Abstract
Parkinson's disease (PD) is a debilitating movement disorder that afflicts >1 million people in North America. Current treatments focused on dopamine-replacement strategies ultimately fail in most patients because of loss of efficacy and severe adverse effects that worsen as the disease progresses. The recent success of surgical approaches suggests that a pharmacological intervention that bypasses the dopamine system and restores balance in the basal ganglia motor circuit may provide an effective treatment strategy. We previously identified the metabotropic glutamate receptor 4 (mGluR4) as a potential drug target and predicted that selective activation of mGluR4 could provide palliative benefit in PD. We now report that N-phenyl-7-(hydroxylimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC) is a selective allosteric potentiator of mGluR4. This compound selectively potentiated agonist-induced mGluR4 activity in cultured cells expressing this receptor and did not itself act as an agonist. Furthermore, PHCCC potentiated the effect of l-(+)-2-amino-4-phosphonobutyric acid in inhibiting transmission at the striatopallidal synapse. Modulation of the striatopallidal synapse has been proposed as a potential therapeutic target for PD, in that it may restore balance in the basal ganglia motor circuit. Consistent with this, PHCCC produced a marked reversal of reserpine-induced akinesia in rats. The closely related analogue 7-(hydroxylimino)cyclopropachromen-1a-carboxamide ethyl ester, which does not potentiate mGluR4, had no effect in this model. These results are evidence for in vivo behavioral effects of an allosteric potentiator of mGluRs and suggest that potentiation of mGluR4 may be a useful therapeutic approach to the treatment of PD.
Parkinson's disease (PD) is a debilitating neurodegenerative disorder that afflicts ≈1% of people older than 55 years. The primary pathology underlying PD is a degeneration of neurons in the substantia nigra pars compacta (1). The finding that these neurons are dopaminergic cells that provide a dense innervation of the striatum (2) led to the development of dopamine-replacement therapies for the treatment of this disease. Drugs such as the dopamine precursor l-dopa and dopamine receptor agonists provide dramatic amelioration of the motor signs of PD at early stages of the disease. However, prolonged treatment with these drugs leads to a loss of reliable efficacy and a variety of motor and cognitive side effects (3). In addition, disagreement still exists as to whether or not l-dopa therapy may actually speed disease progression through increased oxidative damage (for review, see refs. 4 and 5). Therefore, interest has been renewed in the design of therapeutic methods that bypass the dopamine system.
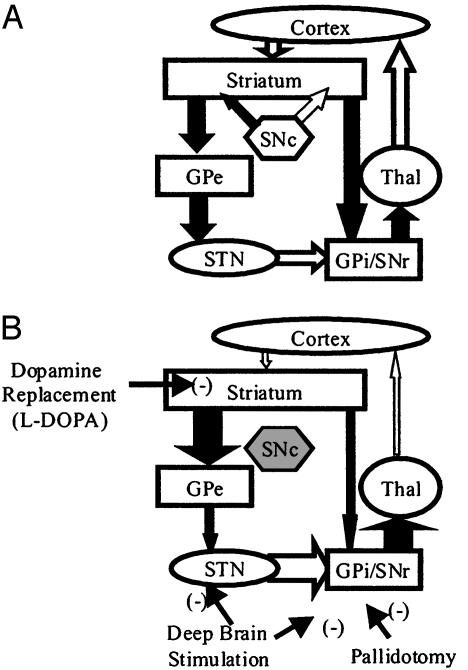
One such method has been suggested by the recent resurgence and advances in surgical interventions such as pallidotomy or deep-brain stimulation. These approaches have led to both dramatic palliative benefits for PD patients and an unprecedented refinement of the model of basal ganglia dysfunction associated with PD (for review, see refs. 6 and 7). In brief, this model suggests that two pathways exist between the striatum and the basal ganglia output nuclei. In PD, loss of striatal dopaminergic tone leads to an imbalance between direct inhibition and indirect excitation, such that an excessive basal ganglia output disrupts motor behavior. As indicated on Fig. 1, surgical interventions bypass the dopamine system and produce a decrease in basal ganglia outflow that results in palliative benefit. Therefore, a pharmacological intervention that mimics these surgical methods could provide palliative benefit to a larger number of patients without the need for invasive surgery. Furthermore, by bypassing the dopamine system, such a treatment should produce fewer side effects and may actually slow the disease process by normalizing overactive glutamatergic input to midbrain dopamine-containing neurons.
Fig. 1.
Model of the basal ganglia motor circuit. (A) In the normal state, the direct and indirect projections from the striatum to the output nuclei are balanced by striatal dopaminergic tone. (B) In PD, the loss of striatal dopamine produces an imbalance in the direct and indirect pathways such that an excessive excitation of the output nuclei leads to a large increase in basal ganglia outflow. The sites of action for dopamine-replacement therapy and for surgical interventions are indicated. Black arrows represent inhibitory GABAergic connections; white arrows represent excitatory glutamatergic connections. GPi, internal globus pallidus; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; Thal, thalamus.
We have been interested in the role of metabotropic glutamate receptors (mGluRs) in the function of the basal ganglia and the potential that these receptors may provide useful targets for the treatment of PD (for review, see ref. 8). Recent studies indicate that mGluR4 decreases GABAergic transmission at the inhibitory striatopallidal synapse within the basal ganglia (9). In PD, increased GABAergic inhibition at this synapse is thought to play an important role in motor dysfunction (Fig. 1). Based on this concept, reduction of γ-aminobutyric acid (GABA) release at the striatopallidal synapse by a selective agonist of mGluR4 has been suggested as an approach for the treatment of PD.
Recently, allosteric modulators of several family C G protein-coupled receptors (including two of the three groups of mGluRs) have been described, which suggested to us that mGluR4 might be amenable to allosteric modulation. We have found that the group I antagonist N-phenyl-7-(hydroxylimino)cyclopropa-[b]chromen-1a-carboxamide (PHCCC) (10) is a potentiator of human and rat mGluR4. PHCCC did not itself exhibit mGluR4 agonist activity, and the closely related analogue 7-(hydroxylimino)cyclopropa[b]chromen-1a-carboxamide ethyl ester (CPCCOEt) (10) had no mGluR4 potentiator activity, suggesting it might be useful as a control for in vivo studies. Characterization of PHCCC revealed that it does not potentiate or activate any other mGluR subtype but acts as an antagonist of some of the mGluRs. In brain-slice electrophysiological studies of the rat striatopallidal synapse, PHCCC was found to potentiate the effect of the mGluR4 agonist l-(+)-2-amino-4-phosphonobutyric acid (L-AP4) in inhibiting transmission. Finally, PHCCC was found to overcome inhibition of movement observed in a dopamine-depletion rat model of PD. Taken together, these studies support the hypothesis that mGluR4 activation may provide therapeutic benefit in PD patients and suggest that allosteric potentiators of this receptor may provide an approach that could be used to increase the activity of this receptor in vivo.
Materials and Methods
Chemicals. PHCCC, CPCCOEt, L-AP4, 6-cyano-7-nitroquinoxaline-2,3-dione, D-(-)-2-amino-5-phosphopentanoic acid, (2S)-3-{[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl}(phenylmethyl)phosphinic acid (CGP 55845), and glutamate were all purchased from Tocris Cookson (Ellisville, MO).
Cell Lines. All cell lines used in these studies stably expressed mGluRs, and, when relevant, exogenous G proteins on separate pIRES/Puro vectors (Clontech). Cell lines expressing mGluR1b [two have been described (11)], mGluR4, 5, and 8 were expressed in Chinese hamster ovary cells, whereas mGluR7 was expressed in human embryonic kidney cells. mGluR4 was coexpressed with the chimeric G protein Gqi5, and mGluR7 and 8 were coexpressed with Gα15 to use Ca2+-sensitive fluorescence assays. Cells were evaluated by using a fluorometric imaging plate reader (FLIPR, Molecular Devices) to measure their ability to mobilize Ca2+ in response to appropriate agonists (i.e., glutamate and L-AP4) as described in O'Brien et al. (12).
Animals. All studies were performed in an American Association for the Accreditation of Laboratory Animal Care-accredited facility in accordance with all applicable guidelines regarding the care and use of animals. Animals were group-housed with access to food and water ad libitum.
Brain-Slice Preparation. All experiments were performed on brain slices from 26- to 30-day-old Sprague-Dawley rats (Taconic Farms). Animals were killed by decapitation, and their brains were rapidly removed and submerged in an ice-cold solution containing the following (in millimolar): choline chloride, 126; KCl, 2.5; NaH2PO4, 1.2; MgCl2, 1.3; MgSO4, 8; glucose, 10; and NaHCO3, 26 equilibrated with 95% O2/5% CO2 (13). The brain was glued to the chuck of a vibrating blade microtome (Leica Microsystems, Nussloch, Germany) and parasagittal slices (300-μm-thick) were obtained. Slices were immediately transferred to a 500-ml holding chamber containing artificial cerebrospinal fluid (in millimolar): NaCl, 124; KCl, 2.5; MgSO4, 1.3; NaH2PO4, 1.0; CaCl2, 2; glucose, 20; and NaHCO3, 26; equilibrated with 95% O2/5% CO2 that was maintained at 32°C. After 20 min at 32°C, the holding chamber was allowed to gradually decrease to room temperature. In all experiments, 5 μM glutathione, 500 μM pyruvate, and 250 μM kynurenic acid were included in the choline chloride buffer and in the holding chamber artificial cerebrospinal fluid.
Electrophysiology. Whole-cell patch-clamp recordings were obtained as described (9). During recording, brain slices were maintained fully submerged on the stage of a 1-ml brain-slice chamber at 32°C and perfused continuously with equilibrated artificial cerebrospinal fluid (2-3 ml/min). Neurons were visualized by using a differential interference contrast microscope and an infrared video system. Patch electrodes were pulled from borosilicate glass on a two-stage puller and had resistances in the range of 3-7 MΩ when filled with the following internal solution (in millimolar): potassium gluconate, 125; NaCl, 4; NaH2PO4, 6; CaCl2, 1; MgSO4, 2; 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-tetrapotassium salt, 10; Hepes, 10; Mg-ATP, 2; Na2-GTP, 0.3 (pH = 7.4).
Inhibitory postsynaptic currents were evoked in the presence of blockers of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (20 μM 6-cyano-7-nitroquinoxaline-2,3-dione), N-methyl-d-aspartate [25 μM D-(-)-2-amino-5-phosphopentanoic acid], and GABA type B (100 nM CGP 55845) receptors as described (9). Inhibitory postsynaptic currents were evoked by single pulses that ranged from 30 to 90 V, 200 to 400 μs, delivered once every 30-60 s from a holding potential of -50 mV. For hippocampal field recordings, a patch electrode filled with artificial cerebrospinal fluid was placed in the dendritic region of CA1 or the dentate gyrus. Field excitatory postsynaptic potentials were isolated and characterized as described (14, 15). Compounds were applied to the bath by using a three-way stopcock and were always applied for 10 min to achieve a plateau concentration.
Behavior. Third-ventricle-cannulated male Sprague-Dawley rats (250-350 g) were purchased from Taconic Farms with the guide cannula implanted such that subsequent placement of an injection cannula allowed for infusion into the third ventricle. These rats were used for intracerebroventricular injection of test compounds within 1 week of arrival at the testing facility. All experiments were carried out during the light cycle (0600-1800 h).
Induction and Measurement of Akinesia. Rats were injected with reserpine (5 mg/kg s.c., dissolved in 1% acetic acid) and kept in their home cages for 1.5-2 h after injection. Activity was measured by placing rats in photocell-activity cages (Hamilton-Kinder, Poway, CA) equipped with 16 × 16 infrared beams. After a 30-min baseline period, rats were given a single intracerebroventricular injection (0.5 μl/min) of either PHCCC (Tocris Cookson; 75 nmol/2.5 μl in vehicle), CPCCOEt (Tocris Cookson; 75 nmol/2.5 μl in vehicle) or vehicle control (2.5 μl of 40% DMSO in 0.85% NaCl). Five minutes after the injection of test compound or vehicle, motor activity was recorded for an additional 30 min for each rat. Motor activity (cumulative beam breaks per 30-min period) was recorded both pre- and postdrug treatment for each rat. Changes in motor activity were analyzed by using a repeated-measures two-factor analysis of variance, where treatment (pre- versus postdrug; within factor) and drug (PHCCC, CPCCOEt, and vehicle; between factor) values were used for each rat. Post hoc comparisons were performed by using the Bonferroni test. Statistical significance was achieved when the P value was <0.05. Data are expressed as mean ± SEM.
Results
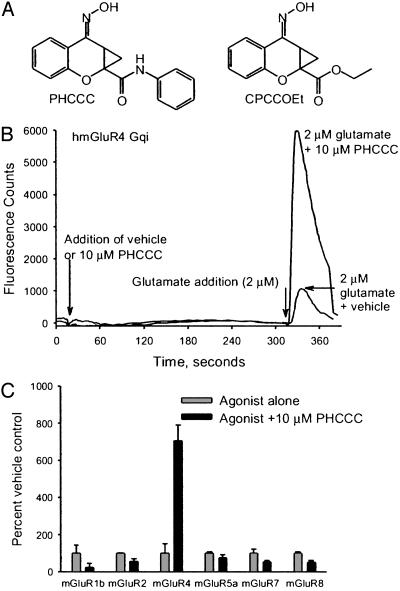
FLIPR Assay. The group I antagonist PHCCC (10 μM) (10) (Fig. 2A) was found to potentiate the response to glutamate (2 μM) 5.6-fold compared with glutamate alone (Figs. 2 B and C and 3A) measured in a FLIPR assay. In the absence of agonist, PHCCC had no effect on the activity of mGluR4 (Fig. 2B). Furthermore, PHCCC (10 μM) did not activate or potentiate responses to any other mGluR subtype examined. However, 10 μM PHCCC partially blocked responses of mGluR1b, mGluR2, mGluR5a, and mGluR8 to glutamate (Fig. 2C and Table 1).
Fig. 2.
(A) Structures of PHCCC and CPCCOEt. (B) Example trace from FLIPR assay demonstrating the effect of 2 μM glutamate on Chinese hamster ovary cells expressing human mGluR4 coupled to the chimeric G protein Gqi. Addition of glutamate in the presence of PHCCC produces a pronounced potentiation. Note the lack of effect of PHCCC alone. (C) Effect of PHCCC on the response of mGluRs to agonist. Cells expressing mGluRs were preincubated with PHCCC or vehicle for 5 min before addition of agonist. Final concentration of PHCCC was 10 μM. Agonist concentrations were as follows: human GluR1b, 100 μM glutamate; human mGluR4, 0.050 μM L-AP4; human mGluR5a, 1 μM glutamate; human mGluR7, 1 mM glutamate; human mGluR8, 0.3 μM glutamate. Response to agonist in the presence of PHCCC is normalized to the response to agonist alone. Each bar represents the mean ± SEM from four to eight independent experiments.
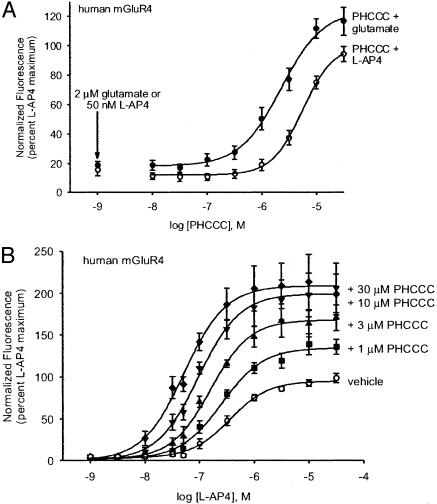
Fig. 3.
Effect of PHCCC on the response of human mGluR4 to L-AP4. Chinese hamster ovary cells expressing human mGluR4 and the chimeric G protein Gqi were preincubated with various concentrations of PHCCC or vehicle for 5 min before addition of agonist. (A) Dose-response relationship of PHCCC determined from studies in which the final agonist concentrations were 50 nM L-AP4 or 2 μM glutamate. Each graph represents the mean ± SEM of data from eight independent experiments. (B) PHCCC shifts the dose-response relationship of L-AP4. Note both the leftward shift in EC50 and the dramatic increase in maximal response. Data represent results obtained in nine independent experiments. All values were normalized to the response to 10 μM L-AP4 alone to allow comparison between conditions.
Table 1. The effects of PHCCC on mGluR responses to agonists.
| mGluRs | PHCCC, IC50 or EC50, μM ± SEM (n) | CPCCOEt, IC50, μM ± SEM (n) |
|---|---|---|
| mGluR1* | Rat 2.7 ± 0.8 (5) | Rat 10.0 ± 2.2 (5) |
| Human 0.32 ± 0.13 (5) | Human 1.2 ± 0.3 (6) | |
| mGluR2† | Human 14.9 ± 1.76 (3) Potentiator EC50 | Human > 100 (3) Antagonist IC50 |
| mGluR4‡ | Rat 3.7 ± 0.9 (3) | Rat > 100 (2) |
| Human 4.1 ± 1.2 (3) | Human > 100 (2) | |
| mGluR5§ | Rat 123 ± 33 (5) | Rat 62.8 ± 28.6 (3) |
| Human 57 ± 4 (6) | Human 43.2 ± 7.8 (5) | |
| mGluR7¶ | Human > 100 | Human > 100 |
| mGluR8∥ | Human 14.7 ± 2.3 (4) | Human > 100 (4) |
Agonist, 300 μM glutamate.
Agonist, 2 μM glutamate.
Agonist, 50 nM L-AP4 for potentiator EC50 values, 500 nM L-AP4 for antagonist IC50 values.
Agonist, 1 μM glutamate.
Agonist, 1 mM glutamate.
Agonist, 300 nM L-AP4.
PHCCC potentiated the response of human mGluR4 to 50 nM L-AP4 with an EC50 value of 4.1 ± 1.2 μM (Fig. 3A and Table 1). Similar values were found by using glutamate as the agonist (EC50 = 3.1 ± 0.9 μM), and for rat mGluR4 by using either L-AP4 or glutamate as the agonist (EC50 values were 3.7 ± 0.9 μM and 2.0 ± 0.5 μM, respectively). L-AP4 concentration-response curves were shifted to the left in the presence of 10 μM PHCCC. EC50 values for L-AP4 activation of human mGluR4 were 484 ± 45 nM (n = 3) in the absence of PHCCC and 71.4 ± 2.9 nM (n = 3) with 10 μM PHCCC, and for rat mGluR4, 832 ± 100 nM (n = 3) without PHCCC and 67.6 ± 5.2 nM (n = 3) with 10 μM PHCCC. The maximal effect of L-AP4 was increased approximately 2-fold in the presence of 10 μM PHCCC, suggesting PHCCC also increases the intrinsic efficacy of agonists (Fig. 3B). Similar effects were observed with glutamate as the agonist (glutamate EC50 values, human mGluR4 = 11.9 ± 1.0 μM; +10 μM PHCCC = 1.0 ± 0.1 μM; rat mGluR4 = 32.1 ± 8.4 μM; +10 μM PHCCC = 1.5 ± 0.1 μM)
The potency of PHCCC in antagonizing mGluR1 agonist responses was similar to or greater than its potency in potentiating mGluR4 agonist responses (Table 1). CPCCOEt, a closely related analogue of PHCCC (Fig. 2 A), has also been reported to have mGluR1 antagonist activity (10). CPCCOEt was tested in the FLIPR assay for its ability to potentiate mGluR4. CPCCOEt did not have any effect on the response of mGluR4 to agonists at concentrations up to 30 μM, although at higher concentrations it appears to be an mGluR4 antagonist (IC50 > 100 μM). CPCCOEt was a potent mGluR1-selective antagonist in the FLIPR assay, consistent with previous reports, and similar to PHCCC in this regard (Table 1). The lack of mGluR4 potentiator activity of CPCCOEt and the similarity of the antagonist activities of this compound to those of PHCCC suggest that CPCCOEt provides an appropriate control for in vivo studies.
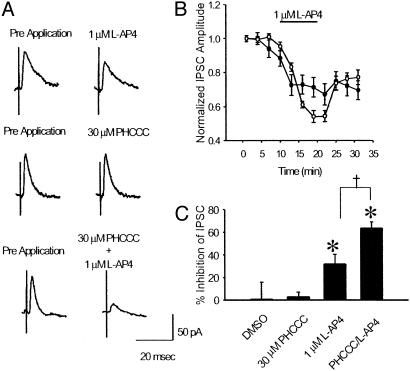
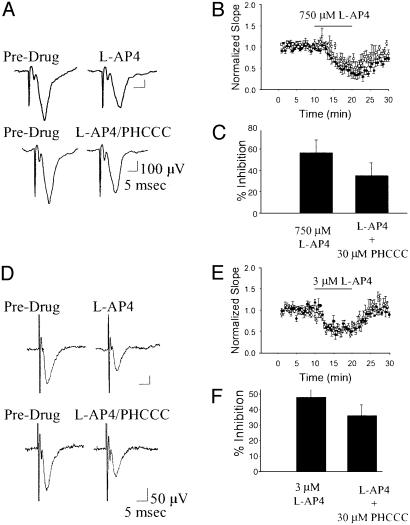
Electrophysiology. Our studies have shown that mGluR4 is presynaptically localized at the striatopallidal synapse (16) and that activation of this receptor produces a marked inhibition of GABAergic transmission at this synapse that likely underlies the antiparkinsonian actions of group III agonists (9). We therefore tested for the ability of PHCCC to potentiate the effects of a low dose of the group III mGluR agonist L-AP4 on striatopallidal transmission. Consistent with our previous findings, application of 1 μM L-AP4 produced a small but significant inhibition of transmission at the striatopallidal synapse (Fig. 4; P < 0.01, paired t test, n = 4). Application of vehicle (1% DMSO) or 30 μM PHCCC alone had no effect on striatopallidal transmission. However, consistent with our findings in recombinant systems, coapplication of 30 μM PHCCC and 1 μM L-AP4 produced a marked inhibition (P < 0.01, paired t test, n = 4). The effect of L-AP4 in the presence of the potentiator was significantly greater than the effect of L-AP4 alone (P < 0.05, ANOVA, Fisher's least significant difference). To determine whether the selectivity for mGluR4 observed in our recombinant studies was evident in the native-slice preparation, we took advantage of two previously characterized synapses in the hippocampus that are known to be modulated by activation of other members of the group III mGluRs. We performed recordings of field excitatory postsynaptic potentials from the Schaffer collateral-CA1 synapse and the lateral perforant path-dentate gyrus synapse as described (14, 15). Previous studies have demonstrated that activation of a group III mGluR at the Schaffer collateral-CA1 synapse produces a presynaptic inhibition of transmission (14). Based on the high level of mGluR7 protein and the low potency of L-AP4 at this synapse, this L-AP4-induced decrease in transmission is likely mediated by mGluR7. In addition, previous reports suggest that the activation of mGluR8 inhibits transmission at the lateral perforant path-dentate gyrus synapse (15, 17). Based on these studies, we chose submaximal concentrations of L-AP4 that produced a significant decrease in the field excitatory postsynaptic potential slope and looked for potentiation of these effects by PHCCC. Consistent with the results obtained in our recombinant studies, PHCCC did not potentiate the L-AP4-induced inhibition of transmission at these two synapses (Fig. 5) and, in fact, produced a slight nonsignificant decrease in the effect of L-AP4. Taken together, these findings suggest that PHCCC acts as a selective potentiator of mGluR4 in this native in vitro preparation.
Fig. 4.
PHCCC potentiates the effects of L-AP4 in a native system. Example traces (A) and summary graphs (B and C) illustrate the effect of PHCCC on L-AP4-mediated inhibition of transmission at the striatopallidal synapse. Whereas a submaximal concentration of L-AP4 produces a small but significant inhibition of transmission at the striatopallidal synapse, the combination of 1 μM L-AP4 and 30 μM PHCCC produces a significantly greater inhibition of transmission. *, Significant drug effect (P < 0.01, paired t test data from four independent experiments per group); †, significant difference between groups (P < 0.05, ANOVA, Fisher's least significant difference, n = 4).
Fig. 5.
PHCCC does not alter L-AP4-induced inhibition of transmission mediated by other group III mGluRs. Example traces (A and D) and summary graphs (B, C, E, and F) illustrate the lack of effect of PHCCC on L-AP4-mediated inhibition of transmission at the Schaffer Collateral-CA1 synapse (A-C) (P = 0.12, Student's t test, n = 6) and the lateral perforant path-dentate gyrus synapse (C and D)(P = 0.10, Student's t test, n = 6). Graphs represents mean ± SEM of data from six independent experiments per group.
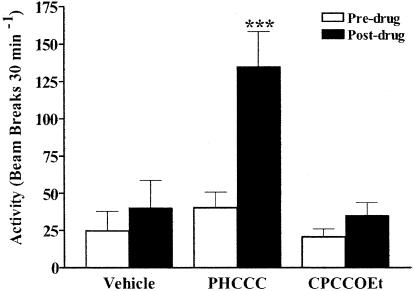
Allosteric Modulation of mGluR4 Produces an Antiparkinsonian Effect in a Dopamine Depletion Akinesia Model. Our previous studies have demonstrated that activation of mGluR4 presynaptically localized at the striatopallidal synapse decreased inhibitory transmission at this synapse. The resultant renormalization of the indirect pathway is believed to underlie the antiparkinsonian actions of L-AP4 in several animal models of PD (9). We therefore tested for the ability of PHCCC to reverse motor deficits in a reserpine-induced akinesia rodent model of PD. As shown in Fig. 6, PHCCC produced a significant increase in locomotor activity, whereas vehicle or CPCCOEt treatment had no effect under the same conditions. This observation was confirmed by the finding of significant main effects for test drug, treatment (pre- versus postdrug), and the interaction between drug and treatment [drug effect, F(2, 9) = 6.53, P < 0.05; treatment effect, F(1, 9) = 30.53, P < 0.001; drug × treatment interaction, F(2, 9) = 12.39, P < 0.01]. Before administering test compounds (predrug), the level of reserpine-induced movement deficits for rats randomly assigned to vehicle, PHCCC, and CPCCOEt treatment groups were similar (F(2, 9) = 1.01, P = 0.40). Post hoc analysis revealed that PHCCC, but not vehicle or CPCCOEt, demonstrated significantly greater activity after treatment (P < 0.001). Taken together, these findings suggest that PHCCC is a positive allosteric modulator of mGluR4 in both recombinant and native systems. The in vivo antiparkinsonian actions of PHCCC lend further support to the hypothesis that activation of mGluR4 represents a viable therapeutic approach for the treatment of PD and provides evidence that allosteric potentiators of the group III mGluRs have therapeutic potential.
Fig.6.
PHCCC produces an antiparkinsonian effect in a rodent model of PD. Shown are the effects of vehicle, PHCCC, and CPCCOEt on reserpine-induced motor deficits in rats (n = 4 per group). *, P < 0.001 for PHCCC posttreatment values compared with the corresponding pretreatment values for the same group.
Discussion
The treatment of PD can be divided into areas of disease modification and symptomatic relief. To date, all available interventions are palliative, and no currently available agents have been demonstrated to halt or reverse the progression of the disease. The current pharmacological treatments for PD are largely limited to dopamine-replacement strategies and are associated with a progressive decline in reliable efficacy and a variety of cognitive and motor side effects. Our previous studies have suggested that a new approach to the treatment of PD may lie in the selective activation of mGluR4 and the subsequent decrease in transmission at the striatopallidal synapse (9). This approach is attractive for several reasons. First, the relatively restricted distribution of mGluR4 (16, 18) suggests that activation of this receptor would produce a somewhat selective decrease in striatopallidal transmission. Second, by targeting the striatopallidal synapse and bypassing the striatal dopamine system, palliative benefit can be achieved while avoiding the severe side effects associated with dopamine-replacement therapy. Finally, by avoiding potential oxidative stress associated with l-dopa therapy, and by decreasing glutamatergic drive through the indirect pathway onto midbrain dopamine neurons, this approach may theoretically lead to a slowing of disease progression (reviewed in ref. 8).
Positive allosteric modulation of G protein-coupled receptors represents an attractive approach to the development of selective compounds both for the characterization of receptor function and for the development of therapeutic compounds. Although the positive allosteric approach has been proven clinically useful in ligand-gated GABA type A receptors, to date no clinically available compounds have been produced that act at an allosteric site on a G protein-coupled receptor.
In the mGluRs, potent orthosteric agonists and antagonists have been discovered (reviewed in ref. 19). However, it has traditionally been very difficult to obtain small molecules acting at the agonist-binding site that achieve true selectivity between members of one of the three groups of mGluRs. Theoretically, it would be expected that allosteric sites not be subjected to the same evolutionary pressure as the orthosteric sites that bind endogenous ligand. Therefore, generation of highly selective allosteric agents might represent a more tractable approach to small-molecule discovery. With the advent of high-throughput functional assays, it has been possible to expand the search for pharmacological tools to include compounds that act on receptors at allosteric sites rather than at the historically targeted orthosteric sites. The first such compounds described for the mGluRs were 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and CPCCOEt, negative allosteric modulators selective for mGluR5 and mGluR1, respectively (20, 21). The development of these compounds provided the first pharmacological tools that allowed definitive assignment of function to a given mGluR subtype and provided surprising insight into the rich diversity of cellular function mediated by closely related receptors (for review see ref. 22). Recently, positive allosteric modulators selective for mGluR1 (23) and mGluR5 (12) also have been identified. The current study represents a continuation of this approach. We have identified PHCCC as a selective allosteric potentiator of mGluR4 that is active in both recombinant and native systems. A recent report by Flor and coworkers confirms our finding that PHCCC potentiates mGluR4 function in recombinant systems.¶
Although PHCCC is a highly selective potentiator of mGluR4, this compound does exhibit antagonist activity at most mGluRs tested. Fortunately, the closely related analog CPCCOEt possesses most of the same antagonist properties and, therefore, serves as a control for the non-mGluR4-mediated actions of PHCCC. This finding is particularly important in the case of mGluR5, because previous studies have suggested that mGluR5 antagonist may produce antiparkinsonian action (24, 25). It should be noted that we found significant antagonism by PHCCC to both mGluR2 and mGluR8 that is not shared with CPCCOEt. However, blockade of either of these receptors is unlikely to produce antiparkinsonian actions. Previous studies have demonstrated an antiparkinsonian action of mGluR2/3 agonists (26, 27), suggesting that mGluR2 antagonists would in fact be proparkinsonian. In mGluR8, the receptor is distributed at a relatively modest level throughout the basal ganglia motor circuit (28) and has not been found to play a role in modulation of any of the key synapses in this circuit (9, 29, 30). Although this finding suggests that the antiparkinsonian effect of PHCCC is due to action on mGluR4, we cannot rule out the possibility that these effects are due to a combined activation of mGluR4 and antagonism of another mGluR.
A recent report by Mathiesen et al. (31) demonstrated allosteric potentiation of mGluR4 by compounds previously described as noncompetitive antagonists of mGluR5. This finding is of interest in light of reports that one of these compounds, MPEP, exhibits antiparkinsonian actions in rodent models (24, 25). However, at the 10 mg/kg dose found effective in these behavioral studies, MPEP occupies <100% of mGluR5 receptors (32). Given that the MPEP has nanomolar affinity for mGluR5 but only potentiates mGluR4 at concentrations >50 μM, it is unlikely that a 10 mg/kg dose will produce potentiation of mGluR4 in vivo. Therefore, the antiparkinsonian actions of MPEP are likely caused by actions at mGluR5.
One of the more attractive aspects of allosteric modulation of G protein-coupled receptors is that this approach may achieve greater selectivity for brain regions in which the receptor is activated by the endogenous ligand. This type of selectivity could lead to both a reduced side-effect profile and a reduction in receptor down-regulation and desensitization that could be produced by chronic treatment with a direct orthosteric agonist. However, this finding could be problematic in that the endogenous ligand must be present at the site of action for the drug to be effective. Because the striatopallidal projection is GABAergic, it is not known if sufficient glutamate will be available to activate mGluR4 at this synapse. However, the external globus pallidus (GPe) receives glutamatergic input from the subthalamic nucleus. Because the subthalamic nucleus is known to be overactive in animal models of PD and in PD patients, we reasoned that, in fact, a significant level of glutamate would be available in the GPe. The finding that PHCCC produced in vivo antiparkinsonian efficacy in the reserpinized rat model suggests that, in fact, a significant level of glutamate is available for the activation of the therapeutically relevant mGluR4. The lack of effect of PHCCC alone in our brain-slice experiments is not surprising because we only maintain a small part of the basal ganglia motor circuit in the brain-slice preparation. We expect that the excitatory drive to the GPe will be diminished by both the cutting of subthalamopallidal fibers and the removal of cortical drive to the subthalamic nucleus that is believed to be involved in the pathophysiology of PD (33).
Taken together, our findings provide a description of a positive allosteric modulator selective for one of the group III mGluRs, provide a pharmacological tool that will allow definitive determination of the role that mGluR4 plays in regulating neuronal function, and lend support to the hypothesis that allosteric potentiators of mGluR4 will be effective agents for the treatment of PD.
Acknowledgments
This work was supported by Merck Research Laboratories. P.J.C. is supported by grants from the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the National Institute of Mental Health.
Abbreviations: CPCCOEt, 7-(hydroxylimino)cyclopropa[b]chromen-1a-carboxamide ethyl ester; GPe, external globus pallidus; L-AP4, l-(+)-2-amino-4-phosphonobutyric acid; PD, Parkinson's disease; PHCCC, N-phenyl-7-(hydroxylimino)cyclopropa[b]chromen-1acarboxamide; FLIPR, fluorometric imaging plate reader; MPEP, 2-methyl-6-(phenylethynyl)-pyridine; mGluR, metabotropic glutamate receptor; GABA, γ-aminobutyric acid.
Footnotes
Flor, P., Maj, J., Dragic, Z., Bruno, V., Battaglia, G., Inderbitzin, W., VanDer Putten, H., Kuhn, R., Nicoletti, F. & Gasparini, F. (2002) Neuropharmacology 43, 286 (abstr.).
References
- 1.Hassler, R. (1938) J. Psychol. Neurol. 48, 387-476. [Google Scholar]
- 2.Erhinger, H. & Hornykiewicz, O. (1960) Klin. Wochenschr. 38, 1236-1239. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic, J. (2002) Neurology 58, 19S-32S. [Google Scholar]
- 4.Fahn, S. (1999) Arch. Neurol. 56, 529-535. [DOI] [PubMed] [Google Scholar]
- 5.Ahlskog, J. E. (2003) Neurology 60, 381-389. [DOI] [PubMed] [Google Scholar]
- 6.Wichmann, T. & DeLong, M. R. (1998) Neurosurg. Clin. N. Am. 9, 223-236. [PubMed] [Google Scholar]
- 7.Lozano, A. & Carella, F. (2002) Parkinsonism Relat. Disord. 8, 455-458. [DOI] [PubMed] [Google Scholar]
- 8.Marino, M., Valenti, O. & Conn, P. J. (2003) Drugs Aging 20, 377-397. [DOI] [PubMed] [Google Scholar]
- 9.Valenti, O., Marino, M. J., Wittmann, M., Lis, E., DiLella, A. G., Kinney, G. G. & Conn, P. J. (2003) J. Neurosci. 23, 7218-7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annoura, H., Fukunaga, A., Uesugi, M., Tatsouka, T. & Horikawa, Y. (1996) Bioorg. Med. Chem. Lett. 6, 763-766. [Google Scholar]
- 11.Lin, F. F., Varney, M., Sacaan, A. I., Jachec, C., Daggett, L. P., Rao, S., Flor, P., Kuhn, R., Kerner, J. A., Standaert, D., et al. (1997) Neuropharmacology 36, 917-931. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien, J. A., Lemaire, W., Chen, T.-B., Chang, R. S. L., Jacobsen, M. A., Ha, S. N., Lindsley, C. W., Schaffhauser, H., Sur, C., Pettibone, D. J., et al. (2003) Mol. Pharmacol. 64, 731-740. [DOI] [PubMed] [Google Scholar]
- 13.Cooper, A. J. & Stanford, I. M. (2001) Neuropharmacology 41, 62-71. [DOI] [PubMed] [Google Scholar]
- 14.Gereau, R. W. & Conn, P. J. (1995) J. Neurosci. 15, 6879-6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macek, T. A., Winder, D. G., Gereau, R. W., Ladd, C. O. & Conn, P. J. (1996) J. Neurophysiol. 76, 3798-3806. [DOI] [PubMed] [Google Scholar]
- 16.Bradley, S. R., Standaert, D. G., Rhodes, K. J., Rees, H. D., Testa, C. M., Levey, A. I. & Conn, P. J. (1999) J. Comp. Neurol. 407, 33-46. [PubMed] [Google Scholar]
- 17.Cai, Z., Saugstad, J. A., Sorensen, S. D., Ciombor, K. J., Zhang, C., Schaffhauser, H., Hubalek, F., Pohl, J., Duvoisin, R. M. & Conn, P. J. (2001) J. Neurochem. 78, 756-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corti, C., Aldegheri, L., Somogyi, P. & Ferraguti, F. (2002) Neuroscience 110, 403-420. [DOI] [PubMed] [Google Scholar]
- 19.Schoepp, D. D., Jane, D. E. & Monn, J. A. (1999) Neuropharmacology 38, 1431-1476. [DOI] [PubMed] [Google Scholar]
- 20.Gasparini, F., Lingenhohl, K., Stoehr, N., Flor, P. J., Heinrich, M., Vranesic, I., Biollaz, M., Allgeier, H., Heckendorn, R., Urwyler, S., et al. (1999) Neuropharmacology 38, 1493-1503. [DOI] [PubMed] [Google Scholar]
- 21.Litschig, S., Gasparini, F., Rueegg, D., Stoehr, N., Flor, P. J., Vranesic, I., Prezeau, L., Pin, J. P., Thomsen, C. & Kuhn, R. (1999) Mol. Pharmacol. 55, 453-461. [PubMed] [Google Scholar]
- 22.Valenti, O., Conn, P. J. & Marino, M. J. (2002) J. Cell. Physiol. 191, 125-137. [DOI] [PubMed] [Google Scholar]
- 23.Knoflach, F., Mutel, V., Jolidon, S., Kew, J. N., Malherbe, P., Vieira, E., Wichmann, J. & Kemp, J. A. (2001) Proc. Natl. Acad. Sci. USA 98, 13402-13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breysse, N., Baunez, C., Spooren, W., Gasparini, F. & Amalric, M. (2002) J. Neurosci. 22, 5669-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ossowska, K., Konieczny, J., Wolfarth, S., Wieronska, J. & Pilc, A. (2001) Neuropharmacology 41, 413-420. [DOI] [PubMed] [Google Scholar]
- 26.Dawson, L., Chadha, A., Megalou, M. & Duty, S. (2000) Br. J. Pharmacol. 129, 541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley, S. R., Marino, M. J., Wittmann, M., Rouse, S. T., Awad, H., Levey, A. I. & Conn, P. J. (2000) J. Neurosci. 20, 3085-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messenger, M. J., Dawson, L. G. & Duty, S. (2002) Neuropharmacology 43, 261-271. [DOI] [PubMed] [Google Scholar]
- 29.Awad-Granko, H. & Conn, P. J. (2001) Neuropharmacology 41, 32-41. [DOI] [PubMed] [Google Scholar]
- 30.Wittmann, M., Marino, M. J., Bradley, S. R. & Conn, P. J. (2001) J. Neurophysiol. 85, 1960-1968. [DOI] [PubMed] [Google Scholar]
- 31.Mathiesen, J. M., Svendsen, N., Brauner-Osborne, H., Thomsen, C. & Ramirez, M. T. (2003) Br. J. Pharmacol. 138, 1026-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban, M. O., Hama, A. T., Bradbury, M., Anderson, J., Varney, M. A. & Bristow, L. (2003) Neuropharmacology 44, 983-993. [DOI] [PubMed] [Google Scholar]
- 33.Magill, P. J., Bolam, J. P. & Bevan, M. D. (2000) J. Neurosci. 20, 820-833. [DOI] [PMC free article] [PubMed] [Google Scholar]