Abstract
Maize (Zea mays L.) shows a high accumulation of silicon (Si), but transporters involved in the uptake and distribution have not been identified. In the present study, we isolated two genes (ZmLsi1 and ZmLsi6), which are homologous to rice influx Si transporter OsLsi1. Heterologous expression in Xenopus laevis oocytes showed that both ZmLsi1 and ZmLsi6 are permeable to silicic acid. ZmLsi1 was mainly expressed in the roots. By contrast, ZmLsi6 was expressed more in the leaf sheaths and blades. Different from OsLsi1, the expression level of both ZmLsi1 and ZmLsi6 was unaffected by Si supply. Immunostaining showed that ZmLsi1 was localized on the plasma membrane of the distal side of root epidermal and hypodermal cells in the seminal and crown roots, and also in cortex cells in lateral roots. In the shoots, ZmLsi6 was found in the xylem parenchyma cells that are adjacent to the vessels in both leaf sheaths and leaf blades. ZmLsi6 in the leaf sheaths and blades also exhibited polar localization on the side facing towards the vessel. Taken together, it can be concluded that ZmLsi1 is an influx transporter of Si, which is responsible for the transport of Si from the external solution to the root cells and that ZmLsi6 mainly functions as a Si transporter for xylem unloading.
Keywords: Influx, Maize, NIP, Root, Silicon, Transporter
Introduction
Silicon is a beneficial element for plant growth. It helps plants to overcome multiple stresses including biotic and abiotic stresses (Epstein 1999, Ma 2004, Ma and Yamaji 2006). For example, Si enhances resistance of different plant species to diseases caused by both fungi and bacteria such as rice blast, powdery mildew, and sheath blight (Fauteux et al. 2005, 2006, Savant et al. 1997). Silicon also suppresses insect pests such as stem borer and brown planthopper (Ma and Yamaji 2008). In addition, Si increases the resistance to lodging, alleviates metal toxicity, salt and drought stresses, and improves nutrient imbalance in a wide of plant species (Ma and Yamaji 2008).
Although all plants contain Si in their tissues, there is a wide variation in Si accumulation; ranging from 0.1% to 10.0% of shoot dry weight. Plants of the families Poaceae, Equisetaceae and Cyperaceae show high Si accumulation (>4% Si), the Cucurbitales, Urticales and Commelinaceae show intermediate Si accumulation (2–4% Si), whereas most other species show low accumulation (Ma and Takahashi 2002, Hodson et al. 2005). These differences in the Si accumulation have been attributed to the ability of the roots to take up Si (Takahashi et al. 1990), but the exact mechanisms involved have been not understood.
Silicon is taken up by the root in the form of silicic acid, an uncharged molecule (Takahashi and Hino 1978). Recently three Si transporters (Lsi1, Lsi2, and Lsi6), which are involved in Si uptake and distribution, have been identified from rice, a typical Si-accumulating species (Ma et al. 2006, Ma et al. 2007, Yamaji et al. 2008). Lsi1 and Lsi6 belong to the nodulin-26 like major intrinsic protein III (NIP III) subgroup of aquaporins and are influx transporters of silicic acid (Ma et al. 2006, Mitani et al. 2008, Yamaji et al. 2008), while Lsi2 is an active efflux transporter of silicic acid (Ma et al. 2007). Lsi1 and Lsi2 are mainly expressed in the roots, but Lsi6 is also expressed in the leaf sheath and blades. In the roots, both Lsi1 and Lsi2 are localized in the exodermis and endodermis, but Lsi1 is on the distal side and Lsi2 is on the proximal side (Ma et al. 2006, 2007). Lsi6 is found in the xylem parenchyma cells of the leaf sheath and leaf blades (Yamaji et al. 2008). Loss of either Lsi1 or Lsi2 caused significant decrease in Si uptake by the roots (Ma et al. 2002, 2007), whereas loss of Lsi6 did not affect the uptake, but resulted in altered distribution of Si in the shoots (Yamaji et al. 2008). These results indicate that Lsi1 and Lsi2 are responsible for Si uptake, while Lsi6 is for transporting Si out of the xylem.
Many plant species in Gramineae family such as maize and wheat also accumulate high Si in their shoots although the extent of accumulation is lower than that of rice (Ma and Takahashi 2002, Tamai and Ma 2003, Liang et al. 2006). However, it is unknown whether these plant species have a similar transport system for Si to that in rice. In the present study, we identified two genes (ZmLsi1 and ZmLsi6) encoding Si influx transporters in maize, an important upland crop. These genes are homologs of OsLsi1 and OsLsi6, respectively, but we found that both the expression pattern and cellular localization of ZmLsi1 is different from those of OsLsi1.
Results
Isolation of rice Lsi1-like genes in maize
Based on homology research using Maize Genomics and Genetics Database (Maize GDB, http://www.maizegdb.org/), there are two homologs (ZmNIP2-1 and ZmNIP2-2, named ZmLsi1 and ZmLsi6 in this paper) of rice Si influx transporter Lsi1 in maize. The presence of these genes in maize has been described previously (Chaumont et al. 2001), but their functions were not understood. We isolated these genes from cDNA of maize roots by PCR. The open reading frame (ORF) is 888 bp long for ZmLsi1 and 885 bp for ZmLsi6 and the deduced protein comprises 295 and 294 amino acids for ZmLsi1 and ZmLsi6, respectively (Fig. 1A). Similarity analysis showed that ZmLsi1 is close to OsLsi1 with 83% identity and ZmLsi6 is close to OsLsi6 with 89% identity at amino acid level (Fig. 1B). Both ZmLsi1 and ZmLsi6 contain two conserved NPAs and four residues (G, S, G, and R) for ar/R selectivity filter identical to OsLsi1 (Fig. 1A). Therefore, ZmLsi1 and ZmLsi6 belong to NIP III subgroup according to recent definition (Mitani et al. 2008).
Fig. 1.
Alignment of the amino acid sequences of ZmLsi1, ZmLsi6, OsLsi1 and OsLsi6 (A) and their phylogenetic relationship (B). The phylogenetic analysis was performed using CrustalW. NPA motifs were boxed and the aromatic/arginine selectivity. lter (H2, H5, LE1, and LE2) are indicated in blue and red letters, respectively. The red open box indicates the peptide sequence that the anti-ZmLsi1 and anti-ZmLsi6 antibody was raised against.
Silicon transport activity of ZmLsi1 and ZmLsi6 in Xenopus laevis oocyte
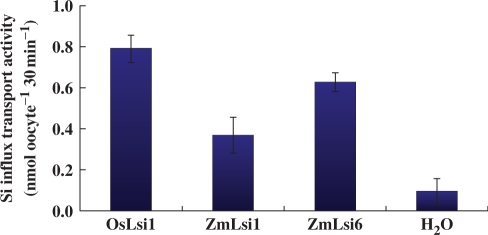
To investigate the silicon transport activity of ZmLsi1 and ZmLsi6, we expressed these genes in Xenopus oocyte as well as OsLsi1 as a positive control. The results showed that the influx transport activity of oocytes expressing either ZmLsi1 or ZmLsi6 was significantly higher than that of oocytes injected with water (control) (Fig. 2), indicating that both ZmLsi1 and ZmLsi6 are permeable to silicic acid as OsLsi1 (Fig. 2).
Fig. 2.
In. ux Si transport activity of ZmLsi1 and ZmLsi6 in Xenopus oocyte. The oocytes injected with cRNA of ZmLsi1, ZmLsi6, OsLsi1 (positive control) or water (negative control) were exposed to 1 mM silicic acid labeled with 68Ge for 30 min. Radioactivity in the oocytes were determined. Means ± SD of biological replicates (n = 3) are shown.
Expression pattern of ZmLsi1and ZmLsi6
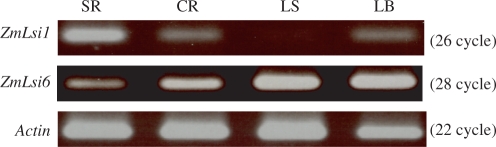
The expression patterns of ZmLsi1 and ZmLsi6 were compared in different tissues and between treatments with Si or without. The mRNA of ZmLsi1 was mainly expressed in the seminal roots including lateral roots, but much less in the crown roots and no expression in the leaf sheaths and blades (Fig. 3). In contrast, the mRNA of ZmLsi6 was expressed less in the seminal roots, but more in the crown roots, and leaf sheathes and blades (Fig. 3).
Fig. 3.
Expression of ZmLsi1 and ZmLsi6 in different tissuse of maize. Transcripts of ZmLsi1 and ZmLsi6 were detected by RT-PCR. Total RNA was isolated from the seminal roots including lateral roots (SR), crown roots (CR), leaf sheaths (LS) and leaf blades (LB) of 20-d-old seedlings grown hydroponically. Actin was used as an internal standard. Numbers in parentheses are PCR cycle.
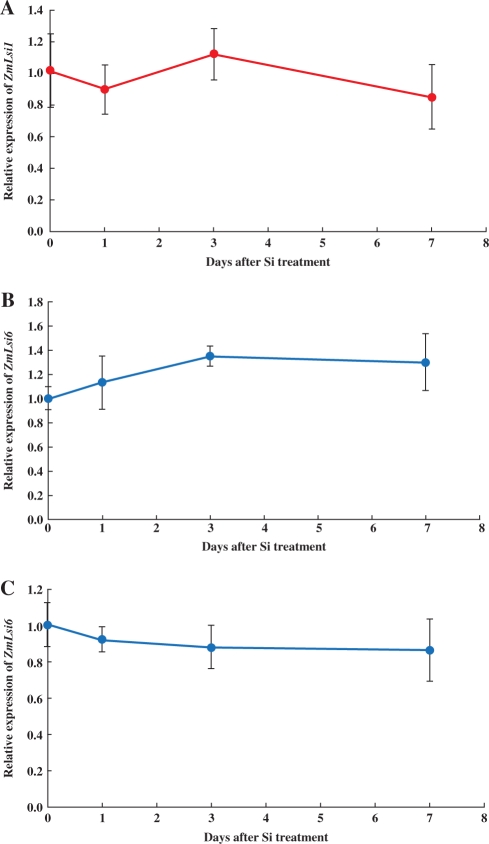
The mRNA expression of ZmLsi1 in the roots was constitutive and its expression was not affected by continuous Si supplement up to 7 d (Fig. 4A). The expression of ZmLsi6 in both the crown roots and leaf blades was also not affected by Si supplement (Fig. 4B, C).
Fig. 4.
Effect of Si supply on ZmLsi1 and ZmLsi6 expression. Seedlings (20-d-old) grown hydroponically were treated with or without 1.0 mM Si for different days and then relative expression levels of ZmLsi1 in the seminal roots (A) and ZmLsi6 in the crown roots (B) and leaf blades (C) were compared by quantitative RT-PCR. Means ± SD of biological replicates (n = 3) are shown.
Subcellular and cellular localization of ZmLsi1 and ZmLsi6
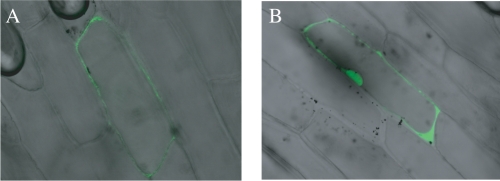
We investigated the subcellular localization of ZmLsi1 by delivering a translational fusion between green fluorescent protein (GFP) and ZmLsi1 into onion epidermal cells by particle bombardment. Cells expressing the GFP-ZmLsi1 fusion showed GFP fluorescence only in the plasma membrane (Fig. 5A), whereas the signal for cells expressing GFP alone was found in the nucleus and cytosol (Fig. 5B). The subcellular localization of ZmLsi6 was not examined due to the difficulties in constructing a GFP-fused gene.
Fig. 5.
Subcellular localization of ZmLsi1. A GFP-ZmLsi1 fusion (A) and GFP alone as control (B) were introduced by particle bombardment into onion epidermal cells.
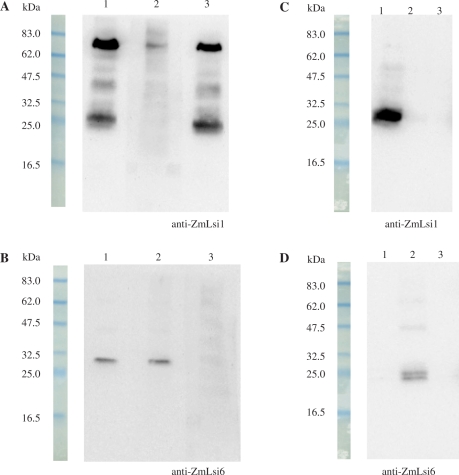
To investigate the localization of ZmLsi1 and ZmLsi6, we raised antibodies against them. The specificity of the antibodies was tested by pre-incubating with the peptide epitopes each other before primary antibody treatment for Western blot analysis. Two major bands for ZmLsi1 were detected in the microsomal fraction of the roots (Fig. 6A, lane 1), which probably corresponds to dimer and monomer of ZmLsi1. When anti-ZmLsi1 was pre-incubated with ZmLsi1 peptide epitope, the signals disappeared (Fig. 6A, lane 2), whereas pre-incubation with ZmLsi6 peptide epitope did not affect the signal (Fig. 6A, lane 3). On the other hand, anti-ZmLsi6 generated a signal in the microsomal fraction of the roots (Fig. 6B, lane 1). When anti-ZmLsi6 was pre-incubated with ZmLsi1 peptide epitope, the signal was not affected (Fig. 6B, lane 2), but pre-incubation with ZmLsi6 peptide epitope resulted in disappearance of the signal (Fig. 6B, lane 3). Furthermore, when ZmLsi1 or ZmLsi6 was expressed in the oocytes, the signal was only detected by their own antibody (Fig. 6C, lane 1, 6D, lane 2). All these results indicate high specificity of each antibody and that there was no cross-reactivity between ZmLsi1 and ZmLsi6.
Fig. 6.
Western blot analysis and subcellular localization. Expression of ZmLsi1 (A) and ZmLsi6 protein (B) in the roots. SDS-PAGE and Western blot analyses of root membrane protein were conducted using anti-ZmLsi1 or anti-ZmLsi6 antibody without (lane 1) or with pre-incubation of ZmLsi1 (lane 2) and ZmLsi6 (lane 3) peptide epitopes. (C–D) Heterologous expression of ZmLsi1 and ZmLsi6 in Xenopus oocytes. Oocytes expressed with ZmLsi1 (lane 1) or ZmLsi6 (lane 2) and without expression (lane 3) were detected with anti-ZmLsi1 (C) or anti-ZmLsi6 (D) antibody.
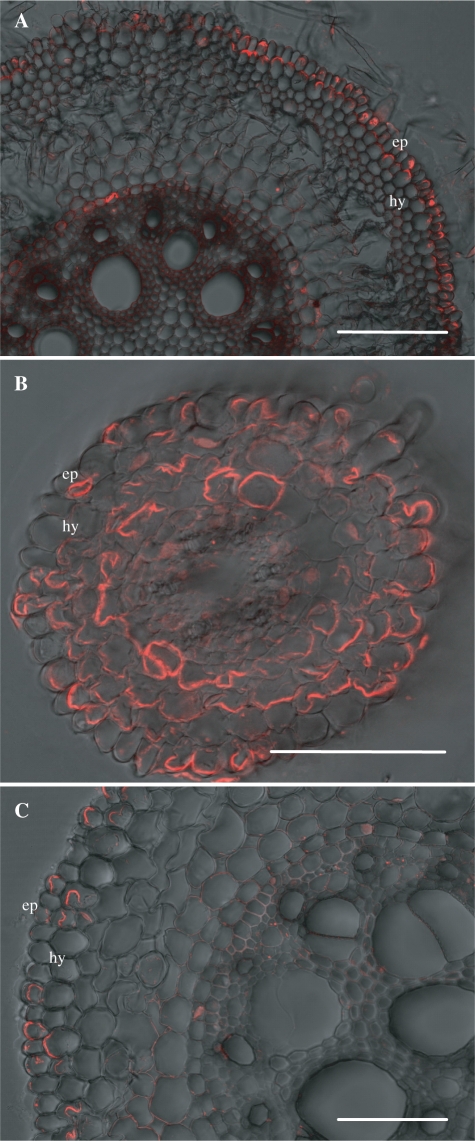
The localization of ZmLsi1 and ZmLsi6 proteins in different maize tissues was examined by immunostaining. ZmLsi1 was localized only in the epidermis and hypodermis cells of seminal roots (Fig. 7A). In contrast, ZmLsi1 was expressed in the epidermal and all cortex cells of seminal lateral roots (Fig. 7B). In crown roots, localization of ZmLsi1 was observed in some of the epidermal and hypodermal cells (Fig. 7C). In all types of roots, ZmLsi1 showed polar localization at the distal side of the cells (Fig. 7).
Fig. 7.
Immunostaining of ZmLsi1 protein in maize roots. The roots were stained with anti-ZmLsi1 polyclonal antibody. (A) Seminal root (30 mm from the tip); (B) lateral root; (C) crown root (15 mm from the tip). Ep, epidermis; Hy, hydrodermis. Scale bar = 100 μm.
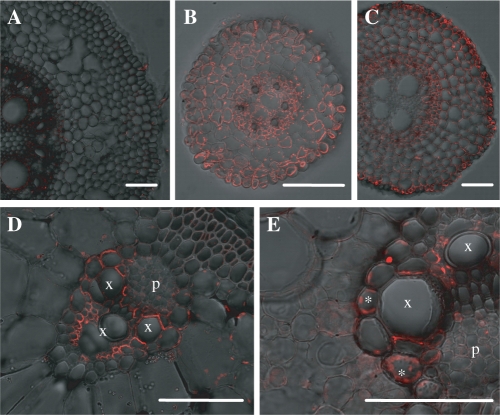
ZmLsi6 was hardly detected in the seminal roots (Fig. 8A), but was found in the lateral roots on the seminal roots and crown roots (Fig. 8B, C). No polar localization of ZmLsi6 was observed in the roots. In the shoot, ZmLsi6 protein was only observed in the xylem parenchyma cells that are adjacent to the vessels in both leaf sheaths and leaf blades (Fig. 8D, E). ZmLsi6 in the leaf sheaths and blades also exhibited polar localization on the side facing toward the vessel (Fig. 8D, E).
Fig. 8.
Cellular localization of ZmLsi6 in maize. The roots and leaf blades and sheaths were stained with anti-ZmLsi6. (A) seminal root (30 mm from the tip); (B) lateral root; (C) crown root (15 mm from the tip); (D) leaf sheath; and (E) leaf blade. Asterisks in (E) indicate auto. uorescence from chloroplasts in mesophyll cells. Xylem and phloem are indicated as x and p, respectively in (D) and (E). Scale bar = 100 μm.
Discussion
In the present study, we identified two genes (ZmLsi1 and ZmLsi6) belonging to NIP III subgroup of aquaporins from maize based on the homology of the rice Si influx transporter gene OsLsi1 (Fig. 1). Heterologous expression analysis showed that both ZmLsi1 and ZmLsi6 gave influx transport activity for silicic acid (Fig. 2), indicating that ZmLsi1 and ZmLsi6 are involved in the uptake and/or distribution of Si in maize. Since ZmLsi1 is only expressed in the seminal roots, but not in the shoots (Fig. 3), it is likely that, similar to OsLsi1, ZmLsi1 functions as an influx transporter of Si in maize roots. However, the cellular localization and expression pattern of ZmLsi1 are different from those of OsLsi1.
OsLsi1 is localized only at the exodermis and endodermis of the roots including seminal, lateral and crown roots (Ma et al. 2006, Yamaji and Ma 2007), whereas ZmLsi1 is localized at the epidermis and hypodermis cells of the seminal roots, and epidermis and cortex cells of the lateral roots (Fig. 7). These differences may be attributed to the differences in the root structure of maize and rice. Rice roots have two Casparian bands at the exodermis and endodermis, which prevents the free passage of minerals into the stele. Furthermore, rice roots are characterized by the formation of the aerenchyma, which is accompanied by the destruction of almost all cortical cells except the exodermis and the endodermis. Therefore, OsLsi1 is required at both the exodermis and endodermis for an efficient transport of Si into the stele (Yamaji and Ma 2007). On the other hand, there is only one Casparian band at the endodermis of maize roots under normal growth condition (Hose et al. 2001). Therefore, Si taken up by ZmLsi1 at the epidermis and hypodermis may reach to the stele by symplastic pathway. Interestingly, in the lateral roots on the seminal roots, ZmLsi1 is localized at all cell layers of cortex in addition to the epidermal cells (Fig. 7). Lateral roots have been demonstrated to play an important role in Si uptake in rice (Ma et al. 2001). In maize, the number of lateral roots is much more than that of seminal roots and crown roots. By taking the lateral root number and the cellular localization of ZmLsi1 into consideration, it is likely that lateral roots play a major role in Si uptake in maize.
The expression level of OsLsi1 is decreased by Si supply (Ma et al. 2006), whereas that of ZmLsi1 was not (Fig. 4). These results suggest that the mechanism regulating expression of ZmLsi1 is different from that of OsLsi1. In the future, it is necessary to compare the promoter region between OsLsi1 and ZmLsi1.
A previous study showed that the uptake capacity of Si by the roots is much higher in rice than in maize (Tamai and Ma 2003). However, ZmLsi1 has influx transport activity for Si similar to OsLsi1 in oocytes (Fig. 2). To address this discrepancy, further studies are required. For example, it is necessary to compare the Si transport activity in oocytes between OsLsi1 and ZmLsi1 based on the expressed protein level. Although we injected the same amount of cRNA into the oocytes, different protein may have different translation efficiency. Alternatively, the expression of ZmLsi1 in a rice mutant defective in Si uptake would be a good way to compare the transport activity of Si between ZmLsi1 and OsLsi1. The different cellular localization of Lsi1 may also be responsible for the difference in root Si uptake capacity. Localization of OsLsi1 at both exodermis and endodermis may be more efficient at transporting Si from the external solution into the xylem. The abundance of ZmLsi1 and OsLsi1 in the roots should also be compared although direct comparison is difficult in different plant species. Finally, the Si uptake by the roots is not only mediated by Lsi1, but other transporters may be involved. In fact, in rice, an efflux transporter OsLsi2 has been identified (Ma et al. 2007). OsLsi2 is responsible for actively transporting Si out of the cell toward to the stele. Therefore, it will be interesting to examine whether maize has a similar efflux transporter in the roots.
In contrast to ZmLsi1, ZmLsi6 showed a higher expression in the shoots and crown roots (Fig. 3). Crown roots form the major part of the adult rootstock and are the basis for lodging resistance of the plants (Hochholdinger et al. 2004). Silicon taken up by the crown roots may increase their mechanical resistance. In the leaf sheaths and blades, ZmLsi6 is localized at the xylem parenchyma cells (Fig. 8). This cellular localization is similar to OsLsi6 (Yamaji et al. 2008). OsLsi6 is responsible for transporting Si out of the xylem. Loss of this gene results in increased Si in the guttation drops and altered Si distribution (Yamaji et al. 2008). Since the knockout line of ZmLsi6 is not available, we could not demonstrate the function of ZmLsi6 directly. However, from the cellular localization and transport activity (Figs. 2, 8), it is likely that ZmLsi6 has similar a function to OsLsi6 in the shoot.
ZmLsi1 showed polar localization at the distal side of the root cells (Fig. 7). This localization is similar to OsLsi1 (Ma et al. 2006). In maize, such polar localization was also reported for water channel ZmPIP2;5 (Hachez et al. 2006). Polar localization regulates the transport direction of a solute, but the mechanism for localization is unknown. ZmLsi6 also showed polar localization in the leaf sheaths and blades (Fig. 8D, E), but not in the roots (Fig. 8A–C). This observation suggests that the protein itself is not involved in the polar localization.
Taken together, it can be concluded that ZmLsi1 is an influx transporter of Si responsible for the transport of Si from the external solution into the root cells. ZmLsi6 mainly functions as a Si transporter for xylem unloading.
Materials and Methods
Plant materials and growth conditions
Seeds of maize (Zea mays L. cv B73) were soaked in water for 3–4 h, and then were placed on the moisture filter paper in a Petri dish and incubated overnight in the refrigerator at 4°C. Seeds were germinated at 25°C in a growth chamber and then transferred onto a net floated on a 0.5 mM CaCl2 solution in a plastic container. On day 7, the seedlings were grown hydroponically in a 1/5000a plastic pot containing continuously aerated one-fifth Hoagland's solution (Mitani and Ma 2005). The nutrient solution was renewed every 2 d.
Cloning of OsLsi1-like genes
The first-strand cDNAs were obtained from the maize roots by reverse transcription. Briefly, the roots of maize (21 d old), prepared as described above, were ground in liquid nitrogen and immediately subjected to RNA extraction with RNeasy™ Plant Mini Kit (QIAGEN). Total RNA (1.5μg) was reverse-transcripted into cDNA using SuperScript™ First-Strand Synthesis System (Invitrogen).
The open-reading frame (ORF) of ZmLsi1 and ZmLsi6 with flanking Bgl II or BamHI site was amplified with the following oligonucleotide primers from the cDNA samples:
ZmLsi1- BglII forward 5′-AAGATCTAATGTCGACCAACTCGAGGTCC-3′,
ZmLsi1-BglII reverse 5′-AAGATCTCACACTTGGATGTGGTCGAGC-3′,
ZmLsi6-BamHI forward 5′-AGGATCCACGCCATGGCC GCCG-3′,
ZmLsi6-BamHI forward 5′-AGGATCCTTTAGACGGTGTCGAACTCGTC-3′.
These amplified ORFs were inserted into TAcloning vector pTA2 (TOYOBO) according to the protocol of the manufacturer. The sequnce of both genes were confirmed by ABI PRISM 310 Genetic Analyzer using BigDye Terminators v3.1 cycle Sequencing Kit.
Silicon transport activity assay in X. laevis oocyte
Oocytes were isolated from adult female X. laevis frogs, and placed in a modified Birth's saline (MBS) solution (Mitani et al. 2008). Procedures for defolliculation, culture condition and selection were the same as described previously (Mitani et al. 2008). For synthesis of capped RNA, the inserts were digested from the pTA2 vector described above with BglII or BamHI and ligated into pXβG-ev1. Capped RNAs were then synthesized from SpeI-linealized pXβG-ev1 plasmids by in vitro transcription with mMASSAGGE mMACHINE High Yield Capped RNA Transcription Kit (Ambion) according to the manufacturer's instructions. Quantities of synthesized RNAs were estimated by denaturing gel electrophoresis. A volume of 50 nl (1 ng nl−1) each of in vitro cRNA transcripts was injected into the selected oocytes using a Nanoject II automatic injector (Drummond Scientific Co.). As a negative control, 50 nl of RNase-free water were also injected. After incubation in MBS at 18°C for 1 d, the oocytes were used for transport activity assay as described previously (Mitani et al. 2008). Briefly, the influx transporter activity for silicic acid was measured by exposing three replicates of Xenopus oocytes (five per replicate) to the MBS solution containing 1 mM silicic acid labeled with 68Ge (Parkin Elmer: 2 MBq mmol−1). After 30 min, the oocytes were washed with MBS and then homogenized with 0.1 N HNO3. Radioactivities of the homogenized oocytes were determined by Liquid Scintillation Analyzer (Perkin Elmer).
Expression pattern
Different tissues including seminal roots, crown roots, leaf blades and sheaths were sampled and subjected to RNA extraction as described above. The expression of ZmLsi1 and ZmLsi6 at different tissues was examined by RT-PCR.
To investigate the effect of Si supply on the gene expression, seedlings (20 d old) were cultured in a one-fifth Hoagland's solution containing 0 or 1 mM silicic acid. At day 0, 1, 3, and 7, different tissues including seminal roots, crown roots, leaf sheaths, and leaf blades were sampled and their RNA was extracted as described above. Quantitative real-time PCR was performed with SYBR premix Ex Taq™ (TaKaRa) using Applied Biosystems 7500. Primer sequences used were as follows:
ZmLsi1 forward 5′-ACATCCAAGTGTGATTCATGCTC-3′,
ZmLsi1 reverse 5′-CATAGACATAGACCATGACTACG-3′,
ZmLsi6 forward 5′- AACACTGTCAGCCTGCAGGTC-3′,
ZmLsi6 reverse 5′- TACATAAACATCACGCTAGCTGC-3′,
Actin forward 5′-GACTCTGGTGATGGTGTCAGC-3′,
Actin reverse 5′-GGCTGGAAGAGGACCTCAGG-3′.
Western blot analysis
Thirty gram of maize roots (14-d-old) were used for preparing membrane protein according to Sugiyama et al. (2007). The protein content of microsomal fraction was measured by the Bradford method (Bio-Rad). Equal amounts of samples were mixed with same volume of sample buffer containing 250 mM Tris–HCl, pH 6.8, 8% (w/v) SDS, 40% (w/v) glycerol, 0.01% (w/v) bromophenol blue, and 200 mM β-mercaptoethanol. The mixture was allowed to incubate at 37°C for 10 min and SDS-PAGE was run using 5% to 20% gradient polyacryl-amide gels (ATTO). The transfer to polyvinylidene difluoride membrane was performed with a semidry blotting system, and the membrane was treated with the purified primary rabbit anti-ZmLsi1 and anti-ZmLsi6 polyclonal antibodies diluted at 1 : 100. To check the antibody specificity, each antibody was pre-incubated with the epitope peptide used for antibody preparation at 75 nmol ml−1 for 1 h at room temperature. Anti-rabbit IgG horseradish peroxidase conjugate (1 : 10,000 dilution; GE Healthcare) was used as a secondary antibody, and ECL plus (GE Healthcare) was used for detection via chemiluminescence.
To further examine the specificity of antibodies, ZmLsi1 or ZmLsi6 cRNA were injected into oocytes. After a 1d incubation, 14 oocytes injected with or without cRNA were homogenized with extraction buffer containing 50 mM Tris–HCl (pH 7.5), 0.3 M sorbitol, 5 mM EDTA, 1 mM DTT, and 1/100 volume of Protease Inhibitor Cocktail (Sigma-Ardrich). After centrifugation at 2,200× g for 5 min at 4°C, the supernatant was subjected to ultracentrifugation at 100,000× g for 20 min at 4°C. Then pellet was resuspended by extraction buffer and subjected to Western blot analysis as described above.
Transient expression of GFP fusion
The open reading frame of ZmLsi1 was amplified by gene-specific primers 5′-TTGTACAAGGGAATGTCGACCAACTCGAGGTCC-3′and 5′-AAGATCTCACACTTGGATGTGGTCGAGC-3′ using high-fidelity KOD plus DNA polymerase (Toyobo) to create BsrGI and BglII sites on each end. The fragment was treated with restriction enzymes and inserted in the frame between the GFP coding sequence and NOS terminator under the control of the 35S promoter in pBluescript vector. The fused gene was transiently introduced into onion epidermal cells by particle bombardment as described previously (Ma et al. 2007).
Immunohistological staining
The synthetic peptides C-SVAVDDDELDHIQV (positions 282–295 of ZmLsi1) and C-QSQLAADEFDTV (positions 283–294 of ZmLsi6) were used to immunize rabbits to obtain antibodies against ZmLsi1 and ZmLsi6, respectively. Obtained antiserums were purified through the peptide affinity column before use. The seminal, crown and lateral roots, and/or leaf sheath and leaf blade of maize were used for immunostaining of ZmLsi1 and ZmLsi6 protein with 1 : 300 diluted anti-ZmLsi1 or anti-ZmLsi6 antibody as described previously (Yamaji and Ma 2007). Fluorescence of secondary antibody (Alexa Fluor 555 goat anti-rabbit IgG; Molecular Probes) was observed with a fluorescence microscope (Axio Imager with Apotome; Carl Zeiss).
Funding
This research was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 17078008 to J. F. M.) and by Sunbor grant and Ohara Foundation for Agricultural Research.
Acknowledgments
We thank Yoshiro Mano for providing the maize seeds and Jared Westbrook for critical reading of this manuscript.
References
- Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001;125:1206–1215. doi: 10.1104/pp.125.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- Fauteux F, Remus-Borel W, Menzies JG, Belanger RR. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005;249:1–6. doi: 10.1016/j.femsle.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Fauteux F, Chain F, Belzile F, Menzies JG, Belanger RR. The protective role of silicon in the Arabidopsis–powdery mildew pathosystem. Proc. Natl Acad. Sci. USA. 2006;103:17554–17559. doi: 10.1073/pnas.0606330103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F. Localization and quantification of plasma membrane aquaporin expression in maize primary root: a clue to understanding their role as cellular plumbers. Plant Mol. Biol. 2006;62:305–323. doi: 10.1007/s11103-006-9022-1. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Ann. Bot. (Lond.) 2004;93:359–368. doi: 10.1093/aob/mch056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson MJ, White PJ, Mead A, Broadley MR. Phylogenetic variation in the silicon composition of plants. Ann. Bot. (Lond.) 2005;96:1027–1046. doi: 10.1093/aob/mci255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W. The exodermis: a variable apoplastic barrier. J. Exp. Bot. 2001;52:2245–2264. doi: 10.1093/jexbot/52.365.2245. [DOI] [PubMed] [Google Scholar]
- Liang Y, Hua H, Zhu YG, Zhang J, Cheng C, Römheld V. Importance of plant species and external silicon concentration to active silicon uptake and transport. New Phytol. 2006;172:63–72. doi: 10.1111/j.1469-8137.2006.01797.x. [DOI] [PubMed] [Google Scholar]
- Ma JF. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci.Plant Nutr. 2004;50:11–18. [Google Scholar]
- Ma JF, Goto S, Tamai K, Ichii M. Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiol. 2001;127:1773–1780. [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Ichii M, Wu K. A rice mutant defective in active Si uptake. Plant Physiol. 2002;130:2111–2117. doi: 10.1104/pp.010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Takahashi E. Amsterdam:: Elsevier Science; 2002. Soil, fertilizer, and plant silicon research in Japan. [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006;11:392–397. doi: 10.1016/j.tplants.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. An efflux transporter of silicon in rice. Nature. 2007;448:209–212. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008 doi: 10.1007/s00018-008-7580-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani N, Ma JF. Uptake system of silicon in different plant species. J. Exp. Bot. 2005;56:1255–1261. doi: 10.1093/jxb/eri121. [DOI] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ma JF. Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Arch. 2008;456:679–86. doi: 10.1007/s00424-007-0408-y. [DOI] [PubMed] [Google Scholar]
- Savant NK, Snyder GH, Datnoff LE. Silicon management and sustainable rice production. Advan. Agron. 1997;58:151–199. [Google Scholar]
- Sugiyama A, Shitan N, Yazaki K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume–Rhizobium symbiosis. Plant Physiol. 2007;144:2000–2008. doi: 10.1104/pp.107.096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Ma JF, Miyake Y. The possibility of silicon as an essential element for higher plants. Comments Agric. Food Chem. 1990;2:99–122. [Google Scholar]
- Takahashi E, Hino K. Silica uptake by plant with special reference to the forms of dissolved silica. J. Soil Sci. Manure Jpn. 1978;49:357–360. [Google Scholar]
- Tamai K, Ma JF. Characterization of silicon uptake by rice roots. New Phytol. 2003;158:431–436. doi: 10.1046/j.1469-8137.2003.00773.x. [DOI] [PubMed] [Google Scholar]
- Yamaji N, Ma JF. Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol. 2007;143:1306–1313. doi: 10.1104/pp.106.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Mitani N, Ma JF. A transporter regulating silicon distribution in rice shoot. Plant Cell. 2008;20:1381–1389. doi: 10.1105/tpc.108.059311. [DOI] [PMC free article] [PubMed] [Google Scholar]