Abstract
The chromophore of visual pigments is 11-cis-retinal and, thus, in its absence, opsin is not photosensitive and no visual function exists. However, in the RPE65 knockout (Rpe65-/-) mouse, where synthesis of 11-cis-retinal does not occur, a minimal visual response from rod photoreceptors is obtained. We have examined if an alternative pathway exists for cis-retinoid generation in the absence of RPE65. Cyclic-light-reared, 2-month-old Rpe65-/- mice were placed in complete darkness. No exogenous retinoids were administered. After 4 weeks, enhanced a- and b-wave amplitudes were obtained, increasing >10-fold for the a-wave and >3-fold for the b-wave as compared with cyclic-light-reared Rpe65-/- mice. Visual-pigment levels increased to ≈10 pmol per retina, compared with no measurable pigment for cyclic-light-reared Rpe65-/- mice. The λmax of the isolated pigment was 487 nm, characteristic for isorhodopsin. Retinoid extractions confirmed the presence of 9-cis-retinal and the absence of 11-cis-retinal. Once the Rpe65-/- mice were returned to cyclic light, within 48 h the electroretinogram function returned to levels found in Rpe65-/- mice maintained in cyclic light. This dark-mediated pathway is also operational in older animals, because 13-month-old Rpe65-/- mice kept in prolonged darkness (12 weeks) had increased isorhodopsin levels and electroretinogram a- and b-wave amplitudes. These studies demonstrate that a pathway exists in the eye for the generation of 9-cis-retinal that is independent of RPE65 and light.
The generation of 11-cis-retinal is critical for visual function. In vitamin A deficiency, the first symptom is night blindness due to the loss of rod function, which in turn is due to a lack of 11-cis-retinal (1). The production of 11-cis-retinal occurs in the retinal pigment epithelium (RPE). One of the more abundant proteins in this tissue is RPE65 and when this protein is mutated or lacking, as in the Rpe65-/- mouse, visual function is impaired (2). Leber's congenital amaurosis, a childhood blinding disorder, results from disruption of a number of genes, but in 12% of the cases, the gene for RPE65 is defective (3-6). RPE65 has been shown to be essential for the conversion of all-trans-retinyl ester to 11-cis-retinol (2), although the mechanism of this reaction is still unknown. The Rpe65-/- mouse is now used in many laboratories as a model for opsin studies and the effects of vitamin A deficiency on the retina.
Vitamin A in its various isomeric and oxidative states has a central role in the visual cycle. In mammals, 11-cis-retinal binds to the rod-and-cone opsins by a lysine residue through the Schiff base linkage, generally protonated (7, 8), with the exception of certain blue-shifted cone opsins (9, 10). 11-cis-Retinal is found only in the retina, RPE, and pineal gland (11).
An isomer of 11-cis-retinal, 9-cis-retinal, has been widely used for vision experiments because of its ease of synthesis in vitro and hence its ready availability. The rod pigment formed with 9-cis-retinal, isorhodopsin, is photosensitive and appears to be very similar to rhodopsin, as determined in numerous in vitro studies and experiments in intact retinae and isolated photoreceptors (12-14). However, endogenous 9-cis-retinal has not been reported in the retina. 9-cis-Retinoids do exist in many tissues, with highest concentrations in liver and kidney, and are essential for gene regulation, and growth and development (15, 16). The high expression of 9-cis-retinol dehydrogenase (RDH4/RDH5) in the RPE (17-19) suggests that 9-cis-retinal could be generated in that tissue.
Although the Rpe65-/- mouse does not generate 11-cis-retinal, a minimal visual response is observed in both the dark-reared (20) and cyclic-light-reared animals (21, 22), and this response has been shown to be generated by the rod rather than the cone photoreceptors (21). In general, the rod pigments are more stable than cone pigments (23), and rods are known to compete favorably with cones for limited amounts of chromophore (24, 25). The quantity of the endogenous pigment in cyclic-light-reared Rpe65-/- mice is believed to be <0.1% of WT (C57BL/6) pigment or <0.2 pmol per retina (26), based on the failure to obtain an absorption measurement of a pigment even with the pooling of 10 animals and by using repetitive scans (R.K.C., M. Kono, and P. Goletz, unpublished data; C. E. Reme, A. Wenzel, and C. Grimmes, personal communication).
The question of the source of the minute rod response found in Rpe65-/- mice in the absence of a system to generate 11-cis-retinal has been puzzling. The hypothesis explored in this study is that a second, less efficient pathway exists for the generation of a cis-retinal that is available for visual-pigment regeneration. In this study, we have examined if maintaining the Rpe65-/- mice in the dark, thereby eliminating (i) any bleaching of small amounts of pigment and (ii) any photoisomerization of retinals, will result in increases in the endogenous visual-pigment formation and rod response.
Materials and Methods
Animals. Rpe65-/- mice were the generous gift of M. Redmond (National Eye Institute, National Institutes of Health) and were genotyped as described (2, 27). Age-matched C57BL/6 (WT) mice were purchased from Harlan Breeders (Indianapolis). Animals were reared under cyclic light (12-h light/12-h dark, with the ambient light intensity at the eye level of the mice being 85 ± 18 lux) until the initiation of the experiment. Rpe65-/- and WT mice (2 months of age) were maintained in complete darkness for various times, and animal husbandry was performed under dim red light (Kodak filter GBX-2). In a separate experiment, 13-month-old Rpe65-/- mice were maintained in the dark for 12 weeks. All experiments were performed in accordance with the policy on the Use of Animals in Neuroscience Research and were approved by the Medical University of South Carolina Animal Care and Use Committee.
Electroretinogram (ERG) Analysis. Mice were anesthetized by using xylazine (20 mg/kg) and ketamine (80 mg/kg). Pupils were dilated with phenylephrine hydrochloride (2.5%) and atropine sulfate (1%). Contact lens electrodes (28) were placed on both eyes with a drop of methylcellose. Full-field ERGs were recorded weekly from both eyes as described (29) by using the universal testing and electrophysiologic system 2000 (UTAS E-2000, LKC Technologies, Gaithersburg, MD). ERGs were recorded in response to 10-μs single flashes of fixed intensity (2.48 photopic m-2·cd·s) under scotopic conditions. This single, intermediate light intensity, rather then a family of light intensities, was chosen to monitor the increase in sensitivity over time, in order not to significantly bleach the newly accumulated pigment. In unpublished experiments, using 11-cis-retinal injections in Rpe65-/- mice, we observed that repeated (every 24 h) comprehensive light-intensity series bleached the newly formed pigment within ≈3 days. The amplitude of the a-wave was measured from the baseline to the lowest negative-going voltage, whereas peak b-wave amplitudes were measured from the trough of the a-wave to the highest peak of the positive b-wave. Data are presented as means ± SE and analyzed by a one-tailed t test, accepting a significance value of P < 0.05.
Extraction of Retinoids and HPLC Analysis. All procedures were performed under dim red light (Kodak filter GBX-2). Retinoids were extracted with modifications of the method described (30, 31). Eyes were removed from dark-adapted animals and rinsed with PBS (pH 7.4). The anterior part of the eyeball was removed and the retina separated from the RPE fraction that contained the choroid and sclera. The retina and RPE fractions were homogenized separately in 200 μl of PBS buffer in a micro-tissue grinder. Three retinae were combined, methanol (300 μl) and hydroxylamine (60 μl, 1 mol/liter in sodium phosphate buffer, pH 7.4) were added, and samples were vortexed (30 s). After standing for 5 min, the samples were mixed well with methylene chloride (300 μl) and centrifuged (16,000 × g, 1 min). The lower phase was dried under argon. Samples were dissolved in the HPLC mobile phase (11.2% ethyl acetate/2.0% dioxane/1.4% octanol in hexane, 90 μl), and retinoids were separated by using a Lichrosphere SI-60, 5-μm column (Alltech Associates) (32). The retinals were quantified by comparison with pure retinoid isomeric standards. The syn-9- and 13-cis-retinal oximes overlap in this system, but the anti forms are cleanly separated, and thus the isomeric identity can be assigned.
Pigment Measurements. Endogenous levels of pigment were determined by extraction of a homogenate of two retinae in 1% dodecylmaltoside (sodium phosphate buffer, pH 7.4). The sample was shaken at 4°C for 2 h, centrifuged (88,000 × g for 10 min), and measured on a Cary 300 spectrophotometer (Varian). The difference spectra were determined from measurements before and after bleaching with white light in the presence of freshly prepared 20 mmol/liter hydroxylamine, pH 7.0. The concentrations of isorhodopsin and rhodopsin were calculated using the following extinction coefficients: ε (rhodopsin) = 40,600 liters·mol-1·cm-1 (33) and ε (isorhodopsin) = 43,000 liters·mol-1·cm-1 (34).
Results
Recovery of Rod Function in Rpe65-/- Mice After Dark Rearing. The impact of dark rearing on rod function was analyzed in vivo using ERG analysis. Scotopic single-flash ERGs, testing rod function, were used. Representative responses from 2-month-old Rpe65-/- mice, which had been maintained in cyclic light from birth and then moved into total darkness for 1 day to 4 weeks, are shown in Fig. 1A. The a-wave responses, known to be correlated with photoreceptor function (35), are shown on an expanded timescale in Fig. 1B. As reported (21, 26), the rod function of Rpe65-/- mice is severely impaired, as measured after one night of dark adaptation. However, after 4 weeks in the dark, the a-wave amplitudes increased significantly from 1.24 ± 1.03 μV to 17.33 ± 1.21 μV (Fig. 1C). The a-wave amplitudes recovered to 10.6% of age-matched WT animals (163.02 ± 5.23 μV) exposed to the same period of dark rearing (Table 1). Likewise, b-wave amplitude increased from 36.7 ± 4.2 μV to 135.33 ± 7.94 μV (Fig. 1D), which represents a 43.6% recovery with respect to WT levels (309.43 ± 7.24 μV). During the 3-4 weeks of dark adaptation, some Rpe65-/- mice exhibited double-peaked b-waves. Similar duplex peaks have been observed in vitamin A-deprived rats (1). Whereas a-wave amplitudes continued to increase during the 4-week dark-adaptation period, b-wave amplitudes saturated after an ≈3.5-fold increase, a phenomenon that could reflect possible pre- and/or postsynaptic changes. When these 4-week dark-reared Rpe65-/- mice were returned to normal cyclic light, after 48 h the amplitudes of both the a- and b-waves decreased to the levels observed in Rpe65-/- mice reared in cyclic light (Fig. 1E).
Fig. 1.
ERG responses in Rpe65-/- mice increase on dark rearing. (A) Full-field scotopic ERG traces in response to a fixed-light intensity (2.48 photopic m-2·cd·s) of cyclic-light-reared Rpe65-/- mice after 1 day (trace a), 1 week (trace b), 2 weeks (trace c), 3 weeks (trace d), and 4 weeks (trace e) of dark rearing. (B) a-waves were plotted on an expanded time base. (C) a-wave amplitude comparisons (n = 22). (D) b-wave amplitude comparisons (n = 22). (E) a-wave amplitude analysis after return of animals to cyclic light (n = 8). Data are presented as means ± SE, the underlines in C-E indicate the time points compared, and * indicates a significant difference (one-tailed t test). DA, dark adaptation; CL, cyclic light.
Table 1. Scotopic ERG amplitudes and pigment levels.
| Animals | a-wave, μV | b-wave, μV | Pigment, pmol per retina |
|---|---|---|---|
| WT, 4-weeks DR | 163.02 ± 5.23 | 309.43 ± 7.24 | 289.41 |
| Rpe65−/−, 1-day DR | 1.24 ± 1.03 | 36.70 ± 4.20 | ND |
| Rpe65−/−, 4-weeks DR | 17.33 ± 1.21 | 135.33 ± 7.94 | 9.6 ± 0.46 |
| Rpe65−/−, 12-weeks DR | 33.61 ± 2.72 | 225.74 ± 37.70 | 36.05 ± 1.75 |
DR, dark reared; ND, not determined.
Endogenous Visual Pigment Increased in Rpe65-/- Mice. Absorption measurement of 1% dodecylmaltoside extracts of the retinae of a 2-month-old animal reared in cyclic light and then in darkness for 1-4 weeks gave definitive difference spectra, characteristic of the 9-cis pigment, isorhodopsin, as shown in Fig. 2. The λmax of the pigment detected in these mice was blue-shifted from 500 to 487 nm, which is the λmax of isorhodopsin (12). The pigment accumulated gradually, reaching a level of 15.7 pmol per retina at 5 weeks, which corresponds to ≈5% of the level of pigment in age-matched WT mice (308 pmol per retina). Dark-reared (up to 8 weeks) WT mice did not show any significant change in endogenous pigment levels as compared with age-matched cyclic-light-reared WT mice (data not shown).
Fig. 2.
Accumulation of endogenous isorhodopsin in long-term dark-adapted Rpe65-/- mice. Absorption spectra of the endogenous pigment of 2-month-old cyclic-light-reared Rpe65-/- mice that were then maintained in the dark for 4 weeks (trace i) and of age-matched WT mice with identical light treatment (trace ii) are shown. Spectra represent the difference spectrum of 1% dodecylmaltoside extractions of two retinae before and after bleaching with white light in the presence of NH2OH (20 mmol/liter). Arrows indicate the respective λmax. Abs, absorbance; KO, knockout.
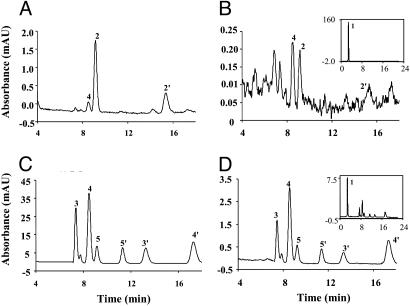
9-cis-Retinal Found in the Rpe65-/- Mouse Retina. To confirm the identity of the chromophore of the pigment formed in the dark and in the absence of RPE65, retinoids were extracted from retina and RPE fractions of dark-reared Rpe65-/- mice and analyzed by HPLC. The extracts were treated with hydroxylamine to ensure clear separation of the isomers (36). Both syn-and anti-9-cis-retinal oximes were identified in the retina extract of Rpe65-/- mice (Fig. 3A) by comparison of retention times and absorption properties (λmax 351.8 nm) of syn- and anti-9-cis-retinal oximes prepared from pure standards (36). In the RPE fractions of Rpe65-/- mice, a minute amount of 9-cis-retinal could be detected (Fig. 3B). The huge retinyl ester peak masked this 9-cis-retinal during the initial examination (Fig. 3B Inset) and, therefore, may be the reason why this isomer has not been reported previously. 11-cis-Retinal oximes were not observed in either the retina or RPE extracts of the Rpe65-/- mice (Fig. 3 A and B). Interestingly, 9-cis-retinal oximes were not observed in retina and RPE extracts of age-matched, 4-week dark-reared WT mice, where syn-11-cis- and syn-all-trans-retinal oximes were dominant (Fig. 3 C and D). The levels of 9-cis-retinal in the Rpe65-/- mice increased with the length of dark-rearing. However, the level of 11-cis-retinal present in 4-week dark-reared WT mice was not significantly changed, compared with age-matched 1-day dark-reared mice (data not shown).
Fig. 3.
Retinoid profiles of Rpe65-/- and WT mice. HPLC chromatograms of retinoid extractions from Rpe65-/- and WT mice (cyclic-light-reared from birth for 2 months, then dark-reared for 4 weeks, three eyes per sample). (A) Rpe65-/- mouse retina. (B) Rpe65-/- mouse RPE fraction. (C) WT mouse retina. (D) WT mouse RPE fraction. (B and D Insets) Full-scale chromatograms. Peaks were identified by comparison to known standards. Peak 1, all-trans-retinyl esters; peak 2, syn-9-cis-retinal oxime; peak 2′, anti-9-cis-retinal oxime; peak 3, syn-11-cis-retinal oxime; peak 3′, anti-11-cis-retinal oxime; peak 4, syn-all-trans-retinal oxime; peak 4′, anti-all-trans-retinal oxime; peak 5, syn-13-cis-retinal oxime; peak 5′, anti-13-cis-retinal oxime. Detection is at 360 nm.
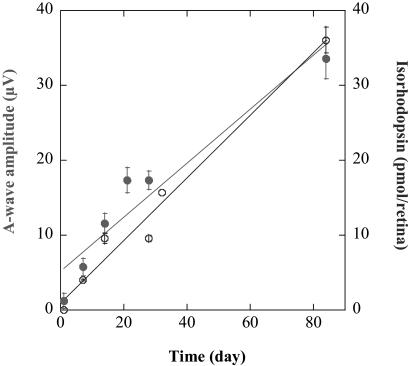
Isorhodopsin Accumulation Is Not Restricted to Early Postnatal Development. To test whether this dark-mediated mechanism for 9-cis production is restricted to early postnatal development, we repeated this experiment in a group of 13-month-old Rpe65-/- mice reared in cyclic light from birth and maintained in complete darkness for 12 weeks. In these animals, pigment levels reached 36.05 ± 1.75 pmol per retina, and the ERG signals measured 33.61 ± 1.92 μV for the a-wave and 225.74 ± 37.70 μV for the b-wave amplitudes, respectively (Table 1). Therefore, the path-sway(s) involved in the generation of the isorhodopsin do not appear to saturate, at least for 12 weeks, and the pigment is available for signal transduction. Fully adult animals (13 months of age) show the accumulation of isorhodopsin, so this mechanism does not seem to be developmentally regulated. Furthermore, the kinetics of the isorhodopsin generation are the same in 2-month-old and adult 13-month-old Rpe65-/- mice, as the pigment concentrations plotted against time in darkness showed a linear fit (R = 0.974). Pigment accumulated at a rate of ≈0.42 pmol per retina per day under these experimental conditions. ERG a-wave amplitudes increased during the same period (Fig. 4).
Fig. 4.
Pigment accumulation and ERG a-wave amplitudes. Pigment accumulation and a-wave responses were plotted against time of complete dark adaptation. Data from 2- and 13-month-old animals were combined for this analysis. Pigment accumulation (○) and the increase in a-wave amplitude (•) were found to be linear over time (R = 0.974 and 0.918, respectively).
Discussion
The Rpe65-/- mouse is a model that has generated great interest, because the lack of this protein blocks the synthesis of 11-cis-retinal. The opsin remaining can be regenerated by administration of retinoids by several delivery methods, even in elderly animals (20, 22, 26, 37). The Rpe65-/- mouse is often referred to as a model of vitamin A deficiency in the retina. However, several facts are often ignored in this interpretation. First, RPE65 has been identified in the cones of several species, both at the gene and protein level [e.g., mouse, rabbit, cow, Xenopus laevis, salamander (38, 39); see also ref. 21]. The role of RPE65 in cone photoreceptors is unknown, as is its role in the iris (40). This unknown role is a complicating factor in considering the Rpe65-/- mouse to be a model of vitamin A deficiency, because it is not clear that its function in cones or the iris is related to the generation of 11-cis-retinal. Second, opsin loss is significantly slower in the Rpe65-/- mouse than in the vitamin-A-deprived rat. The vitamin-A-deprived rat has only 20% of its opsin remaining at 32 weeks (41), and, at 10 months, no response could be generated on administration of retinoid (1, 42). The Rpe65-/- mouse still has 40% regenerable opsin at 12 months of age and 20% regenerable opsin at 18 months of age (22). Third, the retinoid acid is known to have an important role in retinal cell differentiation and development (43). The supplementation of retinoic acid required in vitamin-A-deprived animals to maintain their general health (44) is not necessary in the Rpe65-/- mouse. Therefore, retinoic acid is apparently at a normal level in the Rpe65-/- mouse, whereas it is absent in the vitamin-A-deprived animal. Finally, there is always a small but detectable light-evoked response in these animals, even at 18 months of age (22). This response has been shown to be rod-generated (21, 26), although the pigment responsible for this response has not been identified. We propose, based on the results presented here, that this pigment is isorhodopsin, formed from low levels of 9-cis-retinal, and generated by an unknown mechanism (Fig. 5).
Fig. 5.
Schematic representation of the generation of 9- and 11-cis-retinals.
Although the main role of retinal G protein-coupled receptor (RGR) is proposed to be the photoregeneration of 11-cis-retinal from all-trans-retinal (45) (similar to the squid retinochrome system), a potential role of this isomerase could be the formation of 9-cis-retinal. However, Van Hooser and coworkers (37) reported that ERG amplitudes were indistinguishable between the Rpe65-/-/Rgr-/- and Rpe65-/- mouse. Therefore, it does not appear that RGR is involved in the process of generating 9-cis-retinal.
Another mechanism that has been proposed for the photoresponse in the Rpe65-/- mouse is a light-dependent isomerization of retinoids. Van Hooser et al. (37) reported that a bright flash resulted in the light-dependent isomerization of 2.1 ± 0.6 pmol per eye of 11-cis-retinal (from 4.2 ± 1.1 pmol per eye of all-trans-retinal) in the Rpe65-/- mouse. This generation again was independent of RGR because similar results were obtained in the Rpe65-/-/Rgr-/- mouse. However, because the animals in the experiments reported here were maintained in the dark, a photoisomerization mechanism is not relevant.
Futterman and Rollins (46) reported that some biologically important nucleophiles, including dihydroflavin, dihydrofolate, and dithiols, catalyze the isomerization from the all-trans-retinal to 9-cis-retinal in vitro. On the addition of all-trans-retinal to bleached rod outer-segment preparations, some isorhodopsin was reported to be formed, which was enhanced if reducing agents were present. Therefore, the thermal isomerization of all-trans-retinal to form 9-cis-retinal, possibly through a sulfhydryl-catalyzed mechanism, cannot be ruled out at this time.
Van Hooser and coworkers (37) reported that a single administration of 9-cis-retinal to Rpe65-/- mice supported rod retinal function for 37 days after treatment regardless of whether the animals were kept in darkness or cyclic light. These results differ both with data on the 9-cis-treated vitamin-A-deprived rats, which formed isorhodopsin but, when placed in cyclic light, had little 9-cis-retinal (≈5%) remaining after 10 days (47), and with the data presented here, which show that the isorhodopsin formed in the dark is quickly lost in cyclic light. However, in the vitamin-A-deprived animals, the RPE isomerization machinery is in place, and thus 11-cis-retinal can be generated from any all-trans-retinol generated by bleaching or thermal isomerization. In consideration of our data reported here, it needs to be clarified if the long-lasting effects on rod function reported (20, 37) may have some contribution from the generation of isorhodopsin from endogenous 9-cis-retinal accumulation.
Based on the amount of pigment formed in the Rpe65-/- mice (0.42 pmol per eye per day), if 9-cis-retinal is available to form pigment in the WT animals, the pigment would be <0.2%, in a cyclic-light-reared animal (based on a total pigment level of ≈300 pmol per retina), which is not detectable by the methods used in this study. Further experiments are needed to determine whether indeed a trace of 9-cis-retinal or isorhodopsin exists in the WT retina. The Rpe65-/- mice maintain opsin levels higher than the vitamin-A-deprived rats, suggesting that this small amount of chromophore is sufficient to retard the degeneration of the protein. The source of the 9-cis-retinal is not known at this time. Although we found a trace of 9-cis-retinal in the RPE of the Rpe65-/- mice, it is not clear if this retinal is transported to the retina.
The delivery pathway of 9-cis-retinal to the retina is unknown, but certainly proteins present in the interphotoreceptor matrix bind 9-cis-retinal [e.g., interphotoreceptor retinoid-binding protein (48) and albumin (49)]. It is well established that 9-cis-retinoic acid functions as a ligand for retinoic acid receptors and retinoid X receptors, which are ligand-dependent transcription factors, and have important roles in development and cellular differentiation (43, 50). Several retinal dehydrogenases, including RDH4, RDH5, CRAD3, and CRAD1, are capable of contributing to the production of 9-cis-retinoic acids in embryonic and various nonocular adult tissues (16, 51-57), catalyzing the first step in 9-cis-retinoic acid biosynthesis and converting 9-cis-retinol to 9-cis-retinal. RDH4 has been suggested to have dual tissue-specific functions in the oxidation of 9-cis- and 11-cis-retinol into 9-cis- and 11-cis-retinal, respectively, by depending on substrate availability and expression location (18). High expression of RDH4/RDH5 in the developing and adult mouse eye, particularly in the RPE, has been detected by immunohistochemistry and Northern blotting (18, 19). In long-term dark-adapted Rpe65-/- mice, RDH4/RDH5 might contribute the 9-cis-retinal accumulation when 11-cis-retinal is not available.
All-trans-retinol is known to be the ultimate precursor for biosynthesis of both 9-cis- and all-trans-retinoic acid. Romert and coworkers (17) proposed that 9-cis-retinol is generated from all-trans-retinol by using a conserved mechanism similar to that involved in the generation of 11-cis-retinol in the visual system. The mechanism of formation of 9-cis-retinol from all-trans-retinol is still unclear. However, from the results presented here, the process is independent of light stimulation as well as RPE65.
In summary, this study demonstrates that 9-cis-retinal accumulates in the retina and RPE of Rpe65-/- mice in the dark and forms a functional pigment. This process is independent of the protein RPE65 and most likely RGR, as well as light stimulation. The rate of isorhodopsin accumulation is quite slow (0.42 pmol per retina per day) as shown in Fig. 2B, and the amounts accumulated under normal cyclic light are minute. The source of this 9-cis-retinal and the mechanism underlying its generation are unknown at this time and will require further study.
Acknowledgments
We thank Dr. T. M. Redmond (National Eye Institute) for Rpe65-/- mice and Dr. T. G. Ebrey (University of Washington, Seattle) and Dr. M. Kono (Medical University of South Carolina) for helpful discussions. This work was supported in part by National Institute of Health Grants EY04939 (to R.K.C.), EY13520 (to B.R.), and EY12231 (to J.-x.M.); the Foundation for Fighting Blindness (Owings Mills, MD); and an unrestricted grant to the Department of Ophthalmology at Medical University of South Carolina from Research to Prevent Blindness (RPB; New York). R.K.C. is an RPB Senior Scientific Investigator.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ERG, electroretinogram; RPE, retinal pigment epithelium; Rpe65-/-, RPE65 knockout; RGR, retinal G protein-coupled receptor; RDH, retinal dehydrogenase.
References
- 1.Dowling, J. E. & Wald, G. (1958) Proc. Natl. Acad. Sci. USA 44, 648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redmond, T. M., Yu, S., Lee, E., Bok, D., Hamasaki, D., Chen, N., Goletz, P., Ma, J. X., Crouch, R. K. & Pfeifer, K. (1998) Nat. Genet. 20, 344-351. [DOI] [PubMed] [Google Scholar]
- 3.Gu, S. M., Thompson, D. A., Srikumari, C. R., Lorenz, B., Finckh, U., Nicoletti, A., Murthy, K. R., Rathmann, M., Kumaramanickavel, G., Denton, M. J. & Gal, A. (1997) Nat. Genet. 17, 194-197. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz, B., Gyurus, P., Preising, M., Bremser, D., Gu, S., Andrassi, M., Gerth, C. & Gal, A. (2000) Invest. Ophthalmol. Visual Sci. 41, 2735-2742. [PubMed] [Google Scholar]
- 5.Marlhens, F., Bareil, C., Griffoin, J. M., Zrenner, E., Amalric, P., Eliaou, C., Liu, S. Y., Harris, E., Redmond, T. M., Arnaud, B., et al. (1997) Nat. Genet. 17, 139-141. [DOI] [PubMed] [Google Scholar]
- 6.Thompson, D. A., Gyurus, P., Fleischer, L. L., Bingham, E. L., McHenry, C. L., Apfelstedt-Sylla, E., Zrenner, E., Lorenz, B., Richards, J. E., Jacobson, S. G., et al. (2000) Invest. Ophthalmol. Visual Sci. 41, 4293-4299. [PubMed] [Google Scholar]
- 7.Bownds, D. (1967) Nature 216, 1178-1181. [DOI] [PubMed] [Google Scholar]
- 8.Callender, R. H., Doukas, A., Crouch, R. & Nakanishi, K. (1976) Biochemistry 15, 1621-1629. [DOI] [PubMed] [Google Scholar]
- 9.Ma, J. X., Kono, M., Xu, L., Das, J., Ryan, J. C., Hazard, E. S., III, Oprian, D. D. & Crouch, R. K. (2001) Visual Neurosci. 18, 393-399. [DOI] [PubMed] [Google Scholar]
- 10.Dukkipati, A., Kusnetzow, A., Babu, K. R., Ramos, L., Singh, D., Knox, B. E. & Birge, R. R. (2002) Biochemistry 41, 9842-9851. [DOI] [PubMed] [Google Scholar]
- 11.Okano, T. & Fukada, Y. (1997) J. Pineal Res. 22, 145-151. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard, R. & Wald, G. (1952) J. Gen. Physiol. 36, 269-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepperberg, D. R., Brown, P. K., Lurie, M. & Dowling, J. E. (1978) J. Gen. Physiol. 71, 369-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corson, D. W., Cornwall, M. C., MacNichol, E. F., Mani, V. & Crouch, R. K. (1990) Biophys. J. 57, 109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyman, R. A., Mangelsdorf, D. J., Dyck, J. A., Stein, R. B., Eichele, G., Evans, R. M. & Thaller, C. (1992) Cell 68, 397-406. [DOI] [PubMed] [Google Scholar]
- 16.Paik, J., Vogel, S., Piantedosi, R., Sykes, A., Blaner, W. S. & Swisshelm, K. (2000) Biochemistry 39, 8073-8084. [DOI] [PubMed] [Google Scholar]
- 17.Romert, A., Tuvendal, P., Simon, A., Dencker, L. & Eriksson, U. (1998) Proc. Natl. Acad. Sci. USA 95, 4404-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romert, A., Tuvendal, P., Tryggvason, K., Dencker, L. & Eriksson, U. (2000) Exp. Cell Res. 256, 338-345. [DOI] [PubMed] [Google Scholar]
- 19.Simon, A., Hellman, U., Wernstedt, C. & Eriksson, U. (1995) J. Biol. Chem. 270, 1107-1112. [PubMed] [Google Scholar]
- 20.Van Hooser, J. P., Aleman, T. S., He, Y. G., Cideciyan, A. V., Kuksa, V., Pittler, S. J., Stone, E. M., Jacobson, S. G. & Palczewski, K. (2000) Proc. Natl. Acad. Sci. USA 97, 8623-8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seeliger, M. W., Grimm, C., Stahlberg, F., Friedburg, C., Jaissle, G., Zrenner, E., Guo, H., Reme, C. E., Humphries, P., Hofmann, F., et al. (2001) Nat. Genet. 29, 70-74. [DOI] [PubMed] [Google Scholar]
- 22.Rohrer, B., Goletz, P., Znoiko, S., Ablonczy, Z., Ma, J. X., Redmond, T. M. & Crouch, R. K. (2003) Invest. Ophthalmol. Visual Sci. 44, 310-315. [DOI] [PubMed] [Google Scholar]
- 23.Wald, G., Brown, P. K. & Smith, P. H. (1955) J. Gen. Physiol. 38, 623-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto, H., Tokunaga, F. & Yoshizawa, T. (1975) Biochim. Biophys. Acta 404, 300-308. [DOI] [PubMed] [Google Scholar]
- 25.Shichida, Y., Kato, T., Sasayama, S., Fukada, Y. & Yoshizawa, T. (1990) Biochemistry 29, 5843-5848. [DOI] [PubMed] [Google Scholar]
- 26.Ablonczy, Z., Crouch, R. K., Goletz, P. W., Redmond, T. M., Knapp, D. R., Ma, J. X. & Rohrer, B. (2002) J. Biol. Chem. 277, 40491-40498. [DOI] [PubMed] [Google Scholar]
- 27.Redmond, T. M. & Hamel, C. P. (2000) Methods Enzymol. 316, 705-724. [DOI] [PubMed] [Google Scholar]
- 28.Bayer, A. U., Cook, P., Brodie, S. E., Maag, K. P. & Mittag, T. (2001) Vision Res. 41, 2173-2185. [DOI] [PubMed] [Google Scholar]
- 29.Gresh, J., Goletz, P., Crouch, R. K. & Rohrer, B. (2003) Visual Neurosci. 20, 211-220. [DOI] [PubMed] [Google Scholar]
- 30.Saari, J. C., Garwin, G. G., Van Hooser, J. P. & Palczewski, K. (1998) Vision Res. 38, 1325-1333. [DOI] [PubMed] [Google Scholar]
- 31.Palczewski, K., Van Hooser, J. P., Garwin, G. G., Chen, J., Liou, G. I. & Saari, J. C. (1999) Biochemistry 38, 12012-12019. [DOI] [PubMed] [Google Scholar]
- 32.Landers, G. M. & Olson, J. A. (1988) J. Chromatogr. A 438, 383-392. [DOI] [PubMed] [Google Scholar]
- 33.Wald, G. B. & Brown, P. K. (1953) J. Gen. Physiol. 37, 189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshizawa, T. & Wald, G. B. (1963) Nature 197, 1279-1286. [DOI] [PubMed] [Google Scholar]
- 35.Penn, R. D. & Hagins, W. A. (1969) Nature 223, 201-204. [DOI] [PubMed] [Google Scholar]
- 36.Groenendijk, G. W., De Grip, W. J. & Daemen, F. J. (1980) Biochim. Biophys. Acta 617, 430-438. [DOI] [PubMed] [Google Scholar]
- 37.Van Hooser, J. P., Liang, Y., Maeda, T., Kuksa, V., Jang, G. F., He, Y. G., Rieke, F., Fong, H. K., Detwiler, P. B. & Palczewski, K. (2002) J. Biol. Chem. 277, 19173-19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Znoiko, S. L., Crouch, R. K., Moiseyev, G. & Ma, J. X. (2002) Invest. Ophthalmol. Visual Sci. 43, 1604-1609. [PubMed] [Google Scholar]
- 39.Ma, J., Xu, L., Othersen, D. K., Redmond, T. M. & Crouch, R. K. (1998) Biochim. Biophys. Acta 1443, 255-261. [DOI] [PubMed] [Google Scholar]
- 40.Kociok, N., Heppekausen, H., Schraermeyer, U., Esser, P., Thumann, G., Grisanti, S. & Heimann, K. (1998) Exp. Eye Res. 67, 237-250. [DOI] [PubMed] [Google Scholar]
- 41.Carter-Dawson, L., Kuwabara, T., O'Brien, P. J. & Bieri, J. G. (1979) Invest. Ophthalmol. Visual Sci. 18, 437-446. [PubMed] [Google Scholar]
- 42.Dowling, J. E. & Wald, G. (1960) Proc. Natl. Acad. Sci. USA 46, 587-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyatt, G. A. & Dowling, J. E. (1997) Invest. Ophthalmol. Visual Sci. 38, 1471-1475. [PubMed] [Google Scholar]
- 44.Arens, J. F. & Van Dorp, D. A. (1946) Nature 158, 622-623. [DOI] [PubMed] [Google Scholar]
- 45.Chen, P., Hao, W., Rife, L., Wang, X. P., Shen, D., Chen, J., Ogden, T., Van Boemel, G. B., Wu, L., Yang, M. & Fong, H. K. (2001) Nat. Genet. 28, 256-260. [DOI] [PubMed] [Google Scholar]
- 46.Futterman, S. & Rollins, M. H. (1973) J. Biol. Chem. 248, 7773-7779. [PubMed] [Google Scholar]
- 47.Crouch, R. & Katz, S. (1980) Vision Res. 20, 109-115. [DOI] [PubMed] [Google Scholar]
- 48.Nickerson, J. M., Li, G. R., Lin, Z. Y., Takizawa, N., Si, J. S. & Gross, E. A. (1998) Mol. Vision 4, 33-47. [PubMed] [Google Scholar]
- 49.Li, Z., Zhuang, J. & Corson, D. W. (1999) Photochem. Photobiol. 69, 500-504. [PubMed] [Google Scholar]
- 50.Mangelsdorf, D. J., Kliewer, S. A., Kakizuka, A., Umesono, K. & Evans, R. M. (1993) Recent Prog. Horm. Res. 48, 99-121. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang, R., Lin, M. & Napoli, J. L. (2002) Biochemistry 41, 3477-3483. [DOI] [PubMed] [Google Scholar]
- 52.Chai, X., Zhai, Y. & Napoli, J. L. (1997) J. Biol. Chem. 272, 33125-33131. [DOI] [PubMed] [Google Scholar]
- 53.Su, J., Chai, X., Kahn, B. & Napoli, J. L. (1998) J. Biol. Chem. 273, 17910-17916. [DOI] [PubMed] [Google Scholar]
- 54.Driessen, C. A., Winkens, H. J., Kuhlmann, E. D., Janssen, A. P., van Vugt, A. H., Deutman, A. F. & Janssen, J. J. (1998) FEBS Lett. 428, 135-140. [DOI] [PubMed] [Google Scholar]
- 55.Mertz, J. R., Shang, E., Piantedosi, R., Wei, S., Wolgemuth, D. J. & Blaner, W. S. (1997) J. Biol. Chem. 272, 11744-11749. [DOI] [PubMed] [Google Scholar]
- 56.Wang, J., Chai, X., Eriksson, U. & Napoli, J. L. (1999) Biochem. J. 338, 23-27. [PMC free article] [PubMed] [Google Scholar]
- 57.Gamble, M. V., Shang, E., Zott, R. P., Mertz, J. R., Wolgemuth, D. J. & Blaner, W. S. (1999) J. Lipid Res. 40, 2279-2292. [PubMed] [Google Scholar]