Abstract
Mutations that eliminate DJ-1 expression cause a familial form of Parkinson’s disease (PD). In sporadic PD, and many other neurodegenerative diseases, reactive astrocytes over-express DJ-1 whereas neurons maintain its expression at non-disease levels. Since DJ-1 has neuroprotective properties, and since astrocytes are known to support and protect neurons, DJ-1 over-expression in reactive astrocytes may reflect an attempt by these cells to protect themselves and surrounding neurons against disease progression. We used neuron-astrocyte contact and non-contact co-cultures to show that DJ-1 knock-down in astrocytes impaired their neuroprotective capacity, relative to wild-type astrocytes, against the neurotoxin rotenone. Conversely, DJ-1 over-expression in astrocytes augmented their neuroprotective capacity. Experiments using astrocyte conditioned media on neuron-only cultures suggested that astrocyte-released, soluble factors were involved in the DJ-1-dependent, astrocyte-mediated neuroprotective mechanism. Our findings support the developing view that astrocytic dysfunction, in addition to neuronal dysfunction, may contribute to the progression of a variety of neurodegenerative disorders.
Keywords: astrocyte, glia, Parkinson’s disease, DJ-1, siRNA, rotenone, neuroprotection, PARK7
Introduction
PD and parkinsonism/dementia syndromes are characterized by selective and progressive degeneration of vulnerable neuronal sub-populations within the brainstem, basal ganglia, and cerebral cortex. The development of effective disease-modifying strategies against these disorders will require an improved understanding of the central mechanisms underlying their pathological processes, as well as those through which the brain attempts to protect itself. We propose that the latter process likely involves astrocytes, and that the therapeutic augmentation of mechanisms involved in astrocyte-mediated neuroprotection may represent a novel, and potentially powerful, avenue for the development of new anti-PD/neurodegenerative disease therapies.
Although it is likely that multiple pathological processes contribute simultaneously to PD initiation and progression, oxidative stress is implicated by the abnormal abundance of oxidized proteins, lipids, and nucleic acids in PD brain tissues, and is supported by observations that experimental parkinsonism can be produced in animal models using neurotoxins that induce oxidative stress (Yoritaka et al., 1996; Giasson et al., 2000; Good et al., 1998; Zhang et al., 1999; Castellani et al., 2002; Dexter et al., 1991; Sian et al., 1994; Sherer et al. 2002; Betarbet et al., 2000). Thus, it is conceivable that PD is caused, at least in part, by chronic, low-level exposure to environmental neurotoxins and that the variability seen in PD susceptibility, presentation, and progression is modulated by complex genetic factors that render individuals either more or less vulnerable to them. In support of this, epidemiological studies have associated high pesticide/herbicide exposure rates with a higher risk of developing PD, and genetic mutations that eliminate the expression of putative neuroprotective proteins (e.g. DJ-1) are known to cause familial PD (Ascherio et al., 2006; Brown et al., 2006; Bonifati et al., 2003; Taira et al., 2004; Zhou and Freed, 2005; Xu et al., 2005).
Astrocytes, an abundant group of neuron support cells that survive in PD, are known to harbor a powerful neuroprotective arsenal that includes neurotrophic factors and anti-oxidative stress molecules, and astrocyte-derived anti-oxidant molecules can be utilized by surrounding neurons to reduce their own levels of oxidative stress (Damier et al., 1993; Makar et al., 1994; Gegg et al., 2003; Wang et al., 2000; Sagara et al., 1996; Sagara et al., 1993; Dringen et al., 1999). In sporadic PD brain tissues, reactive astrocytes are robustly immunoreactive for anti-oxidant proteins such as glutathione peroxidase, heme-oxygenase 1, and DJ-1, whereas non-disease astrocytes (and neurons, disease and non-disease) express these proteins at very low levels (Rizzu et al., 2004; Damier et al., 1993; Schipper et al., 1998; Bandopadhyay et al., 2004; Neumann et al., 2004). Taken together, these observations suggest that astrocytes may attempt to protect themselves and their surrounding neurons against PD progression by over-expressing DJ-1 and other neuroprotective molecules.
We endeavored to explore the relevance of astrocyte-derived DJ-1 to astrocyte-mediated neuroprotection against the pesticide/neurotoxin rotenone using neuron-astrocyte co-cultures in which astrocytic, but not neuronal, DJ-1 expression was experimentally manipulated. We report here that astrocyte-mediated neuroprotection against rotenone is significantly impaired when astrocytes under-express DJ-1, that the reverse is true when astrocytes over-express DJ-1, and that a soluble, astrocyte-released factor appears to be involved in the mechanism.
Materials and methods
Cell cultures
Enriched astrocyte primary cultures were prepared from postnatal day 1 CD1 mouse cerebral cortex. Cortex was used due to its broad relevance to human neurodegenerative disease, its material abundance, and the robustness of the effects seen in our experiments. Brain tissues were microdissected and mechanically dissociated into Neurobasal media (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum (FCS, Hyclone, Logan, UT) and 1X antibiotic-antimycotic (ABAM, Invitrogen), passed through sterile 80 μm and 10 μm filters to remove debris, and plated at a density of 7.3 x 104 trypan blue-excluding cells/cm2 in 24-well plates. For contact neuron-astrocyte co-culture experiments, the astrocytes were plated on media pre-equilibrated 12 mm glass coverslips which were placed in the wells. For non-contact neuron-astrocyte co-culture and astrocyte conditioned media experiments, the astrocytes were plated directly on the plastic surface of the wells. The cultures were fed with fresh DMEM/F12 (Sigma, St. Louis, MO)/FCS/ABAM on days in vitro (DIV) 3 and 6, whereas on and after DIV 10 they were maintained in DMEM/F12/ABAM containing 10% calf serum (CS, Invitrogen). Monolayers prepared in this fashion were typically >97% GFAP+ astrocytes at the time of experimental treatments (astrocyte DIV 20).
Enriched neuronal primary cultures were prepared from embryonic day 15 (E15) CD1 mouse cortex tissues that were microdissected, mechanically dissociated, and plated at 4.5 x 104 trypan blue-excluding cells/cm2 using Neurobasal/10% FCS/ABAM media. Contact neuron-astrocyte co-culture experiments utilized neurons plated directly onto astrocyte monolayers that had been previously established on glass coverslips in 24-well plates. Neuronal cultures for neuron-only, non-contact neuron-astrocyte co-culture (ie, each cell type was physically separated from the other but shared the same media), and astrocyte conditioned media experiments were plated onto polyethyleneimine (PEI)-coated, media pre-equilibrated 12 mm glass coverslips in 24-well plates and maintained separately from the astrocyte cultures until the time of experimentation. After an overnight incubation, the media was replaced with serum-free Neurobasal/1X B27 (Invitrogen)/0.5 mM GlutaMAX (Invitrogen)/ABAM. Two days later the conditioned neuronal media was removed, mixed 1:1 with fresh Neurobasal/B27/GlutaMax/ABAM, sterile-filtered, and returned to the cultures to incubate. This procedure consistently produced cell populations of >97% microtubule associated protein 2 (MAP2)+ neurons and <1% GFAP+ astrocytes at the time of experimental treatments. MAP2+ neuronal processes were extended, branched, and expressed synaptophysin distally at the time of experimental treatments, suggesting that synapse formation was occurring between neurons as a sign of early cellular maturity (data not shown).
For the contact co-cultures, E15 cortex preps were seeded directly onto established 14 DIV astrocyte monolayers in Neurobasal/FCS/ABAM, and experiments were performed at astrocyte DIV 20/neuron DIV 6. For the non-contact co-cultures, E15 cortex preps were seeded onto paraffin beaded, PEI-coated coverslips that did not contain astrocytes, incubated in the same fashion as above to allow the neurons to develop, then incubated with astrocyte monolayers for treatments in a manner that did not allow physical contact between the two cell types. Non-contact co-cultures were also used for experiments at astrocyte DIV 20/neuron DIV 6. The beading procedure was not injurious to the cultures, and the co-culture populations were similar in cellular makeup to each other and to the individual enriched cultures at the time of experimentation.
Institutional approval was obtained for all of the animal procedures used here, and adequate measures were taken to minimize animal pain or discomfort.
Immunocytochemistry of cultured mouse cells
Cells were grown on glass coverslips and identified using fluorescence immunocytochemistry (ICC): astrocytes by GFAP and morphology, neurons by MAP2 and morphology, and total cells by DAPI nuclear staining. The cells were lightly fixed using 4% paraformaldehyde in phosphate buffered saline (PBS), permeabilized using 0.2% Triton X-100 in PBS, blocked using 50% normal serum/0.01% Triton X-100 in PBS, and probed with the appropriate primary antibodies in 5% normal serum/0.01% Triton X-100 in PBS. GFAP was identified using a polyclonal rabbit anti-GFAP primary antibody (Dako) at 1:200, MAP2 using a monoclonal anti-rat MAP2 primary antibody (Sigma) at 1:1000, and DJ-1 using an affinity purified polyclonal rabbit anti-human DJ-1 primary antibody (Neuromics, Edina, MN) at 1:100. Appropriately-targeted secondary antibodies, tagged with either Alexa 488 (Molecular Probes/Invitrogen, green) at 1:125 or Cy3 (Jackson Immunoresearch, red) at 1:400, were used for fluorescence detection. The coverslips were mounted using Vectashield with DAPI (Vector). Cell visualization and manual counts were performed using an inverted microscope fitted with an ocular grid and a fluorescent light source.
Western blot analysis
Protein lysates were prepared from cultured cells using a modified Laemmli-sodium dodecyl sulfate (SDS) extraction protocol (Laemmli, 1970). Equal amounts of protein per lane were electrophoretically separated on an SDS-polyacrylamide gel. The separated proteins were then transferred to Immobilon-FL (LiCor, Lincoln, NE) membranes using a Protean 3 transfer apparatus (BioRad, Hercules, CA), the membranes blocked with Odyssey Blocking Buffer (LiCor), and primary antibodies applied in Odyssey Blocking Buffer/0.1% Tween-20. The following primary antibodies were used: rabbit anti-DJ-1 polyclonal (Neuromics) at 1:5,000, anti-DJ-1 monoclonal (StressGen, for human-specific DJ-1 detection) at 1:2,000, and anti-β-actin monoclonal (Sigma) at 1:2,000. The membranes were then washed and incubated with the following secondary antibodies: IRDye 800-conjugated goat anti-mouse (LiCor, green, for β-actin and human-specific DJ-1 detection) and Alexa 680-conjugated goat anti-rabbit (Molecular Probes/Invitrogen, red, for DJ-1 detection), each at 1:20,000 in the same buffer used for the primary antibodies. Direct-to-scanner detection, band visualization, and quantitation were performed using a LiCor Odyssey scanner.
DJ-1 knock-down in astrocytes using siRNA transfections
Anti-mouse DJ-1 siRNA transfections were used to suppress DJ-1 protein levels (DJ-1 knockdown) in cultured mouse astrocytes to ~5% of control levels throughout the entire experimental treatment period. The following non-overlapping, 21 nucleotide, double-stranded Qiagen (Valencia, CA) siRNAs were used: Mm_Park7_1_HP (siDJ#1, ACC GCT TGT TCT CAA AGA CTA, targets 3’ end of open reading frame [ORF]), Mm_Park7_2_HP (siDJ#2, AGG CGC GGC TGC AGT CTT TAA, targets 5’ end of ORF), and Mm_Park7_5_HP (siDJ#5, CTG AAC CTT GCT AGT AGA ATA, targets 3’ end of ORF). A non-silencing Qiagen control siRNA (siNS, AAT TCT CCG AAC GTG TCA CGT), which has no sequence matches based on BLAST analysis, was used as a transfection control. siRNAs were incubated with Hi-Perfect transfection reagent (Qiagen) to form transfection complexes that were added to astrocyte DIV 10 monolayers in DMEM/F12/CS/ABAM media and incubated for 72 hours. Each preparation was used at a ratio of 50 ng siRNA: 3 μl transfection reagent in 0.5 ml total media/well in a 24-well plate. The siRNA transfection media was then completely removed (astrocyte DIV 13), the monolayers washed once with serum-free media, and then fresh DMEM/F12/CS/ABAM media was used for an overnight incubation. The siRNA transfection efficiency was ~80%, as estimated by using a fluorescein-coupled siNS. For the contact co-culture experiments, E15 preparations were seeded directly onto the post-transfected astrocyte monolayers the following day (astrocyte DIV 14). Thus, under all culture conditions, the neurons were never in contact with the transfection reagents or the siRNAs.
DJ-1 over-expression in astrocytes using plasmid transfections
Full-length human (wild type) and mouse (wild-type or non siDJ#2-targeted rescue) DJ-1 cDNA-containing pCMV-SPORT6 mammalian expression plasmid transfections were used to over-express DJ-1 protein in cultured mouse astrocytes using a CMV promoter. This technique resulted in a 2–3 fold elevation in total intracellular astrocytic DJ-1 protein (human exogenous + mouse endogenous or mouse exogenous + mouse endogenous), compared to baseline mouse levels, after transfections with each cDNA for the full duration of the experimental treatment period. Two full-length, sequence-verified DJ-1 cDNA inserts were used in our studies: human wild-type (hDJwt, NCBI ID BC008188, purchased from American Type Culture Collection [ATCC], Manassas, VA) and mouse wild-type (mDJwt, NCBI ID BC002187, ATCC). A mouse siDJ#2 rescue plasmid (mDJres) was prepared by inserting two silent base mutations (…AGG CGG GGC TGC AGC CTT TAA…, compare with siDJ#2 sequence above) that eliminate targeting by this siRNA but do not change the primary amino acid sequence of the resultant DJ-1 protein. DJ-1 cDNA-containing plasmids, or pCMV-SPORT6 vector-only control plasmids, were incubated with Transfectin Lipid Reagent (BioRad) in a 500 ng DNA: 0.75 μl reagent ratio to form transfection complexes. This solution was then added to astrocyte DIV 10 monolayers in DMEM/F12/CS/ABAM media (500 ng DNA/0/75 μl reagent in 0.5 ml/well in a 24-well plate) and allowed to incubate for 4 hours, after which it was removed, the cells washed once with serum-free media, and the monolayers fed with fresh DMEM/F12/CS/ABAM. The DJ-1-transfected astrocytes were then allowed to incubate for three days prior to feeding with fresh DMEM/F12/CS/ABAM on astrocyte DIV 13. The transfection efficiency was ~85%, as estimating by using a green fluorescent protein-expressing cDNA insert in our base vector. For contact co-culture experiments, E15 (neuron) preparation seeding occurred on astrocyte DIV 14, and the same feeding schedule was used as described above up until the time of experimentation. For the siRNA rescue plasmid experiments, astrocytes were first transfected with the mDJres plasmid for 4 hours, then the complexes were removed, the cells washed once, and the monolayers transfected with the siDJ#2 siRNA for 72 hours (each as described above, and both on astrocyte DIV 10). In the rescue experiments only, all combinations of vector plasmid or mDJ1res plasmid plus siNS siRNA or siDJ#2 siRNA were used to control for non-specific astrocytic toxicity induced by the same-day, sequential transfections. As with the siRNA transfections, the neurons were never exposed to the transfection reagents or the plasmids.
Experimental treatments with rotenone
Rotenone treatments were initiated in all experiments at astrocyte DIV 20/neuron DIV 6, and the duration of all experiments was 72 hours based on the results of time-course experiments. Fresh rotenone powder (Sigma, R-8875) was diluted first in dimethyl sulfoxide (DMSO) and then in Neurobasal/1X B27 antioxidant free (Invitrogen)/1X ABAM media for each experimental replicate. Anti-oxidant free B27 (rather than anti-oxidant replete B27) was used in our experiments in an effort to enhance the toxicity of rotenone (an oxidative neurotoxin) and to potentially allow better delineation of the neuroprotective role of astrocyte-expressed DJ-1 (a putative anti-oxidant protein/regulator of anti-oxidant defense mechanisms). The final rotenone concentrations used in each experiment are shown in the figures, and equal concentrations of DMSO vehicle was used as a control in all non-rotenone treated samples. Contact and non-contact co-cultures were treated exactly the same regarding the toxin treatment conditions, that is, in each case both neurons and astrocytes were present simultaneously over the full 72 hour course of the experiment. These conditions could not be fully replicated for the conditioned media studies, however, due to experimental timing constraints. Therefore, DIV 10 transfected astrocytes were fed with fresh Neurobasal/1X B27 antioxidant free/1X ABAM media at DIV 17, and this media was subsequently collected as a 3-day conditioned media (equal to astrocyte DIV 20) and used for rotenone treatments on neuron-only DIV 6 cultures over the following 72 hours.
Data acquisition and analysis
Neuronal survival was assessed by treatment-blinded manual cell counting of the number of MAP2+ neurons present in 10 randomly-selected high power (200X) fields per coverslip, and averaged to arrive at a single number for each treatment group for each replicate experiment. These numbers were then used to determine the mean and standard error for each treatment across 3–6 replicate experiments. MAP2+ neuronal counts correlated with phase neuronal counts under all conditions of treatment, therefore, differences in MAP2+ counts reflected differences in surviving neuron numbers and not simply changes in the intensity of neuronal MAP2+ signal. Independent cultures, transfections, and rotenone treatments were used for each replicate experiment. Statistical comparisons were made between experimental groups by t-tests, and statistical significance for treatment effect was set at p < 0.05.
Results
Astrocyte presence alone is neuroprotective against rotenone
Neuron-only cultures were sensitive to rotenone toxicity with a 50% lethal dose (LD50) of ~2 nM after 72 hour treatment (Figure 1). When neurons were co-cultured in contact with wild-type (WT) astrocytes, and treated for the same amount of time, the rotenone LD50 shifted to ~12 nM and a window of astrocyte-mediated neuroprotection appeared at doses of 5–20 nM. Under these conditions, co-cultured astrocytes were not able to overcome rotenone neurotoxicity at doses above 40 nM, and doses of 1 nM and lower were not significantly neurotoxic (some data not shown).
Figure 1.
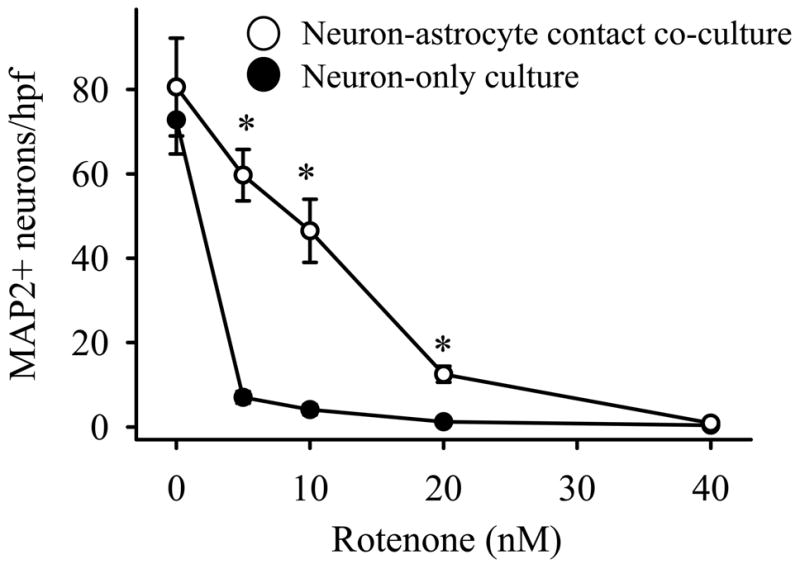
Astrocyte presence alone protects neurons against rotenone toxicity. Cultures were exposed to rotenone at the concentrations shown for 72 hours, immunostained for MAP2 to identify surviving neurons, and the neurons counted in 10 random high power fields (hpf) per treatment. The closed circles (●) represent the average number of surviving neurons in neuron-only cultures, whereas the open circles (○) represent the average number of surviving neurons in neuron-astrocyte contact co-cultures. The mean ± standard error (S.E.) is shown for 5 replicate experiments, and asterisks (*) denote p < 0.01.
DJ-1 deficient astrocytes are impaired in their neuroprotective capacity against rotenone
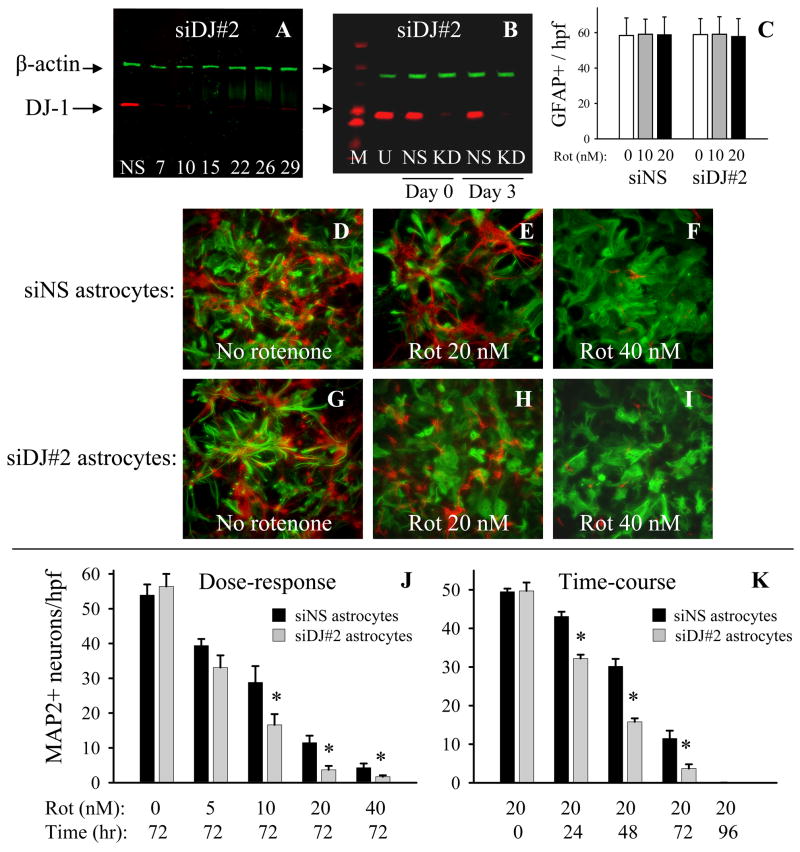
DJ-1 knock-down was used to test the hypothesis that DJ-1 deficient astrocytes would be impaired in their neuroprotective capacity against rotenone relative to DJ-1 replete, wild-type (WT) astrocytes. Three separate anti-mouse DJ-1 siRNAs were tested (siDJ#1, #2, and #5, see Materials and methods), and each suppressed astrocytic DJ-1 protein levels to ~5% of baseline for at least 4 weeks post-transfection (siDJ#2 data shown in Figure 2A–B). These results were used to determine the timing of the co-culture preparation and rotenone treatments, the latter of which corresponded to days 10–13 post-astrocyte transfection. Thus, all co-culture experiments were performed during a period of minimal DJ-1 expression in the astrocytes. None of the anti-DJ-1 siRNAs affected β-actin or α-tubulin levels, and the non-silencing control siRNA (siNS) did not affect DJ-1 or β-actin levels (Figure 2A–B, some data not shown). Furthermore, based on GFAP+ cell counts and cellular morphology, we determined that the transfection reagents/siRNAs were not significantly toxic to the astrocytes, either in the presence or absence of neurotoxic concentrations of rotenone, over the period of our experiments (Figure 2C–I shows the siDJ#2 data, which was similar to that seen for siDJ#1 and siDJ#5). Rotenone treatment did not significantly alter astrocytic DJ-1 protein levels by western blots or enzyme-linked immunosorbent assays, and did not become visibly toxic to the astrocytes until it reached levels >100 nM (data not shown).
Figure 2.
DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection of contact co-cultured neurons against rotenone. (A) Western blot showing the time-course (in days post-siDJ#2 transfection) of astrocytic DJ-1 knock-down. The same pattern was seen for siDJ#1 and siDJ#5 (data not shown), and non-silencing siNS (NS) did not affect DJ-1 protein levels (7 days post-transfection shown). All rotenone experiments were performed between days 10 and 13 post-siRNA transfection, when astrocytic DJ-1 protein levels were nearly undetectable. (B) Western blot showing the levels of DJ-1 protein in untransfected (U) astrocytes and in siNS-transfected (NS) control or siDJ#2 DJ-1 knock-down (KD) astrocytes at times corresponding to the start (Day 0) or the end (Day 3) of all rotenone experiments. The size markers (M) show that the ~21 kDa DJ-1 bands fall between the 20 kDa and 25 kDa marker bands. (C) Treatment with rotenone for 72 hours at 10 or 20 nM did not affect GFAP+ astrocyte numbers when these cells were transfected with siNS or with siDJ#2. The data represent the mean ± S.E. in 3 replicate experiments (similar results were found for siDJ#1 and siDJ#5 transfections, data not shown). (D-I) Astrocytes were transfected with either siNS (WT) or siDJ#2 (DJ-1 knock-down) and then seeded with neurons to produce contact co-cultures. By GFAP immunocytochemistry (green), DJ-1 knock-down astrocytes did not differ in density or morphology from WT astrocytes after 72 hour treatment with rotenone, but clearly did not support neuron (MAP2+, red cells) survival as well as WT astrocytes at a dose of 20 nM rotenone. (J) Dose-response curve showing that siDJ#2-transfected DJ-1 knock-down astrocytes are less protective of contact co-cultured neurons against 10, 20, and 40 nM rotenone after 72 hour treatments than are siNS-transfected WT astrocytes. The data represent the mean ± S.E. in 6 replicate experiments, and asterisks (*) denote p<0.05. (J) Time-course showing that siDJ#2-transfected DJ-1 knock-down astrocytes are less protective of contact co-cultured neurons against 20 nM rotenone after 24, 48, and 72 hour treatments than are siNS-transfected WT astrocytes. The data represent the mean ± S.E. in 4 replicate experiments, and asterisks (*) denote p<0.05.
We next performed dose-response and time-course experiments in contact neuron-astrocyte co-cultures to determine the extent of neurotoxicity allowed by WT (siNS-transfected) vs. DJ-1 knockdown (siDJ#2-transfected) astrocytes over a range of 0–40 nM rotenone (at 72 hours) and 0–96 hours treatment (with 20 nM rotenone), respectively (Figure 2D–K). Visual assessments of these experiments confirmed that DJ-1 knock-down astrocytes were significantly less protective of neurons against rotenone than were DJ-1-replete, WT astrocytes (Figure 2D–I). Quantitative assessments using three distinct anti-DJ-1 siRNAs (siDJ#1, #2, and #5) in separate experiments confirmed these findings, as all three types of DJ-1 knock-down astrocytes were clearly impaired in their neuroprotective capacity against 10 and 20 nM rotenone after 72 hours treatment when compared to siNS-transfected WT astrocytes (Figure 2J and Supplementary Figure 1). In support of these findings, the extent of 20 nM rotenone neurotoxicity was time-dependent, as DJ-1 knock-down astrocytes were less neuroprotective than WT astrocytes at 24, 48, and 72 hours treatment (Figure 2K). By 96 hours, no neuronal cells survived under either co-culture condition, and under all conditions of culture the starting number of neurons were identical.
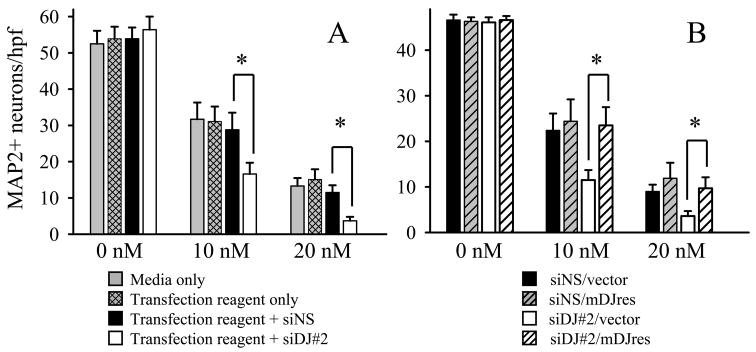
To be sure that the differences between DJ-1 knock-down and WT astrocyte-mediated neuroprotection against rotenone were due to astrocytic DJ-1 deficiency, and not to non-specific astrocytic toxicity produced by the transfection reagents and/or the siRNAs, we employed a number of control experiments. First, we showed that exposure of the astrocytes to media only, transfection reagent alone, transfection reagent + siNS, or transfection reagent + siDJ#2 (DJ-1 knock-down) did not change the number of contact co-cultured neurons present under control (no rotenone) conditions, and that only siDJ#2-mediated DJ-1 knock-down astrocytes were deficient in their capacity to protect neurons against rotenone relative to the other three types of DJ-1-replete astrocytes (Figure 3A). Second, we showed that the reduction in astrocyte-mediated neuroprotection produced by siDJ#2-mediated DJ-1 knock-down in astrocytes was completely reversed, or “rescued,” by co-transfection of the same cells with a non-siDJ#2 targeted mouse DJ-1 cDNA (mDJres) expression plasmid in contact co-cultures (Figure 3B). In this graph only, astrocyte double-transfections were used to justify direct comparisons: siNS/vector plasmid (WT), siNS/mDJres plasmid (DJ-1 over-expression), siDJ#2/vector plasmid (DJ-1 knock-down), and siDJ#2/mDJres plasmid (DJ-1 rescue). Again, and to the same extent as the single-transfection experiments shown in Figure 3A, DJ-1 knock-down astrocytes were significantly less neuroprotective against rotenone than were WT astrocytes. The double-transfected DJ-1 over-expressing astrocytes were slightly more protective of neurons than were the double-transfected WT astrocytes, but not significantly so by statistical analysis, and the modest protection seen in these experiments was not nearly as robust as it was in experiments performed using only single plasmid transfections (Figure 5, discussed below).
Figure 3.
DJ-1 knock-down in astrocytes impairs astrocyte-mediated protection of contact co-cultured neurons against rotenone in a DJ-1 dependent manner. (A) Neurons survive 72 hour rotenone treatments (concentrations listed at bottom of graphs) equally well when they are co-cultured with astrocytes that were previously exposed to media only, the transfection reagent alone, or the transfection reagent + non-silencing siNS. Astrocyte exposure to transfection reagent + siDJ#2 (DJ-1 knock-down) renders the DJ-1-deficient astrocytes less neuroprotective against rotenone than the three forms of DJ-1 replete astrocytes. (B) The impairment of astrocyte-mediated neuroprotection caused by siDJ#2 DJ-1 knock-down was rescued by co-transfection with a non-siDJ#2 targeted DJ-1 rescue plasmid (mDJres). DJ-1 knock-down astrocytes, here transfected with siDJ#2 + vector plasmid, are again less neuroprotective than the DJ-1 replete astrocytes. This effect is rescued to the baseline neuroprotection level of each treatment by co-transfection of siDJ#2 DJ-1 knock-down astrocytes with DJ-1 rescue plasmid (mDJres). The data in each graph represents the mean ± S.E. from 6 replicate experiments, and asterisks (*) denote p<0.05.
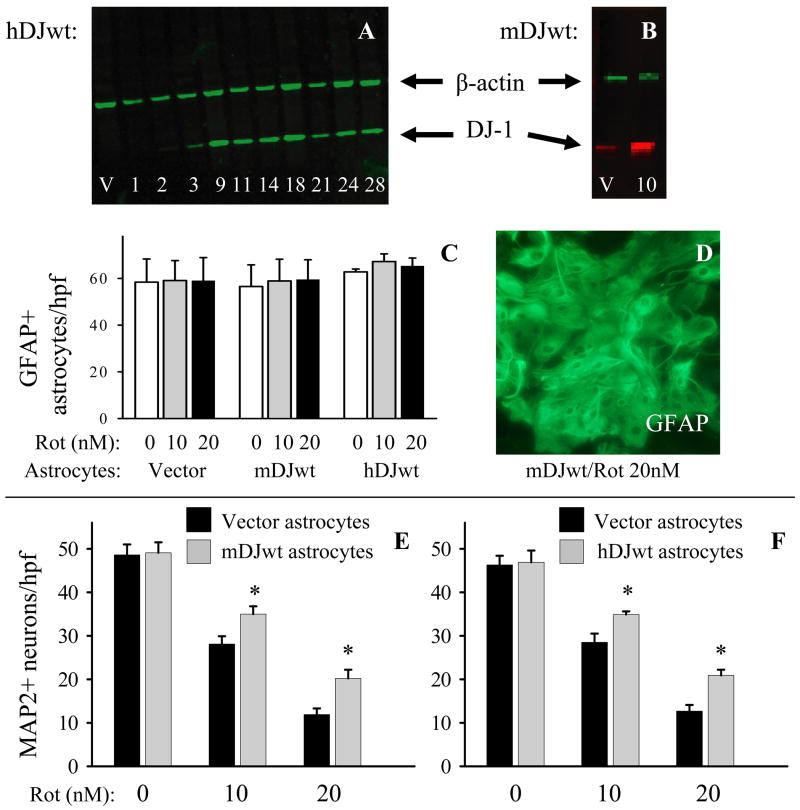
Figure 5.
DJ-1 over-expression in astrocytes augments astrocyte-mediated protection of contact co-cultured neurons against rotenone. (A) Western blot showing the time course (in days post-plasmid transfection) of human DJ-1 protein expression in mouse astrocytes after transfection with wild-type human DJ-1 cDNA (hDJwt). Vector plasmid (no DJ-1 insert, V) transfection did not result in hDJ-1 expression (shown here at 7 days post-transfection). All rotenone experiments were performed between days 10–13 post-transfection, when DJ-1 over-expression levels were maximal. (B) Western blot showing wild-type mouse DJ-1 (mDJwt) over-expression in astrocytes at day 10 post-transfection relative to same-time vector-transfected levels. This time point corresponds to the start time of all rotenone experiments. (C) Treatment with rotenone for 72 hours at 10 or 20 nM did not affect GFAP+ astrocyte numbers compared to controls (0 nM) when these cells were transfected with vector, mDJwt, or hDJwt plasmids. The data represent the mean ± S.E. in 3 replicate experiments. (D) GFAP immunocytochemistry demonstrated that mDJwt-transfected astrocytes treated for 72 hours with 20 nM rotenone retained a normal, healthy-appearing morphology and cellular density (similar results were seen with hDJwt and mDJres transfections, data not shown). (E) Astrocytes that over-expressed mouse DJ-1 (mDJwt-transfected, gray bars) were more neuroprotective against rotenone than were WT astrocytes (vector-transfected, black bars). (F) Astrocytes that over-expressed human DJ-1 (hDJwt-transfected, gray bars) were more neuroprotective against rotenone than were WT astrocytes (vector-transfected, black bars). In both E and F, rotenone was used at the concentrations shown for 72 hours, the graphs represent the means ± S.E. from 5 replicate experiments, and asterisks (*) denote p<0.05. The results of experiments performed with mDJres were similar (data not shown).
Astrocyte-released, soluble factors are involved in the mechanism of the impaired neuroprotective capacity seen in DJ-1 deficient astrocytes
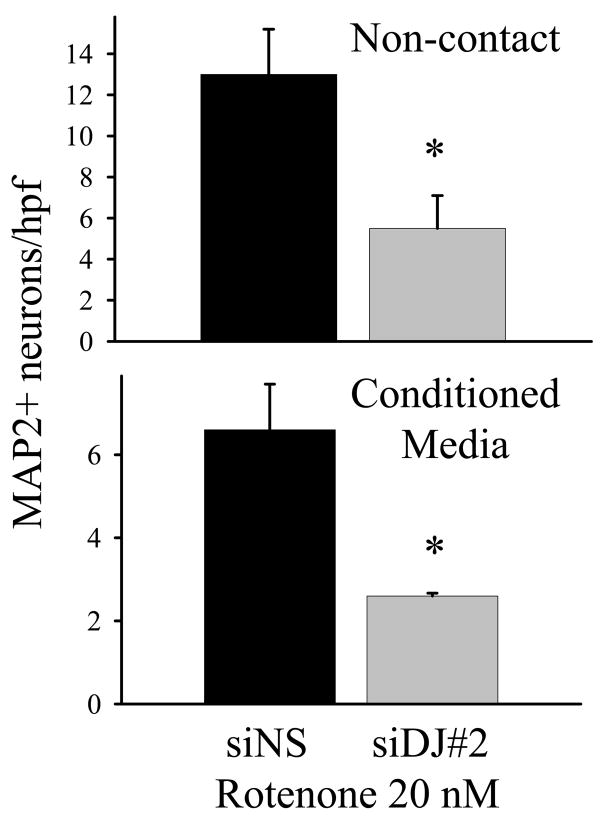
As with the contact co-culture experiments, in which neurons and astrocytes interacted by direct physical contact as well as through shared media, DJ-1 deficient astrocytes (siDJ#1-, siDJ#2-, and siDJ#5-transfected) were less protective of non-contact co-cultured neurons against rotenone than were siNS-transfected, WT astrocytes (Figure 4 top graph, and Supplementary Figure 1). Since physical contact between the two cell types was prevented in these experiments, soluble factors in the media must be involved in the mechanism of altered astrocyte-mediated neuroprotection. Since the source of these factors could be either neuronal or astrocytic, or both, we also evaluated WT vs. DJ-1 knock-down astrocyte conditioned media (Figure 4 bottom graph, and Supplementary Figure 1). As with the contact and non-contact co-culture experiments, the conditioned media from all three types of DJ-1 knock-down astrocytes was less protective of neurons against rotenone than was the conditioned media from WT astrocytes. Since the conditioned media was derived only from astrocytes, astrocyte-released soluble factors must be involved in the mechanism of altered astrocyte-mediated neuroprotection.
Figure 4.
DJ-1 knock-down in astrocytes impairs astrocyte-mediated protection of neurons against rotenone by altering soluble, astrocyte-released factors. In the non-contact neuron-astrocyte co-culture experiments (upper graph), DJ-1 deficient astrocytes (siDJ#2-transfected, gray bar) were less protective of co-cultured neurons against 72 hour treatment with 20 nM rotenone than were DJ-1 replete astrocytes (siNS-transfected, black bar). In the conditioned media experiments (lower graph), conditioned media from DJ-1 deficient astrocytes (gray bar) was less protective of neurons against rotenone than was conditioned media from DJ-1 replete astrocytes (black bar). The data in each graph represents the mean ± S.E. from 5 (non-contact) or 3 (conditioned media) replicate experiments, and asterisks (*) denote p<0.05.
We noted that the number of surviving neurons was lower in the conditioned media experiments than in the co-culture experiments under the same conditions of rotenone treatment, despite the fact that equal numbers of neurons were present at the start of each type of experiment. We suspect that this is related to two issues: astrocyte presence/contact is likely more trophic to neurons than astrocyte conditioned media alone, and the conditioned media was already 3 days old at the time of rotenone treatments (whereas contact and non-contact co-culture media was always fresh).
We were unable to detect soluble, released DJ-1 in the 3-day astrocyte conditioned media (in either unconcentrated or concentrated preparations), suggesting that other astrocyte-released soluble factors are involved in astrocyte-mediated neuroprotection in our system. Further, neuronal DJ-1 protein levels did not change under any conditions of non-contact co-culture with astrocytes, either relative to each other or to neuron-only cultures, supporting the astrocyte-specificity of our culture and transfection methods (data not shown).
DJ-1 over-expressing astrocytes exhibit augmented neuroprotective capacity against rotenone, and astrocyte-released soluble factors appear to be involved in the mechanism
DJ-1 protein over-expression was produced in astrocytes prior to seeding neurons using three separate DJ-1 cDNAs (mDJwt, mDJres, and hDJwt, see Materials and methods) that were incorporated into a mammalian expression plasmid. After transfection, and encompassing the entire period of our co-cultures and rotenone treatments, the extent of DJ-1 protein over-expression in astrocytes was ~2–3 times that of baseline levels (Figure 5A–B). Vector-only plasmid transfections did not cause hDJ-1 to be expressed in mouse astrocytes, nor did it stimulate elevated expression of mDJ-1. Plasmid transfections and rotenone treatments again did not alter astrocyte morphology or cell counts over the period of our co-culture experiments (Figure 5C–D).
Contact neuron-astrocyte co-cultures were used to determine whether DJ-1 over-expressing astrocytes produced using each of the three cDNAs listed above, in separate experiments, were more neuroprotective against rotenone than vector-transfected WT astrocytes. In each case, there was no baseline difference in the number of neurons surviving in the absence of rotenone, and for all three cDNAs the resultant DJ-1 over-expressing astrocytes were more neuroprotective than the WT astrocytes at rotenone concentrations of 10 nM and 20 nM (Figure 5E–F, mDJres not shown but gave similar results). Note that the same window of rotenone sensitivity was seen here as was seen in the siRNA co-culture experiments. In addition, non-contact neuron-astrocyte co-culture and astrocyte conditioned media experiments also showed that DJ-1 over-expressing astrocytes were more neuroprotective than WT astrocytes against rotenone at 20 nM (Figure 6).
Figure 6.
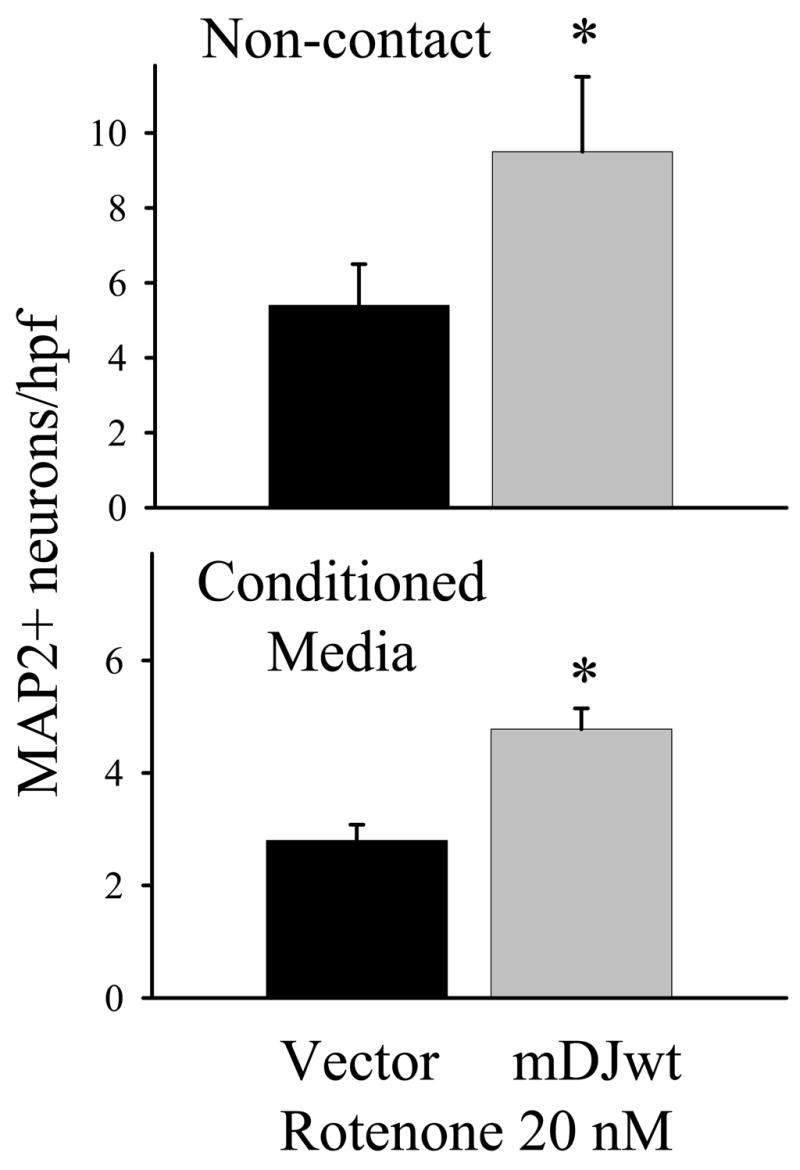
DJ-1 over-expression in astrocytes augments astrocyte-mediated protection of neurons against rotenone by altering soluble, astrocyte-released factors. In the non-contact neuron-astrocyte co-culture experiments (upper graph), DJ-1 over-expressing astrocytes (mDJwt-transfected, gray bar) were more protective of co-cultured neurons against 72 hour treatment with 20 nM rotenone than were WT astrocytes (vector-transfected, black bar). In the conditioned media experiments (lower graph), conditioned media from DJ-1 over-expressing astrocytes (gray bar) was more protective of neurons against rotenone than was conditioned media from WT astrocytes (black bar). The data in each graph represent the mean ± S.E. from 5 (non-contact) or 3 (conditioned media) replicate experiments, and asterisks (*) denote p<0.05.
Discussion
This study is the first to show that the experimental under- and over-expression of a PD-causing gene (DJ-1) in astrocytes renders them less or more neuroprotective, respectively, against a potentially PD-relevant environmental neurotoxin (the pesticide rotenone) that is known to cause experimental parkinsonism in rodents and non-human primates.
Mutations that eliminate human DJ-1 expression are sufficient to cause an early-onset form of familial PD, and sporadic PD tissues show immunohistochemical evidence of robust DJ-1 expression in reactive astrocytes that is out of proportion to that seen in neurons (Bonifati et al., 2003; Rizzu et al., 2004). Reactive astrocytes from many brain regions are also intensely DJ-1 immunoreactive in a variety of parkinsonism/dementia syndromes and in human infarcts, whereas non-disease astrocytes and neurons (both non-disease and disease) are only weakly stained (Rizzu et al., 2004; Bandopadhyay et al., 2004; Neumann et al., 2004; Mullett et al., 2008). Thus, although DJ-1 is clearly expressed in both human neurons and human astrocytes, its expression in the latter cell type appears to be much more vigorously augmented in both acute and chronic forms of neurodegenerative disease. Since DJ-1 has anti-oxidative stress, anti-apoptotic, and other potential neuroprotective properties, is robustly expressed in reactive astrocytes, and since astrocytes may be the major source of anti-oxidant and other trophic support in the brain, DJ-1 may be over-expressed in astrocytes across a broad spectrum of neurodegenerative diseases in an attempt to protect themselves, and their surrounding neurons, against disease progression (Xu et al., 2005; Junn et al., 2005; Inden et al., 2006; Shinbo et al., 2006; Meulener et al., 2006; Clements et al., 2006; Gorner et al., 2007; Andres-Mateos et al., 2007; Aleyasin et al., 2007; Fan et al., 2008). Neuronal selective vulnerability in PD and other degenerative disorders may therefore reflect not only altered neuronal mechanisms, but also altered astrocytic and neuron-astrocyte interaction mechanisms (Rizzu et al, 2004; Damier et al., 1993; Vila et al., 2001).
For these reasons, we hypothesized that DJ-1 deficient astrocytes would exhibit impaired neuroprotective capacity relative to DJ-1 replete WT astrocytes, and that DJ-1 over-expressing astrocytes would be more neuroprotective. To test this, we assayed siRNA-mediated DJ-1 knockdown astrocytes and plasmid-mediated DJ-1 over-expressing astrocytes for their ability to protect neurons against rotenone. We found that WT astrocyte presence alone significantly reduced rotenone toxicity on contact co-cultured neurons, that DJ-1 knock-down in astrocytes impaired their neuroprotective capacity against rotenone under these same conditions, and that DJ-1 over-expression in astrocytes enhanced their neuroprotective capacity. Since the astrocytes remained healthy under all conditions of treatment, the DJ-1 gene was specifically targeted using multiple siRNAs and cDNAs, and since the neurons were never exposed to the transfection complexes, the effects of manipulation of astrocytic DJ-1 expression on rotenone neurotoxicity can be interpreted to reflect primary/initial changes in astrocyte physiology, and thus to be DJ-1-dependent and astrocyte-mediated.
Although single-transfected DJ-1 over-expressing astrocytes were clearly more neuroprotective than WT astrocytes in the contact co-cultures (as well as the non-contact and astrocyte conditioned media experiments), we noted that double-transfected DJ-1 over-expressing astrocytes were only slightly more neuroprotective than their double vector-transfected WT counterparts. This likely represented an increased level of non-specific astrocytic toxicity induced by the double-transfections, which may have rendered the astrocytes less able to mount an enhanced neuroprotective response. It was also clear that the augmentation of astrocyte-mediated neuroprotection produced by DJ-1 over-expressing astrocytes was less robust than the impairment seen in our DJ-1 knock-down astrocyte experiments. This suggests that the high baseline DJ-1 levels seen in WT astrocytes may be sufficient to deliver nearly-optimum astrocyte-mediated neuroprotection, in which case further DJ-1 over-expression may only be able to augment this process slightly, and DJ-1 knock-down would be expected to be much more robust. We did not find any evidence that rotenone, itself, enhanced DJ-1 expression in astrocytes, suggesting that endogenous DJ-1 levels are sufficient for the maintenance of normal astrocytic functioning during exposure to this toxin under our experimental conditions.
To begin to delineate the mechanism of the astrocyte-mediated neuroprotection discovered in our system, we employed non-contact neuron-astrocyte co-cultures to determine whether soluble factors were involved, and astrocyte conditioned media experiments to determine whether these factors were astrocyte-derived. Under each set of transfection conditions (ie, astrocytic DJ-1 under- and over-expression), all three sets of culture systems (ie, contact, non-contact, and astrocyte conditioned media) produced the same results with respect to DJ-1-related alterations in astrocyte-mediated neuroprotection. That is, all three sets of culture conditions showed reduced neuroprotection delivered by DJ-1 deficient astrocytes, and augmented neuroprotection delivered by DJ-1 over-expressing astrocytes. Thus, the separate culture systems served as internal controls for one another, and, when considered together, the experiments using DJ-1 knock-down astrocytes and DJ-1 over-expressing astrocytes proved that in each case a soluble, astrocyte-derived factor (or set of factors) must be involved in the mechanism of astrocyte-mediated neuroprotection.
In aggregate, then, the astrocytic DJ-1 knock-down and over-expression experiments complimented and solidified each other’s findings, even down to the same dose window of rotenone neurotoxicity, and provided strong support for our conclusions that astrocyte-expressed DJ-1 and astrocyte-released soluble factors are central to the mechanism of astrocyte-mediated neuroprotection discovered in our system. Since we were unable to identify DJ-1 itself in the astrocyte conditioned media, either in unconcentrated or concentrated form, or under any condition of treatment, it appears that molecules downstream of DJ-1 are involved in this mechanism. DJ-1 knock-down astrocytes may therefore become less neuroprotective through reduced release of protective substances (such as peptide neurotrophic factors or anti-oxidant molecules), increased release of toxic substances (such as excitotoxins or selected cytokines), or by some combination of these or other processes (such as pH or ionic component management). Likewise, DJ-1 over-expressing astrocytes may become more neuroprotective by the reverse of these processes. Studies are ongoing in the laboratory to further delineate these potentially complex mechanisms.
In summary, we have shown that DJ-1 knock-down in astrocytes (which models aspects of PARK7 mutation hereditary PD) impairs astrocyte-mediated neuroprotection against rotenone, that DJ-1 over-expression in astrocytes (which models aspects of sporadic PD and other neurodegenerative diseases) augments astrocyte-mediated neuroprotection, and that this process likely involves astrocyte-released, soluble factors. It does not appear that DJ-1 itself is released from astrocytes, but rather that intracellular DJ-1 acts as a mediator of other critical downstream mechanisms. These latter effectors may include astrocyte-released anti-oxidant molecules, neurotrophic factors, and cytokines, and/or astrocytic modulation of the ionic, pH, and excitotoxic microenvironment. Our findings here, combined with published human data, suggest that DJ-1 may play a role in the endogenous neuroprotective arsenal employed by astrocytes in PD and other neurodegenerative diseases, and that PARK7 mutation PD may develop because of both neuronal and astrocytic dysfunction. Thus astrocytes, which abundantly survive in most neurodegenerative conditions, may be ideal targets for novel disease-modifying therapies that augment their neuroprotective arsenal, and this may include DJ-1 regulated mechanisms.
Supplementary Material
DJ-1 knock-down in astrocytes using siDJ#1 and siDJ#5 transfections impairs astrocyte-mediated neuroprotection against rotenone, relative to siNS-transfected WT astrocytes, in a manner similar to that seen with siDJ#2 astrocyte transfections. Cultures were treated with rotenone at the concentrations shown for 72 hours and the number of surviving MAP2+ neurons counted in contact neuron-astrocyte co-cultures (5 replicate experiments), non-contact neuron-astrocyte co-cultures (4 replicate experiments), and in neuron-only cultures containing astrocyte conditioned media (4 replicate experiments). Asterisks (*) denote p<0.05.
Acknowledgments
This work was supported by NINDS K08NS055736–01, Pittsburgh Foundation John F. and Nancy A. Emmerling Fund for Medical Research, and Pittsburgh Institute for Neurodegenerative Diseases grants to DH. We acknowledge the technical assistance of Anamika Nath and Beth Gabris.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleyasin H, Rousseaux MWC, Phillips M, Kim RN, Bland RJ, Callaghan S, Slack RS, During MJ, Mak TW, Park DS. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. PNAS. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 Gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. PNAS. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Chen H, Weisskopf MD, O’Reilly E, McCullough ML, Celle EE, Schwarzschild MA, Thun MJ. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature Neurosci. 2000;12:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld G, Krieger E, Dekker MCJ, Squitieri F, Ibanez P, Joose M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease - Is there a link? Env Health Perspectives. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Perry G, Seidlak SL, Nunomura A, Shimohama S, Zhang J, Montine T, Sayre LM, Smith MA. Hydroxynonenal adducts indicate a role for lipid peroxidation in neocortical and brainstem lewy bodies in humans. Neuroscience Letters. 2002;319:25–28. doi: 10.1016/s0304-3940(01)02514-9. [DOI] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JPY. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. PNAS. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alteration in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neuroscience. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Ren H, Jia N, Fei E, Zhou T, Jian P, Wu M, Wang G. DJ-1 decreases Bax expression through repressing p53 transcriptional activity. JBC. 2008;283:4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Beltran B, Salas-Pino S, Bolanos JP, Clark JB, Moncada S. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurons: implications for neuroprotection/neurodegeneration? J Neurochemistry. 2003;86:228–237. doi: 10.1046/j.1471-4159.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IVJ, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Good PF, Hsu A, Werner P, Perl DP, Olanow CW. Protein nitration in Parkinson’s disease. J Neuropath and Exp Neurology. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Gorner K, Holtorf E, Waak J, Pham TT, Vogt-Weisenhorn DM, Wurst W, Haass C, Kahle PJ. Structural determinants of the C-terminal helix-kink-helix motif essential for protein stability and survival promoting activity of DJ-1. J Biol Chem. 2007;282:13680–13691. doi: 10.1074/jbc.M609821200. [DOI] [PubMed] [Google Scholar]
- Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, Takata K, Yanagisawa D, Nishimura K, Taniguchi T, Kiso Y, Yoshimoto K, Agatsuma T, Koide-Yoshida S, Iguchi-Ariga SMM, Shimiohama S, Ariga H. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson’s disease rat model. Neurobiol of Disease. 2006;24:144–158. doi: 10.1016/j.nbd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. PNAS. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head og bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJL. Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: Evidence that astrocytes play an important role in antioxidant processes in the brain. J Neurochemistry. 1994;62:45–53. doi: 10.1046/j.1471-4159.1994.62010045.x. [DOI] [PubMed] [Google Scholar]
- Meulener MC, Xu K, Thompson L, Ischiropoulos H, Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. PNAS. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullett S, Hamilton R, Hinkle D. DJ-1 immunoreactivity in human brain astrocytes is dependent on infarct presence and infarct age. Neuropathology. 2008 doi: 10.1111/j.1440-1789.2008.00955.x. in press. [DOI] [PubMed] [Google Scholar]
- Neumann M, Muller V, Gorner K, Kretzschmar HA, Haas C, Kahle PJ. Pathological properties of the Parkinson’s disease-associated protein DJ-1 in α-synucleinopathies and tauopathies: relevance for multiple system atrophy and Pick’s disease. Acta Neuropath. 2004;107:489–496. doi: 10.1007/s00401-004-0834-2. [DOI] [PubMed] [Google Scholar]
- Rizzu P, Hinkle D, Zhukareva V, Bonifati V, Severijnen LA, Martinez D, Ravid R, Kamphorst W, Eberwine JH, Lee VMY, Trojanowski JQ, Heutink P. DJ-1 co-localizes with pathological tau inclusions: A link between Parkinsonism and dementia. Annals of Neurology. 2004;55:113–118. doi: 10.1002/ana.10782. [DOI] [PubMed] [Google Scholar]
- Sagara J, Makino N, Bannai S. Glutathione efflux from cultured astrocytes. J Neurochemistry. 1996;66:1876–1881. doi: 10.1046/j.1471-4159.1996.66051876.x. [DOI] [PubMed] [Google Scholar]
- Sagara J, Miura K, Bannai S. Maintenance of neuronal glutathione by glial cells. J Neurochemistry. 1996;61:1672–1676. doi: 10.1111/j.1471-4159.1993.tb09802.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos JR, Õvervik E, Ames BN. A marker of oxyradical-mediated DNA damage (8-Hydroxy-2’Deoxyguanosine) is increased in nigro-striatum of Parkinson’s disease brain. Neurodegeneration. 1994;3:197–204. [Google Scholar]
- Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Experimental Neurology. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson’s disease: Linking mitochondrial impairment to altered α-synuclein metabolism and oxidative damage. J Neuroscience. 2002;16:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinbo Y, Niki T, Taira T, Ooe H, Takahashi-Niki K, Maita C, Seino C, Iguchi-Ariga SMM, Ariga H. Proper SUMO-1 conjugation is essential to DJ-1 to exert its full activities. Cell Death and Differentiation. 2006;13:96–108. doi: 10.1038/sj.cdd.4401704. [DOI] [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Annals of Neurology. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SMM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Reports. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Guegan C, Wu DC, Teismann P, Choi DK, Tieu K, Przedborski S. The role of glial cells in Parkinson’s disease. Current Opinion in Neurology. 2001;14:483–489. doi: 10.1097/00019052-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochemistry. 2000;74:1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhong N, Wang H, Elias JE, Kim CY, Woldman I, Pifl C, Gygi SP, Guela C, Yankner BA. The Parkinson’s disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Human Mol Genetics. 2005;14:1231–1241. doi: 10.1093/hmg/ddi134. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenol protein adducts in Parkinson disease. PNAS. 1996;93:3696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DJ, Montine TJ. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. American Journal of Pathology. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibitis A53T α-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DJ-1 knock-down in astrocytes using siDJ#1 and siDJ#5 transfections impairs astrocyte-mediated neuroprotection against rotenone, relative to siNS-transfected WT astrocytes, in a manner similar to that seen with siDJ#2 astrocyte transfections. Cultures were treated with rotenone at the concentrations shown for 72 hours and the number of surviving MAP2+ neurons counted in contact neuron-astrocyte co-cultures (5 replicate experiments), non-contact neuron-astrocyte co-cultures (4 replicate experiments), and in neuron-only cultures containing astrocyte conditioned media (4 replicate experiments). Asterisks (*) denote p<0.05.