Abstract
Boron (B) is an essential micronutrient for vascular plants. However, it remains unclear how B deficiency leads to various metabolic disorders and cell death. To understand this mechanism, we analyzed the physiological changes in suspension-cultured tobacco (Nicotiana tabacum) BY-2 cells upon B deprivation. When 3-day-old cells were transferred to B-free medium, cell death was detectable as early as 12 h after treatment. The B-deprived cells accumulated more reactive oxygen species and lipid peroxides than control cells, and showed a slight but significant decrease in the cellular ascorbate pool. Supplementing the media with lipophilic antioxidants effectively suppressed the death of B-deprived cells, suggesting that the oxidative damage is the immediate and major cause of cell death under B deficiency. Dead cells in B-free culture exhibited a characteristic morphology with a shrunken cytoplasm, which is often seen in cells undergoing programmed cell death (PCD). However, they did not display other hallmarks of PCD such as internucleosomal DNA fragmentation, decreased ascorbate peroxidase expression and protection from death by cycloheximide. These results suggest that the death of tobacco cells induced by B deprivation is not likely to be a typical PCD.
Keywords: Boron deficiency, Cell death, Necrosis, Oxidative damage, Tobacco
Introduction
Boron (B) is an essential micronutrient for vascular plants, and B deficiency is one of the major constraints to crop production worldwide (Shorrocks 1997). Most plant species cannot retranslocate B efficiently, and therefore require a continuous supply of B throughout their life cycle. The symptoms of B deficiency include rapid cessation of root elongation, inhibited growth of young leaves and reduced fertility (Marschner 1995). At the cellular level, B deprivation harms numerous physiological processes, including sugar transport, cell wall synthesis, lignification, cell wall structure, carbohydrate metabolism, RNA metabolism, respiration, IAA metabolism, phenol metabolism and membrane transport (Parr and Loughman 1983), and eventually brings about cell death (Matoh et al. 1992).
In the last decade, much progress has been made in understanding the physiological function of B in plants. We previously reported that B, as a borate, cross-links two rhamnogalacturonan II (RG-II) regions of pectic polysaccharides in cell walls (Kobayashi et al. 1996). Studies using plants with a mutant RG-II structure showed that the B–RG-II complex is necessary for normal plant growth (O’Neill et al. 2001, Iwai et al. 2002, Ahn et al. 2006), and now B has been established as essential for cell wall structure and function (O’Neill et al. 2004). These findings imply that the primary effect of B shortage is to disturb the structural organization of cell walls. Consistent with this assumption, Goldbach's group has reported that the physical properties of squash root cell walls change within minutes after B deprivation (Findeklee and Goldbach 1997, Goldbach et al. 2001). However, it remains unclear how and why B deficiency, and probably the resulting aberrant cell wall structure, leads to such a variety of metabolic disorders and cell death. Elucidating this process is important for a better understanding of the function of B, and for the development of methods to prevent crop loss due to B deficiency.
Most previous studies on B deficiency have been carried out using intact plants. However, the interpretation of experimental results is complicated because intact plant tissues consist of various types of differentiated cells which probably differ in their requirements for B. In addition, since only a proportion of the cells in a tissue are in direct contact with the external solution, most cells might remain unaffected when B is withdrawn from the medium. These experimental problems might be circumvented by the use of suspension-cultured cells, as they are uniform and their surrounding media are easy to control. Cultured tobacco BY-2 cells are especially suitable because they do not aggregate significantly, and essentially all the cells are in direct contact with the external medium. Moreover, their death process has been characterized in detail in recent studies (de Pinto et al. 2002, Vacca et al. 2004, de Pinto et al. 2006). These features would allow us to dissect the process of B deficiency-induced cell death precisely. Here we describe the physiological changes of tobacco BY-2 cells observed upon B deprivation. The results show that oxidative damage is directly involved in the cell death, but the cells do not undergo a typical programmed cell death.
Results
Boron deprivation-induced oxidative damage to tobacco BY-2 cells
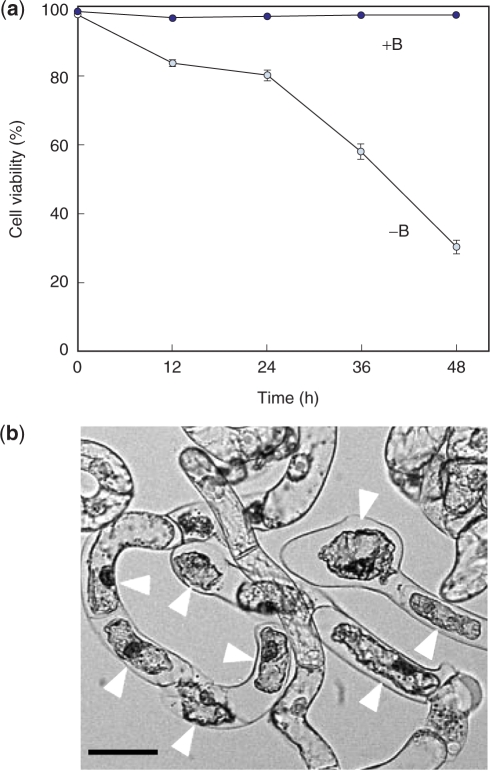
First we examined the effects of B deprivation on the viability of tobacco BY-2 cells. Three-day-old cultures, which were in the log phase of growth, were used in this study. Cells were washed with medium with or without B, and then cultured in the same medium (hereafter referred to as +B or −B treatment, respectively). Cell viability was analyzed by fluorescein diacetate (FDA)/propidium iodide (PI) staining, and a representative result is shown in Fig. 1a. With +B treatment, dead cells comprised <3% of the total cells throughout the experimental period (Fig. 1a). In contrast, with −B treatment, cell death was detectable as early as 12 h after treatment, and the viability decreased to 30% by 48 h (Fig. 1a). When total cells, dead or alive, were counted 24 h after treatment, the −B culture contained 60–70% as many cells as the +B culture did (data not presented). Since the proportion of live proliferating cells in the −B culture had already decreased to approximately 80% at 12 h (Fig. 1a), and since the doubling time of tobacco BY-2 cells is approximately 12 h (Nagata et al. 1992), the −B culture would be predicted to contain around 70% as many cells as the +B culture at 24 h, if cell division is not affected by the treatment. The predicted cell number is consistent with the observed results, suggesting that cell division was not significantly impaired upon B deprivation. Microscopic examination of dead cells in the −B culture revealed a characteristic morphology with a shrunken cytoplasm (Fig. 1b).
Fig. 1.
Cell death induced by B deprivation. (a) The time-dependent change of cell viability. Cells were washed with and cultured in control (+B) or B-free (−B) media and were examined for viability with FDA/PI staining. Values represent the percentage of FDA-stained live cells, which is an average of the results obtained from four randomly selected fields. (b) Morphology of dead cells in −B culture. Cells were observed under bright-field microscopy 48 h after treatment. Arrowheads indicate dead cells. Bar = 50 μm.
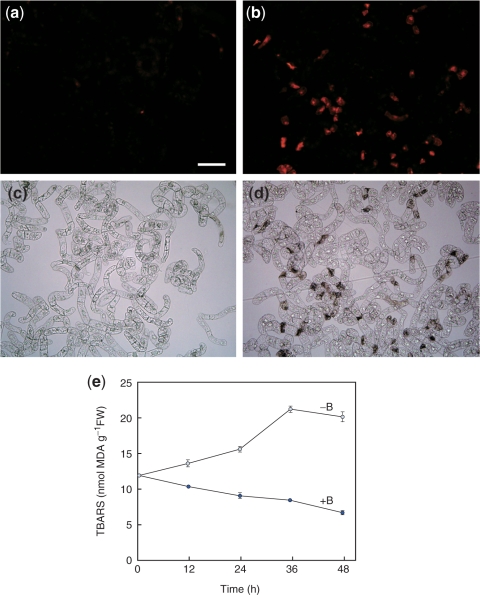
We previously showed that the genes for antioxidant enzymes are up-regulated in low B-acclimated cells, which suggests that oxidative damage is involved in low B stress (Kobayashi et al. 2004). To test this hypothesis, we examined the accumulation of reactive oxygen species (ROS) in −B cells, using dihydroethidium (DHE) as a probe. In the presence of ROS, DHE is oxidized to ethidium that intercalates with DNA to emit red fluorescence. As shown in Fig. 2a–d, the ethidium-derived fluorescence was detectable at 12 h in −B cells, but not in +B cells. This result indicates that ROS accumulated in −B cells. We then analyzed lipid peroxidation in these cells as a measure of oxidative damage, using the thiobarbituric acid reactive substances (TBARS) assay. TBARS are the product of lipid peroxidation, and high levels of these substances correlate with high levels of oxidative damage to membranes (Heath and Packer 1968). As shown in Fig. 2e, more TBARS accumulated in −B cells than in +B cells as early as 12 h, and the levels in −B cells increased even further over time.
Fig. 2.
Oxidative stress induced by B deprivation. (a–d) Accumulation of ROS. At 12 h after treatment, +B (a, c) or −B (b, d) cells were stained with DHE and observed under fluorescence (a, b) or bright-field (c, d) microscopy. Bar = 100 μm. (e) Accumulation of lipid peroxides. Lipid peroxides were quantified by the TBARS method and expressed as nmol malondialdehyde (MDA) per gram fresh weight. Each value is the mean of two replicates and the error bar represents the difference between the replicates.
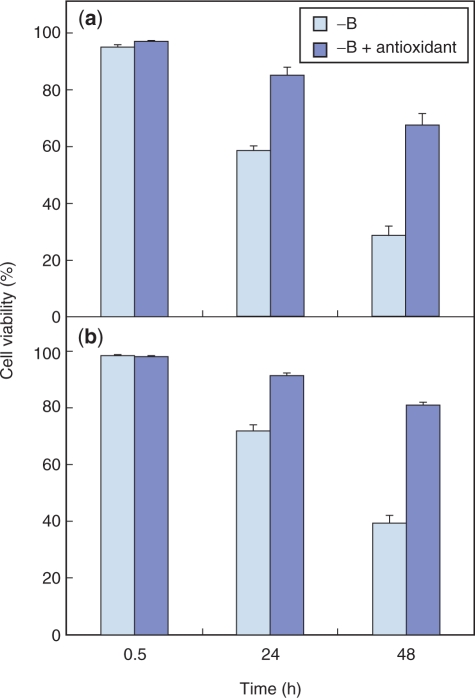
If oxidative damage plays a critical role in cell death induced by B deprivation, then this cell death would be expected to be suppressed by the addition of antioxidants. We therefore examined the effect of butylated hydroxyanisole (BHA), a lipophilic antioxidant that reportedly prevents antimycin A- or aluminum stress-induced ROS accumulation (Maxwell et al. 1999, Yamamoto et al. 2002), upon B deprivation-induced cell death. Supplementing the medium with BHA at 0.1 mM did not affect the viability of +B cells (data not shown). On the other hand, it significantly improved the viability of −B cells (Fig. 3a). α-Tocopherol, another lipophilic antioxidant, also suppressed the death of −B cells (Fig. 3b). Although the cells in Fig. 3b were fed with α-tocopherol prior to the treatment, adding α-tocopherol after B removal was also effective (data not shown). These results were not attributable to contaminating B in the reagents; possible B contamination from BHA or α-tocopherol was estimated to be <0.03 μg B liter–1, which is far lower than the amount remaining in the B-depleted medium (typically 2–5 μg B liter–1).
Fig. 3.
Effect of antioxidant on cell death induced by B deprivation. After pre-culture for 1 h in a standard growth medium supplemented with 0.1 mM of either BHA (a) or α-tocopherol (b), cells were washed with and transferred to a B-free medium supplemented with the same 0.1 mM antioxidant. Cells treated in the same way but without antioxidant were prepared as a control. Each bar shows the mean of results from four randomly selected fields ± SD.
Effects of B resupply
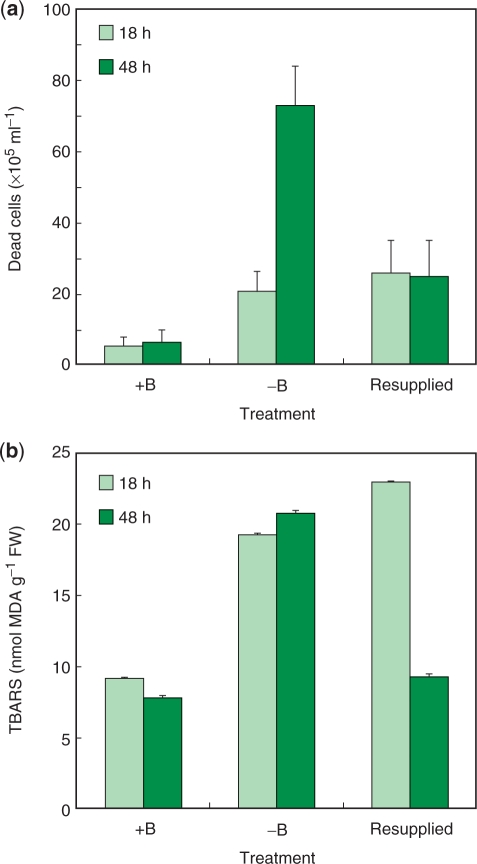
When B (1 mg liter–1 as boric acid) was resupplied to the cells that had been deprived of B for 24 h, the cell viability at 48 h (24 h after resupply) was as high as 68%, significantly higher than for cells remaining in −B medium for the same period (30%; Fig. 1a). We next counted the number of dead cells to investigate the effect of B resupply on cell viability. For this analysis, duplicate cultures were deprived of B, and then supplemented after 18 h with either boric acid at 1 mg B liter–1 (‘resupplied culture’) or water (‘−B culture’). Cells washed with and cultured in control medium were also prepared as a control (‘+B culture’). Cultures that had been deprived of B for 18 h (−B and resupplied cultures) contained significantly more dead cells than the control (+B culture) (Fig. 4a). However, during the subsequent 30 h, while the number of dead cells increased further in the −B culture, it did not increase further in the resupplied culture (Fig. 4a). Resupplying B at 24 h gave essentially the same results; the dead cell number was 33 ± 5 × 105 ml–1 at the time of resupply (24 h), and 32 ± 7 × 105 ml–1 at 24 h after resupply (48 h). These results indicate that the B resupply immediately suppressed further cell death and allowed the surviving cells to resume proliferation.
Fig. 4.
Effects of B resupply on cell death and the accumulation of lipid peroxides. (a) Number of dead cells. Duplicate cultures were deprived of B, and then supplemented after 18 h with either boric acid at 1 mg B liter–1 (‘resupplied culture’) or water (‘−B culture’). Cells washed with and cultured in control medium were also prepared as a control (‘+B culture’). Aliquots of the cell suspensions were withdrawn and dead cells were counted after FDA/PI staining. Each value is an average of the results of six independent counts. (b) Lipid peroxide contents. Cells were treated as in (a) and their lipid peroxides were quantified as in Fig. 2e. Each value is the mean of two replicates, and the error bar represents the difference between replicates.
B resupply also suppressed lipid peroxidation. At 18 h after B deprivation, −B cultures contained twice as much TBARS as +B cultures (Fig. 4b). However, by 48 h, the TBARS in resupplied cultures decreased to the same level as that in the +B cultures, whereas it increased even further in the −B cultures (Fig. 4b). Taken together, these results indicate that the B deprivation-induced oxidative stress was removed immediately upon B resupply.
Antioxidants and antioxidant enzyme activities
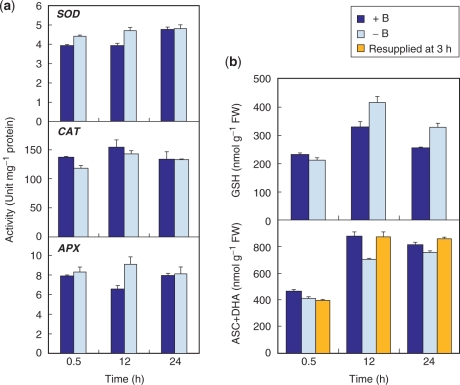
We next explored possible changes in cellular antioxidant systems in cells deprived of B. To detect any change that precedes oxidative injury, the analyses were done at 24 h, when >80% of the cells were still alive (Fig. 1a). When compared with +B cells, −B cells did not show a marked decrease in the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) (Fig. 5a). We then examined changes in the pools of reduced glutathione (GSH) and total ascorbate [ascorbate (ASC) plus dehydroascorbate (DHA)]. The quantity of both antioxidants showed transient increases during the first 12 h in both +B and −B cells, probably in response to the mechanical stress imposed by the cell washing procedure (Fig. 5b). The GSH content of −B cells was not significantly lower than that of +B cells at any of the time points observed (Fig. 5b, upper panel). On the other hand, the total ascorbate pool in 12 h −B cells was decreased by 20% with respect to +B cells (Fig. 5b, lower panel), although the ASC redox state (reduced ASC/total ASC) was unaffected (0.89 for both +B and −B cells). The change in total ascorbate pool appears rather marginal, but may nevertheless be directly involved in the oxidative damage since resupply of B at 3 h restored the total ascorbate pool at 12 h to the same level as that observed in 12 h +B cells (Fig. 5b).
Fig. 5.
Change in antioxidant capacity. (a) Antioxidant enzyme activities. Each value is the mean of three replicates ± SD. (b) Changes in contents of reduced GSH (upper panel) and total ascorbate (lower panel). Total ascorbate was also analyzed in the cells resupplied with B at 3 h. Each value is the mean of four (GSH) and three (total ascorbate) replicates ± SD, respectively.
The mode of cell death induced by B deprivation
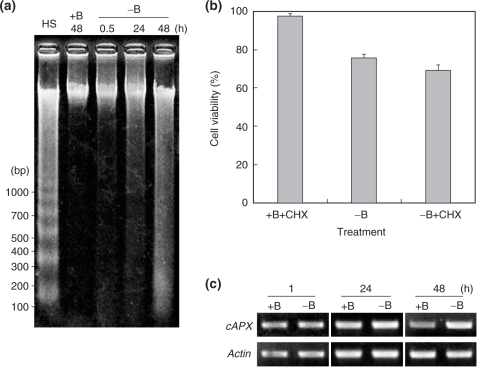
Dead cells in −B culture exhibited a shrunken cytoplasm (Fig. 1b), which is often observed in plant cells undergoing programmed cell death (PCD) (van Doorn and Woltering 2005). This observation prompted us to investigate whether PCD is also involved in the cell death induced by B deprivation. We first examined internucleosomal genomic DNA fragmentation in −B cells, one of the hallmarks of PCD (van Doorn and Woltering 2005). As shown in Fig. 6a, clear laddering was observed with DNA extracted from heat-shocked cells, a positive control for PCD (Vacca et al. 2004). On the other hand, smearing rather than laddering was observed with DNAs extracted from −B cells (Fig. 6a).
Fig. 6.
(a) DNA fragmentation analysis. Genomic DNA was extracted from cells at the indicated times after treatment. As a positive control for DNA laddering, DNA extracted from heat-shocked cells was included in the analysis (lane HS, DNA was extracted 72 h after treatment). The figure shows a representative result in which 400 ng of DNA was run in each lane. (b) Effect of CHX. Cells were treated as in Fig. 3 except that 0.35 μM CHX was used instead of antioxidants. Each bar shows the mean of results on four randomly selected fields ± SD. (c) Gene expression of cAPX. Semi-quantitative RT–PCR for cAPX was performed as described in the text. RT–PCR for the actin gene was also performed to calibrate the quantities of cDNA templates.
We also examined the effect of cycloheximide (CHX) on B deprivation-induced cell death. CHX has been reported to suppress PCD by inhibiting the synthesis of proteins that are required to execute the death program (Solomon et al. 1999, Clarke et al. 2000, Vacca et al. 2004, Duval et al. 2005). If PCD is involved, then supplementing the medium with CHX should suppress death induced by B deprivation. As a positive control for the CHX rescue effect, we confirmed that heat shock-induced PCD could be suppressed by 0.35 μM CHX (cell viability at 24 h was 30 ± 7% and 54 ± 3% in the absence or presence of CHX, respectively). However, the viability of −B cells in the presence of CHX (0.35 μM) was 70 ± 3% at 24 h after B deprivation, not significantly different from the viability in the absence of CHX (76 ± 2%), indicating that CHX did not suppress the death induced by B deprivation (Fig. 6b).
It has been reported that, in tobacco BY-2 cells, different kinds of cell death (PCD or cell necrosis) have different effects on cellular antioxidant systems (de Pinto et al. 2002, Vacca et al. 2004, de Pinto et al. 2006). One of the most distinctive differences is that the gene expression of cytosolic APX (cAPX) is down-regulated during PCD, but not during cell necrosis (de Pinto et al. 2006). We therefore examined cAPX expression as another diagnostic feature of PCD, but found the transcript at similar levels in −B cells and +B cells (Fig. 6c). Taken together, these data suggest that cell death induced by B deprivation is not likely to be a typical PCD.
Discussion
In this report we have described the physiological responses of suspension-cultured tobacco BY-2 cells to B deprivation, and have shown that ROS and lipid peroxides are accumulated in these cells. The results unequivocally demonstrate that B deprivation causes oxidative damage to the cells as a downstream effect. Previous studies have shown that B deficiency symptoms of Lemna are exaggerated under high light conditions (Tanaka 1966), that the cellular antioxidant pool decreases significantly in sunflower or squash under B deficiency (Cakmak et al. 1995, Lukaszewski and Blevins 1996, Cakmak and Römheld 1997) and that oxidative stress-responsive genes are up-regulated in low B-acclimated tobacco cells (Kobayashi et al. 2004). Although these observations are all consistent with the notion that oxidative injuries occur under B deficiency, none of these reports demonstrated that ROS accumulate in the cells. In the present study, we demonstrated the accumulation of ROS and lipid peroxides using DHE staining and TBARS assays. To our knowledge, this is the first direct proof that B-deficient cells suffer from oxidative damage.
Importantly, we furthermore demonstrated that B deprivation-induced cell death was effectively suppressed by lipophilic antioxidants (Fig. 3). This result indicates that oxidative damage is crucial and immediately responsible for the death of tobacco cells induced by B deprivation. Obviously, oxidative damage is not unique to B deficiency, but rather represents a consequential effect of the B deficiency stress. Nonetheless, the results are significant in that we have demonstrated that the oxidative damage is the major cause of death under B deficiency, and therefore plant cells could be less susceptible to B limitation if the oxidative stress can be circumvented. The results also suggest that enhancing the endogenous antioxidant capacity may be one of strategies for engineering B-efficient crops.
It is still debatable whether B plays a direct role(s) in membrane stabilization (Brown et al. 2002). The premise for this hypothesis is that B deprivation makes the plasma membrane depolarized and leaky (Brown et al. 2002). However, given that oxidative damage occurs under B deficiency, the observations of impaired membrane function could be attributed to membrane lipid peroxidation and may not necessarily be the direct effect of B deprivation. In addition, no membrane-associated compound has yet been demonstrated to occur as a complex with B in plant cells. Taken together, we consider that B is unlikely to function primarily as a membrane stabilizer.
Although the dead cells in −B cultures were morphologically similar to those undergoing PCD (Fig. 1b), they did not show other PCD hallmarks such as DNA laddering, decreased cAPX expression or protection from death by CHX. The immediate rescue effect of B resupply also does not support the involvement of PCD; if the death had been ‘programmed’ and initiated in −B cells before the morphological changes became visible, then B resupply at 18 h, when a substantial proportion of the cells are already dead (Fig. 1a), would not be able to suppress the subsequent cell death. In contrast, B resupply was found to be immediately effective (Fig. 4b). Taken together, we conclude that B deprivation-induced death in tobacco BY-2 cells is unlikely to be PCD.
It has been reported that hydrogen peroxide (H2O2) can induce different responses in tobacco BY-2 cells depending on the dose and duration of exposure (de Pinto et al. 2006). Under a threshold value, H2O2 induces a modest rise in cellular antioxidant capacity, such as the GSH pool and APX activity, as a defense mechanism against stress. On the other hand, higher amounts of H2O2 lead to depletion of antioxidant capacity and either PCD or cell necrosis; PCD is triggered if a higher amount of H2O2 is given in a single pulse, whereas cell necrosis occurs under more prolonged exposure to H2O2. In this study, we did not find any marked decrease in antioxidant enzyme activities upon B deprivation (Fig. 5a). We also found that the extracellular H2O2 concentrations were not significantly different between +B and −B cultures (data not shown). These data suggest that, in tobacco BY-2 cells, the B deprivation-triggered overproduction of ROS is not high enough to induce prompt cell death. In fact, the death of −B cells developed rather slowly (Fig. 1a), suggesting a low but continuous production of ROS. In this regard, however, we cannot exclude the possibility that B deprivation may induce more acute damage in some cases. Cakmak and Römheld (1997) reported a marked decrease in the content of ASC and non-protein SH compounds in B-deficient sunflower plants, which was not observed in this study. We assume that the discrepancy reflects the severity of oxidative damage imposed on the cells. In plants, ROS are inevitably produced in chloroplasts during photosynthesis (Foyer et al. 1994). Thus more ROS may accumulate in photosynthesizing green leaves than in heterotrophic cultured tobacco cells, when the redox balance is disturbed under B deficiency.
In this study we have demonstrated the involvement of oxidative damage in the final stage of B deficiency. What remains to be clarified is why B deficiency leads to the ROS overproduction. Considering that > 98% of B in tobacco BY-2 cells localizes in the cell wall (Matoh et al. 1992), and that B deprivation alters the physical properties of squash root cell walls within minutes (Findeklee and Goldbach 1997, Goldbach et al. 2001), it would be reasonable to assume that the B deprivation response begins with aberrations in the cell wall structure. At least two hypotheses could explain the subsequent events. First, when cell growth is arrested due to the inability to organize a normal cell wall, energy consumption would be decreased. This would lead to an over-reduction of the mitochondrial electron transport chain, which favors the generation of superoxide radical anions (Purvis 1997). A second possible mechanism is overaccumulation of ROS that have been produced as a signal for the disturbed cell wall structure. Ryden et al. (2003) demonstrated that the B–RG-II complex contributes significantly to maintaining the tensile strength of the cell wall, in studies using the Arabidopsis mutant mur1 with a defective RG-II structure. Their finding suggests that if the B–RG-II complex is not formed properly due to B deficiency, the cell wall would become weaker and less resistant to turgor pressure. In turn, this could trigger Ca2+ influx through stretch-activated Ca2+ channels in the plasma membrane (Nakagawa et al. 2007), and thereby activate the generation of ROS as signaling molecules (Lecourieux et al. 2006). Similar events may also occur in deprivation experiments. Since B–RG-II complexes are highly stable in vitro (Kobayashi et al. 1997), existing B–RG-II bonding in pectic polysaccharides may be retained even when B is removed from the medium. However, the mechanical strength of squash root cell walls is reduced within minutes after B deprivation (Findeklee and Goldbach 1997, Goldbach et al. 2001), suggesting that significant changes in cell wall structure occur upon B deprivation. This may be either because the B–RG-II bonding in vivo is less stable than in vitro, or because a failure in cross-linking newly secreted polysaccharides is critical. We assume that generation of ROS as a signal is a more plausible hypothesis because we previously found that several ROS-responsive genes are induced within 30 min after B deprivation in tobacco BY-2 cells (Kobayashi et al. 2004). Such a rapid induction is more likely to be triggered by the ROS produced as a signal, rather than the ROS resulting from reduced energy consumption.
Interestingly, the biochemical changes we observed in the B-deprived tobacco cells are similar to those in the aluminum-treated tobacco cells (Yamamoto et al. 2002), in which downstream oxidative stress plays a crucial role in inhibiting growth, and BHA effectively suppresses the damage. Given that the primary target of aluminum toxicity is the cell wall (Ma et al. 2004), these events may represent a general cellular response to the defects in the cell wall. A plant cell wall is a dynamic and responsive structure which is important for the symplast, not only as a mechanical support but also as an interface with the external environment (Hoson 1998). The idea is now emerging that cell walls can generate stress signals that induce cell death. For example, the Arabidopsis mutant cev1 has reduced cellulose content and constitutively expresses stress- and defense-responsive genes (Ellis et al. 2002), and the inhibition of cellulose synthesis by thaxtomin A or isoxaben induces PCD in Arabidopsis (Duval et al. 2005). However, research into the mechanism by which the symplast senses defects in the cell wall, and the pathway through which the signal is transmitted, is still in its infancy. Further studies on the responses of tobacco cells to B deprivation will certainly contribute to a better understanding of the cell wall–symplast interaction.
Materials and Methods
Cell culture and treatments
Suspension-cultured tobacco cells (Nicotiana tabacum L. cv. Bright Yellow-2) were cultured at 25°C as described previously (Nagata et al. 1981) and maintained by transferring a 5 ml aliquot of a 7-day-old culture into 75 ml of fresh medium.
The B-free medium was prepared as described previously (Matoh et al. 1992). Three-day-old cells (150 ml cell suspension) were collected on a 60 μm nylon mesh filter by gravity flow and were divided into two aliquots. Each aliquot was suspended in 400 ml of B-free or control (1 mg B liter–1, Nagata et al. 1981) culture medium for 1 min and was filtered. After repeating the process three times, cells were transferred to a plastic (polymethylpentene) flask containing 75 ml of the corresponding B-free or control medium.
For BHA or α-tocopherol treatment, cells were pre-cultured in the control medium supplemented with 0.1 mM of either BHA (Sigma-Aldrich, St Louis, MO, USA) or α-tocopherol (Nacalai Tesque, Kyoto, Japan) for 1 h, then washed with and transferred to the medium supplemented with 0.1 mM of BHA or α-tocopherol, as above. BHA and α-tocopherol were diluted from a 100 mM stock solution in methanol or ethanol, respectively. For CHX treatment, cells were pre-cultured in control medium supplemented with 0.35 μM CHX (Nacalai Tesque) for 1 h, then washed with and transferred to medium supplemented with 0.35 μM CHX. CHX was diluted from a 0.35 mM stock solution in methanol.
Determination of cell viability
To the cell suspension was added 7.5 μg ml–1 FDA (Sigma-Aldrich) and 22.5 μg ml–1 PI (Wako Pure Chemicals, Osaka, Japan) and they were incubated for 10 min at room temperature. Samples were observed under a fluorescence microscope (BX-51, Olympus, Tokyo, Japan) with a 460–495 nm excitation filter and a 510 nm barrier filter, to distinguish the dead (red) and live (green) cells. Cell viability was calculated from the counts of four randomly selected fields, in which at least 200 cells were present. Cell numbers were counted with a 0.2 mm depth hematocytometer.
Detection of ROS
The assay was performed according to Yamamoto et al. (2002) with some modifications. DHE was added to the cell suspension at a final concentration of 10 μM. After incubation at 25°C for 30 min in the dark, cells were observed for ethidium fluorescence under a fluorescent microscope (Olympus BX-51), with a 510–530 nm excitation filter and a 575 nm barrier filter.
DNA fragmentation analysis
Genomic DNA was prepared by the cetyltrimethylammonium bromide method (Murray and Thompson 1980). Samples were treated with 100 μg ml–1 DNase-free RNase (Nacalai Tesque) for 1 h at 37°C and electrophoresed on a 1.8% (w/v) agarose gel followed by visualization with ethidium bromide. As a positive control for DNA laddering, genomic DNA prepared from heat-shocked cells was run alongside (Vacca et al. 2004).
Determination of lipid peroxides
Lipid peroxides were quantified by the TBARS method as malondialdehyde equivalents, with 1,1,3,3,-tetramethoxypropane as the standard (Yagi 1984, Jagendorf and Takabe 2001). Tobacco cells were harvested by vacuum filtration and were ground in liquid nitrogen to a fine powder with a mortar and pestle. A 50 mg aliquot of the powder was mixed with 1.0 ml of ice-cold 10% (w/v) trichloroacetic acid, left on ice for 1 h and centrifuged at 12,000×g for 20 min at 4°C. A 0.4 ml aliquot of the supernatant was transferred to a glass test tube, mixed with 1.8 ml of 10% (w/v) trichloroacetic acid, 0.01 ml of 2% (w/v) butylated hydroxytoluene in ethanol, and 0.2 ml of 3% (w/v) thiobarbituric acid, then boiled for 60 min. After cooling on ice, the mixture was centrifuged at 700×g for 10 min and the fluorescence of the supernatant was measured at 515 nm excitation and 553 nm emission wavelengths.
Determination of ASC and GSH
Cells were harvested by vacuum filtration and were ground in liquid nitrogen to a fine powder with a mortar and pestle. To determine reduced ASC, 100 mg of the pulverized cells were mixed with 0.4 ml of ice-cold 5% (w/v) metaphosphoric acid and centrifuged at 12,000×g for 10 min at 4°C. The supernatant was filtered through a 0.2 μm filter (GL Chromatodisk 4A, GL Sciences, Tokyo, Japan) and 20 μl aliquots were separated by HPLC (LC-10A HPLC system, Shimadzu, Kyoto, Japan). The column (Unison UK-C18, 4.6×100 mm, Imtakt, Kyoto, Japan) was equilibrated and eluted with 20 mM potassium phosphate (pH 7.0) containing 10 mM tetrabutylammonium bromide at a flow rate of 1 ml min–1. Ascorbate was detected at 265 nm. To determine total ascorbate (ASC + DHA), HPLC samples were added with 5 mM dithiothreitol and were incubated for 30 min in the dark at 25°C before analyses.
GSH was quantified as described by Klapheck et al. (1994) with some modifications. A 50 mg aliquot of the pulverized cells was mixed with 0.8 ml of ice-cold 0.1 M HCl and centrifuged at 12,000×g for 10 min at 4°C. The supernatant was transferred to a new centrifuge tube, and added with trifluoroacetic acid (TFA) to a final concentration of 5% (v/v). The precipitate formed was removed by centrifugation at 12,000×g for 10 min at 4°C, and 20 μl aliquots were separated by HPLC (LC-10A HPLC system). The column (Cosmosil 5C18-PAQ, 5 μm corn size, 4.6×150 mm, Nacalai Tesque) was equilibrated and eluted with 0.05% (v/v) TFA at a flow rate of 0.8 ml min–1. To the column eluent, 75 μM 5,5′-dithiobis(2-nitrobenzoic acid) in 50 mM potassium phosphate (pH 8.0) was added at 0.8 ml min–1, and the mixture was allowed to pass through a reaction loop of 0.5 mm×3 m at 30°C. The derivatized GSH was detected at 410 nm.
Antioxidant enzyme assays
Enzymes were extracted at 0–4°C from 100 mg of pulverized cells with 0.4 ml of one of the following media: SOD (EC: 1.15.1.1), 100 mM potassium phosphate (pH 7.8), 0.1 mM EDTA and 0.1% (v/v) Triton X-100; CAT (EC: 1.11.1.6), 100 mM potassium phosphate (pH 7.0) and 0.1 mM EDTA; APX (EC: 1.11.1.11), 50 mM potassium phosphate (pH 7.8) and 1 mM ASC. The extracts were centrifuged at 12,000×g for 10 min at 4°C, and the supernatants were used for the assays.
SOD activity was determined using a SOD Assay Kit-WST (Dojindo Molecular Technologies, Kumamoto, Japan) according to the manufacturer's instructions. One unit of SOD activity was defined as the amount required to inhibit the reduction of tetrazolium by 50%. CAT activity was determined by following the consumption of H2O2 (extinction coefficient 39.4 mM–1 cm–1) at 240 nm for 30 s (Aebi 1984) in a 3 ml reaction mixture containing 50 mM potassium phosphate (pH 7.0), 10 mM H2O2 and 200 μl of the extract. APX activity was determined by following the consumption of ASC (extinction coefficient 2.8 mM–1 cm–1) at 290 nm for 30 s, in a 1 ml reaction mixture containing 50 mM potassium phosphate (pH 7.0), 0.1 mM EDTA, 0.5 mM ASC, 0.1 mM H2O2 and 4 μl of the extracts (Asada 1984). Soluble protein contents were determined as described previously (Bradford, 1976), with bovine serum albumin as the standard.
Semi-quantitative RT–PCR analysis
Total RNA was isolated by phenol:chloroform extraction and LiCl precipitation (Shirzadegan et al. 1991), and genomic DNA contamination was eliminated by treatment with RNase-free DNase (TAKARA BIO INC., Shiga, Japan). First-strand cDNA was synthesized using ReverTra Ace DNA polymerase (Toyobo, Osaka, Japan) in a 25 μl reaction mixture, containing 1 μg of total RNA and 2.5 pmol (dT)18 primer. The reactions were performed at 42°C for 60 min followed by 95°C for 5 min. The reaction mixture was diluted 4-fold with 10 mM Tris–HCl (pH 8.0) and 1 mM EDTA, and 1 μl aliquots were used for PCR with rTaq DNA polymerase (Toyobo) in a final volume of 25 μl. A 699 bp cDNA fragment of cAPX (accession No. D85912) was amplified using the primers 5′-CACTGTAAGCGAGGAGTACC-3′ and 5′-TGAGCCTCAGCATAGTCAGC-3′ (Vacca et al. 2004). A 493 bp cDNA fragment of actin (accession No. AB158612) was amplified using the primers 5′-AAGGTTACGCCCTTCCTCAT-3′ and 5′-GCCACCACCTTGATCTTCAT-3′, to calibrate the quantities of cDNA templates. PCR was performed with arbitrary cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 30 s. The PCR products were analyzed on a 1% (w/v) agarose gel and were visualized with ethidium bromide.
Funding
The Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Young Scientists, No. 18780046 to M.K.; Grant-in-Aid for Scientific Research on Priority Areas, No. 18056011 to M.K. and T.M.).
Acknowledgments
We thank Dr. Hiroyuki Koyama (Gifu University) for helpful discussions.
Glossary
Abbreviations:
- ASC
ascorbate
- B
boron
- BHA
butylated hydroxyanisole
- CHX
cycloheximide
- DHA
dehydroas-corbate
- DHE
dihydroethidium
- FDA
fluorescein diacetate
- GSH
reduced glutathione
- PCD
programmed cell death
- PI
propidium iodide
- RG-II
rhamnogalacturonan II
- ROS
reactive oxygen species
- RT–PCR
reverse transcription–PCR
- TBARS
thiobarbituric acid reactive substances
- TFA
trifluoroacetic acid.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahn JW, Verma R, Kim M, Lee JY, Kim YK, Bang JW, et al. Depletion of UDP-d-apiose/UDP-d-xylose synthases results in rhamnogalacturonan-II deficiency, cell wall thickening, and cell death in higher plants. J. Biol. Chem. 2006;281:13708–13716. doi: 10.1074/jbc.M512403200. [DOI] [PubMed] [Google Scholar]
- Asada K. Chloroplasts: formation of active oxygen and its scavenging. Methods Enzymol. 1984;105:422–429. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, et al. Boron in plant biology. Plant Biol. 2002;4:205–223. [Google Scholar]
- Cakmak I, Kurz H, Marschner H. Short-term effects of boron, germanium and high light intensity on membrane permeability in boron deficient leaves of sunflower. Physiol. Plant. 1995;95:11–18. [Google Scholar]
- Cakmak I, Römheld V. Boron deficiency-induced impairments of cellular functions in plants. Plant Soil. 1997;193:71–83. [Google Scholar]
- Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ. NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J. 2000;24:667–677. doi: 10.1046/j.1365-313x.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- de Pinto MC, Paradiso A, Leonetti P, De Gara L. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J. 2006;48:784–795. doi: 10.1111/j.1365-313X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- de Pinto MC, Tommasi F, De Gara L. Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco Bright-Yellow 2 cells. Plant Physiol. 2002;130:698–708. doi: 10.1104/pp.005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval I, Brochu V, Simard M, Beaulieu C, Beaudoin N. Thaxtomin A induces programmed cell death in Arabidopsis thaliana suspension-cultured cells. Planta. 2005;222:820–831. doi: 10.1007/s00425-005-0016-z. [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell. 2002;14:1557–1566. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeklee P, Wimmer M, Goldbach HE. Early effects of boron deficiency on physical cell wall parameters, hydraulic conductivity and plasmalemma-bound reductase activities in young C. pepo and V. faba roots. In: Bell RW, Rerkasem B, editors. Boron in Soils and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 221–227. [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol. Plant. 1994;92:696–717. [Google Scholar]
- Goldbach HE, Yu Q, Wingender R, Schulz M, Wimmer M, Findeklee P, et al. Rapid response reactions of roots to boron deprivation. J. Plant Nutr. Soil. Sci. 2001;164:173–181. [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hoson T. Apoplast as the site of response to environmental signals. J. Plant Res. 1998;111:167–177. doi: 10.1007/BF02507163. [DOI] [PubMed] [Google Scholar]
- Iwai H, Masaoka N, Ishii T, Satoh S. A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proc. Natl Acad. Sci. USA. 2002;99:16319–16324. doi: 10.1073/pnas.252530499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagendorf AT, Takabe T. Inducers of glycinebetaine synthesis in barley. Plant Physiol. 2001;127:1827–1835. [PMC free article] [PubMed] [Google Scholar]
- Klapheck S, Fliegner W, Zimmer I. Hydroxymethyl-phytochelatins [(γ-glutamylcysteine)n-serine] are metal-induced peptides of the Poaceae. Plant Physiol. 1994;104:1325–1332. doi: 10.1104/pp.104.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Matoh T, Azuma J. Two chains of rhamnogalacturonan II are cross-linked by borate–diol ester bonds in higher plants cell walls. Plant Physiol. 1996;110:1017–1020. doi: 10.1104/pp.110.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Mutoh T, Matoh T. Boron nutrition of cultured tobacco BY-2 cells. IV. Genes induced under low boron supply. J. Exp. Bot. 2004;55:1441–1443. doi: 10.1093/jxb/erh142. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohno K, Matoh T. Boron nutrition of tobacco BY-2 cells. II. Characterization of the boron–polysaccharide complex. Plant Cell Physiol. 1997;38:676–683. [Google Scholar]
- Lecourieux D, Ranjeva R, Pugin A. Calcium in plant defence-signalling pathways. New Phytol. 2006;171:249–269. doi: 10.1111/j.1469-8137.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Lukaszewski KM, Blevins DG. Root growth inhibition in boron-deficient or aluminum-stressed squash may be a result of impaired ascorbate metabolism. Plant Physiol. 1996;112:1135–1140. doi: 10.1104/pp.112.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Shen R, Nagao S, Tanimoto E. Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol. 2004;45:583–589. doi: 10.1093/pcp/pch060. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. 2nd edn. San Diego: Academic Press; 1995. Boron. pp. 379–396. [Google Scholar]
- Matoh T, Ishigaki K, Mizutani M, Matsunaga W, Takabe K. Boron nutrition of cultured tobacco BY-2 cells. I. Requirement for and intracellular localization of boron and selection of cells that tolerate low levels of boron. Plant Cell Physiol. 1992;33:1135–1141. [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl Acad. Sci. USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cells as the ‘HeLa’ cells in the cell biology of higher plants. Int. Rev. Cytol. 1992;132:1–30. [Google Scholar]
- Nagata T, Okada K, Takebe I, Matsui C. Delivery of tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes). Mol. Gen. Genet. 1981;184:161–165. [Google Scholar]
- Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl Acad. Sci. USA. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MA, Eberhard S, Albersheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Ishii T, Albersheim P, Darvill AG. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 2004;55:109–139. doi: 10.1146/annurev.arplant.55.031903.141750. [DOI] [PubMed] [Google Scholar]
- Parr AJ, Loughman BC. Boron and membrane function in plants. In: Robb DA, Pierpoint WS, editors. Metals and Micronutrients. Uptake and Utilization by Plants. New York: Academic Press; 1983. pp. 87–107. [Google Scholar]
- Purvis AC. Role of the alternative oxidase in limiting superoxide production by plant mitochondria. Physiol. Plant. 1997;100:165–170. [Google Scholar]
- Ryden P, Sugimoto-Shirasu K, Smith AC, Findlay K, Reiter WD, McCann MC. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II–borate complexes. Plant Physiol. 2003;132:1033–1040. doi: 10.1104/pp.103.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirzadegan M, Christie P, Seemann JR. An efficient method for isolation of RNA from tissue cultured plant cells. Nucleic Acids Res. 1991;19:6055. doi: 10.1093/nar/19.21.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorrocks VM. The occurrence and correction of boron deficiency. Plant Soil. 1997;193:121–148. [Google Scholar]
- Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell. 1999;11:431–443. doi: 10.1105/tpc.11.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H. Response of Lemna paucicostata to boron as affected by light intensity. Plant Soil. 1966;25:425–434. [Google Scholar]
- Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, De Gara L. Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiol. 2004;134:1100–1112. doi: 10.1104/pp.103.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ. Many ways to exit? Cell death categories in plants. Trends Plant Sci. 2005;10:117–122. doi: 10.1016/j.tplants.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Yagi K. Assay for blood plasma or serum. Methods Enzymol. 1984;105:328–331. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002;128:63–72. [PMC free article] [PubMed] [Google Scholar]