Abstract
The real-time translocation of iron (Fe) in barley (Hordeum vulgare L. cv. Ehimehadaka no. 1) was visualized using the positron-emitting tracer 52Fe and a positron-emitting tracer imaging system (PETIS). PETIS allowed us to monitor Fe translocation in barley non-destructively under various conditions. In all cases, 52Fe first accumulated at the basal part of the shoot, suggesting that this region may play an important role in Fe distribution in graminaceous plants. Fe-deficient barley showed greater translocation of 52Fe from roots to shoots than did Fe-sufficient barley, demonstrating that Fe deficiency causes enhanced 52Fe uptake and translocation to shoots. In the dark, translocation of 52Fe to the youngest leaf was equivalent to or higher than that under the light condition, while the translocation of 52Fe to the older leaves was decreased, in both Fe-deficient and Fe-sufficient barley. This suggests the possibility that the mechanism and/or pathway of Fe translocation to the youngest leaf may be different from that to the older leaves. When phloem transport in the leaf was blocked by steam treatment, 52Fe translocation from the roots to older leaves was not affected, while 52Fe translocation to the youngest leaf was reduced, indicating that Fe is translocated to the youngest leaf via phloem in addition to xylem. We propose a novel model in which root-absorbed Fe is translocated from the basal part of the shoots and/or roots to the youngest leaf via phloem in graminaceous plants.
Keywords: Barley, Fe translocation, Phloem, Positron-emitting tracer, Real-time imaging, Xylem
Introduction
Iron (Fe) is an essential nutrient for plants because it is required for various cellular functions, including heme and chlorophyll biosynthesis, photosynthesis, and as a component of Fe–S cluster-containing enzymes. Although abundant in soils, Fe often forms insoluble ferric hydroxide precipitates that limit its availability for plants. Therefore, plants have evolved two distinct strategies to solubilize and efficiently take up Fe, i.e. Strategy I and Strategy II (Römheld and Marschner 1986). Strategy I plants (dicots and non-graminaceous monocots) rely on acidification of the rhizosphere by H+-ATPase activity, reduction of Fe3+-chelate to Fe2+ by reductase, and Fe2+ transport across the root cell membrane (Marschner et al. 1986). Genes encoding the Fe3+-chelate reductases, FRO2 (Robinson et al. 1999) and FRO1 (Waters et al. 2002), and the Fe2+ transporters, IRT1 (Eide et al. 1996) and IRT2 (Vert et al. 2001), have been isolated from Arabidopsis. Strategy II plants (graminaceous monocots) secrete mugineic acid family phytosiderophores (MAs), such as 2′-deoxymugineic acid (DMA) and 3-epihydroxymugineic acid (epiHMA), into the rhizosphere, where MAs chelate Fe3+, and the resultant Fe3+–MAs complex is taken up into roots through an Fe3+–MAs complex transporter in the plasma membrane of the root cells (Takagi 1976). The YS1 gene, which encodes an Fe3+–MA transporter, has been isolated using the maize mutant yellow stripe 1 (ys1; Curie et al. 2001).
To date, little is known about how Fe is translocated in intact plants after absorption by the roots. It is assumed that Fe is transported to the shoot via the xylem, driven by the transpiration stream and root pressure, and unloaded from xylem to older leaves. Mori (1998) reported that 59Fe was not transported to aluminum foil-covered leaves of intact barley plants supplied with 59Fe3+–DMA, suggesting that Fe translocation from roots to leaves depends on the transpiration stream in barley. Zhang et al. (1995) reported that the majority of 59Fe translocated to the shoot was present in mature leaves 24 h after the supply of 59Fe3+-EDTA to Fe-sufficient and Fe-deficient French bean. The study suggested that Fe transported via the xylem does not directly reach the shoot apex, which is the site of highest Fe demand, but does so only after remobilization from older leaves. In the case of nitrogen, xylem to phloem transfer was suggested by the enrichment of amino compounds relative to sugar in phloem sap collected from terminal stem tissues, inflorescences or fruit stalks compared with that from petioles in white lupin (Lapinus albus) (Layzell et al. 1981, Pate 1986). Recently, Tanaka et al. (2008) also showed that xylem–phloem transfer mediated by NIP6;1 is required for boric acid transport to the young leaves. However, little is known about Fe trans-location in intact plants after absorption by the roots.
We previously examined the transport of 59Fe in barley (Hordeum vulgare L. cv. Ehimehadaka no. 1) by supplying 59Fe(III)–epiHMA to roots, and detection by autoradiography (Mori 1998). Recently, positron-emitting nuclides have been used in plants to study the distribution and translocation of 18F (Kume et al. 1997), [11C]methionine (Nakanishi et al. 1999, Bughio et al. 2001), 11C (Fujikake et al. 2003, Matsuhashi et al. 2005), 13NO3− (Hayashi et al. 1997, Matsunami et al. 1999, Ohtake et al. 2001), 13N (Kiyomiya et al. 2001b), H215O (Mori et al. 2000, Kiyomiya et al. 2001a, Nakanishi et al. 2002, Tsukamoto et al. 2004), 52Fe (Ishimaru et al. 2006, Suzuki et al. 2006, Ishimaru et al. 2007), 52Mn (Tsukamoto et al. 2006) and 62Zn (Watanabe et al. 2001, Suzuki et al. 2006, Suzuki et al. 2008) using a positron-emitting tracer imaging system (PETIS) (Fujimaki 2007). With this technique, γ-rays produced by positron-emitting nuclides are detected by scintillation detectors, allowing the real-time investigation of the movement of elements in intact plants. In this study, we used PETIS to monitor the real-time translocation of 52Fe in intact barley plants under Fe-deficient or dark conditions and showed that phloem is involved in 52Fe transport to the young leaves.
Results
Fe3+–DMA absorption and translocation in Fe-deficient and Fe-sufficient barley
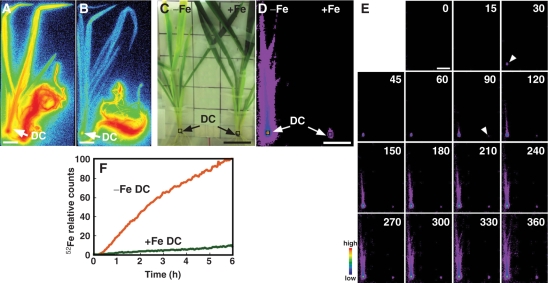
To examine the effect of Fe deficiency on the absorption and translocation of Fe in intact plants, 52Fe3+–DMA was supplied to the roots of both Fe-deficient and Fe-sufficient barley plants, and the translocation of 52Fe was monitored using PETIS and autoradiography. Experiments were independently repeated in triplicate: the results of one experiment are shown in Fig. 1 and the others are shown in Supplementary Figs. S1 and S2. Autoradiography images showed that the shoots of Fe-deficient plants were more strongly labeled than those of Fe-sufficient barley, and the discrimination center (DC), i.e. the basal part of the shoot, was strongly labeled in both Fe-deficient and Fe-sufficient plants (Fig. 1A, B). 52Fe accumulation in the DC was also observed by PETIS (Fig. 1C, D). Fig. 1E shows a montage of PETIS images. In Fe-deficient barley, the signal in the DC (Fig. 1E, left arrowhead) appeared within 30 min, and an image of the leaf sheath appeared within 90 min (Fig. 1E). In contrast, in Fe-sufficient barley, the signal in the DC appeared within 90 min (Fig. 1E, right arrowhead). The radioactivity observed in images of shoots of Fe-deficient barley was higher than that of Fe-sufficient barley shoots in a 6 h experiment, showing that more 52Fe was translocated to shoots in Fe-deficient barley than in Fe-sufficient barley (Fig. 1E, Supplementary Movie 1). Fig. 1F shows the time course change of radioactivity in the DC (indicated in Fig. 1C, D, squares) calculated from the PETIS data. In triplicate experiments, Fe-deficient DCs accumulated 52Fe to >10 times greater levels compared with Fe-sufficient DCs at the end of the experiments. We have established an 52Fe translocation analysis method by PETIS in barley.
Fig. 1.
52Fe translocation from roots to shoots in Fe-deficient (−Fe) and Fe-sufficient (+Fe) barley (Experiment 1). (A and B) Images of 52Fe translocation in Fe-deficient (A) and Fe-sufficient (B) barley using BAS-1500. (C) Gross image of Fe-deficient (left) and Fe-sufficient (right) barley analyzed using PETIS. The same frame was used for D and E. (D) PETIS images of 52Fe accumulation after 6 h. (E) Time course of radioactivity accumulation analyzed using PETIS. The images are shown at 15 and 30 min intervals (0–60 and 60–360 min, respectively). Data were scored every 3 min. Arrowheads indicate the first detection of DC (left arrowhead −Fe, right arrowhead +Fe). (F) Time course of radioactivity accumulation in the DC of Fe-deficient and Fe-sufficient barley (squares in C and D). The maximum measured radioactivity was defined as 100. Activities are shown as percentages of the maximum value. Scale bar = 4 cm. Experiments 2 and 3 are shown in Supplementary Figs. S1 and S2, respectively.
Effect of light on Fe absorption and translocation
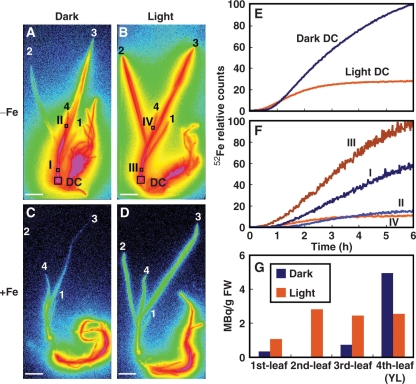
The secretion of MAs from barley follows a distinct diurnal rhythm (Takagi et al. 1984). A peak in secretion occurs just after daybreak. We speculated that light may influence Fe uptake and translocation. The effect of light on 52Fe translocation was examined by performing the same experiments in light and in darkness. In the dark, 52Fe translocation to the DC and leaves was observed (Fig. 2A, C). In the light, the second and the third leaves of Fe-deficient barley accumulated 52Fe to almost the same level (Fig. 2B). However, in the dark, the accumulation of 52Fe in the second leaf was less than in the third leaf, and the youngest leaf (we defined the ‘youngest leaf’ as the newest leaf visible in an intact plant) accumulated more 52Fe than the older leaves (Fig. 2A, Supplementary Movie 2).
Fig. 2.
52Fe translocation in Fe-deficient (−Fe) and Fe-sufficient (+Fe) barley in the dark and in the light (Experiment 1). (A–D) Images of 52Fe translocation in Fe-deficient (A, B) and Fe-sufficient (C, D) barley in the dark (A, C) and in the light (B, D) using BAS-1500. Leaf numbering: 4, youngest leaf (YL); 3, third leaf; 2, second leaf; 1, first leaf. (E and F) Time course of 52Fe translocation in the DC (E) and at the points indicated in A and B (F) of Fe-deficient barley measured by PMPS and PETIS, respectively. The maximum measured radioactivity in PMPS and PETIS was defined as 100. Activities are shown as percentages of the maximum value. (G) 52Fe content in the leaves of Fe-deficient barley after 6 h.
The time course of 52Fe radioactivity in the DC, leaf sheath and the youngest leaf (Fig. 2A, B, squares) was obtained from PETIS and the positron multiprobe system (PMPS) data (Fig. 2E, F). In the dark, radioactivity in the DC (Fig. 2E) was higher than that in the light, while radioactivity in the leaf sheath (point I in Fig. 2A, F) was lower than that in the light (point III in Fig. 2B, F) at the end of the 6 h experiment. Moreover, 52Fe accumulation in the youngest leaf (the fourth leaf) did not change between in the dark (point II in Fig. 2A, F) and in the light (point IV in Fig. 2B, F). The 52Fe content of the youngest leaf after 6 h of absorption was higher (or equal in Supplementary Fig. S3) in darkness, but the 52Fe content of older leaves (third, second and first leaves) was considerably lower (Fig. 2G). The experiments were repeated in triplicate, and similar results were obtained, as shown in Supplementary Figs. S3 and S4.
Effect of steam treatment on Fe translocation
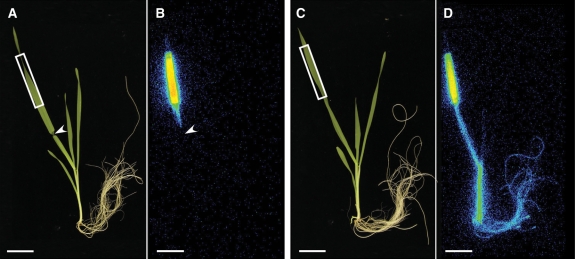
The 52Fe accumulation pattern in the youngest leaf in the dark was different from that in the older leaves. This suggests the possibility that the mechanism and/or pathway of Fe translocation to the youngest leaf may be different from that to the other leaves. Therefore, we used the heat-girdling method (Fisher 2000, Jeannette et al. 2000), which enabled us to distinguish phloem transport from xylem transport. Phloem transport can be blocked without inhibiting xylem transport by appropriate steam treatment. We examined if the heat-girdling method also blocked the phloem transport without affecting the xylem transport in barley under our experimental conditions. First, we checked whether this method blocked phloem transport by supplying 11CO2 via a leaf; 11C signal was detected only in the leaf above the steam-treated position and it was not detected in the other parts of the steam-treated plants (Fig. 3, Supplementary Fig. S5), whereas 11C was detected in leaf sheaths, roots and the youngest leaf in addition to the 11CO2-fed leaf in the untreated plant (Fig. 3). Secondly, to confirm that our steam treatment did not affect the xylem transport, the effect of steam treatment on the relative water content after 5 h incubation in the growth chamber was examined (Table 1). Relative water contents of both steam-treated youngest and older leaves were not significantly different from those of untreated leaves. However, relative water contents of detached youngest or older leaves under the same condition decreased to 24.8 and 49.8%, respectively. These results showed that our heat-girdling effectively inhibited phloem transport, but xylem transport was not significantly affected.
Fig. 3.
Effect of heat-girdling on 11C-labelled photoassimilate translocation from the leaf in barley (Experiment 1). (A and C) Gross images of steam-treated (A) and untreated (C) barley. (B and D) Images of the distribution of 11C in steam-treated (B) and untreated (D) barley detected by a BAS-1500. The arrowhead indicates the position of steam treatment. Rectangles indicate the 11CO2-fed region. Scale bar = 4 cm.
Table 1.
Relative water content (%) of the steam-treated, untreated or detached youngest and older leaves after 5 h of the treatment
| Leaves | Steam treated | Untreated | Detached |
| Youngest | 84.6 ± 11.3a | 92.8 ± 8.6a | 24.8 ± 7.0c |
| Older | 88.2 ± 6.5a | 94.5 ± 2.9a | 49.8 ± 8.6b |
Values are the average ± SD (n = 5–8).
Values followed by different letters were significantly different (P < 0.01).
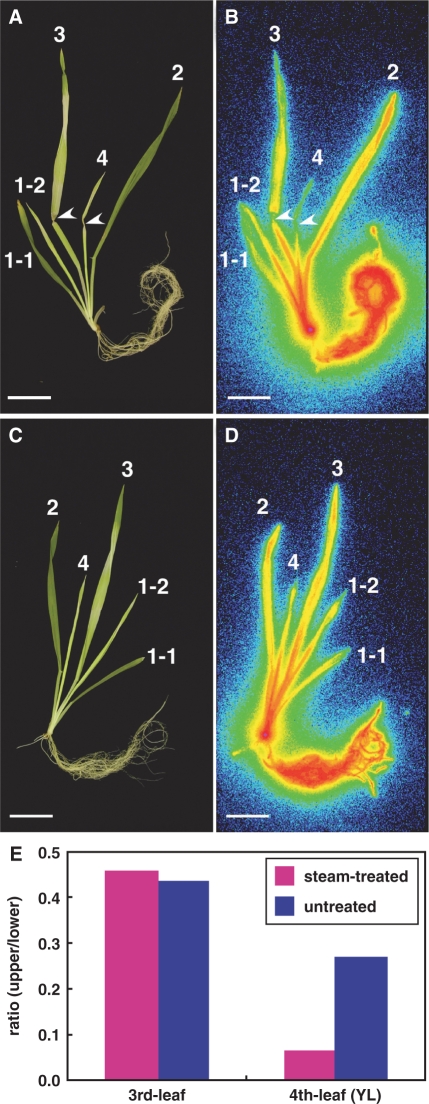
Parts of the youngest leaf (fourth leaf, Fig. 4A) and the most recently expanded leaf (third leaf, Fig. 4A) of an Fe-deficient barley plant were treated with steam, and 52Fe3+–DMA was supplied to the roots for 4 h. In the treated plant, 52Fe translocation above the steam-treated regions of the youngest leaf was severely suppressed, but it was not affected in the expanded third leaf (Fig. 4B). In the untreated plant, 52Fe was translocated to all parts of the plant, including the youngest leaf (Fig. 4C, D). In the youngest leaf, steam treatment reduced the proportion of accumulated 52Fe in the steam-treated region compared with that in the lower region by 75% in this experiment (Fig. 4E). In contrast, the proportion of accumulated 52Fe was not affected in the expanded leaf (Fig. 4E). We repeated this experiment in triplicate and, in all the experiments, steam treatment reduced the 52Fe translocation to the youngest leaves, whereas it did not reduce the 52Fe translocation to the older leaves (Supplementary Figs. S6, S7). In our triplicate experiments, the size of the youngest leaves varied to some extent. We noticed that the ratio of the phloem transport, i.e. the ratio of the inhibition of 52Fe translocation by steam treatment, was higher in small leaves than that in larger leaves; (75, 91 and 61% inhibition of 52Fe translocation by the steam treatment in Fig. 4, Supplementary Figs. S6 and S7, respectively).
Fig. 4.
Effect of heat-girdling on 52Fe translocation from the roots to the shoots in Fe-deficient barley (Experiment 1). (A and C) Gross images of steam-treated (A) and untreated (C) barley. (B and D) Images of the distribution of 52Fe in steam-treated (B) and untreated (D) barley detected by a BAS-1500. Arrowheads indicate the position of steam treatment. Leaf numbering: 4, youngest leaf (YL); 3, third leaf; 2, second leaf; 1-2, youngest leaf of the first tiller; 1-1, first leaf. Scale bar = 4 cm. (E) The ratio of the 52Fe radioactivity of the upper region to that of the lower region after heat-girdling the third and fourth youngest leaves (YL).
Discussion
We traced the time course of 52Fe translocation in intact barley using PETIS and produced dynamic animations of the PETIS images (Supplementary Movies 1 and 2).
Role of the DC in mineral and metabolite transport in graminaceous plants
In all experiments, 52Fe primarily accumulated in the DC, a region that contains the shoot meristem, node and internode (Mori 1998, Itoh et al. 2005). In the node, the structure of the vascular bundles is complicated and they are enlarged (Kawahara et al. 1974, Kawahara et al. 1975). In addition, transfer cells, which play an important role in distributing minerals, are present in the nodes (Zee 1972). Mori (1998) reported that the supply of 59Fe3+–epiHMA or [32P]PO43− to roots or leaves of barley resulted in strong labeling of this region and termed it the DC. Similarly, 52Fe first accumulated in the DC and was then distributed to all plant tissues (Fig. 1E, Supplementary Movie 1). We have also reported that radioactivity accumulated in the DCs of barley and rice, and was then distributed to shoots, in studies using [11C]methionine (Nakanishi et al. 1999, Bughio et al. 2001), 13NH4+ (Kiyomiya et al. 2001b) and H215O (Mori et al. 2000, Kiyomiya et al. 2001a, Nakanishi et al. 2002, Tsukamoto et al. 2004). Therefore, it is likely that not only Fe but also other minerals and metabolites accumulate in the DC after absorption from the roots in graminaceous plants. Our present study further supports the important role of the DC in distributing minerals and metabolites in graminaceous plants.
Effect of Fe status on Fe absorption and translocation from the roots
More 52Fe was translocated to shoots in Fe-deficient barley than in Fe-sufficient barley (Fig. 1D, E, Supplementary Figs. S1, S2, Supplementary Movie 1), suggesting that the demand for Fe affects its translocation. Previous research has shown that Fe starvation increases the 59Fe3+–MAs influx in roots of barley (Römheld and Marschner 1986, Mihashi and Mori 1989, Mori 1998). In the present study, an image of the DC in Fe-deficient barley appeared within 30 min, whereas an image of the DC in Fe-sufficient barley appeared within 90 min (Fig. 1E). This result shows that Fe deficiency enhanced the translocation of 52Fe from the roots to the DC. In roots, the expression of many transporter genes is induced by Fe deficiency. For example, Fe deficiency induces the expression of the Fe3+–MAs transporter gene (YS1) in roots of maize (Curie et al. 2001). Similarly, Fe deficiency induced the expression of the Fe3+–MAs transporter gene in barley (HvYS1; Murata et al. 2006) and rice (OsYSL15; Inoue et al. 2009). Not only the transporters involved in Fe uptake, but also the transporters involved in Fe translocation may be induced under Fe deficiency.
52Fe was preferentially translocated to younger leaves (Fig. 1A, B, Supplementary Figs. S1, S2). Brown et al. (1965) and Mori (1998) reported that Fe accumulation depended on leaf age. Because Fe is required for chlorophyll biosynthesis and photosynthesis, younger leaves function as strong sinks. Indeed, the Fe deficiency symptom, chlorosis, first appears in the youngest leaf. Therefore, it is conceivable that Fe is translocated preferentially to the sink. The DC may play a critical role in this regulation of Fe distribution to the sink organs. In shoots, some Fe and Fe–chelate transporter genes, such as YS1 (Curie et al. 2001), AtNRAMP3 (Thomine et al. 2003), AtNRAMP4 (Thomine et al. 2000) and OsYSL2 (Koike et al. 2004), are up-regulated under Fe deficiency. These transporter genes may be expressed at the DC and regulate the distribution of Fe in plants.
Role of phloem transport in Fe translocation
To investigate the details of Fe translocation to the youngest leaf, phloem transport in the leaf was blocked by steam treatment (Fisher 2000, Jeannette et al. 2000) to distinguish xylem transport from phloem transport. We confirmed that the translocation of 11C-labeled photoassimilates from the treated leaf to other plant parts was inhibited, although the water flow was still active following steam treatment (Fig. 3, Supplementary Fig. S5, Table 1). After steam treatment, 52Fe translocation from the roots to the upper regions of the youngest leaf in Fe-deficient barley was strongly reduced, but that to the fully expanded leaf was not affected (Fig. 4). In addition, translocation to the second leaf was also unaffected by steam treatment (data not shown). These results showed that Fe is mainly translocated to the youngest leaf via phloem and partly via xylem, whereas it is translocated to older leaves mainly via xylem in Fe-deficient barley. As the leaf grows, the pathway of Fe translocation to the leaf appears to change from phloem to xylem. 52Fe translocation to the youngest leaf in the dark was also observed in Fe-sufficient barley (Fig. 2C, D). Therefore, Fe may be translocated to the youngest leaf via phloem, regardless of Fe status. In the case of nitrogen, xylem to phloem transfer occurred in white lupin (Layzell et al. 1981, Pate 1986). In Arabidopsis, xylem–phloem transfer may be involved in boric acid transport to the young leaves (Tanaka et al 2008). Similarly, xylem to phloem transfer of Fe may occur in barley plants.
The location of Fe transfer from xylem to phloem for translocation to the youngest leaf remains unknown. As described earlier, the DC contains a node and internode (Itoh et al. 2005), in which the vascular bundles are complicated and enlarged (Kawahara et al. 1974, Kawahara et al. 1975). OsIRT1, which encodes the Fe2+ transporter in rice, was expressed in the phloem of the DC (Ishimaru et al 2006). Therefore, it is conceivable that Fe may be transferred from xylem to phloem in the DC.
OsNAS1, OsNAS2 and OsNAS3, which encode nicotianamine (NA) synthase in rice, were constitutively expressed in the companion cells of the roots of both Fe-sufficient and Fe-deficient plants (Inoue et al. 2003). This implies that NA is synthesized in the companion cells of the roots and functions in phloem loading or unloading of Fe. Furthermore, genes that participate in DMA synthesis, such as OsNAAT1 (NA aminotransferase gene in rice) and OsDMAS1 (DMA synthase gene in rice), were expressed in these cells, suggesting that DMA in addition to NA is synthesized in these cells (Bashir et al. 2006, Inoue et al. 2008). Therefore, it is possible that Fe may be transferred from xylem to phloem in the roots. Zhang et al. (1995) reported that Fe supplied via the xylem in French bean did not directly reach the apex, which has the highest Fe demand, but did so only after remobilization from older leaves. However, in our experiments 52Fe arrived at the youngest leaf in the light after 1 h (Fig. 2F), which is too short a time for Fe to have been re-translocated from older leaves. Fe transfer from xylem to phloem in older leaves may not contribute to short-term Fe translocation from the roots to the youngest leaf. These results suggest that Fe may be transferred from xylem to phloem in the DC and/or the roots.
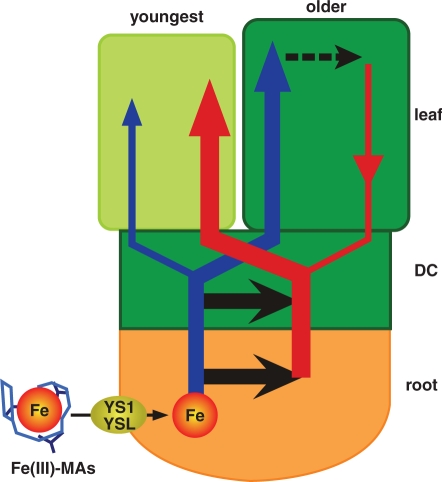
Based on our results and previous knowledge, we propose a novel model in which root-absorbed Fe is translocated from the DC and/or the roots to the youngest leaf via phloem in graminaceous plants (Fig. 5). In graminaceous plants, YS1 and its homologs (e.g. HvYS1 and OsYSL15) are responsible for Fe3+–MAs uptake from the soil (Curie et al. 2001, Murata et al. 2006, Inoue et al. 2009). Fe is then transported from the epidermis of the root to the xylem symplastically, although the form of Fe is still unknown. In xylem, Fe may be transported as an Fe3+–citrate complex ([FeCitrateOH]−1 and [FeCitrate2]−3; Tiffin 1966, López-Millán et al. 2000) and Fe3+–DMA (Mori et al. 1991). Fe may be transferred from xylem to phloem in the DC and/or the roots. In phloem, Fe may be transported mainly as an Fe–NA complex (Koike et al. 2004). The Fe ligand exchange from citrate or DMA to NA may occur during Fe transfer from xylem to phloem in the vascular tissues. Fe is mainly translocated to the youngest leaf via phloem and partly via xylem, whereas Fe is translocated to older leaves mainly via xylem. Fe translocation to younger leaves occurs preferentially compared with translocation to older leaves.
Fig. 5.
A schematic model of root-absorbed Fe transport in graminaceous plants based on our results and previous knowledge. In graminaceous plants, YS1 and its homologs (YSL) are responsible for Fe3+–MAs uptake from the soil. Fe is then transported to the xylem, and then the phloem. Although the exact location of Fe transfer from xylem to phloem remains unknown, Fe transfer may occur in the DC and/or the roots (thick black arrows). Fe is mainly translocated to the youngest leaf via phloem (thick red arrow) and partly via xylem (thin blue arrow), whereas Fe is mostly translocated to older leaves via xylem (thick blue arrow). Fe translocation to younger leaves occurs in preference to older leaves. Our study does not exclude the possibility that re-translocation of Fe from the older leaves has some contribution to the long-term Fe translocation to the youngest leaf (dotted black line and thin red arrow).
Materials and Methods
Plant materials
Barley (Hordeum vulgare L. cv. Ehimehadaka no. 1) seeds were grown hydroponically in modified Kasugai's medium: 0.7 mM K2SO4, 0.1 mM KCl, 0.1 mM KH2PO4, 2.0 mM Ca(NO3)2, 0.5 mM MgSO4, 10 μM H3BO3, 0.5 μM MnSO4, 0.2 μM CuSO4, 0.5 μM ZnSO4, 0.01 μM (NH4)Mo6O24 and 0.1 mM Fe-EDTA, as described previously (Mori and Nishizawa 1987). Fe-deficient plants were generated by culturing in medium lacking Fe for 1 week prior to beginning 52Fe absorption experiments. All absorption experiments were conducted at about 3 weeks after germination, and the sizes and root weights of plants within each experimental set-up were approximately the same.
Production of 52Fe
52Fe (half-life 8.27 h) was produced by the natCr(α, xn)52Fe reaction, in which a 1.5 mm thick chromium foil (natural isotopic composition, 99.9% purity; Goodfellow Metals Ltd., Cambridge, UK) was bombarded with a 100 MeV α beam [generated by the Takasaki Ion Accelerators for Advanced Radiation Application (TIARA) AVF cyclotron; Gunma, Japan; Watanabe et al. 2001). About 1 MBq of 52Fe was produced using a beam current of 3 μA for 2 h. 52Fe was radiochemically separated from the target using the method described by Watanabe et al. (2001). After the pH of the 52Fe3+ solution without cold Fe was adjusted to about pH 3 using 1 M KOH, the 52Fe3+ was chelated with 1.12 μmol of DMA in the dark for 1 h.
Production of 11CO2
The positron-emitting radioisotope 11C (half-life 20.39 min) was produced using a 14N(p, α)11C reaction, in which nitrogen gas was bombarded with a 10 MeV proton beam from the TIARA AVF cyclotron. About 50 MBq of 11C were produced using a beam current of 1 μA for 2 min. 11CO2 was produced from the 11C and oxygen present in the target chamber.
PETIS and PMPS
Plant samples were placed between two opposed two-dimensional block detectors consisting of Bi4Ge3O12 scintillator arrays. The 52Fe and 11CO2 produced as above were used as positron-emitting tracers. Two annihilation γ-rays emitted from the decaying positrons were detected simultaneously (Kume et al. 1997, Uchida et al. 2004). The original position of the annihilation was localized at the intersection of the object plane determined by a line connecting the two detection points on the detectors. The field of view was 143 × 215.6 mm, and the spatial resolution was 2.4 mm. After automatic correction for decay and relative detection efficiencies within the field of view, the resulting image was displayed on a monitor. After the imaging procedure was completed, the regions of interest (DC in Fig. 1, I–IV in Fig. 2 and Supplementary Figs. S3, S4) within the data obtained were set and the radioactivity behavior over time within each region was extracted from the data. Two pairs of PMPS detectors were used for time course tracer analysis of 52Fe (DC in Fig. 2, Supplementary Figs. S1–S4). Although the principles of PMPS are almost the same as those of PETIS, PMPS detectors directly measure the radioactivity time course but bypass the imaging procedure.
Heat-girdling and relative water content of the leaves
Treatment with steam and calculation of relative water contents of leaves were carried out following Jeannette et al. (2000). Steam (∼100°C) was produced using a soldering iron and wet paper towels, and was directed for 5 s towards the leaf at a distance of 1 cm. The tops of the steam-treated leaves were excised, weighed (fresh weight) and placed in distilled water in a 15 ml tube in the dark (4°C) overnight. Rehydrated weight was measured and the sample was then dried. The relative water content was calculated from [(fresh weight – dry weight)/(rehydrated fresh weight – dry weight)] × 100.
Absorption and translocation of 52Fe in plants
To study the absorption of 52Fe by roots, the roots of a single plant were placed in a polyethylene bag containing 15 ml of 5× modified Kasugai's solution lacking Fe. To maintain the geometry, the plant and the bag were placed between two acrylic boards centered between the PETIS detectors in a chamber at 25°C, 65% humidity and a photon flux density of 320 μmol m−2 s−1. 52Fe3+–DMA (0.28–1.14 MBq) in 1 ml of water was added to the culture with gentle aeration for immediate mixing. The PETIS detectors were focused on the shoot. After a 4–6 h trace analysis, the plant was removed from the polyethylene bag and the roots were gently washed for 1 min in 50 ml of 0.01 mM EDTA solution. Next, the plant was exposed to a bio-imaging analyzer system (BAS) imaging plate (Imaging Plate BAS-MP2040S, Fujifilm, Tokyo, Japan) to obtain an autoradiographic image. After 30 min, the plate was scanned using a BAS-1500 (Fujifilm, Tokyo, Japan). In the dark treatment and the steam treatment experiments, the plants were cut into parts after the PETIS and autoradiography analyses. The radioactivity of each part was then determined by γ-ray spectrometry using an HPGe detector (crystal diameter 58.0 mm, length 67.3 mm) coupled to an MCA-7700 multichannel analyzer (Seiko EG & G Co., Ltd., Matsudo, Japan). The peak analysis was performed using Gamma Studio 2nd Edition SP2 (Seiko EG & G Co., Ltd.). The radioactivity of the 52Fe was determined from the peak area at 168 keV. The effects of Fe deficiency (7 d pre-treatment), dark (13 h pre-treatment) and steam treatment of leaves on 52Fe absorption and translocation were examined. In the steam treatment experiment, Fe-deficient barley was used and the experiments were performed under the light condition.
Each experiment was repeated at least three times, and the results of one of the experiments are shown in Figs. 1–4 and those of the other two are shown in the Supplementary figures. Since the absolute radioactivity of 52Fe varied between the experiments, detected signal values cannot to be compared between different experiments. The maximum measured radioactivity in PETIS and PMPS was defined as 100 in each experiment. Activities are shown as percentages of the maximum value in each experiment.
Funding
The Universities and Japan Atomic Energy Research Institute (JAERI) Joint Research Project; the Research Centre for Nuclear Science and Technology, University of Tokyo.
Supplementary Material
Acknowledgments
We express our sincere regrets for the loss of Shoichiro Kiyomiya, who contributed to this work and passed away on August 16, 2001.
Glossary
Abbreviations:
- BAS
bio-imaging analyzer system
- DC
discrimination center
- DMA
2′-deoxymugineic acid
- epiHMA
3-epihydroxymugineic acid
- MAs
mugineic acid family phytosiderophores
- NA
nicotianamine
- PETIS
positron-emitting tracer imaging system
- PMPS
positron multiprobe system.
References
- Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, et al. Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J. Biol. Chem. 2006;281:32395–32402. doi: 10.1074/jbc.M604133200. [DOI] [PubMed] [Google Scholar]
- Brown AL, Yamaguchi S, Leal-Diaz J. Evidence for translocation of iron in plants. Plant Physiol. 1965;40:35–38. doi: 10.1104/pp.40.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bughio N, Nakanishi H, Kiyomiya S, Matsuhashi S, Ishioka NS, Watanabe S, et al. Real-time [11C]methionine translocation in barley in relation to mugineic acid phytosiderophore biosynthesis. Planta. 2001;213:708–715. doi: 10.1007/s004250100552. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl Acad. Sci. USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB. Long-distance transport. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry & Molecular Biology of Plants. RockvilleMD.: American Society of Plant Biologists, John Wiley & Sons; 2000. pp. 730–784. [Google Scholar]
- Fujikake H, Yamazaki A, Ohtake N, Sueyoshi K, Matsuhashi S, Ito T, et al. Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J. Exp. Bot. 2003;54:1379–1388. doi: 10.1093/jxb/erg147. [DOI] [PubMed] [Google Scholar]
- Fujimaki S. The positron emitting tracer imaging system (PETIS), a most-advanced imaging tool for plant physiology. ITE Lett. Batter. New Technol. Med. 2007;8:C1–C10. [Google Scholar]
- Hayashi H, Okada Y, Mano H, Kume T, Matsuhashi S, Ishioka NS, et al. Detection and characterization of nitrogen circulation through the sieve tubes and xylem vessels of rice plants. Plant Soil. 1997;196:233–237. [Google Scholar]
- Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Three rice nicotianamine synthase gene, OsNAS1, OsNAS2 and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J. 2003;36:366–381. doi: 10.1046/j.1365-313x.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, et al. Rice OsYSL15 is an iron-regulated iron(iii)–deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 2009 doi: 10.1074/jbc.M806042200. (in press) [DOI] [PubMed] [Google Scholar]
- Inoue H, Takahashi M, Kobayashi T, Suzuki M, Nakanishi H, Mori S, et al. Identification and localisation of the rice nicotianamine aminotransferase gene OsNAAT1 expression suggests the site of phytosiderophore synthesis in rice. Plant Mol. Biol. 2008;66:193–203. doi: 10.1007/s11103-007-9262-8. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Kim S, Tsukamoto T, Oki H, Kobayashi T, Watanabe S, et al. Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proc. Natl Acad. Sci. USA. 2007;104:7373–7378. doi: 10.1073/pnas.0610555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, et al. Rice plants take up iron as an Fe3+–phytosiderophore and as Fe2+. Plant J. 2006;45:335–346. doi: 10.1111/j.1365-313X.2005.02624.x. [DOI] [PubMed] [Google Scholar]
- Itoh J, Nomura K, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, et al. Rice plant development: from zygote to spikelet. Plant Cell Physiol. 2005;46:23–47. doi: 10.1093/pcp/pci501. [DOI] [PubMed] [Google Scholar]
- Jaennette E, Reyss A, Grégory N, Gantet P, Prioul J.-L. Carbohydrate metabolism in a heat-girdled maize source leaf. Plant Cell Environ. 2000;23:61–69. [Google Scholar]
- Kawahara C, Chonan N, Matsuda T. Studies on morphogenesis in rice plants: 7. The morphology of vascular bundles in the vegetative nodes of the culm. Jpn J. Crop Sci. 1974;43:389–401. (in Japanese) [Google Scholar]
- Kawahara C, Chonan N, Matsuda T. Studies on morphogenesis in rice plants: 8. The morphology of vascular bundles in the dwarf part of stem. Jpn J. Crop Sci. 1975;44:61–67. (in Japanese) [Google Scholar]
- Kiyomiya S, Nakanishi H, Uchida H, Nishiyama S, Tsukada H, Ishioka NS, et al. Light activates H215O flow in rice: detailed monitoring using a positron-emitting tracer imaging system (PETIS). Physiol. Plant. 2001a;113:359–367. doi: 10.1034/j.1399-3054.2001.1130309.x. [DOI] [PubMed] [Google Scholar]
- Kiyomiya S, Nakanishi H, Uchida H, Tsuji A, Nishiyama S, Futatsubashi M, et al. Real time visualization of 13N-translocation in rice under different environmental conditions using positron emitting tracer imaging system. Plant Physiol. 2001b;125:1743–1754. doi: 10.1104/pp.125.4.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, et al. OsYSL2 is a rice metal–nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004;39:415–424. doi: 10.1111/j.1365-313X.2004.02146.x. [DOI] [PubMed] [Google Scholar]
- Kume T, Matsuhashi S, Shimazu M, Ito H, Fujimura T, Adachi K, et al. Uptake and transport of positron-emitting tracer (18F) in plants. Appl. Radiat. Isot. 1997;48:1035–1043. [Google Scholar]
- Layzell DB, Pate JS, Atkins CA, Canvin DT. Partitioning of carbon and nitrogen and the nutrition of root and shoot apex in a nodulated legume. Plant Physiol. 1981;67:30–36. doi: 10.1104/pp.67.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Millán AF, Morales F, Abadía A, Abadía J. Effects of iron deficiency on the composition of the leaf apoplastic fluid and xylem sap in sugar beet. Implications for iron and carbon transport. Plant Physiol. 2000;124:873–884. doi: 10.1104/pp.124.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H, Römheld V, Kissel M. Different strategies in higher plants in mobilization and uptake of iron. J. Plant Nutr. 1986;9:695–713. [Google Scholar]
- Matsuhashi S, Fujimaki S, Uchida H, Ishioka NS, Kume T. A new visualization technique for the study of the accumulation of photoassimilates in wheat grains using [11C]CO2. Appl. Radiat. Isot. 2005;64:435–440. doi: 10.1016/j.apradiso.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Arima Y, Watanabe K, Ishioka NS, Watanabe S, Osa A, et al. 13N-nitrate uptake sites and rhizobium-infectible region in a single root of common bean and soybean. Soil Sci. Plant Nutr. 1999;45:955–962. [Google Scholar]
- Mihashi S, Mori S. Characterization of mugineic acid–Fe transporter in Fe-deficient barley roots using the multicompartment transporter box method. Biol. Metals. 1989;2:146–154. [Google Scholar]
- Mori S. Iron transport in graminaceous plants. In: Sigel A, Sigel H, editors. Iron Transport and Storage in Microorganisms, Plants and Animals. Vol. 35, Metal Ions in Biological Systems. Vol. 35. New York: Marcel Dekker; 1998. pp. 215–237. [Google Scholar]
- Mori S, Kiyomiya S, Nakanishi H, Ishioka NS, Watanabe S, Osa A, et al. Visualization of 15O-water flow in tomato and rice in the light and dark using a positron-emitting tracer imaging system (PETIS). Soil Sci. Plant Nutr. 2000;46:975–979. [Google Scholar]
- Mori S, Nishizawa KN. Methionine as a dominant precursor of phytosiderophores in Graminaceae plants. Plant Cell Physiol. 1987;28:1081–1092. [Google Scholar]
- Mori S, Nishizawa N, Hayashi H, Chino M, Yoshimura E, Ishihara J. Why are young rice plants highly susceptible to iron deficiency? Plant Soil. 1991;130:143–156. [Google Scholar]
- Murata Y, Ma JF, Yamaji N, Ueno D, Nomoto K, Iwashita T. A specific transporter for iron (III)–phytosiderophore in barley roots. Plant J. 2006;46:563–572. doi: 10.1111/j.1365-313X.2006.02714.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Bughio N, Matsuhashi S, Ishioka NS, Uchida H, Tsuji A, et al. Visualizing real time [11C]methionine translocation in Fe-sufficient and Fe-deficient barley using a positron emitting tracer imaging system (PETIS). J. Exp. Bot. 1999;50:637–643. [Google Scholar]
- Nakanishi H, Kiyomiya S, Tsukamoto T, Tsukada H, Uchida H, Mori S. Water (H215O) flow in rice is regulated by the concentration of nutrients as monitored by positron multi-probe system (PMPS). Soil Sci. Plant Nutr. 2002;48:759–762. [Google Scholar]
- Ohtake N, Sato T, Fujikake H, Sueyoshi K, Ohyama T, Ishioka NS, et al. Rapid N transport to pods and seeds in N-deficient soybean plants. J. Exp. Bot. 2001;52:277–283. [PubMed] [Google Scholar]
- Pate JS. Xylem-to-phloem transfer—vital component of the nitrogen-partitioning system of a nodulated legume. In: Cronshaw J, Lucas WJ, Giaquinta RT, editors. Phloem Transport. New York: Alan R. Liss; 1986. pp. 445–462. [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric–chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Römheld V, Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986;80:175–180. doi: 10.1104/pp.80.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Takahashi M, Tsukamoto T, Watanabe S, Matsuhashi S, Yazaki J, et al. Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J. 2006;48:85–97. doi: 10.1111/j.1365-313X.2006.02853.x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Tsukamoto T, Inoue H, Watanabe S, Matsuhashi S, Takahashi M, et al. Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants. Plant Mol. Biol. 2008;66:609–617. doi: 10.1007/s11103-008-9292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S. Naturally occurring iron-chelating compounds in oat- and rice-root washings: activity measurement and preliminary characterization. Soil Sci. Plant Nutr. 1976;22:423–433. [Google Scholar]
- Takagi S, Nomoto K, Takemoto S. Physiological aspect of mugineic acid, a possible phytosiderophores of graminaceous plants. J. Plant Nutr. 1984;7:469–477. [Google Scholar]
- Tanaka M, Wallace IS, Takano J, Robrts DM, Fujiwara T. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell. 2008;20:2860–2875. doi: 10.1105/tpc.108.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomine S, Lelièvre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H. AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 2003;34:685–695. doi: 10.1046/j.1365-313x.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl Acad. Sci. USA. 2000;97:4991–4996. doi: 10.1073/pnas.97.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin LO. Iron translocation: II. Citrate/iron ratios in plant stem exudates. Plant Physiol. 1966;41:515–518. doi: 10.1104/pp.41.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Nakanishi H, Kiyomiya S, Watanabe S, Matsuhashi S, Nishizawa NK, et al. 52Mn translocation in barley monitored using a positron-emitting tracer imaging system. Soil Sci. Plant Nutr. 2006;52:717–725. [Google Scholar]
- Tsukamoto T, Uchida H, Nakanishi H, Nishiyama S, Tsukada H, Matsuhashi S, et al. H215O translocation in rice was enhanced by 10 μM 5-aminolevulinic acid as monitored by positron emitting tracer imaging system (PETIS). Soil Sci. Plant Nutr. 2004;50:1085–1088. [Google Scholar]
- Uchida H, Okamoto T, Ohmura T, Shimizu K, Satoh N, Koike T, et al. A compact planar positron imaging system. Nucl. Instr. Methods A. 2004;516:564–574. [Google Scholar]
- Vert G, Briat JF, Curie C. Arabidopsis IRT2 gene encodes a root–periphery iron transporter. Plant J. 2001;26:181–189. doi: 10.1046/j.1365-313x.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Ishioka NS, Osa A, Koizumi M, Sekine T, Kiyomiya S, et al. Production of positron emitters of metallic elements to study plant uptake and distribution. Radiochim. Acta. 2001;89:853–858. [Google Scholar]
- Waters BM, Blevins DG, Eide DJ. Characterization of FRO1, a pea ferric–chelate reductase involved in root iron acquisition. Plant Physiol. 2002;129:85–94. doi: 10.1104/pp.010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee SY. Transfer cells and vascular tissue distribution in the vegetable nodes of rice. Aust. J. Bot. 1972;20:41–48. [Google Scholar]
- Zhang C, Römheld V, Marschner H. Distribution pattern of root-supplied 59iron in iron-sufficient and iron-deficient bean plants. J. Plant Nutr. 1995;18:2049–2058. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.