Neocortical neurons in vivo exhibit spontaneous subthreshold membrane potential changes, which can evoke spontaneous action potentials (APs). Such spontaneous activity is not only found in association cortex but is also evident in primary sensory areas. Previous studies have shown that both during slow wave sleep and during anesthesia, the brain state is characterized by low frequency, large amplitude spontaneous membrane potential changes (1-3). Dual intracellular recordings have demonstrated that such spontaneous activity can occur synchronously in nearby neurons (4). Voltage-sensitive dye (VSD) imaging has shown complex patterns of spatiotemporal dynamics of the ensemble spontaneous activity (5-7). The nature of this spontaneous activity and how it interacts with sensory responses is poorly understood.
To investigate the relationship between sub- and suprathreshold membrane potential changes of individual neurons in layer 2/3 and the surrounding network, we combined whole-cell (WC) recordings, VSD imaging, and tetrode recordings for in vivo measurements in rodent barrel cortex. Surprisingly, unlike in the anesthetized cat where spontaneous depolarization enhanced responses (6, 8, 9), here we find that both sensory-evoked postsynaptic potentials (PSPs) and sensory-evoked APs are suppressed by ongoing spontaneous activity. This spontaneous activity occurs as both brief localized depolarizations and propagating waves of glutamatergic excitation in layer 2/3.
Methods
Surgical Procedures. Wistar rats or mice C57BL6 aged P21-P35 were anesthetized with urethane (1.5-2 g/kg), ketamine (100 mg/kg)/xylazine (20 mg/kg), or halothane (1.5-2%). Paw withdrawal, whisker movement, and eye blink reflexes were largely suppressed. The head of the animal was fixed either in a stereotaxic apparatus or by gluing a metal post to the skull. A heating blanket maintained the rectally measured body temperature at 37°C.
Training for Awake Recordings. After extensive handling and several training periods of head fixation for ≈30 min each per day, the animal learns to sit quietly for short periods of time. During these periods of quiet wakefulness, stable recordings can be obtained. It is important to note that because each head fixation session is brief (30 min) and the total training is limited to three or four sessions, the animal does not become bored with the experimental scenario. Furthermore, there was only a single experimental day for each animal to allow anatomical identification of the recorded cell. The animal is thus never asleep during the recording session. Instead, the animals were rather tense, made small postural adjustments often, and would react to the visual presence of a nearby approaching object. All experiments were made according to the guidelines of the Max Planck Society and according to permit (Zelluläre Mechanismen assoziativer Lernvorgänge, no. 35-91485.81/26/00).
WC Recordings. Pipettes were advanced into the cortex with a positive pressure until the pipette resistance increased, and then suction was applied to establish a gigaseal followed by the WC configuration, as described (10). WC pipettes had resistances of 5 MΩ filled with a solution containing 135 mM potassium gluconate, 4 mM KCl, 10 mM Hepes, 10 mM phosphocreatine, 4 mM MgATP, 0.3 mM Na3GTP (adjusted to pH 7.2 with KOH), and 2 mg/ml biocytin. WC electrophysiological measurements were made with Axopatch 200 amplifiers (Axon Instruments, Foster City, CA). The membrane potential was filtered at 2 kHz and digitized at 10 kHz in a sweep-based manner by ITC-16 (Instrutech, Mineola, NY) under the control of PULSE (HEKA, Lambrecht/Pfalz, Germany) or IGORPRO (Wavemetrics, Lake Oswego, OR). Individual whiskers were deflected for a 2-ms duration by a computer-controlled piezoelectric wafer attached to the whisker at a distance 10 mm from the snout.
VSD Imaging. RH1691 was applied at 0.1 mg/ml in Ringer's solution for a period of 2 h to the craniotomy followed by rinses to remove unbound dye, as described (10). The cortex was covered with 1% agarose and a glass coverslip placed on top. VSD signals were imaged from a focal plane ≈300 μm below the pia by using a tandem lens arrangement attached to either an Imager 3001 (Optical Imaging, New York; 5 ms per frame) or a Fuji Deltaron HR 1700 camera (2.4 ms per frame). Excitation light was filtered with a 630 ± 15-nm band pass filter, reflected onto the cortex by a 650-nm dichroic, and the epifluorescent image was collected after a 665-nm-long pass filter. Image data were analyzed by using custom-written routines in IGORPRO or MATLAB (MathWorks).
Tetrode Recordings. Methods for tetrode recording were similar to those described (11). Briefly, four Nichrome wires, 12 μm in diameter coated with insulation, were twisted together to form a tetrode. These were then gold plated to reduce the impedance. The tetrode was slowly advanced in the brain by using a micromanipulator until large amplitude (>150 μV) stable clusters of spikes could be discerned. Recordings were done by using an HS-16 preamplifier and a Lynx-8 amplifier (Neuralynx, Tucson, AZ). The spike data were sampled at 33 kHz by using a Neuralynx Cheetah data acquisition system and sorted offline into individual units by using the Neuralynx Spike Sorter program.
Results
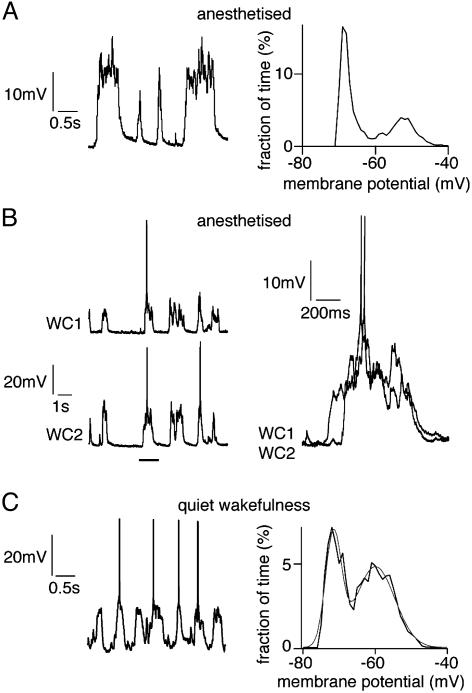
UP and DOWN States in Layer 2/3 Barrel Cortex During Anesthesia and Quiet Wakefulness. WC membrane potential recordings of layer 2/3 pyramidal neurons in barrel cortex under anesthesia showed two stable states (Fig. 1A) separated by almost 20 mV (18 ± 2mV; n = 34 animals). A membrane potential of ≈-75 mV with low variance defines the DOWN state. A distinct second peak in the membrane potential histogram at ≈-55 mV defines the UP state. Large membrane potential fluctuations occur during the UP state creating the broader peak in the membrane potential histogram. Action potentials are generated during UP state at low frequency (0.17 ± 0.1 Hz, range 0 to 1 Hz). The membrane potential remains bistable on injection of hyperpolarizing current (n = 5 animals). Dual WC recordings (n = 5 animals) indicated that all long-lasting spontaneous transitions to UP state occurred nearly synchronously in layer 2/3 barrel cortex neurons (Fig. 1B). During periods of quiet wakefulness in unanesthetized animals, similar but more rapidly changing patterns of spontaneous membrane potential fluctuations in layer 2/3 pyramidal neurons of barrel cortex (UP state separated by 16 ± 3 mV from DOWN state, n = 5 animals) can also be recorded (Fig. 1C).
Fig. 1.
UP and DOWN states during anesthesia and quiet wakefulness. (A)WC recording of the membrane potential of layer 2/3 pyramidal neurons during anesthesia shows two distinct states, which are apparent from inspection of single long traces of spontaneous membrane potential (Left) and from histograms of the time at different membrane potentials calculated from 60 s of data (Right). The presence of two peaks demonstrates two states of the neuronal membrane potential defining two cortical states with a noisy UP state depolarized by ≈20 mV compared with the quiescent DOWN state. (B) Dual WC recordings from layer 2/3 pyramidal neurons display synchronous membrane potential changes (Left). The precise timing of spontaneous events is different in the two neurons, which becomes obvious when examined at a higher time resolution (Right). (C) WC recording of a layer 2/3 pyramidal neuron from an unanesthetized animal during quiet wakefulness. The membrane potential (Left) fluctuates between two states, which can quantitatively represented by the histogram of time spent at each potential calculated from 30 s of data (Right).
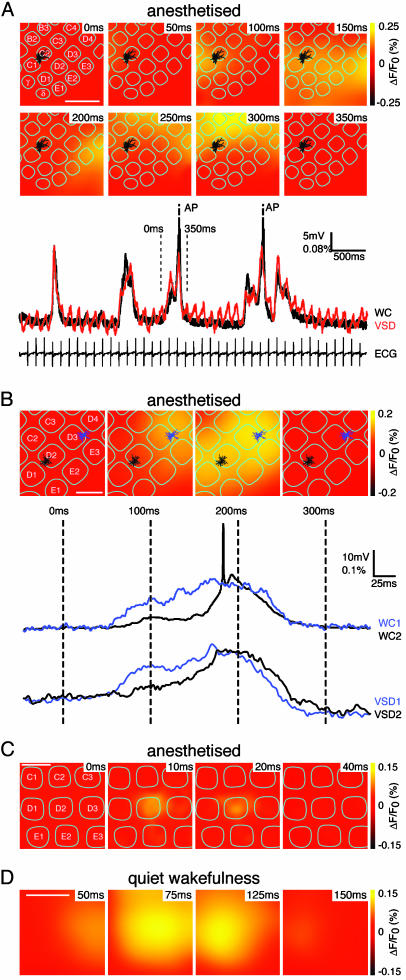
Brief Localized Events and Propagating Waves of Spontaneous Depolarizations. To define the spatiotemporal dynamics of this spontaneous activity, we combined WC recordings with VSD imaging (n = 18 animals). The spontaneous subthreshold membrane potential changes of individual layer 2/3 pyramidal neurons are well correlated with the local changes in VSD signal (Fig. 2A). This provides further support for the notion that neighboring neurons have synchronous spontaneous subthreshold activity, as VSD signals represent the ensemble electrical activity of many neurons. However, spontaneous APs recorded in individual neurons are not correlated with large deflections of the VSD signal, suggesting that APs do not occur simultaneously in a large fraction of neurons (Fig. 2 A and B).
Fig. 2.
Propagating waves of spontaneous activity. (A) Images of propagating waves of spontaneous activity imaged with voltage-sensitive dye (VSD) combined with WC recording. The location of the layer 2/3 pyramidal neuron is indicated by the reconstructed dendritic arborization (black) in the context of the layer 4 barrel pattern (cyan). VSD signals correlating with depolarization are shown in yellow. Activity is initiated in the top left corner of the image (50 ms) and propagates first across the bottom of the image (100-200 ms) and then up along the right side of the image (200-250 ms), finishing at the top (300 ms). The membrane potential of a neuron along with the quantification of the VSD signal immediately surrounding the neuron (200 μm × 200 μm) show synchronous changes, except for the action potentials, which are not reflected in the VSD trace and for the presence of heartbeat-related artifacts in the VSD trace. (Scale bar, 1 mm.) (B) Dual WC recording and VSD imaging of spontaneous activity. A spontaneous wave of activity began at the top right of the image close to the position of one of the layer 2/3 pyramidal neurons from which we are recording (dendrites and soma colored blue located in the D3 barrel). The wave propagates toward the bottom left, where the second WC recording of a layer 2/3 pyramidal neuron is located (soma and dendrites in black located in the D2 barrel) and then disappears. The local VSD signals (quantified in the 200 × 200-μm regions centered on the respective somatic locations of the neurons) correlated closely with the subthreshold membrane potential measurements. Both VSD imaging and dual membrane potential measurements indicate that spontaneous activity occurs as a propagating wave. (Scale bar, 500 μm.) (C) An example of a short duration spontaneous event imaged with VSD lasting <50 ms that remains localized to a single barrel column. (Scale bar, 500 μm.) (D) VSD imaging from an unanesthetized animal during quiet wakefulness. The spontaneous activity occurs in propagating waves, similar to that observed in anesthetized animals. (Scale bar, 1 mm.)
The VSD signals do not occur uniformly across the imaged area of barrel cortex. The spontaneous activity either propagated as waves of excitation (Fig. 2 A and B) or remained localized for short duration events (Fig. 2C; in this example, the spontaneous depolarization remained localized to an individual neocortical barrel column). Spontaneous depolarizations of longer duration are observed when larger areas of neocortex become activated by a propagating wave and, rather than quickly returning to baseline, remain active for several hundred milliseconds. To confirm that these waves observed with VSD imaging are really propagating waves of depolarization, also detectable at the level of individual neurons, we made simultaneous dual WC recordings while imaging VSD signals (Fig. 2B). The spontaneous wave illustrated in Fig. 2B begins in the top right of the image, where one neuron is located (D3 barrel) depolarizing this neuron first. Then, the VSD signal spreads toward the bottom left, where the other WC recording is located (D2 barrel) depolarizing this neuron at a later time. Return to rest in this case occurs uniformly across the entire imaged area. The preferred direction of spread of these spontaneous waves as shown in this example (Fig. 2B) is along the rows of the barrel cortex (63% of waves propagated along the rows, n = 18 animals). This preferred direction of spread of spontaneous excitation is similar to that of the sensory-evoked responses (10). However, more complex patterns of activity are also often observed as in the example above (Fig. 2 A). Propagation velocities ranged from <10 μm/ms to well over 100 μm/ms. Spontaneous subthreshold depolarizations in layer 2/3 barrel cortex therefore predominantly occur in the form of propagating waves of depolarization underlying the broad synchrony observed in spatially separated individual neurons. In further experiments with VSD imaging in awake animals (eight animals), we further found that spontaneous activity during quiet wakefulness is associated with propagating waves of depolarization across the barrel cortex (Fig. 2D). The amplitudes of the spontaneous VSD signals in animals during quiet wakefulness were not different from that observed in anesthetized preparations.
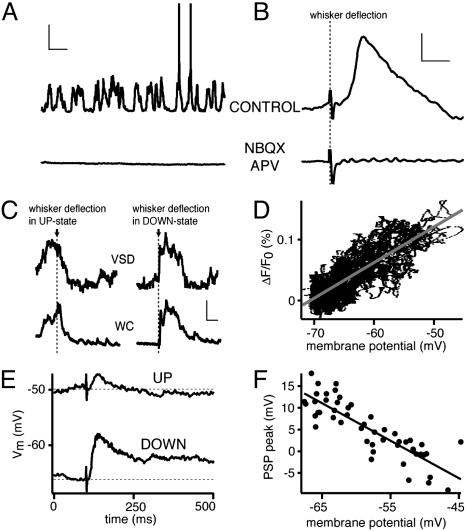
Local Glutamatergic Excitation Drives the UP State, Which Is Strongly Modulated by GABAergic Input. Pharmacological manipulations were performed to examine the relative roles of local glutamatergic and GABAergic synaptic activity. When ionotropic glutamate receptors were blocked by local superfusion of NBQX (10 μM; AMPA receptor blocker) and APV (200 μM; NMDA receptor blocker), neurons were forced into the DOWN state, and both spontaneous and evoked membrane potential changes were abolished (Fig. 3 A and B, n = 5 animals). Blockade of ionotropic GABA receptors by either bicuculline (20 μM, n = 5) or picrotoxin (200 μM, n = 5) increased amplitude of spontaneous membrane potential fluctuations, and spontaneous APs occurred more frequently.
Fig. 3.
Local glutamatergic excitation drives the cortex into the UP state competing with sensory-evoked responses, which become smaller and briefer. (A) The membrane potential of a layer 2/3 pyramidal neuron displaying spontaneous UP and DOWN states. Application of NBQX and APV locally to the craniotomy completely blocked spontaneous activity, locking the neuron into the DOWN state. This indicates that local glutamatergic synaptic activity underlies the UP state (vertical scale, 20 mV; horizontal scale, 1 s). (B) The sensory response evoked by whisker deflection was also blocked in parallel with the block of spontaneous activity (vertical scale, 2 mV; horizontal scale, 20 ms). (C) VSD signal as quantified from a region surrounding the somatic location of a layer 2/3 pyramidal neuron recorded in the WC configuration. Both recording techniques show similar temporal dynamics, indicating that the subthreshold dynamics of many neurons in a given cortical region are similar to what is observed in a single neuron. A whisker was deflected during spontaneous UP state (left traces) and during DOWN state (right traces). There was no significant excitatory sensory response during UP state. However, when the whisker was deflected during the DOWN state, a large prolonged sensory response was evoked (vertical scale, 5 × 10-4, 10 mV; horizontal scale, 100 ms). (D) Plot of VSD signal as a function of membrane potential demonstrating a linear correlation. (E) Sensory responses were evoked by whisker deflection, and each trial was analyzed. The sweeps were separated into two groups depending on the membrane potential immediately preceding the whisker stimulus, such that each group contained half of the sweeps. Sensory responses evoked from more depolarized membrane potentials (UP state) were brief and small compared with those evoked from the DOWN state. (F) The amplitude of responses (quantified at the peak of the averaged response) varies linearly with membrane potential. The trial-to-trial variability of the peak sensory response is thus almost entirely accounted for by its interaction with ongoing spontaneous activity.
Sensory-Evoked PSPs in UP and DOWN States. Membrane potential changes in layer 2/3 pyramidal neurons and VSD signals evoked by whisker deflection have smaller or comparable amplitudes to the spontaneous subthreshold membrane potential changes. Thus, one might expect a profound interaction of evoked and spontaneous activity. We therefore analyzed the trial-by-trial variability of responses to a brief deflection of a single whisker (Fig. 3 C-F). Stimulation of a whisker during the depolarized UP state led to a smaller amplitude subthreshold response [PSP amplitude in the UP state was reduced to 24 ± 7% (n = 34 animals) of the DOWN state] with a briefer time course [PSP half-width duration was decreased to 32 ± 8% (n = 34 animals) of the DOWN state]. This was the case both for the ensemble VSD signal and PSPs recorded from individual neurons (Fig. 3C), which correlated linearly with each other (n = 18 animals, Fig. 3D). Thus the trial-to-trial variability and the reduced amplitude of whisker-evoked responses during UP state is not a phenomenon limited to an individual neuron but a property of the ensemble neural network. The temporal location of the peak of the average PSP was found and used to quantify the PSP amplitude for individual trials. These amplitude measurements of the sensory-evoked PSPs were plotted as a function of the membrane potential immediately preceding whisker deflection. A linear correlation was observed (Fig. 3F), such that at the most depolarized membrane potentials, a hyperpolarizing response was observed.
The decrease in response amplitude during the UP state is likely to result from several mechanisms. At depolarized membrane potentials, the driving force for excitation is reduced, whereas the driving force for inhibition is increased. Thus, depolarization by somatic current injection of a layer 2/3 pyramidal neuron results in a smaller amplitude sensory-evoked response (n = 9 animals, data not shown) (12). Furthermore, the input resistance of layer 2/3 pyramidal neurons is also decreased during UP state (n = 7 animals, data not shown) (2, 13), which will further reduce the amplitude of sensory responses.
However, APs are more readily evoked by somatic current injection during the UP state (n = 7 animals, data not shown), presumably because the neuron is closer to threshold. Thus, despite the smaller subthreshold sensory responses, one might expect that more APs are generated by a sensory stimulus during UP state. In the majority of the WC recordings (67 of 72 animals), action potentials were not evoked by the sensory stimulus in either UP or DOWN states, suggesting a sparse AP encoding of single whisker deflection. However, in a small subset of the layer 2/3 pyramidal neurons from which WC recordings were made (5 of 72 animals), APs were reliably evoked by whisker deflection from the DOWN state (at least one AP was evoked within 50 ms of stimulation in 72% of trials). Surprisingly, APs were less frequently evoked by the same whisker deflection from the UP state in these neurons (at least one AP was evoked within 50 ms of stimulation in only 21% of trials).
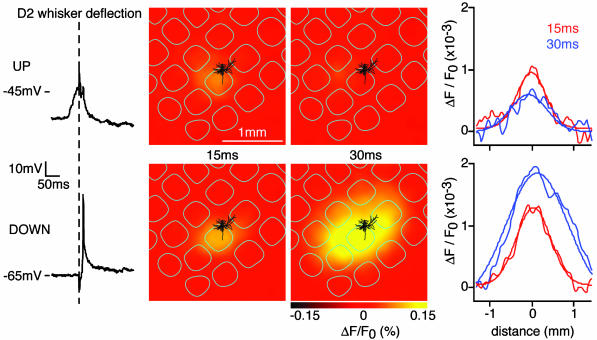
Simultaneous WC recordings and VSD imaging allowed us to investigate the spatiotemporal dynamics of the whisker deflection-evoked responses in the context of the ongoing spontaneous activity (Fig. 4). The earliest response in layer 2/3 to a single whisker stimulus evokes a region of excitation limited to a single barrel column, which over the subsequent milliseconds can spread to excite the entire barrel cortex (10, 14). Based on the membrane potential of the WC recording immediately before the stimulus, sweeps were separated into responses evoked from DOWN states or UP states. Early responses evoked from UP or DOWN state were confined to the same small cortical region covering the principal whisker barrel column [quantified 15 ms poststimulus, the half-width along the row of the sensory-evoked VSD signal was 566 ± 63 μm (n = 18 animals) during UP state and 587 ± 46 μm (n = 18 animals) during DOWN state; for comparison, a layer 4 barrel has a diameter of ≈500 μm]. Responses from DOWN state were not only larger and lasted longer (as described above for WC recordings of individual neurons) but also propagated over large cortical areas in the following milliseconds after the initial response (quantified 30 ms poststimulus, the half-width along the row of the sensory-evoked VSD signal was 1,625 ± 194 μm (n = 18 animals) during DOWN state). Responses from the UP state did not spread and remained localized to the initially excited region for the short duration of the response [quantified 30 ms poststimulus; the half-width along the row of the sensory-evoked VSD signal was 612 ± 87 μm (n = 18 animals) during UP state].
Fig. 4.
Sensory responses evoked during UP state are spatially confined. Sensory responses evoked by whisker deflection were collected, and each trial was analyzed. Responses were divided into those evoked from UP state or DOWN state. The WC recording of a layer 2/3 pyramidal neuron (membrane potential in the left column) indicates small and brief sensory responses from UP state and large responses from DOWN state. In this experiment, four of six responses from DOWN state evoked APs, but no APs were observed in the four trials evoked from the UP state. VSD imaging of the same sweeps indicates that responses from UP and DOWN states are initiated at the same cortical location, with smaller amplitude responses occurring from UP state. In the next milliseconds, the response from DOWN state propagated across a large area of neocortex, whereas the response evoked during UP state remained localized.
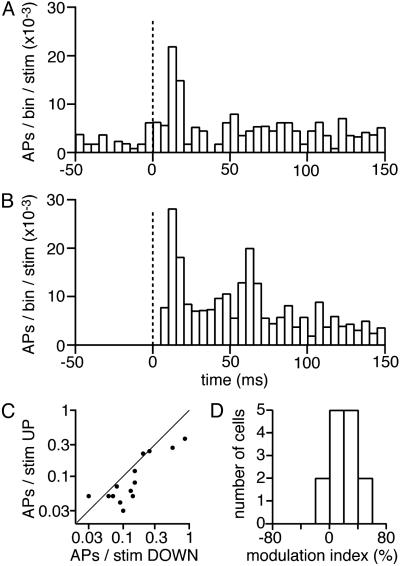
Suprathreshold Sensory Responses in UP and DOWN States. Because very few neurons responded with APs to whisker stimulation in the neuron population sampled with our WC recordings, we made experiments focusing specifically on this issue. The cortical state was imaged with VSD, and neuronal output was simultaneously measured by using tetrodes (11). Action potentials from four to eight well isolated neurons were simultaneously recorded by using a tetrode placed in layer 2/3. A total of 22 cells was recorded in four sessions from three different animals while the principal whisker was stimulated. Eight neurons did not fire any spike within 150 ms of stimulation during the entire session, and hence were excluded from the analyses. The whisker stimuli were separated on the basis of the prestimulus cortical state as reported by the VSD into UP state or DOWN state. The time courses of neuronal suprathreshold responses, characterized by the peristimulus time histograms, were computed separately for the stimuli delivered during UP and DOWN states and averaged across the 14 neurons (Fig. 5 A and B). Ongoing background AP activity can be seen in the peristimulus time histograms before the stimulus onset for the UP state (Fig. 5A) but not in the DOWN state (Fig. 5B). The peak sensory-evoked AP response occurred at about 10 ms poststimulus in both UP and DOWN states with a smaller peak response from the UP state compared with the DOWN state. The largest difference in the sensory-evoked action potential response between the UP and DOWN states appeared between 20 and 70 ms after the stimulus onset, where the DOWN state response clearly showed a second peak but not the UP state response, which returned rapidly to a near prestimulus level of activity. Computed across cells, the sensory-evoked APs during the first 20 ms (after a 7-ms latency, 7-27 ms) and during the first 150 ms were significantly reduced (P < 0.05 Student's paired t test) during the UP state (0.024 ± 0.009 spikes above background firing per stimulus during the first 20 ms; 0.11 ± 0.03 spikes per stimulus during the first 150 ms) compared with the DOWN state (0.081 ± 0.023 spikes above background firing per stimulus during the first 20 ms; 0.20 ± 0.06 spikes per stimulus during the first 150 ms). A cell-by-cell analysis revealed that for each neuron, whisker deflection-evoked APs were either little affected or suppressed during UP state (Fig. 5 C and D).
Fig. 5.
Sensory-evoked APs are suppressed in UP state. (A) The averaged peristimulus time histogram (PSTH, stimulus at 0 ms; 14 neurons) of action potentials in layer 2/3 (bin width 5 ms) computed for stimuli delivered during the UP state. (B) The averaged peristimulus time histograms computed for stimuli delivered during the DOWN state. Note that there was no background activity before the stimulus (from -50 ms to 0 ms) in the DOWN state, whereas significant background activity was found in the UP state. However, neurons responded more vigorously to whisker deflection during DOWN state than UP state. (C) The number of action potentials evoked per stimulus in the first 150 ms was quantified separately for UP and DOWN states of each neuron. The x axis (log scale) shows the DOWN state response, and the y axis (log scale) shows the UP state response of individual neurons (each dot corresponds to a neuron). Individual neurons fire either a similar number of action potentials in UP or DOWN states or a reduced number of action potentials from the UP state. (D) The modulation of sensory response by UP and DOWN states was characterized by computing a modulation index, defined as 100 × (DOWN response - UP response)/(UP response + DOWN response), where response is the number of spikes fired by a neuron within 150 ms after the stimulus. Again, by this measure the sensory-evoked action potential firing activity was either unchanged or reduced during the UP state (modulation index, 20 ± 5%; n = 14 cells).
Discussion
Spatiotemporally Resolved Subthreshold Spontaneous Activity. The spontaneous activity of the neocortex during anesthesia and quiet wakefulness defines two cortical states. One is a quiescent state with hyperpolarized neurons (DOWN state), and the other is an active state where neurons are much closer to AP threshold being depolarized by ≈20 mV (UP state). Within a local cortical region of a few hundred microns, all layer 2/3 pyramidal neurons simultaneously depolarize defining a local UP state. The cortical UP state can remain localized to a single cortical column for brief events (Fig. 2C) or propagate rapidly as waves of depolarization across the somatosensory cortex in simple (Fig. 2B) or complex patterns (Fig. 2 A). Recurrent excitation through local synaptic connections in layer 2/3 is likely to contribute to the propagation because they are blocked by local application of antagonists of ionotropic glutamate receptors. In this context, it is interesting to note that spontaneous waves of depolarization propagated preferentially along the row-axis of barrel cortex, similar to the orientation of sensory-evoked responses and the preferred orientation of the axonal arborizations of layer 2/3 pyramidal neurons (10).
Interaction of Sensory Responses with Spontaneous Activity. In the layer 2/3 rodent somatosensory cortex, sensory responses to simple brief stimuli are strongly suppressed by ongoing spontaneous activity. This is the most important determinant of the sensory response on a trial-to-trial basis. The amplitude, duration, and spatial extent of subthreshold sensory responses are reduced when the stimulus is delivered during the UP state. Decreased driving force for glutamatergic excitation, increased driving force for GABAergic inhibition, and decreased input resistance are factors likely to reduce the amplitude of subthreshold sensory responses during the UP state. Notably, not only were subthreshold sensory responses suppressed by spontaneous activity, but so were suprathreshold AP responses. Thus, although neurons are closer to the AP threshold during UP state and fire spontaneous APs, they respond with less APs to a sensory stimulus. The lower number of APs evoked by a sensory stimulus likely accounts for the lack of propagation of the sensory response during the UP state. Clearly, the underlying neuronal network in layer 2/3 is in a different state during spontaneous depolarizations, with some neurons having already generated APs in the milliseconds before the sensory stimulus was delivered. Thus, short-term depression of excitatory synapses (15-21) may provide additional reduction in the amplitude of sensory responses. In addition, the inhibitory synaptic component of the sensory response may be enhanced during UP state, possibly through short-term facilitation of excitatory synaptic input onto a subset of GABAergic interneurons (17-19). The UP state thus does not correspond to a region of cortex primed to respond more to a sensory stimulus, as one might have imagined. Instead, there seems to be a competition between sensory-evoked responses and ongoing spontaneous activity.
Possible Physiological Function. That sensory responses are regulated by the ongoing spontaneous activity of the neocortex may have physiological implications. That the evoked sensory response during the UP state has a short duration and remains tightly localized to the barrel column isomorphic to the stimulated whisker might improve the ability of the neocortex to discriminate both the temporal and spatial separation of sensory inputs. On the other hand, the propagating sensory responses evoked from the DOWN state might be essential to integrate neocortical activity over longer periods of time and over a larger area. For example, different sensory inputs represented on spatially separated cortical regions might be associated.
In summary, all-or-none spontaneous activity in vivo occurs both in the form of brief localized depolarizations and as propagating waves of excitation. Both sensory-evoked PSPs and APs are inhibited during this spontaneous activity. We thus propose that the representation of the actual sensory periphery competes with ongoing intracortical processing.
Acknowledgments
We thank Drs. Michael Brecht, Fritjof Helmchen, Hartwig Spors, and Jack Waters for technical help and scientific discussion, and Ms. Rina Hildesheim for the kind gift of RH1691.
Abbreviations: AP, action potential; VSD, voltage-sensitive dye; WC, whole cell; PSP, postsynaptic potential.
References
- 1.Steriade, M., Nunez, A. & Amzica, F. (1993) J. Neurosci. 13, 3252-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowan, R. L. & Wilson, C. J. (1994) J. Neurophysiol. 71, 17-32. [DOI] [PubMed] [Google Scholar]
- 3.Timofeev, I., Grenier, F. & Steriade, M. (2001) Proc. Natl. Acad. Sci. USA 98, 1924-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lampl, I., Reichova, I. & Ferster, D. (1999) Neuron 22, 361-374. [DOI] [PubMed] [Google Scholar]
- 5.Arieli, A., Shoham, D., Hildesheim, R. & Grinvald, A. (1995) J. Neurophysiol. 73, 2072-2093. [DOI] [PubMed] [Google Scholar]
- 6.Arieli, A., Sterkin, A., Grinvald, A. & Aertsen, A. (1996) Science 273, 1868-1871. [DOI] [PubMed] [Google Scholar]
- 7.Tsodyks, M., Kenet, T., Grinvald, A. & Arieli, A. (1999) Science 286, 1943-1946. [DOI] [PubMed] [Google Scholar]
- 8.Timofeev, I., Contreras, D. & Steriade, M. (1996) J. Physiol. 494, 265-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azouz, R. & Gray, C. M. (1999) J. Neurosci. 19, 2209-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen, C. C. H., Grinvald, A. & Sakmann, B. (2003) J. Neurosci. 23, 1298-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta, M. R., Quirk, M. C. & Wilson, M. A. (2000) Neuron 25, 707-715. [DOI] [PubMed] [Google Scholar]
- 12.Moore, C. I. & Nelson, S. B. (1998) J. Neurophysiol. 80, 2882-2892. [DOI] [PubMed] [Google Scholar]
- 13.Pare, D., Shink, E., Gaudreau, H., Destexhe, A. & Lang, E. J. (1998) J. Neurophysiol. 79, 1450-1460. [DOI] [PubMed] [Google Scholar]
- 14.Derdikman, D., Hildesheim, R., Ahissar, E., Arieli, A. & Grinvald, A. (2003) J. Neurosci. 23, 3100-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson, A. M. (1997) J. Physiol. 502, 131-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott, L. F., Varela, J. A., Sen, K. & Nelson, S. B. (1997) Science 275, 220-224. [DOI] [PubMed] [Google Scholar]
- 17.Galaretta, M. & Hestrin, S. (1998) Nat. Neurosci. 1, 587-594. [DOI] [PubMed] [Google Scholar]
- 18.Reyes, A., Lujan, R., Rozov, A., Burnashev, N., Somogyi, P. & Sakmann, B. (1998) Nat. Neurosci. 1, 279-285. [DOI] [PubMed] [Google Scholar]
- 19.Markram, H., Wang, Y. & Tsodyks, M. (1998) Proc. Natl. Acad. Sci. USA 95, 5323-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung, S., Li, X. & Nelson, S. B. (2002) Neuron 34, 437-446. [DOI] [PubMed] [Google Scholar]
- 21.Petersen, C. C. H. (2002) J. Neurophysiol. 87, 2904-2914. [DOI] [PubMed] [Google Scholar]