Abstract
The bulk of the research on the neural organization of metaphor comprehension has focused on nominal metaphors and the metaphoric relationships between word pairs. By contrast, little work has been conducted on predicate metaphors using verbs of motion such as “The man fell under her spell.” We examined predicate metaphors as compared to literal sentences of motion such as “The child fell under the slide” in an event-related, functional MRI study. Our results demonstrated greater activation in the left inferior frontal cortex and left lateral temporal lobe for predicate metaphors as compared to literal sentences, while no differences were seen in homologous areas of the right hemisphere. We suggest that the results support a neural organization principle for motion processing in which greater abstraction proceeds along a posterior to anterior axis within the lateral portion of the left temporal cortex.
1. Introduction
Metaphors are used frequently in speech and play a significant role in every day communication. For instance, we easily recognize that the sentence “This job is a jail” does not describe a job that is literally an incarceration for committed crimes, but a job that shares with the notion of jail, the attributes of confinement, punishment and hardship (Bottini et al., 1994). Nominal metaphors like this involve aligning semantically distinct nouns and assume that we understand what a job entails and what a jail is.
In this study, we investigate the processing of another type of metaphor, the predicate metaphor. Predicate metaphors such as “The man fell under her spell” use motion terms figuratively as compared to literal sentences such as “The child fell under the slide” that convey actual physical movement in space. Very little is known about the neural basis of predicate metaphors. Given the differences in what is being processed metaphorically (i.e., object entities/nouns vs. action events/verbs), it is far from obvious that the lessons learned from the nominal metaphor literature generalize to predicate metaphor comprehension. To date, research into the cognitive neuroscience of metaphors has not explicitly considered the fact that not all types of metaphors (i.e., nominal and predicate) may be processed in the same way, nor has it established guiding principles by which one might hypothesize the functional neuroanatomy across different types of non-literal language processing.
At issue in predicate metaphor comprehension is the fact that the level of abstraction in action event and verb processing plays an important role and may be reflected in the anatomical organization. We suggest that motion representations, an important component of action events, follow a posterior-anterior axis of increasingly abstract processing along the lateral temporal cortex. Specifically, we propose a cortical organization of motion knowledge abstraction in which concrete motion percepts are processed in the posterior inferior temporal gyrus, while more abstract motion concepts are processed anteriorly across the posterior middle temporal gyrus (pMTG) and further to the superior temporal gyrus. Predicate metaphors could represent a highly abstract level of motion representation in which physical movement is not being described. In summary, this study considers a functional-anatomical organization principle, based on evidence from perceptual, word, and sentence processing, that suggests a posterior-to-anterior organization for concrete to abstract motion knowledge processing.
2. Methods
2.1. Participants
Fourteen normal, college-age participants were recruited from the University of Pennsylvania community in compliance with the procedures established by the university's Institutional Review Board. All were right-handed, native speakers of English and naïve as to the purposes of the study. The 4 female and 10 male participants had a mean age of 21.5 years and education of 14.6 years.
2.2. Materials
Stimuli materials consisted of three target conditions, each with 35 plausible sentences:
-
Predicate Metaphors (PM):
“The man fell under her spell,”
-
Literal Motion (LM) Sentences:
“The child fell under the slide,”
-
Non-motive (NM) Sentences:
“The merchant was greedy and gluttonous.”
-
Each pair of PM and LM sentence was constructed around a verb and preposition combination that can be interpreted as a metaphor or as concrete, physical motion depending on its context. NM sentences reflected states of being, emotion, etc. These states were chosen to circumvent any potential spatial component confound with size or shape descriptors. Across the list of sentences the same set of subject nouns were used for the non-motive sentences as for the predictae metaphor and literal motion sentences (see Appendix). Finally, in addition to the 35 plausible sentences for each of the three conditions, 20 implausible counterparts were created for the plausibility judgment task by reversing the order of subject and object nouns, for a total of 165 sentences. An additional 55 filler sentences with varying syntactic structures and plausibility were included to reduce the possibility of participant strategy effects.
Sentence stimuli were normed across several factors: (1) Word Length; (2) Written Word Frequency; (3) Noun Concreteness; (4) Noun Imageability; (5) Noun Familiarity; (6) Sentence Figurativeness; (7) Sentence Naturalness; and (8) Sentence Plausibility. Scores for Written Frequency, Concreteness, Imageability, and Familiarity were acquired from the MRC Psycholinguistic Database (http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm). From the database, Written Frequencies are word counts from Kucera and Francis (1967), while noun Concreteness, Imageability and Familiarity are composite scores ranging from 100 to 700. Scores for each sentence were calculated based on the average of the subject and object noun scores. Ratings for sentence Figurativeness, Naturalness and Plausibility were obtained from twenty participants from the University of Pennsylvania community who met the same criteria but did not participate in the neuroimaging experiment. Ratings range from 1 (literal, unnatural, or implausible) to 7 (figurative, natural, or plausible).
To determine the characteristics of the stimuli set seen in Table 1, one-way ANOVAs by items with post-hoc Tukey's tests were carried out for each of the seven factors. Overall, the analyses reveal significant main effects for all factors (ps < .004**), except for marginal effects of Familiarity (F(2,102) = 2.890, MSwithin = 1589, p = .060) and non-significant effects of Word Length (F(2,102) < 1). However, the pattern of results from the post-hoc Tukey's tests reveal that the effects are primarily carried by the NM sentence condition and that PM and LM sentences are not significantly different from each other except for the Figurativeness attribute.
Table 1.
Mean Normative Scores and Ratings (Standard Errors).
| Predicate Metaphors | Literal Motion Sentences | Non-motive Sentences | |
|---|---|---|---|
| Word Length | 6.06 (0.06) | 6.09 (0.06) | 6.17 (0.06) |
| Frequency | 281 (85) | 139 (57) | 125 (54) |
| Concreteness | 524 (18) | 544 (13) | 486 (21) |
| Imageability | 533 (17) | 552 (12) | 507 (19) |
| Familiarity | 571 (14) | 549 (13) | 555 (13) |
| Figurativeness | 5.95 (0.10) | 1.44 (0.07) | 2.54 (0.38) |
| Naturalness | 6.04 (0.13) | 6.47 (0.11) | 6.62 (0.32) |
| Plausibility | 6.04 (0.14) | 6.58 (0.07) | 6.65 (0.06) |
Written Frequencies are word counts from Kucera and Francis (1967). Scores for Concreteness, Imageability, and Familiarity range from 100 to 700 and are based on composite scores from the MRC Database. Ratings for Figurativeness, Naturalness, and Plausibility range from 1 to 7 and were obtained from twenty members of the University of Pennsylvania community who did not participate in the neuroimaging experiment.
In the cases of noun Concreteness and Imageability, the post-hoc tests show that the nouns of the PM condition are not significantly different from LM sentence nouns (p = .257 and p = .246, respectively), though NM sentence nouns are significantly less concrete and imageable than either (ps < .01* and ps < .055, respectively). Familiarity has only a marginal main effect. On post-hoc tests, the nouns of the PM condition are marginally more familiar than LM sentence nouns (p = .059). PM nouns show significantly higher Written Frequency scores than both LM and NM sentence nouns (ps < .01*). However, if Familiarity and Frequency play key roles, then both factors would imply that PMs would be easier to process. We do not find this to be the case in the behavioral pre-test discussed below.
In the case of Figurativeness, PMs are significantly more figurative than LM sentences as designed (p < .001**). Meanwhile, NM sentences fall between PMs and LM sentences in Figurativeness and are significantly different from both (ps < .001**). Finally, PMs are significantly less Natural and Plausible than either LM or NM sentences (ps < .015*). However, all sentence types are highly natural and plausible with scores greater than 6 out of 7. Nevertheless, these attributes are included as noise covariates in the analysis of the functional MRI data such that activation differences can not be explained by first-order effects of these factors.
In addition to the normative scores and ratings, the sentences were piloted in a behavioral experiment designed to mimic the neuroimaging environment. The event-related design included seventeen undergraduate particpants from the University of Pennsylvania who did not participate in either the normalization ratings or the neuroimaging experiment. For each visually presented item, participants were asked to judge the plausibility of the sentence. Reaction times for the plausible items of the three target conditions were not significantly different (Fs < 1), minimizing the possibility of time-on-task confounds.
In summary, all sentence types are of equal Word Length, highly Natural, and highly Plausible. The set of PMs is significantly more Figurative than the set of LM sentences and the two conditions are not significantly different from each other in terms of noun Concreteness, Imageabilty, Familiarity and in participant Reaction Times to the sentences when assessing their plausibilty. The set of NM sentences falls between PMs and LM sentences in Figurativeness, but use significantly less Concrete and Imageable nouns than either. Possible consequences of these differences are addressed in the Discussion section.
2.3. Experimental Design
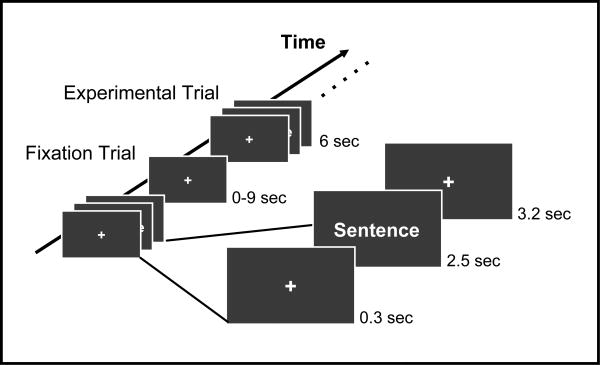
In this event-related experimental design, the sentence stimuli were divided into four separate runs of approximately 6.5 minutes each, such that no matched sentence item appeared in the same run. Approximately 1/3 of the items were presented initially as metaphorical, 1/3 initially as literal, and 1/3 initially as non-motive. Within each run, the sentences were pseudo-randomized such that no two consecutive experimental trials were from the same condition. Figure 1 illustrates that each experimental trial was 6 seconds long and randomly interspersed between experimental trials were random fixation trials of 0, 3, 6, and 9 seconds in length. An individual experimental trial consisted of an initial 0.3 second fixation point, followed by a 2.5 second presentation of the sentence stimuli, and closed by a 3.2 second fixation point.
Figure 1.
Experimental Design. Within each of the four runs, fixation trials of random lengths are interspersed between experimental trials. An individual experimental trial consists of an initial fixation point, followed by the sentence item, and closed by a final fixation point.
Each participant viewed all runs and all items. The order of runs was counterbalanced across participants. Participants were asked to judge whether each sentence was plausible or not; whether the meaning conveyed by the sentence was something they could imagine happening in the world. The correct response for plausible PMs was “plausible.” The plausiblity judgements were made as the sentences were displayed. Task accuracy and reaction times were recorded via a button box and analyzed.
2.4. Scanning Parameters
Participants were scanned in a 3.0T Siemens Trio scanner (Siemens Medical Systems, Iselin, NJ) with a standard Siemens/MRI Devices 8-channel head coil.
High-resolution anatomical images were acquired using a 3-D MPRAGE sequence (TR = 1620 msec, TE = 3.87 msec, TI = 950 msec, and flip angle = 15°). Volumes consisted of 160 slices with an effective thickness of 1 mm. The in-plane resolution was 1 mm × 1 mm (192 × 256 matrix, 187.5 × 250 mm Field of View (FOV)).
The functional volume acquisitions utilized a BOLD EPI sequence (TR = 3000 sec, TE = 30 msec, and flip angle = 90°). The volume was comprised of 45 slices and the interleaved slices were effectively 3 mm thick. The in-plane resolution was 3 mm × 3 mm (64 × 64 matrix, 192 × 192 mm FOV). Each of the four experimental runs consisted of approximately 130 such volume acquisitions for a total of 519 volumes. An initial 9 second (3 TR equivalent) buffer of RF pulse activations, during which no stimulus items were presented and no functional volumes were acquired, was employed to ensure maximal signal during the length of the functional run.
2.5. Group Activation Maps & ROI Analysis
Functional data processing and analysis utilized the VoxBo Data Analysis package (http://www.voxbo.org) developed at the Center for Functional Neuroimaging at the University of Pennsylvania.
Preprocessing routines included standard alignment, smoothing and normalization procedures. For each participant, anatomical and functional volumes were aligned by setting their origins to be equal to each other. Anatomical data was then normalized to the standard MNI T1 template using the SPM2 normalization routine. Functional BOLD data from each particpant was realigned using rigid body alignment and sinc interpolation to register each volume with the participant's first functional volume. Normalization for the functional data then used the same parameters and SPM2 routine as the anatomical data. Finally, smoothing of the functional data used a full-width, half-max (FWHM) kernel of 3×3×3 voxels.
For the data analysis, individual, normalized participant data was modeled using the modified General Linear Model (Worsley & Friston, 1995) and a 1/f estimate of intrinsic autocorrelation (Zarahn et al., 1997). To model the hemodynamic response, each covariate was convolved with a set of eigenvectors derived from a principal components analysis of hemodynamic responses collected across participants in Aguirre et al. (1998b). The set of eigenvectors was designed to serve as a near-basis set to model evoked signal changes up to 16 seconds following the neural event in an event-related design. Group activation maps were then constructed by a random-effects analysis of beta values for each covariate at each voxel. To account for the number of spatial observations, only clusters of 40 or more activated voxels with uncorrected t-value thresholds greater than 3.0 (p < .0051) are reported. Based on modeled data from Forman et al. (1995), these values together approximately correspond to an equivalent voxel-level false positive rate of p < .000001 which in this experiment is comparable to a Bonferroni-type correction with an overall alpha = .05. Noise covariates included the task-related Naturalness and Plausibility ratings of the target sentences as well as the Reaction Times for each individual participant.
Anatomical regions of interest (ROI) were derived from the Automated Anatomical Labeling (AAL) map of the MNI single-subject brain (Tzourio-Mazoyer et al., 2002). After translation to the appropriate file format, regions were resampled to the correct dimensions and manually adjusted to compensate for resampling errors. The anatomical ROIs include: (1) the inferior frontal gyri (663 average voxels); (2) the superior, middle and inferior temporal gyri (1058, 1518, and 411 average voxels respectively); (3) the lateral occipital cortex (529 average voxels); and (4) medial occipital regions including the cuneus, calcarine, lingual, fusiform and retrosplenial cortices (350, 472, 534, 465, and 174 average voxels respectively). The middle temporal gyrus ROI was divided between anterior and posterior portions by a coronal plane defined by the posterior extent of the Sylvian fissure at the supramarginal gyrus (plane defined by Ytal = -32mm in Talairach space).
Analyses followed the same procedure as the whole brain activation map analysis, but only with voxels defined by the ROI contributing to the random-effects group analysis of the beta values.
3. Results
3.1. Task Results
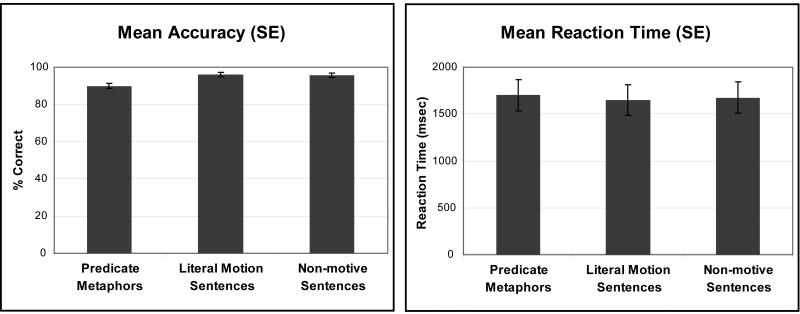
Participants performed the plausibility judgment task with a mean overall accuracy of 91.9% (0.9% SE) and reaction time of 1720 msec (63 msec SE). Analyses include only plausible sentence items.
A one-way ANOVA of accuracy for the three sentence conditions shows a significant main effect (p1 < .003**, p2 < .04*) and post-hoc Tukey's tests demonstrate that Predicate Metaphors are significantly less accurate than both Literal Motion sentences (by participant p < .006**; by item p = .063) and Non-motive sentences (by participant p < .008**; by item p = .075). A similar one-way ANOVA of reaction time for correctly answered sentences shows no significant differences (Fs < 1) in accordance with the behavioral pilot study.
3.2. ROI & Activation Maps
Table 2 demonstrates the regions of significant activation for all contrasts: (1) Predicate Metaphors vs. Literal Motion Sentences (PM vs. LM); (2) Literal Motion vs. Non-motive Sentences (LM vs. NM); (3) Predicate Metaphors vs. Non-motive Sentences (PM vs. NM); and (4) the Figurativeness covariate across all three conditions. Analyses include only plausible sentence items.
Table 2.
ROI Analysis t-Scores.
| Predicate Metaphor vs. Literal Motion | Literal Motion vs. Non-motive Sentences | Predicate Metaphor vs. Non-motive Sentences | Figurativeness | Avg. Voxels | |||||
|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | ||
| IFG | 1.81 | 0.41 | -0.21 | 0.19 | 1.96 | 0.93 | 2.44 | 0.67 | 663 |
| STG | 1.67 | 0.89 | -3.17 | -2.11 | -0.30 | -0.18 | 1.31 | 0.52 | 1058 |
| MTG | |||||||||
| Anterior | 2.32 | 1.85 | -2.83 | -2.64 | 0.53 | 0.45 | 2.20 | 1.58 | 698 |
| Posterior | 1.29 | -0.66 | 0.93 | -0.44 | 2.63 | -0.97 | 1.34 | -1.17 | 821 |
| ITG | -0.20 | 0.39 | -0.03 | -1.12 | -0.21 | -0.60 | -0.48 | 0.13 | 411 |
| LOC | -0.35 | -0.29 | -1.00 | -0.64 | -2.19 | -1.13 | -1.23 | -0.83 | 529 |
| Fusiform | -0.84 | -0.17 | -1.46 | -1.54 | -2.55 | -1.43 | -1.50 | -0.51 | 465 |
| Lingual | 0.54 | 0.48 | -2.95 | -2.52 | -2.68 | -1.51 | -0.32 | -0.18 | 534 |
| Calcarine | -0.09 | 0.34 | -2.46 | -2.47 | -2.53 | -1.75 | -1.29 | -0.71 | 472 |
| Cuneus | -0.32 | 0.00 | -1.43 | -1.53 | -1.68 | -1.40 | -1.57 | -1.08 | 350 |
| Retrosplenial | 1.55 | 0.64 | -0.93 | -0.27 | 0.73 | 0.55 | 1.08 | 0.28 | 174 |
Bolded and shaded t-values are significant (p < .05). Positive t-values indicate activation while negative t-values indicate “deactivation.” Includes only plausible sentences and noise covariates of sentence Naturalness and Plausibility as well as participant Reaction Times. IFG = Inferior Frontal Gyrus; STG = Superior Temporal Gyrus; MTG = Middle Temporal Gyrus; ITG = Inferior Temporal Gyrus; LOC = Lateral Occipital Cortex.
In the case of the PM vs. LM contrast, there are more regions of significant activation in the left hemisphere than the right. In particular, the left inferior frontal gyrus (IFG) ROI and anterior portions of the middle temporal gyrus ROI bilaterally show significant activation. The contrast of LM vs. NM shows significant bilateral deactivation in the superior temporal gyrus ROI, the anterior portions of the middle temporal gyrus ROI, and the lingual and calcarine ROI of the medial occipital cortex. The contrast of PM vs. NM demonstrates significant activity in the left hemisphere. This activity includes activation in the left IFG ROI and the left posterior middle temporal gyrus (pMTG) ROI, as well as deactivation in the left lateral occipital cortex, fusiform, lingual, and calcarine ROIs. Finally, for the Figurativeness covariate across all three sentence conditions, an activation pattern similar to the PM vs. LM contrast is seen, except for only marginally significant activation in the right anterior middle temporal gyrus ROI.
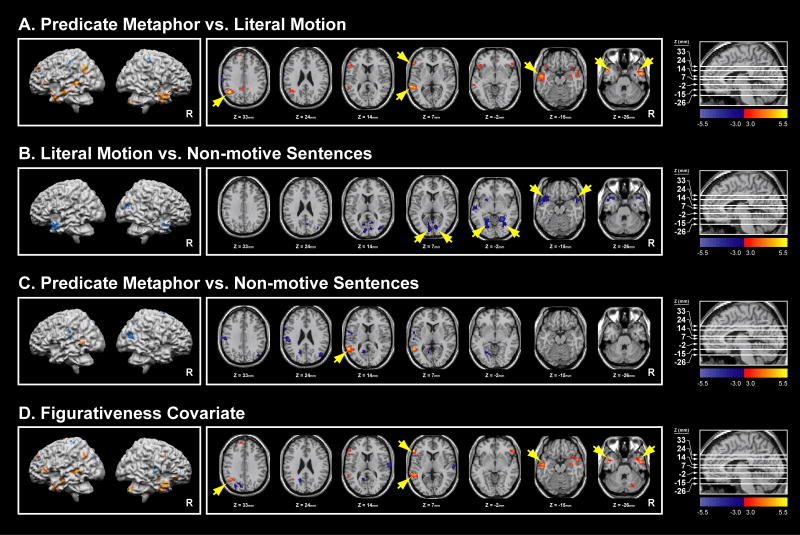
Figure 3 and Table 3 display whole brain activation maps for all contrasts. While the maps largely echo the results described in the ROI analysis (Table 2), some findings of note not captured by the anatomical ROIs were present.
Figure 3.
Activation Maps. (A) Predicate Metaphors vs. Literal Motion Sentences demonstrating left angular gyrus activation at Ztal = 33mm, left inferior frontal gyrus (IFG) and left posterior middle temporal gyrus (pMTG) activation at Ztal = 7mm, left anterior middle temporal gyrus activation at Ztal = -15mm, and bilateral anterior middle temporal gyrus activation at Ztal = -26mm. (B) Literal Motion vs. Non-motive Sentences demonstrating bilateral calcarine deactivation at Ztal = 7mm, bilateral lingual deactivation at Ztal = -2mm, and bilateral anterior temporal lobe deactivation at Ztal = -15mm. (C) Predicate Metaphors vs. Non-motive Sentences demonstrating left pMTG activation at Ztal = 14mm, dorsal and posterior to the pMTG activation seen in the contrast of Predicate Metaphors vs. Literal Motion Sentences (Figure 3A). (D) Figurativeness Covariate for all three conditions demonstrating the same activation pattern as the Predicate Metaphors vs. Literal Motion Sentences contrast. All maps are plotted against the MNI single-subject standard brain with threshold t = 3.0 (p = .0051) and ceiling t = 5.5 (p < .0001), significant for clusters of voxels greater than 40. All maps include plausible sentences only and covariates for Naturalness, Plausibility, and Reaction Time. Surface maps were generated in Brain Voyager for visualization and orientation purposes.
Table 3.
Peak Activation and Cluster Size.
| Left | Right | ||||||
|---|---|---|---|---|---|---|---|
| Peak Coordinate (mm) | Size (voxels) | Activation Direction | Peak Coordinate (mm) | Size (voxels) | Activation Direction | ||
| Predicate Metaphor vs. Literal Motion | IFG | (-53, 18, 7) | 44 | PM > LM | |||
| MTG | |||||||
| Anterior | (-50, 7, -26) | 63 | PM > LM | (53, -1, -25) | 227 | PM > LM | |
| (-53, -9, -15) | 129 | PM > LM | |||||
| Posterior | (-62, -38, 7) | 130 | PM > LM | ||||
| Angular Gyrus | (-42, -57, 33) | 149 | PM > LM | ||||
|
| |||||||
| Literal Motion vs. Non-motive Sentences | Ant. Temporal | (-53, -1, -15) | 404 | LM < NM | (53, 8, -16) | 151 | LM < NM |
| Lingual | (-24, -53, -2) | 331a | LM < NM | (24, -47, -3) | 177 | LM < NM | |
| Calcarine | (-9, -84, 10) | 331a | LM < NM | (9, -75, 9) | 82 | LM < NM | |
|
| |||||||
| Predicate Metaphor vs. Non-motive Sentences | MTG | ||||||
| Posterior | (-56, -49, 11) | 110 | PM > NM | ||||
|
| |||||||
| Figurativeness Covariate | IFG | (-56, 29, 4) | 50 | + | |||
| MTG | |||||||
| Anterior | (-50, 7, -26) | 111b | + | (50, 2, -18) | 227 | + | |
| (-53, -9, -15) | 111b | + | |||||
| Posterior | (-53, -40, 8) | 106 | + | ||||
| Angular Gyrus | (-42, -56 36) | 77 | + | ||||
Peak coordinates are in Talairach space (mm) while sizes of active clusters are in number of voxels. IFG = Inferior Frontal Gyrus; MTG = Middle Temporal Gyrus.
Parts of two separate contiguous clusters.
Parts of two separate contiguous clusters.
In the case of the PM vs. LM contrast (Figure 3a), significant clusters of activation (40 or more voxels with uncorrected ps < .01) are seen in the left angular gyrus ((-42,-57, 33) Talaraich coordinates (mm) of peak activation, 149 voxels), the left IFG ((-53, 18, 7) peak, 44 voxels), the left pMTG ((-62, -38, 7) peak, 130 voxels), and the anterior middle temporal gyrus on the left ((-53, -9, -15) peak, 129 voxels) and bilaterally ((-50, 7, -26) left peak, 63 voxels; (53, -1, -25) right peak, 227 voxels). Of particular note are the clusters of activation in the left pMTG and angular gyrus that were not captured by the ROI analysis. A visual examination of the pMTG with the t-value threshold set to 0 in Figure 4 illustrates the signal trends in the region in which the cluster of significant activation is offset by a large posterior region of non-significant deactivation, averaging to no significant differences for the ROI analysis. It should be emphasized that Figure 4 displays non-significant levels of activation.
Figure 4.
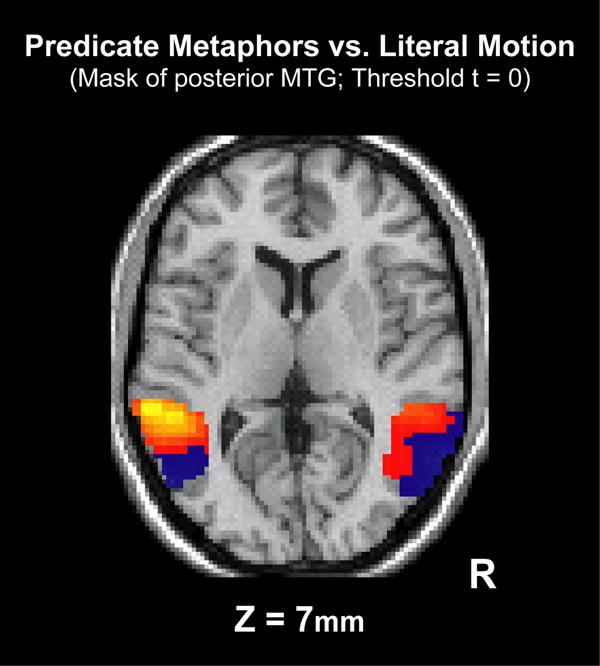
Predicate Metaphors vs. Literal Motion Sentences in the posterior MTG (Ztal = 7mm). Threshold t = 0 to demonstrate signal trends and not a map of significant activation. Otherwise, as Figure 3.
The contrast of LM vs. NM (Figure 3b) shows significant clusters of deactivation bilaterally in the calcarine gyri ((-9, -84, 10) left peak, 331 voxelsa; (9, -75, 9) right peak, 82 voxels), the lingual gyri ((-24, -53, -2) left peak, 331 voxelsa; (24, -47, -3) right peak, 177 voxels), and the anterior temporal lobes ((-53, -1, -15) left peak, 404 voxels; (53, 8, -16) right peak, 151 voxels). The contrast of PM vs. NM (Figure 3c) demonstrates significant activation in the left pMTG ((-56, -49, 11) peak, 110 voxels), both dorsal and posterior to the pMTG activation of the PM vs. LM contrast above ((-62, -38, 7) peak). Finally, for the Figurativeness covariate across all three sentence conditions (Figure 3d), the pattern of activation is nearly identical to that of the PM vs. LM contrast. Significant activation includes the left angular gyrus ((-42, -56, 36) peak, 77 voxels), the left IFG ((-56, 29, 4) peak, 50 voxels), the left pMTG ((-53, -40, 8) peak, 106 voxels), and the anterior middle temporal gyrus on the left ((-53, -9, -15) peak, 111 voxelsb) and bilaterally ((-50, 7, -26) left peak, 111 voxelsb; (50, 2, -18) right peak, 227 voxels).
4. Discussion
The current study examines the anatomical organization of predicate metaphor processing. We consider an anatomical organization principle of metaphor processing based on a posterior-to-anterior organization of abstraction in the knowledge of motion. To summarize the key results, Predicate Metaphors (PM) as compared to Literal Motion (LM) sentences showed significant clusters of activation predominantly in the left hemisphere, including the left inferior frontal gyrus (IFG), the left posterior middle temporal gyrus (pMTG), and the left angular gyrus (Figure 3a). Bilateral activation occurred in anterior portions of the middle temporal gyrus. LM as compared to Non-motive (NM) sentences showed deactivation in medial occipital regions (Figure 3b), an observation we discuss later. Finally, PM as compared to NM sentences showed greater activation in the left pMTG (Figure 3c), both dorsal and posterior to the pMTG activation seen in the PM vs. LM contrast.
Our claim that the abstraction of the visual perception of motion to the conceptions of motion used in language is shifted more anteriorly along the left lateral temporal cortex can be placed in the context of a broader literature on the perception of motion as well as word- and sentence-level studies of motion knowledge. A prominent framework in cognitive neuroscience investigations of semantic knowledge is the sensory-functional theory, according to which the knowledge of concepts are instantiated in or near the sensory-motor areas associated with the relevant percepts (for a brief review, see Simmons & Barsalou, 2003; cf., Mahon & Caramazza, 2003). In the case of visual motion, area MT (middle temporal) and the related area MST (medial superior temporal) of the macaque middle temporal gyrus are selectively sensitive to the percepts of motion. The homologue to area MT/MST in humans is functionally attributed to the junction of the inferior temporal and occipital gyri (Watson et al., 1993; Tootell et al., 1995; Beauchamp et al., 1997). As we have argued elsewhere, concrete visual motion serves as a perceptual point of entry for more abstract motion processing and the conceptual knowledge of actions may be instantiated nearby (Kable & Chatterjee, 2006; Wu, et al., 2007). Simulated motion and implied visual motion with action pictures have all been linked to areas within the posterior lateral temporal cortex (e.g., the pMTG), sometimes including and often just anterior and dorsal to area MT/MST (Oram & Perrett, 1994; Martin et al., 1995; Damasio et al., 2001; Grezes et al., 2001; Grossman et al., 2002; Kable et al., 2002; Martin & Weisberg, 2003; Tranel et al., 2003).
At the word-level, support comes from object vs. action concept processing (Oram & Perrett, 1994; Martin et al., 1995; Damasio et al., 2001; Grezes et al., 2001; Grossman et al., 2002; Kable et al., 2002; Martin & Weisberg, 2003; Tranel et al., 2003; Wallentin et al., 2005a). For instance, Tranel et al. (2003) demonstrated that deficits in recognizing action concepts corresponded to lesions in the left pMTG. Additionally, Damasio et al. (2001) found activation in bilateral pMTG comparing naming actions to naming concrete items displayed in pictures using positron emission tomography. Finally, Kable et al. (2002) explored the functional localization of object and action conceptual knowledge by comparing functional MRI activation in a picture-matching task to a word-matching task. They contrasted picture stimuli of action events to object entities and found greater activity in area MT/MST of the left hemisphere (see also Kable et al., 2005). By contrast, the complementary word stimuli contrast of verbs to nouns activated a region just anterior and dorsal to area MT/MST, within the pMTG and the posterior portion of the superior temporal gyrus. Regions in the lateral occipital-temporal cortex are also involved in the conceptual understanding of actions as judged by the knowledge of thematic roles established by the verb (Wu et al., 2007).
At the sentence-level, the pMTG and the IFG have both been implicated for the processing of motion knowledge in fictive motion sentences (Wallentin et al., 2005a, 2005b). Fictive motion sentences such as “The road runs through the desert” express an abstraction of motion knowledge in that they describe scenes in which the inanimate subject noun cannot actually move. Fictive motion sentences, unlike predicate metaphors however, still describe a spatial scene (Talmy, 1996, 2000). Wallentin et al. (2005a) presented sentences with animate or inanimate subject nouns crossed with dynamic (motion) or static verbs and found greater activation in the left pMTG for dynamic than for static verbs. However, fictive motion sentences (inanimate-dynamic) yielded “no less activation” than sentences of literal motion (animate-dynamic). The lack of significant differences between fictive motion sentences and literal control sentences may have reflected limited statistical power or the possibility that fictive motion sentences, by still describing spatial scenes, are more difficult to distinguish neurally from literal sentences of motion. In a follow-up study, Wallentin et al. (2005b) explored the metaphoric processing of dynamic verbs by presenting sentences with animate or inanimate subject nouns crossed with concrete or abstract object nouns. They found that predicate metaphors (animate-abstract) as compared to the remaining three sentence types significantly activated left lateralized IFG and anterior portions of the lateral temporal cortex.
Taken together, we believe that the literature on action and object processing, verb and noun processing and sentence-level fictive motion support a functional-anatomical organization principle in which more abstract motion knowledge increasingly engages anterior portions of the lateral temporal cortex. This principle is especially relevant for predicate metaphors that center on abstracted conceptual representations of motion terms.
In this study, the hypothesized posterior-to-anterior organization principle for degrees of event abstraction predicts that within the lateral temporal cortex, PMs would produce greater activation than LM sentences anteriorly. The results show this pattern of activation with a significant cluster of activation in the left IFG and pMTG, anterior and dorsal to the human MT/MST homologue (Figure 3a). Additional circumstantial support for this conclusion is provided by Figure 4, in which activation is seen in anterior portions of the pMTG, indicating greater activation for PMs, while deactivation is seen in posterior portions of the pMTG, indicating greater activation for LM sentences. Furthermore, the Figurativeness covariate across all conditions replicated the results of the PM vs. LM contrast (Figure 3d).
Contrary to our expectations, the contrast of LM vs. NM (Figure 3b) does not show significant activation anywhere in the whole-brain analysis. The lack of activation may be the result of unexpected limitations in the design of the NM sentence condition. Specifically, the deactivation in medial occipital cortices may be a reflection of attempts by participants to visualize the emotive states of the subject nouns for the condition in response to the verbs “appearing” and “seemed.” Thus, the NM sentences may have been more visually engaging despite having lower Concreteness and Imageability ratings of the individual words. Despite the this potential limitation of the NM sentences, the contrast of PM vs. NM (Figure 3c) shows significant activation in the pMTG, both dorsal and posterior to the pMTG activation seen in the PM vs. LM contrast, as would be predicted by the posterior-to-anterior organization principle.
The clearest demonstration of such an organization principle would include posterior deactivation in the pMTG for both the PM vs. LM contrast as well as the Figurativeness covariate. The lack of such deactivation in this study could be the result of insufficient statistical power. As such, while the results are not completely unambiguous, we argue that the weight of the evidence from this study and the literature supports our claim for an anatomical organization principle of metaphor processing based on a posterior-to-anterior organization of abstraction in the knowledge of motion. Certainly, more investigation is needed to verify the claim and work is continuing, with better control of the stimulus dimensions and additional stimuli, to replicate and extend the findings.
Returning to the PM vs. LM contrast, other significant clusters of activation include the left IFG, which is likely to represent competition between meanings of spatial terms in the PM condition, including at a minimum the designed literal and the metaphoric interpretations (Thompson-Schill et al., 1997, 1999; Fletcher et al., 2000; Chee et al., 2002; Badre et al., 2005; Moss et al., 2005; Bedny et al., 2007). For instance, Bedny et al. (2007) provide evidence that activation in the left IFG represents top-down selection processes for word-level meanings. In a deficit-lesion study of polysemous and homonymous words, the authors conclude that the left IFG suppresses irrelevant meanings during word comprehension. As such, the competition between the abstract meaning and the literal meaning in predicate metaphor comprehension probably activates the left IFG. Finally, greater activation for predicate metaphor processing was also found in the left angular gyrus. The posterior parietal cortex has been previously shown to mediate knowledge of spatial relationships encoded in prepositions (Tranel & Kemmerer, 2004; Wu et al., 2007). The sentence stimuli used in this study included prepositional terms, such as “jumped at the chance” or “ate up the story.” We suspect that this area may be engaged in the metaphoric uses of prepositional phrases. However, our study was not designed to test this hypothesis directly. Future explorations of this issue might include metaphors such as “reading between the lines” in which the verb does not convey spatial movement, even in its concrete sense.
As a final point, in the introduction we mentioned that the bulk of research in metaphors has focused on nominal metaphors. We raise the possibility that nominal and predicate metaphors might be processed differently with different neural instantiations. The idea that nominal and predicate metaphors might be distinct from each other is in line with recent trends in cognitive neuroscience that recognize that the processing of entities is distinct from events, and the lexical semantics of verbs are distinct from nouns (for example, see Zingeser & Berndt, 1990; Damasio & Tranel, 1993; Warburton et al., 1996; Cappa et al., 1998; Perani et al., 1999; Kable et al., 2002).
Nominal metaphors, according to Structure Mapping Theory (SMT) proposed by Gentner and colleague are processed much like analogies (Gentner & Bowdle, 2001a, 2001b). For example, in the nominal metaphor “Men are wolves,” an analogy is drawn between the target of the metaphor, “men,” and the base of the metaphor, “wolves.” SMT consists of two sequential steps, Alignment and Projection. In Alignment, the semantic attributes of the target and the base are mapped to each other in order to maximize one-to-one, parallel links. In the example, the attributes of men are linked to the attributes of wolves, respectively. In Projection, an inference about the target is made from the relevant attributes of the base. In this case, the predatory attribute of wolves is projected onto men, giving the target its metaphoric meaning. Thus, distinct conceptual entities are selectively conjoined into a common semantic unit in a way that traverses their respective conceptual boundaries.
In predicate metaphors, it is unlikely that the identical Alignment and Projection process occurs in establishing the metaphoric meaning. If one compares the literal sentence to the metaphor in Example (1) (“The man fell under her spell”), no target and base nouns are being aligned. Rather, the phrase “fell under,” is used to describe metaphoric motion based on the context provided by the nouns. The metaphoric meaning is derived, not from mapping across distinct conceptual entities, as much as it is from the bleaching of sensory-motor features from the meaning of “fell under.” Our basic proposal is that the meaning of spatial concepts in verbs of motion includes both sensory-motor as well as more abstract conceptual features. In predicate metaphors, the sensory-motor attributes are shed, and the remaining core abstract conceptual features are highlighted to establish the meaning of the metaphor.
Our proposal that spatial concepts have both sensory-motor and abstract conceptual features is consistent with Jackendoff's view of Conceptual Structure (Jackendoff, 1992, 1999; for related proposals see Pinker, 1989; Levine & Rappaport Hovav, 2005; Van Valin, 2006), which is “an encoding of linguistic meaning that is independent of the particular language” (Jackendoff, 1999, p. 5) with constituent representations of syntactic parts of speech such as Things, Places, Events, etc. Central to our discussion of metaphor processing is that conceptual functions apply to different domains. For instance, Jackendoff demonstrates that the verb GO can apply to a spatial location and literal motion as in “The bird went from the ground to the tree,” or to notions of possession as in “The inheritance went to Phillip,” or to notions of properties as in “The light went from green to red.” In all of these instances, GO shares the same core conceptual representation, a change of state. We suggest that the metaphoric meaning of predicate metaphors is derived from these core abstract conceptual features while eliminating or minimizing the more concrete spatial and motion features of the verb.
In summary, our study demonstrates an anterior bias within the left pMTG and into the left IFG for predicate metaphors. More generally, we suggest that all metaphors might not be processed similarly. We propose that metaphoric processing involves the selection of specific conceptual attributes over the sensory-motor attributes in establishing the non-literal meaning of spatial terms and engages the left hemisphere with an anterior shift from the neural structures of the relevant spatial perceptual processing.
Figure 2.
Task Results. Mean accuracy and reaction times (standard errors) by participant for plausible items only.
Appendix
| Predicate Metaphors | Literal Motion Sentences | Non-motive Sentences |
|---|---|---|
| The man ran for office. | The man ran for the train. | The man was shy but determined. |
| The boy fell for the trick. | The boy fell into the hole. | The boy was loud and energetic. |
| The woman jumped at the chance. | The children jumped in joy. | The children appeared to be afraid. |
| The girl ate up the story. | The children ate up the treats. | The girl was courageous but prudent. |
| The gentleman swept the woman away. | The janitor swept the filth away. | The boss was angered by the situation. |
| The colonel reeled in his officers. | The fisherman reeled in a fish. | The colonel was composed and dignified |
| The champion threw the fight away. | The fisherman threw the net away. | The composer seemed to be talented. |
| The opponent pounded in the point. | The builder pounded in the nail. | The builder was frustrated by the owners. |
| The boss ironed out the details. | The servant ironed out the wrinkles. | The servant was bored by the work. |
| The politician dragged out the speech. | The father dragged out the trash. | The father was loved by his children. |
| The uncle brought up the child. | The servant brought up the bags. | The uncle appeared weak and frail. |
| The man carried out his research. | The husband carried out the garbage. | The husband appeared to be faithful. |
| The man held up the bank. | The champion held up the medal. | The champion was proud of his record. |
| The girl wound up her mother. | The gentleman wound up his watch. | The crowd was cheering with anticipation. |
| The man took back his promise. | The customer took back his gift. | The customer seemed to be wealthy. |
| The student picked up the lesson. | The student picked up the book. | The wife appeared to be delighted. |
| The girl stood up to her boss. | The speaker stood up to the podium. | The speaker pretended to be excited. |
| The man spelled out the plan. | The pupil spelled out the alphabet. | The pupil seemed eager to learn. |
| The woman dove into her work. | The woman dove into the pool. | The woman was happy and radiant. |
| The crowd broke into a dance. | The thief broke into the store. | The thief was silent and quick. |
| The man arrived at his decision. | The traveler arrived at the train station. | The traveler was poor but independent. |
| The lawyer wrapped up the case. | The wife wrapped up the present. | The lawyer was smart and successful. |
| The student flew through the test. | The plane flew through the air. | The plane seemed grand and graceful. |
| The man fell under her spell. | The child fell under the slide. | The merchant was greedy and gluttonous. |
| The composer searched inside her heart. | The musician searched inside her desk. | The musician pretended to be humble. |
| The psychologist picked at the problem. | The child picked at his food. | The psychologist was calm and understanding. |
| The woman walked out on her marriage. | The captain walked out on the deck. | The captain had a powerful build. |
| The student rose to the challenge. | The submarine rose to the surface. | The submarine seemed to be old. |
| The single man picked up the woman. | The garbage man picked up the trash. | The garbage man was completely exhausted. |
| The athlete threw out his back. | The worker threw out his mail. | The worker appeared strong and athletic. |
| The merchant drove up the price. | The car drove up the path. | The car was new and expensive. |
| The man surfed on the web. | The man surfed on the wave. | The politician seemed to be nervous. |
| The professor cleared out his head. | The professor cleared out his office. | The professor was excited by the research. |
| The politician hit below the belt. | The dart hit below the board. | The dart was light and balanced. |
| The speaker stirred up the crowd. | The cook stirred up the soup. | The cook tried to be charming. |
Footnotes
Part of a contiguous cluster.
Part of a contiguous cluster.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre G, Zarahn E, D'Esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Ahrens K, Liu HL, Lee CY, Gong SP, Fang SY, Hsu YY. Functional MRI of conventional and anomalous metaphors in Mandarin Chinese. Brain and Language. 2007;100:163–171. doi: 10.1016/j.bandl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev J, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Beauchamp M, Cox RW, DeYoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing areas. Journal of Neurophysiology. 1997;78:516–520. doi: 10.1152/jn.1997.78.1.516. [DOI] [PubMed] [Google Scholar]
- Bedny M, Hulbert JC, Thompson-Schill SL. Understanding words in context: The role of Broca's area in word comprehension. Brain Research. 2007;1146:101–114. doi: 10.1016/j.brainres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Beeman M. Coarse semantic coding and discourse comprehension. In: Beeman M, Chiarello C, editors. Right hemisphere language comprehension: Perspectives from cognitive neuroscience. Mahwah, NJ: Erlbaum; 1998. [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RSJ, Frith CD. The role of the right hemisphere in the interpretation of figurative aspects of language: A positron emission tomography activation study. Brain. 1994;117:1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Brownell H, Potter HH, Michelow D, Gardner H. Sensitivity to lexical denotation and connotation in brain-damaged patients: A double dissociation? Brain and Language. 1984;22:253–265. doi: 10.1016/0093-934x(84)90093-2. [DOI] [PubMed] [Google Scholar]
- Brownell H, Simpson TL, Bihrle AM, Potter HH, Gardner H. Appreciation of metaphoric alternative word meanings by left and right brain-damaged patients. Neuropsychologia. 1990;28:375–383. doi: 10.1016/0028-3932(90)90063-t. [DOI] [PubMed] [Google Scholar]
- Bryan KL. The right hemisphere language battery. Kibworth: Far Communications; 1989. [Google Scholar]
- Burgess C, Chiarello C. Neurocognitive mechanisms underlying metaphor comprehension and other figurative language. Metaphor and Symbolic Activity. 1996;11:67–84. [Google Scholar]
- Cappa SF, Binetti G, Pezzini A, Padovani A, Rozzini L, Trabucchi M. Object and action naming in Alzheimer's disease and frontotemporal dementia. Neurology. 1998;50:351–355. doi: 10.1212/wnl.50.2.351. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Hon NHH, Caplan D, Lee HL, Goh J. Frequency of concrete words modulates prefrontal activation during semantic judgments. NeuroImage. 2002;16:259–268. doi: 10.1006/nimg.2002.1061. [DOI] [PubMed] [Google Scholar]
- Chomsky N. Lectures of government and binding. Dordrecht: Foris; 1981. [Google Scholar]
- Coulson S, Van Petten C. Conceptual integration and metaphor: An event-related potential study. Memory & Cognition. 2002;30:958–968. doi: 10.3758/bf03195780. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(11):4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Ponto LLB, Hichwa RD, Damasio AR. Neural correlates of naming actions and of naming spatial relations. NeuroImage. 2001;13:1053–1064. doi: 10.1006/nimg.2001.0775. [DOI] [PubMed] [Google Scholar]
- Eviatar Z, Just MA. Brain correlates of discourse processing: An fMRI investigation of irony and conventional metaphor comprehension. Neuropsychologia. 2006;44:2348–59. doi: 10.1016/j.neuropsychologia.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M, Weisper S. Understanding metaphoric sentences in the two cerebral hemispheres. Tennet. 2000;X:186–191. [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. “Sculpting the response space” – An account of left prefrontal activation at encoding. Neuroimage. 2000;12:404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved Assessment of Significant Activation in Functional Magnetic Resonance Imaging (fMRI): Use of a Cluster-Size Threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gardner H, Denes G. Connotative judgments by aphasic patients on a pictorial adaptation of the semantic differential. Cortex. 1973;9:183–96. doi: 10.1016/s0010-9452(73)80027-9. [DOI] [PubMed] [Google Scholar]
- Gentner D, Bowdle BF. Convention, form and figurative language processing. Metaphor & Symbol. 2001a;16:223–247. [Google Scholar]
- Gentner D, Bowdle BF. Metaphor is like analogy. In: Gentner D, Holyoak KJ, Kikinov BN, editors. The analogical mind. Cambridge, MA: MIT Press; 2001b. [Google Scholar]
- Giora R. On the priority of salient meanings: Studies of literal and figurative language. Journal of Pragmatics. 1999;31:919–929. [Google Scholar]
- Giora R, Zaidel E, Soroker N, Batori G, Kasher A. Differential effects of right- and left-hemisphere damage on understanding sarcasm and metaphor. Metaphor & Symbol. 2000;15:63–83. [Google Scholar]
- Glucksberg S. The psycholinguistics of metaphor. TRENDS in Cognitive Sciences. 2003;7:92–96. doi: 10.1016/s1364-6613(02)00040-2. [DOI] [PubMed] [Google Scholar]
- Grezes J, Fonlupt P, Bertenthal B, Delon-Martin C, Segebarth C, Decety J. Does perception of biological motion rely on specific brain regions? NeuroImage. 2001;13:775–785. doi: 10.1006/nimg.2000.0740. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J. Neural representation of verb meaning: An fMRI study. Human Brain Mapping. 2002;15:124–134. doi: 10.1002/hbm.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackendoff RS. Languages of the Mind: Essays on Mental Representation. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- Jackendoff RS. Chapter 1: The Architecture of the Linguistic-Spatial Interface. In: Bloom P, Peterson MA, Nadel L, Farrett MF, editors. Language and Space. Cambridge, MA: MIT Press; 1999. pp. 1–30. [Google Scholar]
- Kable JW, Chatterjee A. The specificity of action representations in lateral occipitotemporal cortex. Journal of Cognitive Neuroscience. 2006;18:1498–1517. doi: 10.1162/jocn.2006.18.9.1498. [DOI] [PubMed] [Google Scholar]
- Kable J, Lease-Spellmeyer J, Chatterjee A. Neural substrates of action event knowledge. Journal of Cognitive Neuroscience. 2002;14:795–805. doi: 10.1162/08989290260138681. [DOI] [PubMed] [Google Scholar]
- Kable JW, Kan IP, Wilson A, Thompson-Schill SL, Chatterjee A. Conceptual representation of action in lateral temporal cortex. Journal of Cognitive Neuroscience. 2005;17:1855–1870. doi: 10.1162/089892905775008625. [DOI] [PubMed] [Google Scholar]
- Kucera, Francis WN. Computational Analysis of Present-Day American English. Providence: Brown University Press; 1967. [Google Scholar]
- Landau B, Jackendoff R. What and where in spatial language and spatial cognition. Behavioral and Brain Sciences. 1993;16:217–238. [Google Scholar]
- Levin B, Rappaport Hovav M. Argument realization. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Mackenzie C, Begg T, Brady M, Lees KR. The effects on verbal communication skills of right hemisphere stroke in middle age. Aphasiology. 1997;11:929–945. [Google Scholar]
- Mackenzie C, Brady M. Communication ability in non-right handers following right hemisphere stroke. Journal of Neurolinguistics. 2004;17:301–313. [Google Scholar]
- Mahon B, Caramazza A. Constraining questions about the organisation and representation of conceptual knowledge. Cognitive Neuropsychology. 2003;20:433–450. doi: 10.1080/02643290342000014. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Martin A, Weisberg J. Neural foundations for understanding social and mechanical concepts. Cognitive Neuropsychology. 2003;20:575–587. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T. The role of the right hemisphere in processing nonsalient metaphorical meanings: Application of Principal Components Analysis to fMRI data. Neuropsychologia. 2005;43:2084–2100. doi: 10.1016/j.neuropsychologia.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung-Beeman M. An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain and Language. 2007;100:115–126. doi: 10.1016/j.bandl.2005.10.005. [DOI] [PubMed] [Google Scholar]
- McIntyre M, Pritchard PB, Lombroso CT. Left and right temporal lobe epileptics: A controlled investigation of some psychological differences. Epilepsia. 1976;17:377–386. doi: 10.1111/j.1528-1157.1976.tb04449.x. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher PC, Bright P, Pilgrim LK, Acres K, Tyler LK. Selecting among competing alternatives: Selection and retrieval in the left inferior frontal gyrus. Cerebral Cortex. 2005;15:1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram M, Perrett DI. Responses of anterior superior temporal polysensory (STPA) neurons to biological motion stimuli. Journal of Cognitive Neuroscience. 1994;6:99–116. doi: 10.1162/jocn.1994.6.2.99. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa S, Schnur T, Tettamanti M, Collina S, Rosa M. The neural correlates of verb and noun processing: A pet study. Brain. 1999;122:2337–2344. doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- Pinker S. Learnability and cognition: The acquisition of argument structure. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- Pynte J, Besson M, Robichon F, Poli J. The time course of metaphor comprehension: An event-related potential study. Brain and Language. 1996;55:293–316. doi: 10.1006/brln.1996.0107. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Azoulai S, Stone L, Srinivasan AV, Bijoy N. Cognitive Neuroscience Society. New York: 2005. Grasping metaphors and thinking with pictures: How brain damage might affect thought and language. [Google Scholar]
- Rapp A, Leube DT, Erb M, Grodd W, Kircher TTJ. Neural correlates of metaphor processing. Cognitive Brain Research. 2004;20:395–402. doi: 10.1016/j.cogbrainres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Leube DT, Erb M, Grodd W, Kircher TTJ. Laterality in metaphor processing: Lack of evidence from functional magnetic resonance imaging for the right hemisphere theory. Brain and Language. 2007;100:142–149. doi: 10.1016/j.bandl.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Rinaldi M, Marangolo P, Baldassarri F. Metaphor comprehension in right brain-damaged patients with visuo-verbal and verbal material: A dissociation (re)considered. Cortex. 2004;40:479–490. doi: 10.1016/s0010-9452(08)70141-2. [DOI] [PubMed] [Google Scholar]
- Rinaldi M, Pizzamiglio L. When space merges into language. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2005.07.007. in press. [DOI] [PubMed] [Google Scholar]
- Schmidt G, DeBuse CJ, Seger CA. Right hemisphere metaphor processing? Characterizing the lateralization of semantic processes. Brain and Language. 2007;100:127–141. doi: 10.1016/j.bandl.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Simmons W, Barsalou LW. The similarity-in-topography principle: Reconciling theories of conceptual deficits. Cognitive Neuropsychology. 2003;20:451–486. doi: 10.1080/02643290342000032. [DOI] [PubMed] [Google Scholar]
- Stanfield R, Zwaan RA. The effect of implied orientation derived from verbal context on picture recognition. Psychological Science. 2001;12:153–156. doi: 10.1111/1467-9280.00326. [DOI] [PubMed] [Google Scholar]
- Stringaris AK, Medford N, Giora R, Giampietro VS, Brammer MJ, David A. How metaphors influence semantic relatedness judgments: The role of the right frontal cortex. NeuroImage. 2006;33:784–793. doi: 10.1016/j.neuroimage.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Talmy L. Fictive motion in language and “ception”. In: Bloom P, Peterson MA, Nadel L, Garett MF, editors. Language and space: Language, speech, and communication. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- Talmy L. Toward a cognitive semantics. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Tanenhaus M, Spivey-Knowlton MJ, Eberhard KM, Sedivy JC. Integration of visual and linguistic information in spoken language comprehension. Science. 1995;268:1632–1634. doi: 10.1126/science.7777863. [DOI] [PubMed] [Google Scholar]
- Tartter V, Gomes H, Dubrovsky B, Molholm S, Stewart RV. Novel metaphors appear anomalous at least momentarily: Evidence from N400. Brain and Language. 2002;80:488–509. doi: 10.1006/brln.2001.2610. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A re-evaluation. Proceedings of the National Academy of Sciences. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Tompkins C. Knowledge and strategies for processing lexical metaphor after right- or left-hemisphere brain damage. Journal of Speech and Hearing Research. 1990;33:307–316. doi: 10.1044/jshr.3302.307. [DOI] [PubMed] [Google Scholar]
- Tootell R, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional-analysis of human MT and related visual cortical areas using magnetic-resonance-imaging. Journal of Neuroscience. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D. Neuroanatomical correlates of locative prepositions. Cognitive Neuropsychology. 2004;21:719–714. doi: 10.1080/02643290342000627. [DOI] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D, Adolphs R. Neural correlates of conceptual knowledge for actions. Cognitive Neuropsychology. 2003;20:409–432. doi: 10.1080/02643290244000248. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Valin RD., Jr . Some universals of verb semantics. In: Mairal R, Gil J, editors. Linguistic universals. Cambridge: Cambridge University Press; 2006. pp. 155–178. [Google Scholar]
- Wallentin M, Lund TE, Ostergaard S, Ostergaard L, Roepstorff A. Motion verb sentences activate left posterior middle temporal cortex despite static context. NeuroReport. 2005a;16:649–652. doi: 10.1097/00001756-200504250-00027. [DOI] [PubMed] [Google Scholar]
- Warburton E, Wise RJ, Price CJ, Weiller C, Hadar U, Ramsay S, et al. Noun and verb retrieval by normal subjects. Studies with pet. Brain. 1996;119(Pt 1):159–179. doi: 10.1093/brain/119.1.159. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Ostergaard S, Lund TE, Ostergaard L, Roepstorff A. Concrete spatial language: See what i mean? Brain and Language. 2005b;92:221–233. doi: 10.1016/j.bandl.2004.06.106. [DOI] [PubMed] [Google Scholar]
- Watson J, Myers R, Frackowiak RSJ, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area-V5 of the human brain – evidence from a combined study using positron emission tomography and magnetic-resonance-imaging. Cerebral Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Winner E, Gardner H. The comprehension of metaphor in brain-damaged patients. Brain. 1977;100:717–729. doi: 10.1093/brain/100.4.717. [DOI] [PubMed] [Google Scholar]
- Worsley K, Friston KJ. Analysis of fMRI time-series revisited – again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Wu D, Waller S, Chatterjee A. The Functional Neuroanatomy of Thematic Role and Locative Relational Knowledge. The Journal of Cognitive Neuroscience. 2007;19:1542–1555. doi: 10.1162/jocn.2007.19.9.1542. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D'Esposito M. Empirical analyses of bold fMRI statistics. NeuroImage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]
- Zingeser LB, Berndt RS. Retrieval of nouns and verbs in agrammatism and anomia. Brain and Language. 1990;39:14–32. doi: 10.1016/0093-934x(90)90002-x. [DOI] [PubMed] [Google Scholar]