Abstract
SAHA, a histone deacetylase inhibitor (HDACi), is clinically approved for treatment of cutaneous T-cell lymphoma. Although the exact underlying mechanisms are unknown, HDACi arrest the cell cycle in rapidly proliferating tumor cells and promote their apoptosis. HDACi were also recently shown to enhance the production and suppressive functions of Foxp3+ regulatory T (Treg) cells in rodents, leading us to begin to investigate the actions of HDACi on rhesus monkey T cells for the sake of potential preclinical applications. In this study, we show that SAHA inhibits polyclonal activation and proliferation of rhesus T cells and that the anti-proliferative effects are due to inhibition of T effector (Teff) cells and enhancement of Treg cells. Cryopreserved rhesus macaque splenocytes were CFSE labeled, stimulated with anti-CD3/anti-CD28 and cultured for 5 days in the presence of varying concentrations of SAHA. Samples were then co-stained to evaluate CD4 and CD8 expression. 10 and 5μM concentrations of SAHA were toxic to splenocytes. Proliferation was inhibited by 57% in CD4 cells and 47% in CD8 cells when unseparated splenocytes were cultured with 3 μM SAHA. Effector cells alone showed a decreased inhibition to proliferation when cultured with 3 μM and 1 μM SAHA when compared to Teff plus Treg cells. Our data suggest that SAHA can be used as part of an immunosuppressive protocol to enhance graft survival by limiting Teff cell proliferation as well as increasing Treg cells, thereby promoting tolerance.
INTRODUCTION
Various histone deacetylase inhibitors (HDACi) in clinical development inhibit the proliferation of transformed cells in culture by inducing cell cycle arrest, differentiation and/or apoptosis, and inhibit tumor growth in animal models.1 HDACi are under intensive study as transcriptionally-based anticancer therapies, given evidence that mutations resulting in constitutive activation of oncogenes, or functional inactivation of tumor suppressor genes, are key tumorigenic events.2,3 Compared to their use in cancer therapy, far less is known regarding the effects of HDACi on the immune system, though HDACi are capable of inducing lymphocyte cell cycle arrest, differentiation or apoptosis in vitro.4–6
HDACi are also being evaluated experimentally for anti-inflammatory or immunosuppressive effects. Administration of the HDACi, suberoylanilide hydroxamic acid (SAHA) after bone marrow transplantation reduced expression of proinflammatory cytokines and decreased intestinal injury, clinical severity, and mortality from acute graft-versus-host disease as compared with vehicle-treated animals.7 Trichostatin A, structurally related to SAHA, attenuated development of asthma-like bronchial hypersensitivity in mice by decreasing expression of Th2 cytokines and IgE production5, blocked production of inflammatory mediators in MRL/lpr mice8, and decreased Th1 cytokine production and demyelination in a murine experimental allergic encephalomyelitis model.9 These beneficial effects were thought due to induction of apoptosis in host inflammatory cells.10 However, recently HDACi therapy was shown to enhance regulatory T (Treg) cell production and suppressive functions in mice, with beneficial consequences in models of homeostatic proliferation, inflammatory bowel disease, and cardiac and islet allograft transplantation.11 In this study, we show that SAHA inhibits proliferation of rhesus T cells, induces expression of FoxP3 and enhances Treg-mediated suppression of effector T cell responses in vitro. Our studies indicate potential therapeutic application of SAHA in prolonging survival of graft function.
MATERIALS AND METHODS
SAHA was purchased from the University of Minnesota Fairview Medical Center Pharmacy and dissolved in (DMSO) at stock concentration of 10 mM. Rhesus splenocytes were labeled with CFSE, resuspended in RPMI/5% FCS and plated in a 96-well plate at 0.5×106 cells/well along with titrating doses of SAHA at final concentrations of 5 μM, 3 μM and 1 μM. Cells were stimulated with 5 μg/ml plate-bound anti-CD3 and 5 μg/ml soluble anti-CD28 mAbs. Media controls received no stimulation. Cells were incubated for 5 d at 37°C, 5% CO2, and then stained with CD4 PerCP and CD8 APC or CD4 PerCP, CD25 PE (BD Biosciences) and Foxp3 APC (eBiosciences) for detection of Treg cells. All flow samples were acquired on a Becton Dickson FACScalibur with CellQuest software and analyzed with FlowJo software. In proliferation assays, splenocytes were stimulated with anti-CD3 and anti-CD28 mAbs, as above. After 5 d, plates were pulsed with 1 mCi/well of thymidine and incubated for 16 hrs. Cells were harvested, mats allowed to dry and counted using a beta-scintillation counter.
CD25− T effector cells were purified from splenocytes using a Treg purification kit from Miltenyi. Briefly, cells were stained with anti-CD25PE antibody, followed by anti-PE magnetic beads and purified using magnetic column. CD25− cells were collected as flow through from the column and CD25+ cells were extracted from the column as Tregs. T effectors were labeled with CFSE and stimulated with anti-CD3 and anti-CD28, as above, either in the presence of SAHA with or without addition of Tregs in 1:0.3 ratio. Cell proliferation was measured at day 5, as above.
RESULTS
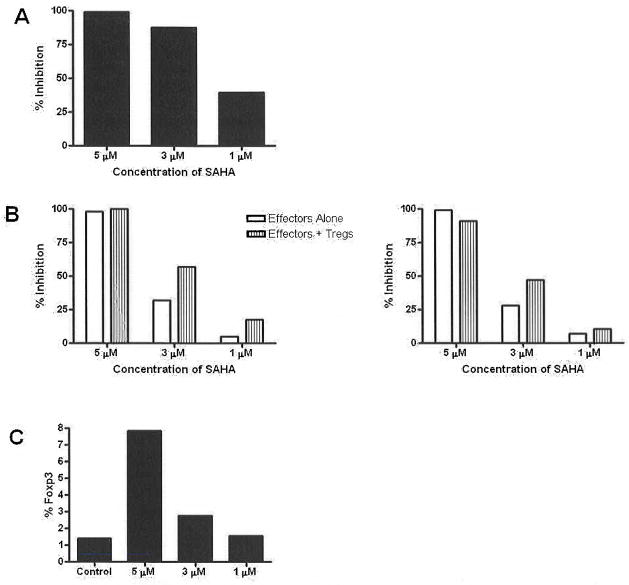
Concentrations of 10 and 5 μM SAHA were toxic to splenocytes as demonstrated by a decrease in lymphocyte population noted on the forward/side scatter (data not shown). The addition of DMSO in varying concentrations did not change the profile of CFSE proliferation from that of the positive control (data not shown). Proliferation was inhibited by 99.2%, 87.4% and 39.5% when splenocytes were cultured with 5 μM, 3μM and 1 μM SAHA, respectively (Fig 1a). When effector cells were cultured alone, CD4 proliferation was inhibited by 98%, 32% and 5% when cultured with 5μM, 3μM and 1 μM SAHA, respectively. When effectors and Tregs were cultured with 5 μM, 3 μM and 1 μM SAHA, CD4 proliferation was inhibited by 100%, 57% and 17.5%, respectively. When effector cells were cultured alone, CD8 proliferation was inhibited by 99%, 28%, and 7% when cultured with 5 μM, 3 μM and 1 μM SAHA, respectively. When effectors and Tregs were cultured with 5 μM, 3 μM and 1 μM SAHA, CD8 proliferation was inhibited by 91%, 47% and 10%, respectively (Fig 1b). Expression of Foxp3 increased in splenocytes cultured in 5 μM and 3 μM when compared to Day 0 baseline (Fig 1c).
Fig 1.
Effects of SAHA on rhesus T cells in vitro. (a) Dose-dependent inhibition of splenocyte proliferation. (b) SAHA inhibited proliferation of both CD4 and CD8 T cells, and effects were enhanced when Tregs were included in the assays. (c) Flow cytometric analysis showed that SAHA increased the numbers of Foxp3+ T cells in splenocyte cultures.
DISCUSSION
Our data show that SAHA inhibits proliferation of rhesus CD4 and CD8 T cells. Furthermore, our data suggest that the effects of SAHA may be two-fold; directly inhibiting proliferation of T effector cells while at the same time increasing the suppressive capabilities of Treg cells through upregulation of FoxP3. Further dose-response and other studies will allow us to dissect the relative importance of these effects in vitro and in vivo. Our ongoing studies are directed towards assessing whether use of SAHA in vivo may enhance outcomes in preclinical rhesus islet transplant models.
Acknowledgments
This work was supported by NIH grant (U19 DK 057958)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richon VM, O’Brien JP. Histone deacetylase inhibitors: a new class of potential therapeutic agents for cancer treatment. Clin Cancer Res. 2002;8:662. [PubMed] [Google Scholar]
- 2.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 3.Kim MS, Kwon HJ, Lee YM, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 4.Lam AL, Pazin DE, Sullivan BA. Control of gene expression and assembly of chromosomal subdomains by chromatin regulators with antagonistic functions. Chromosoma. 2005;114:242. doi: 10.1007/s00412-005-0001-0. [DOI] [PubMed] [Google Scholar]
- 5.Choi JH, Oh SW, Kang MS, Kwon HJ, Oh GT, Kim DY. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin Exp Allergy. 2005;35:89. doi: 10.1111/j.1365-2222.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- 6.Moreira JM, Scheipers P, Sorensen P. The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC Cancer. 2003;3:30. doi: 10.1186/1471-2407-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy P, Maeda Y, Hotary K, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci U S A. 2004;101:3921. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111:539. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camelo S, Iglesias AH, Hwang D, et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164:10. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Glauben R, Batra A, Fedke I, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176:5015. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 11.Tao R, de Zoeten E, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007 doi: 10.1038/nm1652. In press. [DOI] [PubMed] [Google Scholar]