Broca’s area (BA) is crucial for normal speech production, but its role in speech comprehension remains controversial. We used repetitive Transcranial Magnetic Stimulation (rTMS) to reveal that briefly disrupting BA impairs discrimination of visual speech oral movements, but not visual non-speech oral movements. This provides direct evidence that BA is useful in the perception of visual speech information. We demonstrate that the region of the brain responsible for producing speech has a role in the visual perception of speech information, and importantly is not involved in the visual perception of oral movements (gurning) unrelated to speech. Because disrupted performance was observed for speech movements and not nonspeech movements, our data suggests that this form of perception does not benefit from an amodal mirror neuron system general enough to be recruited in the processing of common mouth movements irrespective of their relation to speech. We propose that BA is recruited in the perception of speech because of its role in producing speech.
There is clear evidence that a region of left inferior frontal gyrus is required for normal speech production: permanent injury in it results in non-fluent halting speech (Broca’s Aphasia), while transient disruption using rTMS leads to momentary speech arrest [1]. We refer to this region as “Broca’s area”. In our study BA is functionally defined as the inferior frontal region where rTMS inhibits fluent speech production.
This region’s role in language comprehension remains speculative. For example, individuals with injury to BA can have difficulty comprehending syntactically complex sentences [2,3]. Brain imaging studies routinely show activity in BA during speech perception [4], these techniques cannot inform causality. A TMS pulse applied to the face area of the motor cortex causes the lips to more twitch when an observer is listening to speech. Twitch amplitude was found to correlate with PET activity in BA [5].
There is evidence that motor regions of the left frontal cortex, not specific to language, are critical for speech perception. rTMS of the dorsal premotor area impairs speech perception, but not color or tone discrimination [6]. This is interpreted as supporting a role for mirror neurons in motor speech perception.
We extended these previous findings by directly investigating BA’s role in visual speech perception. We hypothesize that BA is specifically required for visually perceiving speech movements, but not for the same perception of general (non-speech) oral movements. It is possible that BA is involved in both speech and non speech perception because of its mirror neurons’ ability to mirror and then use this information to recognize many types of actions — for example the proposed monkey homologue of BA (area F5) clearly responds to non-linguistic behavior [8]. Alternatively, it is possible that this region is recruited for its specialized role in the perception of speech movements. We asked participants to observe silent videos that showed either speech movements or non-speech movements (gurning). The task was to judge whether two sequentially presented videos showed the same mouth movement. We predicted that disrupting BA would specifically impair performance on trials that benefit from identification of the phonemic content of the speech movements. So, disruption would not hinder the processing of non speech stimuli. Therefore, disruption would be dissociable from the contributions of mimicry, as mimicry would be expected to equally benefit task performance on such similar stimuli irrespective of relatedness to speech.
One potential confound with any TMS study are its side effects: TMS pulses generate a cracking sound and induce contractions of the scalp muscles. Therefore, it can be difficult to determine whether the effects of rTMS reflect brain disruption or the side effects of TMS. In order to optimally control for the confounding effects of these side effects, our sham trials triggered a second TMS wand oriented away from the scalp (mimicking the sound of the TMS trial, but not stimulating the brain) as well as cutaneous electrical shocks that were calibrated to produce the sensation and muscle contractions of the rTMS trial [9]. rTMS trials were randomly interleaved among sham trials. This choice of sham is optimal. Stimulating a different part of the left hemisphere would cause different sensation and muscle contraction. Stimulating the right hemisphere analogue of BA would generate sound and muscle twitching in the opposite hemisphere. Stimulating the right hemisphere analogue would be expected to interfere with BA processing due to the strong connections between these anatomically analogous regions in either hemisphere. And, we had a very strong hypothesis about BA, specifically.
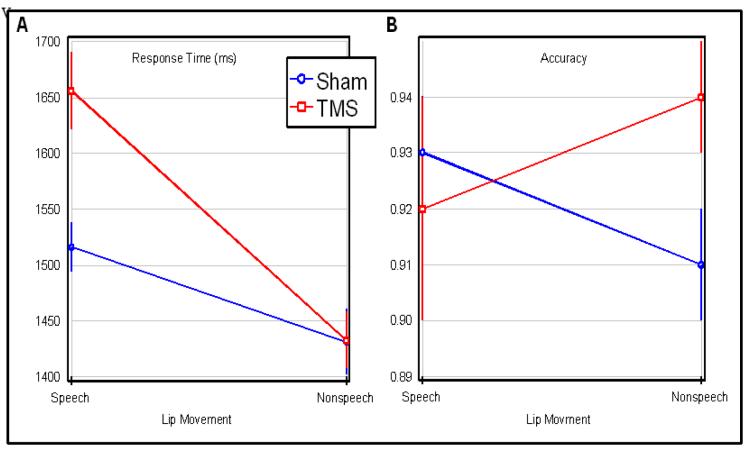
The response times from 18 individuals were analyzed using 2×2 repeated measures ANOVA (all with 1,17 degrees of freedom), with the factors of oral movement (speech vs non-speech) and disruption (TMS vs Sham). A significant main effect was found for oral movement (F=17.1, p<.0007), TMS disruption (F=5.43, p<.0320), as well as an interaction (F=5.29, p=.0340). The interaction effect matches our predictions - with TMS specifically slowing the discrimination of speech relative to non-speech movements (Figure 1). An analogous analysis of errors revealed no effects for oral movement (F=0.06) or disruption type (F= 1.61), and the interaction did not quite achieve significance (F=3.93, p <0.064). Crucially, the trends revealed by this interaction are in the same direction as the significant effect reported for response times, discounting a speed-error tradeoff.
Figure 1.
TMS applied to the Broca’s area specifically impairs the ability to discriminate videos showing speech but not non-speech oral movements. The task was to discriminate if two successive videos showed the same or different lip movement. The horizontal axis shows performance with trials where the videos showed speech movements versus non-speech gurning (with error bars showing the range of standard errors). The vertical axis of the left panel shows response time while the right panel shows accuracy. Broca’s area was disrupted on half the trials (TMS, red lines) and not disrupted on the remaining trials (Sham, blue lines).
Our novel paradigm could be adapted to elucidate this perceptual function and related theory. In particular, future work could contrast stimulation of BA with its right homologue and other cortical areas. While our focus was limited to visual stimuli, future research could contrast speech sounds to non-speech sounds. Another potential improvement would be to better control the difficulty and naturalness of the speech and non-speech stimuli. This would address the potential task difficulty bias in our task, where there was a trend for faster responses for non-speech items. According to this account, our observation reflects the fact that disruption preferentially interferes with a more challenging task. However, we feel this explanation is unlikely. First, the response time trend for sham conditions was not significant (t=2, p < 0.061). Second, the opposite trend was seen in error rates (t=1.07 p<0.3). Therefore, while participants tended to respond more quickly for the non speech stimuli, they also tended to be less accurate for these stimuli.
Our study provides compelling evidence that BA is important for the visual perception of speech movements, and as we predicted, disrupting BA did not significantly effect the perception of non-speech movements. This double dissociation argues against a role for the involvement of an amodal mirror neuron system located in or around or utilizing BA for speech perception. We believe BA evolved to be specialized for the production of speech movements. This intimacy with the motor behavior that gave rise to language is why BA is recruited during speech perception, not its role in mimicry. We believe this facilitation is in line with more traditional motor theories of speech perception [7], and should not be attributed to the venerable mirror neuron [10].
Supplementary Material
References
- 1.Devlin JT, Watkins KE. Stimulating language: insights from TMS. Brain. 2007;130:610–622. doi: 10.1093/brain/awl331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro LP, Gordon B, Hack N, Killackey Verb-argument structure processing in complex sentences in Broca’s and Wernicke’s aphasia. J. Brain Lang. 1993;45:423–447. doi: 10.1006/brln.1993.1053. [DOI] [PubMed] [Google Scholar]
- 3.Zurif E, Swinney D, Prather P, Solomon J, Bushell C. An on-line analysis of syntactic processing in Broca’s and Wernicke’s aphasia. Brain Lang. 1993;45:448–464. doi: 10.1006/brln.1993.1054. [DOI] [PubMed] [Google Scholar]
- 4.Watkins K, Paus T. Modulation of motor excitability during speech perception: the role of Broca’s area. J Cogn Neurosci. 2004;16:978–987. doi: 10.1162/0898929041502616. [DOI] [PubMed] [Google Scholar]
- 5.Campbell R, MacSweeney M, Surguladze S, Calvert G, McGuire P, Suckling J, Brammer MJ, David AS. Cortical substrates for the perception of face actions: an fMRI study of the specificity of activation for seen speech and for meaningless lower-face acts (gurning) Brain Res. Cogn. Brain Res. 2001;12:233–243. doi: 10.1016/s0926-6410(01)00054-4. [DOI] [PubMed] [Google Scholar]
- 6.Meister IG, Wilson SM, Deblieck C, Wu AD, Iacoboni M. The essential role of premotor cortex in speech perception. Current Biol. 2007;17:1692–1696. doi: 10.1016/j.cub.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberman AM, Cooper FS, Shankweiler DP, Studdert-Kennedy M. Perception of the speech code. Psychol Rev. 1967;74:431–461. doi: 10.1037/h0020279. [DOI] [PubMed] [Google Scholar]
- 8.Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- 9.Borckardt J,J, Walker J, Branham RK, Rydin-Gray S, Hunter C, Beeson H, Reeves ST, Madan A, Sackeim H, George MS.Development and evaluation of a portable sham transcranial magnetic stimulation system Brain Stimulation 2008152–59. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iacoboni M. The role of premotor cortex in speech perception: Evidence from fMRI and rTMS. J Physiol Paris. 2008;20:31–34. doi: 10.1016/j.jphysparis.2008.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.