Abstract
Semantic dementia (SD) is a neurodegenerative disease characterized by atrophy of anterior temporal regions and progressive loss of semantic memory. SD patients often present with surface dyslexia, a relatively selective impairment in reading low-frequency words with exceptional or atypical spelling-to-sound correspondences. Exception words are typically ‘over-regularized’ in SD and pronounced as they are spelled (e.g. ‘sew’ is pronounced as ‘sue’). This suggests that in the absence of sufficient item-specific knowledge, exception words are read by relying mainly on subword processes for regular mapping of orthography to phonology. In this study, we investigated the functional anatomy of surface dyslexia in SD using functional magnetic resonance imaging (fMRI) and studied its relationship to structural damage with voxel-based morphometry (VBM). Five SD patients and nine healthy age-matched controls were scanned while they read regular words, exception words and pseudowords in an event-related design. Vocal responses were recorded and revealed that all patients were impaired in reading low-frequency exception words, and made frequent over-regularization errors. Consistent with prior studies, fMRI data revealed that both groups activated a similar basic network of bilateral occipital, motor and premotor regions for reading single words. VBM showed that these regions were not significantly atrophied in SD. In control subjects, a region in the left intraparietal sulcus was activated for reading pseudowords and low-frequency regular words but not exception words, suggesting a role for this area in subword mapping from orthographic to phonological representations. In SD patients only, this inferior parietal region, which was not atrophied, was also activated by reading low-frequency exception words, especially on trials where over-regularization errors occurred. These results suggest that the left intraparietal sulcus is involved in subword reading processes that are differentially recruited in SD when word-specific information is lost. This loss is likely related to degeneration of the anterior temporal lobe, which was severely atrophied in SD. Consistent with this, left mid-fusiform and superior temporal regions that showed reading-related activations in controls were not activated in SD. Taken together, these results suggest that the left inferior parietal region subserves subword orthographic-to-phonological processes that are recruited for exception word reading when retrieval of exceptional, item-specific word forms is impaired by degeneration of the anterior temporal lobe.
Keywords: semantic dementia, dyslexia, parietal lobe, voxel-based morphometry, functional MRI
Introduction
Semantic dementia (SD) is a clinical syndrome characterized by progressive deterioration of semantic knowledge and anatomical damage to anterior and inferior temporal regions (Pick, 1892; Snowden et al., 1989; Hodges et al., 1992; Neary et al., 1998; see Hodges and Patterson, 2007 for review). Patients typically present with a multimodal semantic impairment and profound anomia, but speech fluency, phonology and expressive and receptive syntax are relatively spared (Snowden et al., 1989; Hodges et al., 1992; Gorno-Tempini et al., 2004). Temporal lobe atrophy in SD is usually bilateral, but more extensive in the left hemisphere. Lateral and medial anterior regions are consistently affected, including the perirhinal cortices and fusiform gyri (Mummery et al., 2000; Chan et al., 2001; Galton et al., 2001; Rosen et al., 2002; Gorno-Tempini et al., 2004). Pathologically, SD is most often associated with ubiquitin- and TDP-43-related changes (Davies et al., 2005; Snowden et al., 2007).
Patients with SD frequently present with surface dyslexia; they are selectively impaired in reading words with exceptional spelling-to-sound correspondences such as sew or plaid, whereas they perform well in reading orthographically regular words as well as pseudowords such as doost or bonverse (Patterson and Hodges, 1992; Graham et al., 2000; Gorno-Tempini et al., 2004; Jefferies et al., 2004; Patterson et al., 2006; Woollams et al., 2007). Patients with SD and surface dyslexia typically ‘over-regularize’ exception words by reading them as they are spelled. For example, sew is pronounced as sue and plaid is read as played. Low-frequency exception words are far more affected, but errors can also occur on high-frequency words in more severe cases, as the disease progresses (Woollams et al., 2007). Recent work has suggested that surface dyslexia in SD is not an isolated phenomenon, but it is rather a reflection of a more general loss of semantic information in which idiosyncratic, item-specific knowledge is degraded, especially for less frequent items. This leads to a distinctive error pattern in which the characteristics of prototypical structures of a given domain (e.g. words or objects) are over-extended to idiosyncratic items that were previously retrieved from memory (Patterson et al., 2006).
While SD patients are impaired in reading exception words (surface dyslexia), there are other patients who are selectively impaired in reading pseudowords; this is termed phonological dyslexia (Marshall and Newcombe, 1973). The double dissociation between these two forms of dyslexia suggests that there are at least two types of processes involved in reading: subword processes where regular orthographic-to-phonological mappings are employed and whole-word processes where idiosyncratic item-specific information about the pronunciation of a particular word is retrieved. While there is consensus on this basic distinction, models differ as to whether they postulate two distinct reading-specific pathways (e.g. Coltheart et al., 1993) or a graded division of labour between orthographic-to-phonological and semantic-to-phonological pathways within a language-general architecture (Seidenberg and McClelland, 1989; Patterson and Hodges, 1992; Plaut et al., 1996; Woollams et al., 2007).
Since readers do not know a priori whether a word is regular, irregular, or even a real word, subword and whole-word processes are likely to be employed in parallel, at least initially. However, different classes of words ultimately make differential demands on subword and whole-word processes. Since pseudowords do not have item-specific information, they rely entirely on subword processes. Regular words are likely to tap both kinds of processes since they can be correctly pronounced on the basis of typical orthographic-to-phonological mappings, but can also be retrieved from memory, especially when they are high in frequency. In contrast, exception words can only be pronounced correctly when idiosyncratic word-specific information is retrieved, thus they depend upon whole-word processes. The ‘over-regularization’ of exception words in SD suggests that failure to retrieve item-specific information results in the application of subword processes in cases where idiosyncratic information should have supplanted regular forms (Patterson and Hodges, 1992; Plaut et al., 1996; Graham et al., 2000; Jefferies et al., 2004; Patterson et al., 2006; Woollams et al., 2007).
The double dissociation between surface dyslexia and phonological dyslexia implies that different brain regions within the overall reading network are differentially involved in subword and whole-word processes. Broadly, the available data indicate that left inferior parietal regions and the left posterior inferior frontal gyrus (IFG) are more involved in subword orthographic-to-phonological processes (Pugh et al., 2001; Jobard et al., 2003; Mechelli et al., 2003; Schlaggar and McCandliss, 2007). In contrast, whole-word processes are more dependent on anterior temporal regions, as demonstrated by the prevalence of surface dyslexia in SD, and in particular, correlations between semantic deficits and exception word reading deficits (Patterson and Hodges, 1992; Graham et al., 1994; McKay et al., 2007; Woollams et al., 2007). While the mid-fusiform gyrus (approx. y = –50 to –60) is important for reading in general (Cohen et al., 2000; Price and Devlin, 2003), more anterior fusiform regions (approx. y = –20 to –50) may be differentially involved in semantic and whole-word processes (Cohen et al., 2002; Price and Mechelli, 2005; Mechelli et al., 2005) along with an anterior sector of the IFG (Mechelli et al., 2005).
Only one functional neuroimaging study has examined the neural basis of reading in SD. Price and colleagues used positron emission tomography (PET) to scan a single SD patient reading aloud. They reported increased activity relative to controls in left premotor cortex and reduced activity in the anterior fusiform gyrus and other temporal regions (Price et al., 2003; Price and Mechelli, 2005). Their interpretation was consistent with decreased access to whole-word processes and increased reliance on subword processes.
In this study, we used functional magnetic resonance imaging (fMRI) to scan five SD patients and nine healthy age-matched control subjects as they read regular words, exception words and pseudowords. The pattern of atrophy in the same patients was analysed using voxel-based morphometry (VBM). We hypothesized that, when reading exception words, activity in anatomically spared regions involved in subword orthographic-to-phonological processes would be greater in SD patients than in controls, as patients would make greater demands on subword processes for reading exception words. We also expected to find reduced activity in the fusiform gyrus, since this region is known to be both involved in reading, as well as at least partially atrophied in SD. This experiment is a novel application of functional imaging to a patient population, in that we aimed to explore the neural basis of a cognitive function (exception word reading) that is neither simply impaired nor spared, but rather is systematically abnormal in a potentially informative way.
Methods
Subjects
Five patients with SD were successfully scanned with fMRI within an 18-month period. Patients were recruited through the Memory and Aging Center at the University of California, San Francisco (UCSF) and were diagnosed with SD based on published criteria (Neary et al., 1998) by a multi-disciplinary team of neurologists, neuropsychologists, neuropsychiatrists and nurses after a comprehensive evaluation including neurological history and examination, and neuropsychological testing of memory, executive function, visuospatial skills, language and mood. Neuroimaging results were not used to make the SD diagnosis, but were used to exclude other causes of brain damage, such as strokes or tumours. Besides being diagnosed with SD, patients were required (i) to score at least 15 out of 30 on the Mini-Mental Status Exam (MMSE); (ii) to be fluent in English; (iii) to read regular words and pseudowords better than exception words, as assessed by the Psycholinguistic Assessments of Language Processing in Aphasia (PALPA; Kay et al., 1992); and (iv) to be otherwise sufficiently functional to be scanned.
Nine SD patients met the first two criteria and were thus considered for the fMRI study. All the nine patients showed a pattern of surface dyslexia and thus met the third criterion. However, one patient was judged too behaviourally impaired to undergo scanning, and one was unable to be scheduled. Functional imaging data were acquired for the seven remaining patients; however, among these patients the data were unusable for two due to technical problems. Thus, imaging data were successfully acquired and analyzed for five SD cases. Demographic information and neuropsychological data for these five patients are shown in Table 1. All patients were fluent in English; four were native speakers, whereas one was a native speaker of German who had attended an English-speaking school in her teens and spoke fluently with only a slight accent.
Table 1.
Demographic, functional and neuropsychological characteristics of the subjects
| Characteristics | SD (N = 5) | Control (N = 9) |
|---|---|---|
| Demographic | ||
| Age | 61.4 (4.8) | 65.7 (11.8) |
| Sex (F/M) | 4/1 | 7/2 |
| Education | 14.2 (1.8)* | 18.0 (2.2) |
| Handedness (R/L) | 4/1 | 7/2 |
| Status | ||
| MMSE (30) | 24.2 (4.8)* | 29.7 (0.7) |
| CDR total | 0.8 (0.3)* | 0.0 (0.0) |
| Years from first symptom | 5.0 (1.7) | N/A |
| Language production | ||
| Phonemic fluency | 7.4 (2.1)* | 14.7 (3.1) |
| Semantic fluency | 5.4 (3.6)* | 24.9 (5.6) |
| Boston naming test (15) | 2.8 (0.8)* | 15.0 (0.0) |
| Speech fluency (WAB, 10) | 8.8 (0.8) | |
| Apraxia of speech rating (7) | 0 (0) | |
| Dysarthria rating (7) | 0 (0) | |
| Repetition (WAB, 100) | 79.2 (21.5) | |
| Language comprehension | ||
| Word recognition (WAB, 60) | 54.6 (3.4) | |
| Sequential commands (WAB, 80) | 76.0 (3.3) | |
| Syntactic comprehension (CYCLE, 55) | 50.8 (5.0) | |
| Pyramids and palm trees— pictures (52) | 39.6 (6.3) | |
| Reading | ||
| PALPA regular words (30) | 26.2 (4.1) | |
| PALPA exception words (30) | 19.6 (4.4) | |
| PALPA pseudowords (24) | 17.8 (4.9) | |
| Visuospatial function | ||
| Modified Rey-Osterrieth copy (17) | 14.8 (1.9) | 15.6 (0.9) |
| Visual memory | ||
| Modified Rey-Osterrieth delay (17) | 7.0 (6.0) | 12.2 (3.2) |
| Verbal memory | ||
| CVLT-MS trials 1–4 | 12.2 (6.6)* | 30.5 (5.4) |
| CVLT-MS 30 s free recall | 1.0 (1.7)* | 8.3 (1.2) |
| CVLT-MS 10 min free recall | 1.0 (1.7)* | 7.7 (2.3) |
| Executive | ||
| Digit span backwards | 4.4 (1.1) | 4.8 (0.7) |
| Modified trail lines per minute | 28.1 (12.8) | 43.9 (14.3) |
| Calculation | 4.6 (0.5) | 4.8 (0.4) |
Scores shown are mean (standard deviation). Asterisks indicate values significantly different from controls (P < 0.05). MMSE = Mini-Mental State Exam; CDE = Clinical Dementia Rating; WAB = Western Aphasia Battery; CYCLE = Curtiss-Yamada Comprehensive Language Examination; PALPA = Psycholinguistic Assessments of Language Processing in Aphasia; CVLT-MS = California Verbal Learning Test––Mental Status.
Nine healthy age-matched control subjects were also scanned with fMRI. Control subjects were verified as normal on the basis of a neurological exam, neuropsychological testing and MRI. Demographic information and neuropsychological data for the control subjects are also shown in Table 1. Although control subjects had significantly more education than SD patients, this would not account for the dramatic disparities observed in reading abilities that were subsequently observed.
VBM was performed by comparing the five SD patients who were included in the functional imaging study to a larger control group of 48 subjects screened similarly [mean age 61.5 years (s.d. 10.3); 38 females, 10 males].
All participants gave written informed consent according to the Declaration of Helsinki, and the study was approved by the Committee on Human Research at UCSF.
Experimental design
Participants lay supine in the scanner and viewed a screen through a mirror. There were two functional runs, each 379.5 s in duration. During each run, the subjects were presented with 20 regular words, 20 exception words, 10 pseudowords and 10 false font strings in a rapid event-related design. These words were presented in large white type on a black background. The subjects were instructed to read each word out loud and to say ‘yes’ when they saw the false font strings. It was emphasized that words should be read as quickly as possible, but that head movement should be minimized. Each word was presented for 3.5 s. The time between word onsets varied randomly and ranged from 4.5 to 10.5 s. Stimuli were presented by means of a PC laptop using E-prime (Psychology Software Tools, Inc., Pittsburgh, PA).
Stimuli
The stimuli consisted of 40 regular words (in which pronunciation was entirely predictable based on the rules of English orthography), 40 exception words (in which pronunciation did not follow directly from the orthography), 20 pseudowords (which could be read by applying orthographic rules) and 20 false font strings (i.e. sequences of non-orthographic symbols). The false font condition was not ultimately used in the analyses reported (see below).
The regular words and exception words were divided into high-frequency (HF) and low-frequency (LF) sets, each containing 20 items. Frequency (Kucera and Francis, 1967) was matched for the regular and exception words [HF: T(38) = 0.07; P = 0.94; LF: T(38) = 0.15; P = 0.88], and length was matched for all categories [F(5, 114) = 0.34; P = 0.89]. Examples of the stimuli in each category, along with frequency and length measures, are shown in Table 2.
Table 2.
Characteristics of stimuli
| Condition | Abbreviation | Frequency | Letters | Examples |
|---|---|---|---|---|
| Regular high frequency | Reg HF | 316.7 (228.5) | 5.9 (1.8) | mouth, problem |
| Regular low frequency | Reg LF | 6.9 (5.4) | 6.1 (1.6) | coil, friction |
| Exception high frequency | Exc HF | 311.4 (223.9) | 6.0 (2.1) | once, although |
| Exception low frequency | Exc LF | 7.3 (8.8) | 5.9 (1.4) | plaid, chassis |
| Pseudowords | Pseudo | N/A | 5.7 (1.5) | doost, bonverse |
| False font strings | FF | N/A | 5.5 (1.1) | N/A |
Values shown are mean (standard deviation). The frequency measure is the Kucera–Francis written frequency count (Kucera and Francis, 1967).
The pseudowords were generated by changing one or two letters in the regular words, thus matching them closely to the regular words in terms of phonotactic structure.
Prior to scanning, participants were trained on a similar but non-overlapping set of stimuli.
Behavioural data
The responses of the subjects were recorded using a dual-channel scanner-compatible microphone (Optoacoustics, Or-Yehuda, Israel) and the bundled software (OptiMRI), which was used to filter out the scanner noise. After filtering, the responses were clearly intelligible, and reaction times and responses were determined manually using Audacity (http://audacity.sourceforge.net).
The responses to the regular and exception word stimuli were scored as correct if they were entirely correct. Incorrect responses to the exception words were further classified as either ‘over-regularizations’, or as other errors. Over-regularizations were defined as responses that were clearly prejudiced by the orthographic form of the word. They were not required to reflect an exact application of regular orthographic-to-phonological rules to the irregular word form. For instance, a reading of ghoul as [goʊl] (to rhyme with hole) would be counted as an over-regularization, even though orthographic-to-phonological rules dictate that ou should be pronounced as [aʊ], not [oʊ]. The responses to the pseudowords were also counted as correct even when they had minor deviations from the expected forms. For instance, pronunciation of bome as [bum] was considered correct even though it should be [boʊm]; this is because the correct and substituted vowels are similar, both being back rounded vowels. However, more substantial errors such as pronouncing reept as [rǝspit] were counted as errors.
The statistical analysis of percent correct and reaction time was assessed with JMP (SAS Institute, Cary, NC) using repeated-measures ANOVAs (analysis of variances).
The false font condition was intended as a baseline for the other reading conditions. However, despite training, some patients did not respond ‘yes’ to these stimuli as instructed. One patient did not respond at all, while another produced gibberish responses in an apparent attempt to depict the unintelligible visual stimulus. Therefore, in our analyses, we did not use data from the false font condition. Binder et al. (2005) also presented subjects with false font stimuli, but did not find it informative to report the data from this condition.
Image acquisition
Functional imaging data were acquired with a 3T GE Signa scanner (GE, Milwaukee, WI) at the UCSF Department of Radiology. The manufacturer's head coil was equipped with a backprojection screen and first surface mirror for presentation of visual stimuli, and the head of the subject was thoroughly padded in the coil to reduce head motion. An automated high-order shimming method based on spiral acquisitions was employed to reduce B0 heterogeneity. For each run, 232 functional T2*-weighted images were acquired with the following parameters: 23 axial slices in a sequential (bottom to top) order; slice thickness = 4 mm with 1 mm gap; field of view (FOV) = 220 mm; matrix 64 × 64; voxel size 3.4 × 3.4 × 5 mm3; repetition time (TR) = 1650 ms; echo time (TE) = 30 ms. Images were acquired using a spiral-in/out pulse sequence (Glover and Law, 2001; Preston et al., 2004).
Structural images were acquired on a 1.5T Siemens Magnetom Vision system (Siemens, Iselin, NJ) equipped with a standard quadrature head coil. A volumetric magnetization prepared rapid gradient echo (MPRAGE) sequence was used to acquire T1 images of the whole brain (164 coronal slices; slice thickness = 1.5 mm; FOV = 256 mm; matrix 256 × 256; voxel size 1.0 × 1.5 × 1.0 mm3; TR = 10 ms; TE = 4 ms; flip angle = 15°). The larger group of 48 control subjects, who were used for the VBM analysis, were also scanned on this structural sequence.
VBM
To identify regions of atrophy, the five SD patients were compared to 48 normal control subjects using VBM, implemented in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5; Friston et al., 2007) running under MATLAB 7.4 (Mathworks, Natick, MA). All T1 structural images were segmented, bias corrected and spatially normalized to MNI space using a unified segmentation procedure (Ashburner and Friston, 2005). The VBM analysis was based on modulated grey matter images, whose grey matter value in each voxel was multiplied by the Jacobian determinant derived from the spatial normalization in order to preserve the total amount of grey matter from the original images. These modulated grey matter images were smoothed with a Gaussian kernel (8 mm FWHM). A general linear model (GLM) was then fit at each voxel, with one variable of interest (group), and three confounds of no interest: sex, age and total intracranial volume (calculated by summing across the grey matter, white matter and CSF images, all modulated). The resulting statistical parametric map (SPM) was thresholded at voxel-wise P < 0.001, and then corrected for multiple comparisons at P < 0.05 based on cluster extent and Gaussian Random Field (GRF) theory. A correction for non-stationary smoothness was applied (Hayasaka et al., 2004) using the implementation of this method in the VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm), since this is necessary to avoid false positives with VBM (Ashburner and Friston, 2000).
All results (for VBM as well as for the fMRI analyses described below) were displayed with MRIcron (version beta 9/6/07, http://www.sph.sc.edu/comd/rorden/mricron). Functional data were overlaid on a high resolution T1 image of a single subject, and anatomical labels in tables were determined based on visual inspection of the data with reference to the atlas of Duvernoy (1999).
Analysis of functional imaging data
Preprocessing
The functional data were preprocessed using standard methods implemented in SPM5. The first two volumes from each run were discarded to allow for T1 equilibrium effects. Slice-timing correction was used to resample all slices to the acquisition time of the reference (middle) slice. To account for head motion, realignment was performed using six-parameter rigid body transformations, and the three translation and three rotation parameters for each volume were saved. The data were also ‘unwarped’ to account for susceptibility-by-motion interactions. The mean functional image was then coregistered with the structural image using a rigid body transformation. Structural images were segmented, bias corrected and spatially normalized to Montreal Neurological Institute (MNI) space using a unified segmentation procedure (Ashburner and Friston, 2005). The resulting parameters were then applied to the functional images to normalize them to MNI space. Finally, functional images were smoothed with a Gaussian kernel (8 mm FWHM).
Model fitting
For each subject, a GLM was fit to the data at each voxel using SPM5. The two functional runs were high-pass filtered at 128 s to account for slow drift, then concatenated. The design matrix contained one explanatory variable (EV) per run for each condition (Reg HF, Reg LF, Exc HF, Exc LF, Pseudo, FalseFont; see Table 2 for abbreviations of condition names). Each EV was convolved with a canonical hemodynamic response function (HRF). In addition, there were 12 motion-related covariates (six from each run, saved during the realignment step), and two variables encoding the means of the two runs, none of which were convolved with the HRF. Model parameters were estimated using restricted maximum likelihood (ReML) using an autoregressive AR(1) model to correct for non-sphericity arising from serial correlations.
Basic activation maps for reading in the control and SD groups separately
We first analysed signal increases for each of the five conditions versus rest in the control and SD groups separately. These analyses identified the overall pattern of activations in controls and patients and were used to confirm that our experimental paradigm produced, especially in controls, results consistent with those of previous studies. Random effects analyses were used to reveal regions that were reliably activated in each population, while fixed effects analyses were also included to emphasize similarities in the networks activated in the two groups.
Random effects models were based on the coefficients derived from the first-level analysis of each subject. SPMs were first thresholded at P < 0.01 at the voxel level. The correction for multiple comparisons was based on cluster extent. Five bilateral a priori regions-of-interest (ROIs) that have been frequently activated in prior fMRI studies on single word reading (Turkeltaub et al., 2002; Jobard et al., 2003; Binder et al., 2005; Price and Mechelli, 2005) were selected; these regions were occipital cortex, mid-fusiform gyrus, sensorimotor cortex, superior temporal gyrus (STG) and inferior parietal cortex. For each of these regions, we identified the relevant cluster and determined its probability based on spatial extent, following the method of Friston (1997) for testing anatomically specified regional effects. Specifically, the relevant cluster was identified as the cluster nearest to the peak reported by Binder et al. (2005); this is because this study had the design most similar to the present one. These P-values were then corrected for multiple comparisons (i.e. the 10 comparisons: one for each ROI) at P < 0.05 using the false discovery rate procedure (Benjamini and Hochberg, 1995). In other brain regions, clusters were required to be significant at P < 0.05 corrected based on GRF theory.
Fixed effects analyses were carried out in each group by concatenating the data across subjects. Fixed effects SPMs were thresholded at P < 0.001 voxel-wise, and then corrected based on cluster size for both the groups at P < 0.05 based on GRF theory. A high voxel-wise threshold is possible because of the additional power afforded by concatenating runs across subjects, although the results cannot be generalized beyond the subjects studied.
Group differences in activation for reading in general
Differences in signal change between SD patients and controls for reading in general (i.e. irrespective of word type) were examined with ROI analyses of four left hemisphere regions: occipital cortex, the mid-fusiform gyrus, sensorimotor cortex and the STG. These four regions were selected because each had been frequently activated in prior studies on single word reading (Turkeltaub et al., 2002; Jobard et al., 2003; Binder et al., 2005; Price and Mechelli, 2005) and each was prominently activated under all conditions in the control group in the present study. The local maximum in each region was identified in the contrast of all words versus rest in the control group. Then, the signal change was extracted and plotted as a function of group and word type using custom MATLAB scripts. Statistical significance was assessed with JMP. A whole-brain random effects analysis of the difference between groups for the contrast of all words versus rest was also carried out to ensure that there were no additional regions with reliable between-group differences.
Interaction of group by word type
Our primary aim was to identify regions that are important for subword processes and that are differentially recruited by patients with SD when reading exception words. This was examined with a random effects contrast for the interaction of group (control, SD) by word type (Exc LF, Pseudo). Pseudowords were compared with exception words rather than regular words; this is because exception words must be retrieved from memory and cannot be read correctly only on the basis of subword processes, whereas regular words are ambiguous because either they could be read by subword processes or their phonological representations could be retrieved from memory. Low-frequency exception words in particular were used since the reading of these words is more comparable in reaction time (and presumably difficulty) to pseudowords (Binder et al., 2005). This interaction contrast was examined only in areas where (i) Pseudo > Exc LF (in controls) and (ii) Exc LF (in SD) > Exc LF (in controls). These two inclusive masks ensured that the interaction would be driven by (i) subword processes in controls and (ii) increased responses in SD patients for exception word reading, respectively. The two masks were thresholded at voxel-wise P < 0.05 uncorrected, with a minimum cluster size of 100 voxels, and then they were applied to the main contrast, which was subsequently thresholded at voxel-wise P < 0.01, and cluster size P < 0.05 corrected based on GRF theory. The correction was based on the volume of the Pseudo > Exc LF mask (in controls) (129 136 mm3), since the search was restricted to that region by the mask. Note that the second mask was not used to restrict the search volume since that would be circular as the second mask, unlike the first, depends upon the patient data. In the region revealed by this contrast, signal change was extracted and plotted as a function of group and word type as abovementioned.
For the SD patients, a second analysis was performed where trials were categorized based on actual recorded responses. Correct trials were modelled as described above, but incorrect trials were modelled with one of two additional EVs, that were added to the model: one for over-regularizations and one for miscellaneous other errors. Signal change was then plotted for each of the five word types when correct as well as for these two additional categories of trials.
Additional ROI analysis of the inferior frontal gyrus
Because different inferior frontal regions have been identified in prior studies as important for both subword and whole-word processes (Mechelli et al., 2005), we carried out additional ROI analyses to examine responses in these areas in SD patients and in controls. In each case, ROIs were identified solely based on the data from the control group. Regions within the IFG that were potentially differentially involved in subword processes were identified with the contrast (in controls) of Pseudo > Exc LF, inclusively masked with Pseudo > rest. IFG regions that were potentially more involved in whole-word reading processes were identified with the contrast of Exc LF > Reg LF, inclusively masked with Exc LF > rest.
Results
Behavioural data
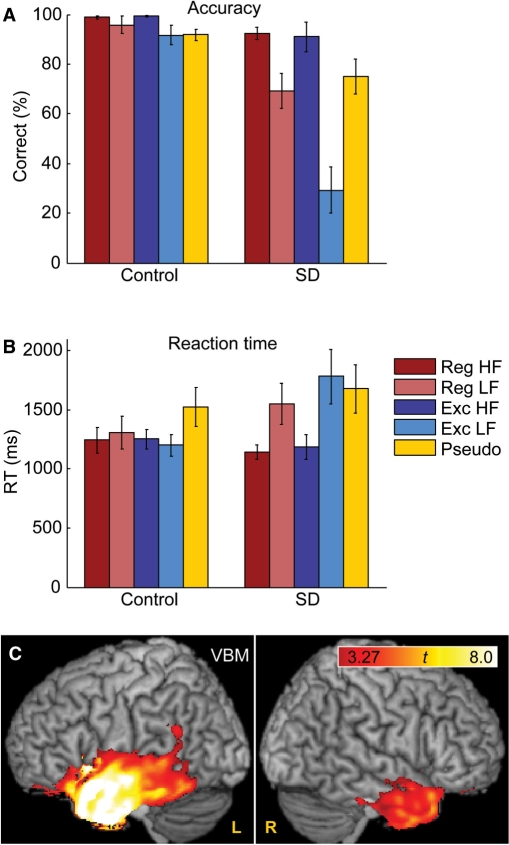
All the responses of the subjects in the scanner were recorded and transcribed, and accuracy (Fig. 1A) and reaction time (Fig. 1B) were compared across groups and conditions. For accuracy, there were significant main effects of group [F(1, 14) = 29.92, P < 0.0001] and condition [F(4, 14) = 20.17, P < 0.0001], and a significant interaction of group by condition [F(4, 14) = 14.84, P = 0.0002]. This was driven primarily by patients’ poor performance on low-frequency exception words, as expected. All patients made numerous over-regularization errors on low-frequency exception words, whereby exception words were pronounced based on their orthographic forms as if they were regular. The mean number of such errors was 9.6 out of 20 (s.d. 3.8, range 5–15). In contrast, few such errors were made with high-frequency exception words (mean 0.8, s.d. 0.8, range 0–2). Presumably, high-frequency words have more redundant neural representations and are less vulnerable to atrophy of crucial regions. Performance on atypical items in many domains is strongly modulated by frequency in SD (Patterson et al., 2006). Control subjects made very few over-regularization errors (mean 0.4, s.d. 0.5, range 0–1), all of which were on low-frequency words. Patients were also worse than controls at reading low-frequency regular words and pseudowords, although these deficits were much less severe than their difficulties with low-frequency exception words.
Fig. 1.
Behavioural data and VBM. (A) Accuracy in single word reading in each condition, in controls and patients with SD. See Table 2 for abbreviations of condition names. (B) Reaction time in each condition, in controls and patients with SD. (C) Regions showing significantly reduced grey matter volumes in SD patients relative to 48 normal controls, as revealed by VBM.
For reaction time, neither the main effects of group (P = 0.52) nor condition (P = 0.25) nor their interaction (P = 0.29) were significant. In controls, there was a trend for pseudoword trials to be slower than the other four trial types, and in patients, there was a trend for slower responses to pseudowords and both low-frequency word types. Reaction times in both groups were considerably slower than those typically observed in behavioural studies (e.g. Strain et al., 1998); this likely reflects the unfamiliar scanner environment, and the fact that subjects had been asked to minimize head movement, which could have slowed their responses (c.f. Binder et al., 2005).
VBM
SD patients had reduced grey matter volumes in a number of regions bilaterally including the temporal poles, amygdala, hippocampus, anterior STG, anterior fusiform gyrus, insula and caudate nucleus (Fig. 1C, Table 3). Atrophy in each of these regions was more extensive in the left hemisphere than in the right hemisphere. In the left hemisphere, it extended caudally to the mid-fusiform gyrus.
Table 3.
Regions of significant atrophy in the SD group
| Brain region | MNI coordinates |
Extent (mm3) | P | Max T | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Bilateral temporal, insula and subcortical regions | 181 288 | <0.001 | ||||
| Bilateral amygdala/anterior hippocampus | −26 | −4 | −18 | 12.67 | ||
| 24 | 4 | −16 | 6.44 | |||
| Bilateral temporal pole | −46 | 4 | −42 | 10.89 | ||
| −26 | −8 | −38 | 12.41 | |||
| 52 | −2 | −36 | 5.48 | |||
| 30 | 18 | −38 | 5.92 | |||
| Bilateral anterior superior temporal gyrus | −48 | 12 | −16 | 10.24 | ||
| 44 | 20 | −24 | 5.77 | |||
| Bilateral anterior fusiform gyrus | −32 | −26 | −26 | 9.18 | ||
| 58 | −32 | −26 | 4.29 | |||
| Left mid-fusiform gyrus | −34 | −50 | −16 | 3.72 | ||
| Bilateral insula | −36 | −8 | −2 | 9.32 | ||
| 40 | −4 | −8 | 4.52 | |||
| Left caudate nucleus | −6 | 8 | −2 | 7.53 | ||
| Left medial frontal | −8 | 54 | 16 | 1368 | 0.035 | 4.71 |
P-values are corrected based on cluster extent, whereas Max T is the maximum T statistic of each local maximum.
Functional imaging data
Basic activation maps for reading in the control and SD groups separately
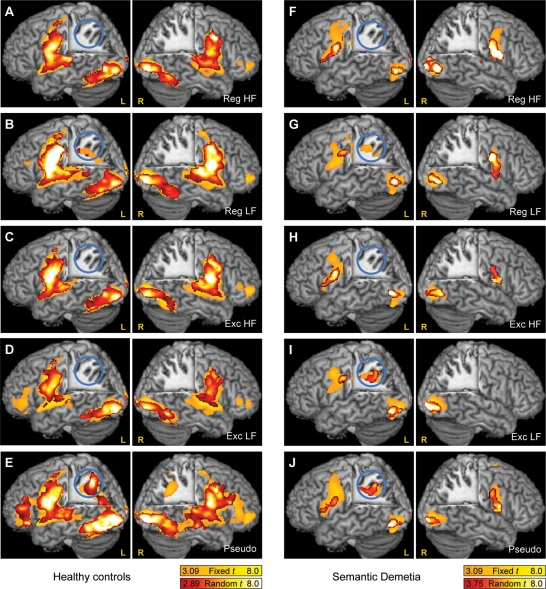
First, the activation for each condition versus rest was examined in two separate analyses for the control group and the SD patients. Random effects analyses (red to white) were used to reveal reliably activated regions (Fig. 2, Table 4), while fixed effects analyses (orange to yellow) were also included to emphasize the similarities in the networks activated in the two groups (Fig. 2).
Fig. 2.
Basic networks activated by reading single words of each type in controls (A–E) and SD patients (F–J). Fixed effects analyses are shown in the yellow-to-orange colour scale, and random effects analyses in the red-to-white colour scale. Although fixed effects do not allow for inference to the general population, fixed effects results are presented to emphasize the similarities in reading networks in the two groups. The cut-out region is defined by the planes x = ±28, y = –26 and z = +18. The blue circle shows the left intraparietal sulcus.
Table 4.
Regions activated by reading each word type in the control and SD groups
| Brain area | Control group | SD group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates | Extent (mm3) | P | Max T | MNI coordinates | Extent (mm3) | P | Max T | |||||
| x | y | z | x | y | z | |||||||
| Reg HF | ||||||||||||
| Left sensorimotor | −54 | −8 | 30 | 24 240 | <0.001 | 8.73 | −52 | −6 | 28 | 3768 | <0.001 | 10.45 |
| Left superior temporal | −60 | −26 | 14 | " | " | 8.78 | ||||||
| Right sensorimotor | 60 | −2 | 44 | 18 088 | <0.001 | 18.17 | 62 | 2 | 16 | 4760 | <0.001 | 25.51 |
| Right superior temporal | 68 | −26 | 18 | " | " | 5.70 | 42 | −32 | 6 | 856 | 0.019 | 24.46 |
| Left occipital | −32 | −94 | −6 | 18 072 | <0.001 | 10.35 | −38 | −90 | −6 | 1224 | 0.006 | 12.63 |
| Left mid-fusiform | −28 | −62 | −18 | " | " | 7.41 | ||||||
| Right occipital | 26 | −92 | −4 | 11 568 | <0.001 | 10.05 | 38 | −86 | 2 | 2616 | <0.001 | 33.81 |
| Right mid-fusiform | 36 | −60 | −16 | " | " | 6.84 | ||||||
| Reg LF | ||||||||||||
| Left sensorimotor | −56 | −8 | 28 | 52 360 | <0.001 | 19.25 | −36 | −14 | 38 | 1384 | 0.012 | 6.46 |
| Left superior temporal | −50 | −28 | 10 | " | " | 7.25 | ||||||
| Left intraparietal sulcus | −46 | −40 | 28 | " | " | 6.28 | ||||||
| Right sensorimotor | 56 | −8 | 44 | 36 336 | <0.001 | 16.68 | 46 | −10 | 42 | 3696 | <0.001 | 20.45 |
| Right superior temporal | 66 | −24 | 18 | " | " | 9.21 | ||||||
| Left occipital | −30 | −94 | −8 | 22 120 | <0.001 | 8.39 | −38 | −90 | −2 | 1160 | 0.019 | 26.47 |
| Left mid-fusiform | −36 | −50 | −16 | " | " | 6.34 | ||||||
| Right occipital | 22 | −94 | −2 | 15 736 | <0.001 | 8.98 | 38 | −84 | −4 | 1904 | 0.004 | 18.70 |
| Right mid-fusiform | 34 | −60 | −18 | " | " | 5.81 | ||||||
| Mid-cingulate | −4 | −2 | 38 | 7888 | 0.009† | 6.34 | ||||||
| Exc HF | ||||||||||||
| Left sensorimotor | −62 | −4 | 24 | 26 616 | <0.001 | 10.20 | −50 | −6 | 28 | 2496 | 0.001 | 9.94 |
| Left superior temporal | −50 | −24 | 8 | " | " | 6.81 | ||||||
| Right sensorimotor | 58 | −10 | 20 | 21 992 | <0.001 | 11.90 | 62 | −6 | 18 | 1920 | 0.002 | 8.89 |
| Right superior temporal | 60 | −28 | 12 | " | " | 5.41 | ||||||
| Left occipital | −28 | −96 | −8 | 20 456 | <0.001 | 10.34 | −40 | −84 | 0 | 984 | 0.020 | 21.93 |
| Left mid-fusiform | −36 | −46 | −18 | " | " | 7.93 | ||||||
| Right occipital | 28 | −92 | −4 | 10 920 | <0.001 | 8.29 | 46 | −82 | 0 | 1952 | 0.002 | 9.33 |
| Right mid-fusiform | 32 | −58 | −18 | " | " | 6.35 | ||||||
| Exc LF | ||||||||||||
| Left sensorimotor | −52 | −10 | 28 | 16 088 | <0.001 | 7.22 | −38 | −14 | 38 | 944 | 0.022 | 6.80 |
| Left superior temporal | −40 | −36 | 10 | " | " | 3.44 | ||||||
| Left intraparietal sulcus | −38 | −36 | 32 | 2888 | <0.001 | 9.93 | ||||||
| Right sensorimotor | 60 | −2 | 44 | 11 784 | <0.001 | 6.91 | ||||||
| Right superior temporal | 64 | −24 | 14 | " | " | 3.35 | ||||||
| Left occipital | −28 | −96 | −8 | 15 336 | <0.001 | 11.39 | −38 | −84 | −8 | 1952 | 0.002 | 12.29 |
| Left mid-fusiform | −34 | −48 | −18 | " | " | 6.43 | ||||||
| Right occipital | 28 | −94 | −4 | 9304 | <0.001 | 8.12 | 18 | −100 | −2 | 3384 | <0.001 | 16.57 |
| Right mid-fusiform | 44 | −70 | −12 | " | " | 8.90 | ||||||
| Pseudo | ||||||||||||
| Left sensorimotor | −48 | 4 | 20 | 32 688 | <0.001 | 10.51 | −36 | −14 | 36 | 2480 | 0.002 | 5.84 |
| Left superior temporal | −68 | −30 | 8 | " | " | 6.16 | ||||||
| Right sensorimotor | 56 | −10 | 20 | 30 472 | <0.001 | 9.07 | 62 | 0 | 18 | 2592 | 0.001 | 8.68 |
| Right superior temporal | 60 | −30 | 12 | " | " | 7.29 | ||||||
| Left occipital | −32 | −94 | −6 | 63 592 | <0.001 | 16.90 | −40 | −84 | −10 | 1304 | 0.015 | 14.82 |
| Left mid-fusiform | −34 | −48 | −16 | " | " | 12.67 | ||||||
| Right occipital | 22 | −94 | −4 | " | " | 13.25 | 20 | −98 | −6 | 1840 | 0.005 | 10.01 |
| Right mid-fusiform | 40 | −56 | −16 | " | " | 6.63 | ||||||
| Left intraparietal sulcus | −36 | −50 | 34 | " | " | 7.75 | −26 | −58 | 44 | 2720 | 0.001 | 10.67 |
| Left dorsal parietal | −26 | −60 | 48 | " | " | 11.80 | ||||||
| Left IFG, pars orbitalis | −48 | 30 | −8 | 13 720 | <0.001 | 9.35 | ||||||
| pars triangularis | −46 | 36 | 12 | " | " | 6.23 | ||||||
| Left anterior insula | −32 | 34 | 6 | " | " | 5.70 | ||||||
| Left basal ganglia | −18 | 8 | 6 | " | " | 6.36 | ||||||
P-values were based on cluster extent. In ten a priori regions of interest, P-values were significant for FDR = 0.05. The cluster marked † was not in an ROI and so was significant after whole-brain correction for multiple comparisons. Max T is the maximum T statistic of each local maxima. IFG = inferior frontal gyrus.
In the control group, there were bilateral activations in occipital cortex, the mid-fusiform gyrus, sensorimotor cortex and the STG for each of the five word types. For pseudowords and low-frequency regular words, there was an additional activated region in the left intraparietal sulcus (IPS). This pattern of activation suggests that the left IPS might be differentially involved in subword processes. A large region of left inferior frontal cortex was activated only by pseudowords.
In the SD group, there were bilateral activations in occipital cortex and sensorimotor cortex for each of the five word types (with the exception of the right sensorimotor region, which did not reach significance under the Exc LF condition). There were no activations observed in the mid-fusiform gyrus or the STG either in the random effects or the fixed effects analyses (with the exception of a right superior temporal activation under the Reg HF condition). As in the control group, there were activations in the left IPS for pseudowords and low-frequency regular words (the latter only in the fixed effects analysis). However, unlike the control group, the SD group also showed a statistically significant activation of the left IPS for low-frequency exception words, suggesting possible recruitment of this subword region for exception word reading.
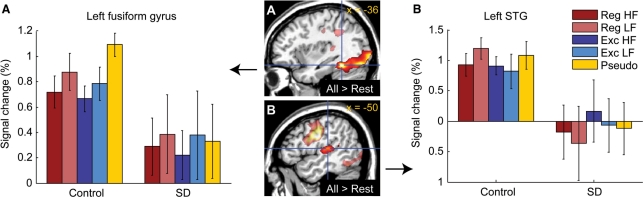
Group differences in activation for reading in general
Signal change was examined as a function of group (control, SD) and word type (Reg HF, Reg LF, Exc HF, Exc LF and Pseudo) in four left hemispheric ROIs consistently activated in prior studies on single word reading. There were significant main effects of group in the mid-fusiform gyrus [Fig. 3A; F(1, 12) = 5.11, P = 0.043] and STG [Fig. 3B; F(1, 12) = 6.72, P = 0.024]. Both regions showed reduced signal in patients, and neither peak voxel was activated above rest in patients [mid-fusiform: F(1, 4) = 1.59, P = 0.28; STG: F(1, 4) = 0.06, P = 0.82]. In contrast, there were no main effects of group in occipital cortex [F(1, 12) = 0.10, P = 0.76] or sensorimotor cortex [F(1, 12) = 3.90, P = 0.07], although the latter showed a trend toward reduced signal in patients. There were no word type effects in any of these regions, nor any interactions of group by word type (all P values were > 0.15), although there was a trend towards greater activation for pseudowords in the mid-fusiform, as has been observed in some previous studies (Mechelli et al., 2003).
Fig. 3.
Group differences in activation for reading in general. (A) The left mid-fusiform gyrus (peak –36, –48, –16) was activated by all words versus rest in controls, but was not activated in SD patients. (B) The left STG (peak –50, –26, 10) was activated by all words versus rest in controls, but was not activated in SD patients.
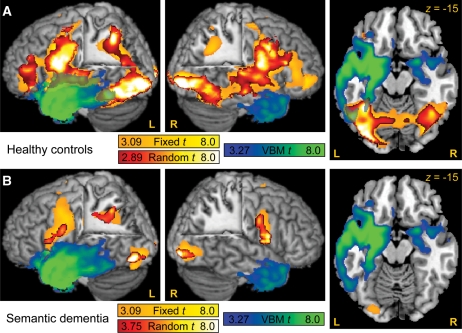
Comparison of VBM and fMRI results revealed that activations in control subjects in the mid-fusiform gyrus and left STG overlapped regions of atrophy in the patient group (Fig. 4A). Likely owing to the degeneration of these regions, the activation in patients did not overlap with regions of atrophy (Fig. 4B). In contrast, occipital and sensorimotor regions were not only functionally normal but appeared to be structurally intact in SD patients.
Fig. 4.
Comparison of VBM and fMRI results. (A) Regions of atrophy in SD patients (blue-to-green colour scale) and regions activated by reading pseudowords in the control group (colour scales as in Fig. 2). The pseudoword condition was selected for illustrative purposes, since this is the only condition in which all regions of interest, including the intraparietal sulcus, were activated in the control group. Overlap was observed in the left mid-fusiform gyrus, and left STG. Note that these two regions were also activated in controls in all real-word conditions of the fMRI study (Fig. 2). (B) Regions of atrophy in SD patients (blue-to-green colour scale) and regions activated by reading pseudowords in the SD group (colour scales as in Fig. 2). There was no overlap, because atrophic regions were not activated in the patient group.
A whole-brain random effects analysis of the difference between groups for the contrast of all words versus rest did not reveal any additional regions that had different overall levels of activation across the two groups, after correction for multiple comparisons.
Interaction of group by word type
Our primary aim was to identify regions important for subword reading processes that are recruited by patients with SD when reading exception words. This was examined by a contrast for the interaction of group (control, SD) by word type (Exc LF, Pseudo), masked with Pseudo > Exc LF (in controls) and Exc LF (in SD) > Exc LF (in controls). The sole region revealed by this contrast was the left intraparietal sulcus (Fig. 5A; MNI peak coordinates =−36, −50, 38; volume = 3992 mm3; cluster P = 0.050; maximum T = 6.57). Signal change in this region was plotted as a function of group and word type (Fig. 5B). By definition, the region showed a significant interaction of group by word type, driven by activation for pseudowords in controls, and an increase in activation under the Exc LF condition in patients relative to controls. Notably, in control subjects, there was also greater signal increase for Reg LF than Exc LF words [F(1, 8) = 9.1115; P = 0.017], although the region was not defined with reference to the Reg LF condition. This provides further support for a role for this region in subword processes because regular words make a greater demand on subword processes than exception words.
Fig. 5.
Interaction of group by word type in the left intraparietal sulcus. (A) The left IPS was the only significant cluster in a whole-brain analysis of regions showing a significant interaction of group (control, SD) by word type (Exc LF, Pseudo). (B) Signal change in each condition in the two groups in this region. (C) Signal change in SD patients for correct responses to the five conditions, over-regularization responses to exception words, and other miscellaneous errors.
We next examined signal in this left IPS region in SD patients after dividing trials based on responses of the subjects (Fig. 5C). There was a trend towards increased signal in the left IPS during those trials on which over-regularization errors occurred, however significance could not be assessed as the number of subjects was less than the number of conditions.
These results suggest that the left IPS is the region that underlies the imperfect compensatory process whereby patients with SD rely to a greater extent on spared subword processes in reading exception words, after access to item-specific information has been lost. Comparison of VBM with fMRI revealed that atrophy did not extend into the parietal lobe, so the left IPS in particular was structurally intact (Fig. 4).
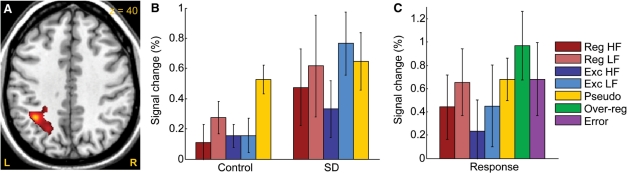
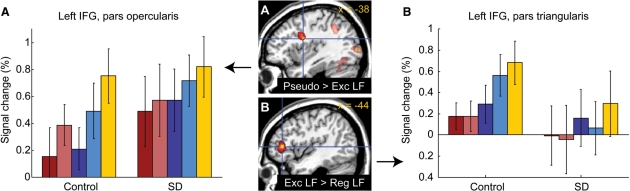
Additional ROI analysis of the inferior frontal gyrus
The contrast of Pseudo > Exc LF (masked with Pseudo > rest) in control subjects was used to identify regions in the IFG potentially involved in subword processes. There was a cluster in the pars opercularis of the IFG and the ventral precentral gyrus (MNI peak coordinates = –38, 2, –24; volume = 4552 mm3; cluster P = 0.069; maximum T = 5.92), which although only marginally significant, was consistent with numerous prior studies on pseudoword reading (Mechelli et al., 2003). In this region, there was a significant main effect of word type [F(4, 9) = 9.81, P = 0.0024], but no main effect of group [F(1, 12) = 0.66, P = 0.43] and no-interaction of group by word type [F(4, 9) = 1.72, P = 0.23]. The pattern of activity across conditions suggests a task difficulty effect, since low frequency words resulted in more activation for both regular and exception words, and pseudowords produced the most activity of all (Binder et al., 2005). The lack of any main effect of group, or any group by word type interaction, suggests that this region is functionally intact in SD patients, and VBM showed that the region is structurally intact (Fig. 4).
To identify regions potentially involved in whole-word processes, we used the contrast Exc LF > Reg LF (masked with Exc LF > rest) in control subjects. There was one small cluster located in the pars triangularis of the IFG (MNI peak coordinates = –44, 36, 6; volume = 496 mm3; cluster P = 1.00; maximum T = 3.32). This cluster was not significant after correction, consistent with prior studies which have failed to consistently identify any regions for similar contrasts (Mechelli et al., 2003). However, it was the only fair-sized cluster in the brain identified by this contrast, and it was close to the locations of activations for similar contrasts in some previous studies (Binder et al., 2005; Mechelli et al., 2005). Signal change was plotted for the peak voxel of this region (Fig. 6B). Neither the main effects of group [F(1, 12) = 1.03, P = 0.33] nor word type [F(4, 9) = 2.42, P = 0.12] were significant, nor was the interaction [F(4, 9) = 0.47, P = 0.75]. However, there was a trend for the activation for low-frequency exception words, apparent in control subjects, to be absent in patients [F(1, 12) = 2.35, P = 0.15]. If this region is involved in retrieval of whole-word phonological forms from the lexicon, this lack of activation in patients could reflect their failure to perform this process normally.
Fig. 6.
(A) The left posterior IFG (peak –38, 2, 24) was activated by Pseudo > Exc LF in controls, suggesting a possible role in subword processes. But this region showed a similar pattern of activation in patients. (B) The left IFG, pars triangularis (peak –44, 36, 6) was activated by Exc LF > Reg LF in controls, a contrast designed to identify regions potentially involved in whole-word processes. This region was less activated in patients, but not significantly so.
Discussion
The pattern of surface dyslexia frequently observed in SD suggests that these patients have impaired whole-word reading of exception words, but relative sparing of subword processes that allow relatively accurate reading of regular words and pseudowords. In this study, we investigated the patterns of functional activation in response to reading different word types and their relationship to structural damage in five SD patients with surface dyslexia. The results showed that SD patients recruited a structurally intact region in the left intraparietal sulcus for reading exception words, whereas controls recruited this area only for pseudowords and low-frequency regular words. Secondly, the left mid-fusiform gyrus and the left STG were activated in controls for reading in general, but were atrophied and not activated in SD patients. We argue below that the left inferior parietal region subserves subword processes that are recruited for exception word reading when anterior temporal atrophy causes deficits in retrieval of exceptional, item-specific word forms.
Role of the left intraparietal sulcus in subword reading processes
The left intraparietal sulcus was the only brain region that showed an interaction of group by word type that was driven by increased signal for pseudowords in the control group and increased signal for low-frequency exception words in the SD group. The pattern of activity in controls suggests that this region is important for subword orthographic-to-phonological processes in reading, since pseudowords, by definition, cannot be retrieved from memory and thus make the most demands on subword processes. The signal increase for exception words in this region in SD patients suggests that the IPS may constitute the neural substrate for their abnormal over-reliance on subword processes in reading these words. Further supporting this interpretation, the analysis of trial-by-trial responses in the SD group showed a trend toward more activity on trials where over-regularization errors occurred, i.e. those trials in which patients must have relied on subword processes. The IPS was not significantly atrophied in SD. Taken together, these findings suggest that the IPS is a crucial region underlying a compensatory process in which patients with SD employ a spared function (reading via the subword orthographic-to-phonological mechanism) as a substitute for a lost function (retrieval of exceptional spelling-to-sound associations), resulting in systematic over-regularization errors.
There is substantial neuropsychological and neuroimaging evidence that inferior parietal regions are important for reading, and for subword processes in particular. The study of the neural basis of reading dates back to the pioneering work of Dejerine (1891, 1892), who reported two patients: the first with alexia and agraphia associated with a lesion of the left angular gyrus (Dejerine, 1891), and the second with alexia without agraphia, and a lesion to left occipital cortex (Dejerine, 1892). Dejerine surmised that the angular gyrus contained a ‘visual word centre’ necessary for reading and that the occipital lesion disconnected visual input from this region. There has been much subsequent neuropsychological work supporting this basic model (Geschwind, 1965), with significant refinements (Binder and Mohr, 1992). Moreover, recent studies on acute stroke patients have confirmed the crucial role of the supramarginal gyrus and/or angular gyrus in reading (Hillis et al., 2001, 2005; Philipose et al., 2007). Inferior parietal regions have emerged less consistently in functional neuroimaging studies (for review, see Turkeltaub et al., 2002; Price et al., 2003; Price and Mechelli, 2005), possibly due to factors such as regularity, frequency and length (Fiez et al., 1999); however, there have nevertheless been numerous functional imaging studies demonstrating the activation of parietal regions for reading (Price et al., 1994; Moore and Price, 1999; Mechelli et al., 2003; Binder et al., 2005; Church et al., 2008; for review see Jobard et al., 2003).
The role for left inferior parietal regions in subword reading processes in particular suggested by our results is supported by several lines of evidence. First, in functional imaging studies on healthy subjects, activations have been observed in this region for reading pseudowords versus words, a contrast that should isolate subword processes (Joubert et al., 2004; Binder et al., 2005, although c.f. Mechelli et al., 2003), and for rhyme judgments of pseudowords versus words (Xu et al., 2001). Secondly, developmental studies on reading suggest that children rely more on inferior parietal regions than adults do, reflecting their dependence on subword orthographic-to-phonological rules in reading as compared to skilled adult readers (Schlaggar and McCandliss, 2007; Church et al., 2008). Thirdly, functional imaging studies have shown that effortful letter-by-letter reading in patients with alexia caused by occipital/fusiform lesions or tumour resection is supported by left fronto-parietal regions including the supramarginal gyrus (Cohen et al., 2003, 2004). Similarly, in an fMRI study on patients with primary progressive aphasia likely of the logopenic variant, Sonty et al. (2003) speculated that increased activation in the left intraparietal sulcus observed in tasks that required reading could reflect laborious mapping between graphemes and phonemes, resulting from decreased efficiency of the language network. Finally, the IPS was more activated in the present study in control subjects for reading low-frequency regular words compared to low-frequency exception words, similar to previous findings (Fiez et al., 1999). We interpret this as evidence for subword processing because only regular words can be correctly read via this mechanism. Unlike pseudowords, regular words also have the potential to be stored, since they are encountered repeatedly. The demands that regular words make on subword and whole-word mechanisms appear to depend on frequency (Woollams et al., 2007), which is consistent with the frequency-dependent modulation of signal we observed in the IPS. Taken together, our results and previous studies support a role for the left IPS in subword orthographical-to-phonological reading processes.
Similar left inferior parietal regions have consistently been activated by a variety of verbal working memory paradigms (for review, see Owen et al., 2005). We suggest that the kinds of computations that the left IPS appears to be specialized for (i.e. manipulation of verbal material, application of discrete symbolic rules, etc.) ideally situate it to play a functionally specific role in subword processes in the context of reading, since these processes involve piecemeal and serial processing of the letter string. The greater activation of the left IPS in reading low-frequency regular words versus low-frequency exception words argues against an explanation of our finding in terms of verbal working memory or difficulty per se, since regular words are, if anything, less demanding to read than exception words (Binder et al., 2005). In other words, signal in the left IPS was modulated by demands on subword processes rather than verbal working memory.
Atrophy and reduced activation in temporal lobe regions in SD patients
The left mid-fusiform gyrus and the left STG were activated in the control group but not in patients with SD. Both of these regions overlapped with areas of atrophy in SD, as revealed by VBM, so it seems likely that the functional abnormalities of these regions follow from the structural abnormalities. It remains unclear whether one, both, or neither of these regions are specifically important for the retrieval of phonological forms from memory, i.e. whole-word reading processes. Neither region showed preferential activation for exception words in the control group, which argues against such a role, although null results in fMRI are not very strong inferentially since there is no reason to presume that every important process will result in a signal increase.
The pervasive surface dyslexia observed in SD constitutes strong evidence that one or more temporal lobe regions are crucial for knowledge of exceptional word forms (Patterson and Hodges, 1992; Graham et al., 2000; Jefferies et al., 2004; Patterson et al., 2006; Woollams et al., 2007). Recent cognitive studies have shown that severity of surface dyslexia is correlated with the degree of semantic impairment (Patterson and Hodges, 1992, Woollams et al., 2007), that at the item level, loss of semantic knowledge and reading errors for exception words are associated (Graham et al., 1994; McKay et al., 2007) and that deficits in exception word reading along with deficits in other non-semantic domains may follow from a primary semantic memory impairment affecting item-specific knowledge (Patterson et al., 2006). These findings suggest that reading of exception words may require mediation by semantic areas in the anterior temporal lobe, which is compromised in SD.
In imaging studies that have compared word reading to pseudoword reading, a contrast that might highlight whole-word processes, no consistent regions have emerged across studies (see Mechelli et al., 2003; Price and Mechelli, 2005 for review). However, in one PET study, Herbster et al. (1997) reported increased activation for words relative to pseudowords in left anterior fusiform gyrus, a plausible region given the SD findings, and similar effects have been observed at reduced thresholds (Brunswick et al., 1999; Owen et al., 2004; Mechelli et al., 2005). Unfortunately, ventral temporal regions are difficult to study with fMRI because the adjacent air-filled sinuses create magnetic susceptibility gradients that cause static magnetic field inhomogeneities and reduce BOLD sensitivity (Devlin et al., 2000). The spiral in/out pulse sequence employed in this study was designed to reduce susceptibility artefacts, but such artefacts were only partially alleviated (Glover and Law, 2001; Preston et al., 2004).
Our findings of reduced activation in temporal regions in SD are consistent with two previous functional neuroimaging studies on SD patients. In a study on reading in a single SD patient, Price et al. (2003; see also Price and Mechelli, 2005) found reduced activation in the left anterior fusiform gyrus and left superior temporal sulcus among other areas. Mummery et al. (1999) scanned six SD patients on a semantic task (not reading) and found reduced activity in a left posterior ITG region quite close to the mid-fusiform area in which we found reduced activation in patients. In that study, atrophy did not appear to extend so far posteriorly, and it was argued that the lack of posterior temporal activation might reflect reduced input from atrophied anterior temporal areas. VBM results in our patient group instead demonstrated atrophy extending as far caudally as the mid-fusiform gyrus, suggesting that the lack of functional activation in this region may follow directly from structural damage.
Two subregions in the inferior frontal gyrus
Different subregions of the IFG have been implicated in both subword and whole-word processes in previous studies (for review, see Mechelli et al., 2005). In the posterior IFG (pars opercularis), we observed increased activation for pseudowords relative to low-frequency exception words, suggestive of a subword role. The evidence for the role for the pars opercularis in subword reading includes its consistent activation in imaging studies that have contrasted pseudoword reading to word reading (for review, see Mechelli et al., 2003), increased activation for pseudowords in lexical decision tasks (Fiebach et al., 2002; Binder et al., 2003), and recruitment for letter-by-letter reading in alexia (Cohen et al., 2003, 2004). The only previous study on reading in a single SD patient found increased activity for reading in this region (Price et al., 2003; Price and Mechelli, 2005). However, the possibility has also been raised that activations in the IFG are related to task difficulty (Binder et al., 2005). Supporting this position, we observed that activity was greater for low-frequency than high-frequency words, but did not depend on regularity. The SD patients showed the same pattern of activity as the control group, suggesting that whether the function of this region in reading is executive, phonological, or some combination, this posterior IFG region is functionally intact in SD.
For whole-word reading, we found weak evidence for the involvement of a more anterior sector of the left IFG, the pars triangularis, in retrieval of exception words, consistent with several prior studies (Binder et al., 2005; Mechelli et al., 2005). This activation was decreased in SD patients, which might be consistent with their failure to retrieve stored lexical forms, although the difference between groups was not statistically significant. We do not suggest that exception words are stored in the IFG pars triangularis; rather this region might be involved in retrieving them from temporal storage sites. It should be noted that this region was activated most of all by reading pseudowords. Therefore, even if it is involved in retrieval of exception words, it must also have an orthogonal role in some component of the pseudoword reading process.
Limitations
Future studies, involving a greater number of patients, will be able to further clarify the roles of the left intraparietal sulcus, STG and fusiform regions that have been identified as crucial for different reading mechanisms in this study. In particular, a larger patient sample will allow direct correlations between specific error types, regional activity and grey matter volumes. However, our sample size was comparable to the few previous functional neuroimaging studies on SD and was a practical consequence of the rareness of this disease. Furthermore, our sample was adequate to show significant effects in expected regions within the normal reading network. In the future, PET studies and improved fMRI pulse sequences that reduce susceptibility artefacts may allow the identification of specific anterior temporal regions that are differentially activated for exception word reading. We predict that such regions would demonstrate abnormal activity in SD patients.
Conclusion
The central finding of this study is that patients with SD and surface dyslexia recruited a left inferior parietal region for the reading of low-frequency exception words, which they frequently over-regularize. In control subjects, this region was most active for reading pseudowords and low-frequency regular words, suggesting that its role is in subword mapping of orthographic to phonological representations. These findings reveal the neural basis for the employment of a spared function (reading via subword processes) to substitute for a lost function (retrieval of exceptional word forms).
Our study illustrates a novel way of making use of functional neuroimaging in a neurological patient population, in which specific abnormal responses are analyzed in the context of current cognitive models, in this case the highly developed models of reading that have emerged from psycholinguistic research (Seidenberg and McClelland, 1989; Coltheart et al., 1993; Plaut et al., 1996). The central paradox in designing imaging studies on patients is the choice of task (Price et al., 2006). If the task can be performed by the patient group, then it presumably relies on spared structures, so studying patients may offer no advantage over studying normal subjects. On the other hand, if the task cannot be performed by patients, then activation data relating to failure to perform the task are almost impossible to interpret. We studied a cognitive process (exception word reading) where patients with SD neither completely succeed nor simply fail. Rather, they rely more on an alternative mechanism (subword reading processes), which results in systematically incorrect but logical pronunciations. Our identification of the left IPS as the region underlying this behaviour not only contributes to understanding the neural basis of surface dyslexia, but also strengthens the evidence for the involvement of this region in subword processes in reading.
Funding
National Institutes of Health (NINDS R01 NS050915, NIA P50 AG03006, NIA P01 AG019724); State of California (DHS 04-35516); Alzheimer's Disease Research Center of California (03-75271 DHS/ADP/ARCC); Larry L. Hillblom Foundation; John Douglas French Alzheimer's Foundation; Koret Family Foundation; McBean Family Foundation.
Acknowledgements
We thank Victoria Beckman, Lovingly Quitania, Linda Isaac, Jung Jang and Matthew Growdon for administrative support; Nina Dronkers, Jomar Suarez and Serena Amici for assistance in the design, implementation and analysis of the experiment; Argye Hillis, Karalyn Patterson and one other reviewer for helpful suggestions; and all of the patients and healthy volunteers who participated in the study.
Glossary
Abbreviations
- fMRI
functional magnetic resonance imaging
- IFG
inferior frontal gyrus
- PET
positron emission tomography
- SD
Semantic dementia
- VBM
voxel-based morphometry
References
- Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Binder JR, Mohr JP. The topography of callosal reading pathways. A case-control analysis. Brain. 1992;115(Pt 6):1807–26. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, et al. Neural correlates of lexical access during visual word recognition. J Cogn Neurosci. 2003;15:372–93. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–93. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke's Wortschatz? Brain. 1999;122(Pt 10):1901–17. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol. 2001;49:433–42. [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A Developmental fMRI Study of Reading and Repetition Reveals Changes in Phonological and Visual Mechanisms Over Age. Cereb Cortex. 2008;18:2054–65. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–69. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehericy S, Samson Y, Obadia M, et al. Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cereb Cortex. 2003;13:1313–33. doi: 10.1093/cercor/bhg079. [DOI] [PubMed] [Google Scholar]
- Cohen L, Henry C, Dehaene S, Martinaud O, Lehericy S, Lemer C, et al. The pathophysiology of letter-by-letter reading. Neuropsychologia. 2004;42:1768–80. doi: 10.1016/j.neuropsychologia.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: dual-route and parallel distributed-processing approaches. Psychol Rev. 1993;100:589–608. [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain. 2005;128:1984–95. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Sur un cas de cécité verbale avec agraphie, suivi d'autopsie. Comptes Rendus Hebdomodaires des Séances et Mémoires de la Société de Biologie. 1891;3:197–201. [Google Scholar]
- Dejerine J. Contribution à l'Etude Anatomo-Pathologique et Clinique des Differentes Variétés de Cécité Verbale. Comptes Rendus Hebdomodaires des Séances et Mémoires de la Société de Biologie. 1892;4:61–90. [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, et al. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. Wien: Springer; 1999. [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–18. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Testing for anatomically specified regional effects. Hum Brain Mapp. 1997;5:133–6. doi: 10.1002/(sici)1097-0193(1997)5:2<133::aid-hbm7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel S, Nichols T, Penny W, editors. Statistical parametric mapping. London: Academic Press; 2007. [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology. 2001;57:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–94. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–22. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Hodges JR, Patterson K. The relationship between comprehension and oral reading in progressive fluent aphasia. Neuropsychologia. 1994;32:299–316. doi: 10.1016/0028-3932(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Graham KS, Simons JS, Pratt KH, Patterson K, Hodges JR. Insights from semantic dementia on the relationship between episodic and semantic memory. Neuropsychologia. 2000;38:313–24. doi: 10.1016/s0028-3932(99)00073-1. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Hum Brain Mapp. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kane P, Barker N, Beauchamp N, Wityk R. Neural substrates of the cognitive processes underlying reading: evidence from magnetic resonance perfusion imaging in hyperacute stroke. Aphasiology. 2001;15:919–31. [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker P, Herskovits E, Degaonkar M. The roles of the “visual word form area” in reading. Neuroimage. 2005;24:548–59. doi: 10.1016/j.neuroimage.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6:1004–14. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA, Jones R, Bateman D, Patterson K. Surface dyslexia in semantic dementia: a comparison of the influence of consistency and regularity. Neurocase. 2004;10:290–99. doi: 10.1080/13554790490507623. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Joubert S, Beauregard M, Walter N, Bourgouin P, Beaudoin G, Leroux JM, et al. Neural correlates of lexical and sublexical processes in reading. Brain Lang. 2004;89:9–20. doi: 10.1016/S0093-934X(03)00403-6. [DOI] [PubMed] [Google Scholar]
- Kay J, Lesser R, Coltheart M. Psycholinguistic assessment of language processing in aphasia. Hove, UK: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Kucera H, Francis W. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Marshall JC, Newcombe F. Patterns of paralexia: a psycholinguistic approach. J Psycholinguist Res. 1973;2:175–99. doi: 10.1007/BF01067101. [DOI] [PubMed] [Google Scholar]
- McKay A, Castles A, Davis C, Savage G. The impact of progressive semantic loss on reading aloud. Cogn Neuropsychol. 2007;24:162–86. doi: 10.1080/02643290601025576. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J Cogn Neurosci. 2003;15:260–71. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, et al. Dissociating reading processes on the basis of neuronal interactions. J Cogn Neurosci. 2005;17:1753–65. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999;10:181–92. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJ, Vandenberghe R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122(Pt 1):61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Owen WJ, Borowsky R, Sarty GE. FMRI of two measures of phonological processing in visual word recognition: ecological validity matters. Brain Lang. 2004;90:40–6. doi: 10.1016/S0093-934X(03)00418-8. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Hodges JR. Deterioration of word meaning: implications for reading. Neuropsychologia. 1992;30:1025–40. doi: 10.1016/0028-3932(92)90096-5. [DOI] [PubMed] [Google Scholar]
- Patterson K, Lambon Ralph MA, Jefferies E, Woollams A, Jones R, Hodges JR, et al. “Presemantic” cognition in semantic dementia: six deficits in search of an explanation. J Cogn Neurosci. 2006;18:169–83. doi: 10.1162/089892906775783714. [DOI] [PubMed] [Google Scholar]
- Philipose LE, Gottesman RF, Newhart M, Kleinman JT, Herskovits EH, Pawlak MA, et al. Neural regions essential for reading and spelling of words and pseudowords. Ann Neurol. 2007;62:481–92. doi: 10.1002/ana.21182. [DOI] [PubMed] [Google Scholar]
- Pick A. Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Medische Wochenschrift. 1892;17:165–7. [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: computational principles in quasi-regular domains. Psychol Rev. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. Neuroimage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Watson JD, Patterson K, Howard D, Frackowiak RS. Brain activity during reading. The effects of exposure duration and task. Brain. 1994;117(Pt 6):1255–69. doi: 10.1093/brain/117.6.1255. [DOI] [PubMed] [Google Scholar]
- Price CJ, Gorno-Tempini ML, Graham KS, Biggio N, Mechelli A, Patterson K, et al. Normal and pathological reading: converging data from lesion and imaging studies. Neuroimage. 2003;20(Suppl 1):S30–41. doi: 10.1016/j.neuroimage.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–81. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. Reading and reading disturbance. Curr Opin Neurobiol. 2005;15:231–8. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Price CJ, Crinion J, Friston KJ. Design and analysis of fMRI studies with neurologically impaired patients. J Magn Reson Imaging. 2006;23:816–26. doi: 10.1002/jmri.20580. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Neurobiological studies of reading and reading disability. J Commun Disord. 2001;34:479–92. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychol Rev. 1989;96:523–68. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol. 1989;2:167–82. [Google Scholar]
- Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–8. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Thompson CK, Johnson NA, Weintraub S, Parrish TB, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol. 2003;53:35–49. doi: 10.1002/ana.10390. [DOI] [PubMed] [Google Scholar]
- Strain E, Patterson K, Graham N, Hodges JR. Word reading in Alzheimer's disease: cross-sectional and longitudinal analyses of response time and accuracy data. Neuropsychologia. 1998;36:155–71. doi: 10.1016/s0028-3932(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Ralph MA, Plaut DC, Patterson K. SD-squared: on the association between semantic dementia and surface dyslexia. Psychol Rev. 2007;114:316–39. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, et al. Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cereb Cortex. 2001;11:267–77. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]