Abstract
The physiology of control and suppression of natural urges is not well understood. We used [15O]H2O positron-emission tomography imaging to identify neural circuits involved in suppression of spontaneous blinking as a model of normal urges. Suppression of blinking was associated with prominent activation of bilateral insular-claustrum regions, right more than left; activation was also found in bilateral anterior cingulate cortex (ACC), supplementary motor areas, and the face area of the primary motor cortex bilaterally. These results suggest a central role for the insula possibly together with ACC in suppression of blinking.
Keywords: blinking, insula, PET, suppression, urges, visceral homunculus
Introduction
Suppression phenomena are usually considered within the domains of affect, cognition, and behavior and are necessary for people's social and individual well-being. Disordered suppressive mechanisms may contribute to neuropsychiatric disorders where individuals cannot control unwanted memories, impulses, behaviors, and emotions. In this regard, the majority of past studies focused on revealing neural networks responsible for suppressing emotions (Beauregard et al. 2001; Ochsner et al. 2002; Levesque et al. 2003; Phan et al. 2005), thoughts, and memories (Wyland et al. 2003; Anderson et al. 2004) and different aspects of behavior including response suppression (Garavan et al. 1999; Liddle et al. 2001; Rubia et al. 2003; Blasi et al. 2006; Li et al. 2006; Chevrier et al. 2007). The most frequent finding of these studies, independent of paradigm, was involvement of limbic and paralimbic structures including anterior cingulate, orbitofrontal cortex, and insula as well as the prefrontal cortices.
There are only a few neuroimaging studies that examined neural networks involved in control and suppression of natural urges. Neuroimaging studies of air hunger and suppression of breathing showed strong right insular activation (Banzett et al. 2000); however, studies of urge and suppression of voiding yielded variable results. In different studies, the urge to void activated cingulate gyrus and insula (Kuhtz-Buschbeck et al. 2005; Seseke et al. 2006), pre–supplementary motor areas (Seseke et al. 2006), supplementary motor areas (SMA), and cerebellum (Kuhtz-Buschbeck et al. 2005) and deactivated bilaterally cingulate, premotor cortices, and hypothalamus (Athwal et al. 2001), whereas suppression of the urge activated bilateral SMA and putamen, right parietal cortex, parahippocampal gyrus and cerebellum (Zhang et al. 2005), and left superior frontal lobe (Kuhtz-Buschbeck et al. 2005).
Neural circuits involved in control and suppression of natural urges might play a key role in cognitive and behavioral suppression as well; in fact, they might be part of a common network engaged in suppressive behavior. Thus, knowledge about brain structures involved in control of urge and suppression phenomena may facilitate understanding the abnormalities underlying many neurological and psychiatric disorders in which the natural suppression network fails, as in obsessive compulsive disorder, tics and Tourette syndrome, hyperactivity and impulse control disorders, posttraumatic stress disorder, and addictions. We investigated the neural network involved in suppression of spontaneous blinking because it is a common occurrence.
Methods
Subjects
We studied 14 healthy volunteers, aged 21–47 (7 women and 7 men). A portion of the data from 9 subjects in relation to a comparison of the conditions rest versus sleep was presented earlier (Lerner et al. 2007). All subjects had normal neurological examinations. Demographic information include the following: 12 subjects were right-handed and 2 were left-handed and nobody was a smoker; as to educational level, 1 person had high school, 8 had college, and 5 were in graduate schools. The study was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke, and all subjects gave their informed consent.
Conditions
There were 2 experimental conditions: 1) spontaneous blinking at rest, when subjects were allowed to blink, and 2) suppression of blinking at rest; each condition lasted 60 s. We instructed subjects to perform suppression of blinking, and in case of accidental blinks, we told them to return immediately to the mode of suppression of blinking. The suppression of blinking condition had 2 measures: 1) objective, the observation of number of blinks, and 2) subjective, namely subjects’ feedback of their performance and evaluation of urge as either present or absent. All subjects experienced urge to blink. The majority did not blink at all or blinked no more than 2 times during the 60-s period; on only few occasions, subjects blinked more than twice, and a few subjects experienced some discomfort during suppression of blinking.
The final data are the sum of 14 positron-emission tomography (PET) scanning sessions (1 for each subject) during which subjects were suppressing blinking and experienced the urge to blink. The experimental paradigm consisted of 3 scans with suppression of blinking and 3 scans with spontaneous blinking when blinking was allowed; these conditions were administered in a pseudorandomized order. At the beginning of each scan marked by injection of intravenous (i.v.) bolus of [15O]H2O, subjects were instructed to either start suppression of blinking or to blink spontaneously and continue for 60 s, which is the duration of the PET scan; the next scan would occur after the rest period of 9 min. Eleven subjects underwent the study in the early morning, and 3 in the early afternoon.
PET Procedure
Because we used the same method as in the previous paper (Lerner et al. 2007) to acquire, process, and analyze the PET data, we abbreviated the description of PET procedure and analysis sections. Subjects were scanned using a General Electric Advance Scanner in 3D acquisition mode; 35 contiguous slices were obtained with 4.25-mm plane separation and 15-cm field of view. The 3D resolution of reconstructed PET images resulted in a matrix size of 128 × 128 and a final voxel size of 2 × 2 × 4.25 mm; spatial resolution of raw PET images was 6–7 mm full width at half maximum (FWHM). All subjects received an i.v. bolus of 10 mCi of [15O]H2O in 10-min intervals. The distribution of cerebral radioactivity was measured in a 60-s emission scan following the bolus injection.
Data Analysis
Image processing and analysis were performed on Sun Unix workstation (Sun Microsystems Solaris 7, Palo Alto, CA), using Statistical Parametric Mapping SPM2 implemented in Matlab. The images were realigned to the first volume. The resliced volumes were normalized to a standard PET template based on the Montreal Neurological Institute reference brain (Ashburner and Friston 1999) in Talairach space (Talairach and Tournoux 1988). The normalized images of 2 × 2 × 2 mm3 voxels were smoothed with 12-mm FWHM isotropic Gaussian kernel. Global Cerebral Blood Flow (CBF) was adjusted to an arbitrary value of 50, and an effect of the different global CBF values in different scans was removed by analysis of covariance. We performed 2 analyses using contrasts: 1) suppression of blinking minus spontaneous blinking and 2) spontaneous blinking minus suppression of blinking. For all analyses, height threshold was set at P = 0.05 false discovery rate corrected for multiple comparisons.
Results
Brain Areas Activated during Suppression of Blinking
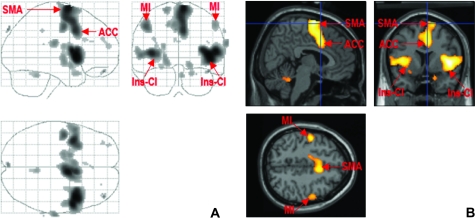
The analysis of the contrast suppression of blinking minus spontaneous blinking showed robust activation in the bilateral insular-claustrum regions, right more than left. On the right side, maximal activation was seen in the midinsula and the large cluster of activity also extended into the right precentral gyrus, opercular area (area 6), and motor area 4; on the left side, maximum activity was seen in midclaustrum and midinsula and activity extended into the left temporal operculum (area 22). Activation was also found in the anterior cingulate cortex (ACC), area 24, right more than left, bilateral primary motor cortex, face area, and SMA, (Figs 1 and 2; Table 1).
Figure 1.
Panel (A): glass brain shows all brain areas activated during suppression of blinking (contrast: suppression minus spontaneous blinking). Panel (B): suppression of blinking: activations are seen in bilateral insular-claustrum regions (Ins-CL), right more than left; ACC, area 24, bilateral primary motor cortex, face area (M1), and SMA.
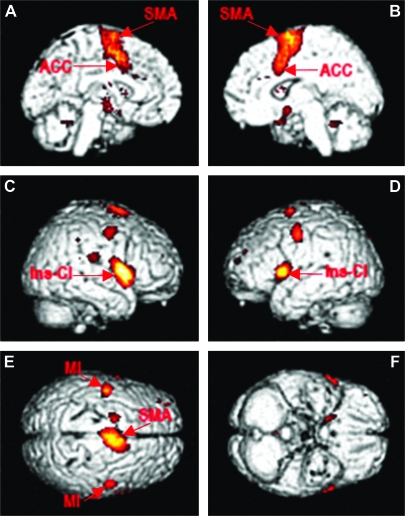
Figure 2.
The panels (A–F) show cortical areas activated during suppression of blinking: left and right anterior cingulate and SMA (panel A and B), left and right insula-claustrum and primary motor cortex (panel C and D); SMA and both motor areas M1 (panel E). For both Figures 1 and 2, activations are statistically significant at P = 0.05 false discovery rate corrected for multiple comparison.
Table 1.
Brain areas activated during the suppression of blinking as compared with spontaneous blinking
| Cluster size | Regions (Brodmann areas) | x | y | z | Z value |
| 1872 | Right insula and claustrum, with maximum in midinsula | 40 | 4 | 3 | 5.52 |
| Precentral gyrus, opercular area (areas 4 and 6) | 48 | 1 | 9 | 5.28 | |
| 2197 | Right superior frontal gyrus (area 6) | 8 | −5 | 63 | 5.21 |
| Right and left anterior cingulate (area 24) | 6 | 4 | 35 | 4.75 | |
| 989 | Left claustrum and insula, with maxima in midclaustrum and midinsula | −33 | 3 | 5 | 4.90 |
| Left operculum/superior temporal gyrus (area 22) | −47 | 4 | 3 | 4.03 | |
| 293 | Left primary motor cortex (area 4) | −40 | −11 | 40 | 4.30 |
| 93 | Right superior temporal gyrus (area 42) | 62 | −25 | 18 | 3.86 |
| 95 | Left frontal dorsal gyrus (area 6) | −15 | −3 | 61 | 3.76 |
| 160 | Right primary motor cortex (area 4) | 48 | −7 | 43 | 3.70 |
Note: cluster size = number of voxels; x, y, and z = stereotaxic coordinates in Talairach space.
Brain Areas Deactivated during Suppression of Blinking
Significant deactivation during blinking suppression (contrast: spontaneous blinking minus suppression of blinking) was seen in the bilateral occipital cortices, areas 17 and 19 and precuneus (Table 2).
Table 2.
Brain areas deactivated during the suppression of blinking as compared with rest with open eyes
| Cluster size | Regions (Brodmann areas) | x | y | z | Z value |
| 7481 | Right superior occipital gyrus (area 19) | 18 | −67 | 21 | 5.04 |
| Left and right cuneus (area 17) | −10 | −69 | 12 | 4.84 | |
| Right, left precuneus (area 7) | 11 | −54 | 32 | 4.78 | |
| 851 | Right medial occipital gyrus (area 19) | 43 | −67 | 19 | 4.35 |
| 237 | Left fusiform gyrus (area 37) | −47 | −60 | −12 | 3.62 |
| 142 | Left medial occipital gyrus (area 19) | −41 | −75 | 15 | 3.34 |
Note: cluster size = number of voxels; x, y, and z = stereotaxic coordinates in Talairach space.
Discussion
Neural Correlates of Suppression of Blinking
We identified strong activation of 4 structures during the suppression of the urge to blink: bilateral insula-claustrum region, primary motor cortex, face areas, ACC, and SMA. The new, most striking finding of our study is bilateral robust activation of the insular-claustrum regions during suppression of blinking. We think that activation of bilateral primary motor cortex, face area, and SMA might be explained by activation of motor circuitry involved in maintaining forced opening of eyes to suppress blinking, which involved muscles of the upper face, in particular, the frontalis muscle and levator palpebrae. Activation of the ACC could be due to few factors: 1) activation of motor areas subserving facial muscles, namely rostral cingulate motor area (M3) (Morecraft et al. 2004; Sohn et al. 2004), and 2) attention to task and performance monitoring; areas 24 and 32 have been implicated in attention (Vogt et al. 1992), cognitive control (Wyland et al. 2003; Anderson et al. 2004), and behavior including suppression (Braver et al. 2001; Liddle et al. 2001). Additionally, some activation of the ACC and insula can be attributed 3) to the unpleasantness of the experience as it was noted in previous pain-evoking studies (Peyron et al. 2000; Rainville 2002); however, only on few occasions subjects reported some discomfort during suppression condition. Furthermore, during pain experience there is usually prominent activation of secondary somatosensory area (SII), which was absent in our results. We could distinguish between these regions because, even though SII lies in the close proximity of insula and occupies the lower aspect of the parietal operculum, the most caudal activation of the insula occurs at y-axis = 0 of Montreal Neurological Institute and y = −3.3 in Talairach space, which is clearly anterior to the anterior parts of the parietal operculum and SII (Talairach and Tournoux 1988; Eickhoff et al. 2006). Finally, ACC could contribute and participate in the experience of urge and its suppression together with insula because both these structures are anatomically and functionally integrated in control of emotional, motivational, and autonomic states. The ACC receives similar interoceptive input as does the insula (Craig 2002; Critchley et al. 2003) and participates in visceromotor control (Kaada and Jasper 1952; Devinsky et al. 1995; Critchley 2005). The dorsal ACC was shown to participate in the control and integration of peripheral autonomic responses during volitional and cognitive as well as noncognitive behaviors (Critchley et al. 2003; Critchley 2005).
Deactivation in our study was seen mainly bilaterally in the cuneus (area 17), occipital cortex (area 19), and precuneus (area 7). Deactivation of the primary and secondary visual cortices in the contrast, spontaneous blinking minus suppression of blinking, could be due to differential activation of visual cortices in both conditions, with longer time of light exposure occurring in the suppression condition. Also, many subjects kept their eyes wide open to suppress blinking; this could contribute to greater activation of visual cortices in the blinking suppression condition when compared with rest. Deactivation of precuneus is a common finding in functional imaging studies where an active condition is compared with a rest condition (Gusnard et al. 2001). Precuneus and occipital cortex, area 19, belong to the network of brain structures that shows task-independent decreases in signal during such paradigms. It has been postulated that these structures are functionally active during the rest condition and are probably involved in continuous processing of information from the world around us. Thus, goal-oriented action of the task condition would attenuate this resting state activity (Gusnard et al. 2001).
Two previous studies of blink inhibition showed activation of bilateral orbitofrontal areas (Tsubota et al. 1999) and medial/superior frontal, precentral, cingulate, and superior temporal gyrus (Chung et al. 2006). In the first study, these somewhat different results possibly can be explained by the small number of subjects (6) and different paradigm (rest with eyes closed was used as a baseline). The second study used only 20- to 30-s blink inhibition, which may not be sufficiently strong to engage the whole network of urge and suppressive mechanisms.
A possible weakness of this study is a lack of correlation analysis with behavioral ratings, which potentially could even better distinguish degree of involvement of particular neural components of urge–suppression experience. However, our emphasis in this experimental paradigm was to identify brain areas responsible for suppression of the natural urge, and use of behavioral data carries sometimes undesired bias of subject's lack of insight and/or lack of concentration on the task. In addition, we attempted to avoid any mental preprocessing of behavioral components so as not to engage other cortical areas and thus acquire a pure substrate of urge–suppression experience.
Insula, a Visceral Homunculus
Suppression of natural urges is a frequent phenomenon in our culture. Deeply ingrained knowledge of appropriateness of social rules and behaviors forces us to suppress, postpone, and delay execution of physiological urges. Suppression is generally associated with some discomfort and stress. The mechanism of suppression must include a structure or network of structures in the brain, which 1) senses the urge, 2) evaluates appropriateness of the behavior in given circumstances according to the situation, and 3) makes and executes a decision about suppressing or releasing the behavior. The insula, by virtue of its anatomical organization and pattern of connectivity, seems to be in an ideal position to function in the role of sensor and executor of physiological urges and together with other paralimbic areas, in particular, ACC, is thought to be functionally responsible “for behaviors which require integration between extrapersonal stimuli and internal milieu” (Mesulam and Mufson 1982a).
Functional division of insula into an anterior part that has autonomic–olfactory–gustatory function and a posterior part that is specialized for auditory–somesthetic–skeletomotor function is reflected in their patterns of connectivity (Mesulam and Mufson 1982b). The anterior insula is connected to the paralimbic areas, olfactory, and gustatory cortex and autonomic centers of the brainstem, whereas the posterior insula has reciprocal connections with auditory, somatosensory, and paramotor cortices.
The influence that the insula has on the function of internal organs was first noticed during stimulation of the insular cortex, which elicited cardiovascular, respiratory, and gastric motor responses (Kaada and Jasper 1952; Penfield and Faulk 1955). The insula was also shown to be involved in conscious experience of the status of internal organs and proposed to be a cortical site for integrated interoception where information about all bodily sensations converges. These sensations include pain, temperature, local metabolic states (pH, hypoxia, hypoglycemia, hypo-osmolarity), itch, and sensual touch, which are carried by the lamina 1 spinothalamocortical pathway, whereas visceral and gustatory sensations are carried by the nucleus and tractus solitarius (Craig 2002; Saper 2002). Neuroimaging and neurophysiological studies have identified cortical representation in insula of lung and heart (King et al. 1999), baroreceptors (Zhang et al. 1998), upper and lower digestive tract (Drewes et al. 2006), and taste sensation (Yaxley et al. 1990). This evidence confirms earlier suggestions (Mesulam and Mufson 1982b) that the insula is an integration center for visceral sensory and motor function. In fact, the insula probably could be called a visceral sensory and motor homunculus, the name that was first used to describe viscerotopic representation of pain sensation in the gastrointestinal tract in the insula (Drewes et al. 2006). As such, insula seems to serve as an interface between the external world with its emotional and cognitive aspects and bodily function. This exchange of information probably operates in both directions: not only are function and dysfunction of inner organs first consciously perceived and experienced at the level of the insula (King et al. 1999; Verne et al. 2003; Kuhtz-Buschbeck et al. 2005; Drewes et al. 2006) but also psychological and emotional circumstances might affect the insula to modify the function of internal organs. The insula could be a site where thoughts and emotions influence health or disease of inner organs. The insula has been postulated to participate in stress-induced cardiovascular dysfunction (Cechetto 1994) and was shown to affect neural–cardiac coupling in threat-evoked anxiety (Dalton et al. 2005) and to modulate effect of emotions on visceral perception (Phillips et al. 2003). This evidence might suggest that function of inner organs can be consciously affected and modified.
However, it should be stated that interoception is just one of the large spectrum of insular functions. Insula has been implicated in emotional processing (Phillips et al. 1997; Damasio et al. 2000; Schienle et al. 2002) and executive control (Dove et al. 2000), anxiety (Paulus and Stein 2006; Stein et al. 2007), speech (Ackermann and Riecker 2004), singing (Perry et al. 1999), and drug craving and addictions (Wang et al. 1999; Kilts et al. 2001; Brody et al. 2002; Naqvi et al. 2007).
Proposed Model of Insular Interoceptive Function
Our proposed model of the insula as a central structure responsible for control, release, and suppression of spontaneously developing urges can be even better appreciated considering it within the broader scope of interoceptive functions (Craig 2002, 2003; Critchley et al. 2004). According to this model, the insula is a sensor, which registers the urge to blink (due in part to somatosensory input), processes the information to follow the instruction “not to blink” in spite of the strong urge, and then executes the suppression of blinking. During this suppression, which is in essence a motor to prevent the blinking, the insula probably recruits motor cortices through the activation of insular ascending projections to the areas controlling muscles of the upper face, including the ACC (M3) (Morecraft et al. 2004; Sohn et al. 2004) and SMA (M2). These regions then engage upper facial muscles to achieve forced and prolonged eye opening. We think that a similar mechanism involving 3 aspects of insular function, namely sensor, processor, and executor, could be active in suppressing other urges, such as the urge to breathe (Banzett et al. 2000) and urge to smoke. As reported by Naqvi et al. (2007), insular damage lead to cessation of smoking addiction; we hypothesize that the functional area of sensing the urge to smoke was destroyed.
The ACC probably also contributes to and participates in the control and suppression of natural urges, in particular in the motivational aspect of suppression. A similar conceptual model has been presented by Craig (2002) who suggested that the insula and ACC can be viewed as limbic sensory and motor cortices, respectively. In this model, the insula would have a role of “feeling the urge” and ACC would act upon the “urge as motivation.” Their joint activation would be required for monitoring and controlling spontaneous behaviors. The predominance of activation of the right insula and ACC in our experiment is consistent with the theory of lateralization of perception and processing of internal stimuli (urge to blink and its suppression) activating higher order rerepresentation of sympathetic homeostatic system and associated with stress response as proposed by Craig (2005).
Conclusions
Possible Role of Insula in Suppressive Mechanisms
We propose that insula probably together with ACC constitutes a neural substrate for the control and suppression of physiological urges. Additionally, we suggest that it is possible that the role of insula and ACC in suppressive phenomena extends beyond regulation of physiological urges and includes control of addictive behaviors (urge to smoke and cocaine craving) (Brody et al. 2002; Gray and Critchley 2007; Naqvi et al. 2007), behavioral inhibition (Garavan et al. 1999), and cognitive control of thoughts and thought suppression (Wyland et al. 2003; Anderson et al. 2004) and affects modulation (Ochsner et al. 2002; Phan et al. 2005). There is increasing evidence of insular involvement in neuropsychiatric disorders where suppressive mechanisms fail such as Obsessive Compulsive Dissorder (Pujol et al. 2004) and phobias (Rauch et al. 1995), Tourette syndrome (Weeks et al. 1996; Bohlhalter et al. 2006; Lerner et al. 2007), Attention Deficit Hyperactivity Disorder (Tian et al. 2006), and schizophrenia (Kim et al. 2003).
Funding
Intramural Research Programs of the National Institute of Neurological Disorders and Stroke and the National Institute of Mental Health with the National Institutes of Health.
Acknowledgments
The authors thank the staff of the National Institutes of Health PET Department for successful completion of the scanning studies and Devera Schoenberg, MSc, for editorial work. Conflicts of interest: None declared.
References
- Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89:320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JD. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, Frackowiak RS, Fowler CJ. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11:2117–2120. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, Bertolino A, Callicott JH, Weinberger DR, Mattay VS. Brain regions underlying response inhibition and interference monitoring and suppression. Eur J Neurosci. 2006;23:1658–1664. doi: 10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, Wurzman R, Hallett M. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Cechetto DF. Identification of a cortical site for stress-induced cardiovascular dysfunction. Integr Physiol Behav Sci. 1994;29:362–373. doi: 10.1007/BF02691356. [DOI] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Yoon HW, Song MS, Park H. Event related fMRI studies of voluntary and inhibited eye blinking using a time marker of EOG. Neurosci Lett. 2006;395:196–200. doi: 10.1016/j.neulet.2005.10.094. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. J Cogn Neurosci. 2005;17:969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Drewes AM, Dimcevski G, Sami SA, Funch-Jensen P, Huynh KD, Le Pera D, Arendt-Nielsen L, Valeriani M. The “human visceral homunculus” to pain evoked in the oesophagus, stomach, duodenum and sigmoid colon. Exp Brain Res. 2006;174:443–452. doi: 10.1007/s00221-006-0480-0. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum 2: Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54:183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Kaada BR, Jasper H. Respiratory responses to stimulation of temporal pole, insula, and hippocampal and limbic gyri in man. AMA Arch Neurol Psychiatry. 1952;68:609–619. doi: 10.1001/archneurpsyc.1952.02320230035004. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Youn T, Lee JM, Kim IY, Kim SI, Kwon JS. Morphometric abnormality of the insula in schizophrenia: a comparison with obsessive-compulsive disorder and normal control using MRI. Schizophr Res. 2003;60:191–198. doi: 10.1016/s0920-9964(02)00306-7. [DOI] [PubMed] [Google Scholar]
- King AB, Menon RS, Hachinski V, Cechetto DF. Human forebrain activation by visceral stimuli. J Comp Neurol. 1999;413:572–582. [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Junemann KP. Cortical representation of the urge to void: a functional magnetic resonance imaging study. J Urol. 2005;174:1477–1481. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Boudreau EA, Hanakawa T, Pagan F, Mari Z, Bara-Jimenez W, Aksu M, Garraux G, Simmons JM, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007;68:1979–1987. doi: 10.1212/01.wnl.0000264417.18604.12. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey 1: architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982a;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey 3: efferent cortical output and comments on function. J Comp Neurol. 1982b;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. The motor cortex and facial expression: new insights from neuroscience. Neurologist. 2004;10:235–249. doi: 10.1097/01.nrl.0000138734.45742.8d. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Penfield W, Faulk ME., Jr The insula; further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- Perry DW, Zatorre RJ, Petrides M, Alivisatos B, Meyer E, Evans AC. Localization of cerebral activity during simple singing. Neuroreport. 1999;10:3979–3984. doi: 10.1097/00001756-199912160-00046. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Gregory LJ, Cullen S, Coen S, Ng V, Andrew C, Giampietro V, Bullmore E, Zelaya F, Amaro E, et al. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain. 2003;126:669–684. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L, Breiter HC, Fischman AJ, Manzo PA, Moretti C, Jenike MA. A positron emission tomographic study of simple phobic symptom provocation. Arch Gen Psychiatry. 1995;52:20–28. doi: 10.1001/archpsyc.1995.03950130020003. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, Sammer G, Vaitl D. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. 2002;13:2023–2026. doi: 10.1097/00001756-200211150-00006. [DOI] [PubMed] [Google Scholar]
- Seseke S, Baudewig J, Kallenberg K, Ringert RH, Seseke F, Dechent P. Voluntary pelvic floor muscle control—an fMRI study. Neuroimage. 2006;31:1399–1407. doi: 10.1016/j.neuroimage.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Voller B, Dimyan M, St Clair Gibson A, Hanakawa T, Leon-Sarmiento FE, Jung HY, Hallett M. Cortical control of voluntary blinking: a transcranial magnetic stimulation study. Clin Neurophysiol. 2004;115:341–347. doi: 10.1016/j.clinph.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart, Germany: Georg Thieme; 1988. [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, et al. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400:39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Kwong KK, Lee TY, Nakamura J, Cheng HM. Functional MRI of brain activation by eye blinking. Exp Eye Res. 1999;69:1–7. doi: 10.1006/exer.1999.0660. [DOI] [PubMed] [Google Scholar]
- Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Weeks RA, Turjanski N, Brooks DJ. Tourette's syndrome: a disorder of cingulate and orbitofrontal function? QJM. 1996;89:401–408. doi: 10.1093/qjmed/89.6.401. [DOI] [PubMed] [Google Scholar]
- Wyland CL, Kelley WM, Macrae CN, Gordon HL, Heatherton TF. Neural correlates of thought suppression. Neuropsychologia. 2003;41:1863–1867. doi: 10.1016/j.neuropsychologia.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls ET, Sienkiewicz ZJ. Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol. 1990;63:689–700. doi: 10.1152/jn.1990.63.4.689. [DOI] [PubMed] [Google Scholar]
- Zhang H, Reitz A, Kollias S, Summers P, Curt A, Schurch B. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. Neuroimage. 2005;24:174–180. doi: 10.1016/j.neuroimage.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Dougherty PM, Oppenheimer SM. Characterization of baroreceptor-related neurons in the monkey insular cortex. Brain Res. 1998;796:303–306. doi: 10.1016/s0006-8993(98)00268-6. [DOI] [PubMed] [Google Scholar]