Abstract
To compare the clinical outcomes of older (age ≥ 55 years) non-Hodgkin’s lymphoma (NHL) patients with younger NHL patients (< 55 years) receiving autologous hematopoietic cell transplantation (HCT) while adjusting for patient-, disease-, and treatment-related variables. We compared autologous HCT outcomes in 805 NHL patients age ≥ 55 years to 1,949 NHL patients < 55 years during the years 1990–2000 using data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). In multivariate analysis, older patients with aggressive histologies were 1.86 times [95% confidence interval (CI) 1.43–2.43, p<0.001] more likely than younger patients to experience treatment-related mortality. Relative death risks were 1.33 times (CI 1.04–1.71, p=0.024) and 1.50 times (CI 1.33–169, p<0.001) higher in older compared to younger patients with follicular grade I/II and aggressive histologies, respectively. Autologous HCT in older NHL patients is feasible but most disease-related outcomes are statistically inferior to younger patients. Studies addressing supportive care particular to older patients who are most likely to benefit from this approach are recommended.
Key Words/phrases: non-Hodgkin’s lymphoma, autologous HCT, relapse, second complete remission, elderly
INTRODUCTION
In the USA, over 55,000 NHL patients are diagnosed each year and the majority of the patients are over 55 years of age; furthermore, incidence rates have risen with each year of age above 55 years, with the rate of increase larger among each successively older age group1,2. Older age is a well-recognized poor prognostic factor 3,4. Reluctance to offer autologous hematopoietic cell transplantation (HCT) to older patients with hematologic malignancies is reinforced by a high treatment-related mortality. Several studies published more than a decade ago showed a direct correlation with increased age and higher likelihood for hepatic veno-occlusive disease, interstitial pneumonitis and other fatal complications 5,6. Additionally, Weaver and co-workers 7 reported a large study of community cancer center patients receiving autologous HCT for various malignant disorders where 9.5 % of patients older than 60 years died of treatment-related causes within 100 days of HCT compared to 3% of younger patients. It is unclear what selection criteria were used when considering HCT in the elderly population included in this study. The median age of autologous HCT in several recent series is 35–45 years6,8–12.
We performed a multi-center retrospective study using an observational database to determine the effect of age (i.e., <55 versus ≥ 55 years old) on the short-term and long-term outcomes of NHL patients who have undergone an autologous HCT. Although the literature commonly reports age 60 years as a cutoff, in part reflecting the prognostic index derived from a non-transplant data set3, we chose 55 years as the optimal value in order to demonstrate the largest differences for individuals from two age groups (vide infra). Further, some reports for NHL HCT procedures combine results for indolent and aggressive histologies. Our main study objective was to compare overall survival, disease-free survival, treatment-related mortality and relapse rates between younger and older patients, for patients with indolent (follicular center cell grade I and II) and aggressive lymphoma (follicular III, diffuse large cell and immunoblastic). We also sought to identify patient-, disease-, and treatment-related factors correlated with outcome. These data will provide important information for treatment decisions for NHL patients being considered for autologous HCT.
PATIENTS AND METHODS
CIBMTR
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP) that comprises a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on consecutive allogeneic and autologous transplants to a Statistical Center at the Health Policy Institute of the Medical College of Wisconsin in Milwaukee or the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all consecutive transplants; compliance is monitored by on-site audits. Subjects are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are done with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
The CIBMTR collects data at two levels: registration and research. Registration data include disease type, age, sex, pretransplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow- and/or blood-derived stem cells), high-dose conditioning regimen, post-transplant disease progression and survival, development of a new malignancy and cause of death. Requests for data on progression or death for registered patients are at six-month intervals. All CIBMTR teams contribute Registration data. Research data are collected on a subset of registered patients selected using a weighted randomization scheme and include detailed disease, and pre- and post-transplant clinical information. Based on data collected in the Centers for Disease Control Hospital Surveys 13,14 and the U.S. Government Accounting Office 15,16 and worldwide surveys of transplant activity, approximately 40% of allogeneic transplants worldwide and more than 50% of autologous HCTs in North and South America are registered with the CIBMTR.
Patients
Between January 1, 1990 and December 31, 2000, 8244 NHL (histology limited to indolent and aggressive) patients who received autologous HCT were registered with the CIBMTR. Of these, a total of 2754 (33%) NHL patients have complete Research Data and were included in the study. Forty-eight patients were excluded because they were younger than 18 years prior to transplantation. 1,949 patients were less than age 55 years at time of transplantation, while 805 were at least 55 years old. Patients were reported to the CIBMTR by 176 centers in 10 different countries. To assure that the Research patients were representative of all registered patients, demographics, relapse and survival rates between Research and Registered patients were compared; no differences were noted. Median follow-up of survivors after autologous HCT was 92 months (range: <1–198 months) for patients < 55 years and 83 months (range: 2–196 months) for patients ≥ 55 years.
Study Endpoints
Primary outcomes studied were treatment-related mortality, relapse, treatment failure (inverse of disease-free survival) and overall survival. Treatment-related mortality was defined as death in continuous complete remission or any death occurring less than 28 days after transplant. Patients who never achieved complete remission (CR) were considered to relapse at day 28. Patients with recurrent lymphoma were censored for treatment-related mortality at the time of relapse. Likewise, those alive in remission were censored for relapse at the last follow-up evaluation. For disease free survival (DFS), patients were considered treatment failures at the time of relapse or at the time of death from any cause. Patients alive in continuous complete remission were censored at last follow-up evaluation. Overall survival was defined as the interval between transplant and death from any cause. Surviving patients were censored at the date of last contact.
Statistical Methods
Univariate probabilities of treatment-related mortality and relapse were computed using cumulative incidence to accommodate competing risks. Univariate probabilities of treatment failure (inverse of disease-free survival) and overall survival were computed using the Kaplan-Meier estimator 17.
Statistical techniques, i.e., Contal and O’Quigley 18 and maximum likelihood theory, were used to determine the optimal categorization of age groups among cutpoints including ages 50, 55, 60 and 65 years. The choice of 55 years produced the optimal age cutoff value based on these statistical methods; optimal in the sense that 55 maximizes the likelihood function and yields the largest difference between individuals from the two age groups (data not shown). Because the literature commonly reports categories based around 60 years of age, we also analyzed the data using age 60 as the cutpoint. These analyses produced similar results (data not shown). Comparisons of the two age groups and assessment of other potential risk factors for outcomes of interest were done using multivariate Cox proportional hazards regression analysis19. Age group (≥ 55 vs < 55 years) was forced into all Cox models. Other variables considered in the analysis included gender, Karnofsky performance score at transplant (<90% versus ≥ 90%), disease stage at diagnosis (stage I/II versus III/IV), presence versus absence of B symptoms, disease status at transplant, interval from diagnosis to transplant (<12 months versus ≥ 12 months), type of graft (bone marrow versus peripheral blood), use of involved-field radiation, conditioning regimen (no TBI versus TBI), year of transplant, use of purging and use of G-CSF or GM-CSF to promote engraftment (defined as initiation of these therapies within 7 days of HCT).
Overall completeness index follow-up is 92% (<55=91%; ≥ 55=94%). To accommodate the physiologic differences between histologies, separate analyses were performed for indolent and aggressive lymphoma histologies. For all outcomes of interest, the assumption of proportional hazards was tested using time-dependent covariates and graphical methods20. For relapse and treatment failure, all covariates considered in the multivariate analyses satisfied the proportionality assumption, for both histology types. For overall survival, non-proportional hazards were identified for Karnofsky performance score at transplant (indolent histology patients) and interval from diagnosis to transplant (aggressive histology patients). Cox regression models for overall survival were thus stratified by the Karnofsky performance score or interval from diagnosis to transplant, according on histology type. For treatment-related mortality, non-proportional hazards were identified for type of graft (indolent histology patients) and use of G-CSF or GM-CSF (aggressive histology patients). Therefore, type of graft was entered into the Cox model for treatment-related mortality for indolent NHL model as a time varying covariate, with early (<12 months) and late (≥ 12 months) effects for peripheral blood. Similarly, G-CSF or GM-CSF to promote engraftment was entered into the treatment-related mortality for aggressive NHL model as a time varying covariate, with early (< 8 months) and late (≥ 8 months) effects for recipients who received growth factors. The eight Cox models were built using a forward stepwise selection process and covariates that attained a p-value ≤0.05 were considered statistically significant and held in the final model (again with the exception that age group was forced into all models). For all outcomes of interest, interactions between age group and all covariates were tested before and after the model building. For relapse, there was a significant interaction between year of transplant and the effect of age for indolent NHL. In other words, age had a different effect depending on whether the patient was transplanted between 1990–1994, 1995–1996, 1997–1998 or 1999–2000. Therefore, the comparisons between age groups for this model are presented separately for each year of transplantation (see table 3A). Overall covariate effects were tested using Wald test. All computations were made using the procedures PHREG and TPHREG in the statistical package SAS Version 9.1 for Unix. All multivariate models were examined for center effects using a random effects or frailty model 21; there were no significant center effects.
Table 3.
| Table 3A. Multivariate analysis of relapse for follicular grade I/II NHL | |||
|---|---|---|---|
| Variables: | N | Relative Risk of relapse (95% CI) | P-value |
| Main effect of agea: | |||
| Year of transplant: 1990–1994 Age ≥ 55 vs <55 | 375 | 1.35 (0.95 – 1.94) | 0.10 |
| Year of transplant: 1995–1996 Age ≥ 55 vs <55 | 170 | 0.76 (0.48 – 1.21) | 0.25 |
| Year of transplant: 1997–1998 Age ≥ 55 vs <55 | 143 | 1.12 (0.69 – 1.82) | 0.64 |
| Year of transplant: 1999–2000 Age ≥ 55 vs <55 | 75 | 2.66 (1.32 – 5.37) | 0.006 |
| Other significant covariates: | |||
| Disease status at transplantb | |||
| (1) CR1 | 97 | 1.00 | <0.001c |
| (2) PIF-sensitive | 141 | 1.66 (1.13 – 2.45) | 0.010 |
| (3) PIF-resistant | 22 | 3.23 (1.79 – 5.81) | <0.001 |
| (4) REL-sensitive | 209 | 1.93 (1.35 – 2.77) | <0.001 |
| (5) REL-resistant | 46 | 2.58 (1.62 – 4.11) | <0.001 |
| (6) CR2+ | 125 | 1.16 (0.77 – 1.75) | 0.47 |
| (7) REL-untreated/unknown | 29 | 1.44 (0.82 – 2.53) | 0.20 |
| (8) PIF-untreated/unknown | 10 | 1.80 (0.70 – 4.59) | 0.22 |
| (9) Unknown | 84 | 1.35 (0.86 – 2.11) | 0.19 |
| Table 3B. Multivariate analysis of relapse for follicular grade III/diffuse large B cell/immunoblastic NHL | |||
|---|---|---|---|
| Variables: | N | Relative Risk of relapse (95% CI) | P-value |
| Main effect of age: | |||
| <55 | 1294 | 1.00 | 0.002 |
| ≥ 55 | 615 | 1.22 (1.08 – 1.38) | |
| Other significant covariates: | |||
| Disease status at transplanta | |||
| (1) CR1 | 221 | 1.00 | <0.001b |
| (2) PIF-sensitive | 293 | 2.28 (1.76 – 2.95) | <0.001 |
| (3) PIF-resistant | 98 | 4.07 (2.98 – 5.55) | <0.001 |
| (4) REL-sensitive | 570 | 2.34 (1.85 – 2.97) | <0.001 |
| (5) REL-resistant | 155 | 4.08 (3.07 – 5.43) | <0.001 |
| (6) CR2+ | 324 | 1.34 (1.03 – 1.74) | 0.031 |
| (7) REL-untreated/unknown | 75 | 2.44 (1.72 – 3.46) | <0.001 |
| (8) PIF-untreated/unknown | 15 | 1.72 (0.75 – 3.93) | 0.20 |
| (9) Unknown | 158 | 2.62 (1.97 – 3.48) | <0.001 |
Additional tests:
Overall 1 degree of freedom test for age (≥55 vs <55): p-value=0.006
Overall 3 degree of freedom test for year of transplant: p-value=0.002
Overall 3 degree of freedom test for age × year of transplant: p-value=0.027
There is a significant interaction between the effects of age and year of transplant on the risk of relapse (p=0.03) such that the effect age differs with the year of transplant.
Other significant pairwise comparisons: P23 = 0.016; P26 = 0.040; P36 = <0.001; P39 = 0.004; P46 = 0.001; P52 = 0.036; P56 = <0.001; P59 = 0.007; P73 = 0.020; P75 = 0.049.
Eight degrees of freedom
Other significant pairwise comparisons: P23 = <0.001; P26 = <0.001; P36 = <0.001; P39 = 0.004; P43 = <0.001; P45 =<0.001; P46 = <0.001; P52 = <0.001; P56 = <0.001; P58 = 0.040; P59 = 0.001; P73 = 0.005; P75 = 0.003; P76 = <0.001; P83 = 0.04; P96 = <0.001;
Eight degrees of freedom
RESULTS
Table 1 shows the patient-, disease-, and transplant-related characteristics of the 2754 patients included in the study according to age group (≥ 55 versus < 55 years) and histology type. The median age in the two age groups was 61 years (range, 55–73) and 45 years (range, 18–55) respectively, and younger patients were more likely to have follicular lymphoma (32% versus 21%). Combining patients from the two histology types, karnofsky performance score at transplant did not differ significantly, but younger patients were more likely to have, B symptoms at diagnosis (38% versus 31%), have primary refractory disease (24% versus 15%), receive bone marrow rather than peripheral blood as the graft source (30% versus 19%), and undergo a TBI-containing regimen (31% versus 22%).
Table 1.
Characteristics of NHL patients undergoing autologous HCT from 1990 to 2000 and reported to the CIBMTR.
| Follicular grade I/II |
Follicular grade III DLBCL Immunoblastic NHL |
||||
|---|---|---|---|---|---|
| < 55yr | ≥ 55yr | < 55yr | ≥ 55yr | ||
| Variable | N (%) | N (%) | N (%) | N (%) | P-valuea |
| Number of patients | 615 | 173 | 1334 | 632 | |
| Age, median (range), years | 46 (19 – 55) | 60 (55 – 72) | 44 (18 – 55) | 61 (55 – 73) | |
| Male sex | 339 (55) | 105 (61) | 776 (58) | 367 (58) | 0.48 |
| Karnofsky performance score at transplant | <0.001 | ||||
| <90 | 145 (25) | 41 (24) | 475 (37) | 237 (38) | |
| ≥ 90 | 440 (75) | 132 (76) | 819 (63) | 381 (62) | |
| Missing | 30 | 0 | 40 | 14 | |
| Disease stage at diagnosis | <0.001 | ||||
| I or II | 87 (14) | 41 (24) | 479 (36) | 221 (35) | |
| III or IV | 520 (85) | 129 (74) | 822 (62) | 402 (64) | |
| Unknown | 8 (1) | 3 (2) | 33 (2) | 9 (1) | |
| B symptoms at diagnosis | <0.001 | ||||
| Absent | 383 (62) | 112 (65) | 691 (52) | 362 (57) | |
| Present | 193 (32) | 39 (22) | 552 (41) | 211 (34) | |
| Unknown | 39 (6) | 22 (13) | 91 (7) | 59 (9) | |
| Disease status at transplant | <0.001 | ||||
| CR1 | 87 (16) | 14 (9) | 178 (15) | 50 (8) | |
| CR2+ | 98 (18) | 31 (21) | 217 (18) | 113 (19) | |
| PIF-sensitive | 118 (21) | 27 (18) | 236 (19) | 69 (12) | |
| PIF-resistant | 18 (3) | 4 (3) | 78 (6) | 22 (4) | |
| PIF-untreated/unknown | 10 (2) | 0 | 13 (1) | 3 (1) | |
| REL-sensitive | 165 (30) | 53 (35) | 343 (28) | 243 (42) | |
| REL-resistant | 30 (5) | 17 (11) | 102 (9) | 57 (10) | |
| REL-untreated/unknown | 28 (5) | 4 (3) | 53 (4) | 25 (4) | |
| Missing | 61 | 23 | 114 | 50 | |
| Chemosensitivity at transplant | 0.01 | ||||
| Sensitivity | 479 (78) | 137 (79) | 976 (73) | 498 (79) | |
| Resistant | 56 (9) | 22 (13) | 184 (14) | 74 (12) | |
| Untreated/not evaluable/unknown | 80 (13) | 14 (8) | 174 (13) | 60 (9) | |
| Interval from diagnosis to transplant | <0.001 | ||||
| <12 months | 126 (20) | 34 (20) | 613 (46) | 172 (27) | |
| ≥12 months | 489 (80) | 139 (80) | 721 (54) | 460 (73) | |
| Graft type | <0.001 | ||||
| Bone marrow | 191 (31) | 38 (22) | 398 (30) | 113 (18) | |
| Peripheral blood | 424 (69) | 135 (78) | 936 (70) | 519 (82) | |
| Use of involved-field radiation | 34 (6) | 7 (4) | 61 (5) | 16 (3) | 0.06 |
| Use of TBI | 280 (46) | 59 (34) | 320 (24) | 120 (19) | <0.001 |
| Conditioning regimen | <0.001 | ||||
| TBI | 280 (46) | 59 (34) | 320 (24) | 120 (19) | |
| Cy+VP16 | 46 (7) | 9 (5) | 132 (10) | 52 (8) | |
| BCNU-based: | |||||
| BEAM/BEAC | 170 (28) | 64 (37) | 643 (48) | 315 (50) | |
| Platinum based (no Cy) | 24 (4) | 10 (6) | 64 (5) | 29 (5) | |
| Others | 95 (15) | 31 (18) | 175 (13) | 116 (18) | |
| Year of transplantation | <0.001 | ||||
| 1990–1994 | 327 (53) | 56 (32) | 528 (39) | 168 (27) | |
| 1995–1996 | 130 (21) | 44 (25) | 334 (25) | 165 (26) | |
| 1997–1998 | 104 (17) | 46 (27) | 302 (23) | 174 (27) | |
| 1999–2000 | 54 (9) | 27 (16) | 170 (13) | 125 (20) | |
| In vitro purging performed | 142 (23) | 34 (20) | 99 (7) | 45 (7) | <0.001 |
| G-CSF or GM-CSF to promote engraftment | 450 (73) | 129 (75) | 1007 (75) | 496 (78) | 0.18 |
| New malignancy | 0.02 | ||||
| MDS/AML | 8 (1) | 1 (1) | 9 (1) | 6 (1) | |
| Other leukemia | 0 | 0 | 1 (<1) | 0 | |
| Solid tumor | 4 (1) | 1 (1) | 4 (<1) | 8 (1) | |
| Skin cancer | 2 (<1) | 1 (1) | 0 | 1 (<1) | |
| New malignancy, not specified | 47 (8) | 13 (7) | 55 (4) | 30 (5) | |
| None | 553 (90) | 156 (90) | 1261 (95) | 584 (93) | |
| Missing | 1 | 1 | 4 | 3 | |
| Median follow-up of survivors, months | 90 (3 – 180) | 81 (2 – 155) | 93 (1 – 198) | 84 (3 – 196) | |
Abbreviations: CR = Complete Remission; PIF=primary induction failure TBI = Total Body Irradiation; Cy = cyclophosphamide; GF = growth factors; G-CSF = granulocyte-colony stimulating factor; GM-CSF = granulocyte-macrophage colony stimulating factor.
The chi-square test was used for discrete covariates; the Kruskal-Wallis test was used for continuous covariates.
Follow-up completeness index = 92% (Overall); 91% (<55 yr); 94% (≥55 yr).
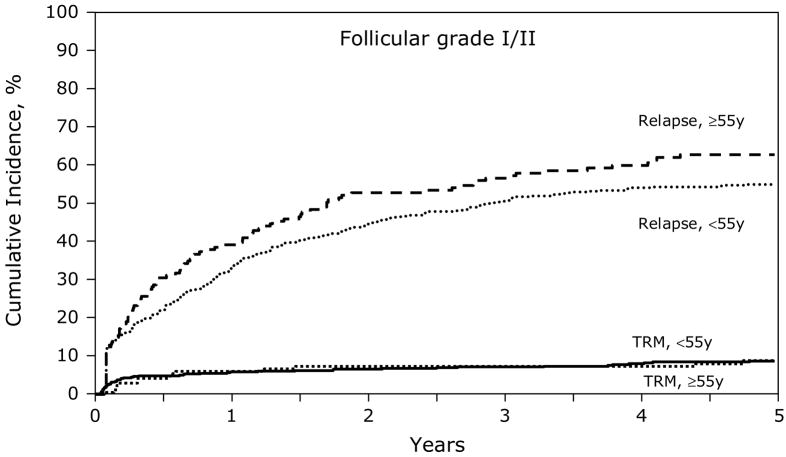
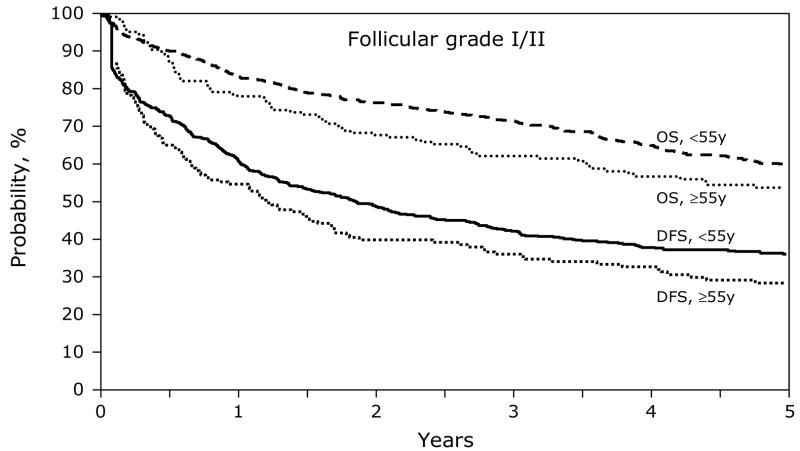
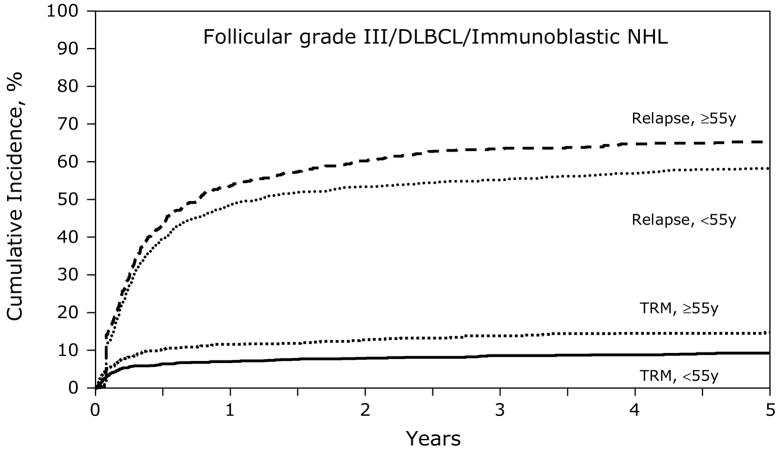
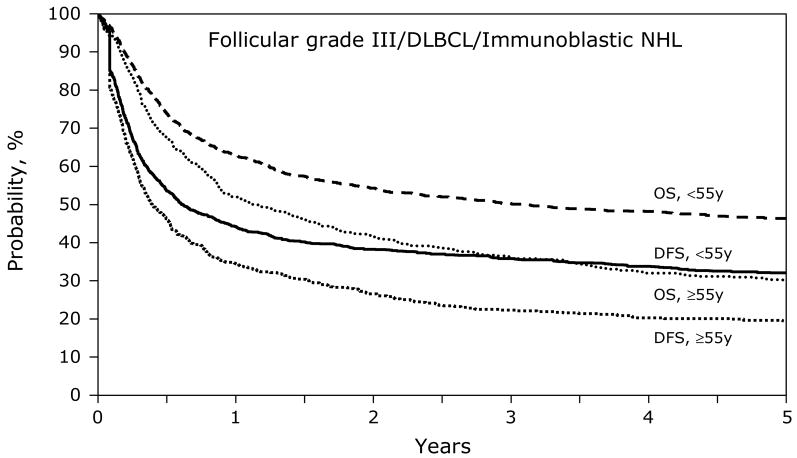
Figures 1 and 3 show the univariate probabilities of all outcomes of interest after transplantation according to age group for indolent histology patients. At one-, three- and five-years after transplant younger patients had a lower probability of relapse and a higher probability of disease-free and overall survival. At five-years after transplant, treatment-related mortality did not differ significantly between age groups but relapses were significantly higher, 8% versus 7% and 55% versus 63%, for subjects < 55 years versus ≥ 55 years, respectively. Disease-free and overall survival rates at five-years also favored younger patients, 37% versus 29% and 60% and 54%, respectively, Similarly, the younger aggressive histology patients had a lower probability of treatment-related mortality and relapse and a higher probability of disease-free and overall survival compared to subjects age greater than 55 years (Figures 2 and 4). Specifically, at five-years treatment-related mortality rates were significantly lower in younger patients (9% versus 15%) as were relapse rates (59% versus 66%), respectively. Correspondingly, disease-free and overall survival rates were superior in the younger patient population, 32% versus 19% and 47% versus 30%, respectively.
Figure 1.
Cumulative incidence of treatment-related mortality (TRM) and relapse after autologous HCTs for follicular grade I/II NHL patients age < 55 years versus ≥ 55 years.
Figure 3.
Cumulative incidence of disease-free survival and overall survival after autologous HCTs for follicular grade I/II NHL patients age < 55 years versus ≥ 55 years.
Figure 2.
Cumulative incidence of treatment-related mortality (TRM) and relapse after autologous HCTs for follicular grade III/diffuse large B cell/immunoblastic NHL patients age < 55 years versus ≥ 55 years.
Figure 4.
Cumulative incidence of disease-free survival and overall survival after autologous HCTs for follicular grade III/diffuse large B cell/immunoblastic NHL patients age < 55 years versus ≥ 55 years.
Tables 2A and 2B show the multivariate analysis of treatment-related mortality for older versus younger patients in both histologic subgroup types respectively. After adjusting for other covariates, aggressive histology patients 55 years and older were 1.86 times more likely to have treatment-related mortality than younger patients (95% confidence interval (CI) 1.43–2.43, p<0.001). Age, however, was not a factor in the indolent histology group (p=0.54). Other factors found to be associated with an increased treatment-related mortality in the more aggressive histology patients were poor-performance status, chemo-resistant disease before transplant, more than 12 months duration from diagnosis to transplant, and use of purging. For the indolent histology patients, significant covariates for increased treatment-related mortality included use of bone marrow rather than blood as the graft source (however this effect was no longer statistical significant in patients surviving more than 12 months post-transplant) and a TBI-containing conditioning regimen.
Table 2.
| Table 2A. Multivariate analysis of treatment-related mortality for follicular grade I/II NHL | |||
|---|---|---|---|
| Variables: | N | Relative Risk of TRM (95% CI) | P-value |
| Main effect of age: | |||
| <55 | 595 | 1.00 | 0.54 |
| ≥ 55 | 168 | 1.18 (0.69 – 2.02) | |
| Other significant covariates: | |||
| Type of graft | <0.001a | ||
| Bone marrow | 221 | 1.00 | |
| Peripheral bloodb | |||
| Within first 12 months after transplant | 235 | 0.26 (0.14 – 0.48) | <0.001 |
| Beyond first 12 months after transplant | 307 | 1.26 (0.61 – 2.60) | 0.54 |
| Conditioning regimen | |||
| No TBI | 434 | 1.00 | 0.014 |
| TBI | 329 | 1.75 (1.12 – 2.72) | |
| Table 2B. Multivariate analysis of treatment-related mortality for follicular grade III/diffuse large B cell/immunoblastic NHL | |||
|---|---|---|---|
| Variables: | N | Relative Risk of TRM (95% CI) | P-value |
| Main effect of age: | |||
| <55 | 1294 | 1.00 | <0.001 |
| ≥ 55 | 615 | 1.86 (1.43 – 2.43) | |
| Other significant covariates: | |||
| Karnofsky performance score at transplanta | |||
| (1) ≥90% | 1167 | 1.00 | 0.003b |
| (2) <90% | 692 | 1.59 (1.20 – 2.04) | 0.001 |
| (3) Missing | 50 | 1.04 (0.50 – 2.17) | 0.26 |
| Disease status at transplantc | |||
| (1) CR1 | 221 | 1.00 | <0.001d |
| (2) PIF-sensitive | 293 | 1.35 (0.77 – 2.38) | 0.30 |
| (3) PIF-resistant | 98 | 1.39 (0.61 – 3.14) | 0.44 |
| (4) REL-sensitive | 570 | 1.17 (0.69 – 1.98) | 0.56 |
| (5) REL-resistant | 155 | 3.35 (1.88 – 5.95) | <0.001 |
| (6) CR2+ | 324 | 1.65 (0.96 – 2.81) | 0.07 |
| (7) REL-untreated/unknown | 75 | 2.17 (1.07 – 4.39) | 0.032 |
| (8) PIF-untreated/unknown | 15 | 4.43 (1.52 – 12.96) | 0.007 |
| (9) Unknown | 158 | 1.70 (0.92 – 3.14) | 0.09 |
| Time from diagnosis to transplant | |||
| <12 months | 759 | 1.00 | 0.040 |
| ≥12 months | 1150 | 1.41 (1.02 – 1.95) | |
| Use of purging | |||
| No | 1771 | 1.00 | 0.008 |
| Yes | 138 | 1.77 (1.16 – 2.68) | |
| G-CSF or GM-CSF to promote engraftment | |||
| No | 449 | 1.00 | 0.017b |
| Yese | |||
| Within first 8 months after transplant | 800 | 0.69 (0.49 – 0.98) | 0.039 |
| Beyond first 8 months after transplant | 660 | 1.70 (0.99 – 2.92) | 0.054 |
Two degrees of freedom test
Time dependent covariates. The effect of peripheral blood graft type on outcome differs with the length of time after transplant. The risk of TRM is lower for recipients of peripheral blood grafts within the first 12 months following HCT compared to bone marrow recipients, but no different in the period beyond 12 months after HCT.
Other pairwise comparisons: P23 = 0.91.
Two degrees of freedom
Other significant pairwise comparisons: P45 =<0.001; P48 = 0.012; P52 = 0.001; P53 = 0.028; P56 = 0.003; P59 = 0.019; P74 = 0.046; P82 = 0.027.
Eight degrees of freedom
Time dependant covariates. The effect of G-CSF or GM-CSF to promote engraftment differs with the length of time after transplant. The risk of TRM is lower for recipients with G-CSF or GM-CSF to promote engraftment within the first 8 months following HCT compared to recipients who did not receive G-CSF or GM-CSF, but no different in the period beyond 8 months after HCT.
Tables 3A and 3B show the multivariate analysis of relapse. There was a statistically significant increase in risk of relapse for older patients (≥ 55 years) among patients with more aggressive NHL histologies (relative risk (RR) 1.22, 95% CI 1.08–1.38, p=0.002). However, older patients with indolent histologies had an increased risk of relapse only if they were transplanted in the time period of 1999–2000. After adjusting for other covariates, both the indolent and the aggressive lymphoma histology patients with primary induction failure (PIF) and relapsed disease were at increased risk for lymphoma recurrence.
Similar results were noted for treatment failure (i.e. inverse of disease-free survival) for both histologic groups for the effect of age (a consistent effect confined to the aggressive subtype) and disease status at transplant. For the indolent histology group, age did not affect treatment failure (inverse of disease-free survival) but disease status at transplant was the major determinate of outcome. The relative risk of treatment failure (95% CI) was significantly higher for patients with primary induction failure-sensitive [1.64 (1.15 – 2.32) times, p=0.006], primary induction failure-resistant [2.74 (1.59 – 4.73) times, p<0.001], relapse-sensitive [1.93 (1.39 – 2.68) times, p<0.001] and relapse-resistant [2.55 (1.66 – 3.93) times, p<0.001]. On the other hand, older age aggressive NHL patients were 1.32 (1.18–1.48) times more likely to fail than younger patients. Similar to the indolent NHL population, disease status at transplant again was a major determinant of outcome. The relative risk of treatment failure (95% CI) was significantly higher for patients with primary induction failure-sensitive [2.03 (1.61 – 2.56) times, p<0.001], primary induction failure-resistant [3.43 (2.57 – 4.58) times, p<0.001], relapse-sensitive [2.11 (1.71 – 2.61) times, p<0.001] relapse-resistant [3.89 (3.01 – 5.02) times, p<0.001] and second complete remission or beyond [1.47 (1.16 – 1.86) times, p=0.001]. The use of TBI in the conditioning regimen and poor performance status were associated with a statistically significant increase in treatment failures in the aggressive lymphoma subgroup [1.16 (1.02 – 1.31), p=0.027].
Tables 4A and 4B show the multivariate analysis of overall survival for the main effect of age. The relative risk of death was higher in older patients (≥ 55 years) in indolent histology as well as in aggressive NHL patients. After adjusting for other covariates, risk of mortality was statistically significantly increased in patients whose disease was not controlled (relapse or primary induction failure). Again, the use of TBI in the conditioning regimen and poor performance status were associated with a statistically significant increase in mortality in the aggressive lymphoma subgroup
Table 4.
| Table 4A. Multivariate analysis of overall survival for follicular grade I/II NHLa | |||
|---|---|---|---|
| Variables: | N | Relative Risk of death (95% CI) | P-value |
| Main effect of age: | |||
| <55 | 615 | 1.00 | 0.024 |
| ≥ 55 | 173 | 1.33 (1.04 – 1.71) | |
| Other significant covariates: | |||
| Disease status at transplantb | |||
| (1) CR1 | 101 | 1.00 | <0.001c |
| (2) PIF-sensitive | 145 | 1.39 (0.90 – 2.14) | 0.14 |
| (3) PIF-resistant | 22 | 2.89 (1.53 – 5.46) | 0.001 |
| (4) REL-sensitive | 218 | 1.82 (1.23 – 2.71) | 0.003 |
| (5) REL-resistant | 47 | 3.27 (1.99 – 5.39) | <0.001 |
| (6) CR2+ | 129 | 1.44 (0.92 – 2.23) | 0.11 |
| (7) REL-untreated/unknown | 32 | 1.48 (0.83 – 2.65) | 0.19 |
| (8) PIF-untreated/unknown | 10 | 3.31 (1.44 – 7.58) | 0.005 |
| (9) Unknown | 84 | 2.09 (1.32 – 3.33) | 0.002 |
| Year of transplantd | |||
| (1) 1999–2000 | 81 | 1.00 | 0.005e |
| (2) 1990–1994 | 383 | 2.08 (1.32 – 3.28) | 0.002 |
| (3) 1995–1996 | 174 | 1.54 (0.95 – 2.51) | 0.080 |
| (4) 1997–1998 | 150 | 1.74 (1.05 – 2.87) | 0.030 |
| Table 4B. Multivariate analysis of overall survival for follicular grade III/diffuse large B cell/immunoblastic NHLa | |||
|---|---|---|---|
| Variables: | N | Relative Risk of death (95% CI) | P-value |
| Main effect of age: | |||
| <55 | 1334 | 1.00 | <0.001 |
| ≥ 55 | 632 | 1.50 (1.33 – 1.69) | |
| Other significant covariates: | |||
| Karnofsky performance score at transplantb | |||
| (1) ≥ 90% | 1200 | 1.00 | <0.001c |
| (2) <90% | 712 | 1.35 (1.20 – 1.54) | <0.001 |
| (3) Missing | 54 | 0.93 (0.67 – 1.32) | 0.038 |
| Disease status at transplantd | |||
| (1) CR1 | 228 | 1.00 | <0.001e |
| (2) PIF-sensitive | 305 | 1.60 (1.23 – 2.07) | <0.001 |
| (3) PIF-resistant | 100 | 3.09 (2.27 – 4.21) | <0.001 |
| (4) REL-sensitive | 586 | 1.92 (1.50 – 2.45) | <0.001 |
| (5) REL-resistant | 159 | 3.94 (2.98 – 5.21) | <0.001 |
| (6) CR2+ | 330 | 1.54 (1.17 – 2.02) | 0.002 |
| (7) REL-untreated/unknown | 78 | 2.51 (1.79 – 3.52) | <0.001 |
| (8) PIF-untreated/unknown | 16 | 1.85 (0.96 – 3.57) | 0.07 |
| (9) Unknown | 164 | 2.36 (1.78 – 3.15) | <0.001 |
| Conditioning regimen | |||
| No TBI | 1526 | 1.00 | 0.009 |
| TBI | 440 | 1.20 (1.05 – 1.37) | |
| Year of transplantf | |||
| (1) 1999–2000 | 295 | 1.00 | 0.008g |
| (2) 1990–1994 | 696 | 1.36 (1.13 – 1.65) | 0.002 |
| (3) 1995–1996 | 499 | 1.24 (1.02 – 1.52) | 0.032 |
| (4) 1997–1998 | 476 | 1.14 (0.93 – 1.40) | 0.20 |
This Cox model was stratified on Karnofsky performance score at transplant (i.e. ≥90% and <90%).
Other significant pairwise comparisons: P23 = 0.014; P29 = 0.045; P36 = 0.021; P45 = 0.004; P52 = <0.001; P56 = <0.001; P75 = 0.008; P82 = 0.03; P86 = 0.040.
Eight degrees of freedom
Other pairwise comparisons: P23 = 0.027; P24 = 0.24; P34 = 0.49.
Three degrees of freedom
This Cox model was stratified on interval from diagnosis to transplant (i.e. ≥12 months and <12 months).
Other pairwise comparisons: P23 = 0.70.
Two degrees of freedom
Other significant pairwise comparisons: P23 = <0.001; P29 = 0.002; P36 = <0.001; P43 = <0.001; P45 = <0.001; P46 = 0.016; P52 = <0.001; P56 = <0.001; P58 = 0.022; P59 = <0.001; P72 = 0.004; P75 = 0.004; P76 = 0.001; P96 < 0.001.
Eight degrees of freedom
Other pairwise comparisons: P23 = 0.22; P24 = 0.025; P34 = 0.31.
Three degrees of freedom
We further explored outcome in the oldest patient population, i.e. N=149 subjects age over 65 years (Table 5). Compared to those patients < 65 years (N=2605), older individuals were statistically more likely to have a lower performance status (p=0.044), have aggressive rather than indolent histologies (p<0.001), have more advanced disease stage (p=0.032) yet more sensitive disease (p=0.002), and undergo HCT beyond 12 months after diagnosis (p=0.002). Table 6 shows the univariates for the 4 outcomes of interest for the older patient group. At 5 years after HCT, probabilities of treatment-related mortality and relapse were 14% (95% CI 9–20) and 66%(95% CI 58–73), respectively, These data translate into 5-year disease-free survival and overall survival probabilities of 20% (95% CI 14–27) and 29% (95% CI 22–37), respectively. Table 7 shows causes of death for all patients using age 55 years as the breakpoint. The major cause of death in both age groups was recurrent lymphoma.
Table 5.
Characteristics of NHL patients > age 65 years undergoing autologous HCT from 1990 to 2000 and reported to the CIBMTR.
| Variable | N (%) |
|---|---|
| Number of patients | 149 |
| Age, median (range), years | 67 (65 – 73) |
| Male sex | 71 (48) |
| Karnofsky performance score at transplant | |
| <90 | 61 (41) |
| ≥ 90 | 87 (59) |
| Missing | 1 |
| Histology | |
| Follicular grade I/II | 21 (14) |
| Follicular grade III DLBCL/Immunoblastic NHL | 128 (86) |
| Disease stage at diagnosis | |
| I or II | 59 (40) |
| III or IV | 87 (58) |
| Unknown | 3 (2) |
| B symptoms at diagnosis | |
| Absent | 91 (61) |
| Present | 40 (27) |
| Unknown | 18 (12) |
| Disease status at transplant | |
| CR1 | 10 (7) |
| CR2+ | 33 (24) |
| PIF-sensitive | 18 (13) |
| PIF-resistant | 2 (1) |
| REL-sensitive | 48 (35) |
| REL-resistant | 15 (11) |
| REL-untreated/unknown | 13 (9) |
| Missing | 10 |
| Chemosensitivity at transplant | |
| Sensitivity | 109 (73) |
| Resistant | 24 (16) |
| Untreated/not evaluable/unknown | 16 (11) |
| Interval from diagnosis to transplant | |
| <12 months | 34 (23) |
| ≥12 months | 115 (77) |
| Graft type | |
| Bone marrow | 17 (11) |
| Peripheral blood | 132 (89) |
| Use of involved-field radiation | 6 (4) |
| Use of TBI | 19 (13) |
| Year of transplantation | |
| 1990–1994 | 21 (14) |
| 1995–1996 | 32 (22) |
| 1997–1998 | 54 (36) |
| 1999–2000 | 42 (28) |
| In vitro purging performed | 10 (7) |
| G-CSF or GM-CSF to promote engraftment | 134 (90) |
| New malignancy | |
| Solid tumor | 3 (2) |
| Skin cancer | 1 (1) |
| New malignancy, not specified | 5 (3) |
| None | 140 (94) |
| Median follow-up of survivors, months | 69 (3 – 139) |
Abbreviations: CR = Complete Remission; PIF=primary induction failure TBI = Total Body Irradiation; GF = growth factors; G-CSF = granulocyte-colony stimulating factor; GM-CSF = granulocyte-macrophage colony stimulating factor.
Table 6.
Univariate probabilities of outcomes for NHL patients > age 65 years undergoing autologous HCT from 1990 to 2000.
| Outcome event | N | Prob (95% CI) |
|---|---|---|
| Treatment-related mortality | 147 | |
| @ 1 year | 11 (7 – 17) | |
| @ 3 years | 13 (8 – 19) | |
| @ 5 years | 14 (9 – 20) | |
| Relapse | 147 | |
| @ 1 year | 60 (51 – 67) | |
| @ 3 years | 66 (58 – 73) | |
| @ 5 years | 66 (58 – 73) | |
| Disease-free survival | 147 | |
| @ 1 year | 29 (22 – 36) | |
| @ 3 years | 21 (15 – 28) | |
| @ 5 years | 20 (14 – 27) | |
| Overall survival | 149 | |
| @ 1 year | 50 (42 – 58) | |
| @ 3 years | 35 (28 – 43) | |
| @ 5 years | 29 (22 – 37) |
Abbreviations: PROB = probability; CI = confidence interval.
Probabilities of treatment-related mortality and relapse were calculated using the cumulative incidence estimate. Disease-free survival and overall survival were calculated using the Kaplan-Meier product limit estimate.
Table 7.
Causes of death for NHL patients undergoing autologous HCT from 1990 to 2000 comparing <55 vs >55 years of age.
| ≤55 years | >55 years | |
|---|---|---|
| Causes of death | N (%) | N (%) |
| Number of patients | 1032 | 544 |
| Primary disease | 729 (71) | 370 (68) |
| New malignancy | 23 (2) | 18 (3) |
| Graft vs host disease | 5 (<1) | 0 |
| Interstitial pneumonia | 48 (5) | 32 (6) |
| Infection | 48 (5) | 30 (6) |
| Organ failure | 55 (5) | 41 (8) |
| Other cause | 124 (12) | 53 (9) |
DISCUSSION
We report the outcomes and prognostic factors for 2754 patients with NHL who received autologous stem cell transplant between 1990 and 2000 and were reported to the CIBMTR, based on age groups older or less than 55 years. In multivariate analysis, older patients with more aggressive NHL histologies were 1.86 times more likely than younger patients to experience treatment-related mortality. Outcomes reported in this study appear better than the considerably smaller series of patients with aggressive NHL histologies reported in the literature 22–29, some of which included aggressive 21–23,27 versus mixed indolent and aggressive 25–27,29 histologies. It should be noted that in many of these reports, including those by Bitran and colleagues28 and Moreau and co-workers 24, the transplant was performed only if the patient had relapsed disease that was sensitive to salvage therapy. With the exception of Sweetenham 22, all these authors used 60 years as their age cutoff. Although 55 years is a more optimal choice for our data, the analyses of tables 2A/B-4A/B were repeated with 60 years as the age threshold. While the point estimates for the effect of age varied slightly, the overall effect of age remained the same, as did the other significant covariates (data not shown). Further, in our series, treatment-related mortality at up to 5 years did not exceed 8% for either age group for follicular grade I/II NHL patients.
Our observational database collects information prospectively and such data likely are a more representative reflection of the practice of HCT in the community. It is difficult to make effective comparisons between our results and those reported in the literature for these patients. Published results from single centers studies are often unadjusted (univariate outcomes), and study entry criteria, treatment and attribution of cause of death are likely to vary across centers and studies. As well, the observational data collected by CIBMTR may include patients previously reported in single center experiences. Our reported 6% treatment-related mortality at one year and 7% at three years for the indolent histology group for both older and younger patients compares favorably with the experiences reported in the two largest series 22,27 although these communications included mostly aggressive histologies. In the aggressive NHL patients, treatment-related mortality rates at three years and five years after transplant of 14% (95% CI 11–17) and 15% (95% CI 12–18), respectively, in the over 55 year age group compare favorably with the 22.4% reported by Gopal et al 27. Those investigators reported both infectious and non-infectious events as causes of death, the former postulated to be due to a protracted time to immune reconstitution in the older patients. Further, for patients over age 55 years, the lower treatment-related mortality in the indolent population compared to the aggressive histology group may reflect an inherent selection bias, i.e. other therapeutic options may be available for elderly indolent NHL patients. As anticipated, poor performance status at transplant and a longer time from diagnosis to transplant was associated with a toxic death. The adverse effect of hematopoietic growth factor use in this patient population has been previously described 30. Data from the European Group for Blood and Marrow Transplantation (EBMT) and reported by Sweetenham et al 22 described a 38% treatment-related mortality for patients age >55 years. A comparison within the EBMT database for the patients age < 55 years showed a significantly lower treatment-related mortality, 12% versus 38% (p=0.03). Other hand, in our series those subjects < age 55 years had a reduced treatment-related mortality of 9% at three years (95% CI 7–10) as well as at five years (95% CI 7–11). Use of bone marrow rather than blood as the stem cell source and use of a TBI-containing regimen portended for treatment-related mortality in patients with the aggressive lymphoma histology. The EBMT also reported that TBI-based preparative regimens contributed to a higher toxic death rate 22.
We also demonstrated that risk of relapse was greater for all older patients in the more aggressive histologic group, but only for older patients transplanted between 1999 and 2000 in the indolent histologic group. As anticipated, advanced or persistent disease in both indolent and aggressive histologic patient populations was associated with an increased risk of relapse compared to remission. Similarly, chemotherapy-resistance prior to transplant was associated with an increased risk of relapse.
These data show that older patient age was associated with a statistically significant increased risk of treatment failure only in the aggressive histology subset (1.32 fold increase, p<0.001) compared to the indolent histology group; however, in both groups age ≥ 55 years was associated with increased mortality (RR 1.50, p<0.001 and RR 1.33, p=0.024, respectively). Other factors associated with treatment failure and increased death in both patient populations included persistent, relapsed or chemo-resistant disease. Gopal and colleagues 27 reported superior survival in patients with responsive, relapsed disease as overall survival at 4 years was 39% in sensitive disease versus only 15% in resistant NHL. In the aggressive histologic group, poor performance status as well as use of TBI in the preparative regimen significantly increased the risk of treatment failure and reduced overall survival. Our data did not indicate that blood rather than bone marrow as the graft source was associated with an improved overall survival, in contrast to the European experience generated in advanced Hodgkin’s and high-grade NHL patients 31.
Limitations of these analyses include inability to account for patients who may not have been considered for HCT, i.e. careful selection and exclusion of older patients deemed unfit for HCT. Also, perhaps other inherent selections biases are in operation such as offering HCT only for follicular NHL patients with highly aggressive disease who are young versus designating the older patients for other therapies, e.g. age and disease biologic behavior discrimination. Another limitation of this report is the observation that various histologic classifications were in use during this long period of patient accrual and follow-up. All studies are subject to changing lymphoma classification over time, but the histologies noted were those reported by each institution and the diagnoses are consistent within the era of HCT. Pathology materials are not routinely subject to secondary review.
After adjusting for other important characteristics, older patients transplanted between 1990 and 2000 have a greater risk of adverse outcomes than those less than age 55 years. Although changes in transplantation have allowed more advanced age patients to be considered for HCT, these patients have worse outcomes compared to their younger counterparts. Despite this observation, some older patients still should be considered for potential cure using HCT. Buadi and colleagues 32 at the Mayo Clinic reported a series of 93 intermediate-grade NHL patients at least 60 years of age (including 24 over age 70 years) who underwent HCT. Treatment-related mortality was 5.4% and 4-yr event-free survival was 38%, results that did not differ from a cohort less than age 60 years (2.2% and 42%, p=0.1, respectively). While a small series from a single institution, this group showed that good results can be obtained in older patients using careful patient selection and a non-TBI regimen. Another recent single institution trial reported by Wilde and colleagues33 showed similar toxicities and survival for patients older than 60 years compared to younger patients. These investigators observed that after controlling for age, comorbidities significantly influenced overall survival.
The 149 patients age > 65 years described herein were more likely to have a worse performance status, more advanced disease and a more aggressive histology compared to their younger counterparts in our data set. Such information may help account for the 14% (95% CI 11–19) 5-year treatment-related mortality. This patient group also had lower 5-year disease-free survivals and overall survivals, 20% (95% CI 14–27) and 29% (95% CI 22–37), respectively. Seventy percent of elderly patients died due to lymphoma, a rate essentially the same as in the younger patients (69%). A series of 99 consecutive relapsed NHL patients age older than 65 years reported recently by Hosing and coworkers34 showed an 8% cumulative nonrelapse mortality at 26 months and 61% 3-year overall survival. They found that even elderly patients with a comorbidity index > 2 had acceptable outcomes but were at higher risk for developing significant toxicity.
Additional strategies to reduce these risks for treatment-related mortality and relapse should be explored. Possible strategies could include individual patient dosing as employed with busulfan-containing regimens in the allograft setting 35, and use of targeted radioimmunoconjugates, which may facilitate effective delivery of radiation to tumor cells without causing excessive toxic effect to normal tissues 36,37. Ultimately, these and other approaches in older patients require further study.
Acknowledgments
Supported by Public Health Service Grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research; Health Resources and Services Administration (DHHS); and grants from AABB; Aetna; American International Group, Inc.; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; BioOne Corporation; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Bristol-Myers Squibb Company; Cangene Corporation; Celgene Corporation; CellGenix, GmbH; Cerus Corporation; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; EKR Therapeutics; Enzon Pharmaceuticals, Inc.; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Gift of Life Bone Marrow Foundation; GlaxoSmithKline, Inc.; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; MultiPlan, Inc.; National Marrow Donor Program; Nature Publishing Group; Oncology Nursing Society; Osiris Therapeutics, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Roche Laboratories; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; SuperGen, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; University of Colorado Cord Blood Bank; ViaCell, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Reilly SE, Connors JM, Macpherson N, et al. Malignant lymphomas in the elderly. Clin Geriatr Med. 1997;13:251–63. [PubMed] [Google Scholar]
- 2.Devesa SS, Fears T. Non-Hodgkin’s lymphoma time trends: United States and international data. Cancer Res. 1992;52:5432s–5440s. [PubMed] [Google Scholar]
- 3.Shipp MA, Anderson JR, et al. A Predictive Model for Aggressive Non-Hodgkin’s Lymphoma. The Internationl Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 4.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 5.Miller CB, Piantadosi S, Vogelsang GB, et al. Impact of age on outcome of patients with cancer undergoing autologous bone marrow transplant. J Clin Oncol. 1996;14:1327–32. doi: 10.1200/JCO.1996.14.4.1327. [DOI] [PubMed] [Google Scholar]
- 6.Kusnierz-Glaz CR, Schlegel PG, Wong RM, et al. Influence of age on the outcome of 500 autologous bone marrow transplant procedures for hematologic malignancies. J Clin Oncol. 1997;15:18–25. doi: 10.1200/JCO.1997.15.1.18. [DOI] [PubMed] [Google Scholar]
- 7.Weaver CH, Schwartzberg LS, Hainsworth J, et al. Treatment-related mortality in 1000 consecutive patients receiving high-dose chemotherapy and peripheral blood progenitor cell transplantation in community cancer centers. Bone Marrow Transplant. 1997;19:671–8. doi: 10.1038/sj.bmt.1700713. [DOI] [PubMed] [Google Scholar]
- 8.Freedman AS, Neuberg D, Mauch P, et al. Long-term follow-up of autologous bone marrow transplantation in patients with relapsed follicular lymphoma. Blood. 1999;94:3325–33. [PubMed] [Google Scholar]
- 9.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan A, Bhatia S, Slovak ML, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–93. [PubMed] [Google Scholar]
- 11.Stiff PJ, Dahlberg S, Forman SJ, et al. Autologous bone marrow transplantation for patients with relapsed or refractory diffuse aggressive non-Hodgkin’s lymphoma: value of augmented preparative regimens--a Southwest Oncology Group trial. J Clin Oncol. 1998;16:48–55. doi: 10.1200/JCO.1998.16.1.48. [DOI] [PubMed] [Google Scholar]
- 12.Bierman PJ, Vose JM, Anderson JR, et al. High-dose therapy with autologous hematopoietic rescue for follicular low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 1997;15:445–50. doi: 10.1200/JCO.1997.15.2.445. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services PHS, Centers for Disease Control. National Hospital Discharge Survey for 1990 and 1991. Hyattsville, MD: National Center for Health Statistics; Hospital Care Statistics Branch; Hyattsville, MD: [Google Scholar]
- 14.Graves E. Detailed diagnoses and procedures, national Hospital Discharge Survey, 1989. 1991 [PubMed] [Google Scholar]
- 15.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 16.Silberman G, Crosse MG, Peterson EA, et al. Availability and appropriateness of allogeneic bone marrow transplantation for chronic myeloid leukemia in 10 countries. N Engl J Med. 1994;331:1063–7. doi: 10.1056/NEJM199410203311606. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from in complete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Contal CJOQ. An application of change point methods in studying the effect of age on survival in breast cancer. Computational Statistics and Data Analysis. 1999;30:253–270. [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 20.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer-Verlag; New York: 2000. [Google Scholar]
- 21.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–500. doi: 10.1002/(sici)1097-0258(19990630)18:12<1489::aid-sim140>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Sweetenham JW, Pearce R, Philip T, et al. High-dose therapy and autologous bone marrow transplantation for intermediate and high grade non-Hodgkin’s lymphoma in patients aged 55 years and over: results from the European Group for Bone Marrow Transplantation. The EBMT Lymphoma Working Party. Bone Marrow Transplant. 1994;14:981–7. [PubMed] [Google Scholar]
- 23.Stamatoullas A, Fruchart C, Khalfallah S, et al. Peripheral blood stem cell transplantation for relapsed or refractory aggressive lymphoma in patients over 60 years of age. Bone Marrow Transplant. 1997;19:31–5. doi: 10.1038/sj.bmt.1700604. [DOI] [PubMed] [Google Scholar]
- 24.Moreau P, Milpied N, Voillat L, et al. Peripheral blood stem cell transplantation as front-line therapy in patients aged 61 to 65 years: a pilot study. Bone Marrow Transplant. 1998;21:1193–6. doi: 10.1038/sj.bmt.1701272. [DOI] [PubMed] [Google Scholar]
- 25.Mazza P, Palazzo G, Amurri B, et al. Analysis of feasibility of myeloablative therapy and autologous peripheral stem cell (PBSC) transplantation in the elderly: an interim report. Bone Marrow Transplant. 1999;23:1273–8. doi: 10.1038/sj.bmt.1701804. [DOI] [PubMed] [Google Scholar]
- 26.Jantunen E, Mahlamaki E, Nousiainen T. Feasibility and toxicity of high-dose chemotherapy supported by peripheral blood stem cell transplantation in elderly patients (>/=60 years) with non-Hodgkin’s lymphoma: comparison with patients <60 years treated within the same protocol. Bone Marrow Transplant. 2000;26:737–41. doi: 10.1038/sj.bmt.1702577. [DOI] [PubMed] [Google Scholar]
- 27.Gopal AK, Gooley TA, Golden JB, et al. Efficacy of high-dose therapy and autologous hematopoietic stem cell transplantation for non-Hodgkin’s lymphoma in adults 60 years of age and older. Bone Marrow Transplant. 2001;27:593–9. doi: 10.1038/sj.bmt.1702833. [DOI] [PubMed] [Google Scholar]
- 28.Bitran JD, Klein L, Link D, et al. High-dose myeloablative therapy and autologous peripheral blood progenitor cell transplantation for elderly patients (greater than 65 years of age) with relapsed large cell lymphoma. Biol Blood Marrow Transplant. 2003;9:383–8. doi: 10.1016/s1083-8791(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 29.Leger CS, Bredeson C, Kearns B, et al. Autologous blood and marrow transplantation in patients 60 years and older. Biol Blood Marrow Transplant. 2000;6:204–10. doi: 10.1016/s1083-8791(00)70044-9. [DOI] [PubMed] [Google Scholar]
- 30.Vose JM, Rizzo DJ, Tao-Wu J, et al. 2004:116–27. [Google Scholar]
- 31.Schmitz N, Linch DC, Dreger P, et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353–7. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- 32.Buadi FK, Micallef IN, Ansell SM, et al. 2006:1017–22. doi: 10.1038/sj.bmt.1705371. [DOI] [PubMed] [Google Scholar]
- 33.Wildes TM, Augustin KM, Sempek D, Zhang QJ, Vij R, Dipersio JF, Devine SM. Comorbidities, not age, impact outcomes in autologous stem cell transplant for relapsed non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:840–6. doi: 10.1016/j.bbmt.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Hosing C, Saliba RM, Okoroji GJ, et al. High-dose chemotherapy and autologous hematopoietic progenitor cell transplantation for non-Hodgkin’s lymphoma in patients >65 years of age. Ann Oncol. 2008;19:1166–71. doi: 10.1093/annonc/mdm608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radich JP, Gooley T, Bensinger W, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood. 2003;102:31–5. doi: 10.1182/blood-2002-08-2619. [DOI] [PubMed] [Google Scholar]
- 36.Press OW, Eary JF, Gooley T, et al. A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood. 2000;96:2934–42. [PubMed] [Google Scholar]
- 37.Gopal AK, Rajendran JG, Petersdorf SH, et al. High-dose chemo-radioimmunotherapy with autologous stem cell support for relapsed mantle cell lymphoma. Blood. 2002;99:3158–62. doi: 10.1182/blood.v99.9.3158. [DOI] [PubMed] [Google Scholar]