Abstract
Boron (B) is an essential element for plants, and B deficiency is a worldwide agricultural problem. In B-deficient areas, B is often supplied as fertilizer, but excess B can be toxic to both plants and animals. Generation of B deficiency-tolerant plants could reduce B fertilizer use. Improved fertility under B-limiting conditions in Arabidopsis thaliana by overexpression of BOR1, a B transporter, has been reported, but the root growth was not improved by the BOR1 overexpression. In this study, we report that enhanced expression of NIP5;1, a boric acid channel for efficient B uptake, resulted in improved root elongation under B-limiting conditions in A. thaliana. An NIP5;1 activation tag line, which has a T-DNA insertion with enhancer sequences near the NIP5;1 gene, showed improved root elongation under B limitation. We generated a construct which mimics the tag line: the cauliflower mosaic virus 35S RNA promoter was inserted at 1,357 bp upstream of the NIP5;1 transcription initiation site. Introduction of this construct into the nip5;1-1 mutant and the BOR1 overexpresser resulted in enhanced expression of NIP5;1 and improved root elongation under low B supply. Furthermore, one of the transgenic lines exhibited improved fertility and short-term B uptake. Our results demonstrate successful improvement of B deficiency tolerance and the potential of enhancing expression of a mineral nutrient channel gene to improve growth under nutrient-limiting conditions.
Keywords: Arabidopsis thaliana, Boron, Channel, Deficiency tolerance, Nodulin 26-like intrinsic proteins, Transporter
Introduction
Boron (B) is an essential element for plants (Marschner 1995). B is mainly present in cell walls and cross-links rhamnogalacturonan-II, a pectic polysaccharide (Kobayashi et al. 1996, Ishii and Matsunaga 1996, O’Neill et al. 1996). Reduction of cross-linked rhamnogalacturonan-II resulted in suppressed growth of Arabidopsis thaliana (O’Neill et al. 2001), demonstrating that B is required as one of the cell wall components. B deficiency is reported in >80 countries (Shoroccks 1997). B-deficient symptoms first appear in the growing point of plants, and typical symptoms include inhibition of root elongation, expansion of leaves and fertilization (Dell and Huang 1997). Therefore, a continuous supply of B is important for normal growth through vegetative and reproductive stages. In the B-deficient areas, B is supplied as fertilizer, but the usage of B fertilization needs to be tightly controlled because B accumulation potentially causes toxicity problems. Generation of B deficiency-tolerant plants could reduce B fertilizer use and potential toxicity problems. Regulation of B transport is among the strategies to generate such plants.
Understanding of B transport mechanisms at the molecular level has advanced greatly in the last several years (for a review, see Takano et al. 2008). Two different types of transporters, BOR1 and NIP5;1, were identified as B transport molecules required for efficient B translocation under B-limited conditions in A. thaliana (Takano et al. 2002, Takano et al. 2006).
BOR1 is a boric acid/borate transporter for xylem loading (Takano et al. 2002) identified as a causual gene for the bor1-1 mutant of A. thaliana. The bor1-1 mutant exhibited severe shoot growth inhibition and reduced fertility under low B conditions (Noguchi et al. 1997) and is defective in B translocation from roots to shoots (Noguchi et al. 2000). Overexpression of BOR1 under the control of the cauliflower mosaic virus 35S RNA (CaMV 35S) promoter resulted in improved shoot growth and fertility under B-deficient conditions (Miwa et al. 2006).
NIP5;1 is a member of the major intrinsic protein (MIP) family and has been identified as a boric acid channel required for plant growth under low B conditions in A. thaliana (Takano et al. 2006). MIPs are channels for water and/or small non-charged molecules (Wallace et al. 2006). MIPs are grouped into four subfamilies: the plasma membrane intrinsic protein (PIPs), the tonoplast intrinsic proteins (TIPs), the nodulin 26-like intrinsic proteins (NIPs) and the small basic intrinsic proteins (SIPs). NIP5;1 belongs to the NIP subfamily and mediates boric acid transport (Takano et al. 2006). Under low B conditions, NIP5;1 is up-regulated at the mRNA level and NIP5;1 mutants exhibited severe growth reduction both in shoots and roots.
As overexpression of BOR1 under the control of the CaMV 35S promoter improved shoot growth and fertility under B deficiency (Miwa et al. 2006), we tried the same approach with NIP5;1 without success (data not shown). However, we succeeded in generating plants with elevated tolerance to low B levels by inserting the CaMV 35S enhancer into the promoter region of NIP5;1.
Results
Root growth was improved in the NIP5;1 activation tag lines under limited B conditions
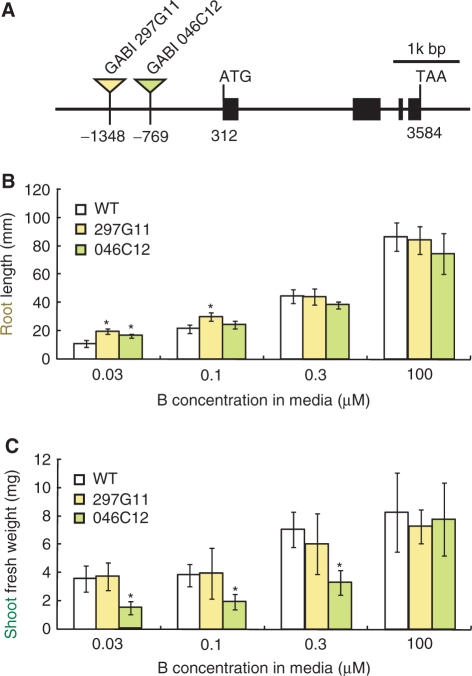
Because overexpression of NIP5;1 under the control of the CaMV 35S promoter did not result in improved growth under low B supply (data not shown), we tried another approach. Two independent NIP5;1 activation tag lines (GABI 297G11 and 046C12), which carry T-DNA insertions upstream of the initiation codon of the NIP5;1 gene (Rosso et al. 2003), were obtained (Fig. 1A). NIP5;1 mRNA levels in roots were increased by 2-fold in both lines compared with wild-type plants (Supplementary data 1). The activation tag lines were grown on solid medium containing various concentrations of B, and shoot fresh weights and primary root lengths were measured (Fig. 1B, C). In GABI 297G11, root length was 1.8 and 1.4 times longer than that of the wild-type plants on medium containing 0.03 and 0.1 μM B, respectively. The root length of GABI 297G11 was similar to that of the wild type at 0.3 and 100 μM B. There was no significant difference in shoot fresh weight compared with the wild-type plants under all conditions examined. In GABI 046C12, root lengths on medium containing 0.03 μM B were 1.5 times longer than those of the wild-type plants; however, shoot fresh weight was reduced to about 50% of that of wild-type plants under B limitation.
Fig. 1.
Growth of NIP5;1 activation tag lines. (A) Schematic representation of a gene model of NIP5;1 and T-DNA insertion sites of activation tag lines based on SIGnAL T-DNA express. Black boxes represent exons. T-DNAs are not drawn to scale. The first nucleotide of full-length cDNA (NM_117106) was defined as + 1. (B) and (C) Growth of NIP5;1 activation tag lines. Plants were grown on solid medium containing various concentrations of boric acid. Primary root lengths (B) and shoot fresh weights (C) were measured. The averages and SDs are shown (n = 7–10). Asterisks indicate significant differences from the wild-type plants (Student's t-test P < 0.05).
These observations established that in the two enhancer tag lines, root growth was improved under low B conditions. Wild-type plants and two NIP5;1 activation tag lines grew similarly on media containing 100 μM B, suggesting that growth enhancement of roots is specific to the low B condition. It is not clear why shoot fresh weight was reduced in GABI 046C12 but not in GABI 297G11, while both lines have a similar level of NIP5;1 transcript accumulation in roots (Supplementary data 1). Possible explanations include second site mutations and/or loss of tissue-specific expression.
Enhanced expression of NIP5;1 resulted in improved root elongation under low B conditions in the nip5;1-1 mutant
The sites of T-DNA insertion in the two tag lines were both upstream of the NIP5;1 gene, and the T-DNA in the GABI lines includes the CaMV 35S promoter. As shown in Supplementary data 1, NIP5;1 transcription levels were elevated in these lines. It is possible that the root growth enhancement observed only under low B conditions is due to enhancement of NIP5;1 expression.
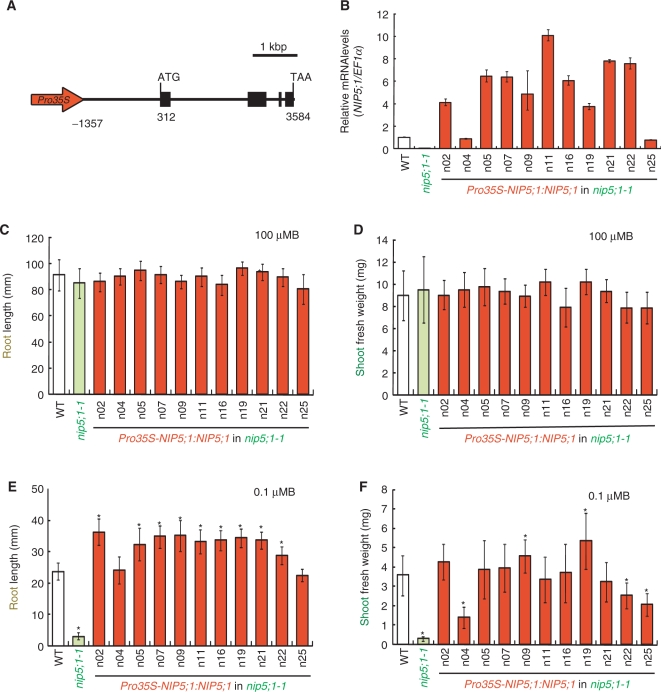
To verify this hypothesis, we generated a DNA construct which has the CaMV 35S promoter at 1,357 bp upstream of the NIP5;1 transcription initiation site (Fig. 2A). This DNA construct was designed to mimic the NIP5;1 promoter in GABI 297G11 and will be referred to as Pro35S-NIP5;1:NIP5;1. GABI 297G11 was selected because, unlike the case of GABI 046C12, it exhibited growth enhancement without inhibitory effects on shoot growth (Fig. 1B, C). This DNA construct was introduced into nip5;1-1, a NIP5;1 mutant line (Takano et al. 2006). Eleven independent transgenic lines (named n02–n25) were obtained and T3 homozygous plants were established. The relative expression level of NIP5;1 in roots was measured under B-limiting conditions (Fig. 2B). Nine out of the 11 transgenic lines had 3.7- to 10-fold more accumulation of NIP5;1 mRNA than the wild-type plants. Accumulation of NIP5;1 mRNA in n04 and n25 was 0.88 and 0.76 times lower than in the wild-type plants.
Fig. 2.
Generation and growth characterization of Pro35S-NIP5;1:NIP5;1 transgenic plants in the nip5;1-1 background. (A) The schematic representation of the DNA construct which has the CaMV 35S promoter at 1,357 bp upstream of the NIP5;1 transcription initiation site. (B) Relative mRNA level of NIP5;1 in roots of the transgenic plants. Plants were grown on plates containing 100 μM B for 12 d. Then the plants were transferred to plates containing 0.1 μM B and incubated for 1 d. Total RNAs were extracted from roots of 7–10 plants. The averages and SDs are presented for independent reverse transcription reactions followed by real-time PCR (n = 3). (C–F) Growth of the transgenic plants. Plants were grown on solid medium containing 100 or 0.1 μM boric acid for 12 d. Shoot fresh weights and primary root lengths were measured. Averages and SDs are shown (n = 9–10). Asterisks indicate significant differences from the wild-type plants (Student's t-test P < 0.05). (C) Primary root length under 100 μM B conditions. (D) Shoot fresh weight under 100 μM B conditions. (E) Primary root length under 0.1 μM B conditions. (F) Shoot fresh weight under 0.1 μM B conditions.
To investigate the effect of enhanced expression of NIP5;1, the wild type, nip5;1-1 and the transgenic nip5;1-1 lines carrying Pro35S-NIP5;1:NIP5;1 were grown on medium containing 0.1 or 100 μM B. Shoot fresh weight and primary root length were measured (Fig. 2C–F). Growth of the transgenic lines under 100 μM B supply was similar to that of the wild type except for a slight reduction of root length in n25 (Fig. 2C, D). Under 0.1 μM B supply, the root length of transgenic plants which have enhanced expression of NIP5;1 was 1.2- to 1.5-fold longer than that of the wild-type plants (Fig. 2E). Root lengths of n04 and n25, which have lower NIP5;1 mRNA levels than the wild-type plants, were not significantly different from that of the wild-type plants. Shoot fresh weights were similar to that of the wild-type plants in seven transgenic lines (Fig. 2F). The shoot fresh weights of n09 and n19 were 1.3 and 1.5 times larger than that of wild-type plants, respectively (Fig. 2F). These results indicate that enhanced expression of NIP5;1 resulted in improved root elongation under low B supply.
Enhanced expression of NIP5;1 improved root elongation in plants overexpressing BOR1 under low B supply
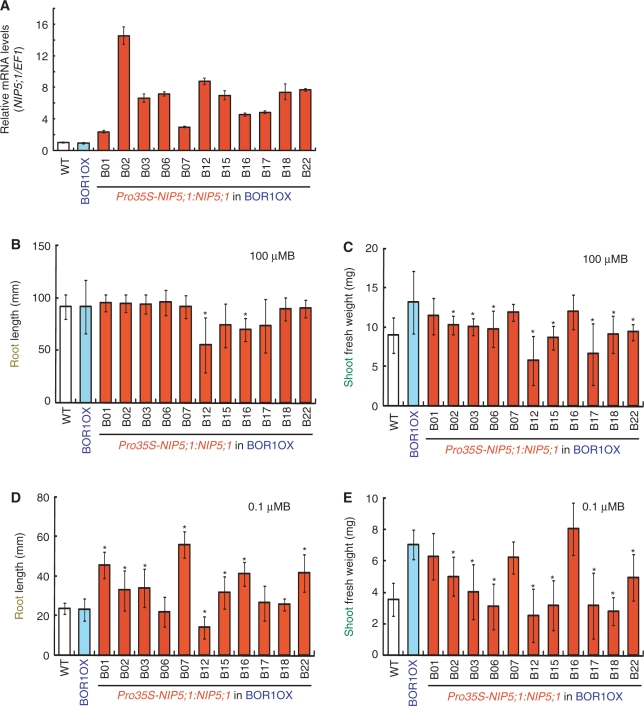
Overexpression of BOR1 improved shoot growth but not root growth (Miwa et al. 2006). To confer further B deficiency tolerance, we introduced Pro35S-NIP5;1:NIP5;1 into the transgenic line overexpressing BOR1 (BOR1OX, line18, Takano et al. 2005). Eleven independent transgenic lines were obtained and T3 homozygous plants in terms of Pro35S-NIP5;1:NIP5;1 insertion were established. The relative expression levels of NIP5;1 in roots were measured under B-limiting conditions (Fig. 3A). Transgenic plants had 2.3- to 15-fold more accumulation of NIP5;1 mRNA than the wild-type plants.
Fig. 3.
Generation and growth characterization of Pro35S-NIP5;1:NIP5;1 transgenic plants in the BOR1OX background. (A) Relative mRNA level of NIP5;1 in roots of the transgenic plants. Plants were grown on plates containing 100 μM B for 12 d. The plants were then transferred to plates containing 0.1 μM B and incubated for 1 d. Total RNAs were extracted from roots of 7–10 plants. The averages and SDs are presented for independent reverse transcription reactions followed by real-time PCR (n = 3). (B–E) Growth of the transgenic plants. Plants were grown on solid medium containing 100 or 0.1 μM boric acid for 12 d. Shoot fresh weight and root length were measured. Averages and SDs are shown (n = 8–10). Asterisks indicate significant differences from the BOR1OX plants (Student's t-test P < 0.05). (B) Primary root length under 100 μM B conditions. (C) Shoot fresh weight under 100 μM B conditions. (D) Primary root length under 0.1 μM B conditions. (E) Shoot fresh weight under 0.1 μM B conditions.
To investigate the effect of elevated NIP5;1 expression, wild-type, BOR1OX and the transgenic plants were grown on solid media containing 0.1 or 100 μM B, and shoot fresh weight and root length were measured (Fig. 3B–E). Under 100 μM B supply, growth of the transgenic lines was not improved compared with the BOR1OX plants (Fig. 3B, C). With 0.1 μM B, root lengths were 1.4–2.4 times increased in seven lines (Fig. 3D), but no line showed improved shoot growth compared with the BOR1OX plants (Fig. 3E). These results show that enhanced expression of NIP5;1 improves root elongation under low B supply in the BOR1OX background.
In the several transgenic lines carrying both Pro35S-BOR1-GFP and Pro35S-NIP5;1:NIP5;1, reduced shoot growth was observed (Fig. 3C, E), presumably due to mutation/variations induced during the transformation processes.
Pro35S-NIP5;1:NIP5;1 plants exhibited remarkable improvement of root elongation on media without B supply
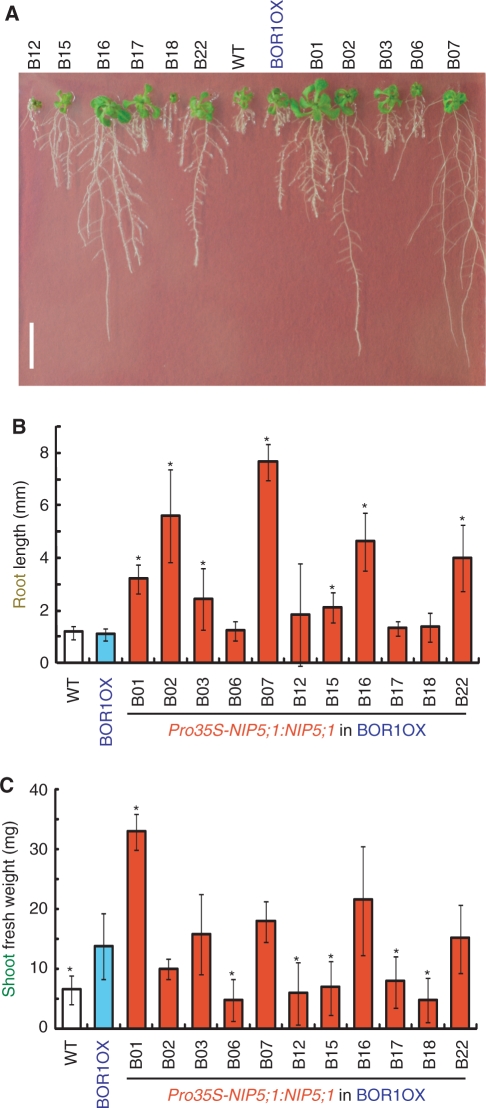
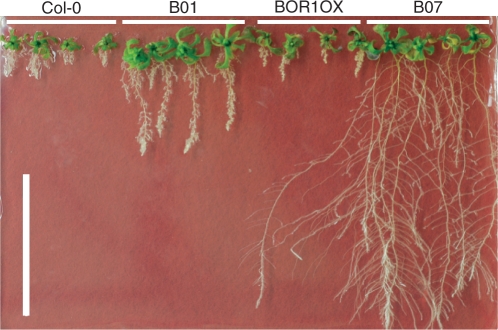
We grew the transgenic BOR1OX lines carrying Pro35S-NIP5;1:NIP5;1 on solid medium without supplemental B (Fig. 4A). Several transgenic plants exhibited remarkable improvement of root elongation under these severe B-deficient conditions. Wild-type and BOR1OX plants under these conditions exhibited a disorder of root morphology, as has been observed in the nip5;1-1 mutant line under low B conditions (Takano et al. 2006). The root tips of these plants were stunted and root hair density increased dramatically. However, the roots of B02, B07, B16 and B22 were apparently normal and similar to those of plants grown with sufficient B supply. Shoot growth was also improved in several lines, especially in B01.
Fig. 4.
Growth of Pro35S-NIP5;1:NIP5;1 transgenic plants in the BOR1OX background on solid medium without supplemental B. (A) Plants were grown on solid media without B supply for 16 d. Scale bar, 10 mm. (B) Root lengths and (C) shoot fresh weights were measured. Averages and SDs are shown (n = 7). Asterisks indicate significant differences from the BOR1OX plants (Student's t-test P < 0.05).
The shoot fresh weights and root lengths of the transgenic plants were measured (Fig. 4B, C). Primary root lengths were 2.0–7.0 times longer in seven transgenic lines compared with the original BOR1OX (Fig. 4B). Shoot fresh weight of B01 was 2.4 times more than that of the original BOR1OX lines (Fig. 4C). Statistically significant improvement of shoot growth was observed only in B01, but lines B03, B07, B16 and B22 showed a tendency to higher shoot fresh weight.
Growth improvement was further observed after an extended growth period (Fig. 5). Lines B01 and B07 continued to grow for 23 d and the difference in growth was much more evident after the extended period of growth.
Fig. 5.
Growth of Pro35S-NIP5;1:NIP5;1 transgenic plants after an extended period of time on solid medium without supplemental B. Plants of the wild type (WT, Col-0), BOR1OX and the transgenic lines B01 and B07 (four plants each) were grown on plates without supplemental B for 23 d. Scale bar, 50 mm.
Improved fertility of a BOR1OX line carrying Pro35S-NIP5;1:NIP5;1 under very low B
To reveal the effect of enhanced expression of NIP5;1 on the reproductive growth stage, the wild type, BOR1OX and the transgenic lines (B01, B07, B16 and B22) were grown hydroponically supplied with 0.3 μM B for 65 d (Fig. 6). Wild-type plants did not set seeds but BOR1OX did under these conditions. This improvement in fertility in the BOR1OX line was consistent with the report of Miwa et al. (2006). Among the lines tested, B01 developed the highest numbers of branches and set seeds more vigorously than the BOR1OX line. In other lines, fertility was similar to that of BOR1OX (data not shown).
Fig. 6.
Improved fertility of a BOR1OX line carrying Pro35S-NIP5;1:NIP5;1 under B-limiting conditions. The wild type, BOR1OX and the B01 line were grown hydroponically supplied with 0.3 μM B for 68 d. Scale bar, 100 mm.
Increased short-term B uptake and/or translocation in a BOR1OX line carrying Pro35S-NIP5;1:NIP5;1
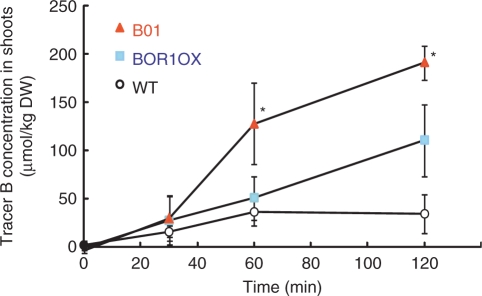
To determine the physiological reason for improved fertility of B01, we measured short-term B uptake using a stable isotope of B (Fig. 7). The plants were first grown on solid medium containing 100 μM 11B-enriched boric acid for 22 d. Next, the plants were incubated in liquid medium containing 0.1 μM 11B-enriched boric acid for 2 d and then exposed to liquid medium containing 10 μM 10B-enriched boric acid. The amount of B absorbed in shoots was determined (Fig. 7). When the time of exposure to 10B was 30 min, no significant difference in B uptake was detected among the wild type, BOR1OX and B01. BOR1OX exhibited greater B uptake than wild-type plants when incubated for 120 min. Remarkably, B01 exhibited higher B uptake than the BOR1OX plants when exposed for 60 or 120 min.
Fig. 7.
Short-term boron uptake of a BOR1OX line carrying Pro35S-NIP5;1:NIP5;1. The wild type, BOR1OX and the B01 line were first grown on solid medium containing 100 μM 11B-enriched boric acid for 22 d. The plants were subsequently incubated in liquid medium containing 0.1 μM 11B-enriched boric acid for 2 d and then exposed to liquid medium containing 10 μM 10B-enriched boric acid. The amount of B absorbed in shoots was determined. The averages and SDs are shown (n = 3–4). Asterisks indicate significantly larger values than the value for BOR1OX plants (Student's t-test P < 0.05).
Discussion
In this study, we demonstrated that enhanced expression of NIP5;1 leads to improved root elongation under B-limiting conditions. There are several reports on improvement of growth by modulating expression of MIPs (for a review, see Hachez et al. 2006). Overexpression of an A. thaliana PIP, PIP1b, resulted in an increased plant growth rate, transpiration rate, stomatal density and phytosynthetic efficiency in tobacco plants (Ahron et al. 2003). Stress-induced overexpression of OsPIP1;3/OsRWC3 leads to higher root osmotic hydraulic conductivity, leaf water potential and transpiration, but does not change morphology in rice plants (Lian et al. 2004). These studies report changes of growth properties related to water conditions, but there has been no report of enhanced growth under nutrient deficiency by modulating expression of a MIP. This study represents the first successful demonstration of improved growth under nutrient deficiency by enhancement of MIP expression. Recently some MIPs have been shown to mediate transport of various physiologically important molecules, such as CO2, H2O2, silicon or lactic acid (Choi and Roberts 2007, for a review, see Maurel 2007). Enhancement of expression of these MIPs may improve plant growth under various conditions.
In the case of NIP5;1 the situation is different from previous work in that introduction of NIP5;1 under the control of the CaMV 35S promoter did not improve plant growth under B-limiting conditions (data not shown). The CaMV 35S promoter is a constitutive promoter that induces gene expression in all cell types and strongly in vascular tissues (Benfey and Chua 1990), and its expression is not induced by B deficiency (Takano et al. 2005). NIP5;1 is expressed in epidermal, cortical and endodermal cells, but weakly in stellar cells in roots, and it is strongly induced by low B conditions (Takano et al. 2006). Incompatibility between cell type specificity and/or low B induction may be a reason why Pro35S:NIP5;1 did not improve plant growth under B deficiency. Native promoter regions may be important to maintain specific expression patterns required for NIP5;1 function in B transport. In support of this hypothesis, induction of NIP5;1 expression under B deficiency was maintained in Pro35S-NIP5;1:NIP5;1 plants. As shown in Supplementary data 1, NIP5;1 expression in Pro35S-NIP5;1:NIP5;1 plants (n05, n19, B01, B02 and B22) was 9.1- to 13.0-fold higher under B-limiting conditions than under adequate B supply. This induction was similar to the case of wild-type plants which exhibited 14.5-fold induction.
NIP5;1 is a boric acid channel required for efficient import of B into roots (Takano et al. 2006). The NIP5;1 mutants exhibited severe growth defects in both roots and shoots under limited B supply (Takano et al. 2006), suggesting that NIP5;1 is important for overall B intake into plants. In this study, we demonstrated that enhanced expression of NIP5;1 improved root growth more effectively than shoot growth under B limitation, suggesting that NIP5;1 is important for supplying B to roots to be used for their elongation.
This observation also implies that enhanced expression of NIP5;1 is not sufficient to enhance shoot growth. It is likely that xylem loading needs to be improved for shoot growth in the transgenic plants with enhanced expression of NIP5;1. BOR1 is responsible for xylem loading of B under low B conditions in the wild-type plants. Overexpression of BOR1 resulted in enhanced xylem loading of B and improved shoot growth but not root growth under low B (Miwa et al. 2006). Given the different roles of NIP5;1 and BOR1 in B transport, we expected that enhancement of NIP5;1 expression in the BOR1 overexpression background, in which xylem loading activity is enhanced, would result in shoot growth improvement. In our study we obtained such a line (line B01) with higher levels of both BOR1 and NIP5;1 expression. This line exhibited greatly enhanced shoot growth and improved fertility under low B conditions (Fig. 6). B translocation into shoots was increased more in B01 compared with BOR1OX (Fig. 7) and this is likely to cause development of the branches and increased seed yields. We fully acknowledge that results from a single transgenic line cannot be conclusive, but highly expressed NIP5;1, a boric acid channel to facilitate the passive transport of boric acid from the soil to root cells, is likely to facilitate B flow effectively through the concentration gradient generated by BOR1 across the root diameter. We propose that collective enhancement of NIP5;1 and BOR1 is an effective strategy to improve B flow from soil to xylem across roots.
In conclusion, we demonstrated successful improvement of B deficiency tolerance by enhanced expression of B transport proteins. The approach to enhance expression of transporters while keeping their cell type specificity or response to nutrient conditions might generate transgenic plants tolerant to many types of nutrient deficiency and toxicity around the world.
Materials and Methods
Plant material and plasmid constructions
Arabidopsis thaliana (L.) Heynh. Col-0, nip5;1-1 (Takano et al. 2006) and Pro35S:BOR1-GFP (BOR1OX, line 18) (Takano et al. 2005) were from our laboratory stock. Two NIP5;1 activation tag lines (GABI 297G11 and GABI 046C12) were obtained from the Max Planck Institute für Züchtungsforschung (Köln, Germany), and T3 homozygous plants were established by PCR analysis using primers specific to genome DNA and T-DNA. Information about the T-DNA insertion site was obtained from the SIGnAL database (Alonso et al. 2003). Primers used are as follows: 5′-CCCATTTGGACGTGAATCTAGACAC-3′for T-DNA; 5′-GACTTACACAAAGGGCCAACTT-3′ and5′-ATGCCTACGTTAACACTGAACAAA-3′ for GABI 046C12;and 5′-AGAGGGGGAGGTCATAGGAA-3′ and 5′-TGTGCCAAAATCTTAAACATCACT-3′ for GABI 297G11.
Pro35S-NIP5;1:NIP5;1 was constructed as follows. The genomic fragment containing the 1,357 bp region upstream of the transcription initiation site of NIP5;1 and the NIP5;1 gene was PCR amplified from bacterial artificial chromosome (BAC) clone F24G24 (obtained from the Arabidopsis Biological Resource Center at Ohio State University, Columbus, OH, USA) using primers 5′-TAAAGTCGACAAAAATCAAGCCACTAACACG-3′ and 5′-TAAAGTCGACACAACACATTACACATGCCATA-3′. The amplified fragment was A-tailed and cloned into the pGEM-T Easy vector (Promega, Madison, WI, USA). The set of the NIP5;1 promoter region and the NIP5;1 gene was subcloned into pPTbar. The pPTbar vector has the CaMV 35S promoter and rbcS terminator and is derived from pPZP212 (Hajdukiewicz et al. 1994). The resulting construct Pro35S-NIP5;1:NIP5;1 has the CaMV 35S promoter located at 1,357 bp upstream of the NIP5;1 initiation site. The nip5;1-1 mutant and BOR1OX plants were transformed via the Agrobacterium tumefaciens-mediated floral dip method (Clough and Bent 1998). Plant lines homozygous for the T-DNA were established by observation of the resistance to Basta herbicide.
Plant growth conditions
Plant growth media were prepared according to Fujiwara et al. (1992) and supplied with various concentrations of boric acid. Solid medium contained 2% sucrose and 1.5% gellan gum. To observe vegetative growth, surface-sterilized seeds were sown on the plates and incubated at 22°C under a 16 h light/8 h dark cycle as described previously (Takano et al. 2001). To observe reproductive growth, plants were grown hydroponically at 22°C under a 16 h light/8 h dark cycle as described previously (Takano et al. 2001).
Quantification of transcripts by real-time reverse transcription–PCR
Plants were grown for 12 d on solid medium containing 100 μM boric acid and transferred to plates containing 0.1 or 100 μM boric acid. They were then incubated for 1 d. Total RNA was extracted from roots with an RNeasy mini kit (QIAGEN, Hilden, Germany) following the manufacturer's procedure. A 400 ng aliquot of total RNA was subjected to reverse transcription with the Primescript RT reagent kit (TAKARA SHUZO CO. LTD., Kyoto, Japan). Real-time PCR was conducted using Thermal Cycler Dice (TAKARA SHUZO CO. LTD) with SYBR Premix Ex Taq II (TAKARA SHUZO CO. LTD). The NIP5;1 transcript levels were standardized to the levels of the EF1 a. The sequences of the primers used for NIP5;1 and EF1a were described by Takano et al. (2006).
Determination of B accumulation and tracer experiment
The digestion of samples and B isotope determination by inductively coupled plasma mass spectroscopy were as described previously (Takano et al. 2002). Determination of the amount of B absorbed during the exposure to tracer B was calculated as described by Miwa et al. (2006). In brief, the fraction of B derived from the tracer (Bdft) was determined using the following equation:
The subscripts s, p and t refer to the atomic percentages of 10B in the treated sample, the hydroponic solution used for the pre-culuture and the hydroponic solution used for the tracer experiments, respectively.
Supplementary data
Supplementary data are available at PCP online.
Funding
The Ministry of Education, Culture, Sports, Science and Technology of Japan Grant-in-Aid for Scientific Research on Priority Areas (to T.F.) and the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics for Agricultural Innovation Grant IPG-0005 to T.F.).
Supplementary Material
Acknowledgments
The T-DNA insertion lines were kindly provided by GABI-Kat at Bielefeld University, Germany. We acknowledge K. Schumacher (Universität Tübingen) for providing the pPTbar vector. We thank Ms. Y. Kawara and K. Aizawa for excellent technical assistance, and Dr. H. Hanaoka for discussion.
Glossary
Abbreviations:
- CaMV 35S promoter
cauliflower mosaic virus 35S RNA promoter
- MIP
major intrinsic protein
- NIP
nodulin 26-like intrinsic protein
- PIP
plasma membrane intrinsic protein
- RT–PCR
reverse transcription–PCR.
References
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G. Overexpression of a plasma membrane aquaporin in trans-genic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell. 2003;15:439–447. doi: 10.1105/tpc.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen HM, Shinn P, et al. Genome-wide insertional mutagenesis of. Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- Benfey P.N., Chua N.H. The cauliflower mosaic virus 35S promoter: combinatorial regulation of transcription in plants. Science. 1990;250:959–966. doi: 10.1126/science.250.4983.959. [DOI] [PubMed] [Google Scholar]
- Choi WG, Roberts DM. Arabidopsis NIP2;1, a major intrinsic protein transporter of lactic acid induced by anoxic stress. J. Biol. Chem. 2007;282:24209–24218. doi: 10.1074/jbc.M700982200. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of. Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Dell B, Huang LB. Physiological response of plants to low boron. Plant Soil. 1997;193:103–120. [Google Scholar]
- Fujiwara T, Hirai MY, Chino M, Komeda Y., Naito S. Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol. 1992;99:263–268. doi: 10.1104/pp.99.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Zelazny E., Chaumont F. Modulating the expression of aquaporin genes in planta: a key to understand their physiological functions? Biochim. Biophys. Acta. 2006;1758:1142–1156. doi: 10.1016/j.bbamem.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z., Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 1994;25:989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- Ishii T., Matsunaga T. Isolation and characterization of a boron–rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydr. Res. 1996;284:1–9. [Google Scholar]
- Kobayashi M, Matoh T, Azuma J. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 1996;110:1017–1020. doi: 10.1104/pp.110.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian HL, Yu X, Ye Q, Ding XS, Kitagawa Y, Kwak SS, et al. The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol. 2004;45:481–489. doi: 10.1093/pcp/pch058. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. 2nd. New York: Academic Press; 1995. [Google Scholar]
- Maurel C. Plant aquaporins: novel functions and regulation properties. FEBS Lett. 2007;581:2227–2236. doi: 10.1016/j.febslet.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Miwa K, Takano J., Fujiwara T. Improvement of seed yields under boron-limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in Arabidopsis thaliana. Plant J. 2006;46:1084–1091. doi: 10.1111/j.1365-313X.2006.02763.x. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Dannel F, Pfeffer H, Romheld V, Hayashi H., Fujiwara T. Defect in root–shoot translocation of boron in Arabidopsis thaliana mutant bor1-1. J. Plant Physiol. 2000;156:751–755. [Google Scholar]
- Noguchi K, Yasumori M, Imai T, Naito S, Matsunaga T, Oda H, et al. bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol. 1997;115:901–906. doi: 10.1104/pp.115.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MA, Eberhard S, Albersheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, et al. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. In vitro conditions for the formation and hydrolysis of the dimer. J. Biol. Chem. 1996;271:22923–22930. doi: 10.1074/jbc.271.37.22923. [DOI] [PubMed] [Google Scholar]
- Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B. An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol. Biol. 2003;53:247–259. doi: 10.1023/B:PLAN.0000009297.37235.4a. [DOI] [PubMed] [Google Scholar]
- Shorrocks V.M. The occurrence and correction of boron deficiency. Plant Soil. 1997;193:121–148. [Google Scholar]
- Takano J, Miwa K., Fujiwara T. Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci. 2008;13:451–457. doi: 10.1016/j.tplants.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Takano J, Miwa K, Yuan LX, von Wiren N, Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl Acad. Sci. USA. 2005;102:12276–12281. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, et al. Arabidopsis boron transporter for xylem loading. Nature. 2002;420:337–340. doi: 10.1038/nature01139. [DOI] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wiren N., Fujiwara T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell. 2006;18:1498–1509. doi: 10.1105/tpc.106.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Yamagami M, Noguchi K, Hayashi H, Fujiwara T. Preferential translocation of boron to young leaves in Arabidopsis thaliana regulated by the BOR1 gene. Soil Sci. Plant Nutr. 2001;47:345–357. [Google Scholar]
- Wallace IS, Choi W.G., Roberts DM. The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim. Biophys. Acta. 2006;1758:1165–1175. doi: 10.1016/j.bbamem.2006.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.