Abstract
AMPA receptors (AMPA-R) are major mediators of synaptic transmission and plasticity in the developing and adult central nervous system. Activity-dependent structural plasticity mediated by dynamic changes in the morphology of spines and dendrites is also essential for the formation and tuning of neuronal circuits. RhoA and Rac1 are known to play important roles in the regulation of spine and dendrite development in response to neuronal activity. These Rho GTPases are activated by guanine nucleotide exchange factors (GEFs). In this study, we identified GEF-H1/Lfc as a component of the AMPA-R complex in the brain. GEF-H1 is enriched in the postsynaptic density and is colocalized with GluR1 at spines. GEF-H1 activity negatively regulates spine density and length through a RhoA signaling cascade. In addition, AMPA-R-dependent changes in spine development are eliminated by down-regulation of GEF-H1. Altogether, these results strongly suggest that GEF-H1 is an important mediator of AMPA-R activity-dependent structural plasticity in neurons.
Keywords: glutamate receptor, GTPase, learning and memory, structural plasticity, synaptic plasticity
Dendritic spines are small actin-rich protrusions from neuronal dendrites with a globular head and thin neck. As a basic functional unit of the excitatory synapse, dendritic spines are critical for most excitatory synaptic transmission in the brain. Because dynamic changes in the shape, size, and number of spines are major forms of structural synaptic plasticity, the molecular mechanism underlying the regulation of spine development, maintenance, and dynamics has been an active research area in neuroscience. Spines are rich in F-actin and actin dynamics modulate the morphological plasticity of spines. In various cell types including neurons, actin dynamics is regulated by Rho GTPases in response to external cues (1).
The Rho family of small GTPase consists of a large number of proteins including Rho, Rac, and Cdc42. These proteins are binary switches that cycle between GDP-bound inactive and a GTP-bound active state. In response to various extracellular signals, this switch is turned on or off by regulatory proteins. In neurons, Rho GTPases have been implicated in the cytoskeletal dynamics for structural plasticity of excitatory synapses. Particularly, RhoA and Rac1 have been known as key players for the regulation of spine and dendrite development and dynamics (2).
The regulatory proteins of the Rho GTPases include guanine nucleotide exchange factors (GEFs), GTPase activating proteins, and guanine nucleotide dissociation inhibitors. GEFs catalyze the exchange of GDP for GTP to generate the active state of Rho GTPases. In contrast, GTPase activating proteins and guanine nucleotide dissociation inhibitors inactivate Rho GTPases. The expression of GEF proteins is tissue-specific, providing a molecular mechanism for tissue-specific modulation of Rho GTPases (3). Tiam1 is known as a neuronal GEF involved in NMDA receptor activity-dependent structural plasticity (4). However, neuronal GEFs involved in AMPA receptor (AMPA-R) activity-dependent structural plasticity have not been reported.
Modulation of the trafficking and activity of AMPA-R by NMDA receptor activity is a major mechanism for synaptic plasticity (5). Recent studies have identified several AMPA-R interacting proteins that can modulate the activity and trafficking of AMPA-Rs (5). In this study, we immunopurified the AMPA-R from rat brain and found that the RhoA GEF, GEF-H1, is a significant component of the AMPA-R complex in vivo. Truncated GEF-H1/Lfc was first identified as an oncogene (p40/Lfc) involved in cell proliferation (6–8). The full-length GEF-H1 was found as a 120-kDa protein consisting of 985 aa (9). Interestingly, GEF-H1 binding to microtubule regulates its enzymatic activity, suggesting GEF-H1 could mediate cross-talk between microtubules and actin (9, 10). Our functional analysis of GEF-H1 demonstrated that GEF-H1 negatively regulates the density and length of spines through a RhoA signaling cascade. In addition, AMPA-R activity-dependent changes in spine development were eliminated by inhibition of GEF-H1. Together, this study strongly suggests that GEF-H1 is an AMPA-R associated protein that is important for activity-dependent structural plasticity.
Results
Identification of GEF-H1 as a Component of the AMPA-R Complex.
Through proteomic screening of AMPA-R-binding proteins in rat brains, GEF-H1, a GEF, was identified as a component of the AMPA-R complex in the brain [Fig. 1 and supporting information (SI) Fig. S1]. AMPA-R complexes were purified from rat brains using wheat germ agglutinin (WGA)-chromatography and large-scale immunoprecipitation (IP). WGA-chromatography was adapted to enrich for glycosylated mature membrane proteins and to exclude immature AMPA-R complexes in the endoplasmic reticulum and Golgi apparatus. To distinguish specific binding from nonspecific binding of proteins to IgG, Protein A, and Sepharose during the large-scale IP, the eluant from WGA-chromatography was divided into 2 pools and 1 pool was used as a negative control. In the negative control group, the GluR1 antibody was blocked with the synthetic peptide used for the generation of the antibody (see Fig. 1A, Pep +). The isolation of many of the copurifying proteins was inhibited by preabsorbing the GluR1 antibody with the antigenic peptide, indicting their specific association with AMPA-Rs. As shown in Fig. 1A, this procedure specifically isolated a major protein band with a molecular weight of 105 kDa, which contains the AMPA-R subunits GluR1–4, and several other protein bands detected by silver-staining. One of the proteins (see Fig. 1A, arrow) around the 120-kDa region was excised from the protein gel and applied to liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis as described in Methods and the SI Methods. Protein database searches identified 3 peptides as a part of Rho/Rac GEF 2 (see Fig. S1A). Fig. S1B shows MS/MS spectra of one of the peptides. Further sequence analysis indicated that the Rho/Rac GEF 2 is a rat homolog of human GEF-H1 and mouse Lfc identified in previous studies (6–9).
Fig. 1.
Proteomic and Western blot analysis of GEF-H1 association with AMPA-R complex. (A) Silver staining of cortical AMPA-R complex resolved by SDS/PAGE. LC-MS/MS analysis of a band (arrow) around the 120-kDa region revealed GEF-H1 (GEF), an interactor of AMPA-R. Western blot analyses with anti-GEF-H1 antibody confirm the association of GEF-H1 with AMPA-Rs (B–D). The immunoprecipitation (IP) was performed with (+) or without (–) peptide block (Pep or Peptide) with the peptide used to develop the anti-GluR1 antibody (A and C). Western blot analyses were performed with protein samples from each step of the AMPA-R complex purification (B), from cortical neuronal culture (C), or from HEK293T cells (D). Sol, solubilized brain lysate; Stg, stargazin; WGA, wheat-germ agglutinin. (D) GEF-H1 tagged with GFP (GEF) was coexpressed with myc-tagged GluR1, GluR2, or C-terminal truncated form of GluR1 (R1CtΔ). Pull-down of the GEF-H1 with anti-GFP antibodies specifically co-immunoprecipitated these GluRs. Molecular-mass of standards are indicated in kDa on the left (A–D).
The specificity of GEF-H1 association with AMPA-R complex was confirmed through Western blot analyses with protein samples from rat brains, cultured cortical neurons, and heterologous cells (see Fig. 1 B, C and D). First, protein samples from each step of the purification of AMPA-Rs were probed with anti-GEF-H1 antibodies using Western blot analysis (see Fig. 1B). GEF-H1 was detected in solubilized brain lysates at around 120 kDa, similar in size to its human and mouse homologous. Interestingly, GEF-H1 was detected in the eluant of WGA-chromatography as much as in the void, suggesting that significant amounts of GEF-H1 bind to mature membrane proteins. The enrichment and specific detection of the GEF-H1 protein in the eluant of the large-scale AMPA-R IP confirmed the result of LC-MS/MS analysis (see Fig. 1B). In small-scale IPs of endogenous AMPA-R complexes using cortical neuronal cultures, GEF-H1 as well as stargazin (a known interactor of AMPA-Rs) was specifically coimmunoprecipitated with GluR1 as a part of AMPA-R complex in neurons. Neither GEF-H1 nor stargazin was detected in this co-IP when GluR1 antibody was blocked by the synthetic peptide (see Fig. 1C, Peptide +). For further analysis of GEF-H1 association with AMPA-R complex, GFP-tagged GEF-H1 (GFP-GEF-H1) was coexpressed with myc-tagged AMPA-R subunits in heterologous cells, and the GFP-GEF-H1 was immunoprecipitated using an anti-GFP antibody (see Fig. 1D). Both GluR1 and GluR2 were specifically coimmunoprecipitated with GFP-GEF-H1. However, the efficiency of this co-IP with heterologous cell lysate was much lower than that with brain or neuronal lysates, suggesting that the interaction may be stabilized by other proteins or by posttranslational modifications in neurons. Surprisingly, a GluR1 mutant lacking the cytoplasmic C-terminal (R1CtΔ) was also coimmunoprecipitated with GFP-GEF-H1, indicating that the C-terminal of AMPA-R is not required for GEF-H1 binding (see Fig. 1D). On the other hand, GluR6 (a subunit of kainate receptors) was not coimmunoprecipitated with GFP-GEF-H1, indicating that the interaction between AMPA-Rs and GEF-H1 is specific (Fig. S2).
GEF-H1 Negatively Regulates the Development of Dendritic Spines.
Our biochemical and immunocytochemical analyses of GEF-H1 showed localization of GEF-H1 in postsynaptic density (PSD) and spines (Figs. S3A and S4C). In addition, GEF-H1 was highly expressed in the brain during the period of active spine development (Fig. S3B). Given that a GEF-H1 could regulate actin dynamics through the activation of Rho GTPases, we hypothesized that GEF-H1 could modulate spine development. Because previous studies showed that dendritic spine development starts after 14 days in vitro (DIV) in cultured dissociated neurons (11), our experiments were designed to manipulate the GEF-H1 activity during 14 to 16 DIV. We first examined the effects of GEF-H1 loss of function on spine development using a dominant-negative form of GEF-H1 and shRNA knock-down of GEF-H1 expression. Both of these manipulations demonstrated that GEF-H1 regulates spine density and length (Figs. 2 and 3). For quantitative analysis in this study, a spine was defined as a small protrusion from dendrite with GluR1 staining. Therefore, neurons were costained with antibodies recognizing transfected GFP-GEF-H1 and endogenous GluR1. In addition, either GFP or mCherry was used as morphological marker. Through this triple immunofluorescence staining of cultured hippocampal neurons, the number and length of spines only from GFP or GFP-GEF-H1 transfected neurons could be measured.
Fig. 2.
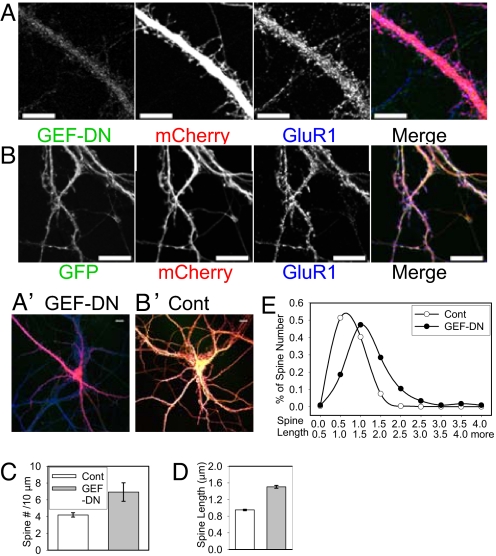
Dominant-negative form of GEF-H1 increases the density and length of dendritic spines. (A) Hippocampal neurons (13 DIV) were transfected with mCherry and a dominant-negative form of GEF-H1 tagged with GFP (GEF-DN), and stained then with antibodies against GFP and GluR1 at 16 DIV. (B) As negative control, the same batch of neurons were transfected with mCherry and GFP and stained then same way as above. (A′ and B′) Images of the whole neurons presented in (A) and (B), respectively. (Scale bars, 10 μm.) (C) The effect of GEF-DN on spine density was analyzed quantitatively by plotting number (#) of spines per 10 μm of dendrite. Compared with GFP-transfected neurons (Cont), the neurons transfected with GEF-DN had significantly more number of spines. (D) Compared with Control, GEF-DN caused significant increase in spine length. The label of bars is same as in (C). See Table S1 for values and statistical analysis for (C) and (D). (E) The effect of GEF-DN on spine length was also analyzed quantitatively by plotting frequency (%) distribution of spine length.
Fig. 3.
shRNA mediated down-regulation of endogenous GEF-H1 increases density and length of dendritic spines and changes the activity of RhoA and Rac1. (A) Hippocampal neurons (9 DIV) were infected with lentivirus carrying shRNA of GEF-H1 and GFP, and stained then with antibodies against GFP, GluR1, and MAP2 at 16 DIV. (B) As negative control, the same batch of neurons were infected with lentivirus carrying GFP only, and stained then same way as above. (A′ and B′) Image of the whole neurons presented in (A) and (B), respectively. (Scale bars, 10 μm.) (C–E) The density and length of spines were analyzed quantitatively as explained in Fig. 2. Compared with neurons infected with GFP only (Cont), neurons infected with GFP and shRNA of GEF-H1 (RNAi) showed significant increase in spine density and length. See Table S1 for values and statistical analysis. (F) Cortical neurons (DIV 9) were infected with lentivirus carrying shRNA of GEF-H1 and GFP (Ri) or GFP only (Vec). At DIV 16, neurons were harvested and solubilized proteins were then applied to Western analyses with antibodies against GEF-H1, GluR1, and neurofilament. Infection of the shRNA significantly down-regulated the expression of endogenous GEF-H1. (G) Cortical neurons were infected and harvested as above and solubilized proteins were applied to RhoA or Rac1 assay. Compared with the infection of lentivirus carrying GFP only (Vec), the infection of lentivirus carrying GFP and GEF-shRNA (Ri) significantly increased and decreased active RhoA and Rac1, respectively. See Table S2 for values and statistical analysis.
A dominant-negative form of GEF-H1 was generated by introducing a point mutation (T247A) on the catalytic domain (Rho GEF domain) of GEF-H1 (see SI Methods for detail). A group of 13 DIV hippocampal neurons was cotransfected with the GFP-tagged dominant-negative GEF-H1 (GEF-DN) and mCherry, fixed at 16 DIV, and stained with antibodies against GFP and GluR1. The neurons transfected with GEF-DN (see Fig. 2 A and A′) have more spines and longer spines compared with the neurons transfected with GFP (see Fig. 2 B and B′). The change on spine density was analyzed quantitatively by counting the number of spines per 10 μm of dendrite. Over-expression of GEF-DN significantly increased spine density (see Fig. 2C and Table S1). The change in spine length was analyzed quantitatively by plotting average and frequency (%) distribution of spine length. Over-expression of GEF-DN significantly increased average spine length (see Fig. 2D and Table S1). The length increase was the result of an increase in percentage of longer spines (see Fig. 2E). On the other hand, over-expression of GEF-H1 decreased the length of dendritic spines (Fig. 4 A and E, and see Table S1). The length decrease is because of an increase in the percentage of shorter spines (Fig. 4F) without increase in spine number (see Fig. 4D and Table S1).
Fig. 4.
The negative effect of GEF-H1 over-expression on spine length is eliminated by an inhibitor of RhoA or ROCK. (A) Hippocampal neurons (13 DIV) were transfected with mCherry and GFP (Cont) or mCherry and GEF-H1 tagged with GFP (GEF), and then stained with antibodies against GFP and GluR1 at 16 DIV. (B) Hippocampal neurons were transfected the same way as above, treated with a RhoA inhibitor C3 transferase (C3T, 1.0 μg/ml) overnight (14–16 h) at 15 DIV, and stained as above. (C) Hippocampal neurons were transfected the same way as above, treated with a ROCK inhibitor (Y27632, 100 μM) for 3 days, and stained as above. Only GFP or GFP-GEF and mCherry images are shown here for comparison (A–C). (Scale bars, 10 μm.) (D–F) The effect of drug or GEF-H1 on the density and length of spines were analyzed quantitatively as explained in Fig. 2. Compared with untreated neurons (Cont), neurons treated with C3T (C3T) or Y27632 (Y27) showed significant increases in spine density. Spine density did not changed by GEF-H1 over-expression (GEF +). (E) Compared with untransfected neurons (first bar from the left), neurons over-expressing GEF-H1 (second bar) showed significant decrease in spine length. The change of spine length by GEF-H1 over-expression is eliminated by treatment of C3T (fourth bar) or Y27632 (sixth bar). The drug treatments only (third or fifth bar) significantly increased spine length. The label of bars is the same as in (D). (F) The change of spine length by GEF-H1 over-expression was eliminated by the drug treatments. See Table S1 for values and statistical analysis (D and E). (G) Cortical neurons were treated with C3T or Y27632 as above. At 16 DIV, neurons were harvested and solubilized proteins were applied to RhoA or Rac1 assay. C3T treatment significantly decreased and increased the activity of RhoA and Rac1, respectively. See Table S2 for values and statistical analysis.
To study this in more detail, a lentivirus that expresses GEF-H1 shRNA and GFP under the control of 2 promoters was generated, and examined as described in the SI Methods and Fig. S5. First, the knock-down efficiency of the shRNA of GEF-H1 in neurons was quantitatively analyzed using cortical neuronal culture. Four days after viral infection, GFP signals begin to appear and reached a plateau 7 days after the infection. Therefore, 9 DIV hippocampal neurons were infected to knock-down endogenous GEF-H1 expression during initial period for spine development (13 to 16 DIV). The infection of the lentivirus carrying GEF-H1 shRNA knocked down most of endogenous GEF-H1 expression in cortical neurons (86 ± 1.0%, n = 4) (see Fig. 3F). The inhibition of GEF-H1 expression also significantly down-regulated GluR1 expression (21 ± 3.0%, n = 4) (see Fig. 3F).
For immunocytochemical analysis, hippocampal neurons were infected with the lentivirus at 9 DIV, fixed at 16 DIV, and stained with antibodies against GFP, GluR1, and MAP2 that were used as markers for morphology, the postsynaptic membrane, and dendrite, respectively. Compared with the neurons infected with lentivirus carrying GFP only (see Fig. 3 B and B′), the neurons infected with lentivirus carrying shRNA of GEF-H1 and GFP (see Fig. 3 A and A′) showed dramatic changes in spine development similar to the neurons transfected with GEF-DN. Inhibition of GEF-H1 expression by shRNA significantly increased the spine density (see Fig. 3C and Table S1) and spine length (see Fig. 3D and Table S1). The length increase was because of an increase in percentage of longer spines (see Fig. 3 C and E), which is consistent with the result of similar analysis with GEF-DN (see Fig. 2). Together, the effects of dominant-negative GEF-H1 and GEF-H1 shRNA and over-expressed GEF-H1 on spine development clearly showed that GEF-H1 negatively regulates spine development.
Early studies of GEF-H1 suggested that GEF-H1 could activate both RhoA and Rac1 (7, 12, 13). However, recent studies of GEF-H1 homologs consistently demonstrated that GEF-H1 is a RhoA-specific GEF in non-neuronal cells (9, 14–16). To test if GEF-H1 could activate either RhoA or Rac1 in neuronal cells, the activity of RhoA and Rac1 was measured by pulling down activated RhoA or Rac1 after infection of the lentivirus carrying shRNA of GEF-H1. In agreement with the recent studies from non-neuronal cells, RhoA activity was significantly decreased by knock-down of endogenous GEF-H1 (see Fig. 3G and Table S2), indicating that GEF-H1 is a GEF for RhoA in neurons. On the other hand, Rac1 activity was increased by knock-down of GEF-H1 (see Fig. 3G and Table S2). Given that RhoA could inhibit Rac1 activity in neurons (17), the decrease of RhoA activity by the shRNA of GEF-H1 could result in the increase of Rac1 activity. This result demonstrated that GEF-H1 could negatively regulate Rac1 activity by activating RhoA in neurons. Previous studies demonstrated that RhoA could negatively regulate spine density and length (18) and that Rac1 could positively regulate spine density (11, 19, 20). Therefore, it is likely that the up-regulation of spine density and length by GEF-H1 shRNA is a result of down-regulation of RhoA activity accompanying up-regulation of Rac1 activity.
GEF-H1 Regulates the Development of Dendritic Spines Through RhoA Signaling Cascade.
As mentioned above, data in Fig. 3 imply that RhoA signaling pathway is involved in the regulation of spine development by GEF-H1. To test this hypothesis, we attempted to interfere with the negative regulation of spine development by over-expressed GEF-H1 through pharmacological inhibition of the RhoA signaling pathway. As expected, the inhibition of the RhoA signaling pathway eliminated the effect of GEF-H1 over-expression on spine development (see Fig. 4).
Treatment of neurons with a RhoA inhibitor, C3T, significantly increased the spine density and length of cultured neurons (see Fig. 4 B, D, and E, and Table S1). This result is consistent with previous study using brain-slice cultures (18). Moreover, treatment of neurons with Y27632, an inhibitor of the kinase ROCK, a downstream effecter of RhoA, also significantly increased the spine density and length of cultured neurons (see Fig. 4 C–E, and Table S1). The Y27632 effect on spine length is consistent with a previous study using brain-slice culture, although the effect on spine density was not observed in this previous study (21). In our experiments, the effect of Y27632 on spine length was significantly stronger than that of C3T (see Fig. 4 E and F, and Table S1), which may be because of the relatively longer treatment of neurons with Y27632 (3 days) than that of C3T (14–16 h).
Over-expression of GEF-H1 showed a dosage effect on spine development as well as on dendritic arbor development. Neurons with large amount of GEF-H1 expression had poor spine development (see Fig. 4A). However, a neuron with low or mild expression of GEF-H1 did not show any significant changes in the development of spines (see Fig. S4C). As mentioned above, a neuron over-expressing GEF-H1 always showed strong colocalization of GEF-H1 with microtubules (Fig. S4 B and E). Therefore, for our quantification of spine development shown in Fig. 4, only the neurons showing microtubule-like structure of GEF-H1 were selected as neurons over-expressing GEF-H1.
The negative effect of GEF-H1 over-expression on spine length was completely eliminated by the inhibitor of RhoA or ROCK (see Fig. 4 B, C, E, and F, and Table S1). The combination of GEF-H1 over-expression and inhibition of RhoA-ROCK signaling pathway clearly demonstrated that GEF-H1 could modulate the spine development through the RhoA-ROCK signaling pathway (see Fig. 4 and Fig. S6).
The effect of the drugs on the RhoA signaling pathway was confirmed through RhoA and Rac1 assays with cortical neurons (see Fig. 4G and Table S2). Most RhoA activity was inhibited by C3T. However, Y27632 did not decrease RhoA activity, confirming that ROCK is downstream of RhoA. RhoA activity was slightly increased by Y27632, possibly because of negative feedback. Furthermore, Rac1 activity was significantly increased by the RhoA inhibitor but not by the ROCK inhibitor. This result is also consistent with the increase of Rac1 activity by shRNA of GEF-H1 (see Fig. 3G and Table S2), confirming that Rac1 activity is inhibited by RhoA activity in neurons. All together, these results demonstrated that GEF-H1 could regulate spine development through the RhoA signaling cascade including ROCK and Rac1.
GEF-H1 Mediates the AMPA-R Activity-Dependent Regulation of Spine Development.
It has been previously reported that AMPA-R activity regulates the development of dendrites through Rho GTPases (2, 22). Previous studies also demonstrated that AMPA-R activity is involved in the stabilization of spines (2, 22). Blocking AMPA-R activity with NBQX significantly reduced spine density (23) and spine motility was inhibited by application of AMPA-R agonists (24). However, the molecular mechanism underlying this regulation is not clear. Our data demonstrating the association of GEF-H1 with the AMPA-R complex and the regulation of spine development by GEF-H1, suggest that GEF-H1 could play a role in the AMPA-R-dependent regulation of spine development. To test this hypothesis we first examined AMPA-R-dependent regulation of spine development after shRNA knock-down of GEF-H1 expression.
As shown previously with organotypic hippocampal slice cultures (23), treatment of cultured neurons with the AMPA-R antagonist NBQX for 7 days significantly decreased spine density because of elimination of immature spines (Fig. 5 B and E, and see Table S1). Most of the remaining spines after NBQX treatment are mushroom spines that have a medium length and round head (see Fig. 5 B and E and Table S1). This negative effect of NBQX on spine density was completely eliminated by the down-regulation of GEF-H1 expression (see Fig. 5 C and E and Table S1). The combination of NBQX treatment and down-regulation of GEF-H1 demonstrated that GEF-H1 is critical for the AMPA-R regulation of spine development.
Fig. 5.
The negative effect of NBQX on spine density is eliminated by shRNA of GEF-H1. (A) A group of hippocampal neurons (9 DIV) was infected with lentivirus carrying GFP only, then stained with antibodies against GFP, GluR1, and MAP2 at 16 DIV. (B) The same batch of neurons were infected same way as above and treated with an AMPA-R blocker (NBQX, 20 μM) for 7 days. (C) The same batch of neurons were infected with lentivirus carrying shRNA of GEF-H1 and GFP and treated with NBQX (20 μM). (D) The same batch of neurons was infected with lentivirus carrying shRNA of GEF-H1 and GFP. (B–D) Staining was done same way as in (A). (Scale bars, 10 μm.) (E) The effect of drug and shRNA of GEF-H1 on spine density was analyzed quantitatively by plotting number (#) of spines per 10 μm of dendrite. Compared with untreated neurons (Cont,), NBQX treatment (NBQX) significantly decreased spine density. The change of spine density by NBQX was eliminated by infection of shRNA of GEF-H1 (NB+Ri). (F) Cortical neurons were infected with the lentivirus (RNAi or Ri) and treated with NBQX (NBQX or NB) as above. At 16 DIV, neurons were harvested and solubilized proteins were applied to the RhoA or Rac1 assay. NBQX treatment significantly increased and decreased the activity of RhoA and Rac1, respectively. However, the NBQX effect was eliminated by shRNA of GEF-H1 (NB+Ri). See Table S2 for values and statistical analysis.
To further examine the effect of NBQX on spine development, the activity of RhoA and Rac1 were measured with or without NBQX treatment using cortical neuronal cultures (Fig. 5F and see Table S2). NBQX treatment significantly increased RhoA activity and decreased Rac1 activity. This activity change of Rho GTPases by NBQX was completely eliminated by the down-regulation of GEF-H1 expression (see Fig. 5F and Table S2). Therefore, it is likely that the decrease of spine density by NBQX is a result of an increase of RhoA activity by activation of GEF-H1. All together, these results strongly suggest that inhibition of AMPA-R activity results in the activation of GEF-H1 and the modulation of spine development.
Discussion
Recent studies have implicated AMPA-Rs in the regulation of activity-dependent structural plasticity (2). However, the molecular details of the intracellular signaling pathways for AMPA-R-dependent structural plasticity are largely unknown. In this study, we have shown that GEF-H1 is associated with the AMPA-R complex and that GEF-H1 is a link from AMPA-R activity changes to the regulation of structural plasticity in neurons. Many numbers of neurological disorders, such as mental retardation, are associated with defects in dendritic spine morphology and number of dendritic spines (25). Therefore, our study provides additional insights into the AMPA-R function and synaptic plasticity in general that may be relevant to brain development, learning and memory, and neurological disorders.
A previous study of GEF-H1 in neurons showed that GFP-GEF-H1 was localized in the dendritic shaft and translocated to spines only after KCl-dependent depolarization or electrical stimulation (26). As mentioned above, our results showed that over-expressed GFP-GEF-H1 was not observed in spines but in dendritic shafts. However, we observed GEF-H1 puncta in spines from neurons expressing low levels of GFP-GEF-H1 that could be similar to the expression level of endogenous GEF-H1. Our Western blot analysis showed that endogenous GEF-H1 is highly enriched in PSD III fraction from the brains, strongly suggesting that endogenous GEF-H1 is in spines where most excitatory synapses are located. Our neurons were transfected with GFP-GEF-H1 at DIV 13 and examined at DIV 16. In the previous study (26), neurons were transfected with GFP-GEF-H1 at DIV 7 and 8 and examined at DIV 16 to 21. This difference in expression time of exogenous GFP-GEF-H1 might be responsible for the different subcellular location of GEF-H1. In addition, the previous study (26) used rat GEF-H1 cDNA (encode 958 aa) that is shorter than our rat GEF-H1 cDNA (encode 985 aa). Compared to their shorter cDNA, our full-length cDNA encode 27 aa more in the N-terminal region. Previous studies showed that full-length human GEF-H1 and mouse Lfc have the same size as our GEF-H1 cDNA (9, 15).
Data in this study consistently demonstrated that GEF-H1 negatively regulates the development of spines through a RhoA signaling cascade. Our data also strongly indicate that AMPA-R activity negatively regulates GEF-H1 activity, resulting in inhibition of the synaptic RhoA signaling cascades. These are consistent with the general conclusion derived from the studies of activity-dependent dendrite arbor growth in vivo using a Xenopus tadpole's visual system. Glutamate receptor activation by visual stimulation negatively regulated the RhoA signaling pathway, resulting in dendritic arbor growth (2). Furthermore, it has been reported that AMPA-Rs regulate experience-dependent dendritic arbor growth in vivo (27). Similarly, for spine development, AMPA-R activation by local presynaptic inputs could negatively regulate the RhoA-signaling pathway, resulting in an increase in spine density and strengthening local connections with the presynaptic inputs. Through similar mechanisms, the RhoA signaling pathway could be activated when spine development needs to be limited because of a low level of synaptic inputs.
GEF-H1 is the first GEF identified as a mediator of AMPA-R activity-dependent regulation of spine development. AMPA-Rs and Rho GTPases have been known as key players for functional and structural plasticity, respectively. In that regard, our finding of GEF-H1 as a linker between these two could give us important insight into the molecular mechanisms for synaptic development and plasticity. Because structural and functional plasticity seems to be coordinated, there may be common regulators of functional and structural plasticity that coordinate these 2 aspects of synaptic plasticity. GEF-H1 may be one of the common regulators because AMPA-R trafficking is regulated by actin dynamics that is regulated by GEF-H1 activity. It has been suggested that synaptic Ca2+ influx through NMDA receptors during long-term potentiation induction triggers Rho GTPase-meditated actin polymerization, resulting in AMPA-R trafficking to synapses (28). Regulation of the RhoA signaling pathway by GEF-H1 could also be involved in this NMDA receptor-mediated AMPA-R trafficking in and out of synapses.
Methods
Detailed experimental methods are described in SI Methods. The use and care of animals in this study follows the guideline of the Institutional Animal Care and Use Committee at the Johns Hopkins University.
Biochemical Analyses of AMPA-R Complex from Rat Brains and Cells.
Preparations of rat brain lysate, fractionation, and solubilization are described in the SI Methods in detail. For the purification of AMPA-R complex from rat brain, matured membrane proteins were then enriched as described in a previous study (29). AMPA-R complexes were then purified through large-scale IP followed by Mass Spectrometry analysis as described in the SI Methods and a previous study (30) in detail. Purification of synaptosome and postsynaptic density were performed based on a method described previously (31). Western blot analyses were performed as described previously (32).
cDNA Subcloning, Mutagenesis, and Preparation of Lentiviral shRNA.
The EST clone of Rho/Rac GEF2 was obtained from Integrated Molecular Analysis of Genomes and their Expression (IMAGE) Consortium and subcloned into mammalian-expression vectors. Based on a previous study (33), the dominant-negative form of GEF-H1 (GEF-DN) was designed and generated. The short hairpin RNA (shRNA) of GEF-H1 was designed based on the sequences of short interference RNA (siRNA) used in previous studies of GEF-H1 homologous (33, 34), and the shRNA was subcloned into lentiviral vector, FUGW (35). See the SI Methods for details.
Neuronal Cell Culture, Immunocytochemistry, Microscopy Image Analysis, and Statistics.
Cortical and hippocampal neuron cultures were prepared, maintained, and analyzed as previously described (36). See SI Methods for details.
Supplementary Material
Acknowledgments.
We thank Min Dai and Da-ting Lin for technical assistance. This work was supported by the National Institutes of Health Grants R01NS036715 (to R.L.H.) and N01-HV-28180 (to Y.G.). R.L.H. is an investigator of the Howard Hughes Medical Institute. M.-G.K. was supported by an Epilepsy Foundation Postdoctoral Fellowship.
Footnotes
Conflict of interest statement: Under a licensing agreement between Millipore and the Johns Hopkins University, R.L.H. is entitled to a share of royalty received by the university on sales of products described in this article. R.L.H. is a paid consultant to Millipore. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812861106/DCSupplemental.
References
- 1.Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- 2.Van Aelst L, Cline HT. Rho GTPases and activity-dependent dendrite development. Curr Opin Neurobiol. 2004;14:297–304. doi: 10.1016/j.conb.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 4.Tolias KF, et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2006;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 6.Reddy AB, Chatterjee A, Rothblum LI, Black A, Busch H. Isolation and characterization of complementary DNA to proliferating cell nucleolar antigen P40. Cancer Res. 1989;49:1763–1767. [PubMed] [Google Scholar]
- 7.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273:34954–34960. doi: 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead I, Kirk H, Tognon C, Trigo-Gonzalez G, Kay R. Expression cloning of Lfc, a novel oncogene with structural similarities to guanine nucleotide exchange factors and to the regulatory region of protein kinase C. J Biol Chem. 1995;270:18388–18395. doi: 10.1074/jbc.270.31.18388. [DOI] [PubMed] [Google Scholar]
- 9.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 10.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci. 2005;118:1861–1872. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- 11.Wiens KM, Lin H, Liao D. Rac1 induces the clustering of AMPA receptors during spinogenesis. J Neurosci. 2005;25:10627–10636. doi: 10.1523/JNEUROSCI.1947-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaven JA, Whitehead I, Bagrodia S, Kay R, Cerione RA. The Dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J Biol Chem. 1999;274:2279–2285. doi: 10.1074/jbc.274.4.2279. [DOI] [PubMed] [Google Scholar]
- 13.Glaven JA, Whitehead IP, Nomanbhoy T, Kay R, Cerione RA. Lfc and Lsc oncoproteins represent two new guanine nucleotide exchange factors for the Rho GTP-binding protein. J Biol Chem. 1996;271:27374–27381. doi: 10.1074/jbc.271.44.27374. [DOI] [PubMed] [Google Scholar]
- 14.Aijaz S, D'Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–786. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Benais-Pont G, et al. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J Cell Biol. 2003;160:729–740. doi: 10.1083/jcb.200211047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuzawa T, Kuwae A, Yoshida S, Sasakawa C, Abe A. Enteropathogenic Escherichia coli activates the RhoA signaling pathway via the stimulation of GEF-H1. EMBO J. 2004;23:3570–3582. doi: 10.1038/sj.emboj.7600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 18.Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the Rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penzes P, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 21.Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci. 2004;26:429–440. doi: 10.1016/j.mcn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 23.McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2(1):44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- 24.Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci. 2000;3:887–894. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- 25.Halpain S, Spencer K, Graber S. Dynamics and pathology of dendritic spines. Prog Brain Res. 2005;147:29–37. doi: 10.1016/S0079-6123(04)47003-4. [DOI] [PubMed] [Google Scholar]
- 26.Ryan XP, et al. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron. 2005;47(1):85–100. doi: 10.1016/j.neuron.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci USA. 2006;103:12127–12131. doi: 10.1073/pnas.0602670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matus A. Growth of dendritic spines: a continuing story. Curr Opin Neurobiol. 2005;15(1):67–72. doi: 10.1016/j.conb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Kang MG, et al. Biochemical and biophysical evidence for gamma 2 subunit association with neuronal voltage-activated Ca2+ channels. J Biol Chem. 2001;276:32917–32924. doi: 10.1074/jbc.M100787200. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y, Ma SF, Grigoryev D, Van Eyk J, Garcia JG. 1-DE MS and 2-D LC-MS analysis of the mouse bronchoalveolar lavage proteome. Proteomics. 2005;5:4608–4624. doi: 10.1002/pmic.200500052. [DOI] [PubMed] [Google Scholar]
- 31.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 32.Boehm J, et al. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Birukova AA, et al. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006;290:L540–L548. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- 34.Bakal CJ, et al. The Rho GTP exchange factor Lfc promotes spindle assembly in early mitosis. Proc Natl Acad Sci USA. 2005;102:9529–9534. doi: 10.1073/pnas.0504190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 36.Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.