Abstract
It is often assumed that neural activity in face-responsive regions of primate cortex correlates with conscious perception of faces. However, whether such activity occurs without awareness is still debated. Using functional magnetic resonance imaging (fMRI) in conjunction with a novel masked face priming paradigm, we observed neural modulations that could not be attributed to perceptual awareness. More specifically, we found reduced activity in several classic face-processing regions, including the “fusiform face area,” “occipital face area,” and superior temporal sulcus, when a face was preceded by a briefly flashed image of the same face, relative to a different face, even when 2 images of the same face differed. Importantly, unlike most previous studies, which have minimized awareness by using conditions of inattention, the present results occurred when the stimuli (the primes) were attended. By contrast, when primes were perceived consciously, in a long-lag priming paradigm, we found repetition-related activity increases in additional frontal and parietal regions. These data not only demonstrate that fMRI activity in face-responsive regions can be modulated independently of perceptual awareness, but also document where such subliminal face-processing occurs (i.e., restricted to face-responsive regions of occipital and temporal cortex) and to what extent (i.e., independent of the specific image).
Keywords: fMRI, fusiform face area, implicit memory, priming, subliminal perception
Introduction
There is now considerable evidence that face processing involves a circumscribed set of brain regions within occipitotemporal cortex, as evidenced by single-cell recording in primates (Perrett et al. 1982), intracranial recording in humans (McCarthy et al. 1999) and neuropsychological deficits such as prosopagnosia (De Renzi 1986). Human functional imaging studies have localized these face-sensitive regions to lateral occipital cortex (LOC), superior temporal sulcus (STS) and, more selectively, part of the middle fusiform gyrus, which has been labeled the fusiform face area (FFA) (Kanwisher et al. 1997). Indeed, the FFA shows a high degree of domain specificity (Grill-Spector et al. 2004; Tsao et al. 2006), responding more strongly to faces than any other type of stimulus yet found (Kanwisher 2006; Kanwisher and Yovel 2006).
Whether activity in such face-responsive regions always correlates with the perceptual awareness of faces, however, remains unclear. According to some, activity in the ventral stream constitutes the neural correlate of visual consciousness (Milner and Goodale 1995; Fang and He 2005). Accordingly, several studies have found that brain activity in face-responsive regions like the FFA shows a strong correlation with the detection or identification of faces under normal viewing conditions (Grill-Spector et al. 2004), during binocular rivalry (Tong et al. 1998), identification of ambiguous figures (Kleinschmidt et al. 1998) and the recognition of masked objects (Bar and Biederman 1999). Moreover, the FFA is responsive to the subjective experience of faces induced by other objects (e.g., houses), rather than face stimuli per se (Summerfield et al. 2006). As a consequence, a common interpretation of this literature is that neural activity in the FFA and face awareness go hand-in-hand, such that face-selective activity in FFA might be thought not to occur without awareness of a face.
However, although perceptual awareness of faces may imply modulation of neural activity in face-responsive regions, modulation of neural activity in such regions may not imply perceptual awareness. In other words, neural activity in these regions may be necessary but not sufficient for perceptual awareness of faces. Moreover, it is possible that neural activity in some of these face-responsive regions (e.g., FFA) implies perceptual awareness of a face, but activity in others (e.g., OFA) can occur without such awareness.
Some studies have reported FFA activity when attention is drawn away from a face, such that it cannot be reported anymore. For instance, residual FFA activity has been observed for extinguished faces in neglect patients (Rees, Wojciulik, et al. 2002) and, in normal participants, for unnoticed face changes (Beck et al. 2001). Further functional magnetic resonance imaging (fMRI) studies have found evidence for greater FFA activity for faces than houses when they are supposedly ignored (e.g., Henson and Mouchlianitis 2007). However, others have not (e.g., Furey et al. 2006), and a persistent problem is whether such manipulations of attention are sufficiently strong to prevent limited or occasional attention (and awareness) of ignored faces, for example, whether the attentional “load” is high enough (Rees et al. 1999; Pessoa et al. 2002; Yi et al. 2004). A 2nd problem of course is the difficulty in claiming no face-specific processing of unattended stimuli, particularly given the variable sensitivity of different fMRI analysis techniques (Haynes and Rees 2005).
Other studies have used the binocular rivalry paradigm, where awareness for the unattended stimulus can be indicated directly. Although activity for unperceived faces has been found in the amygdala, in particular in relation to emotional face processing (Pasley et al. 2004; Williams et al. 2004), activity in the FFA is almost entirely eliminated (see Tong et al. 2006, for a recent review). Indeed, studies have found increased activity for faces relative to houses in the FFA for the attended rivalrous stimulus, but not for the unattended stimulus (Tong et al. 1998; Pasley et al. 2004). One notable recent exception to this view used continuous flash suppression, which allows longer suppression of unattended percepts. Although FFA activity was drastically reduced for unattended faces (relative to scrambled faces), it could still be reliably observed (Jiang and He 2006). Interestingly, participants in this study showed no evidence of being able discriminate real from scrambled faces when suppressed, contrary to the general pattern from standard rivarly experiments (Tong et al. 2006). However, it remains unclear whether the residual FFA activity results from longer processing of the unattended faces compared to classical methods of binocular rivalry, or whether it reflects the use of a different control stimuli (i.e., scrambled images rather than houses), and thus a domain-general effect of object recognition rather than face-processing per se.
Thus the data from attentional and rivalry paradigms are mixed on the question of whether face-related activity in face-responsive regions can occur without awareness. Importantly, participants in such studies are distracted from perceiving a stimulus that would be perfectly visible if attention was drawn toward it. This aspect is crucial to recent neurobiological accounts of consciousness, which assume that perception without attention and perception without awareness (i.e., subliminal perception) involve qualitatively different processes (Koch and Tsuchiya 2007; Kouider and Dehaene 2007): Although subliminal stimuli genuinely induce unconscious processing, perception without attention is closer to conscious perception (Dehaene et al. 2006), if not a certain form of “phenomenal” conscious perception on its own (Block 2005; Lamme 2006). The only study of which we are aware that found face-specific FFA activity in the absence of awareness, without using an obvious attentional manipulation, is that of (Moutoussis and Zeki 2002). This study used binocular fusion, in which 1 eye was shown a red face on a green background whereas the other eye was shown a green face on a red background, such that the overall perception was a yellow color field. We revisit this study in the Discussion.
An alternative paradigm that can render stimuli invisible, and that is quite different from paradigms using attentional/binocular manipulations, is the masked priming method (Kouider and Dehaene 2007). In this paradigm, a stimulus can be made difficult if not impossible to perceive by presenting it briefly, preceded and succeeded by “forward” and “backward” pattern masks—so-called “sandwich masking.” Here, processing of a stimulus in the absence of awareness is inferred from the effects of a masked prime stimulus on the response to a subsequent same or different target stimulus, even when the prime itself is invisible (see e.g., Fig. 1a). Importantly, because the prime is in close spatial and temporal proximity to the target, it will receive the same degree of selective attention as the task-relevant target (Naccache et al. 2002). Another important advantage of this paradigm is that it avoids potential confounds due to the use of different control stimuli, because exactly the same stimuli are contrasted in the primed and unprimed condition.
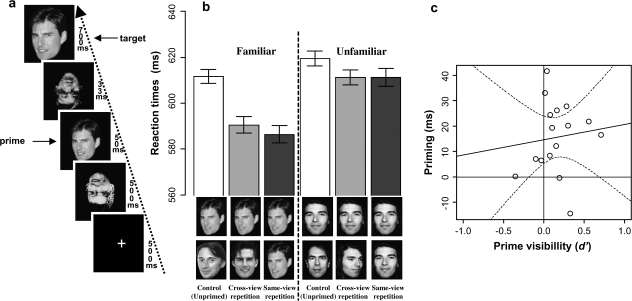
Figure 1.
Schematic description of the subliminal face priming method and behavioral results. (a) Each trial consisted in the sequential presentation of a fixation cross, a forward mask, a prime, a backward mask and the target. Participants were presented with familiar and unfamiliar faces and were instructed to perform a fame-judgment task on the target. Masks were constructed from overlays of inverted faces. (b) Mean reaction times for the 6 priming conditions. The experiment involved a 2 × 3 factorial design including famous and nonfamous target faces preceded by a prime that could depict the same person in the same view (same-view conditions), the same person in a different view (cross-view conditions) or a different person (control condition). (c) Regression of priming on prime visibility. Each data point represents a participant. The regression functions (dotted lines indicate 95% confidence intervals) show the association between the global priming effect found for famous faces and prime visibility. Priming is interpreted as subliminal when the curve representing the lowest value in the confidence interval passes above the origin.
This paradigm has been used previously in conjunction with fMRI to measure subliminal processing of words. Analogous to the tight association between faces and the FFA, evidence suggests a tight association between processing of legal orthographic strings and the “visual words form area” (VWFA), also in fusiform gyrus (Cohen et al. 2000; Cohen and Dehaene 2004). A reduction in VWFA activity, as well as in some other occipito-temporal regions, has been found to accompany masked word priming (Dehaene et al. 2001; Kouider et al. 2007). Furthermore, this reduction in VWFA activity is unaffected by whether the prime and target are in the same or different cases (e.g., radio–radio vs. radio–RADIO), implying that this region achieves orthographic invariance in the absence of awareness (Dehaene et al. 2001, 2004).
To investigate whether and to what extent face-processing can occur without awareness we used a novel masked priming paradigm similar to the one used for words, but optimized for faces (Fig. 1a). To pre-empt, we find that neural activity in several ventral and lateral temporal regions, as measured by fMRI, is modulated by the prime-target relationship, and that this modulation cannot be explained by awareness of the prime. All the classic face-responsive regions (i.e., FFA, OFA, and STS) show this effect. Moreover, this modulation of activity, at least in FFA and OFA, is not restricted to the same face image, but generalizes across different photographs of the same person; nor is it restricted to faces for which the participant is pre-experimentally familiar. These data confirm not only that activity in face-responsive regions need not imply awareness of a face, but also document where such processing can occur, and to what extent.
Finally, we contrast these data from masked priming with those from a long-lag priming, a paradigm using visible primes and commonly employed in implicit memory research (Henson 2003). Data from the long-lag priming paradigm have revealed reduced fusiform responses to primed faces relative to unprimed faces, at least when the faces are familiar (Henson et al. 2000). However, although such paradigms are used to measure behavioral evidence of “implicit” (unconscious) memory, by virtue of the fact that the instructions make no reference to the primes at the time of presentation of the targets, it is likely that imaging data from these paradigms include effects of (incidental) conscious memory for the primes (Naccache and Dehaene 2001; Henson 2003). We therefore contrast fMRI data from this paradigm, when participants consciously perceive both prime and target, with those from the masked priming paradigm in which participants have minimal awareness of the prime. We show that, unlike the masked paradigm, the long-lag priming paradigm produces repetition-related activity reductions that extend beyond occipitotemporal cortex, in addition to repetition-related increases in more dorsal regions.
Materials and Methods
Participants
A total of 16 right-handed British volunteers gave written consent to participate in the study (8 female, mean age 23 ± 2 years). All volunteers reported themselves to be in good health, with no history of neurological illness. The study was approved by the Joint Ethics Committee of the National Hospital and Institute of Neurology, London.
Experimental Protocols
Participants took part in 4 successive phases during the same day: masked priming (3 fMRI sessions of ∼10 min each), face-localizer (1 fMRI session of ∼7 min), long-lag priming (1 fMRI session of ∼10 min) and prime visibility measure (no fMRI acquisition). The entire protocol, instruction and training included lasted about an hour.
During the masked priming phase (Fig. 1a), participants received 480 trials with the following structure: a fixation cross for 500 ms, a 1st (forward) mask for 500 ms, a prime face for 50 ms, a different (backward) mask for 33 ms and finally the target face for 700 ms. The participant's task was to decide, as quickly as possible, whether the target face belonged to a famous or nonfamous (unfamiliar) person, whereas ignoring the preceding strange pictures of scrambled face parts (i.e., the masks). We used this fame-judgment task because it has been shown to produce large behavioral priming effects, at least for famous faces (Ellis et al. 1990; Henson et al. 2002). Participants were not informed about the presence of the primes. They indicated their response with either their left or right index finger, counterbalanced across participants. On each trial, prime faces were from either the same person as the target (primed condition) or a different person (“unrelated” control condition). To avoid response congruity interpretations (Damian 2001), the unrelated prime and the target were always both famous or both unfamiliar (and of the same sex). To investigate the level of unconscious processing of subliminal faces, we also compared same-view repetitions (i.e., same person, same photo) and cross-view repetitions (same person, different photo) (see Fig. 1b). Thus, prime and target corresponded either to the same view (same-view condition), to 2 different views of the same person (cross-view condition) or to different persons (control condition).
Two sets (A and B) of 80 grayscale photographs of faces (half male, half female; half famous; half unknown) were matched for image size, and cropped to show face and hair only. Each participant received only 1 of the 2 sets of faces (set A or set B). This assignment was counterbalanced across participants and allowed us measuring long-lag priming in the long-lag phase (see below). There were 2 different photos of each of the 80 faces, with no explicit control of the differences across the 2 images of each face: The 2 photographs could be taken from different perspectives (though the majority were between frontal and three-quarters views), and involve different facial expressions and/or differences in lighting conditions, or hairstyles.
The photos of the 80 persons appeared, across trials, in each of the 3 priming conditions, and as both a prime and a target (leading to 480 trials in total). The assignment of face images to conditions was thus fully counterbalanced within participants. In addition, in order to avoid potential behavioral or neural confounds related to the masks, each target face was preceded by the same pair of forward and backward masks across the 3 priming conditions. The 80 masks were created by overlaying 4 upside-down faces (half famous, half female). Upside-down faces were chosen because they constitute a better mask than conventional noise masks (Loffler et al. 2005), but they were overlaid to avoid them from being perceived as a face and hence interfering with the task performed on the targets. Brightness reduction (−30%) was applied to the masks such that they would appear with the same brightness as the original faces. In order to minimize pixel overlap with the target face, the prime was scaled to be 80% smaller than the target (masks received the same reduction for masking improvement reasons).
In the 2nd phase of the experiment, face-responsive regions were mapped in each participant by a separate localizer scan comparing images of faces and scrambled faces. A new set of faces was used (half familiar/half unfamiliar), and scrambled versions of these stimuli were created by randomly permuting the Fourier phase information (see e.g., Eger et al. 2005). The contrast of faces versus scrambled faces therefore controls for low-level visual differences, such as the spatial frequency power spectrum (unlike a contrast of faces vs. houses). This contrast also allowed us to isolate other “face-processing” regions (such as the occipital face area, hereafter OFA), which other researchers have argued are also “face-selective” (Rossion, Caldara, et al. 2003; Rossion, Schiltz, et al. 2003; Steeves et al. 2006). Four conditions (familiar faces, unfamiliar faces, scrambled familiar faces, scrambled unfamiliar faces) were presented in a blocked design (12 stimuli/block, each presented for 500 ms with 500 ms inter-stimulus interval, with 6 s of fixation baseline between blocks. Participants performed a 1-back repetition detection task on the stimuli (with 2 stimulus repetitions occurring at random positions within each block).
In the 3rd phase, we measured more conventional long-lag priming using the same stimuli. This was in order to compare any subliminal effects with the more established neural changes associated with repetition of stimuli perceived consciously on their initial occurrence (Henson 2003). Participants made a fame judgment on 160 trials corresponding to 1 of the 2 views of the 80 persons from set A and the 80 persons from set B. For half the participants, faces from set A appeared in the masked priming phase (primed condition), whereas faces from set B did not (unprimed condition). Conversely, for the remaining participants, it was faces from set B that were primed and faces from set A that were unprimed. This design allowed us to measure long-lag repetition priming, although we could only compare same-view repetitions vs. unprimed faces. Which of the 2 views was presented during this test phase was also counterbalanced across participants. Each trial consisted of a fixation cross for 500 ms followed by a target face for 700 ms for fame judgment. Otherwise, the procedure was identical to the masking priming phase.
In the final “prime visibility” phase, participants received stimulus presentation conditions identical to the masked priming phase and had to perform a forced-choice fame-judgment task now on the masked primes rather than on the targets (64 trials). The only difference in the composition of the trials was that, for half of them, the fame of the prime and target faces differed, so that the fame of the target could not be used to infer the fame of the prime. Participants were told that only accuracy mattered and they were not scanned during this phase, although they remained in the scanner to ensure that the visual conditions of the prime visibility phase did not differ from the masked priming phase (see Hannula et al. 2005) This measure was performed after the masked priming phase (rather than before or during) in order to avoid under-estimating visibility due to training and adaptation to the display conditions (see e.g., Kouider and Dehaene 2007) and so that participants need not be alerted the presence of primes prior to the masked priming phase. They also received a practice session with 200 ms prime administered as many times as required to ensure that they understood the prime visibility task. We analyzed the masked and long-lag priming experiments in terms of reaction time (excluding fame-judgment errors and trials with reaction times above 1000 ms).
Basic Analysis Strategy
The same basic analysis was performed on the reaction times, fMRI whole-brain data and functional region of interest (fROI) signal change estimates, and consisted of 2, 2 × 2 repeated-measures analyses of variance (ANOVAs): The 1st one crossed familiarity (familiar vs. unfamiliar) and repetition (unrelated vs. repeated, collapsing across view, or “global priming”). The 2nd ANOVA crossed familiarity with view change (within vs. cross-view repetitions).
fMRI Acquisition
A 3-T Allegra system (Siemens) was used to acquire blood oxygenation level–dependent (BOLD) gradient echoplanar images. For each volume, we acquired 32 2-mm thick slices (64 × 64 × 3 × 3 mm pixels, time echo = 30 ms) with a pitch of 30° up at the front in order to reduce susceptibility artifacts in temporal cortices (Deichmann et al. 2003. The repetition time was of 2080 ms in all 5 sessions. The 4th session (face-localizer) comprised 185 volumes, whereas 285 volumes were acquired during the other sessions.
Event-Related fMRI Analysis
After image reconstruction, the functional images were processed using the SPM2 software (Wellcome Department of Cognitive Neurology, London, UK). Five initial volumes were discarded to eliminate nonequilibrium effects of magnetization. Images were realigned (Friston et al. 1996), unwarped (Andersson et al. 2001), normalized to the Montreal Neurological Institute template brain (3 mm voxel resampling) and spatially smoothed with an isotropic Gaussian filter (8 mm full width half maximum). The time series for each voxel was high-pass filtered at 1/128 Hz. Statistics were computed in 2 steps for both types of priming. First, a parameter estimate image for each of the conditions (6 for masked priming and 4 for long-lag priming) was computed by fitting each voxel time series with a time course created by convolving delta functions at the onset of each target with a canonical hemodynamic response function (HRF) and its time and dispersion derivatives (though this model captures the total neural activity induced by the prime, masks and target, given the poor temporal resolution of the BOLD response, any differences between conditions must reflect the prime-target relationship, given that the faces and masks were matched across conditions). In the 2nd step, group-based statistical inferences were made using a random effect model (Friston et al. 1999) and performing the ANOVAs described above on the canonical HRF parameter estimate images of all participants with voxel-wise P < 0.001 and a cluster extent of 20 or more contiguous voxels.
Functional Localizer and fROI Analyses
For the face-localizer, preprocessing and 1st-level statistics were analogous to the priming data, except that only a canonical HRF was used for this block-based design. For each of the 16 participants, we tested for regions showing more activation for real compared to scrambled faces (at a voxel-wise threshold of P < 0.001, uncorrected) and identified the coordinates of the peaks in bilateral occipital and mid-fusiform cortex (OFA and FFA). Using the MarsBar software (http://marsbar.sourceforge.net/), we defined 8-mm spheres surrounding each peak and averaged percent signal change within these participant-specific ROIs (the choice of a 8 mm radius was based on previous experience of the likely spatial scale of activations in such regions of interest).
Results
Behavioral Results
Subliminal Priming
We performed 2 orthogonal 2 × 2 ANOVAs (see “Materials and Methods,” Basic analysis strategy). For the 1st ANOVA, the factors were familiarity (familiar vs. unfamiliar) and “global priming” (unrelated vs. repeated, collapsing across view change). This revealed a significant interaction (F1,15 = 9.81, P < 0.01), with greater priming for familiar than unfamiliar faces. The 2nd ANOVA compared within- and cross-view priming conditions and revealed no significant differences (all Fs < 1), suggesting that masked priming generalizes across view changes. Indeed, planned comparisons showed that priming for familiar faces was significant for both same-view (F1,15 = 21.26, P < 0.0005) and cross-view conditions (F1,15 = 20.76, P < 0.0005), whereas priming for unfamiliar faces was only marginal for same-view (F1,15 = 2.78, P = 0.12) and cross-view conditions (F1,15 = 4.52, P = 0.06). These results suggest that face repetition effects with masked primes are highly reliable for familiar faces, but small and not reliable for unfamiliar faces, consistent with the literature on masked word priming during lexical decision tasks (Forster 1998; Kouider and Dupoux 2001, 2005).
Visibility of the Masked Primes
Debriefing participants before the prime visibility test revealed that none of them noticed the presence of the prime stimuli, nor did they notice repetitions of the same face or person within a trial. The forced-choice fame-judgment task on the primes confirmed that our masking method rendered the primes largely invisible, as performance was close to chance (mean percentage correct = 52.3% (SD = 5.5), mean d′ = 0.16 (SD = 0.25)). Although the mean d′ measure of discriminability was significantly above zero (T(15) = 2.53, P < 0.05, 2-tailed), crucially, priming was still reliable when the prime discrimination task was extrapolated to null performance (i.e., P < 0.005 for the intercept of the regression of priming against d′, Fig. 1c) (see Greenwald et al. 1995; Hannula et al. 2005; Kouider and Dupoux 2005 for justification of this method).
Long-Lag Priming
For the long-lag priming phase (in which there was only 1 primed condition, i.e., same-view), a 2 × 2 ANOVA showed an interaction between familiarity and priming (F1,15 = 43.15, P < 0.0001). In agreement with previous results using this task (Ellis et al. 1990; Henson et al. 2002), long-lag same-view priming was found for familiar faces (82 ms; F1,15 = 45.50, P < 0.0001) but not for unfamiliar faces (4 ms; F < 1).
fMRI Whole-Brain Analyses
Subliminal Priming
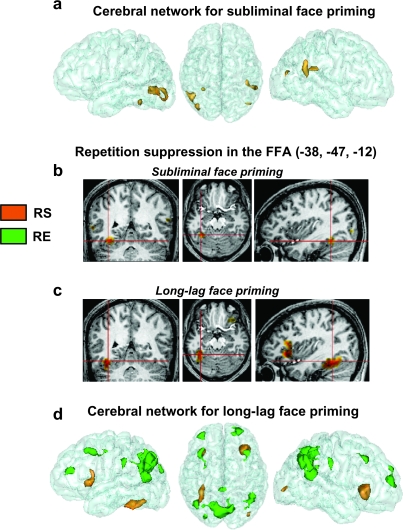
The 1st ANOVA model (see Materials and Methods, Basic analysis strategy) allowed us to identify regions showing a global priming effect (i.e., a difference between unrelated and repeated trials collapsing across view). Global priming effects were found in several regions, though unlike the behavioral data, no region showed a reliable interaction between familiarity and priming. These regions showing a subliminal priming effect were restricted to occipitotemporal cortex, and all showed “repetition suppression” (Grill-Spector et al. 2006), that is, reduced responses to repeated relative to control conditions (Fig. 2a). They included a region in left, mid-fusiform gyrus (Fig. 2b), as well as a large cluster extending from the LOC to the posterior middle temporal gyrus (Table 1). In the right hemisphere, repetition suppression was found in 3 lateral temporal regions, including posterior middle temporal gyrus and superior temporal gyrus STS. Surprisingly, no effect reached significance in right fusiform or ventral occipital cortices in this whole-brain analysis (though see functional ROI analyses below). Moreover, a 2nd analysis contrasting the 2 primed conditions (i.e., a difference between same- and cross-view repetitions) failed to find any regional differences, suggesting that repetition suppression was not specific to 1 view of a face. We return to these 2 points in the Discussion section.
Figure 2.
Cerebral bases of subliminal and long-lag priming. The neural activity differences related to subliminal priming (a) led to repetition suppression only and were restricted to occipitotemporal areas (in the FFA, MTG, and STS). For long-lag priming, repetition suppression was found in the same FFA cluster, but it also extended to ventral frontal cortex (b and c). Contrary to subliminal priming, long-lag priming showed also repetition enhancement (d).
Table 1.
Results for masked and long-lag priming for the fMRI whole-brain analyses (n = 16)
| Condition | Region | Talairach coordinates |
Z score | ||
| x | y | z | |||
| Masked priming | |||||
| Repetition suppression collapsed across familiarity | Right superior temporal gyrus and sulcus | 59 | −40 | 19 | 4.35 |
| 56 | −40 | 10 | 3.87 | ||
| Left LOC and posterior middle temporal gyrus | −45 | −72 | 9 | 4.25 | |
| −50 | −55 | 6 | 4.11 | ||
| −56 | −64 | 6 | 4.06 | ||
| Left mid-fusiform gyrus | −36 | −50 | −10 | 4.17 | |
| Right superior temporal gyrus | 48 | −28 | 18 | 3.91 | |
| Right posterior middle temporal gyrus | 48 | −69 | 20 | 3.75 | |
| 53 | −72 | 12 | 3.50 | ||
| Long-lag priming | |||||
| Repetition suppression for familiar faces | Right inferior frontal gyrus | 33 | 29 | −9 | 5.57 |
| 42 | 32 | −2 | 4.26 | ||
| Left mid-fusiform gyrus | −39 | -47 | −15 | 5.10 | |
| −39 | −56 | −15 | 4.15 | ||
| −42 | −62 | −7 | 4.01 | ||
| Left inferior frontal gyrus | −36 | 26 | −6 | 4.90 | |
| −39 | 21 | 7 | 3.94 | ||
| Right lateral occipital | 39 | −84 | 2 | 3.73 | |
| Repetition enhancement collapsed across familiarity | Medial posterior parietal and precuneus | −18 | −66 | 25 | 5.30 |
| −3 | −68 | 42 | 5.10 | ||
| −9 | −59 | 53 | 4.72 | ||
| Left frontopolar | −42 | 53 | 3 | 4.48 | |
| Left inferior parietal lobule | −45 | −50 | 47 | 4.24 | |
| −53 | −59 | 36 | 3.22 | ||
| Right frontopolar | 27 | 59 | 11 | 4.08 | |
| 21 | 60 | 25 | 3.93 | ||
| 15 | 64 | 2 | 3.57 | ||
| Right dorsolateral prefrontal | 45 | 28 | 37 | 4.02 | |
| 39 | 20 | 43 | 3.80 | ||
| Right inferior parietal lobule | 48 | −51 | 38 | 3.91 | |
| 48 | −45 | 27 | 3.86 | ||
| 33 | −51 | 36 | 3.43 | ||
| Left dorsolateral prefrontal | −38 | 31 | 37 | 3.89 | |
| −30 | 37 | 34 | 3.57 | ||
| −30 | 13 | 35 | 3.41 | ||
| Right inferior parietal | 50 | −38 | 52 | 3.61 | |
| Posterior cingulate | −3 | −30 | 35 | 3.56 | |
| 3 | −30 | 40 | 3.48 | ||
| Left superior parietal lobule | −33 | −80 | 37 | 3.54 | |
| −30 | −74 | 42 | 3.44 | ||
Note: Clusters that exceed an extend threshold of 20 voxels at P < 0.001, uncorrected, are reported.
Long-Lag Priming
The neuronal bases of long-lag priming differed from those of subliminal priming in 3 major ways. First, long-lag priming led to repetition suppression in several regions, but only for familiar faces, mimicking the behavioral results and in accord with past fMRI studies using this priming method (Henson et al. 2000; Henson et al. 2002, 2003). As there was no repetition suppression for unfamiliar faces, we report below the results of simple effects for familiar faces (note that all regions showing repetition suppression we report below also showed a reliable interaction between familiarity and priming). Repetition suppression was found in left mid-fusiform gyrus and right LOC (Fig. 2c). As can be seen on Figure 2b,c, the left fusiform region found for subliminal priming overlapped with the one for long-lag priming. Secondly, repetition suppression for familiar faces extended beyond occipitotemporal cortex, occurring also in lateral ventral prefrontal cortex bilaterally. Thirdly, priming was not restricted to repetition suppression but also led to repetition enhancement in an extensive set of regions, for both familiar and unfamiliar faces (as confirmed by the main effect of priming, with no region showing a reliable interaction between repetition enhancement and familiarity) (Fig. 2d). These included large clusters in medial and bilateral inferior parietal, bilateral frontopolar and dorsolateral prefrontal cortices, precuneus, and posterior cingulate (see Table 1).
Functional Region of Interest Analyses in Occipitotemporal Cortex
Although our preceding whole-brain analyses are useful to detect differences in neural activity within common anatomical locations across participants (to the extent that brains can be matched on anatomy), they may obscure differences that are functionally common across participants, but expressed in anatomically different (or at least nonspatially overlapping) regions. We therefore examined a subset of brain regions, FFA and OFA, respectively, that were defined in individual participants by the face versus scrambled face contrast in our 2nd, “functional localizer” phase (Eger et al. 2005). When focusing on fusiform and LOC, we managed to identify left and right FFA in 15/16 participants, right OFA in 14/16 participants, and left OFA in 13/16 participants. Differential levels of neural activity for these 4 regions are depicted in Figure 3.
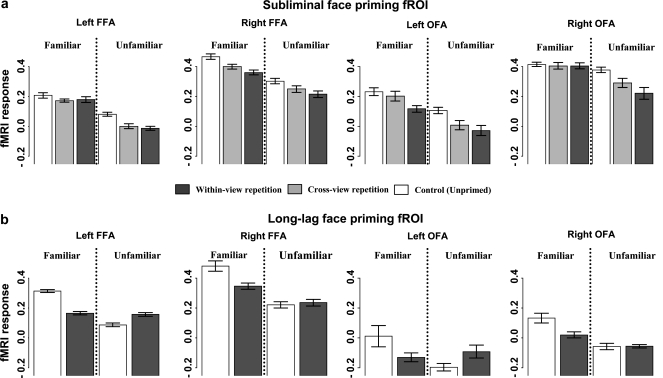
Figure 3.
fMRI response in terms of % signal change for the subliminal and long-lag priming conditions in 4 occipitotemporal regions of interest. Note that zero-value of signal change is arbitrary (only differences between conditions are estimated efficiently in this design).
Subliminal Priming
For the subliminal conditions, region-specific ANOVAs revealed a significant global priming effect (i.e., collapsing across view) in all 4 fROIs (all Ps < 0.03), with no evidence of an interaction with familiarity (all Ps > 0.12). In the 2nd analysis of view effects, no significant difference was found between the within- and cross-view conditions in any fROI, apart from a marginal trend in the left OFA for greater repetition suppression for the same-view condition (P = 0.06). Thus, contrary to the preceding whole-brain analyses, priming effects were found in fusiform and occipital regions of the right, as well as left, hemisphere (suggesting perhaps greater anatomically variability across participants in the right FFA/OFA). This reinforces the importance of allowing for variability in the functional–anatomical mapping across individuals (Friston and Henson, 2006; Saxe et al. 2006).
An omnibus ANOVA that included all 4 fROIs (for those 13/16 participants for which all 4 regions were reliably located), factorized by region, familiarity, and global priming, showed a global priming effect (F1,12 = 8.93, P < 0.02) that did not interact with familiarity, nor with region (all Ps > 0.16). The ANOVA on view effects likewise showed no same-view advantage, nor an interaction with region or familiarity (all Fs < 1). Further post hoc comparisons, collapsing across familiar/unfamiliar, confirmed significant priming for both same-view (F1,12 = 6.86, P < 0.03) and cross-view conditions (F1,12 = 5.54, P < 0.04). In sum, the left and right OFA and left and right FFA showed the same general pattern: repetition suppression for subliminal primes regardless of familiarity and view.
Even though the ability to discriminate the primes was small, the behavioral results suggest that a few participants may have been able to perceive at least a few primes during the subsequent visibility test (Fig. 1c). In order to deal with this possibility, we performed further linear regressions of each participant's global repetition suppression effect against their d′ measure of prime visibility, for each of the 4 occipitotemporal fROIs. There was no reliable relationship in any of the 4 target regions (all Ts < 1). Moreover, when extrapolated to chance-level prime visibility, as we did for the behavioral priming (see Behavioral Results), repetition suppression remained significant in 3 of the fROIs, and still marginally so in the left FFA (all Ps < 0.07). Thus, the occipitotemporal repetition suppression effects associated with masked face priming in this study are likely to reflect genuinely subliminal effects of repetition, supporting the idea that these ventral regions are involved in an unconscious form of perceptual processing.
Long-Lag Priming
For the long-lag manipulation, a similar omnibus ANOVA including all 4 regions showed a marginal priming-by-familiarity interaction (F1,12 = 4.43, P = 0.06), and a marginal priming effect for familiar faces (F1,12 = 3.19, P < 0.10), but no reliable effect for unfamiliar faces (F < 1.5). This pattern of repetition suppression for familiar but not unfamiliar faces is consistent with previous studies of long-lag priming (Henson et al. 2000, 2003). Again, no reliable interactions with region were found (F < 1). Thus again, unlike the masked conditions, repetition suppression in the long-lag conditions, like the behavioral priming, appeared sensitive to face familiarity.
Discussion
Using a novel masked priming paradigm with faces, we found evidence of repetition-related hemodynamic response decreases (i.e., repetition suppression) in several regions of the occipitotemporal cortex. Both whole-brain and individual fROI analyses revealed repetition suppression in fusiform and occipital regions (FFA and OFA) that are believed to play an important role in face processing (e.g., Haxby et al. 2000). These data provide evidence that face processing can occur in face-processing regions within the ventral visual stream in the absence of perceptual awareness. This is because repetition suppression was obtained under conditions where most participants failed to discriminate the masked primes, and even when we took into account the possibility that some participants were conscious of the primes, extrapolating the BOLD data back to zero discriminability still left reliable repetition suppression in most, if not all, of the fROIs.
Measures of Perceptual Awareness
Here, the absence of perceptual awareness was based on participants' difficulty in classifying whether the prime face belonged to a famous person. We chose this fame-classification task to measure awareness because it is the same task (and trial procedure) that was used to measure behavioral priming (and therefore, constitutes a conservative test if participants had access to the same information that supports their behavioral priming). However, 1 potential caveat with this measure is that participants may have had partial awareness of the prime stimulus (e.g., awareness of face parts), which may have caused the neural effects, even if such partial awareness was insufficient to support accurate fame-classification. In other words, it remains possible that our measure underestimated prime awareness (e.g., Kouider and Dupoux 2004) for a discussion of the impact of partial awareness on masked priming]. To deal with this possibility, we ran another behavioral version of this experiment on a new group of participants (N = 11), using the same masked priming method but now followed by a 2-alternative forced-choice (2-AFC) task for the prime, rather than a fame-judgment task. Each trial comprised the same sequence of masks and stimuli as in the priming experiment and, in addition, a pair of choices presented simultaneously after the target, 1 left of fixation and the other right of fixation. One of the 2 alternatives always corresponded to the prime, whereas the other was a different face. Participants were asked to indicate which one corresponded to the prime within the preceding event sequence, by pressing the left button for the face on the left or the right button for the face on the right. The 2 alternatives remained on the screen until a response was made. Because the 2-AFC task can be performed on the basis of face parts, it takes into account the potential influence of partial awareness on priming (see Kouider et al. 2007 for a discussion on the beneficial use of this type of 2-AFC procedures). With this alternative measure of awareness, we still observed the same pattern of results: Priming occurred for both same and different view (both Ps < 0.0001) and to the same extent (interaction: F < 1), and although d′ was again slightly above 0 and significant (d′ = 0.34; F1,10 = 14.58, P < 0.005), the same regression analysis showed that extrapolation to null performance on the 2-AFC task led to a significant intercept of 19 ms (P < 0.0001) and no significant correlation between priming and prime awareness (T < 1). These additional data provide further support for a genuinely subliminal locus of the priming method used in this study.
Unconscious Face Processing, Attention, and the Ventral Visual Stream
Previous research has provided 2 conflicting accounts on the cerebral distinction between conscious and unconscious processes. On the one hand, it has been claimed that the ventral stream conveys visual consciousness, whereas unconscious patterns of neural activity are to be found in parietal areas along the dorsal stream and in subcortical pathways (Milner and Goodale 1995; Ohman 2002; Pasley et al. 2004; Fang and He 2005; Tong et al. 2006). On the other hand, it has been proposed that neural activity in the occipitotemporal cortex can reflect unconscious analysis of visual stimuli (Rees, Kreiman, et al. 2002). According to this latter account, activity in the dorsal stream/parietal cortex reflects, along with late synchronous activation of prefrontal and cingulate areas, the cerebral basis of conscious processing (Dehaene and Naccache 2001; Rees, Kreiman, et al. 2002; Koivisto and Revonsuo 2003; Gross et al. 2004; Koch 2004; Kouider et al. 2007). As explained in the introduction, whereas the literature on visual word recognition provides unequivocal support for the involvement of occipitotemporal/fusiform regions during unconscious perception, the literature on face recognition has not reached the same conclusion. One possible reason for this discrepancy rests on the fact that although studies using words relied on the processing of attended stimuli and masked priming, those on face processing focused primarily on the processing of unattended stimuli, and in particular on binocular rivalry. Yet, as outlined in the Introduction, using conditions of inattention poses 2 major problems.
Firstly, the unattended signal, particularly in binocular rivalry paradigms, can be suppressed in precortical regions as early as the lateral geniculate nucleus (Haynes et al. 2005) which might explain why it is usually totally suppressed in occipitotemporal regions (Tong et al. 2006). One exception to this rule is the recent study by (Jiang and He 2006) using the more promising approach of combining continuous flash suppression and binocular rivalry. They found reduced but still reliable activity in the right FFA. Nevertheless, Jiang and He compared the processing of real versus scrambled images of faces, contrary to other studies that compared faces versus other types of objects (i.e., houses) and found no effect at all (e.g., Tong et al. 1998; Pasley et al. 2004). This aspect is important because although the FFA is more responsive to faces than other objects, it is also more responsive to nonface objects (e.g., cars, houses, etc.) than to nonobjects (e.g., texture patterns) (Grill-Spector et al. 2004). As a result, the residual activity found when comparing face and nonobject stimuli cannot be taken as evidence for unconscious face processing per se. In addition, as acknowledged by the authors, the absence of attention might explain why neural activity associated with recognition of (neutral) faces was still reliable in the FFA but absent in STS. Because the priming approach has the advantage of avoiding potential confounds related to low-level differences with the baseline, given that the same images of faces are used in the primed and unprimed condition, our current data provide strong evidence for face-specific unconscious perception in both the FFA and STS. In addition, the fact that priming obtained even when the view of the face (in the primed conditions) differed between prime and target suggests that faces can be processed in the ventral stream to some level of abstraction. Although some previous studies have reported view-independent priming in the FFA with visible primes (Pourtois et al. 2005), here we show that this is the case even under conditions of invisibility. This is consistent with previous subliminal priming fMRI studies using words (Dehaene et al. 2001, 2004; Devlin et al. 2004; Nakamura et al. 2005; Kouider and Dehaene 2007; Kouider et al. 2007).
Our results are consistent with a previous study by Moutoussis and Zeki (2002), which relied on binocular fusion instead of rivalry (Moutoussis and Zeki 2002). These authors showed that presenting a picture of a red face on a green background to 1 eye, and a picture of the same face in green on a red background to the other eye, makes the face disappear and leads instead to the perception of a uniform yellow field. Under such conditions of fusion, they found an increase in FFA activity for invisible faces compared to invisible houses. As discussed by these authors, the strong correlation usually found between conscious face perception and FFA activity might actually be restricted to binocular rivalry paradigm.
The 2nd problem associated with conditions of inattention is that there are both empirical and theoretical reasons why inattention should not be equated with invisibility. Indeed, there is now mounting evidence that attention and consciousness have distinct neural and functional properties (Koch and Tsuchiya 2007), and that perception without attention is qualitatively different from subliminal perception (Kouider and Dehaene 2007). Indeed, perception without attention has been defined as “preconscious” (Dehaene et al. 2006), an intermediate state between subliminal processing and conscious access, which has also been considered as a certain form of (“phenomenal”) consciousness (Block 2005; Lamme 2006). Thus an important advance of the present study is that evidence for unconscious processing of faces was found even when the critical stimulus (i.e., the prime) was invisible but within the focus of attention (Naccache et al. 2002).
Because subliminal face priming was restricted to the occipitotemporal cortex, our results argue against the claim that unconscious processing of objects/faces is deserved solely by dorsal and/or subcortical pathways (Milner and Goodale 1995; Fang and He 2005; Tong et al. 2006). More specifically, it does not support an account according to which conscious identification of faces is directly related to activity in FFA (Grill-Spector et al. 2004; Kanwisher and Yovel 2006). Although neural activity in ventral steam might be necessary, it does not appear to be a sufficient condition for the perceptual awareness of faces. It remains an open question whether the perceptual awareness of faces arises from early activity and/or local loops within occipito-temporal regions (Lamme 2003; Zeki 2003) or rather through the later involvement of fronto-parietal regions (Crick and Koch 1998; Rees, Kreiman, et al. 2002; Koch 2004). In favor of the latter account, both fMRI (Lumer et al. 1998; Beck et al. 2001) and TransMagnetic Stimulation (TMS) studies (Beck et al. 2006) have reported a strong association between awareness of face changes and dorsal areas in parietal cortex. Yet, as stated earlier, this type of manipulation involves condition of inattention, which may not be sufficient to prevent any form of consciousness (Block 2005; Lamme 2006).
Note that the strong relation between subliminal face processing and occipitotemporal activity might hold only for identification tasks such as the fame decision task used in this study. Indeed, it is plausible that the neural activity induced by subliminal stimuli is not fully automatic but depends, at least to some extent, on the conscious task and strategies adopted by the participants (Dehaene and Naccache 2001; Kouider and Dehaene 2007). Under this perspective, brain regions associated with subliminal face priming might vary, depending on the “automatization” of task-specific neural pathways. As a consequence, with other tasks that are more likely to involve the dorsal pathway (e.g., visuo-motor tasks), it is conceivable that subliminal face priming will be found in dorsal regions. Support for this hypothesis comes from a recent study by Nakamura et al. (2006) who used TMS to disrupt the processing of subliminal primes and found that behavioral priming can be selectively eliminated: applied to middle temporal cortex, TMS removed detectable priming in a recognition task, but crucially not during a naming task. Conversely, TMS applied to the inferior parietal lobe removed priming only in the naming task, suggesting that the cerebral bases of subliminal priming are task-specific, and that they can be found in the dorsal stream.
Subliminal versus Long-Lag Neural Priming
Although long-lag repetition priming effects are sometimes taken to reflect an implicit/unconscious form of memory, their neural correlates can be “contaminated” by explicit/conscious memory processes, even if these do not necessarily affect the concurrent behavioral measure of priming (see Henson 2003). Indeed, it is often assumed that the implicit component of long-lag priming is associated with repetition suppression whereas the explicit component is associated with repetition enhancement. Nevertheless, a direct verification of this assumption is currently lacking (though see Schott et al. 2005). Indeed, a “pure” index of implicit priming, unconfounded by explicit memory contamination, is difficult to achieve (Shanks and St John 1994; Butler and Berry 2001; Berry et al. 2006). One notable exception is priming under subliminal processing conditions, as here, where the relation between the prime and target cannot be explicitly established by the participant.
Consistent with the hypothesis that repetition suppression is associated with implicit processing, we found that subliminal priming was restricted to repetition suppression. Furthermore, the present study showed that, whereas such repetition suppression was confined to occipitotemporal cortex for subliminal priming, long-lag priming showed repetition suppression in both occipitotemporal and ventral prefrontal regions (see Fig. 2). In addition, long-lag priming showed repetition enhancement in a large set of parietal and dorsal–prefrontal regions (see Fig. 2d). Consistent with the hypothesis that repetition enhancement reflects explicit memory contamination in long-lag “priming” paradigms, these fronto-parietal regions have been reported in several studies using explicit memory tasks (e.g., Henson et al. 2002). In particular dorsal regions (e.g., the posterior parietal cortex) have been more recently argued to be directly associated with conscious memory (Shannon and Buckner 2004; Wagner et al. 2005).
Note that we are not assuming here that repetition suppression reflects the same neural mechanisms in masked/subliminal paradigms as the more conventional long-lag/implicit memory paradigms. Because the former results from the processing of invisible stimuli, whereas the latter results from the implicit processing of visible stimuli, the 2 probably originate from different mechanisms. Indeed, the neural response to masked stimuli has been shown to reflect an early component in the feedforward sweep of object processing (e.g., Super et al. 2001; Lamme 2003; Dehaene et al. 2006) whereas, by contrast, repetition suppression in long-lag face priming is believed to reflect later, possibly re-entrant activity in the occipitotemporal cortex (Henson 2003). Different mechanisms might also explain why repetition suppression in the fROIs was found for both familiar and unfamiliar faces under subliminal priming, but only for familiar faces in the long-lag priming.
One unexpected results concerning our subliminal data is the difference between the pattern of repetition suppression in the fROI and the pattern of behavioral priming: as noted above, repetition suppression did not differ for familiar and unfamiliar faces, yet behavioral priming was significantly larger for familiar faces (indeed, only borderline for unfamiliar faces). One possibility is that subliminal repetition suppression reflects short-lived, visual representations of the prime that facilitate perceptual processing of the subsequent target. For familiar faces, this facilitation would speed the fame decision to the target. For unfamiliar faces though, there may be a tendency for any awareness of the facilitation itself (“ease of processing”) to be falsely attributed to the target face being famous (cf. the “false fame effect”; Jacoby et al. 1989). Thus, any speed-up due to perceptual facilitation might be counteracted by interference during the decision process, reducing the amount of priming. Although an appealing hypothesis, this clearly requires further investigation.
In conclusion, our findings show that unconscious face perception can occur in face-responsive regions of the ventral stream, at least when using a fame-classification task, even under conditions of spatial and temporal attention. Our data therefore demonstrate that one can observe activity in face-responsive regions related to invisible stimuli, and therefore such activity need not to necessarily correlate with the conscious perception of face stimuli. Understanding the mechanisms that differentiate conscious and unconscious processing in such ventral (or dorsal) visual-processing pathways remains and important question for future research.
Funding
Fyssen Foundation; UK Medical Research Council (WBSE U.1055.05.012.00001.01); and UK Wellcome Trust.
Acknowledgments
Conflict of Interest: None declared.
References
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Bar M, Biederman I. Localizing the cortical region mediating visual awareness of object identity. Proc Natl Acad Sci USA. 1999;96:1790–1793. doi: 10.1073/pnas.96.4.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DM, Muggleton N, Walsh V, Lavie N. Right parietal cortex plays a critical role in change blindness. Cereb Cortex. 2006;16:712–717. doi: 10.1093/cercor/bhj017. [DOI] [PubMed] [Google Scholar]
- Beck DM, Rees G, Frith CD, Lavie N. Neural correlates of change detection and change blindness. Nat Neurosci. 2001;4:645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- Berry CJ, Shanks DR, Henson RN. On the status of unconscious memory: Merikle and Reingold (1991) revisited. J Exp Psychol Learn Mem Cogn. 2006;32:925–934. doi: 10.1037/0278-7393.32.4.925. [DOI] [PubMed] [Google Scholar]
- Block N. Two neural correlates of consciousness. Trends Cogn Sci. 2005;9:46–52. doi: 10.1016/j.tics.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Butler LT, Berry DC. Implicit memory: intention and awareness revisited. Trends Cogn Sci. 2001;5:192–197. doi: 10.1016/s1364-6613(00)01636-3. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. Consciousness and neuroscience. Cereb Cortex. 1998;8:97–107. doi: 10.1093/cercor/8.2.97. [DOI] [PubMed] [Google Scholar]
- Damian MF. Congruity effects evoked by subliminally presented primes: automaticity rather than semantic processing. J Exp Psychol Hum Percept Perform. 2001;27:154–165. doi: 10.1037//0096-1523.27.1.154. [DOI] [PubMed] [Google Scholar]
- De Renzi E. Prosopagnosia in two patients with CT scan evidence of damage confined to the right hemisphere. Neuropsychologia. 1986;24:385–389. doi: 10.1016/0028-3932(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words: behavioral and neuroimaging evidence. Psychol Sci. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Matthews PM, Gonnerman LM. Morphology and the internal structure of words. Proc Natl Acad Sci USA. 2004;101:14984–14988. doi: 10.1073/pnas.0403766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E, Schweinberger SR, Dolan RJ, Henson RN. Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. Neuroimage. 2005;26:1128–1139. doi: 10.1016/j.neuroimage.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ellis AW, Young AW, Flude BM. Repetition priming and face processing: priming occurs within the system that responds to the identity of a face. Q J Exp Psychol A. 1990;42:495–512. doi: 10.1080/14640749008401234. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat Neurosci. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- Forster KI. The pros and cons of masked priming. J Psycholinguist Res. 1998;27:203–233. doi: 10.1023/a:1023202116609. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1996;2:165–189. [Google Scholar]
- Friston KJ, Henson RN. Commentary on: divide & conquer: a defense of functional localizers. Neuroimage. 2006;30:1097–1099. doi: 10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Furey ML, Tanskanen T, Beauchamp MS, Avikainen S, Uutela K, Hari R, Haxby JV. Dissociation of face-selective cortical responses by attention. Proc Natl Acad Sci USA. 2006;103:1065–1070. doi: 10.1073/pnas.0510124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG, Klinger MR, Schuh ES. Activation by marginally perceptible (“subliminal”) stimuli: dissociation of unconscious from conscious cognition. J Exp Psychol Gen. 1995;124:22–42. doi: 10.1037//0096-3445.124.1.22. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci USA. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Simons DJ, Cohen NJ. Imaging implicit perception: promise and pitfalls. Nat Rev Neurosci. 2005;6:247–255. doi: 10.1038/nrn1630. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception [Record Supplied by Publisher] Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005;438:496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JD, Rees G. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nat Neurosci. 2005;8:686–691. doi: 10.1038/nn1445. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RN, Goshen-Gottstein Y, Ganel T, Otten LJ, Quayle A, Rugg MD. Electrophysiological and haemodynamic correlates of face perception, recognition and priming. Cereb Cortex. 2003;13:793–805. doi: 10.1093/cercor/13.7.793. [DOI] [PubMed] [Google Scholar]
- Henson RN, Mouchlianitis E. Effect of spatial attention on stimulus-specific haemodynamic repetition effects. Neuroimage. 2007;35:1317–1329. doi: 10.1016/j.neuroimage.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Gorno-Tempini M-L, Dolan RJ. Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cereb Cortex. 2002;12:178–186. doi: 10.1093/cercor/12.2.178. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Kelley C, Brown J, Jasechko J. Becoming famous overnight: limits on the ability to avoid unconscious influences of the past. J Pers Soc Psychol. 1989;56:326–338. [Google Scholar]
- Jiang Y, He S. Cortical responses to invisible faces: dissociating subsystems for facial-information processing. Curr Biol. 2006;16:2023–2029. doi: 10.1016/j.cub.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Neuroscience. What's in a face? Science. 2006;311:617–618. doi: 10.1126/science.1123983. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A, Buchel C, Zeki S, Frackowiak RS. Human brain activity during spontaneously reversing perception of ambiguous figures. Proc Biol Sci. 1998;265:2427–2433. doi: 10.1098/rspb.1998.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. The quest for consciousness: a neurobiological approach. Denver (CO): Roberts & Co; 2004. [Google Scholar]
- Koch C, Tsuchiya N. Attention and consciousness: two distinct brain processes. Trends Cogn Sci. 2007;11:16–22. doi: 10.1016/j.tics.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Revonsuo A. An ERP study of change detection, change blindness, and visual awareness. Psychophysiology. 2003;40:423–429. doi: 10.1111/1469-8986.00044. [DOI] [PubMed] [Google Scholar]
- Kouider S, Dehaene S. Levels of processing during non-conscious perception: a critical review of visual masking. Philos Trans R Soc Lond B Biol Sci. 2007;362:857–875. doi: 10.1098/rstb.2007.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouider S, Dehaene S, Jobert A, Le Bihan D. Cerebral bases of subliminal and supraliminal priming during reading. Cereb Cortex. 2007;17:2019–2029. doi: 10.1093/cercor/bhl110. [DOI] [PubMed] [Google Scholar]
- Kouider S, Dupoux E. A functional disconnection between spoken and visual word recognition: evidence from unconscious priming. Cognition. 2001;82:B35–49. doi: 10.1016/s0010-0277(01)00152-4. [DOI] [PubMed] [Google Scholar]
- Kouider S, Dupoux E. Partial awareness creates the “illusion” of subliminal semantic priming. Psychol Sci. 2004;15:75–81. doi: 10.1111/j.0963-7214.2004.01502001.x. [DOI] [PubMed] [Google Scholar]
- Kouider S, Dupoux E. Subliminal speech priming. Psychol Sci. 2005;16:617–625. doi: 10.1111/j.1467-9280.2005.01584.x. [DOI] [PubMed] [Google Scholar]
- Lamme VA. Why visual attention and awareness are different. Trends Cogn Sci. 2003;7:12–18. doi: 10.1016/s1364-6613(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Lamme VA. Towards a true neural stance on consciousness. Trends Cogn Sci. 2006;10:494–501. doi: 10.1016/j.tics.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Loffler G, Gordon GE, Wilkinson F, Goren D, Wilson HR. Configural masking of faces: evidence for high-level interactions in face perception. Vision Res. 2005;45:2287–2297. doi: 10.1016/j.visres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lumer ED, Friston KJ, Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Belger A, Allison T. Electrophysiological studies of human face perception. II: Response properties of face-specific potentials generated in occipitotemporal cortex. Cereb Cortex. 1999;9:431–444. doi: 10.1093/cercor/9.5.431. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. The visual brain in action. New York: Oxford University Press; 1995. [Google Scholar]
- Moutoussis K, Zeki S. The relationship between cortical activation and perception investigated with invisible stimuli. Proc Natl Acad Sci USA. 2002;99:9527–9532. doi: 10.1073/pnas.142305699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L, Blandin E, Dehaene S. Unconscious masked priming depends on temporal attention. Psychol Sci. 2002;13:416–424. doi: 10.1111/1467-9280.00474. [DOI] [PubMed] [Google Scholar]
- Naccache L, Dehaene S. The priming method: imaging unconscious repetition priming reveals an abstract representation of number in the parietal lobes. Cereb Cortex. 2001;11:966–974. doi: 10.1093/cercor/11.10.966. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Dehaene S, Jobert A, Le Bihan D, Kouider S. Subliminal convergence of Kanji and Kana words: further evidence for functional parcellation of the posterior temporal cortex in visual word perception. J Cogn Neurosci. 2005;17:954–968. doi: 10.1162/0898929054021166. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hara N, Kouider S, Takayama Y, Hanajima R, Sakai K, Ugawa Y. Task-guided selection of the dual neural pathways for reading. Neuron. 2006;52:557–564. doi: 10.1016/j.neuron.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Ohman A. Automaticity and the amygdala: nonconscious responses to emotional faces. Curr Dir Psychol Sci. 2002;11:62–66. [Google Scholar]
- Pasley BN, Mayes LC, Schultz RT. Subcortical discrimination of unperceived objects during binocular rivalry. Neuron. 2004;42:163–172. doi: 10.1016/s0896-6273(04)00155-2. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Exp Brain Res. 1982;47:329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: an event-related fMRI study. Neuroimage. 2005;24:1214–1224. doi: 10.1016/j.neuroimage.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Rees G, Russell C, Frith CD, Driver J. Inattentional blindness versus inattentional amnesia for fixated but ignored words. Science. 1999;286:2504–2507. doi: 10.1126/science.286.5449.2504. [DOI] [PubMed] [Google Scholar]
- Rees G, Wojciulik E, Clarke K, Husain M, Frith C, Driver J. Neural correlates of conscious and unconscious vision in parietal extinction. Neurocase. 2002;8:387–393. doi: 10.1076/neur.8.4.387.16190. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- Rossion B, Schiltz C, Crommelinck M. The functionally defined right occipital and fusiform “face areas” discriminate novel from visually familiar faces. Neuroimage. 2003;19:877–883. doi: 10.1016/s1053-8119(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: a defense of functional localizers. Neuroimage. 2006;30:1088–1096. doi: 10.1016/j.neuroimage.2005.12.062. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- Schott BH, Henson RN, Richardson-Klavehn A, Becker C, Thoma V, Heinze HJ, Duzel E. Redefining implicit and explicit memory: the functional neuroanatomy of priming, remembering, and control of retrieval. Proc Natl Acad Sci USA. 2005;102:1257–1262. doi: 10.1073/pnas.0409070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks DR, St John MF. Characteristics of dissociable human learning systems. Behav Brain Sci. 1994;17:367–447. [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves JK, Culham JC, Duchaine BC, Pratesi CC, Valyear KF, Schindler I, Humphrey GK, Milner AD, Goodale MA. The fusiform face area is not sufficient for face recognition: evidence from a patient with dense prosopagnosia and no occipital face area. Neuropsychologia. 2006;44:594–609. doi: 10.1016/j.neuropsychologia.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Egner T, Mangels J, Hirsch J. Mistaking a house for a face: neural correlates of misperception in healthy humans. Cereb Cortex. 2006;16:500–508. doi: 10.1093/cercor/bhi129. [DOI] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VA. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) Nat Neurosci. 2001;4:304–310. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends Cogn Sci. 2006;10:502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Tong F, Nakayama K, Vaughan JT, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron. 1998;21:753–759. doi: 10.1016/s0896-6273(00)80592-9. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Williams MA, Morris AP, McGlone F, Abbott DF, Mattingley JB. Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. J Neurosci. 2004;24:2898–2904. doi: 10.1523/JNEUROSCI.4977-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi DJ, Woodman GF, Widders D, Marois R, Chun MM. Neural fate of ignored stimuli: dissociable effects of perceptual and working memory load. Nat Neurosci. 2004;7:992–996. doi: 10.1038/nn1294. [DOI] [PubMed] [Google Scholar]
- Zeki S. The disunity of consciousness. Trends Cogn Sci. 2003;7:214–218. doi: 10.1016/s1364-6613(03)00081-0. [DOI] [PubMed] [Google Scholar]