Abstract
Phosphoinositide turnover and calcium mobilization are fundamental determinants of acute and chronic opioid effects. Phosphoinositide-specific phospholipase C (PLC) are key signaling enzymes that play a pivotal role in mediating opioid modulation of inositol trisphosphate production and cytosolic calcium distribution, substrates for many acute and chronic opioid effects. Notably, phosphorylation of the β isoforms of PLC, by kinases that are up-regulated after chronic morphine, is a potent modality for their regulation. Direct assessment of PLCβ1 and PLCβ3 phosphorylation in the guinea pig longitudinal muscle myenteric plexus tissue revealed substantial alterations after the induction of opioid tolerance. Notably, the direction of this modulation is isoform-specific. Phosphorylation of PLCβ1 is significantly reduced, whereas that of PLCβ3 is substantially augmented, changes not accompanied by altered content of PLCβ1 or PLCβ3 protein. In contrast to chronic morphine, acute morphine treatment of opioid naïve longitudinal muscle myenteric plexus tissue attenuates PLCβ3 phosphorylation, an effect also manifested by endogenous opioids that is reflected by the ability of acute naloxone to substantially augment PLCβ3 phosphorylation. This indicates that PLCβ phosphorylation is dynamically regulated. PLCβ1 and PLCβ3 activities are negatively modulated by phosphorylation. Thus, their concomitant reciprocal phosphorylation would alter the relative contribution of these isoforms to PLC/Ca2+ signaling, a significant shift in light of their differential regulatory characteristics. Reciprocal modulation of the phosphorylation (activity) of two isoforms within the same subclass of signaling enzyme, proteins that have a high degree of structural similarity and subserve the same biological function, represents an adaptation modality to chronic morphine that has heretofore not been recognized.
Keywords: opioid tolerance, phosphorylation
Opioids are known to produce a plethora of neurochemical perturbations. These include inhibition of adenylyl cyclase (1, 2), activation of inwardly rectifying K+ channels (3, 4), and inhibition of voltage-activated calcium (Ca2+) channels, predominantly of the N and P/Q types (5, 6). Additionally, there is growing evidence that modulation of phosphoinositide-specific phospholipase C (PLC), and consequently altered formation of inositol 1,4,5-trisphosphate/diacylglycerol/Ca2+ signaling, plays a pivotal role in mediating opioid effects.
PLC is one of only two signaling effector enzymes (the other being adenylyl cyclase) whose activity is directly modulated by opioids. PLC is positively regulated by opioids (7-11), and several physiological studies have implicated PLC-linked pathways in a diverse range of opioid-modulated events. These include pain regulation (12, 13), manifestation of opioid tolerance (14), and opioid withdrawal (15). More recently, facilitative interactions between vasoactive intestinal polypeptide and the δ-opioid [D-Pen2,5] enkephalin to enhance cAMP accumulation in spinal tissue have been shown to involve the PLC signaling cascade (16).
PLC is the enzyme responsible for generating two intracellular second messengers via the hydrolysis of phosphatidylinositol 4,5-bisphosphate (17). Inositol 1,4,5-trisphosphate partitions in the cell cytosol and is considered to be universal Ca2+ mobilizing second messenger. Diacylglycerol remains in the membrane and is an activator of PKC. Molecular cloning and biochemical characterizations have revealed the existence of four types of PLC: β, γ, δ, and ε. These isoforms range in molecular mass (kDa) from 230 to 260 (ε) and 150 (β and γ) to 85 (δ), which are immunologically distinct and products of separate genes. One ε, four β, two γ, and four δ isoforms have been described in mammalian systems (18).
Phosphorylation state is a critical regulatory parameter of PLCβ isoforms, which are substrates for protein kinase A (PKA) and PKC. Phosphorylation by these kinases negatively modulates the activity of PLCβ isoforms (see refs. 19 and 20 and references therein). Regulation of PLCβ isoform activity by a PKA-/PKC-mediated phosphorylation suggests their modulation during opioid tolerance, a hallmark feature of which is enhanced phosphorylation of key components of signaling cascades. Up-regulation of adenylyl cyclase, i.e., cAMP overshoot (21, 22), and protein kinases, which include PKA (23-28) and PKC (29-31), which results in the augmented phosphorylation of multiple specific proteins (24, 26, 32, 33), are among the most frequently used neurochemical markers of opioid tolerance/dependence.
Based on the importance of (augmented) PKA and PKC activity to the transduction of chronic opioid effects, the ability of these kinases to also phosphorylate and thereby regulate PLCβ activity and the established importance of phosphoinositide turnover and calcium mobilization to chronic opioid sequelae, we hypothesized that PLCβ phosphorylation would be augmented as a function of opioid tolerance. The present results reveal, using the guinea pig longitudinal muscle myenteric plexus preparation (LMMP), that PLCβ1 and PLCβ3 phosphorylation is significantly altered after induction of opioid tolerance. Notably, however, the change in phosphorylation (and presumably activity) qualitatively differs between these PLCβ isoforms. This is a demonstration of chronic morphine-induced reciprocal regulation among isoforms of an identified protein.
Materials and Methods
Materials. Morphine pellets were supplied by the National Institute on Drug Abuse. 32P-labeled phosphoric acid (32Pi) was obtained from Perkin-Elmer. All protein kinase and phosphatase inhibitors were purchased from LC Laboratories (Woburn, MA) except for cypermethrin, which was purchased from Calbiochem. All protease inhibitors were purchased from Sigma except for Complete Protease Inhibitor mixture, which was purchased from Roche Molecular Biochemicals. Antibodies against PLCβ1, PLCβ3, and their blocking peptides were obtained from Santa Cruz Biotechnology. Anti-phosphoPLCβ3 (Ser-1105) and its blocking peptide were obtained from Cell Signaling Technology (Beverly, MA).
LMMP Preparation and Incubation with 32Pi. Studies were carried out in accordance with the guide for the care and use of laboratory animals as adopted and promulgated by the National Institutes of Health. Morphine tolerance/dependence was induced by s.c. implantation of five morphine pellets (each containing a 75-mg morphine base) into male albino guinea pigs (375-450 g) as described (33, 34). For tolerant/dependent LMMP tissue, morphine (100 nM) was included in the buffer. LMMP strips were then incubated with 1 mCi/ml 32Pi in phosphate-free Krebs (2 h at 35°C) with continuous gassing. To determine the effect of acute opioid treatment on phosphorylation of PLCβ1 and PLCβ3, opioid naïve LMMP strips were incubated with morphine (100 nM) during the last 5 min of 32Pi incubation. The kinase(s)/phosphatases required for chronic morphine-induced change in phosphorylation of PLCβ3 and PLCβ1 were inferred from the effect of inhibitors on the incorporation of 32P into LMMP protein in tissue obtained from morphine-treated animals. LMMP tissue from the same ileum was randomly divided into two samples, one of which contained a kinase or phosphatase inhibitor. Each was added during the last 30 min of the 2-h 32P-labeling period. Concentrations of inhibitor were selected to maximize discrimination between classes of kinase or phosphatase.
After incubation with 32Pi, LMMP tissues were washed extensively with phosphate-free Krebs buffer and homogenized in Tris buffer (10 mM, pH 7.6) containing 10% sucrose, 2 mM DTT, 5 mM EDTA, 1 mM EGTA, 10 mM sodium pyrophosphate, protease inhibitors (Bacitracin, 100 mg/liter; 20 mg/liter each of chloromethyl ketone, Nα-p-tosyl-l-lysine chloromethyl ketone, and phenylmethylsulfonyl fluoride; 3.2 mg/liter each of leupeptin and soybean trypsin inhibitor; 1.0 mg/liter Aprotinin, 1 mM Benzamidine, and complete inhibitor mixture, one tablet per 50 ml), protein phosphatase inhibitors, 25 nM calyculin A; 0.5 μM okadaic acid, 10 mM sodium fluoride and 100 μM sodium orthovanadate. Supernatant obtained from a low-speed centrifugation (1,000 × g, 4°C, 10 min) was subjected to a high-speed centrifugation (30,000 × g, 4°C, 30 min). Membranes obtained were resuspended in 50 mM Tris buffer containing 1 mM DTT, 10 mM sodium pyrophosphate, and the above-mentioned protease and phosphatase inhibitors.
Autoradiography of PLCβ1 and PLCβ3. Immunoprecipitation of PLCβ1 and PLCβ3 used rabbit polyclonal antibodies generated against the carboxyl terminus of bovine PLCβ1 or rat PLCβ3, respectively. Membranes were solubilized in buffer containing phosphatase and protease inhibitors, as described above, and 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol, and 150 mM NaCl (60 min on ice), agitated, and centrifuged (at 14,000 × g for 30 min). Protein amounts in solubilized supernatants were determined by Bradford method (35) using BSA as a standard. Solubilized membrane protein (1 mg) from each sample was incubated with either 4 μg of anti-PLCβ1 or PLCβ3 antibodies (each for 60 min at 4°C), after which immunoprecipitation was performed as described (32). Samples were separated by NUPAGE (using 7% Tris-acetate gels) electrophoresis (Invitrogen). Gels were dried and exposed to PhosphorImager screens that were scanned in a Phosphor-Imager Storm 860 (Molecular Dynamics). The amount of 32P incorporated into the phosphorylated protein bands was determined by using densitometric analysis (imagequant, Molecular Dynamics).
Western Blot Analysis. Standard procedures were used for Western analyses as used previously by this laboratory (36). Proteins from gels were electrophoretically transferred onto nitrocellulose membranes. Selected lanes were incubated (overnight at 4°C) with anti-PLCβ1, PLCβ3, phosphoPLCβ3 (all at 1:1,000), or antibodies in the presence of their respective blocking peptides (1:1; preadsorbed controls). The secondary antibody used was a peroxidase-labeled anti-rabbit antibody. Antibody-substrate complex was visualized by using an enhanced chemiluminescence detection kit (Amersham Pharmacia). Sample pairs, obtained from opioid naïve and chronic morphine-treated LMMP tissues, were processed, electrophoresed, and blotted in parallel, after which they were exposed concomitantly to the same Kodak X-Omat film (Perkin-Elmer). Intensity of signal was quantified by using NIH imaging software.
Statistical Analysis. Significance of autoradiographic and Western analyses was assessed by using a paired Student's t test.
Results
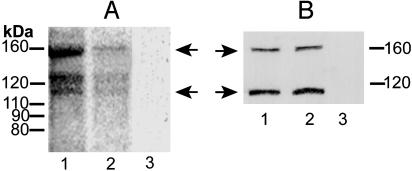
Effect of Chronic Morphine on PLCβ1 Phosphorylation and Protein Content. Immunoprecipitate (IP) was obtained by using PLCβ1 antibodies (PLCβ1 IP) from 32P-radiolabeled opioid naïve and chronic morphine-treated LMMP tissue and subjected to autoradiographic analysis (Fig. 1A, lanes 1 and 2, respectively). PLCβ1 IP obtained from both preparations contained phosphorylated proteins of predominantly three molecular masses (≈155, 130, and 118 kDa). These radiolabeled proteins were not observable in IP obtained by using preadsorbed anti-PLCβ1-antibody (Fig. 1 A, lane 3), underscoring the specificity of the immunoprecipitation. The ≈155-kDa radiolabeled band is consistent with previously established values for the PLCβ1 isoform, whereas the ≈130-kDa radiolabeled species is consonant with the molecular mass of its reported splice variant (20, 37). The radiolabeled signal of ≈118 kDa most likely represents a degradation product of the ≈155- or ≈130-kDa single and was not visible in all experiments. Therefore, quantification of changes in PLCβ1 phosphorylation as a function of morphine treatment was confined to the ≈155- and 130-kDa radiolabeled molecular mass species.
Fig. 1.
Chronic morphine attenuates PLCβ1 phosphorylation without altering its protein content. (A) LMMP tissue was obtained from opioid naïve (lane 1) and chronic morphine-treated (lane 2) guinea pigs and incubated with 32P for 2 h. Thereafter, membranes were prepared, solubilized, and immunoprecipitated by using antibodies selective for PLCβ1 or its preadsorbed control (lane 3). IPs were subjected to NUPAGE electrophoresis, and radiolabeled proteins were visualized by autoradiography (≈18-h exposure). Quantitative densitometric analysis was used to assess magnitude of 32P incorporation. Chemical identity of proteins was based on electrophoretic molecular mass, calculated from a prestained protein ladder (10-200 kDa) that was included in each electrophoresis in combination with loss of signal after antibody preadsorbtion (representative of nine experiments). (B) LMMP membranes (50 μg) obtained from opioid naïve and chronic morphine-treated guinea pigs (lanes 1 and 2, respectively) were processed as described for A and subjected to Western blot analysis by using anti-PLCβ1 antibodies. Lane 3 represents Western blot analysis of LMMP membranes (50 μg) obtained from chronic morphine-treated tissue by using preadsorbed anti-PLCβ1 antibodies (representative of three experiments). Antibody-substrate complex was visualized by using ECL detection. Left arrows in A indicate phosphoPLCβ1, and right arrows in B indicate PLCβ1 protein. Chronic systemic morphine markedly reduces phosphorylation of PLCβ1 but does not alter its content.
PLCβ1 IP obtained from opioid naïve and morphine-treated LMMP tissue contained analogous molecular mass profiles of radiolabeled proteins. However, the intensity of radiolabeling differed substantially among these two preparations. There was a substantial reduction in the magnitude of 32P incorporated into the ≈155 (63 ± 1.73%; P < 0.005; n = 9) and 130-kDa proteins (43 ± 2.77%; P < 0.05; n = 9) present in PLCβ1 IP obtained from morphine-treated vs. opioid naïve tissue (Fig. 1 A, compare lanes 1 and 2). These observations reveal that chronic morphine decreases PLCβ1 phosphorylation.
To investigate whether changes in PLCβ1 phosphorylation were accompanied by changes in protein content, quantitative Western analyses were performed. Two bands of ≈155 and 118 kDa were visualized (Fig. 1B, lanes 1 and 2), comparable to that observed in autoradiography. However, the ≈130-kDa band, visible in autoradiography, was not detected by Western analysis, possibly reflecting that it is present in minor amounts. Specificity of Western signals is indicated by their absence in Western blots by using preadsorbed antibody (Fig. 1B, lane 3). In contrast to 32P incorporation, quantitative Western analyses revealed that the content of PLCβ1 protein (≈155 kDa) does not differ among LMMP membranes obtained from opioid naïve and chronic morphine-treated animals (93 ± 9% of control; Fig. 1B, lane 1 vs. lane 2; n = 4).
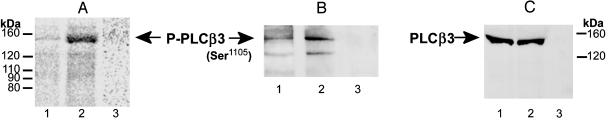
Effect of Chronic Morphine on PLCβ3 Phosphorylation and Protein Content. Analogous experiments were performed by using antibodies selective for PLCβ3 (Fig. 2 A and B). PLCβ3 IP obtained from opioid naïve and chronic morphine-treated 32P-labeled LMMP tissue contained radiolabeled proteins of predominantly ≈150 and 130 kDa (Fig. 2 A, lanes 1 and 2). Neither was visible in autoradiographs of IP obtained by using preadsorbed anti-PLCβ3 antibody (Fig. 2 A, lane 3). The ≈130-kDa species is a relatively minor constituent. It was not observable in PLCβ3 Western analyses of LMMP membranes (Fig. 2C) unless the amount of membrane protein loaded was increased from 100 to 150 μg or protein was immunoprecipitated by using anti-PLCβ3 antibody (thereby concentrating PLCβ3 protein) before commencing Western analysis (data not shown). Therefore, quantification of changes in PLCβ3 phosphorylation as a function of morphine treatment was confined to the ≈150-kDa radiolabeled molecular mass species.
Fig. 2.
Chronic morphine augments phosphorylation of PLCβ3 without altering its protein content. (A) Aliquots of solubilized LMMP membranes, prepared for experiments described in Fig. 1 A, were immunoprecipitated by using antibodies selective for PLCβ3 and subjected to autoradiographic analysis concomitantly with that of PLCβ1 IP. Lanes 1 and 2 represent IPs from LMMP tissue obtained from opioid naïve vs. chronic morphine-treated animals, respectively. Lane 3 represents IP obtained from opioid tolerant LMMP tissue by using preadsorbed anti-PLCβ3 antisera (representative of 10 experiments). (B and C) LMMP membranes obtained from opioid naïve and chronic morphine-treated guinea pigs (lanes 1 and 2, respectively) were processed as described for autoradiography and subjected to Western blot analysis by using either antibodies specific for phosphoPLCβ3Ser1105 (150 μg of membrane, B) or PLCβ3 (100 μg of membrane, C). Lane 3 in B and C represents Western analysis of chronic morphine-treated LMMP membranes using preadsorbed antibodies against phosphoPLCβ3 and PLCβ3, respectively. B and C are representative of four experiments each. Chronic morphine augments the phosphorylation of PLCβ3, assessed by 32P incorporation or phosphoPLCβ3 Western analysis, but does not alter the content of PLCβ3. P-PLCβ3, phosphorylated PLCβ3.
Chronic systemic morphine also had a substantial effect on the magnitude of 32P incorporated into the ≈150-kDa immunoprecipitated molecular mass species. Notably, however, the direction of the modulation was opposite from that observed for proteins present in PLCβ1 IP. Chronic morphine substantially increased (227 ± 19% of control) the phosphorylation of PLCβ3 (P < 0.05; n = 10).
Consonant results were obtained when the phosphorylation state of PLCβ3 was assessed by using a PLCβ3 phosphospecific antibody. As was observed in autoradiographic analysis, quantitative Western analysis of PLCβ3 IP, obtained from control and chronic morphine-treated LMMP tissue, using an antibody selective for phospho-Ser-1105PLCβ3, revealed signals of ≈150 and 130 kDa (Fig. 2B, lanes 1 and 2). Neither was observed in Western analysis using preadsorbed phosphoPLCβ3 antibody (Fig. 2B, lane 3). Higher amounts of protein (150 vs. 100 μg) were used in the Westerns that used the anti-phosphoPLCβ3 antibody than did those using an anti-PLCβ3 common antibody, which would account for the presence of the ≈130-kDa band in the former but not the latter (Fig. 2 B vs. C). Notably, the intensity of the ≈150-kDa signal was significantly augmented (150 ± 10% of control, P < 0.05; n = 4) in phosphoPLCβ3 Western analysis of PLCβ3 IP obtained from chronic morphine-treated LMMP tissue. The differential magnitude of the increment in PLCβ3 phosphorylation observed by autoradiographic vs. Western analysis most likely indicates the chronic morphine-induced phosphorylation of sites in addition to Ser-1105, which would contribute to the autoradiographic but not the Western signal. These data reaffirm that chronic morphine augments PLCβ3 phosphorylation and underscore that potential metabolic effects of morphine on the specific activity of ATP pools do not confound interpretation of the chronic morphine-induced enhancement of 32P incorporation into PLCβ3. Furthermore, they reveal that it results, at least in part, from increased phosphorylation at Ser-1105, the site recognized by the anti-phosphoPLCβ3 antibody.
Western analysis employing an anti-PLCβ3 common antibody also revealed a ≈150-kDa molecular mass signal, analogous to that observed in autoradiographic and phosphoPLCβ3 Western analyses (Fig. 2C, lanes 1 and 2). This signal was obliterated in blots probed with preadsorbed antisera (Fig. 2C, lane 3). As was observed for PLCβ1, the content of PLCβ3 protein does not differ between LMMP membrane obtained from opioid naïve vs. chronic morphine-treated animals (91 ± 7% of control; Fig. 2C, lane 1 vs. lane 2; n = 4).
Multiple considerations indicate that data interpretation is not confounded by cross reactivity of the anti-PLCβ1 and anti-PLCβ3 antibodies used for IP. First, chronic morphine-induced enhanced phosphorylation of PLCβ3 was demonstrated by using two different methodologies, which employ two different anti-PLCβ3 antibodies recognizing different epitopes. Second, because the direction of change in chronic morphine-induced phosphorylation of PLCβ1 is opposite that of PLCβ3, any residual cross reactivity would result in an underestimate of the magnitude of effect. Last, the peptide sequences used to generate anti-PLCβ1 and anti-PLCβ3 do not share any homology with PLCβ3 and PLCβ1, respectively.
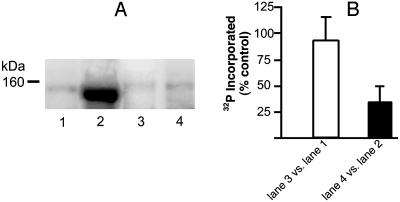
PKC Inhibition Abolishes the Chronic Morphine-Induced Increment in PLCβ3 Phosphorylation. The effect of PKC inhibition on the chronic morphine-induced increment in PLCβ3 phosphorylation is represented in Fig. 3. Pretreatment with the PKC-selective inhibitor chelerythrine failed to produce a significant reduction in 32P incorporation into PLCβ3 obtained from opioid naïve tissue (Fig. 3A, lane 3 vs. lane 1). However, chelerythrine abolished the chronic morphine-induced increment in PLCβ3 phosphorylation (Fig. 3A, compare lanes 4 and 2), suggesting its mediation via PKC. In like fashion, we determined the effect of protein phosphatase inhibitors on the chronic morphine-induced decrement in PLCβ1 phosphorylation to ascertain whether or not enhanced phosphatase activity was a contributory factor. Neither okadaic acid, an inhibitor of protein phosphatases 1 and 2A (38), nor cypermethrin, an inhibitor of protein phosphatase 2B (39), altered the magnitude of the diminution of PLCβ1 phosphorylation.
Fig. 3.
Selective inhibition of PKC, but not PKA, abolishes the chronic morphine-induced increment in PLCβ3 phosphorylation. LMMP tissue from the same control and chronic morphine-treated guinea pig was randomly divided such that 32P incorporation into immunoprecipitated protein could be concomitantly determined in the absence as well as the presence of chelerythrine (PKC-selective inhibitor, which was added during the last 30 min of the 2-h 32P-labeling period). Thereafter, membranes were prepared, solubilized, and immunoprecipitated by using anti-PLCβ3 antibodies. IPs were subjected to electrophoresis, and radiolabeled protein was visualized by autoradiography (≈18-h exposure) by using storage phosphor imaging techniques. Quantitative densitometric analysis was used to assess magnitude of 32P incorporation into an ≈150-kDa signal. Radiolabeling of PLCβ3 in opioid naïve tissue is depicted without (lane 1) and with (lane 3) chelerythrine. Radiolabeling of PLCβ3 in chronic morphine-treated tissue is shown without (lane 2) and with (lane 4) PKC inhibition. The magnitude of 32P incorporation was determined concomitantly in the presence vs. the absence of kinase inhibitor (n = 3). Chelerythrine abolished the increment in PLCβ3 phosphorylation produced by chronic morphine.
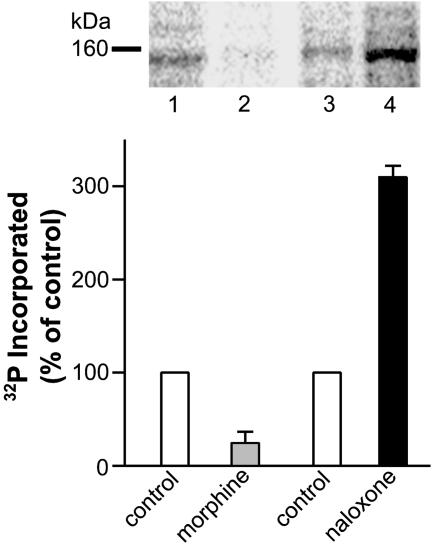
Acute Treatment with Either Morphine or Naloxone Inhibits and Augments, Respectively, PLCβ3 Phosphorylation. To determine whether augmented PLCβ3 phosphorylation is a neurochemical sequela specifically associated with chronically administered morphine, the effect of its acute (5 min) administration on PLCβ3 phosphorylation was ascertained. In contrast to chronically administered morphine, its acute application (100 nM) inhibited PLCβ3 phosphorylation by 74 ± 14% (n = 3; Fig. 4, lane 2 vs. lane 1, gray bar). No effect of acute application of morphine was observed on phosphorylation of PLCβ1 (n = 2; data not shown). On the other hand, acute (30 min) treatment with 10 or 1 μM of the opioid antagonist naloxone substantially augmented phosphorylation of PLCβ3 (309 ± 11% of control; n = 2; Fig. 4, lane 4 vs. lane 3, solid bar) and 257% of control, respectively (data not shown). These data indicate that morphine and its endogenous counterparts can acutely modulate (albeit negatively) PLCβ3 phosphorylation, but the augmentation of PLCβ3 phosphorylation that is observed after the development of tolerance requires their chronic administration.
Fig. 4.
Acute treatment with morphine and naloxone reciprocally modulates PLCβ3 phosphorylation. LMMP tissue from opioid naïve guinea pigs was randomly divided such that 32P incorporation into immunoprecipitated protein could be determined concomitantly in the absence and presence of either morphine (100 nM; added during the last 5 min of the 2-h 32P-labeling period) or naloxone (10 μM; added during the last 30 min of the 2-h 32P-labeling period). Thereafter, tissues were processed and analyzed as described for Fig. 3. The effect of acute opioid receptor activation on PLCβ3 phosphorylation is derived from comparing the intensity of signal in lane 1 (without morphine) vs. the signal present in lane 2 (with morphine) (n = 3); the effect of acute opioid receptor blockade on PLCβ3 phosphorylation is derived from comparing the intensity of signal in lane 3 (without naloxone) vs. the signal present in lane 4 (with naloxone). n = 2 in Lower. P < 0.05 for each comparison, which was determined in LMMP tissue obtained from separate animals. Data are expressed as percent control 32P incorporation. Acute morphine substantially attenuates, whereas acute naloxone substantially enhances, PLCβ phosphorylation.
Discussion
The present study reveals that chronic morphine alters the phosphorylation state of two PLCβ isoforms, PLCβ1 and PLCβ3. Strikingly, their phosphorylation state is modulated in reciprocal fashion. Phosphorylation of PLCβ1 is diminished, whereas that of PLCβ3 is significantly augmented. There is a growing list of signaling proteins whose phosphorylation is modulated in response to chronic morphine. For example, in the locus coeruleus, the (cAMP-dependent) phosphorylation state of proteins of multiple molecular masses is augmented after chronic morphine (26). More recently, using the LMMP preparation, this laboratory demonstrated that chronic systemic morphine augments the phosphorylation of four signaling proteins, adenylyl cyclase (32), β-arrestin, G protein receptor kinase 2/3, and the Gβ subunit of G proteins (33). The diminished phosphorylation of another signaling protein, mitogen-activated protein kinase, has also been demonstrated during precipitated opioid withdrawal (40). The current demonstration of the reciprocal modulation of the phosphorylation of two isoforms within the same subclass of signaling enzyme (PLCβ), proteins that not only have a high degree of structural similarity and amino acid identity (50%) but also subserve the same biological function, represents an adaptation modality that has heretofore not been recognized.
The augmented phosphorylation that results in response to chronic morphine is mediated via PKC. This is evidenced by the ability of chelerythrine, a PKC-selective inhibitor, to abolish the chronic morphine-induced phosphorylation increment. This is consonant with the reported up-regulation of PKC in spinal cord (29, 30) and LMMP preparation (41) during opioid tolerance development. Notably, the chronic morphine-induced augmented phosphorylation of adenylyl cyclase, G protein receptor kinase2/3, β-arrestin, and Gβ, previously reported by this laboratory, is also PKC-mediated (32, 33).
The mechanism(s) underlying the chronic morphine-induced diminution in phosphorylation of PLCβ1 remains to be elucidated. Pretreatment with an inhibitor of either protein phosphatase 1/2A or 2B, classes of phosphatase that act on Ser/Thr phosphorylation sites and should therefore counter the action of PKA and PKC, failed to diminish the decrement in PLCβ1 phosphorylation. This notwithstanding, both okadaic acid and cypermethrin produced a proportionately similar increase (2-3-fold) in 32P incorporation into PLCβ1 obtained from either opioid naïve or morphine-treated LMMP preparations, indicating their effectiveness as phosphatase inhibitors under the current experimental conditions (data not shown). Thus, it seems unlikely that phosphatase activation is involved in this phenomenon, although the participation of other classes of protein phosphatase remains a viable possibility. Alternatively, the ability of G protein βγ subunits to antagonize PKC-dependent phosphorylation of PLCβ1 in vitro (42) could be applicable to regulating the phosphorylation state of this isoform in vivo. Negative modulation of PLCβ1 phosphorylation by Gβγ would be augmented in the opioid tolerant state because chronic morphine augments phosphorylation of Gβ, which decreases its association with GRK2/3 and thus enhances the availability of Gβγ for interaction with its varied effectors (33).
G protein mediation, pertussis toxin-insensitive (via the α subunit of Gq) or sensitive (via the Gβγ subunit of Gi/Go) is a prerequisite for receptor activation of PLCβ isoforms, a property not observed for δ or γ types (19, 20). Gα, Gβγ, or both subunits bind to PLCβ1 and PLCβ3, increasing their catalytic activity. This mechanism of activation of the PLCβ isoforms is of particular relevance to opioid neurochemical sequelae, as all known opioid effects are G protein mediated.
PLCβ1 and PLCβ3 are both negatively modulated by phosphorylation. PKC phosphorylates PLCβ1 (43), which inhibits its Ca2+-dependent stimulation (44) as well as its activation via Gβγ subunits (42). PKC can also phosphorylate, in vivo, PLCβ3 (45, 46), which inhibits activation by Gαq as well as Gβγ (46). Inhibition of activation by Gαq by PKC is attributable to phosphorylation of Ser-1105, whereas inhibition of Gβγ stimulated activity results from phosphorylation at another site (46). Thus, concomitant reciprocal phosphorylation of PLCβ1 and PLCβ3 by PKC, such as that which occurs after chronic systemic morphine, would be expected to alter the relative contribution of these isoforms to PLC signaling.
Exchange of one isoform for another with comparable regulatory and catalytic properties might not be expected to have notable physiological consequences. The chronic morphine-induced shift in the relative predominance of PLCβ1 vs. PLCβ3, however, should have significant opioid tolerance-associated signaling consequences because each manifests several unique regulatory characteristics. PLCβ1 and PLCβ3 are both activated by Gαq/11 subunits (derived from Gq), which as expected, is insensitive to PTX. The concentration dependence of the activation, however, differs among these isoforms. Activation of PLCβ1 by Gαq/11 subunits occurs over a broad concentration range, whereas its activation of PLCβ3 exhibits a relatively steep dependence on concentration (47). It is interesting to note that although all mammalian PLCβ isoforms are Gq GTPase-activating proteins, GTPase-activating protein activity varies among isoforms. PLCβ1 increases GTPase activity about twice as much as PLCβ3 (G. Biddlecome and E. Ross, personal communication). This underscores potential PLCβ isoform-specific differences in signaling.
Efficacy of activation of PLCβ1 and PLCβ3 by Gβγ subunits is also isoform-selective. Gβγ is clearly more effective in stimulating PLCβ3 than PLCβ1 (ED50 for Gβγ activation of PLCβ3 and PLCβ1 is 90 nM and >300 nM, respectively) (47). Additionally, concomitant regulation by Gαq and Gβγ subunits is also β-isoform subtype-selective. Gαq/11 activation of PLCβ1 and PLCβ3 is reduced in the presence of Gβγ. However, whereas Gαq/11 fails to activate the β1 isoform in the presence of Gβγ, activation of PLCβ3 remains detectable. Moreover, the less than additive regulation of PLCβ3 by Gαq/11 and Gβγ becomes additive or even synergistic at higher Mg2+ concentrations (≈5 mM), whereas concomitant G protein subunit regulation of PLCβ1 remains less than additive at all Mg2+ concentrations (47). These observations indicate that PLCβ3, in contrast to PLCβ1, is able to integrate coincident signaling via pathways coupled to Gq and Gi/Go.
These isoform-specific differences indicate that chronic morphine-induced diminution of the activity of PLCβ3 (via its increased state of phosphorylation) concomitant with augmented activity of PLCβ1 (via its diminished phosphorylation) results in the prevalence of PLCβ signaling that is more muted and not able to act as a coincident signal detector. Chronic morphine shifts PLCβ signaling from a high gain Gαq-/Gβγ-regulated PLC isoform to one that is more graded in G protein subunit responsiveness. The sustained enhanced activity of PKC that has been associated with opioid tolerance might be more compatible with a new steady state in which there are less precipitous fluctuations in intracellular calcium content. Alternatively, or additionally, PLCβ1 and PLCβ3 isoforms may differ their associated assembly and organizational components that have been shown to be members of the PLC signaling family (48). These could include A-kinase anchoring protein, caveolin, and postsynaptic density disk-large ZO-1 proteins. These recognize specific motifs in various signaling components such as tyrosine and Ser/Thr protein kinases, phosphatases, ion channels, etc. Consequently, the temporal and spatial aspects of signaling as well as their relationship to disparate signaling pathways could differ between PLCβ1 and PLCβ3. These possibilities notwithstanding, the specific relevance of PLC isoform-specific adaptations to the formation of opioid tolerance requires further elucidation. The necessity of PLC activity for the tolerance development, albeit isoform-specific, is underscored by previous observations that i.c.v. application of ET-18-OCH3, a selective inhibitor of PLC, reversed tolerance formation (14).
Neither the phosphorylation of PLCβ3 nor that of PLCβ1 was augmented or diminished, respectively, after acute morphine (5-min treatment), as was observed after its chronic administration. Thus, tolerant-associated changes in PLCβ3 and PLCβ1 phosphorylation represent adaptations unique to the chronic administration of morphine. Acute morphine did, however, diminish PLCβ3 phosphorylation. This is consistent with the established ability of opioids to stimulate PLCβ (7-10, 12, 40, 49, 50) and suggests the relevance of dual mechanisms underlying the opioid activation, i.e., generation of Gαq and Gβγ subunits as well as acute negative modulation of PLCβ3 phosphorylation, which would augment its stimulatory responsiveness to them.
Interestingly, the opioid antagonist naloxone was also able to modulate PLCβ3 phosphorylation, but the direction was opposite to that of morphine, i.e., acute naloxone treatment augmented PLCβ3 phosphorylation. This indicates that negative modulation of PLCβ3 phosphorylation by exogenous opioids also occurs via their endogenous counterparts and that physiological levels of PLCβ3 phosphorylation are dynamically regulated, i.e., constrained, by tonic opioid activity.
Comparison of acute morphine responsiveness of PLCβ3-null mice with their wild-type counterpart has suggested that PLCβ3 constitutes an intrinsic negative modulatory pathway by which opioid responsiveness is constrained (13). This conclusion would seem to be the antithesis of the current demonstration that diminution of PLCβ3 activity, i.e., its increased phosphorylation (concomitant with enhanced activity of PLCβ1), is associated with tolerance formation. This apparent dichotomy underscores that divergent mechanisms subserve adaptations to acute vs. long-term exposure to opioids.
Chronic morphine also induces adenylyl cyclase isoform-specific changes in the LMMP tissue (36, 51, 52) and locus coeruleus (53). These observations, in combination with present findings of a reciprocal shift in PLCβ isoform activity in response to chronic morphine, suggest that altered activity among isoforms of signaling proteins could have more generalized applicability to opioid tolerance-producing mechanism than has thus far been appreciated. Such considerations underscore that opioid tolerance is not simply the offset of acute, analgesic mechanisms but involves the emergence of new signaling strategies.
Abbreviations: PLC, phosphoinositide-specific phospholipase C; LMMP, longitudinal muscle myenteric plexus; PKA, protein kinase A; IP, immunoprecipitate.
References
- 1.Childers, S. R. (1991) Life Sci. 48, 1991-2003. [DOI] [PubMed] [Google Scholar]
- 2.Sharma, S. K., Nirenberg, M. & Klee, W. A. (1975) Proc. Natl. Acad. Sci. USA 72, 590-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong, D. L. & White, R. E. (1992) Trends Neurosci. 15, 403-408. [DOI] [PubMed] [Google Scholar]
- 4.North, R. A., Williams, J. T., Surprenant, A. & Christie, M. J. (1987) Proc. Natl. Acad. Sci. USA 84, 5487-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moises, H. C., Rusin, K. I. & Macdonald, R. L. (1994) J. Neurosci. 14, 5903-5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder, J. E., Fischbach, P. S., Zheng, D. & McCleskey, E. W. (1991) Neuron 6, 13-20. [DOI] [PubMed] [Google Scholar]
- 7.Smart, D., Smith, G. & Lambert, D. G. (1994) J. Neurochem. 62, 1009-1014. [DOI] [PubMed] [Google Scholar]
- 8.Smart, D., Smith, G. & Lambert, D. G. (1995) Biochem. J. 305, 577-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin, W., Lee, N. M., Loh, H. H. & Thayer, S. A. (1994) J. Neurosci. 14, 1920-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyamae, T., Fukushima, N., Misu, Y. & Ueda, H. (1993) FEBS Lett. 333, 311-314. [DOI] [PubMed] [Google Scholar]
- 11.Smart, D. & Lambert, D. G. (1996) J. Neurochem. 66, 1462-1467. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Blazquez, P. & Garzon, J. (1998) J. Pharmacol. Exp. Ther. 285, 820-827. [PubMed] [Google Scholar]
- 13.Xie, W., Samoriski, G. M., McLaughlin, J. P., Romoser, V. A., Smrcka, A., Hinkle, P. M., Bidlack, J. M., Gross, R. A., Jiang, H. & Wu, D. (1999) Proc. Natl. Acad. Sci. USA 96, 10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith, F. L., Lohmann, A. B. & Dewey, W. L. (1999) Br. J. Pharmacol. 128, 220-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrini-Giampietro, D. E., Ruggiero, M., Giannelli, S., Chiarugi, V. P. & Moroni, F. (1988) Eur. J. Pharmacol. 149, 297-306. [DOI] [PubMed] [Google Scholar]
- 16.Liu, N. J. & Gintzler, A. R. (2003) Brain Res. 959, 103-110. [DOI] [PubMed] [Google Scholar]
- 17.Berridge, M. J. & Irvine, R. F. (1989) Nature 341, 197-205. [DOI] [PubMed] [Google Scholar]
- 18.Rhee, S. G. (2001) Annu. Rev. Biochem. 70, 281-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher, S. K. (1995) Eur. J. Pharmacol. 288, 231-250. [DOI] [PubMed] [Google Scholar]
- 20.Rebecchi, M. J. & Pentyala, S. N. (2000) Physiol. Rev. 80, 1291-1335. [DOI] [PubMed] [Google Scholar]
- 21.Sharma, S. K., Klee, W. A. & Nirenberg, M. (1975) Proc. Natl. Acad. Sci. USA 72, 3092-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma, S. K., Klee, W. A. & Nirenberg, M. (1977) Proc. Natl. Acad. Sci. USA 74, 3365-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kogan, J. H., Nestler, E. J. & Aghajanian, G. K. (1992) Eur. J. Pharmacol. 211, 47-53. [DOI] [PubMed] [Google Scholar]
- 24.Nestler, E. J. (1992) J. Neurosci. 12, 2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duman, R. S., Tallman, J. F. & Nestler, E. J. (1988) J. Pharmacol. Exp. Ther. 246, 1033-1039. [PubMed] [Google Scholar]
- 26.Guitart, X. & Nestler, E. J. (1989) J. Neurosci. 9, 4371-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nestler, E. J. & Tallman, J. F. (1988) Mol. Pharmacol. 33, 127-132. [PubMed] [Google Scholar]
- 28.Self, D. W., McClenahan, A. W., Beitner-Johnson, D., Terwilliger, R. Z. & Nestler, E. J. (1995) Synapse 21, 312-318. [DOI] [PubMed] [Google Scholar]
- 29.Mao, J., Price, D. D., Phillips, L. L., Lu, J. & Mayer, D. J. (1995) Brain Res. 677, 257-267. [DOI] [PubMed] [Google Scholar]
- 30.Mayer, D. J., Mao, J. & Price, D. D. (1995) Pain 61, 365-374. [DOI] [PubMed] [Google Scholar]
- 31.Mayer, D. J., Mao, J. & Price, D. D. (1995) NIDA Res. Monogr. 147, 269-298. [PubMed] [Google Scholar]
- 32.Chakrabarti, S., Wang, L., Tang, W.-J. & Gintzler, A. R. (1998) Mol. Pharmacol. 54, 949-953. [DOI] [PubMed] [Google Scholar]
- 33.Chakrabarti, S., Oppermann, M. & Gintzler, A. R. (2001) Proc. Natl. Acad. Sci. USA 98, 4209-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, L. & Gintzler, A. R. (1997) J. Neurochem. 68, 248-254. [DOI] [PubMed] [Google Scholar]
- 35.Bradford, M. M. (1976) Anal. Biochem. 72, 248-254. [DOI] [PubMed] [Google Scholar]
- 36.Chakrabarti, S., Rivera, M., Yan, S.-Z., Tang, W.-J. & Gintzler, A. R. (1998) Mol. Pharmacol. 54, 655-662. [PubMed] [Google Scholar]
- 37.Bahk, Y. Y., Lee, Y. H., Lee, T. G., Seo, J., Ryu, S. H. & Suh, P. G. (1994) J. Biol. Chem. 269, 8240-8245. [PubMed] [Google Scholar]
- 38.Ishihara, H., Martin, B. L., Brautigan, D. L., Karaki, H., Ozaki, H., Kato, Y., Fusetani, N., Watabe, S., Hashimoto, K. & Uemura, D. (1989) Biochem. Biophys. Res. Commun. 159, 871-877. [DOI] [PubMed] [Google Scholar]
- 39.Enan, E. & Matsumura, F. (1992) Biochem. Pharmacol. 43, 1777-1784. [DOI] [PubMed] [Google Scholar]
- 40.Schulz, S. & Hollt, V. (1998) Eur. J. Neurosci. 10, 1196-1201. [DOI] [PubMed] [Google Scholar]
- 41.Wang, L., Medina, V. M., Rivera, M. & Gintzler, A. R. (1996) Brain Res. 723, 61-69. [DOI] [PubMed] [Google Scholar]
- 42.Litosch, I. (1997) Biochem. J. 326, 701-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryu, S. H., Kim, U. H., Wahl, M. I., Brown, A. B., Carpenter, G., Huang, K. P. & Rhee, S. G. (1990) J. Biol. Chem. 265, 17941-17945. [PubMed] [Google Scholar]
- 44.Litosch, I. (1996) Recept. Signal Transduct. 6, 87-98. [PubMed] [Google Scholar]
- 45.Ali, H., Fisher, I., Haribabu, B., Richardson, R. M. & Snyderman, R. (1997) J. Biol. Chem. 272, 11706-11709. [DOI] [PubMed] [Google Scholar]
- 46.Yue, C., Ku, C. Y., Liu, M., Simon, M. I. & Sanborn, B. M. (2000) J. Biol. Chem. 275, 30220-30225. [DOI] [PubMed] [Google Scholar]
- 47.Smrcka, A. V. & Sternweis, P. C. (1993) J. Biol. Chem. 268, 9667-9674. [PubMed] [Google Scholar]
- 48.Pawson, T. & Scott, J. D. (1997) Science 278, 2075-2080. [DOI] [PubMed] [Google Scholar]
- 49.Johnson, P. S., Wang, J. B., Wang, W. F. & Uhl, G. R. (1994) NeuroReport 5, 507-509. [DOI] [PubMed] [Google Scholar]
- 50.Yeo, A., Samways, D. S., Fowler, C. E., Gunn-Moore, F. & Henderson, G. (2001) J. Neurochem. 76, 1688-1700. [DOI] [PubMed] [Google Scholar]
- 51.Rivera, M. & Gintzler, A. R. (1998) Mol. Brain Res. 54, 165-169. [DOI] [PubMed] [Google Scholar]
- 52.Gintzler, A. R. & Chakrabarti, S. (2000) Mol. Neurobiol. 21, 21-33. [DOI] [PubMed] [Google Scholar]
- 53.Lane-Ladd, S. B., Pineda, J., Boundy, V. A., Pfeuffer, T., Krupinski, J., Aghajanian, G. K. & Nestler, E. J. (1997) J. Neurosci. 17, 7890-7901. [DOI] [PMC free article] [PubMed] [Google Scholar]