Abstract
To gain a molecular understanding of kidney functions, we established a high-resolution map of gene expression patterns in the human kidney. The glomerulus and seven different nephron segments were isolated by microdissection from fresh tissue specimens, and their transcriptome was characterized by using the serial analysis of gene expression (SAGE) method. More than 400,000 mRNA SAGE tags were sequenced, making it possible to detect in each structure transcripts present at 18 copies per cell with a 95% confidence level. Expression of genes responsible for nephron transport and permeability properties was evidenced through transcripts for 119 solute carriers, 84 channels, 43 ion-transport ATPases, and 12 claudins. Searching for differences between the transcriptomes, we found 998 transcripts greatly varying in abundance from one nephron portion to another. Clustering analysis of these transcripts evidenced different extents of similarity between the nephron portions. Approximately 75% of the differentially distributed transcripts corresponded to cDNAs of known or unknown function that are accurately mapped in the human genome. This systematic large-scale analysis of individual structures of a complex human tissue reveals sets of genes underlying the function of well-defined nephron portions. It also provides quantitative expression data for a variety of genes mutated in hereditary diseases and helps in sorting candidate genes for renal diseases that affect specific portions of the human nephron.
The human kidney consists of one million nephrons functioning in parallel to ensure efficient body fluid homeostasis. This vital function rests on sequential blood filtration by the glomerulus and specific transport processes accomplished by the successive nephron segments. Because of this axial functional segmentation of the nephron, studies carried out at the whole-kidney level cannot define sites and mechanisms of physiological processes. Physiological and biochemical methods that allow study of well-delineated nephron portions, including human ones (1, 2), were set up long ago. More recently, molecular methods completed these approaches through the cloning and characterization of the expression pattern along the nephron of a number of genes essential for a variety of kidney functions. Besides providing decisive progress for elucidating the mechanisms of physiological processes, the genetic and molecular strategies led to the identification of genes mutated in inherited kidney or kidney-dependent diseases. Strikingly, several such genes are expressed in discrete nephron portions (3).
Despite this progress, a remaining challenge is obtaining a complete overview of the genes expressed in the different nephron portions, especially in humans. With the availability of the human genome sequence (4, 5), the first level of genetic complexity has now been deciphered. The second level of complexity, usually referred to as the transcriptome (6), can be studied by using a variety of techniques. Large-scale EST (7) and cDNA (8) sequencing projects provide a framework for transcriptome analysis, but they are not quantitative enough to make possible the accurate comparison of gene expression patterns in different cell populations. In addition, when carried out at the level of single nephron portions, they provided only a partial picture through the analysis of ≈1,000 transcripts (9). Hybridization to arrayed cDNAs or oligonucleotides offers the opportunity of studying more transcripts and comparing their expression levels in different tissue samples and has already enabled the analysis of thousands of mRNAs in large human kidney zones (10) and cultured renal cells (11) but has not permitted up to now a systematic survey of the different human nephron portions. The method of serial analysis of gene expression (SAGE) (6) is potentially the most exhaustive one for characterizing transcriptomes, because it measures the expression of both known and unknown genes. SAGE relies on sequencing short diagnostic 10-bp tags recovered in a cDNA library proportionally to their abundance in the native tissue sample. Although SAGE initially required large amounts of tissue, a microassay compatible with the analysis of microdissected nephron segments has been set up. It was previously used to analyze 15,000 mRNA tags retrieved from two nephron portions of the mouse kidney (12). We now report on the analysis of >400,000 mRNAs tags isolated from most portions of the human nephron.
Methods
Kidney Microdissection. The Necker Hospital ethical committee approved our study, and we obtained informed consent from all patients. The nine donors [seven males and two females; age (years): 59 ± 10 (SD)] were all devoid of AIDS and hepatitis B and C viral infection and had undergone surgery for removal of kidney tumors. After nephrectomy, a healthy kidney fragment was perfused via a branch of the renal artery with EuroCollins (Fresenius Kabi, Sèvres, France), immersed in ice-cold Euro-Collins, and transferred to Saclay within 30 min. On arrival in the laboratory, the kidney fragment was perfused with 20 ml of Hanks' modified microdissection solution (13) supplemented with 0.24% wt/vol collagenase (Serva) and 0.05% lissamine green (2). The perfused zone of the kidney, as judged by the presence of the dye, was excised, cut into small pyramids, and incubated 45-60 min at 35°C in gassed microdissection solution containing 0.12% wt/vol collagenase. The pyramids were then thoroughly rinsed and transferred into Petri dishes for micro-dissection, carried out at ice-cold temperature under stereomicroscopic observation by using anatomical and morphological criteria (2). Once isolated, the structures were transferred by pipetting into another Petri dish and dragged individually for counting, evaluating tubular length, and removing any residual debris.
Generation of SAGE Libraries. Pools of identical structures from each kidney were transferred into 400 μl of microdissection solution and centrifuged for 5 min at 1,000 × g. The cell pellet was dissolved in 100 μl of lysis-binding buffer (Dynal, Oslo) containing 20 μg of glycogen (Roche, Basel), and stored at -80°C. SAGE libraries were generated by using 1,000 glomeruli or 300-600 mm of tubular segments (105 to 2 × 105 cells) isolated from three to nine kidneys. Libraries were obtained as described (12, 14) by using Sau3A I as anchoring enzyme.
DNA Sequences Analysis. Génoscope (Evry, France) performed plasmid minipreparations and automatic DNA sequencing. Sequence files were analyzed with the help of sadelab (CEA Saclay, France), a web-based integrated platform dedicated to the management of SAGE projects. Ditags consisting of nucleotides that all displayed a PHRED score >16 or <10 were automatically accepted or rejected, respectively. Ditags of intermediate quality were individually checked and eventually accepted after corrections for erroneous base calling. With single-pass sequencing, a 1% error rate is routinely obtained, which translates to a SAGE tag error rate of 9.56% (1-0.9910) (15). Because sequencing errors are essentially random, they do not substantially affect tag abundance but likely inflate the number of different transcripts detected. Tag extraction, counting and library comparisons were performed by using sadelab. Tags for linker-derived sequences were discarded, and those originating from duplicate ditags were counted only once (6).
SAGE Data Analysis. Mitochondrial SAGE tags were inferred from the sequence of Anderson et al. (16) and from the database for human mitochondrial genome polymorphisms (www.genpat.uu.se/mtDB). For all other tags, identification and chromosomal mapping were initiated on Unigene clusters by using SAGEmap (www.ncbi.nlm.nih.gov/SAGE) and carried out until March 31, 2003. By using our SAGE protocol, each polyadenylated transcript is expected to be detected through a single tag adjacent to the most 3′ Sau3A I site of the corresponding cDNA. The reliability of the identification procedure thus critically depends on the possibility of assessing that canonical tags are obtained. Therefore, Unigene clusters referred to as reliable matches in SAGEmap were all explored for the location and correct orientation of the tag, as well as the presence of a polyadenylation signal and/or poly(A) tail in the most 3′ sequence. Reliably matched sequences belonging to different clusters were aligned to assess the accuracy of the clustering process, allowing in a few instances resolution of two clusters in a single one. When a tag reliably matched sequences belonging to two unrelated clusters, both entries were recorded. When more than two reliable matches were obtained, the ambiguous tag to gene mapping was referred to as multiple matches (see Results). When no entry or no reliable entry was obtained in SAGEmap, additional resources were used. First, we considered the consensus sequence of the TIGR database (www.tigr.org), which in some instances allowed extending the cDNA sequence up to the poly(A) tail. When this procedure was inefficient, a BLAST search (www.ncbi.nlm.nih.gov/BLAST) was performed on human ESTs recorded in Gen-Bank, and the retrieved sequences were analyzed as described above to check for matching accuracy. Such reliable entries were recorded as EST matches or attributed a gene name when mapping information on the human genome indicated overlapping with a known gene. Tags matching ESTs in the reverse orientation or ESTs that could not be ascertained as 3′ sequences, as well as tags matching only the human genome, were considered not reliably matched. Tags without reliable match were discarded from the comparative analysis when they did not exceed by a factor of five the expected abundance due to a sequencing error in a more abundant tag. As reported (12, 15), the expression of several genes was detected through more than one tag in a pattern consistent with alternative mRNA splicing or polyadenylation. In the absence of systematic information on the different transcripts generated from each human gene, the different tags for a single gene were numbered alphabetically according to their abundance in the libraries of the present study.
Gene to Tag Mapping. For analyzing the expression of genes belonging to a same family (e.g., solute carriers, channels, or claudins), the web site of the Human Genome Organisation nomenclature committee (www.gene.ucl.ac.uk/nomenclature) was used to search for all registered members. Then, mRNA sequences recorded in the corresponding UniGene clusters (www.ncbi.nlm.nih.gov/UniGene) were analyzed, and the most 3′ tag was validated by its presence either in the reviewed RefSeq record or in sequences of the UniGene or TIGR clusters. When present in our SAGE nephron database, the tag was further controlled for correct gene identification and possible match to several UniGene clusters by using SAGEmap. The same kind of analysis was performed to analyze the expression of disease genes selected from the Online Mendelian Inheritance in Man database (www.ncbi.nlm.nih.gov/Omim).
Quantitative RT-PCR. Pools of glomeruli or isolated segments were transferred onto a concave bacteriological glass slide and photographed for counting or tubular length measurement, respectively (13). Total RNAs were extracted as described (13), and cDNA synthesis was primed by using oligo(dT)12-18. Duplicate aliquots corresponding to cDNA amounts generated from half a glomerulus or 0.5 mm of tubular length were analyzed by real-time quantitative PCR. Amplification was performed in an ABI prism 7000 SDS by using Sybr Green PCR master mix, according to recommendations of the manufacturer (Applera, Foster City, CA), and 300 nM each primer. Aliquots of the same cDNA sample were used to study the expression of the different targets analyzed. The amplification rate of each target, evaluated from experiments carried out on whole-kidney cDNAs, was used to calculate expression differences from one tissue sample to another. Peptidylprolyl isomerase A (PPIA), which was found to be nearly similarly expressed in all structures [tag counts for 50,000 tags: glomerulus, 8; proximal convoluted tubule, 12; proximal straight tubule, 9; medullary thick ascending limb of Henle's loop, 17; cortical thick ascending limb of Henle's loop, 13] was used for normalization. The primers used for quantitative RT-PCR are available from the authors on request.
Results
Healthy parts of human kidneys were obtained from donors undergoing tumorectomy. We isolated by microdissection eight nephron portions (Fig. 1), and these native tissue samples were processed for the generation of SAGE libraries.
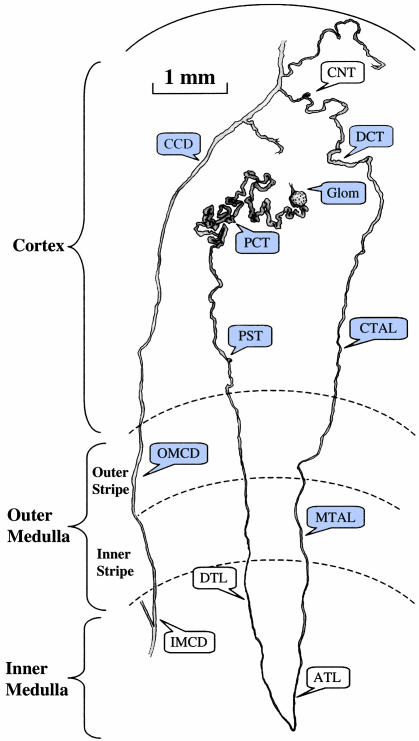
Fig. 1.
A microdissected human nephron. Blue boxes indicate the eight structures analyzed in the present study. Solid and dotted arcs indicate the kidney surface and limits of kidney zones, respectively. Glom, glomerulus; PCT, proximal convoluted tubule; PST, proximal straight tubule; DTL, descending thin limb; ATL, ascending thin limb; MTAL, medullary thick ascending limb of Henle's loop; CTAL, cortical thick ascending limb of Henle's loop; CNT, connecting tubule; CCD, cortical collecting duct; OMCD, outer medullary collecting duct; and IMCD, inner medullary collecting duct; DCT, distal convoluted tubule.
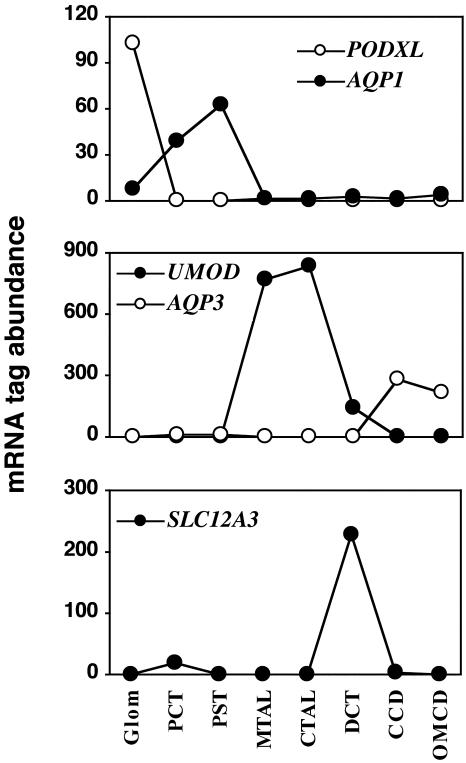
We sequenced ≈50,000 tags from each library (Table 3, which is published as supporting information on the PNAS web site). With 50,000 mRNA tags, a 95% confidence level is obtained for detecting transcripts present at 0.006% of the total RNA mass (i.e., ≥18 copies per cell) (14). The relevance of the libraries is supported by the expression pattern of several genes. For example, known markers for glomerular epithelial cells (PODXL) (17), proximal tubules (AQP1) (18), thick ascending limbs (UMOD) (19), distal convoluted tubules (SLC12A3) (20), and collecting ducts (AQP3) (20, 21) were evidenced in the appropriate libraries and in most cases undetected in all others (Fig. 2). Uromodulin expression was detected in the distal convoluted tubule (DCT) but reached a considerably lower level than in either the cortical [cortical thick ascending limb of Henle's loop (CTAL)] or the medullary (medullary thick ascending limb of Henle's loop) portion of the thick ascending limb of Henle's loop. This expression pattern is consistent with the observation that the initial DCT portion consists of CTAL-like cells (22). On the other hand, the absence of AQP3 tags in the DCT library demonstrates that DCTs were microdissected free of connecting tubule pieces (20).
Fig. 2.
Expression pattern of markers for different nephron portions. Tag abundance indicates mRNA tag counts in libraries normalized to 50,000 tags. PODXL, podocalyxin-like; AQP1, aquaporin 1; UMOD, uromodulin; AQP3, aquaporin 3; SLC12A3, thiazide-sensitive Na-Cl cotransporter.
Comparative analysis of libraries was performed to identify sets of genes that support the specific functions of the successive nephron portions. Stringent criteria (P < 0.01 and ≥7-fold difference) were used for assessing statistically significant differences. Indeed, Monte-Carlo simulations indicate that the 0.01 level of confidence requires a ≥7-fold difference for low-abundance tags (15). To further delineate specific expression patterns despite functional kinships between adjacent nephron portions, differences obtained with at least three libraries were considered. The comparative analysis revealed differential abundances for 998 tags (Fig. 3, and Table 4, which is published as supporting information on the PNAS web site). As shown in Fig. 3, they do not equally partition among the structures. Clustering analysis disclosed various extents of similarity between nephron portions. Proximal convoluted tubule and proximal straight tubule emerge as the closest related structures, being both enriched for 85 of the differentially distributed tags. The two collecting duct portions (cortical collecting duct and outer medullary collecting duct) also share a number of specific tags (n = 31). Strikingly, the cortical thick ascending limb of Henle's loop, medullary thick ascending limb of Henle's loop, and DCT are very close to each other. These three structures are more closely related to the collecting duct than to the proximal tubule. Finally, the more distinctive pattern is obtained for the glomerulus, which connects to the clustered tubular structures rather than to a peculiar nephron segment. The specific gene-expression signature of the glomerulus is consistent with its special cell constitution (22) and is highlighted further by the number of tags detected only in the glomerulus library [n = 34, whereas nephron segments display only one to two tags specific for a single structure (Table 4)]. The paucity of mitochondrial transcripts, which goes along with the absence of active ion transports, also sets the glomerulus apart from the tubular segments (Fig. 5, which is published as supporting information on the PNAS web site).
Fig. 3.
Overview of mRNA tag differential distribution. The number of mRNA tags displaying a significant differential distribution (P < 0.01, and ≥7-fold difference as compared with three libraries) is indicated for each library. The sum of all differences (n = 998) corresponds to 773 unique tags because of overlapping between libraries. The y axis indicates the number of differentially distributed tags common to clustered structures.
The differentially distributed tags consist of 773 unique tags. The majority of them were reliably matched to a single characterized gene (58.7%) or to anonymous cDNAs and ESTs (13.8%) (Table 5, which is published as supporting information on the PNAS web site). Because the data were obtained from a human tissue, it is of special interest to look for the expression of disease genes. We found 75 genes mutated in inherited human diseases to be differentially expressed along the nephron. Examples of such genes are displayed in Table 1, and the complete list is available online (Table 6a, which is published as supporting information on the PNAS web site). Although a number of them are not currently related to kidney diseases, we believe it important to deliver all the information that may help in refining phenotypic analysis. For genes mutated in kidney or kidney-dependent diseases, the expression patterns display variable degrees of tissue specificity that are consistent with the syndromes. For example, NPHS2, which encodes the glomerular protein podocin and is mutated in a steroid-resistant nephrotic syndrome (23), is indeed expressed only in the glomerulus. By contrast, the Na-K-2Cl cotransporter (SLC12A1), the chloride channel CLCNKB, and hydroxysteroid (11-β) dehydrogenase 2 (HSD11B2), which are all mutated in blood pressure disorders resting on salt reabsorption in the distal nephron (24-26), display significant predominant expression in several structures. Table 1 also shows that genes detected through more than one tag were repeatedly encountered. For ALDOB, the minor tag and the predominant one are relevant for a short and a long transcript, terminating at a proximal and a distal polyadenylation site, respectively (27). For SLC12A1, such detailed information is not available from the literature, but a similar mechanism may exist because the minor tag, which displays a consensus polyadenylation signal (AATAAA), locates upstream to the major one. Turning to AQP2, the published cDNA sequence corresponds to a 1.6-kb mRNA, but a more abundant transcript was detected at 4.2 kb by Northern hybridization (28). The human genome sequence now makes it feasible to conclude that the Northern and SAGE data are both consistent with alternative transcripts terminating at either the proximal or the distal polyadenylation site of the last AQP2 exon. Additional alternative transcripts may also exist, because four other SAGE tags match the AQP2 sequence (Table 4).
Table 1. Examples of disease genes differentially expressed along the human nephron.
| mRNA tag abundance
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease (gene) | Mapping | Tag | Glom | PCT | PST | MTAL | CTAL | DCT | CCD | OMCD |
| Nephrotic syndrome steroid-resistant (NPHS2, Podocin) | 1q25-q31 | CCTCACTGAA | 68 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glomerulosclerosis, focal segmental, 1 (ACTN4) | 19q13 | ATGGCGGGGC | 21 | 1 | 2 | 2 | 5 | 2 | 4 | 7 |
| Wilms tumor, type 1 (WT1) | 11p13 | TTACAAGATA | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hereditary fructose intolerance (ALDOB)* | 9q21.3-q22.2 | AAATTTCACA | 0 | 288 | 273 | 3 | 6 | 26 | 2 | 7 |
| GTGGTGGGAA | 0 | 19 | 46 | 1 | 1 | 8 | 0 | 1 | ||
| Renal tubular acidosis, proximal (SLC4A4) | 4q21 | AACATGGTGG | 0 | 29 | 10 | 2 | 3 | 1 | 10 | 1 |
| Hypophosphatemia (SLC34A1, NPT2) | 5q35 | AGCATTGAGA | 1 | 23 | 12 | 0 | 0 | 3 | 0 | 0 |
| Fructosuria (KHK) | 2p23.3-p23.2 | CGGGTGTCCG | 0 | 19 | 12 | 1 | 0 | 0 | 0 | 0 |
| Dihydropyrimidinuria (DPYS) | 8q22 | TTCATTTTAA | 0 | 16 | 3 | 0 | 0 | 3 | 0 | 0 |
| Alkaptonuria (HGD) | 3q21-q23 | GCCAAGTACC | 0 | 2 | 14 | 0 | 0 | 1 | 1 | 1 |
| Bartter syndrome type 1 (SLC12A1)* | 15q15-q21.1 | TGAGCAATCA | 0 | 0 | 1 | 222 | 149 | 29 | 1 | 2 |
| TCAATAAATG | 0 | 0 | 0 | 7 | 4 | 3 | 0 | 0 | ||
| Bartter syndrome type 3 (CLCNKB) | 1p36 | CTGGTGGGCA | 0 | 8 | 3 | 51 | 42 | 42 | 20 | 6 |
| Hypomagnesemia, primary (CLDN16) | 3q29 | ATTGTTCTAT | 0 | 0 | 0 | 6 | 14 | 3 | 0 | 1 |
| Hypomagnesemia (FXYD2, ATP1G1) | 11q23 | TTCGCTGGAC | 0 | 50 | 44 | 115 | 83 | 156 | 7 | 7 |
| Diabetes insipidus, nephrogenic (AQP2)* | 12q12-q13 | ACACACACCA | 0 | 1 | 33 | 2 | 3 | 3 | 157 | 156 |
| GGACCCCTGG | 0 | 0 | 4 | 0 | 0 | 0 | 34 | 39 | ||
| Apparent mineralocorticoid excess (HSD11B2) | 16q22 | CCCCAAGTGT | 0 | 0 | 4 | 5 | 11 | 31 | 45 | 21 |
| Renal tubular acidosis-osteopetrosis syndrome (CA2) | 8q22 | TACCTTGGTG | 0 | 4 | 3 | 1 | 0 | 14 | 20 | 16 |
| Liddle syndrome (SCNN1G) | 16p12 | TTCCCACTTC | 0 | 1 | 1 | 0 | 0 | 3 | 8 | 4 |
Values indicate mRNA tag abundance for libraries normalized to 50,000 total tags. The gene symbol from the Human Genome Organisation nomenclature, indicated in parentheses, is eventually followed by a usual alternate symbol. See Fig. 1 legend for definitions of abbreviations.
This gene was detected through more than one tag.
Identifying genes preferentially expressed in well-delineated structures is potentially useful to progress toward the characterization of diseases resting on a specifically located gene expression (29). Examples of renal diseases lacking a molecular characterization are type 2A pseudohypoaldosteronism (PHA2A), and IgA nephropathy. PHA2A is a syndrome of hypertension with hyperkalemia. As reviewed by Lifton et al. (3), all elucidated Mendelian forms of blood pressure disturbance concern genes that control renal salt reabsorption, and several of them are chiefly expressed in the distal nephron. PHA2A has been mapped to 1q31-q42 (30), which contains ≈400 genes, but analyzing this region for genes preferentially expressed in the distal nephron focused on four candidates (Table 2). The same kind of analysis was carried out for IgA nephropathy, a common form of end-stage renal disease with proliferation of the glomerular mesangium. IgA nephropathy has been linked to 6q22-q23 (31). The syndrome outcome points to the glomerulus as a relevant target, from which we tentatively sorted three candidates.
Table 2. Candidate genes for pseudohypoaldosteronism type 2A (PHA2A) and IgA nephropathy.
| mRNA tag abundance
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Disease and candidate genes | Tag | Glom | PCT | PST | MTAL | CTAL | DCT | CCD | OMCD |
| PHA2A (1q31-q42) | |||||||||
| Hypothetical protein DKFZp761N1114 | CAACTTTTTT | 0 | 0 | 0 | 14 | 5 | 3 | 0 | 1 |
| BTG family member 2 (BTG2)* | CCTTTGAGAG | 5 | 1 | 1 | 6 | 2 | 5 | 51 | 39 |
| E74-like factor 3 (ELF3)* | TATTTTTTCT | 0 | 6 | 1 | 3 | 4 | 1 | 13 | 24 |
| Ladinin 1 (LAD1) | TGATAAACTC | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 7 |
| IgA nephropathy (6q22-q23) | |||||||||
| Connective tissue growth factor (CTGF)* | AGTTTTTTCA | 239 | 2 | 0 | 30 | 4 | 12 | 5 | 9 |
| Transcription factor 21, podocyte-expressed (TCF21) | ATAGGATAGC | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Gap junction protein alpha1 43kD (GJA1) | ATGTGTTCTG | 8 | 0 | 1 | 0 | 0 | 1 | 0 | 2 |
Values indicate mRNA tag abundance for libraries normalized to 50,000 total tags. See Fig. 1 legend for definitions of abbreviations.
This gene was detected through more than one tag. Only the most abundant one is indicated.
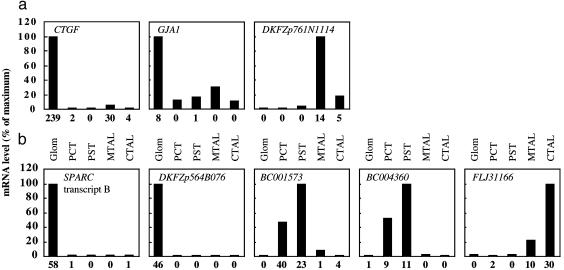
To check for representative tag sampling and correct gene identification, we further analyzed by quantitative RT-PCR a set of transcripts displaying different expression patterns. We studied three candidates for renal diseases and validated all of them (Fig. 4a). Fig. 4 also shows that the specific distribution of transcripts known only through anonymous cDNAs was corroborated by RT-PCR.
Fig. 4.
Quantitative RT-PCR analysis of mRNAs distribution along the nephron. RNAs were extracted from the five indicated structures, obtained in sufficient amounts to perform both SAGE and RT-PCR validations. RT-PCR data are displayed relative to the structure where expression is maximal. The value below each column indicates the result of the SAGE analysis and corresponds to tag counts for 50,000 tags. (a) Expression of candidate genes for IgA nephropathy (CTGF, GJA1) or PHA2A (DKFZp761N1114). (b) Selected examples of genes predicted by SAGE to be predominantly expressed in the glomerulus (SPARC, DKFZp564B076), the proximal tubule (BC001573, BC004360), or the thick ascending limb (FLJ31166).
The information gathered in this study goes far beyond the identification of differentially expressed genes. The human nephron gene expression database includes >90,000 tags, among which 10,705 were detected at least five times (Table 7, which is published as supporting information on the PNAS web site). As human gene and cDNA sequences are deciphered with increasing accuracy, this database offers the opportunity of updated identification of human kidney mRNAs by linking each tag to the SAGEmap resource (32). It is also anticipated to serve as a standard for future comparisons, including comparisons across species, as well as for the comprehensive analysis of gene families. As an illustration of this later possibility, we screened the human nephron database for the expression of genes that confer tissue transport and permeability properties (Table 8, which is published as supporting information on the PNAS web site). We detected tags for 258 such genes (119 solute carriers, 84 water or ion channels, 43 ion-transport ATPases, and 12 claudins). Genes from these families that are mutated in hereditary diseases are displayed in Table 6 a or b, according to the specificity of their expression pattern.
Discussion
The feasibility of analyzing gene expression patterns in well-delineated nephron portions was previously demonstrated by studies providing 1,000-15,000 ESTs or SAGE tags from microdissected tubular segments (9, 12). By sequencing >400,000 SAGE tags, we increase by several orders of magnitude the accuracy of gene expression profiling in the mammalian nephron. Moreover, our study was carried out on fresh human tissue pieces, which makes it possible to directly assess the compendium of genes expressed in native human nephron portions.
Several lines of evidence support the reliability of the data. First, known markers for specific nephron portions predominated in the corresponding libraries; second, when gene expression was evidenced through a tag that did not match the original cDNA sequence (e.g., AQP2, SLC12A1, or CLDN16), we repeatedly observed that this sequence either corresponded to a short transcript variant or was incomplete; and third, we were able to confirm by RT-PCR the SAGE data for mRNAs that had not been studied previously along the nephron.
We document huge differences from one nephron portion to another for several hundred transcripts. Although substantial, this number is expected to be a minimal estimate, because we used stringent criteria for defining differences. The majority (75%) of the differentially distributed tags were reliably matched to cDNAs of known or unknown function that are accurately mapped in the human genome. The mapping information provides a series of beacons to survey genomic regions held to contain gene(s) for renal diseases that affect specific nephron portions. Linkage studies usually highlight chromosomal domains containing hundreds of candidate genes, a number that can be substantially reduced by implementing transcriptome data to the screening procedure. This integrated strategy was explored to progress toward the molecular analysis of PHA2A and IgA nephropathy, but it could also be used for other renal diseases.
Clustering analysis revealed kinships between nephron portions that largely agree with those drawn from morphological and physiological studies. The nephron segments indeed partitioned into three groups consisting of proximal, a thick ascending limb-DCT, and a collecting duct cluster. However, the observation that the number of similarities between mated structures varies greatly from one cluster to another was rather unexpected. The number of tags predominantly expressed in both the proximal convoluted tubule and straight tubule is especially high. The difference with other clusters turns out to be robust, because it stands when we consider either the absolute (n = 85) or relative number of tags (45-60%) shared between the two proximal segments. On the other hand, clusters of tubular structures are much more related to each other than to the glomerulus, a notion consistent with the distinct functions of the glomerular and tubular portions of the nephron.
For obvious reasons, segmental analysis of nephron function is most often carried out on laboratory animals. However, as outlined previously (2, 20), caution is required when extrapolating to human beings observations made on the kidneys of animals. Comparison of the present results to those obtained in the mouse kidney (12, 33) confirms that marked differences indeed are present between species. For example, the mRNA levels for AQP2 and AQP3 are heavily different in the mouse outer medullary collecting duct (OMCD), reaching a 30:1 ratio (33), whereas in the human OMCD we found similar mRNA abundances for both aquaporins. It was the purpose of the present study to help characterize gene expression patterns in the human kidney. With a freely accessible database for most nephron segments, a number of queries pertinent to human kidney physiology can now be addressed without inferences from model systems.
Supplementary Material
Acknowledgments
We thank O. Gontcharevskaia for dissecting complete human nephrons (Fig. 1); P. Lesavre and D. Chauveau for help in starting this project and for advice about renal diseases; G. Deschênes for fruitful discussions; N. Caudy, S. Jounier, and H. Moysan for technical assistance; I. Bordelais for colony picking; and all those from Génoscope who contributed to DNA sequencing. This work was supported by Commissariat à l'Energie Atomique (CEA) grants to the Département de Biologie Joliot-Curie, and CEA and Centre National de la Recherche Scientifique grants to Unité de Recherche Associée 1859.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SAGE, serial analysis of gene expression; DCT, distal convoluted tubule.
Data deposition: SAGE data for the libraries described here are available at GEO (www.ncbi.nlm.nih.gov/geo) (accession nos. GSM10419 and GSM10423-GSM10429).
References
- 1.Abramow, M. & Dratwa, M. (1974) Nature 250, 492-493. [DOI] [PubMed] [Google Scholar]
- 2.Chabardès, D., Gagnan-Brunette, M., Imbert-Teboul, M., Gontcharevskaia, O., Montégut, M., Clique, A. & Morel, F. (1980) J. Clin. Invest. 65, 439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lifton, R. P., Gharavi, A. G. & Geller, D. S. (2001) Cell 104, 545-556. [DOI] [PubMed] [Google Scholar]
- 4.International Human Genome Sequencing Consortium. (2001) Nature 409, 860-921. [DOI] [PubMed] [Google Scholar]
- 5.Venter, J. C., Adams, M. D., Myers, E. W., Li, P. W., Mural, R. J., Sutton, G. G., Smith, H. O., Yandell, M., Evans, C. A., Holt, R. A., et al. (2001) Science 291, 1304-1351. [DOI] [PubMed] [Google Scholar]
- 6.Velculescu, V. E., Zhang, L., Zhou, W., Vogelstein, J., Basrai, M. A., Bassett, D. E., Jr., Hieter, P., Vogelstein, B. & Kinzler, K. W. (1997) Cell 88, 243-251. [DOI] [PubMed] [Google Scholar]
- 7.Adams, M. D., Kerlavage, A. R., Fleischmann, R. D., Fuldner, R. A., Bult, C. J., Lee, N. H., Kirkness, E. F., Weinstock, K. G., Gocayne, J. D., White, O., et al. (1995) Nature 377, 3-174. [PubMed] [Google Scholar]
- 8.Strausberg, R. L., Feingold, E. A., Grouse, L. H., Derge, J. G., Klausner, R. D., Collins, F. S., Wagner, L., Shenmen, C. M., Schuler, G. D., Altschul, S. F., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 16899-16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takenaka, M., Imai, E., Kaneko, T., Ito, T., Moriyama, T., Yamauchi, A., Hori, M., Kawamoto, S. & Okubo, K. (1998) Kidney Int. 53, 562-572. [DOI] [PubMed] [Google Scholar]
- 10.Yano, N., Endoh, M., Fadden, K., Yamashita, H., Kane, A., Sakai, H. & Rifai, A. (2000) Kidney Int. 57, 1452-1459. [DOI] [PubMed] [Google Scholar]
- 11.Hishikawa, K., Oemar, B. S. & Nakaki, T. (2001) J. Biol. Chem. 276, 16797-16803. [DOI] [PubMed] [Google Scholar]
- 12.Virlon, B., Cheval, L., Buhler, J.-M., Billon, E., Doucet, A. & Elalouf, J.-M. (1999) Proc. Natl. Acad. Sci. USA 96, 15286-15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabardès, D., Firsov, D., Aarab, L., Clabecq, A., Bellanger, A.-C., Siaume-Perez, S. & Elalouf, J.-M. (1996) J. Biol. Chem. 271, 19264-19271. [DOI] [PubMed] [Google Scholar]
- 14.Cheval, L., Virlon, B. & Elalouf, J.-M. (2000) in Functional Genomics, eds. Hunt, S. P. & Livesey, J. P. (Oxford Univ. Press, Oxford), pp. 139-163.
- 15.Zhang, L., Zhou, W., Velculescu, V. E., Kern, S. E., Hruban, R. H., Hamilton, S. R., Vogelstein, B. & Kinzler, K. W. (1997) Science 276, 1268-1272. [DOI] [PubMed] [Google Scholar]
- 16.Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H. L., Coulson, A. R., Drouin, J., Eperon, I. C., Nierlich, D. P., Roe, B. A., Sanger, F., et al. (1981) Nature 290, 457-465. [DOI] [PubMed] [Google Scholar]
- 17.Kershaw, D. B., Beck, S. G., Wharram, B. L., Wiggins, J. E., Goyal, M., Thomas, P. E. & Wiggins, R. C. (1997) J. Biol. Chem. 272, 15708-15714. [DOI] [PubMed] [Google Scholar]
- 18.Denker, B. M., Smith, B. L., Kuhajda, F. P. & Agre, P. (1988) J. Biol. Chem. 263, 15634-15642. [PubMed] [Google Scholar]
- 19.Hession, C., Decker, J. M., Sherblom, A. P., Kumar, S., Yue, C. C., Mattaliano, R. J., Tizard, R., Kawashima, E., Schmeissner, U., Heletky, S., et al. (1987) Science 237, 1479-1484. [DOI] [PubMed] [Google Scholar]
- 20.Biner, H. L., Arpin-Bott, M. P., Loffing, J., Wang, X., Knepper, M., Hebert, S. C. & Kaissling, B. (2002) J. Am. Soc. Nephrol. 13, 836-847. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi, K., Sasaki, S., Fushimi, K., Uchida, S., Kuwahara, M., Saito, H., Furukawa, T., Nakajima, K., Yamaguchi, Y., Gojobori, T., et al. (1994) Proc. Natl. Acad. Sci. USA 91, 6269-6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kriz, W. & Kaissling, B. (2000) in The Kidney, Physiology and Pathophysiology, eds. Seldin, D. W. & Giebisch, G. (Lippincot Williams & Wilkins, Philadelphia), 3rd Ed., Vol. 1, pp. 587-654. [Google Scholar]
- 23.Boute, N., Gribouval, O., Roselli, S., Benessy, F., Lee, H., Fuchshuber, A., Dahan, K., Gubler, M. C., Niaudet, P. & Antignac, C. (2000) Nat. Genet. 24, 349-354. [DOI] [PubMed] [Google Scholar]
- 24.Simon, D. B., Karet, F. E., Hamdan, J. M., DiPietro, A., Sanjad, S. A. & Lifton, R. P. (1996) Nat. Genet. 13, 183-188. [DOI] [PubMed] [Google Scholar]
- 25.Simon, D. B., Bindra, R. S., Mansfield, T. A., Nelson-Williams, C., Mendonca, E., Stone, R., Schurman, S., Nayir, A., Alpay, H., Bakkaloglu, A., et al. (1997) Nat. Genet. 17, 171-178. [DOI] [PubMed] [Google Scholar]
- 26.Mune, T., Rogerson, F. M., Nikkila, H., Agarwal, A. K. & White, P. C. (1995) Nat. Genet. 10, 394-399. [DOI] [PubMed] [Google Scholar]
- 27.Sakakibara, M., Mukai, T., Yatsuki, H. & Hori, K. (1985) Nucleic Acids Res. 13, 5055-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki, S., Fushimi, K., Saito, H., Saito, F., Uchida, S., Ishibashi, K., Kuwahara, M., Ikeuchi, T., Inui, K.-I., Nakajima, K., et al. (1994) J. Clin. Invest. 93, 1250-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwaenepoel, I., Mustapha, M., Leibovici, M., Verpy, E., Goodyear, R., Liu, X. Z., Nouaille, S., Nance, W. E., Kanaan, M., Avraham, K. B., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 6240-6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansfield, T. A., Simon, D. B., Farfel, Z., Bia, M., Tucci, J. R., Lebel, M., Gutkin, M., Vialettes, B., Christofilis, M. A., Kauppinen-Makelin, R., et al. (1997) Nat. Genet. 16, 202-205. [DOI] [PubMed] [Google Scholar]
- 31.Gharavi, A. G., Yan, Y., Scolari, F., Schena, F. P., Frasca, G. M., Ghiggeri, G. M., Cooper, K., Amoroso, A., Viola, B. F., Battini, G., et al. (2000). Nat. Genet. 26, 354-357. [DOI] [PubMed] [Google Scholar]
- 32.Lash, A. E., Tolstoshev, C. M., Wagner, L., Schuler, G. D., Strausberg, R. L., Riggins, G. J. & Altschul, S. F. (2000) Genome Res. 10, 1051-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elalouf, J.-M., Aude, J.-C., Billon, E., Cheval, L., Doucet, A. & Virlon, B. (2002) Exp. Nephrol. 10, 75-81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.