Abstract
Unlike semantic degradation disorders, the mechanisms and the anatomical underpinnings of semantic access disorders are still unclear. We report the results of a case series study on the effects of temporal lobe gliomas on semantic access abilities of a group of 20 patients. Patients were tested 1–2 days before and 4–6 days after the removal of the tumour. Their semantic access skills were assessed with two spoken word-to-picture matching tasks, which aimed to separately control for rate of presentation, consistency and serial position effects (Experiment 1) and for word frequency and semantic distance effects (Experiment 2). These variables have been held to be critical in characterizing access in contrast to degraded-store semantic deficits, with access deficits characterized by inconsistency of response, better performance with slower presentation rates and with semantically distant stimuli, in the absence of frequency effects. Degradation deficits show the opposite pattern. Our results showed that low-grade slowly growing tumours tend not to produce signs of access problems. However, high-grade tumours especially within the left hemisphere consistently produce strong semantic deficits of a clear access type: response inconsistency and strong semantic distance effects in the absence of word frequency effects were detected. However, effects of presentation rate and serial position were very weak, suggesting non-refractory behaviour in the tumour patients tested. This evidence, together with the results of lesion overlapping, suggests the presence of a type of non-refractory semantic access deficit. We suggest that this deficit could be caused by the disconnection of posterior temporal lexical input areas from semantic system.
Keywords: semantic access, brain tumours, refractoriness, dysphasia, temporal lobes
Introduction
After the first formal distinction made by Tulving in 1972 between episodic and semantic memory, the first selective impairment of semantic knowledge was reported by Warrington in 1975. Warrington (1975) described three patients with cerebral atrophy (probable semantic dementia) and selective progressive difficulties in comprehending the meaning of words and the significance of objects in spite of a fluent and generally syntactically correct speech. These patients were highly consistent in their likelihood of retrieving a given concept and were strongly affected by the frequency of the target word. They behaved as if the semantic representations underlying concepts had been degraded.
Since this first report, degradation of semantic memory has almost always been associated with widespread damage to the neocortex of the temporal lobes as, for example, that produced by Alzheimer's disease (Chertkow and Bub, 1990; Lambon Ralph et al., 1997) or herpes simplex virus encephalitis (Warrington and Shallice, 1984; Gitelman et al., 2001) or semantic dementia (Snowden et al., 1989; Hodges et al., 1992), a subtype of the fronto-temporal lobar degeneration, typically involving anterior portions of the neocortex of the temporal lobes, mainly on the left (Mummery et al., 1999, 2000; Noppeney et al., 2007).
In contrast to these disorders held to cause the degradation of semantic memory representations, Warrington and Shallice (1979) and then Warrington and McCarthy (1983, 1987) described patients whom they argued have problems in accessing the semantic representations they still retained; they were inconsistent in whether a concept could be activated and were at most only weakly affected by word frequency. Moreover, Warrington and McCarthy (1983, 1987) showed that the probability of correctly recognizing a target stimulus was influenced by the semantic distance between the target word and the distractors and by the rate at which the items were presented. They re-defined access conditions as due to a temporary unavailability of the stored representations due to abnormal refractoriness within the semantic system. Refractoriness was defined as ‘the reduction of the ability to utilize the system for a certain period of time following activation’ (Warrington and McCarthy, 1983; p. 874).
Since these first reports, however, the appropriateness of the distinction between deficits of semantic access and semantic degradation has been questioned on both theoretical and empirical grounds. Rapp and Caramazza (1993) pointed out that the criteria proposed to distinguish the two syndromes had never been assessed in the same fashion on both groups of patients. In fact, patients of the two types had been studied with different procedures and materials. They also argued from a theoretical point of view that there was no theoretical account available to explain the phenomena putatively held to co-occur in the semantic access syndrome.
In an attempt to respond to the first of these concerns, Warrington and Cipolotti (1996), using the same tests and materials, contrasted the performance obtained by a group of patients with a putative semantic degradation syndrome (four patients with probable semantic dementia) and that of two patients putatively affected by semantic access syndrome (one stroke and one left temporal high-grade tumour). In the word-to-picture matching tasks administered, ‘degradation’ patients performed consistently on whether they could access concepts and were also sensitive to the lexical frequency of the target item but not to the semantic distance between the target and the distractors. Moreover, they were not affected by changes in the response-stimulus-interval (RSI). By contrast, ‘semantic access’ patients were very inconsistent in whether they could access concepts and were strongly influenced by semantic distance, whereas word frequency had only a very weak effect. Manipulation of the rate of presentation had a dramatic effect on their performance with ‘access’ patient A2 who showed a serial position effect. The sensitivity of the patient to the rate of presentation variable was then held to be a crucial factor in the definition of a ‘refractory’ syndrome: in addition, the performance of the patients should deteriorate progressively when the same stimulus is subsequently re-presented (a serial position effect) (Warrington and McCarthy, 1983, 1987).
Since 1996, the only group study conducted to assess the proposed distinction between access and degradation deficits is that of Jefferies and Lambon Ralph (2006). This confirmed (although with different tasks) the complementarity of the performance between a group of 10 patients affected by semantic dementia (who showed degradation of semantic representations) and a group of 10 fronto-temporal or temporo-parietal stroke patients (who showed access difficulties). However, individual case studies showed that not all patients held to be of access type are sensitive to temporal factors and so cannot be characterized as being of a refractory type. Thus, Warrington and Leff (2000) failed to find rate effects in the reading aloud performance of a jargon dyslexic patient; similarly, Gotts et al. (2002) did not find rate effects in the naming performance of their patient. However, in these patients, the locus of the impairment could be attributed to a post-semantic (lexical selection) stage of processing.
Few formal attempts have been made to model the properties empirically claimed to hold for semantic access dysphasia. In 2002, however, Gotts and Plaut put forward a comprehensive computational model of access to the semantic system in order to account for different types of syndromes on the access/degradation spectrum. Their basic idea was that although degradation of semantic representations could be due to damage involving cortical neurons within the semantic system itself (encoding information itself), access deficits could be due to damage involving neuromodulatory white matter fibre systems implicated in the efficient regulation of normal refractory processes within the cortical semantic network (Gotts and Plaut, 2002).
Their model has, as a central concept, that of synaptic depression, the typical reduction in the activity of synapses after repetitive firing [see, for example, Varela et al. (1999)]. To reduce the effects of synaptic depression and so ensure efficiency in repeatedly stimulated synapses, neuromodulatory systems, in particular, cholinergic, play a key role in reducing the probability of transmitter release in the pre-synaptic neurons (e.g., Hasselmo and Bower, 1992; Hasselmo, 1995 for review) and so reducing the adaptation of the firing rate. The largest set of cholinergic fibres comes from the basal forebrain nuclei of Meynart (nbM-Ch4), which spreads throughout the neocortex including the temporal lobes (Selden et al., 1998). They can in principle be selectively damaged by different pathologies. In their model, Gotts and Plaut (2002) hypothesize that vascular accidents in the territory of the middle cerebral artery could in principle cause a large neuromodulatory breakdown within the temporal lobes, causing abnormal levels of synaptic depression that would lead to refractoriness in the semantic system.
More recently, Jefferies et al. (2007) proposed a somewhat different account of refractory semantic access disorders. They assessed the semantic abilities of a group of left hemisphere stroke patients (the same patients as in the 2006 study) and found an overall refractory behaviour in those patients whose lesion involved the left inferior prefrontal cortex as well as the temporal lobes. This was a more consistent effect in naming than in matching tasks and quite variable in magnitude across different patients. Jefferies and colleagues argue that lesions in this area may lead to a failure in frontal control processes, which are held to be required to assure adequate and flexible semantic access especially when dealing with highly demanding tasks such as naming when stimuli are quickly presented. When several semantically related competitors are repeatedly activated at a fast rate, activation will spread among them without having time to fully decay between trials, leading to summation effects worsening the performance over time [see also Schnur et al. (2006) for a similar account]. Control processes were argued to come into play in these situations. An interesting additional finding was that two of the patients reported by Jefferies and colleagues, who had left posterior temporo-parietal lesions, did not show any sign of refractoriness at all.
Aim of the study
Rapp and Caramazza (1993) criticized the early empirical characterizations of the claimed functional syndromes of semantic access disorders as insufficiently solidly based. With the exception of the study of Jefferies et al. (2006), both the earlier and later characterizations of the functional syndrome have relied on individual case studies of patients selected for their pattern of performance, the standard methodology of cognitive neuropsychology. However, the study of Woollams et al. (2007) on the preservation of word reading in semantic dementia has shown that the methodology is subject to the potential danger of selection artefacts. The alternative methodology, these authors propose, is the case series in which non-behavioural criteria are used to select the patients whose performance, though, can be assessed individually. The one application of this methodology to the semantic access set of disorders—that of Jefferies et al. (2006) on stroke patients—suggests that the patients so-characterized may not all present with the same functional syndrome.
Individual patients who have been held to manifest semantic access disorders have included patients with temporal tumours as well as stroke patients. However, although stroke patients have been extensively investigated on semantic access, patients with tumour have very rarely been studied. Brain tumours, indeed, tend to induce lesions that are more circumscribed and restricted to the white matter. Therefore, tumours can give better chances to localize a pathological behaviour both functionally and anatomically. We have therefore investigated the behaviour of a series of patients with temporal lobe tumours on tasks derived from those used initially by Warrington and McCarthy (1983, 1987) using a case series methodology. The principal aim was to confront the critique made by Rapp and Caramazza (1993) of the empirical adequacy of semantic access disorder as a unitary functional syndrome. The secondary aim was to assess the theoretical accounts of the disorder presented by Warrington and McCarthy (1983, 1987), Gotts and Plaut (2002), and Jefferies et al. (2006). Our study involved the four main variables thought to distinguish semantic access from degradation disorders. The patients were, though, not selected on the basis of the presence of semantic difficulties. The only inclusion criteria were the presence of a glioma of either high or low grade within the left or right temporal lobe.
Material and Methods
Subjects
Tumour patients’ group
This study involved a consecutive series of 20 patients with a glioma located within the temporal lobes. The selection of the patients followed a clinical criterion: regardless of their cognitive level or neuropsychological picture, patients were selected on the basis of the presence of a glioma either exclusively or mainly within the left or the right temporal lobe. All patients gave their consent to participate in the study; the study was approved by the ethical committee of SISSA-ISAS (International School for Advanced Studies, Trieste). Ten of the patients were affected by high-grade malignant gliomas (glioblastoma) and 10 by low-grade gliomas. Thirteen patients had a left and seven patients had a right hemisphere lesion. Basic demographic information is summarized in Table 1. All the patients were tested before the surgical removal of the mass, 15 of them being also available for re-testing post-operatively. All patients underwent the complete resection of the tumour except for patient LL5. No cases were treated differently from a medication point of view. Patients were usually tested the day before and from 3 to 6 days after the operation, in a session lasting about 2 h. Because of the strictly limited time available, in addition to tests assessing their semantic abilities, the patients were administered with brief baseline neuropsychological tasks in order to monitor their basic visuo-perceptive, semantic and attentive/executive skills. The results of the baseline screening as well as neurological data are reported in Table 1.
Table 1.
Baseline assessment and neurological data of the group of tumour patients
| Patient |
Age (years) | Edu | Tumour type | Tumour location | Semantic fluencies (1 min) |
BORBa (n/25) |
VOSPb (n/20) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animals |
Objects |
Proper names |
Minimal features |
Forshort views |
Incomplete letters |
Object decision |
Visual searchc |
||||||||||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||||||
| 1. | LH1 | 61 | 8 | Glioblastoma | Left temporal | 3d | 4d | 2d | 0d | 8d | 9d | 23 | 24 | 23 | 23 | 16 | 10e | 15 | 15 | 26e | 33 |
| 2. | LH2 | 66 | 5 | Glioblastoma | Left temp.-insular | 0d | – | 0d | – | 0d | – | 0e | – | NA | – | 0e | – | 9e | – | NA | – |
| 3. | LH3 | 63 | 7 | Glioblastoma | Left temporal | 17 | 19 | 8 | 11 | 18 | 19 | 22 | 22 | 22 | 24 | 6e | 5e | 12e | 13e | 41 | 42 |
| 4. | LH4 | 70 | 5 | Glioblastoma | Left sup-post temp. | 16 | 5d | 10 | 2d | NA | NA | 22 | 22 | 23 | 22 | 19 | 17 | 15 | 10e | NA | NA |
| 5. | LH5 | 81 | 5 | Glioblastoma | Left post. temp.-par. | 2d | 3d | 2d | 2d | 6 | 10 | 18e | 18e | 12e | 15e | 2e | 0e | 11e | 6e | 32 | 29 |
| 6. | LH6 | 48 | 13 | Glioblastoma | Left post. temporal | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 7. | LH7 | 69 | 17 | Glioblastoma | Left temporal | NA | – | NA | – | NA | – | NA | – | NA | – | NA | – | NA | – | NA | – |
| 8. | LL1 | 45 | 13 | Anapl Astrocyt | Left sup-post temp. | 19 | NA | 23 | NA | 25 | NA | 25 | NA | 24 | NA | 19 | NA | 16 | NA | 57 | NA |
| 9. | LL2 | 36 | 17 | Grd II Astrocyt | Left inf.-post. temp. | 23 | 22 | 21 | 15d | 25 | 23 | 24 | 24 | 25 | 25 | 19 | 19 | 16 | 15 | 53 | 59 |
| 10. | LL3 | 38 | 17 | Grd II Astrocyt | Left ant. med. temp. | 29 | 21 | 16d | 21 | 23 | 20 | 25 | 25 | 24 | 25 | 18 | 18 | 18 | 18 | 57 | 56 |
| 11. | LL4 | 38 | 9 | Grd II Astrocyt | Left frontal-temp. | 18 | 14d | 21 | 14d | 27 | 15d | 25 | 25 | 24 | 25 | 19 | 20 | 12e | 14e | 48 | NA |
| 12. | LL5 | 25 | 17 | Grd II Astrocyt | Left frontal-temp. | 26 | – | 21d | – | 12d | – | 24 | – | 25 | – | 20 | – | 17 | – | 58 | – |
| 13. | LL6 | 46 | 17 | Grd II Astrocyt | Left ant. med. temp. | 25 | 21 | 23 | 16d | 21 | 22 | 25 | 25 | 25 | 25 | 19 | 19 | 18 | 19 | 54 | 60 |
| 14. | RH1 | 65 | 5 | Glioblastoma | Right temp.-insular | 15 | 11 | 16 | 15 | 16 | NA | 24 | NA | 19 | NA | 6e | 0e | 12e | 14 | NA | 24e |
| 15. | RH2 | 71 | 5 | Glioblastoma | Right temp.-insular | 12 | 12 | 10 | 9 | 15 | 21 | 19 | 20 | NA | NA | NA | NA | NA | NA | 26e | NA |
| 16. | RH3 | 72 | 8 | Glioblastoma | Right ant. temporal | 16 | 12 | 12 | 9 | 19 | 21 | 22 | 19 | 19 | 22 | 14e | 11e | 12e | 11e | 37 | 40 |
| 17. | RL1 | 68 | 5 | Grd II Astrocyt | Right frontal-temp. | 16 | – | 10 | – | NA | – | 24 | – | 23 | – | NA | – | NA | – | NA | – |
| 18. | RL2 | 30 | 13 | Grd II Astrocyt | Right anterior temp. | 12d | – | 12d | – | 16d | – | 25 | – | 25 | – | 19 | – | 14e | – | 50 | – |
| 19. | RL3 | 57 | 13 | Grd II Astrocyt | Right temp.-polar | 21 | 29 | 24 | 30 | 28 | 32 | 25 | 25 | 25 | 23 | 17 | 18 | 13e | 14 | 58 | 58 |
| 20. | RL4 | 63 | 13 | Grd II Astrocyt | Right post. temp. | 20 | 17 | 21 | 24 | 23 | 22 | 25 | 25 | 24 | 24 | 19 | 18 | 10e | 13e | 56 | 54 |
Control patients
To check whether the tasks developed could potentially provide evidence on semantic degradation effects as well as semantic access ones and to test the procedures developed also on a patient affected by the aetiology traditionally associated with refractory semantic access disorders, we administered both experiments to three control patients. The first two patients should in theory show degradation effects as they had sustained primary damage to the cortex. Patient MU is a herpes encephalitis patient (see Borgo and Shallice, 2001) whose semantic memory skills were gravely degraded after his illness. Patient MG is a 78-year-old right-handed retired metalworker, showing signs of cortical atrophy on CT scan. The third patient, SV, suffered from a stroke involving the left basal ganglia and the left anterior frontal-temporal areas. Patient SV was tested with the same battery of tasks on two separate occasions. (Further details about neurological history of MG and SV are provided in the Supplementary material.)
Healthy controls sample
The performance of the patients in the experimental tasks was compared to that of a group of 20 control subjects divided into two age groups (below and above 50 years of age) and two education groups (below and above 10 years of schooling). Age and education cut-offs were determined on the basis of demographic characteristics of a group of similar patients (Vallesi et al., 2007). Thus, the performance of four subgroups of five subjects each could be compared with that of each tumour patient matched for age and education at the single-case level of analysis. At the group level, however, all control subjects were collapsed into an overall group of 20 subjects.
General experimental procedures
Unlike the previous studies on semantic access disorders, which used a single task for testing all the variables of interest at the same time, we were forced to split the assessment of semantic access skills of the tumour patients into two separate tasks because of the time constraints in testing the patients. When possible, all the patients were tested with both the tasks on two separate occasions. Both tasks used a spoken-word-to-picture matching technique and were implemented for computer presentation using E-prime software (Psychology Software Tools). After hearing the target item from the computer loudspeakers, the patient was required to identify and touch the appropriate picture among the four simultaneously presented on a touch screen. The RSI was controlled by the software. The tasks were designed to control for the typical variables thought to be critical in the definition of semantic access deficits and to distinguish them from degradation deficits: semantic distance, word frequency (Experiment 1), rate of presentation and consistency of response (and possible serial position effects) (Experiment 2). The general procedures were basically the same as used in previous works on this topic [see, for example, Cipolotti and Warrington (1995) and Warrington and Cipolotti (1996)].
Experiment 1: rate-consistency matching task
This first task was designed in order to control the consistency of patients’ responses and to investigate whether possible serial position effects occurred. The rate of presentation was strictly controlled.
Materials
Stimuli for this task consisted of 16 coloured digital pictures of manipulable objects. Each picture was sized to a resolution of 400 × 300 pixels and arranged in four arrays of four items on a 1024 × 768-pixel touch-screen display. Each array was built with the following properties: (i) low frequency: to produce the higher level of difficulty possible (mean frequency: 3.94); (ii) closely related distance: to produce a higher level of semantic interference (mean semantic distance: 2.28). The complete list of stimuli with frequency and semantic distance ratings is reported in the Supplementary material.
Procedure
The task consisted of a fast and a slow presentation rate conditions. In the fast condition, the name of the target stimulus was first acoustically presented from the computer to the patient together with a fixation point in the centre of the screen for 1500 ms. After the auditory presentation, an array of four items was presented on the screen and lasted until the response was made by touching the screen. After the response was collected, the ‘same’ array was pseudo-randomly rearranged after an RSI of 1000 ms, and a second target from the same array was presented. The order of presentation was pseudo-random, the position of the target and other stimuli in each array being constantly varied. Target position was balanced across each of the four possible screen positions. This procedure was repeated until all four stimuli were presented as targets and until each target was presented three times. Then the array was replaced by another composed of four other objects. The fast and slow conditions therefore involved a total amount of 48 presentations each (four stimuli × four arrays ×three times). The same order of presentation was used across subjects. The slow condition was identical to the fast one with the exception of the adoption of 10 s interval between the stimuli (RSI). The two conditions were administered in separate blocks.
Patients LH1 and LH2
These two patients were administered with a slightly different version of Experiment 1, which basically involved four more stimuli but only two (instead of three) presentations of the same target (further details about the precise procedure can be found in the Supplementary material). Because of this difference, their data are reported at the single-case level of analysis; but at the group level, their data were not included.
Experiment 2: frequency–distance matching task
In this second task, the word frequency of the target stimuli and the semantic distance between them were manipulated in order to assess their possible effects on the performance of the patients.
Materials
The stimuli consisted of 80 coloured digital pictures of manipulable objects divided into four sessions of 20 items each. Each picture had a resolution of 400 × 300 pixels and was arranged in a four-item array. There were five blocks for each session. Arrays were presented on a 1024 × 768 touch-screen display. Each block was built in order to fit the following criteria:
Low frequency, closely related (20 stimuli);
Low frequency, distant (20 stimuli);
High frequency, closely related (20 stimuli);
High frequency, distant (20 stimuli).
Unlike previous investigations, in this task, stimuli differed between close and distant and low- and high-frequency conditions. This was done to avoid excessive stimuli repetitions in the same session of testing. Given the use of different stimuli in the close and distant conditions, also other possible confounding variables (visual complexity and familiarity) were taken into account and carefully controlled (see Supplementary material for further details).
Procedure
The general procedure for each trial was as follows: the name of the target stimulus was first acoustically presented by the computer together with a fixation point in the centre of the screen for 1500 ms. Then an array of four items was presented until the patient responded. After the response, the procedure started again with a different array belonging to the same frequency/distance block. Each stimulus was presented only once in a pseudo-random order. The position of stimuli belonging to each array was changed across trials, as was target position. Target position was moreover balanced across each of the four possible screen positions. The same order of presentation was maintained across subjects. A standard 1 s RSI timing was adopted. The target stimuli were presented only once, without stimulus repetition.
General procedures for the analysis of the results
We analysed the performance of the patients both at a single case (Supplementary material: tables C–F) and at a group level (Tables 2, 3, 5 and 6).
Table 2.
Mean accuracy raw scores across all the sub-groups of patients, in each of the tasks
| Group | No. of subj. | Presentation rate |
Semantic distance |
Word frequency |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fast |
Slow |
Close |
Distant |
Low |
High |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| (n/48) | (n/48) | (n/40) | (n/40) | (n/40) | (n/40) | ||||||||
| Before surgery | |||||||||||||
| Controls | 20 | 47.6 | 0.6 | 47.3 | 0.8 | 39.2 | 0.9 | 39.9 | 0.2 | 39.7 | 0.6 | 39.5 | 0.8 |
| High grade | 10 | 36.9 | 10.1 | 39.6 | 7.7 | 29.0 | 8.5 | 35.5 | 4.5 | 31.6 | 5.5 | 32.9 | 6.9 |
| Low grade | 10 | 46.8 | 2.1 | 47.3 | 2.2 | 39.3 | 1.3 | 39.9 | 0.3 | 39.6 | 0.7 | 39.6 | 1.0 |
| Left hem | 13 | 40.9 | 10.3 | 42.8 | 8.0 | 32.5 | 9.4 | 37.3 | 4.4 | 34.7 | 6.6 | 35.2 | 7.0 |
| Right hem | 7 | 44.7 | 3.4 | 45.6 | 2.9 | 37.1 | 2.5 | 38.4 | 2.4 | 37.3 | 2.8 | 38.3 | 1.9 |
| Left high gr. | 7 | 33.0 | 11.0 | 36.6 | 8.4 | 26.4 | 9.1 | 35.0 | 5.1 | 30.3 | 6.2 | 31.1 | 7.6 |
| Left low gr. | 6 | 47.5 | 0.8 | 48.0 | 0.0 | 39.7 | 0.8 | 40.0 | 0.0 | 39.8 | 0.4 | 39.4 | 1.3 |
| Right high gr. | 3 | 43.3 | 3.8 | 44.7 | 2.3 | 35.0 | 1.0 | 36.7 | 3.1 | 34.7 | 2.1 | 35.3 | 2.1 |
| Right low gr. | 4 | 45.8 | 3.2 | 46.3 | 3.5 | 38.8 | 1.9 | 39.8 | 0.5 | 39.3 | 1.0 | 39.3 | 1.5 |
| After surgery | |||||||||||||
| Controls | 20 | 47.6 | 0.6 | 47.3 | 0.8 | 39.2 | 0.9 | 39.9 | 0.2 | 39.7 | 0.6 | 39.5 | 0.8 |
| High grade | 8 | 39.4 | 6.8 | 43.1 | 4.7 | 32.0 | 4.4 | 36.9 | 2.0 | 35.0 | 1.7 | 34.5 | 3.7 |
| Low grade | 7 | 47.7 | 0.5 | 47.9 | 0.4 | 39.1 | 1.2 | 40.0 | 0.0 | 39.4 | 1.1 | 39.7 | 0.5 |
| Left hem | 10 | 42.8 | 7.6 | 44.8 | 4.8 | 34.6 | 5.7 | 38.3 | 2.2 | 37.1 | 2.8 | 36.3 | 4.2 |
| Right hem | 5 | 45.0 | 3.0 | 46.8 | 1.6 | 36.8 | 2.1 | 38.4 | 2.2 | 37.0 | 2.5 | 38.2 | 2.4 |
| Left high gr. | 5 | 36.5 | 7.6 | 41.0 | 5.2 | 30.0 | 4.4 | 36.6 | 1.9 | 34.8 | 1.6 | 32.8 | 3.1 |
| Left low gr. | 5 | 47.8 | 0.4 | 47.8 | 0.4 | 39.2 | 1.3 | 40.0 | 0.0 | 39.8 | 0.4 | 39.8 | 0.4 |
| Right high gr. | 3 | 43.3 | 3.2 | 46.0 | 1.7 | 35.3 | 1.2 | 37.3 | 2.3 | 37.0 | 1.7 | 37.3 | 2.9 |
| Right low gr. | 2 | 47.5 | 0.7 | 48.0 | 0.0 | 39.0 | 1.4 | 40.0 | 0.0 | 39.5 | 0.7 | 39.5 | 0.7 |
Table 3.
Accuracy group analysis—experiment 1: Kruskal–Wallis ANOVA and post hoc comparisons: presentation rate (slow–fast condition)
| Contrast | Main effect | P-level | Contrast | Post hoc | P-level |
|---|---|---|---|---|---|
| Presentation rate: accuracy | |||||
| Before surgery | |||||
| Ctrls (n = 20) versus | H(2,38) = 9.89 | 0.007* | Ctrls versus High gr. | Z= 2.92 | 0.010° |
| High gr. (n = 8) versus | Ctrls versus Low gr. | Z = 1.64 | 0.302 | ||
| Low gr. (n = 10) | High gr. versus Low gr. | Z = 1.26 | 0.617 | ||
| Ctrls (n = 20) versus | H(2,28) = 7.05 | 0.029 | Ctrls versus Left high | Z = 2.32 | 0.061 |
| Left high (n = 5) versus | Ctrls versus Right high | Z = 1.54 | 0.372 | ||
| Right high (n = 3) | Left high versus Right high | Z = 0.29 | 1 | ||
| Ctrls (n = 20) versus | H(2,38) = 8.16 | 0.017* | Ctrls versus Left hem | Z= 2.45 | 0.042° |
| Left hem (n = 11) versus | Ctrls versus Right hem | Z = 1.95 | 0.152 | ||
| Right hem (n = 7) | Left hem versus Right hem | Z = 0.14 | 1 | ||
| Ctrls (n = 20) versus | H(2,31) = 6.76 | 0.03 | Ctrls versus Left high | Z= 2.51 | 0.037° |
| Left high (n = 5) versus | Ctrls versus Left low | Z = 0.52 | 1 | ||
| Left low (n = 6) | Left high versus Left low | Z = 1.68 | 0.27 | ||
| After surgery | |||||
| Ctrls (n = 20) versus | H(2,33) = 12.88 | 0.002* | Ctrls versus High gr. | Z= 3.43 | 0.002° |
| High gr. (n = 7) versus | Ctrls versus Low gr. | Z = 0.84 | 1 | ||
| Low gr. (n = 7) | High gr. versus Low gr. | Z = 2.17 | 0.088 | ||
| Ctrls (n = 20) versus | H(2,26) = 11.17 | 0.004 | Ctrls versus Left high | Z= 2.67 | 0.023° |
| Left high (n = 4) versus | Ctrls versus Right high | Z = 2.25 | 0.071 | ||
| Right high (n = 3) | Left high versus Right high | Z = 0.09 | 1 | ||
| Ctrls (n = 20) versus | H(2,33) = 8.31 | 0.016* | Ctrls versus Left hem | Z = 1.97 | 0.146 |
| Left hem (n = 9) versus | Ctrls versus Right hem | Z= 2.41 | 0.048° | ||
| Right hem (n = 5) | Left hem versus Right hem | Z = 0.74 | 1 | ||
| Ctrls (n = 20) versus | H(2,24) = 8.13 | 0.017 | Ctrls versus Right high | Z= 2.64 | 0.025° |
| Right high (n = 3) versus | Ctrls versus Right low | Z = 1.01 | 0.93 | ||
| Right low (n = 2) | Right high versus Right low | Z = 0.89 | 0.97 | ||
*Bonferroni correction: P = 0.025; °significant corrected post hoc contrast; Bold and Italic values depict a significant contrast.
Table 5.
Accuracy group analysis—Experiment 2: Kruskal–Wallis ANOVA and post hoc comparisons: semantic distance (distant–close condition)
| Contrast | Main effect | P-level | Contrast | Post hoc | P-level | |
|---|---|---|---|---|---|---|
| Semantic distance: accuracy | ||||||
| Before surgery | ||||||
| Ctrls (n = 20) versus | H(2,40) = 12.25 | 0.002* | Ctrls versus High gr. | Z= 2.93 | 0.010° | |
| High gr. (n = 10) versus | Ctrls versus Low gr. | Z = 0.47 | 1 | |||
| Low gr. (n = 10) | High gr. versus Low gr. | Z= 2.99 | 0.008° | |||
| Ctrls (n = 20) versus | H(2,30) = 13.08 | 0.001 | Ctrls versus Left high | Z= 3.52 | 0.001° | |
| Left high (n = 7) versus | Ctrls versus Right high | Z = 0.59 | 1 | |||
| Right high (n = 3) | Left high versus Right high | Z = 1.73 | 0.24 | |||
| Ctrls (n = 20) versus | H(2,40) = 3.44 | 0.178* | ||||
| Left hem (n = 13) versus | – | |||||
| Right hem (n = 7) | ||||||
| After surgery | ||||||
| Ctrls (n = 20) versus | H(2,35) = 11.19 | 0.004* | Ctrls versus High gr. | Z= 3.07 | 0.006° | |
| High gr. (n = 8) versus | Ctrls versus Low gr. | Z = 0.06 | 1 | |||
| Low gr. (n = 7) | High gr. versus Low gr. | Z= 2.47 | 0.041° | |||
| Ctrls (n = 20) versus | H(2,28) = 12.38 | 0.002 | Ctrls versus Left high | Z= 3.35 | 0.002° | |
| Left high (n = 5) versus | Ctrls versus Right high | Z = 1.06 | 0.85 | |||
| Right high (n = 3) | Left high versus Right high | Z = 1.41 | 0.47 | |||
| Ctrls (n = 20) versus | H(2,35) = 4.97 | 0.083* | ||||
| Left hem (n = 10) versus | – | |||||
| Right hem (n = 5) | ||||||
*Bonferroni correction: P = 0.025; °significant corrected post hoc contrast; Bold and Italic values depict a significant contrast.
Table 6.
Accuracy group analysis—experiment 2: Kruskal–Wallis ANOVA and post hoc comparisons: word frequency (low–high condition)
| Contrast | Main effect | P-level | Contrast | Post hoc | P-level |
|---|---|---|---|---|---|
| Word frequency: accuracy | |||||
| Before surgery | |||||
| Ctrls (n = 20) versus | H(2,40) = 6.36 | 0.041* | – | – | – |
| High gr. (n = 10) versus | |||||
| Low gr. (n = 10) | |||||
| Ctrls (n = 20) versus | H(2,40) = 4.19 | 0.12* | – | – | – |
| Left Hem (n = 13)versus | |||||
| Right Hem (n = 7) | |||||
| After surgery | |||||
| Ctrls (n = 20) versus | H(2,35) = 1.89 | 0.38* | – | – | – |
| High gr. (n = 8) versus | |||||
| Low gr. (n = 7) | |||||
| Ctrls (n = 20) versus | H(2,35) = 2.35 | 0.31* | – | – | – |
| Left Hem (n = 10) versus | |||||
| Right Hem (n = 5) | |||||
*Bonferroni correction: P = 0.025.
Group analysis procedure
As a dependent variable, the differences between the mean scores obtained by each patient were used on each of the two levels of the three independent variables: semantic distance (distant–close: i.e. subtracting accuracy on close from accuracy on distant arrays), word frequency (high–low) and presentation rate (slow–fast). Kruskal–Wallis non-parametric ANOVAs (analysis of variances) were then carried out to investigate group differences between patients and controls together with the attendant post hoc comparisons [see Siegel and Castellan (1988) for details]. We were interested in investigating two main types of effect, namely the location (left or right hemisphere) and histology (high- or low-proliferation grade) of the tumour, together with possible interactions between these two variables. Since non-parametric ANOVAs do not allow the direct determination of interactions, the following logic was adopted in the analysis of the data: Kruskal–Wallis ANOVAs were carried out on the results of the patients after being separately grouped in parallel according to both the location and the histology of the lesion. As two parallel statistical analyses were carried out, a Bonferroni correction was adopted: the P-level threshold was set at 0.025 (i.e. 0.05/2). If a significant effect was detected in either parallel confrontation, then the effect was further investigated in terms of whichever variable had been significant, location or histology, using post hoc comparisons, to assess which of the groups was significantly different from the others (see Tables 3, 5 and 6). For instance, if in the comparison of controls versus high- versus low-grade patients, the Kruskal-Wallis ANOVA gave a significant effect of histology, and post hoc comparisons highlighted high-grade patients as the source of this effect, then another ANOVA was carried out comparing controls versus left high grade versus right high-grade patients to assess the effect of laterality given the critical histology.
Single-case procedure
Fisher's exact chi squared test was adopted when analysing accuracy scores for each patient.
Consistency analysis
The consistency of responding was computed by analysing the performance obtained by patients in the ‘fast’ presentation rate condition of Experiment 1. We used the same procedure as Warrington and Cipolotti (1996) (see Supplementary material for details about the procedure). With P < 0.05, the pattern of performance exhibited was considered to be significantly more consistent than the chance-response expectation. The results of the consistency analyses are reported in Table 4. In addition to this procedure, we also analysed consistency by means of consistency coefficient φ calculation and logistic regression [see Jefferies and Lambon Ralph (2006) and Jefferies et al. (2007)]; results are provided in Supplementary Tables K and L.
Table 4.
Experiment 1—consistency calculation by patient: left high, low grade and right hemisphere tumours
| Tumour type | Pat. | Consistent (vvv/xxx) | Inconsistent (vvx/vxx) | Signif. (χ2a) | |
|---|---|---|---|---|---|
| Before surgery | |||||
| Left high gr. | LH1a | Expected | 10 | 10 | n.s. |
| Observed | 9 | 11 | |||
| Left high gr. | LH2a | Expected | 10 | 10 | n.s. |
| Observed | 8 | 12 | |||
| Left high gr. | LH3 | Expected | 13 | 3 | n.s.b |
| Observed | 12 | 4 | |||
| Left high gr. | LH4 | Expected | 13 | 3 | n.s. |
| Observed | 12 | 4 | |||
| Left high gr. | LH5 | Expected | 6 | 10 | n.s. |
| Observed | 7 | 9 | |||
| Left high gr. | LH6 | Expected | 4 | 12 | n.s. |
| Observed | 5 | 11 | |||
| Left high gr. | LH7 | Expected | 4 | 12 | n.s. |
| Observed | 3 | 13 | |||
| Left low gr. | LL1 | n.c. | n.c. | n.c. | n.c. |
| Left low gr. | LL2 | n.c. | n.c. | n.c. | n.c. |
| Left low gr. | LL3 | n.c. | n.c. | n.c. | n.c. |
| Left low gr. | LL4 | n.c. | n.c. | n.c. | n.c. |
| Left low gr. | LL5 | n.c. | n.c. | n.c. | n.c. |
| Left low gr. | LL6 | n.c. | n.c. | n.c. | n.c. |
| Right high gr. | RH1 | n.c. | n.c. | n.c. | n.c. |
| Right high gr. | RH2 | Expected | 9 | 7 | n.s. |
| Observed | 11 | 5 | |||
| Right high gr. | RH3 | Expected | 13 | 3 | n.s. |
| Observed | 14 | 2 | |||
| Right low gr. | RL1 | Expected | 10 | 6 | P < 0.05 |
| Observed | 15 | 1 | |||
| Right low gr. | RL2 | n.c. | n.c. | n.c. | n.c. |
| Right low gr. | RL3 | n.c. | n.c. | n.c. | n.c. |
| Right low gr. | RL4 | n.c. | n.c. | n.c. | n.c. |
| After surgery | |||||
| Left high gr. | LH1 | Expected | 10 | 10 | n.s. |
| Observed | 14 | 6 | |||
| Left high gr. | LH2 | N.T. | N.T. | N.T. | N.T. |
| Left high gr. | LH3 | n.c. | n.c. | n.c. | n.c. |
| Left high gr. | LH4 | Expected | 9 | 7 | n.s. |
| Observed | 9 | 7 | |||
| Left high gr. | LH5 | Expected | 5 | 11 | n.s. |
| Observed | 7 | 9 | |||
| Left high gr. | LH6 | Expected | 5 | 11 | n.s. |
| Observed | 3 | 13 | |||
| Left high gr. | LH7 | N.T. | N.T. | N.T. | N.T. |
| Left low gr. | LL1 | n.c. | n.c. | n.c. | n.c. |
| Left low gr. | LL2 | n.c. | n.c. | n.c. | n.c. |
| Left low gr. | LL3 | n.c. | n.c. | n.c. | n.c. |
| Left low gr. | LL4 | n.c. | n.c. | n.c. | n.c. |
| Left low gr. | LL5 | N.T. | N.T. | N.T. | N.T. |
| Left low gr. | LL6 | n.c. | n.c. | n.c. | n.c. |
| Right high gr. | RH1 | n.c. | n.c. | n.c. | n.c. |
| Right high gr. | RH2 | Expected | 11 | 5 | n.s. |
| Observed | 12 | 4 | |||
| Right high gr. | RH3 | Expected | 10 | 6 | P < 0.05 |
| Observed | 14 | 2 | |||
| Right low gr. | RL1 | N.T. | N.T. | N.T. | N.T. |
| Right low gr. | RL2 | n.c. | n.c. | n.c. | n.c. |
| Right low gr. | RL3 | n.c. | n.c. | n.c. | n.c. |
| Right low gr. | RL4 | n.c. | n.c. | n.c. | n.c. |
a Patients LH1 and LH2 were administered with a different version of exp1 (see page 1 of the Supplementary material for further details).
b n.s. = non significant. Significant results indicate a performance more consistent than the expected. N.T. = not tested. n.c. = not computed (≤3 errors in the condition).
Serial position effects
To examine whether serial position effects occur in Experiment 1, the number of times that the first probe was correct and either the second or the second and the third were missed by the patients was contrasted with the number of times the complementary pattern of responding was found. A binomial test was performed in order to assess the significance of this difference. The results of this analysis are reported in Supplementary Table D.
Results
Presentation rate effects (Experiment 1: slow–fast condition)
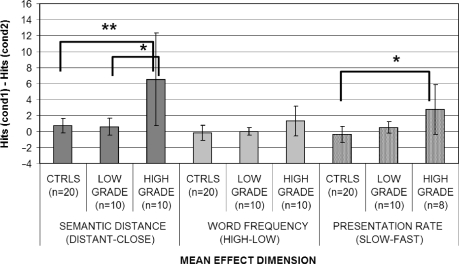
Grouping the patients initially on the basis of the histology (high- versus low-grade tumours versus controls; Table 3) led to significant effect of presentation rate on group (Kruskal–Wallis ANOVA: P = 0.007 before and P = 0.002 after the surgery). The performance of high-grade patients, in particular, was significantly more influenced by the presentation rate, with respect to controls both before (P = 0.01) and after (P = 0.002) surgery. On the other hand, performance of low-grade patients (see Figs 1 and 2) did not, meaning that high-grade patients, as a group, were significantly worse in identifying target stimuli when presented at a faster presentation rate. Low-grade patients on the other hand did not differ significantly from the controls.
Fig. 1.
Effects of semantic distance, word frequency and presentation rate on high versus low-grade tumour patients before the surgery: asterisks indicate the presence of effects in post hoc comparisons after significant main effect: *P < 0.05; **P < 0.01.
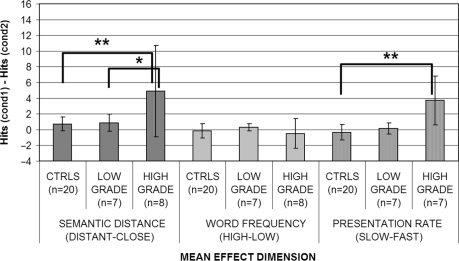
Fig. 2.
Effects of semantic distance, word frequency and presentation rate on high versus low-grade tumour patients after the surgery: asterisks indicate the presence of effects in post hoc comparisons after significant main effect: *P < 0.05; **P < 0.01.
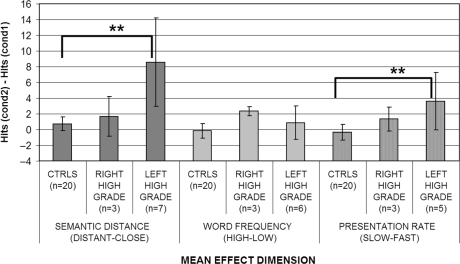
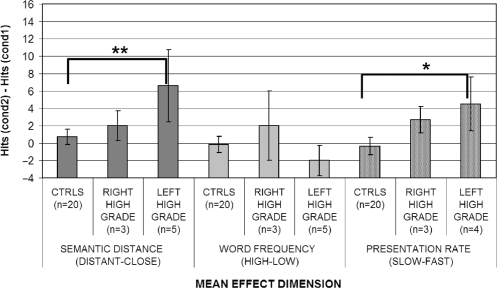
To examine this finding in further detail, left high-grade patients were compared with right high-grade ones and controls. An effect of lateralization was found (Kruskal–Wallis ANOVA: P = 0.029 before and P = 0.004 after the surgery). Post hoc comparisons showed that the effects of presentation rate tended to be higher for left high-grade patients (see Figs 3 and 4) with respect to controls especially after the surgery (P = 0.061 before and P = 0.023 after surgery); whereas, for right high-grade patients, the difference was never significant. No significant difference was however found in the direct comparison of left and right high-grade patients.
Fig. 3.
Effects of semantic distance, word frequency and presentation rate on left versus right high-grade tumour patients before the surgery: asterisks indicate the presence of effects in post hoc comparisons after significant main effect: *P < 0.05; **P < 0.01.
Fig. 4.
Effects of semantic distance, word frequency and presentation rate on left versus right high-grade tumour patients after the surgery: asterisks indicate presence of effects in post hoc comparisons after significant main effect: *P < 0.05; **P < 0.01.
When the patients were initially grouped on the basis of lateralization of the lesion alone, a significant main effect of presentation rate was found both before (Kruskal–Wallis ANOVA: P = 0.017) and after the surgery (P = 0.016). Post hoc comparisons however showed that before surgery the performance of left hemisphere patients was significantly more influenced by presentation rate (P = 0.042) than the controls, whereas the right hemisphere patients were not. To examine this finding in further detail, left high-grade patients were compared with the left low-grade ones and controls. An effect of lateralization was found (Kruskal–Wallis ANOVA: P = 0.030 before and P = 0.017 after surgery). Post hoc comparisons showed that the effects of presentation rate were significant for left high-grade patients (P = 0.037) with respect to controls, whereas left low-grade patients completely overlapped to controls. After surgery, however, post hoc comparisons investigating the source of the group effect showed that the presentation rate had a significant effect for right hemisphere patients (P = 0.048) with respect to controls. Comparing right high- and low-grade tumour patients with controls, an overall rate of presentation effect was found again (Kruskal–Wallis ANOVA: P = 0.017). Post hoc comparison showed that the effect was attributable to right high-grade patients being more affected by presentation rate with respect to controls (P = 0.025).
Single-case analysis (experiment 1): rate, consistency and serial position
In Experiment 1, high-grade patients had great difficulties, being constantly below the range of controls (Table C; Supplementary material). Considering the findings at a single-case level of analysis, however, the effects of presentation rate are weak. Although almost all patients showed better performance with slower presentation rates, the effect did never reach significance in any patient except for patient LH5 who showed a marginally significant effect before surgery. On the other hand, low-grade tumour patients constantly performed at ceiling level with respect to accuracy.
However, with only one exception (patient RH3 after surgery), all high-grade tumour patients who had difficulties in the task (seven of eight) showed an inconsistent pattern of responding (P > 0.05), suggesting that they have difficulties in accessing the concept rather than in storage per se (see Table 4). Once again, nearly all low-grade patients (nine of ten) almost always scored at ceiling. Finally, only for patient LH6, there was a significant serial position effect; in his case, both before and after surgery (Table D; Supplementary material).
Semantic distance and word frequency effects (Experiment 2: distant–close; high–low frequency)
When the performance of the tumour patients group was compared initially on the basis of the histology of the gliomas (high grade versus low grade versus controls) (Figs 1 and 2), a significant main effect of semantic distance was found both before (Kruskal–Wallis ANOVA P = 0.002) and after the surgery (Kruskal–Wallis ANOVA P = 0.004) (see Table 5). These significant effects were attributable to the high-grade patients being both significantly different from the controls (P = 0.01 before and P = 0.0006 after) and from low-grade patients (P = 0.008 before and P = 0.041 after). On the other hand, low-grade patients did not differ significantly from controls. To investigate semantic distance effects for high-grade patients further, a Kruskal–Wallis ANOVA (left high grade versus right high grade versus controls) was performed to assess whether, within high-grade patients, semantic distance had a larger effect on left rather than right hemisphere patients (Figs 3 and 4). A significant main effect of hemisphere was found both before (P = 0.025) and after (P = 0.002) surgery. Once again, the source of this effect was due to the worse performance of left hemisphere high-grade patients (P = 0.001 before and P = 0.002 after surgery) with respect to controls. Right hemisphere high-grade patients did not significantly differ from either controls or left hemisphere high-grade patients.
Regardless of the histology, a parallel grouping by tumour location was then carried out. No main effect either of semantic distance (Table 5) or word frequency (Table 6) was found for any of the variables either before (P = 0.17) or after the surgery (P = 0.083). This may have been due to the increase in variability resulting from the combining of high- and low-grade patients who showed very different patterns of behaviour.
In contrast with all these effects of semantic relatedness, no effect whatsoever was obtained for word frequency (Table 6) in any of the contrasts.
Single-case analysis (Experiment 2)
The semantic relatedness effect is even clearer when results are examined on the single-case level of analysis: many (seven of ten) of the high-grade patients (especially left hemisphere ones: six of seven) were significantly affected by semantic relatedness (Table E; Supplementary material) at a single-case level. On the other hand, word frequency (Table F; Supplementary material) did not show a significant effect for any of the patients (with the exception of patient RH2 after the surgery). Almost all (eight of ten) low-grade patients again performed at ceiling.
Effects of surgery
A direct comparison of the performance of the patients before and after the surgical removal of the tumour was carried out in order to assess the effects of the operation on the patients. Again, as dependent variables, we used the differences between the mean scores obtained on each of the two levels of the three independent variables (semantic distance, word frequency and presentation rate) by each patient (for example, the difference between the scores obtained in the distant versus the close condition). The obtained scores were then compared with the ones obtained after surgery by the same patients using the Wilcoxon matched pairs test. The analysis did not reveal any significant difference between the two testing sessions in the effects of semantic distance, word frequency or presentation rate for any of the groups considered or where there any significant differences when comparing accuracy in each of the individual conditions before and after surgery. Low-grade patients tended to show ceiling performance in each condition both before and after surgery. Roughly, the same number of high-grade patients improved and worsened (see also Supplementary Tables C, E, F).
Control patients
Patients MU and MG
In Experiment 2, neither of the cortical damaged patients showed an effect of semantic distance on accuracy (see Table G; Supplementary material), but they had significantly worse scores on low frequency compared to high-frequency arrays (MU: P = 0.05; MG: P < 0.05). In Experiment 1, MU unlike nearly all the tumour patients performed significantly more consistently than chance (see Table H; Supplementary material), suggesting that items not recognized had degraded semantic representations. MG was tested with the same version of Experiment 1 as tumour patients LH1 and LH2. In this version of the task, MG also performed significantly more consistently than chance (P < 0.01; see Table H; Supplementary material) and was not influenced by the presentation rate being even better with fast than with slow presentation rates. These results indicate that the particular experimental paradigms used were potentially sensitive to effects associated with semantic degradation effects (i.e. word frequency).
Patient SV
Stroke patient SV (see supplementary Table I), in Experiment 1, behaved as a typical refractory semantic access patient, showing inconsistency of response and being significantly influenced by presentation rate in both testing occasions. She was moreover showing the classical serial position effect in the first testing session (P < 0.01). In Experiment 2, SV again behaved as expected from a refractory semantic access patient, being influenced by semantic distance more than by word frequency. However, this time semantic distance effects were milder than the effects of temporal factors and were significant only in the first testing session (being however always larger than word frequency effects). These results clearly suggest that the task procedures were sensitive also to temporal variables, and that, therefore, the non-refractory behaviour shown by tumour patients was genuine.
Lesion mapping
Mapping of lesion sites was carried out to investigate which brain areas were responsible for the pattern of results obtained. Lesion reconstruction was performed on the scans of the patients who showed a clear semantic access pattern of performance, namely six of seven of the left hemisphere high-grade tumour patients. The seventh patient (LH3) was excluded because of his clinical history and because he did not have any apparent semantic deficit on the tasks. He had suffered a left temporal lobe glioblastoma, but this was in the same area in which he had been operated some years before for the removal of an arterio venous malformation. It is in principle possible that the arterio venous malformation could have influenced the organization of his semantic memory, as they have sometimes been reported to induce a shifting in the cortical organization of the underlying cognitive functions [see, for example, Duffau et al., 2000)].
The pre-operative location of the tumour was determined using digital format T1-weighted MRI scans. Only pre-operative MRI scans were used for reconstruction purposes, as in post-operative scans, lesion locus is usually at least partially replaced by healthy neighbouring tissue. The 3D reconstruction of lesions was drawn as regions of interest using each slice of the MRI scan of each patient on the horizontal plane, using MRIcro software (Rorden and Brett, 2000). Regions of interests included both the lesion boundaries and oedema (given that oedema has been found to commonly cause cognitive deficits). Each patient's MRI scan underwent spatial normalization using SPM2 software in order to match and align images on a common Talairach space (Talairach and Tournoux, 1988). Normalized 3D reconstructed lesions were then overlapped on a common Montreal Neurological Institute template.
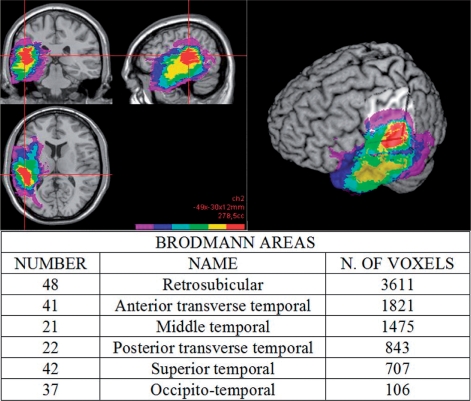
Figure 5 shows a common region of involvement shared by all the left high-grade patients reporting semantic access difficulties. This region is confined to the posterior superior portion of the left temporal lobe. Superimposing these data on an automated anatomical labelling template (Tzourio et al., 2002), which shows a macroscopic anatomical parcellation of the Montreal Neurological Institute template, the region of maximum overlap was found to mainly involve posterior portions of the superior and middle temporal gyri (areas 21 and 22) and also the transverse temporal cortex (areas 41 and 42). The largest region of lesion overlap (reported in detail in Fig. 5), however, involves area 48 (retrosubicular cortex), which cytoarchitectonically also includes the insula.
Fig. 5.
3D lesion reconstruction highlights a subcortical common area of involvement in the posterior part of the left superior and middle temporal gyri for patients showing semantic access difficulties. The red colour indicates the area of maximum overlap (six of six subjects). The table reports proportions of the Brodmann areas involved in this region.
It is worth noting, as shown in Fig. 5, that this area is largely subcortical.
Discussion
Although there is now widespread agreement on the disease processes and cognitive mechanisms underlying the degradation of semantic representations, many questions still remain open in the field of the semantic access disorders. It still remains unclear whether semantic access disorders constitute a functionally unitary syndrome or not. Moreover, no consensus has been found on the functional locus of damage, whether it lies within the semantic system itself (Warrington and Cipolotti, 1996), or in the failure of neuromodulatory mechanisms acting on semantic memory (Gotts and Plaut, 2002), or in the failure of frontal selection mechanisms (Jefferies et al., 2007), or, finally, a simple disconnection between lexical input and semantic representation areas.
In this study, we have developed two spoken word-to-picture matching tasks, which were aimed to assess consistency, rate of presentation and serial position effects (Experiment 1) and semantic distance and word frequency (Experiment 2), in a series of patients selected only by aetiology and general localization of the lesions (temporal lobes). We analysed the findings both at a single case and at a group level of analysis. Single-case comparisons were carried out by directly comparing the performance of each patient with an appropriate small group of age and education matched control subjects. Group analysis was carried out by means of a series of hierarchically organized comparisons between the patients (grouped in parallel according to lateralization or histology of the tumour) and the overall collapsed control group.
Our findings show that in brain tumour patients, who had lesions affecting the temporal lobes, semantic impairments emerged in a considerable number of cases. We have shown that the performance of high-grade tumour patients, with the sole exception of patient RH1 after surgery in Experiment 1, was always outside the accuracy cut-off scores of control subjects. Deficits were especially severe in left hemisphere patients. Low-grade temporal tumours, either of the left or the right hemisphere, on the other hand, did not produce semantic deficits on our tests (with occasional exceptions such as patients RL1 in both experiments and LL2 in Experiment 1 before surgery and patient LL6 in Experiment 2 after surgery; these patients, however, performed only slightly below the normal range).
Whenever semantic deficits emerged in the current series of patients, they were qualitatively of a clear ‘access’ type. Patients having difficulties in performing the comprehension tasks (all high-grade tumour patients) were found indeed to be inconsistent in whether they were correct or not (Experiment 1). The only exceptions were patients RL1 before and RH3 after operation, who were consistent. In addition, all left hemisphere high-grade tumour patients, in at least one of the two testing sessions and normally in both (except for patient LH3) were affected by the semantic distance between the target and distractors (Experiment 2). At a group level, both before and after surgery, high-grade tumour patients were significantly more affected by semantic distance than both the low-grade tumour patients and the controls with the latter two groups giving similar types of performance. Left high-grade tumour patients were the source of this effect, being significantly more influenced by semantic relatedness than either the right high-grade tumour patients or control subjects. By contrast, word frequency effects never reached significance in any of the patients, either at a single case or a group level of analysis, with the one exception of patient RH2 after surgery.
Surprisingly, in Experiment 1, only two patients showed a significant serial position effect in the whole series of patients tested (patient LH6 both before and after surgery and patient LH1 but only after surgery). In addition, the rate of presentation variable had a much milder effect than would be expected from a refractory access disorder. None of the individual high- or low-grade tumour patients tested, either left or right, showed a significant rate of presentation effect. However, at a group level of analysis, this effect was found to be significant for high-grade tumour patients. In particular, the effect was attributable to left high-grade tumour patients who were significantly more influenced by rate of presentation than the right high-grade tumour group or the controls before surgery. After surgery, however, right high-grade tumour patients seemed to be more prone to the presentation rate effects.
Tumour histology and cognitive impact
Our results are in accord with the findings on the different cognitive impact of high- and low-grade tumour lesions (see Supplementary material for a detailed discussion on the topic): indeed not all types of temporal lobe tumour regularly produced semantic memory impairments on these tests. In this study, high-grade aggressive tumours (such as glioblastomas) regularly impaired access to the semantic representations, but low-grade tumours did not. The performance of low-grade patients was always in the range of the controls in both tasks. An obvious explanation of the difference is in terms of the different developmental dynamics of high- and low-malignancy rate tumours. The slow rate of growth of low-grade tumours (typically grades I or II astrocytomas) means that the compressed areas could well have time to adapt to the presence of an abnormal mass by reorganizing the underlying functions in neighbouring vicarious areas [see Desmurget et al. (2007) for review].
On the other hand, the presence of a high-grade glioma (if left-sided) almost invariably leads to semantic deficits that bore the hallmarks of the access syndrome. Highly aggressive tumours such as glioblastoma could indeed produce a sudden damage to the white matter fibres leaving no time for reorganization of function to occur.
Refractoriness and brain tumours
In the ‘Introduction’ section, we defined refractory access deficits as a subtype of access deficits characterized by sensitivity of the patients to ‘temporal factors’ (presentation rate). Indeed, within the cases characterized as ‘semantic access’ deficits, most of the patients previously described have been sensitive to this variable, and therefore the main theoretical accounts for this type of deficit have involved refractoriness. A striking feature of the performance of the current group of patients was, instead, that at a single-case level of analysis, none was significantly influenced by the rate of presentation of the stimuli. Over the left high-grade tumour patients group as a whole, there was an advantage for the more slowly presented stimuli that resulted in a significant effect. However, the effect was weaker than would have been expected on the traditional refractory account. It is conceivable that this lack of effect is due to the minor changes we made in procedure compared to previous studies and that the patients showed some degree of refractoriness that resolved after a very short period. However, given that a deficit still exists at a 10 s interval, the pattern of performance is more plausibly attributable to a qualitative difference from previously described refractory patients. These results do not fit with the predictions of Gotts and Plaut's neural network simulation: their model gave rise to strong effects of rate of presentation even with mild neuromodulatory damage; whereas, in general, semantic distance effects were milder at each level of neuromodulatory damage (see their Fig. 8). The performance of the left high-grade tumour patients on the contrary shows a different pattern of effects.
The weakness of any observed rate effect in the context of strong semantic distance effects suggests that the semantic problems showed by the glioblastoma patients could be qualitatively different from those of most of the previously studied patients. In fact, our stroke patient SV showed a clearly significant rate effect. Critically, the lack of significant rate effects does not mean that the comprehension problems shown by these tumour patients are not of an access type, because all were highly inconsistent in retrieving semantic information. It seems likely that left temporal high-grade tumours can give rise to a specific different type of semantic access syndrome in which temporal factors play a secondary role in comparison with the stronger semantic relatedness effects.
Overall, the syndrome we are describing shows features similar to those reported by Jefferies et al. (2007) in two of the stroke patients they described. Although the group of anterior fronto-temporal stroke patients described by the authors showed refractory behaviour, two of their patients were not sensitive to temporal factors at all. Moreover, these patients were sensitive to semantic relatedness, but not word frequency. They also had a more posterior lesion, compatible in lesion location with that obtained in the current tumour patients. Although no detailed anatomical report was provided, lesion location seems to be much more similar to the one we found in our tumour patients.
Jefferies et al. (2007), however, suggest that the differences in behaviour between anterior and posterior patients may not be critical and that the failure of cognitive selection mechanisms may account for both behaviours. According to Jefferies and colleagues, prefrontal cortex, together with temporo-parietal attentional areas, may constitute a complex cognitive control network with an important role in tasks with high level of selection demands, the higher the competition, the higher the demands, the more critical the role of selection mechanisms [see also Peers et al. (2005)]. With repetitive presentations of the same ‘high-demand’ array of objects (semantically close arrays), failure of such mechanisms would lead to summation effects and progressive deterioration of performance (serial position effects). However, this is clearly not happening to posterior patients. If, as suggested by Jefferies and colleagues (but also by Peers et al., 2005), this high-level function is supported by a complex network of separate but interconnected areas such as lateral inferior prefrontal cortex and temporo-parietal junction [see also tractography studies: Parker et al. (2005) and Powell et al. (2006)], then damage to either of these areas should produce a similar behavioural failure with increasing difficulties with increased task demands. This is however not the case in our current group of patients.
The patients described here present a slightly different syndrome: the left high-grade patients (as well as the Jefferies et al.'s posterior patients) show weaker refractory behaviour. This suggests that the origin of such behaviour may differ between the two syndromes.
An alternative account for tumour-induced semantic access syndrome
As shown by the lesion-mapping results, the common region of maximum overlap in the patients with semantic access effects mainly involves a subcortical white matter area located in the posterior superior part of the left temporal lobe. This area, which is located in the territory of Wernicke's region, has traditionally been associated with word comprehension, but its function has been linked more to the lexical pre-semantic components of this process [see, for example, Friederici and Kots (2003) and Miozzo and Gordon (2005)]. In contrast, more ventral anterior parts of the temporal lobes have more often been associated with semantic processing (see, for example, Mummery et al., 1999, 2000; Devlin et al., 2002; Thompson-Schill, 2003; Bright et al., 2004; Moss et al., 2005; Patterson et al., 2007).
One possibility is that functionally the critical damage could be due to the connections linking lexical processing regions in the superior posterior left temporal area to the semantic processing areas (Scott and Johnsrude, 2003). Anatomical evidence, discussed by Scott and Johnsrude (2003), suggests that the pathways involved in auditory comprehension may run from both rostral and caudal parabelt auditory cortices anteriorly towards STS, but also to more posteriorly to the inferior temporal areas. Indeed, the white matter tracts, underlying the left posterior parabelt areas, are involved in the region of maximum overlap of lesions found in this study, and their location is therefore compatible with the functional hypothesis of (possibly partial) disconnection of lexical processing regions (or phonological-to-semantic hidden units) from semantic units.
An important issue to deal with, with respect to this hypothesis, is whether semantic distance effects could arise due to disconnections at this level of processing. The current functional syndrome can be thought of as the auditory verbal correspondence of the semantic access dyslexia syndrome originally described by Warrington and Shallice (1979) in the acquired dyslexic patient AR or of the form of pure alexia with partially spared comprehension (Shallice and Saffran, 1986; Coslett and Saffran, 1989; Coslett et al., 1993). Thus, for AR, word frequency effects were weak as in the left hemisphere high-grade tumour patients reported here. Semantic distance effects were not directly addressed in the original investigation of AR; however, he often produced semantic errors in word reading, which represented confusions between closely related word pairs (e.g. ‘peach’ for ‘apricot’). Moreover, AR was still able to categorize stimuli, which suggests a preserved ability to discriminate between semantically distant stimuli. These two complementary phenomena suggest the presence of a semantic distance effect in AR.
Hinton and Shallice (1991) put forward a multi-layer neural network model to implement the mapping of written words onto semantic representations [see also Plaut and Shallice (1993)]. After training, the network was able to produce a final correct target semantic pattern, given a particular pattern of activation of input units (letters). The trajectory of semantic access in the space state of the network was realized through attractor basins. For the correct semantic target to be reached, the initial semantic representation produced by the input had to fall roughly within the correct basin. The operation of part of the network then enabled it to ‘clean up’ initially somewhat distorted patterns of semantic activation in order to allow them to activate the correct target semantic representation. Lesioning the connections between the graphemic level and hidden units or between hidden and semantic units led to the occurrence of semantic errors. Moreover, the network was able to correctly select the superordinate category an item was in, when it could not identify it explicitly. This implies a semantic distance effect. Noise in a network where an intact clean-up system is partially disconnected from its input would produce inconsistency of responding.
Caramazza and Hillis (1990) had independently made somewhat analogous proposals about the output system, namely that semantic errors could occur as a result of damage to the lexical level as well as within the semantic system itself. Those lesions, subsequent to the semantic system on the output side, could also lead to ‘access-type’ deficits, which are less sensitive to temporal factors and this would fit the behaviour of certain other patients (Warrington and Leff, 2000; Gotts et al., 2002).
As far as the current patients are concerned, the possible influence of impairments to temporo-parietal junction attentional systems in the pattern of performance of the left temporal high-grade tumour patients cannot be excluded. Indeed, some cannot solely have input problems as they had low scores in fluency tasks. Our theoretical account relates specifically to their word-picture matching performance.
Overall, we would suggest that patients described as having a semantic access disorder are not functionally unitary. Refractoriness is clearly a major factor in many such patients, possibly due to a failure of frontal control mechanisms or possibly through inappropriate regulation of cholinergic neuromodulatory mechanisms. However, in certain of the patients described here, the relative weakness of refractory effects in the presence of effects of semantic distance, but not frequency, suggests an alternative cause. To conclude, we believe that our study, together with the works by Jefferies and Lambon Ralph (2006) and Jefferies et al. (2007) provide complementary evidence for the better understanding of brain bases of semantic access syndromes.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We thank Dr. Tamara Ius and Dr. Francesco Tuniz for valuable discussions, Dr. Marco Del Giudice (PhD) for his statistical advice, and all the three referees for the helpful and detailed comments. This research was facilitated by a PRIN grant to T.S.
References
- Borgo F, Shallice T. When living things and other “sensory quality” categories behave in the same fashion: a novel category specificity effect. Neurocase. 2001;7:201–20. doi: 10.1093/neucas/7.3.201. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss H, Tyler LK. Unitary vs multiple semantics: PET studies of word and picture processing. Brain Lang. 2004;89:417–32. doi: 10.1016/j.bandl.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE. Where do semantic errors come from? Cortex. 1990;26:95–122. doi: 10.1016/s0010-9452(13)80077-9. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D. Semantic memory loss in dementia of Alzheimer type: what do the various measures measure? Brain. 1990;113:397–417. doi: 10.1093/brain/113.2.397. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Warrington EK. Towards a unitary account of access dysphasia: a single case study. Memory. 1995;3:309–32. doi: 10.1080/09658219508253155. [DOI] [PubMed] [Google Scholar]
- Consiglio nazionale delle Ricerche (CNR) (unpublished) Roma, Italy: Dizionario di Frequenza della Lingua Italiana. [Google Scholar]
- Coslett HB, Saffran EM. Evidence for preserved reading in ‘pure alexia’. Brain. 1989;112:327–59. doi: 10.1093/brain/112.2.327. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Saffran EM, Greenbaum S, Schwartz H. Reading in pure alexia: the effect of strategy. Brain. 1993;116:21–37. doi: 10.1093/brain/116.1.21. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130:898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Moore CJ, Mummery CJ, Gorno-Tempini ML, Phillips JA, Noppeney U, et al. Anatomic constraints on cognitive theories of category specificity. NeuroImage. 2002;15:675–85. doi: 10.1006/nimg.2001.1002. [DOI] [PubMed] [Google Scholar]
- Duffau H, Sichez JP, Lehèricy S. Intraoperative unmasking of brain redundant motor sites during resection of a precentral angioma: evidence using direct cortical stimulation. Ann Neurol. 2000;47:132–5. [PubMed] [Google Scholar]
- Friederici DA, Kots SA. The brain basis of syntactic processes: functional imaging and lesion studies. NeuroImage. 2003;20:8–17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Ashburner J, Friston KJ, Tyler LK, Price CJ. Voxel-based morphometry of Herpes Simplex Hencephalitis. Neuroimage. 2001;13:623–31. doi: 10.1006/nimg.2000.0734. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Incisa della Rocchetta A, Cipolotti L. Mechanisms underlying perseveration in aphasia: evidence from a single case study. Neuropsychologia. 2002;40:1930–47. doi: 10.1016/s0028-3932(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Plaut DC. The impact of synaptic depression following brain damage: a connectionist account of “access/refractory” and “degraded store” semantic impairments. Cogn Affect Behav Neurosci. 2002;2:187–213. doi: 10.3758/cabn.2.3.187. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J Neurophysiol. 1992;67:1222–9. doi: 10.1152/jn.1992.67.5.1222. [DOI] [PubMed] [Google Scholar]
- Hinton GE, Shallice T. Lesioning an attractor network: investigations of acquired dyslexia. Psychol Rev. 1991;98:74–95. doi: 10.1037/0033-295x.98.1.74. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnel E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Baker SS, Doran M, Lambon Ralph MA. Refractory effects in stroke aphasia: a consequence of poor semantic control. Neuropsychologia. 2007;45:1065–79. doi: 10.1016/j.neuropsychologia.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph AM. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–47. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson H, Hodges JR. The relationship between naming and semantic knowledge for different categories in dementia of Alzheimer's type. Neuropsychologia. 1997;35:1251–60. doi: 10.1016/s0028-3932(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Miozzo M, Gordon P. Facts, events, and inflection: when language and memory dissociate. J Cogn Neurosci. 2005;17:1074–86. doi: 10.1162/0898929054475163. [DOI] [PubMed] [Google Scholar]
- Moss HE, Rodd JM, Stamatakis EA, Bright P, Tyler LK. Anteromedial temporal cortex supports fine-grained differentiation among objects. Cereb Cortex. 2005;15:616–27. doi: 10.1093/cercor/bhh163. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiack RSJ, Hodges JR. A voxel based morphometry of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJS, Vanderbergh R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122:61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss H, Stamatakis EA, Bright P, et al. Temporal lobe lesions and semantic impairment: a comparison of Herpes simplex encephalitis and semantic dementia. Brain. 2007;130:1138–47. doi: 10.1093/brain/awl344. [DOI] [PubMed] [Google Scholar]
- Parker GJM, Luzzi S, Alexander DC, Wheeler-Kingshott CAM, Clecarelli O, Ralph MAL. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–66. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–99. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peers PV, Ludwig CJH, Rorden C, Cusack R, Bonfiglioli C, Bundesen C, et al. Attentional functions of parietal and frontal cortex. Cer Cor. 2005;15:1469–84. doi: 10.1093/cercor/bhi029. [DOI] [PubMed] [Google Scholar]
- Plaut DC, Shallice T. Deep dyslexia: a case study of connectionist neuropsychology. Cogn Neuropsychol. 1993;10:377–500. [Google Scholar]
- Rapp B, Caramazza A. On the distinction between deficits of access and deficits of storage: a question of theory. Cogn Neuropsychol. 1993;10:113–41. [Google Scholar]
- Riddoch MJ, Humphreys GW. BORB: the Birmingham Object Recognition Battery. Hove, UK: Lawrence Erlbaum; 1993. [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: evidence from aphasia. J Memory Lang. 2006;54:199–227. [Google Scholar]
- Scott S, Johnsrude I. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26:100–7. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121:2249–57. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Shallice T, Saffran EM. Lexical processing in the absence of explicit word identification: evidence from a letter-by-letter reader. Cogn Neuropsychol. 1986;63:429–58. [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric statistics for the behavioural sciences. New York: McGraw-Hill; 1988. p. 213. [Google Scholar]
- Snowden J, Goulding P, Neary D. Semantic dementia: a form of circumscribed brain atrophy. Behav Neurol. 1989;2:167–82. [Google Scholar]
- Spinnler H, Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici. Ital Ital J Neurol Sci. 1987;8(Suppl 6):1–120. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A co-planar sterotactic atlas of the human brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Thompson-Schill S. Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia. 2003;41:280–92. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Mussoni A, Mondani M, Budai R, Skrap M, Shallice T. The neural basis of temporal preparation: insights from brain tumor patients. Neuropsychologia. 2007;45:2755–63. doi: 10.1016/j.neuropsychologia.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Varela JA, Song S, Turrigiano GG, Nelson SB. Differential depression at excitatory and inhibitory synapses in visual cortex. J Neurosci. 1999;19:4293–304. doi: 10.1523/JNEUROSCI.19-11-04293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27:635–57. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Cipolotti L. Word comprehension: the distinction between refractory and storage deficits. Brain. 1996;119:611–25. doi: 10.1093/brain/119.2.611. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. VOSP: Visual object and space perception battery. Bury St Edmunds, UK: Thames Valley Test Company; 1991. [Google Scholar]
- Warrington EK, Leff A. Jargon dyslexia: a single case study of intact reading comprehension in a jargon dysphasic. Neurocase. 2000;6:499–507. [Google Scholar]
- Warrington EK, McCarthy RA. Categories of knowledge: further fractionation and an attempted integration. Brain. 1987;110:1273–96. doi: 10.1093/brain/110.5.1273. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. Category specific access dysphasia. Brain. 1983;106:859–78. doi: 10.1093/brain/106.4.859. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Semantic access dyslexia. Brain. 1979;102:43–63. doi: 10.1093/brain/102.1.43. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107(Pt 3):829–54. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Lambon Ralph MA, Plaut DC, Patterson K. SD-squared: on the association between semantic dementia and surface dyslexia. Psychol Rev. 2007;114:316–39. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.