Abstract
Inorganic sulfate is required for numerous functions in mammalian physiology, and its circulating levels are proposed to be maintained by the Na+-SO42- cotransporter, (NaSi-1). To determine the role of NaSi-1 in sulfate homeostasis and the physiological consequences in its absence, we have generated a mouse lacking a functional NaSi-1 gene, Nas1. Serum sulfate concentration was reduced by >75% in Nas1-/- mice when compared with Nas1+/+ mice. Nas1-/- mice exhibit increased urinary sulfate excretion, reduced renal and intestinal Na+-SO42- cotransport, and a general growth retardation. Nas1-/- mouse body weight was reduced by >20% when compared with Nas1+/+ and Nas1+/- littermates at 2 weeks of age and remained so throughout adulthood. Nas1-/- females had a lowered fertility, with a 60% reduction in litter size. Spontaneous clonic seizures were observed in Nas1-/- mice from 8 months of age. These data demonstrate NaSi-1 is essential for maintaining sulfate homeostasis, and its expression is necessary for a wide range of physiological functions.
Inorganic sulfate (SO42-) is an abundant anion in mammalian plasma and is essential for numerous physiological functions (1). SO42- conjugation is an important step in the biotransformation of xenobiotics such as steroids, antiinflammatory agents, adrenergic stimulants and blockers, analgesics, and, in most cases, leads to an increase in their urinary excretion (2). SO42- is also required for the activation of many endogenous compounds such as heparin, heparan sulfate, dermatan sulfate, and bile acids (3). In addition, sulfation of structural components such as glycosaminoglycans and cerebroside sulfate is essential for the maintenance of normal structure and function of tissues (4). Disturbances of SO42- metabolism and transport have been associated with human syndromes and diseases, including metachromatic leukodystrophy, Hunter's syndrome, Morquio's syndrome, Maroteaux-Lamy syndrome, multiple-sulfohydrolase deficiency, and osteochondrodysplasias (5, 6). However, despite its importance in the body, SO42- levels are rarely measured in a clinical setting and there remains areas in which knowledge of its significance is still lacking, or is at most, minimal.
In humans, SO42- absorption is initiated in the small intestine and its homeostasis is proposed to be maintained through renal tubular mechanisms (7). SO42- is freely filtered in the glomerulus and is actively reabsorbed in the proximal tubule. Renal proximal tubular SO42- reabsorption is mediated by entry through the brush-border membrane (BBM) by a Na+-SO42- cotransporter (NaSi-1), and exit through the basolateral membrane by an anion exchanger, Sat-1 (1). The NaSi-1 transporter is expressed primarily in the kidney and intestine (8) and has been proposed to play a major role in maintaining serum SO42- concentrations within the normal physiological range of 0.33-0.47 mmol/liter in humans (9-11). In recent years, a growing body of experimental evidence has demonstrated that various dietary and hormonal conditions alter serum SO42- levels by means of the regulation of NaSi-1 (1, 12). The positive and negative regulatory effects of these factors on NaSi-1 mRNA and protein levels and Na+-SO42- cotransport demonstrate that NaSi-1 is highly regulated by its external environment and most likely plays an important role in maintaining SO42- homeostasis.
Recently, we cloned both the mouse and human NaSi-1 genes, designated Nas1 and NAS1, respectively (13, 14). These genes are structurally similar, with each containing 15 exons and 14 introns spread over ≈80 kb. The Nas1 gene was mapped to mouse chromosome 6, in a region of conserved synteny with the human NAS1 gene on chromosome 7q31-q32. Interestingly, other SO42- transporter genes including DRA (down-regulated in colonic adenomas and adenocarcinomas) (15) and SUT-1 (a Na+-SO42- cotransporter from human high endothelial venules) (16), are present in close proximity to NAS1, suggesting this genomic region may be important for coordinating the regulation of SO42- homeostasis.
To define the role of NaSi-1 in the maintenance of SO42- homeostasis, we have generated mice in which the NaSi-1 gene was disrupted by targeted mutagenesis.
Methods
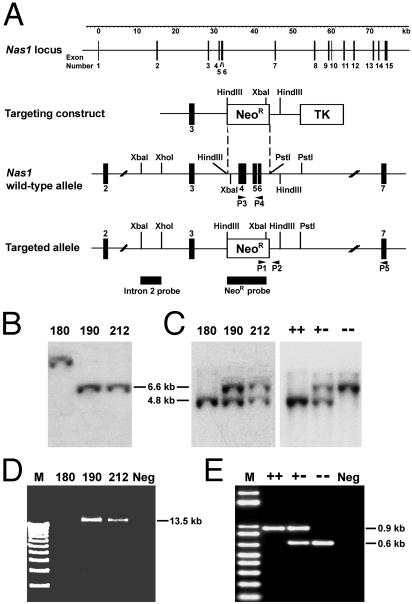
Targeting Vector Construction. Fragments of Nas1 were isolated from a genomic clone (λ5) (13) and used to create the targeting vector shown in Fig. 1A. A 3.7-kb XhoI-HindIII fragment containing exon 3 and a 2.3-kb PstI fragment of intron 6 were subcloned on either side of the phosphoglycerate kinase-neomycin resistance (Neor) expression cassette of the pPNT vector (17). On homologous recombination, this vector deleted ≈3 kb of Nas1 genomic sequences, including exons 4-6. Deletion of exons 4-6 introduced a subsequent frameshift and premature stop codon (Fig. 2A).
Fig. 1.
Targeted disruption of Nas1. (A) Nas1 targeting strategy. Exons (black boxes), restriction sites, probes, and primers (P1-P5) are shown. NeoR, neomycin resistance. TK, thymidine kinase sequences. (B) Southern analysis of XbaI-digested DNA from ES cell clones. The NeoR probe detected a 6.6-kb fragment from clones 190 and 212, but, presumably, a random insertion in clone 180. (C) Southern analysis of XbaI-digested DNA from ES cell clones and from Nas1+/+, Nas1+/-, and Nas1-/- mice. The intron 2 probe detected 4.8-kb wild-type and 6.6-kb targeted allele fragments. (D) Forward (P1) and reverse (P5) primers amplified a 13.5-kb product in ES cell clones 190 and 212. (E) PCR genotyping of Nas1+/+, Nas1+/-, and Nas1-/- mice. Primers P3 and P4 amplified a 0.9-kb wild-type fragment; P1 and P2 amplified a 0.6-kb targeted allele product.
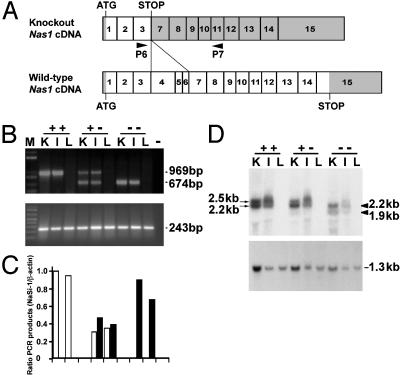
Fig. 2.
Analysis of NaSi-1 mRNA. (A) A schematic of NaSi-1 cDNA and the predicted knockout cDNA lacking exons 4-6. Exons 1-15 (boxes) and protein coding sequences (white portions) of wild-type (1,779 nucleotides) and NaSi-1 knockout (363 nucleotides) cDNA. (B) Total RNA from kidney (K), ileum (I), and liver (L) was RT-PCR-amplified. A 969-bp wild-type product and a 674-bp knockout NaSi-1 cDNA product (Upper) were amplified by using primers P6 and P7. A 243-bp product (Lower) was amplified by using β-actin primers. M, molecular mass ladder. -, negative control. (C) Densitometric analysis of the NaSi-1 PCR products in B, representative of two other experiments with similar data. White bars, wild-type product; filled bars, knockout product. (D) Northern analysis of RNA from kidney, ileum, and liver of Nas1+/+, Nas1+/-, and Nas1-/- mice. RNA was hybridized sequentially with a 32P-labeled NaSi-1 cDNA (Upper) showing the two Nas1+/+ transcripts (arrows, 2.5 and 2.2 kb) and the two Nas1-/- truncated transcripts (arrowheads, 2.2 and 1.9 kb), and GAPDH cDNA (Lower).
Generation and Identification of Nas1-/- Mice. The targeting construct was electroporated into C1368 mouse embryonic stem (ES) cells (a gift from Dr. G. Kay, Queensland Institute of Medical Research, Brisbane, Queensland, Australia). Transfectants were selected in medium containing 300 μg/ml G418 and 0.2 μM 1-(2′-deoxy-2′-fluoro-β-d-arabinofuranosyl)-5-iodouracil for 5 days and then for 3 more days with G418 alone. Clones that had undergone homologous recombination were identified by Southern blot analysis by using an external 1.1-kb XbaI-XhoI fragment of intron 2 as a probe (Fig. 1A). The intron 2 probe detected a 4.8-kb XbaI wild-type allele fragment and a 6.6-kb XbaI recombinant allele fragment. Positive clones were further analyzed by using PCR to amplify a 13.5-kb recombinant allele fragment. PCR was performed by using primer P1 (5′-GAATGGGCTGACCGCTTCCT-3′) in the Neor gene cassette and antisense primer P5 (5′-CATCTTTCTTGACACTTTCCCTTT-3′) in exon 7 (Fig. 1A). Positive ES cell clones (3 of 300 clones) were injected into C57BL/6J blastocysts that were implanted into foster mothers (Transgenic Animal Service of Queensland, Queensland, Australia, and Queensland Institute of Medical Research, Brisbane, Queensland, Australia). Chimeric mice were generated from two different clones, and the F2 generation mice were used in the present study. Tail biopsies were genotyped by PCR and Southern blot analysis (see below). To produce Nas1-/- mice, heterozygous offspring were intercrossed. Studies were performed on mice with a mixed genetic background (129Sv and C57BL/6J). Mice were maintained on a 12-h light-dark cycle and fed ad libitum on standard rodent chow (diet no. AIN93G; Glen Forrest Stockfeeders, Glen Forrest, Western Australia) in accordance with the guidelines of the University of Queensland Animal Ethics Committee.
Genotyping of Mice. Mice were genotyped by PCR and Southern blot analysis. PCR was performed by using primer P1 and antisense primer P2 (5′-CTGGATTAGACAGAAGCAAC-3′) in intron 6 to amplify a 0.6 kb fragment of the recombinant Nas1 allele (Fig. 1A). Primer P3 (5′-CACTGCCTTCTTATCTATGTGG-3′) in exon 4 and antisense primer P4 (5′-CATCTTTCTTGACACTTTCCCTTT-3′) in exon 6 were used to amplify a 0.9-kb fragment of the wild-type Nas1 allele (Fig. 1A). Cycle parameters were: 95°C for 10 seconds; followed by 35 cycles of 95°C for 10 seconds, 60°C (P1/P2) or 55°C (P3/P4) for 30 seconds, and 68°C for 5 min. Genomic DNA was digested with XbaI and analyzed by Southern blot hybridization using intron 2 probe (Fig. 1A). Expected sizes of fragments for the wild-type and disrupted alleles are 4.8 and 6.6 kb, respectively.
Analysis of RNA. Total RNA (10 μg) was separated on a 1% agarose formaldehyde gel in Mops buffer and transferred to Hybond-XL nylon membranes (Amersham Pharmacia). The blots were probed with a 1.2-kb NaSi-1 cDNA and a 0.6-kb mouse GAPDH cDNA probe. For RT-PCR, total RNA (2 μg) was reverse transcribed by using random hexamers and Moloney murine leukemia virus reverse transcriptase (Progen, Brisbane, Queensland, Australia) as recommended by the manufacturer. Primer P6 (5′-CTTTCACCTTCTGCTAATTGGA-3′) in exon 3 and antisense primer P7 (5′-GTCATTTTTGTCAGTTTCTTGGC-3′) in exon 11 (Fig. 2A) were used to amplify NaSi-1 cDNA fragments. Primers 5′-CGTGGGCCGCCCTAGGCACCA-3′ and 5′-TTGGCCTTAGGGTTCAGGGGGG-3′ were used for detection of β-actin cDNAs (18).
BBM Vesicle (BBMV) Preparation and Transport Studies. BBMVs were isolated from mouse kidneys and ilea by using described methods (19). The uptakes of SO42- (0.1 mM) and glucose (0.1 mM), each performed in quadruplicate, were measured at 10 s and 90 min in media containing either 100 mM NaCl or 100 mM KCl by the rapid filtration technique (19).
Blood and Urinary Analysis. Serum and urine SO42-, PO42-, Ca2+, and creatinine concentrations were assayed on an Hitachi 917 automatic analyzer (Hitachi, Tokyo). The fractional excretion index (FEI) of SO42-, PO42-, and Ca2+ was calculated as follows: (serum creatinine × urine SO42-, PO42-, or Ca2+)/(urine creatinine × serum SO42-, PO42-, or Ca2+). The serum concentration of insulin-like growth factor I (IGFI) was measured by using a radioimmunoassay kit (Bioclone, Marrickville, New South Wales, Australia). Serum Na+, K+, and Cl- concentrations were assayed by a commercial pathology laboratory (Sullivan Nicolaides Pathology, Taringa, Queensland, Australia) using a Vitros 250 chemistry analyzer (Ortho-Clinical Diagnostics, New York). Whole blood and urine pH was measured with an ISFET pH meter (Shindengen, Camarillo, CA). Urine total protein levels were quantitated by using a Microprotein-PR kit (Sigma). Serum bile acids were HPLC-separated by using a Waters X-Terra MS C18 3.5-μm2.1 × 150-mm column at a flow rate of 0.2 ml/min. Unpaired Student's t tests were used to analyze all blood and urinary data. Systolic blood pressure was measured three to four times per mouse by using an MLT125/M computerized tail-cuff apparatus (ADInstruments, Castle Hill, New South Wales, Australia).
Histopathological Analysis. Tissues were dissected into ≈50 volumes of 10% buffered formalin and fixed for 3 days before paraffin embedding. Tibia samples were decalcified in Gooding and Stewart's solution (2% formalin and 10% formic acid) at 4°C for 4-7 days before embedding in paraffin. Embedded tissue was sectioned, hematoxylin/eosin stained, and examined by light microscopy. Bone length was measured from digital images of excised femurs by using NIH IMAGE software.
Sulfotransferase (ST) Assays. Liver cytosol preparations and phenol ST assays were performed as described (20). Livers were homogenized on ice in 5 volumes of 10 mM phosphate buffer (pH 7.4) containing 1 mM DTT and 10% glycerol. Liver homogenates were centrifuged at 100,000 × g for 1 h at 4°C. The supernatant fraction (cytosol) was removed and assayed for protein concentration (21). The cytosolic fractions were diluted 10-fold in 10 mg/ml BSA. Phenol ST activity was assayed in 10 mM potassium phosphate (pH 7.4) at 37°C for 30 min with 5 and 25 μM p-nitrophenol and 8 μM [35S]3′-phosphoadenosine 5′-phosphosulfate by using the barium precipitation procedure (22).
Results
Inactivation of Nas1. Our targeting strategy (Fig. 1A) replaced Nas1 exons 4-6 with a neomycin-resistance cassette. Genomic Southern analysis using both neomycin and external probes (Fig. 1 B and C) and PCR analysis (Fig. 1D) confirmed the targeted disruption of Nas1 in ES cells. The genotypes of 435 mice were determined by PCR and Southern blot analysis (Fig. 1 C and E): 117 were Nas1+/+ (27%), 209 Nas1+/- (48%), and 109 Nas1-/- (25%), which is close to the Mendelian ratio (1:2:1), indicating that loss of Nas1 is not embryonically lethal. Northern hybridization revealed the presence of the two NaSi-1 mRNA transcripts (2.5 and 2.2 kb) that are normally present in Nas1+/+ kidney and ileum (13), and the two truncated (≈300 nucleotides smaller) transcripts (2.2 and 1.9 kb) in Nas1-/- mice (Fig. 2D). Sequence analysis of the truncated RT-PCR products from Nas1-/- mice (Fig. 2B) revealed sequence skipping from exon 3 to exon 7, with an in-frame stop codon at the junction of exons 3 and 7 (Fig. 2A). The truncated cDNA encodes the first 121 amino acids of the NaSi-1 protein, which was unable to induce any Na+-SO42- cotransport in Xenopus oocytes (data not shown). Quantitation of the NaSi-1 RT-PCR products, relative to β-actin, indicated that Nas1+/- mice had ≈50% of both wild-type and truncated NaSi-1 mRNAs (Fig. 2C). The level of truncated NaSi-1 mRNA in Nas1-/- mice, determined by densitometric analysis, was similar to the level of wild-type NaSi-1 mRNA in Nas1+/+ mice.
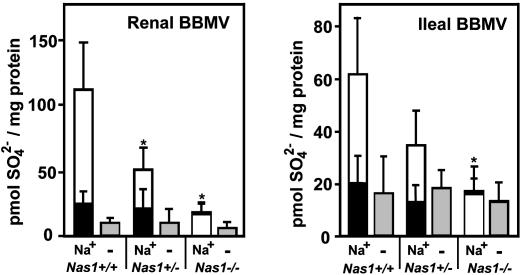
Characterization of the Nas1 Knockout Mice. Membrane transport. To determine the effect of the Nas1 null allele on functional activity, BBMVs were isolated from tissues where NaSi-1 is highly expressed (renal cortex and ileal mucosa) from Nas1+/+, Nas1+/-, and Nas1-/- mice and flux transport measurements were performed. Na+-dependent SO42- uptake was abolished in ileal BBMVs and reduced (by 90%) in renal BBMVs from Nas1-/- mice, whereas an ≈50% reduction was observed in renal and ileal BBMVs from Nas1+/- mice, when compared with Nas1+/+ mice (Fig. 3). Na+-independent SO42- uptake (Fig. 3) and Na+-dependent glucose uptake (data not shown) was similar in Nas1+/+, Nas1+/-, and Nas1-/- mice. These data suggest that the reduced Na+-SO42- cotransport was due to the loss of a functional NaSi-1 protein in the Nas1-/- mice. Our data also indicate that there is no compensation of the active Nas1 allele in the Nas1+/- mice to compensate for the loss of one functional Nas1 allele.
Fig. 3.
Na+-SO42- uptake in renal and ileal BBMVs from Nas1+/+, Nas1+/-, and Nas1-/- mice. SO42- uptake was measured in 100 mM NaCl for 10 s (white bars) and 90 min (black bars) or in 100 mM KCl for 10 s (gray bars). Each bar represents the mean ± SD of four measurements derived from each BBM preparation (12 Nas1+/+, 12 Nas1+/-, and 12 Nas1-/- mice) and is representative of two independent experiments. *, P < 0.05 when compared with Nas1+/+.
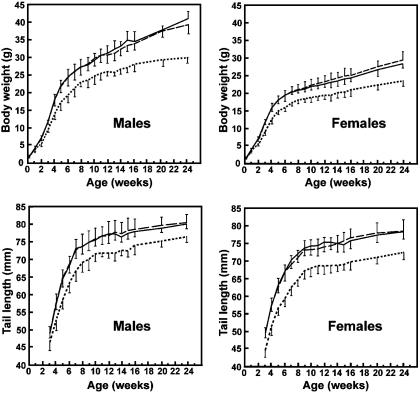
Growth retardation. As an indicator of growth, we measured body weight and tail length of mice up to 24 weeks of age (Fig. 4). At birth, Nas1-/- males and females resembled Nas1+/+ and Nas1+/- littermates in body weight. Nas1-/- mice were generally smaller than Nas1+/+ and Nas1+/- mice at 1 week of age, although significant (P < 0.05) differences were not detected until week 2. From 19 litters, the average weights of Nas1-/- males and females at 3 weeks of age were 75 ± 3% (P < 0.001) and 76 ± 3% (P < 0.001), respectively, of the Nas1+/+ and Nas1+/- littermates, and remained so for at least 5 months thereafter (Fig. 4). Tail lengths of Nas1-/- mice were shorter (by ≈10%) than Nas1+/+ and Nas1+/- littermates (Fig. 4). In addition, at 4 weeks of age, the femoral length was decreased in Nas1-/- mice (0.91 ± 0.04 cm, n = 8; P < 0.01) when compared with Nas1+/+ mice (1.03 ± 0.03 cm, n = 8). Taken together, these data demonstrated a growth retardation in Nas1-/- mice.
Fig. 4.
Growth of Nas1+/+, Nas1+/-, and Nas1-/- mice. Body weights (Upper) and tail lengths (Lower) were determined for male and female mice up to 24 weeks of age. Each point represents the mean ± SD derived from 15-30 measurements of Nas1+/+ (solid line), Nas1+/- (long dashed line), and Nas1-/- (dotted line) mice.
Reduced fertility. Nas1-/- male mice grew to become fertile animals, whereas female Nas1-/- mice showed a lower fertility than Nas1+/+ and Nas1+/- littermates, with the average litter size for Nas1-/- females being 4.1 ± 3.9 (n = 19, P < 0.001), compared with Nas1+/+ (10.7 ± 2.5, n = 15) and Nas1+/- (10.9 ± 1.8, n = 18) mice. In addition, blood spotting or miscarriages were observed in seven pregnancies from three of the Nas1-/- females at 14.6 ± 1.5 days postcoitum.
Seizures. We observed seizures in Nas1-deficient mice at ≈8 months of age. The stress of simply handling the mice could elicit a seizure in some of them. These episodes frequently included the mouse turning over onto a side position with clonic seizures, from which the mouse usually recovered within 2 min. The seizures were mostly observed in Nas1-/- mice (27%, n = 26), but were also observed in a small number of Nas1+/- mice (5%, n = 43).
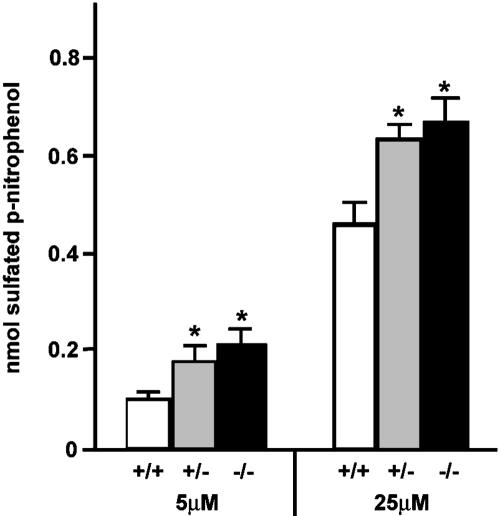
Histology and organ phenotypes. Gross histological analysis of tissue sections of kidney, ileum, liver, heart, spleen, uterus, seminal vesicles, adrenals, bone, and skin from Nas1-/- and Nas1+/+ mice at 4 and 48 weeks of age, respectively, showed no structural peculiarities (data not shown). However, we detected a small but significant increase in the liver:body weight ratio of Nas1-/- mice (5.31 ± 0.47%, n = 14; P < 0.01) when compared with Nas1+/- (4.67 ± 0.50%, n = 18) and Nas1+/+ (4.57 ± 0.22%, n = 9) mice. Liver cytosolic ST activity. ST activity was assayed in the Nas1-deficient mice to determine whether their low serum SO42- levels were associated with changes in ST activity. The level of phenol ST activity was significantly (P < 0.01) increased in Nas1-/- (1.5- to 2.0-fold) and Nas1+/- (1.4- to 1.7-fold) mice when compared with their Nas1+/+ littermates (Fig. 5). There was a small but nonsignificant increase in the average ST activity from the Nas1-/- mice (1.1-fold increase) when compared with the Nas1+/- mice (Fig. 5). Blood and urinary data. Several blood and urine parameters of Nas1-/- mice were significantly different from their Nas1+/+ littermates (Table 1). At all ages examined, the serum SO42- concentration in Nas1-/- mice was markedly reduced by >75% (P < 0.001), whereas Nas1+/- mice exhibited an intermediate serum SO42- concentration that was lower by >40% (P < 0.001) than that of Nas1+/+ mice. Urine SO42-:creatinine ratio and FEI for SO42- were elevated in Nas1-/- mice when compared with Nas1+/+ mice. The FEI for SO42- was also increased in Nas1+/- mice. Serum creatinine levels were constant between genotypes (data not shown). Serum levels of PO42-, Ca2+, Na+, K+, and Cl-, and urine levels of PO42- and Ca2+ were similar in the Nas1-/-, Nas1+/-, and Nas1+/+ mice (Table 1), showing that Nas1 ablation specifically disrupted SO42- homeostasis without affecting the homeostasis of other ions such as PO42-, Ca2+, Na+, K+, and Cl-. Furthermore, there is no increase in urinary protein excretion in Nas1-/- mice (Table 1), indicating that a generalized tubulopathy was not responsible for the renal SO42- leak in Nas1-/- mice. The values obtained for whole blood and urine pH were not different between the genotypes (Table 1). Consistent with the growth retardation of Nas1-/- mice, the serum level of IGFI was significantly reduced by >30% (P < 0.001) in the Nas1-/- mice when compared with the Nas1+/+ mice (Table 1). Systolic blood pressure was not different (P = 0.46) between the Nas1-/- (132.4 ± 4.2 mmHg, n = 7) and Nas1+/+ mice (132.9 ± 10.1 mmHg, n = 7).
Fig. 5.
Elevation of hepatic ST activity in Nas1-deficient mice. Phenol ST activities (mean ± SD, n = 5) in liver cytosol from Nas1+/+ (white bars), Nas1+/- (gray bars), and Nas1-/- (black bars) mice. The concentrations of p-nitrophenol in the assays were 5 and 25 μM. *, P < 0.01 when compared with Nas1+/+.
Table 1. Blood and urine profiles for Nas1+/+, Nas1+/−, and Nas1−/− mice.
| Age, mo | Nas1+/+ (n) | Nas1+/− (n) | Nas1−/− (n) | |
|---|---|---|---|---|
| Serum SO2−4, mM | 2 | 0.99 ± 0.23 (9) | 0.56 ± 0.15 (8) ** | 0.22 ± 0.05 (11) ** |
| 4 | 0.98 ± 0.10 (7) | 0.57 ± 0.08 (8) ** | 0.22 ± 0.04 (12) ** | |
| Serum PO2−4, mM | 2 | 2.39 ± 0.26 (9) | 2.39 ± 0.30 (8) | 2.37 ± 0.33 (11) |
| 4 | 1.97 ± 0.23 (7) | 2.06 ± 0.18 (8) | 2.08 ± 0.26 (12) | |
| Serum total Ca2+, mM | 2 | 2.22 ± 0.14 (9) | 2.22 ± 0.14 (8) | 2.20 ± 0.16 (11) |
| 4 | 2.15 ± 0.06 (7) | 2.18 ± 0.31 (8) | 2.24 ± 0.26 (12) | |
| Urine SO2−4/creatinine | 2 | 8.91 ± 0.74 (9) | 9.10 ± 0.88 (8) | 10.01 ± 1.09 (11) * |
| 4 | 8.92 ± 0.75 (7) | 9.06 ± 0.91 (8) | 10.07 ± 1.03 (12) * | |
| Urine PO2−4/creatinine | 2 | 12.47 ± 4.18 (9) | 12.47 ± 4.09 (8) | 14.02 ± 4.81 (11) |
| 4 | 13.58 ± 4.07 (7) | 11.54 ± 4.70 (8) | 13.45 ± 5.00 (12) | |
| Urine Ca2+/creatinine | 2 | 1.04 ± 0.30 (9) | 0.96 ± 0.18 (8) | 1.00 ± 0.30 (11) |
| 4 | 1.07 ± 0.32 (7) | 0.96 ± 0.18 (8) | 1.00 ± 0.29 (12) | |
| FEI SO2−4 | 2 | 0.21 ± 0.05 (9) | 0.38 ± 0.09 (8) ** | 1.09 ± 0.34 (11) ** |
| 4 | 0.20 ± 0.03 (7) | 0.37 ± 0.05 (8) ** | 1.07 ± 0.25 (12) ** | |
| FEI PO2−4 | 2 | 0.12 ± 0.05 (9) | 0.12 ± 0.04 (8) | 0.13 ± 0.05 (11) |
| 4 | 0.15 ± 0.05 (7) | 0.13 ± 0.05 (8) | 0.15 ± 0.05 (12) | |
| FEI Ca2+ | 2 | 0.010 ± 0.003 (9) | 0.010 ± 0.002 (8) | 0.010 ± 0.003 (11) |
| 4 | 0.011 ± 0.003 (7) | 0.010 ± 0.002 (8) | 0.011 ± 0.004 (12) | |
| Urine total protein, ng/μl | 2 | 2.62 ± 0.98 (9) | 2.52 ± 0.81 (11) | 2.01 ± 1.06 (6) |
| Urine pH | 2 | 6.5 ± 0.6 (7) | 7.1 ± 0.6 (7) | 6.6 ± 0.6 (5) |
| Whole blood pH | 2 | 7.4 ± 0.2 (7) | 7.5 ± 0.1 (7) | 7.3 ± 0.2 (5) |
| Serum IGF-I, ng/ml | 2 | 421.9 ± 23.5 (4) | ND | 280.6 ± 12.3 (4) ** |
| 4 | 512.3 ± 37.9 (4) | ND | 388.6 ± 15.1 (4) ** | |
| Serum Na+, mM | 2 | 144 ± 4 (7) | ND | 143 ± 3 (7) |
| K+, mM | 2 | 6.9 ± 0.8 (7) | ND | 6.3 ± 1.1 (6) |
| Cl−, mM | 2 | 111 ± 3 (7) | ND | 110 ± 2 (6) |
Results are means ± SD. ND, test not performed. *, P < 0.05 when compared with Nas1+/+ mice. **, P < 0.001 when compared with Nas1+/+ mice. FEI, ratio between {serum creatinine (mM) × urine SO2−4, PO2−4, or Ca2+ (mM)} / {urine creatinine (mM) × serum SO2−4, PO2−4, or Ca2+ (mM)}.
Because bile acids require SO42- conjugation for normal function (4), we measured serum bile acid levels in the Nas1-/-, Nas1+/-, and Nas1+/+ littermates. Elevated levels of several different bile acids were found in the Nas1-/- and Nas1+/- mice (Table 2). Serum levels of cholic, hyodeoxycholic, murocholic, chenocholic, glycoursodeoxycholic, glycolithocholic, and taurocholic acid were significantly increased by ≈2- to 4-fold in the Nas1-/- mice, when compared with the Nas1+/+ mice. The serum concentration of murocholic and taurocholic acids was increased by 2- and >4-fold, respectively, in Nas1+/- mice when compared with their Nas1+/+ littermates.
Table 2. Serum bile acid profiles for Nas1+/+, Nas1+/−, and Nas1−/− mice.
| Bile acid, nM | Age, mo | Nas1+/+ (n) | Nas1+/− (n) | Nas1−/− (n) |
|---|---|---|---|---|
| Cholic | 4 | 800 ± 540 (4) | 3461 ± 2107 (4) | 2987 ± 191 (4)* |
| Lithocholic | 4 | 47.3 ± 14.2 (4) | 70.9 ± 19.8 (4) | 82.7 ± 29.6 (3) |
| Hyodeoxycholic | 4 | 24.0 ± 2.5 (3) | 40.5 ± 8.2 (3) | 54.4 ± 9.4 (4)* |
| Ursodeoxycholic | 4 | 345.7 ± 214.1 (4) | 436.3 ± 4.7 (4) | 296.2 ± 157.0 (4) |
| Murocholic | 4 | 98.13 ± 28.0 (4) | 204.5 ± 20.8 (4)** | 290.1 ± 40.9 (4)** |
| Chenodeoxycholic | 4 | 145.9 ± 64.2 (4) | 241.4 ± 31.1 (4) | 415.5 ± 72.1 (4)* |
| Deoxycholic | 4 | 623 ± 326 (4) | 1267 ± 637 (4) | 904 ± 272 (4) |
| Glycoursodeoxycholic | 4 | 2.6 ± 0.4 (3) | 5.4 ± 1.9 (3) | 6.6 ± 0.8 (4)* |
| Glycolithocholic | 4 | 1.8 ± 0.7 (4) | 1.3 ± 0.4 (3) | 7.5 ± 3.5 (4)* |
| Taurocholic | 4 | 5608 ± 4281 (4) | 31442 ± 353 (4)* | 24052 ± 3134 (4)* |
| Taurochenodeoxycholic | 4 | 728 ± 339 (4) | 785 ± 437 (4) | 1157 ± 642 (4) |
| Taurodeoxycholic | 4 | 287 ± 156 (4) | 557 ± 429 (4) | 740 ± 513 (4) |
Results are means ± SD. *, P < 0.05 when compared with Nas1+/+ mice. **, P < 0.01 when compared with Nas1+/+ mice.
Discussion
We have generated a Nas1-deficient mouse, which, to our knowledge, is the first model of an SO42- transporter knockout. We demonstrated that the Nas1-deficient mice exhibited increased urinary SO42- excretion and hyposulfatemia, together with increased serum bile acid levels. These features were associated with normal serum levels of PO42-, Ca2+, Na+, K+, and Cl- and urine PO42-:creatinine and Ca2+:creatinine ratios and no changes in systolic blood pressure. In addition, the Nas1-/- mice have a growth retardation associated with decreased IGFI serum levels and display seizures in older mice.
The phenotypic features that characterize the Nas1-/- mice are likely the direct consequence of impaired SO42- absorption and reabsorption, which is secondary to the loss of NaSi-1 function in the intestine and kidney, respectively. This conclusion is based on our previous study (13), demonstrating that Nas1 is strongly expressed in the ileum and kidney. Here, we demonstrate that Na+-SO42- cotransport is completely abolished in ileal BBMVs and reduced by 90% in renal BBMVs from Nas1-/- mice, suggesting that NaSi-1 is the predominant Na+-SO42- cotransporter in kidney and intestine, and that other molecular entities that may contribute to SO42- homeostasis, do not compensate for the lack of NaSi-1. Because Nas1-/- mice have very low serum SO42- levels (>75% reduction) and a 5-fold increase in FEI SO42- when compared with wild-type littermates, the loss of the renal NaSi-1 is most likely the primary cause of the hyposulfatemia in the Nas1-/- mice. However, the precise contribution of the intestinal NaSi-1, relative to the renal NaSi-1, to the serum SO42- levels, will come from future studies that aim to measure the level of excreted sulfate in the stools of Nas1-/- mice and their Nas1+/+ and Nas1+/- littermates. Although disruption of NaSi-1 leads to a significant reduction in the circulating concentration of SO42-, it is interesting to note that this level is not completely abolished in the Nas1-/- mice. The remaining SO42- level could possibly be obtained directly from the diet by other SO42- transporters, including DRA (15) or DTDST (23). This finding is consistent with our BBMV data that shows a residual level of SO42- transport, which may be due to DRA (1) or other transporters, which have yet to be identified. Alternatively, the remaining SO42- could be derived from the sulfur-containing amino acids, cysteine and methionine (3). Nonetheless, our data demonstrate that Nas1 plays a major role in the maintenance of serum SO42- levels.
The Nas1-/- mice appear normal at birth, and the frequency of Nas1 genotypes among the progeny from Nas1+/- × Nas1+/- matings is consistent with Mendelian inheritance. This finding shows that Nas1 is not required for embryonic development, which is consistent with a gene array study of the developing rat kidney that showed an initial induction of NaSi-1 mRNA levels at birth with increasing levels in the postnatal period (24). It is also consistent with intestine and kidney development, which occur late in gestation. An analysis of postnatal growth revealed a decrease of ≈25% in mean body weight and ≈10% in tail length of both male and female Nas1-/- mice. Growth retardation appears within the first few weeks after birth and is not compensated in adulthood. Consistent with the growth retardation in Nas1-/- mice is the 30% reduction in their serum IGFI levels. Lupu et al. (25) demonstrated the important role of IGFI in postnatal growth, mainly between 15 and 40 days of mouse age, which is consistent with the reduced IGFI levels and growth retardation of Nas1-/- mice between 2-6 weeks of age. Also consistent with the reduced IGFI level in the Nas1-/- mice is an ≈10% decrease in their femoral length when compared with Nas1+/+ mice. Reductions in serum IGFI and femoral length were also observed in the liver IGFI-deficient (LID) mouse, acid labile subunit knock-out (ALSKO) mouse, and in the double gene disrupted LID plus ALSKO mouse (26). IGFI was reported to play a role in up-regulating the steady-state levels of NaSi-1 mRNA and Na+-SO42- cotransport in cultured renal cells (27), and stimulating cartilage SO42- uptake in Xenopus tadpoles (28). However, to our knowledge, the present study is the first to report that hyposulfatemia is associated with reduced levels of circulating IGFI.
We found significant increases in the levels of serum cholic, hyodeoxycholic, murocholic, chenocholic, glycoursodeoxycholic, glycolithocholic, and taurocholic acids in Nas1-/- mice, which indicates a disturbance in bile acid homeostasis. Bile acids play an important role in the intestinal absorption of lipids and lipid-soluble nutrients, with SO42- conjugation being an important modification for maintaining normal levels of bile acids in the intestine (4). SO42- conjugation was also shown to protect ursodeoxycholic acid from bacterial degradation in the intestine (29), which may explain why this particular bile acid was not elevated in the serum from Nas1-/- mice. Consistent with the 16% increase in liver:body weight ratio in the Nas1-/- mice, when compared with Nas1+/+ mice, Repa et al. (30) described hepatomegaly and disturbed bile acid homeostasis in cyp27-/- mice. We speculate that the low availability of SO42- in Nas1-/- mice may disturb normal bile acid homeostasis and lead to hepatomegaly.
Nas1-/- female mice showed a reduced fertility, which was not seen in the male Nas1-/- mice. Our data shows that litter sizes of female Nas1-/- mice at birth were ≈60% lower than litters from either Nas1+/+ or Nas1+/- mice. Early studies (31, 32) showed that serum SO42- levels increased during pregnancy, which were correlated with an increased renal reabsorption of SO42- (33). More recently, BBMV Na+-SO42- cotransport was observed to be higher in pregnant guinea pigs when compared with nonpregnant animals (34). We observed blood spotting and miscarriages in some Nas1-/- mice at ≈14 days gestation, which corresponds to the beginning of the third trimester. These observations could be ascribed to the low sulfatemia in the Nas1-/- mice. A number of changes of sulfated compounds take place in the endometrium during pregnancy, including increased turnover of heparan sulfate proteoglycans (35), delocalization of syndecan (36), and a change in the cholesterol sulfate levels (37). In addition, Mi et al. (38) demonstrated the importance of the sulfated lutropin (LH) receptor, mannose/N-acetylgalactosamine-4-SO4, in maintaining normal serum levels of LH during pregnancy. More recently, tyrosylprotein ST-1-deficient mice were shown to have smaller litter sizes as a result of increased postimplantation fetal death (39). These observations, together with the findings of the present study, indicate that SO42- is important for female reproductive physiology.
The seizure susceptibility phenotype of the Nas1-deficient mice underscores the important, yet poorly understood role of SO42- in mammalian neurology. Seizures are a common feature in some human syndromes of disturbed SO42- metabolism. These include metachromatic leukodystrophy (40) and Hunter's syndrome (41), which are caused by a deficiency in cerebroside sulfatase and iduronate-2-sulfatase activity, respectively. In addition, catecholamines display anticonvulsant properties (reviewed in ref. 42), and SO42- conjugation is required for maintaining their circulating levels. Strobel et al. (43) showed that catecholamine sulfates exhibit a plasma half-life of ≈3 h, which is in contrast to free catecholamines with a half-life of <3 min. The mode of inheritance of seizures in mammals is variable, with evidence for both Mendelian and more complex patterns of inheritance (44). Our observation of seizures in a small number (≈5%) of Nas1+/- mice indicates a complex pattern of inheritance, which may be influenced by the mixed genetic background of the mice in this present study. The intriguing finding of seizures in hyposulfatemic mice represents an important area for future research.
The phenotype of the Nas1+/- mice is of great interest. Nas1+/- mice grow normally, despite the ≈50% reduction in their Na+-SO42- cotransport activity, leading to a 50% reduction in their serum SO42- levels. These findings indicate that the normal Nas1 allele in Nas1+/- mice does not compensate for the loss of one functional Nas1 allele. Although Nas1 is weakly expressed in other tissues including testis, adrenal, and adipose tissue (13), these tissues do not appear to contribute to the circulating levels of SO42-. The subtle phenotype of the Nas1+/- mice, in comparison to the Nas1-/- mice, implies that the level of sulfatemia should reach a threshold between 0.22 and 0.56 mM before it has an affect on growth and fertility.
Interestingly, our data show an increase of liver phenol ST activity in both Nas1-/- and Nas1+/- mice. Although glucocorticoids and some clinical conditions such as diabetes have been shown to increase ST gene expression (45), no studies to date have examined the effect of reduced SO42- levels in the body on ST activity. Our data indicate that ST activity is sensitive to changes in blood SO42- levels and is positively regulated, even with intermediate reductions of serum SO42- levels, as shown in the Nas1+/- mice.
In summary, the generation and characterization of a Nas1-/- mouse has underscored the essential role of the NaSi-1 transporter in maintaining SO42- homeostasis, normal development and growth, and highlights the importance of SO42- in mammalian biology. The Nas1 knockout mouse now provides an animal model for defining the effects of disturbed sulfate homeostasis on a variety of organ systems where sulfate plays an important physiological role.
Acknowledgments
We thank Dr. I. Wilkie (School of Veterinary Science, University of Queensland) for the histopathological analyses, Dr. P. Noakes and N. Hemple (School of Biomedical Sciences, University of Queensland) for valuable discussions, and Associate Professor C. Liddle and S. Coulter (Department of Clinical Pharmacology and Storr Liver Unit, Westmead Millennium Institute, University of Sydney, Sydney) for performing the bile acid measurements. This work was supported in part by the Australian Research Council and the National Health and Medical Research Council (D.M., L.B., and P.A.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NaSi-1, Na+-sulfate cotransporter; BBM, brush-border membrane; BBMV, BBM vesicle; ES, embryonic stem; Neor, neomycin resistance; IGFI, insulin-like growth factor I; ST, sulfotransferase.
References
- 1.Markovich, D. (2001) Physiol. Rev. 81, 1499-1534. [DOI] [PubMed] [Google Scholar]
- 2.Falany, C. N. (1997) FASEB J. 11, 206-216. [DOI] [PubMed] [Google Scholar]
- 3.Mulder, G. J. (1981) in Sulfation of Drugs and Related Compounds, ed. Mulder, G. J. (CRC, Boca Raton, FL), pp. 32-52.
- 4.Mulder, G. J. & Jakoby, W. B. (1990) in Conjugation Reactions in Drug Metabolism: An Integrated Approach: Substrates, Cosubstrates, Enzymes, and Their Interactions In Vivo and In Vitro, ed. Mulder, G. J. (Taylor and Francis, London), pp. 107-161.
- 5.Hastbacka, J., Superti-Furga, A., Wilcox, W. R., Rimoin, D. L., Cohn, D. H. & Lander, E. S. (1996) Am. J. Hum. Genet. 58, 255-262. [PMC free article] [PubMed] [Google Scholar]
- 6.Tallgren, L. (1980) Acta Med. Scand. Suppl. 640, 1-100. [PubMed] [Google Scholar]
- 7.Murer, H., Manganel, M. & Roch-Ramel, F. (1992) in Handbook of Physiology, ed. Winhager, E. (Oxford Univ. Press, London), Vol. 2, pp. 2165-2188. [Google Scholar]
- 8.Markovich, D., Forgo, J., Stange, G., Biber, J. & Murer, H. (1993) Proc. Natl. Acad. Sci. USA 90, 8073-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker, E. L., Heinemann, H. O., Igarashi, K., Holdler, J. E. & Gershberg, H. (1960) J. Clin. Invest. 39, 1909-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berglund, F. & Sorbo, B. (1960) Scand. J. Clin. Lab. Invest. 12, 147-153. [DOI] [PubMed] [Google Scholar]
- 11.Krijgsheld, K. R., Scholtens, E. & Mulder, G. J. (1980) Comp. Biochem. Physiol. 67, 683-686. [Google Scholar]
- 12.Dawson, P. A. & Markovich, D. (2002) Cell Biochem. Biophys. 36, 175-182. [DOI] [PubMed] [Google Scholar]
- 13.Beck, L. & Markovich, D. (2000) J. Biol. Chem. 275, 11880-11890. [DOI] [PubMed] [Google Scholar]
- 14.Lee, A., Beck, L. & Markovich, D. (2000) Genomics 70, 354-363. [DOI] [PubMed] [Google Scholar]
- 15.Hoglund, P., Haila, S., Socha, J., Tomaszewski, L., Saarialho-Kere, U., Karjalainen-Lindsberg, M., Airola, K., Holmberg, C., de la Chappelle, A. & Kere, J. (1996) Nat. Genet. 14, 316-319. [DOI] [PubMed] [Google Scholar]
- 16.Girard, J. P., Baekkevold, E. S., Feliu, J., Brandtzaeg, P. & Amalric, F. (1999) Proc. Natl. Acad. Sci. USA 96, 12772-12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tybulewicz, V., Crawford, C., Jackson, P., Bronson, R. & Mulligan, R. (1991) Cell 65, 1153-1163. [DOI] [PubMed] [Google Scholar]
- 18.Watson, A. J., Hogan, A., Hahnel, A., Wiemer, K. E. & Schultz, G. A. (1992) Mol. Reprod. Dev. 31, 87-95. [DOI] [PubMed] [Google Scholar]
- 19.Tenenhouse, H. S. & Scriver, C. R. (1978) Can. J. Biochem. 56, 640-646. [DOI] [PubMed] [Google Scholar]
- 20.Falany, J. L., Greer, H., Kovavs, T., Sorscher, E. J. & Falany, C. N. (2002) Biochem. J. 364, 115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford, M. M. (1976) Anal. Biochem. 72, 248-325. [DOI] [PubMed] [Google Scholar]
- 22.Falany, C. N., Vazquez, M. E., Heroux, J. A. & Roth, J. A. (1990) Arch. Biochem. Biophys. 278, 312-318. [DOI] [PubMed] [Google Scholar]
- 23.Hastbacka, J., de la Chapelle, A., Mahtani, M., Clines, G., Reeve-Daly, M., Daly, M., Hamilton, B., Kusumu, K., Trivedi, B., Weaver, A., et al. (1994) Cell 78, 1073-1087. [DOI] [PubMed] [Google Scholar]
- 24.Stuart, R. O., Bush, K. T. & Nigam, S. K. (2001) Proc. Natl. Acad. Sci. USA 98, 5649-5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lupu, F., Terwilliger, J. D., Lee, K., Segre, G. V. & Efstratiadis, A. (2001) Dev. Biol. 229, 141-162. [DOI] [PubMed] [Google Scholar]
- 26.Yakar, S., Rosen, C. J., Beamer, W. G., Ackert-Bicknell, C. L., Wu, Y., Liu, J. L., Ooi, G. T., Setser, J., Frystyk, J., Boisclair, Y. R. & LeRoith, D. (2002) J. Clin. Invest. 110, 771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, H. J., Sagawa, K., Shi, W., Murer, H. & Morris, M. E. (2000) Proc. Soc. Exp. Biol. Med. 225, 49-57. [DOI] [PubMed] [Google Scholar]
- 28.Schneider, A. & Hanke, W. (1997) Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 117, 317-322. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues, C. M., Kren, B. T., Steer, C. J. & Setchell, K. D. (1995) Gastroenterology 109, 2036-2038.7498674 [Google Scholar]
- 30.Repa, J. J., Lund, E. G., Horton, J. D., Leitersdorf, E., Russell, D. W., Dietschy, J. M. & Turley, S. D. (2000) J. Biol. Chem. 275, 39685-39692. [DOI] [PubMed] [Google Scholar]
- 31.Morris, M. E. & Levy, G. (1983) J. Pharm. Sci. 72, 715-716. [DOI] [PubMed] [Google Scholar]
- 32.Cole, D. E., Baldwin, L. S. & Stirk, L. J. (1985) Clin. Chem. 31, 866-867. [PubMed] [Google Scholar]
- 33.Cole, D. E., Baldwin, L. S. & Stirk, L. J. (1985) Obstet. Gynecol. 66, 485-490. [PubMed] [Google Scholar]
- 34.Lee, H. J., Balasubramanian, S. V. & Morris, M. E. (1999) Proc. Soc. Exp. Biol. Med. 221, 336-344. [DOI] [PubMed] [Google Scholar]
- 35.Morris, J. E., Potter, S. W. & Gaza-Bulseco, G. (1988) J. Biol. Chem. 263, 4712-4718. [PubMed] [Google Scholar]
- 36.Potter, S. W. & Morris, J. E. (1992) Anat. Rec. 234, 383-390. [DOI] [PubMed] [Google Scholar]
- 37.Momoeda, M., Taketani, Y., Mizuno, M., Iwamori, M. & Nagai, Y. (1991) Biochem. Biophys. Res. Commun. 178, 145-150. [DOI] [PubMed] [Google Scholar]
- 38.Mi, Y., Shapiro, S. D. & Baenziger, J. U. (2002) J. Clin. Invest. 109, 269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouyang, Y. B., Crawley, J. T., Aston, C. E. & Moore, K. L. (2002) J. Biol. Chem. 277, 23781-23787. [DOI] [PubMed] [Google Scholar]
- 40.Bostantjopoulou, S., Katsarou, Z., Michelakaki, H. & Kazis, A. (2000) Acta Neurol. Scand. 102, 192-195. [DOI] [PubMed] [Google Scholar]
- 41.Wraith, J. E., Cooper, A., Thornley, M., Wilson, P. J., Nelson, P. V., Morris, C. P. & Hopwood, J. J. (1991) Hum. Genet. 87, 205-206. [DOI] [PubMed] [Google Scholar]
- 42.Weinshenker, D. & Szot, P. (2002) Pharmacol. Ther. 94, 213-233. [DOI] [PubMed] [Google Scholar]
- 43.Strobel, G., Friedmann, B., Jost, J. & Bartsch, P. (1994) Am. J. Physiol. 267, E537-E543. [DOI] [PubMed] [Google Scholar]
- 44.Kaneko, S., Okada, M., Iwasa, H., Yamakawa, K. & Hirose, S. (2002) Neurosci. Res. 44, 11-30. [DOI] [PubMed] [Google Scholar]
- 45.Runge-Morris, M. A. (1997) FASEB J. 11, 109-117. [DOI] [PubMed] [Google Scholar]