Abstract
The posterior parietal cortex (PPC) has been proposed to play a critical role in exerting top-down influences on occipital visual areas. By inducing activity in the PPC (angular gyrus) using transcranial magnetic stimulation (TMS), and using the phosphene threshold as a measure of visual cortical excitability, we investigated the functional role of this region in modulating the activity of the visual cortex. When triple-pulses of TMS were applied over the PPC unilaterally, the intensity of stimulation required to elicit a phosphene from the visual cortex (area V1/V2) was reduced, indicating an increase in visual cortical excitability. The increased excitability that was observed with unilateral TMS was abolished when TMS was applied over the PPC bilaterally. Our results provide a demonstration of the top-down modulation exerted by the PPC on the visual cortex and show that these effects are subject to interhemispheric competition.
Keywords: phosphenes, posterior parietal cortex, top-down modulation, transcranial magnetic stimulation, V1/V2
Introduction
Attention can bias neural responses toward an attended target stimulus within a receptive field, and diminish responses to distractors (Kastner and Ungerleider 2000). There is a great deal of evidence that the frontoparietal network is the likely source of this attentional modulation (cf. Latto and Cowey 1971; Gitelman et al. 1999; Hopfinger et al. 2000; Giesbrecht et al. 2003). Firstly, the neuropsychological phenomena of neglect and extinction, namely reduced attention and awareness for stimuli in the contralesional hemifield, particularly in the presence of ipsilateral stimuli, strongly implicates the frontoparietal cortex (e.g., Mesulam 1981, 1999; Robertson and Marshall 1993) in visual attention. Secondly, in neurologically normal observers, neuroimaging studies typically implicate the frontoparietal network in attention (e.g., Corbetta and Shulman 2002). Thirdly, a number of transcranial magnetic stimulation (TMS) studies have demonstrated the necessity of frontoparietal areas and in tasks such as spatial orienting of attention, visual search, visuo-spatial priming, and change detection (Cowey and Latto 1971; Rushworth et al. 2001; Grosbras and Paus 2002; Muggleton et al. 2003; O'Shea et al. 2004; Turatto et al. 2004; Hung et al. 2005; Beck et al. 2006; O'Shea and Walsh 2006; Campana et al. 2007). In addition, studies using microstimulation and single-cell recordings in macaques have reported reciprocal pathways between the frontoparietal network cortex and occipital visual areas (Moore and Armstrong 2003), and the rapid neural responses to visual stimulation in this network enable modulation of ongoing processing in the visual cortex (Raiguel et al. 1989; Bisley et al. 2005).
Recently, a number of studies have provided direct causal evidence for the view that the frontoparietal network exerts top-down modulation on visual cortical activity. The first such study was conducted by Taylor et al. (2007) who applied TMS over the FEF during a covert orienting task and simultaneously measured occipital visual evoked potentials (ERPs). Their results showed changes in visual cortical activity resulting from the FEF TMS. Using the phosphene threshold as a measure of cortical excitability, Silvanto et al (2006) showed that stimulation of the frontal eye fields increases the excitability of the visual cortex. Subsequently, Ruff et al. (2006, 2007) investigated the top-down influence exerted by the frontoparietal network by applying TMS over parietal and frontal regions during functional magnetic resonance imaging. Their findings confirmed those of Taylor et al. (2007) and Silvanto et al. (2006) on the modulatory influence of the FEF on visual cortical activation. They also found that TMS applied over the intraparietal sulcus affected the blood oxygenation level–dependent signal in areas V1–V4 and in V5/MT. However, although these studies provide strong evidence for parietal modulation of visual cortex, because the experiments were carried out in passive observers, they do not reveal the perceptual significance of this modulation or address the effects of posterior parietal cortex (PPC) TMS on visual cortex sensitivity.
The objective of the present study was to study the perceptual consequence of the top-down modulation exerted by the PPC on the visual cortex. We induced activity in the PPC via TMS and by locally inducing phosphenes from V1/V2, we obtained a perceptual/functional measure of the consequences of top-down modulation on the sensitivity of a specific region in the visual cortex. Our results show that stimulation of PPC leads to a decrease in the intensity of stimulation required eliciting a visual percept implying that PPC TMS modulates excitability of the visual cortex. Secondly, to determine whether parietal top-down modulation is affected by interhemispheric competition, we applied TMS bilaterally over the PPC while inducing phosphenes from the visual cortex. Our results show that the increase in visual cortical excitability that was observed with unilateral PPC TMS was lost with bilateral stimulation of the parietal regions. This demonstrates that the parietal top-down modulation is affected by interhemispheric competition.
Methods
Subjects
Seven subjects took part in the investigation, six of whom were naïve to its purpose. The other subject was the author J.S. The study was approved by the local ethics committee of University College London, and subjects gave informed consent. All subjects had previously participated in studies of phosphene perception, the advantage being that their phosphene thresholds are stable.
Transcranial Magnetic Stimulation
TMS was administered with three Magstim Super Rapid stimulators (Magstim Company, UK). The pulses were triggered remotely using a computer that controlled both stimulators, using E-Prime software. Fifty-millimeter figure-8 coils were used over all sites. For PPC TMS, stimulation was applied over locations that corresponded with the anatomical delineation of left and right angular gyrus by structural magnetic resonance imaging (MRI) in each subject (see Muggleton et al. 2006 for details). The stimulation sites were identified on each subject's T1-weighted MRI scan and coregistered with scalp coordinates. For V1/V2 an additional criterion was the induction of small phosphenes near fixation in the contralateral visual field (see Walsh and Pascual-Leone 2003). The mean Talaraich coordinates of the stimulation sites were left PPC: −42, −64, 36; right PPC: 47, 61, 37; left V1/V2: −12, −73, 14; right V1/V2: 10, −70, 15. Vertex was used as a control stimulation site to control for nonspecific effects of TMS.
Stimulation strength was always the same over the PPC and the control site (Vertex), 65%. To determine phosphene thresholds for each TMS condition the intensity of V1/V2 pulse was varied according to a modified binary search (MOBS, Tyrell and Owens 1988), an adaptive threshold finding algorithm. The TMS intensity was increased or decreased according to the subject's report on the previous trial. The original upper boundary of the stimulation was 100% of stimulator output and the lower limit 0%. After each TMS delivery, subjects reported verbally whether or not they had perceived a phosphene. The number of trials required for setting a threshold depends on the consistency of the subject's reports and in this experiment was between 6 and 15 trials. Intertrial interval was five seconds per trial. The mean values of the baseline thresholds were 71.5% and 73.8% of the maximum stimulator output for left and right V1/V2, respectively.
TMS Conditions
The experiment consisted of two types of PPC stimulation: unilateral and bilateral. In both types of conditions, three coils were placed on the subjects’ head, so that any differences in results could not simply be due the discomfort of having three coils on one's scalp. In the bilateral conditions, the PPC was stimulated in both the left and right hemisphere. In the unilateral conditions, the PPC was stimulated in either the left or right hemisphere, with one coil stimulating the Vertex. The third coil was used to induce a phosphene from either the left or right V1/V2. The PPC and Vertex were stimulated with pulse trains consisting of three pulses (with a pulse gap of 50 ms). V1/V2 was stimulated with a single pulse of TMS that was administered in the middle of the PPC/Vertex pulse trains. The V1/V2 TMS pulse was applied in the middle of the PPC pulse train so that the PPC top-down modulation would preactivate V1/V2 prior to application of V1/V2 TMS and also overlap with the visual cortical activity induced by the V1/V2 TMS pulse. Additionally, a control condition was carried out in which a pulse train was administered over the vertex, whereas phosphenes were induced from either the left or right V1/V2 with single pulse TMS.
In summary, the TMS conditions were 1) left PPC TMS + vertex TMS 2) right PPC TMS + vertex TMS; 3) left PPC TMS + right PPC TMS; 4) vertex TMS.
For each condition, phosphene threshold was determined for both the left and right V1/V2 using the MOBS paradigm (described above). In addition, two baseline phosphene threshold measurements were conducted. The order of blocks was randomized. Each condition was repeated twice.
Procedure
Subjects were seated on a chair and placed their head on a chinrest. The coils were fixed in place using “magic arm” (Manfrotto) coil holders. Subjects’ eyes were covered throughout the experiment and they were instructed to report whether or not they had perceived a phosphene after each TMS pulse. Phosphene threshold was measured twice for each condition. In addition, the baseline threshold (in which TMS was applied only over left or right V1/V2) was measured twice in each session, one at the beginning and the other at the end of each session. In order to investigate any changes in phosphene appearance resulting from the PPC stimulation, subjects were also asked to draw their percept after each TMS condition.
Results
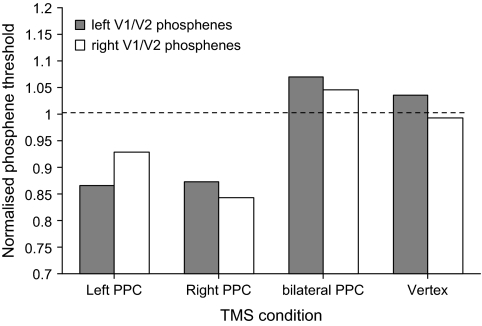
Subjects’ phosphene thresholds in each condition were measured as a percentage of the maximum output of the stimulator unit. To obtain a relative measure of the phosphene thresholds, subjects’ absolute phosphene threshold in each TMS condition was normalized relative to their baseline threshold level. Figure 1 shows the subjects’ mean phosphene thresholds as a function of the PPC TMS condition.
Figure 1.
The normalized (n = 7) phosphene thresholds for left and right V1/V2 as a function of the TMS condition. Right PPC TMS significantly lowered the intensity of stimulation required to induce a phosphene from both the left and right V1/V2. Left PPC TMS significantly lowered the phosphene threshold of phosphenes induced from the left V1/V2 TMS; for right V1/V2 there was a trend which did not reach statistical significance. These decreases in phosphene thresholds reflect increased excitability of the early visual cortex. With bilateral PPC TMS, the reduction of phosphene thresholds that was observed with unilateral PPC TMS was abolished.
The Effect of PPC TMS on the Excitability of Right V1/V2
Repeated measures analysis of variance (ANOVA) with TMS condition as the main factor (right PPC, left PPC, bilateral PPC, Vertex) indicated a significant effect (F(3) = 10.056, P = 0.0002). Pairwise comparisons (with the P = value adjusted to 0.0125 to account for four t-tests that were carried out) revealed that the application of TMS over the right PPC decreased the phosphene threshold relative to the vertex condition (t(6) = 4.339; P = 0.0049). In contrast, phosphene threshold in the bilateral PPC TMS condition did not differ from that in the Vertex condition (t(6) = 0.669; P = 0.529). Moreover, in the bilateral PPC condition the phosphene thresholds were significantly higher than in the right PPC condition (t(6) = 3.842; P = 0.009). With left PPC TMS, there was a trend toward a significant reduction in phosphene thresholds relative to the Vertex condition but it did not reach statistical significance (t(6) = 2.753; P = 0.033).
The Effect of PPC TMS on the Excitability of the Left V1/V2
Repeated measures ANOVA with TMS condition as the main factor (right PPC, left PPC, bilateral PPC, Vertex) indicated a significant effect (F(3) = 15.75; P = 0.0001). Pairwise comparisons (with the P = value adjusted to 0.0125 to account for multiple comparisons) revealed that the application of TMS over both the left and right PPC decreased the phosphene threshold relative to the vertex condition (left PPC vs. Vertex: t(6) = 5.583; P = 0.0014; right PPC vs. Vertex: t(6) = 5.737; P = 0.0012). In contrast, phosphene threshold in the bilateral PPC TMS condition did not differ from that in the Vertex condition (t(6) = 0.675; P = 0.525). In the bilateral PPC condition the phosphene thresholds were significantly higher than in the right PPC condition (t(6) = 5.430; P = 0.0016) and the left PPC condition (t(6) = 3.63; P = 0.01).
The Effect of PPC TMS on Phosphene Appearance
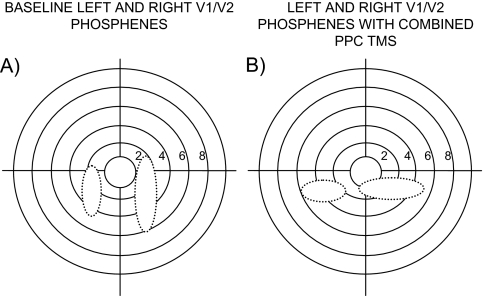
In addition to determining the effect of PPC TMS on phosphene threshold, we also investigated the effect of this stimulation on phosphene appearance by asking subjects to draw the phosphenes that they perceived. Figure 2 shows phosphene appearance in one representative subject. In this subject, the baseline V1/V2 phosphene appeared as a vertical bar (Fig. 2A). However, when TMS was applied over the PPC (in this case the right PPC), the shape of the phosphene changed and it now appeared as a horizontal bar (Fig. 2B). This change in phosphene shape was also induced with bilateral PPC TMS, even though phosphene thresholds were not affected in this condition. Vertex TMS had no effect on phosphene appearance. With left PPC TMS, a change in phosphene shape was observed in five of the seven subjects for left V1/V2 phosphenes and in two of the subjects for right V1/V2 phosphenes. With right PPC TMS, this was observed in four subjects for left V1/V2 phosphenes and in five subjects for right V1/V2 phosphenes.
Figure 2.
Examples of phosphenes induced from V1/V2 in the right and left hemisphere, based on the drawing of a representative subjects. (A) Phosphene appearance when TMS is applied over the left/right V1/V2 only. (B) Phosphene appearance in the same subject when TMS is additionally applied over the PPC. The PPC TMS induced a change in phosphene shape in majority of subjects. The numericals indicate the degree of eccentricity.
Discussion
Our results show that the PPC exerts perceptually significant top-down influence on early visual cortex. When TMS was applied over the right PPC, the level of stimulation required for inducing a phosphene from both the left and right V1/V2 decreased, indicating an increase in cortical excitability. With left PPC TMS a significant increase in excitability was only observed for phosphenes induced from the same hemisphere, although statistically nonsignificant trend was present also for phosphenes induced from the right V1/V2. This pattern of results is consistent with the established hemispheric asymmetry in attentional functions in humans according to which the right hemisphere is more commonly concerned with processing information in both visual fields, whereas the left hemisphere is concerned only with the right visual field (e.g., Mesulam 1981).
TMS applied over the PPC not only affected phopshene intensity (as reflected in the phosphene threshold), but also the phosphene appearance. A common finding was that a phosphene with a shape of a horizontal bar became a vertical bar when PPC were stimulated. This suggests that stimulation of PPC does not lead to a uniform increase in visual cortical excitability; rather, neurons encoding certain parts of the visual field are facilitated and neurons encoding other parts are inhibited, leading to change in phosphene shape. This is likely to reflect the difference in the visual field maps between the PPC and V1/V2. Effectively, the top-down modulation induced by PPC TMS imposes the retinotopy of the visual map in PPC on the pattern of V1/V2 activation, and this is reflected in the appearance of the phosphene.
Importantly, when TMS was applied bilaterally over the PPC, level of stimulation that was required to induce a phosphene from the visual cortex increased relative to the unilateral PPC conditions. In other words, the increase in visual cortical excitability that was observed with unilateral PPC TMS was abolished when TMS was applied over both the left and right PPC. This result cannot simply due to the presence of three coils over the subjects’ head, as subjects were stimulated with three coils also in the unilateral PPC TMS conditions, where decreases in phosphene thresholds were observed.
This effect of bilateral PPC TMS on visual cortical excitability reveals the role of interhemispheric competition in top-down modulation. It has been proposed that a dynamic balance exists between the two hemispheres in orienting attention toward the contralateral hemispace (Kinsbourne 1977, 1994). After a unilateral lesion, the unaffected hemisphere becomes hyperactivated following the release of reciprocal inhibition by the lesioned hemisphere, and this breakdown of interhemispheric balance produces neglect. The hyperactivation in the unaffected hemisphere could lead to enhanced inhibition of the affected hemisphere. This view is supported by a finding that disruption of the undamaged hemisphere in patients using TMS reduces the level of contralesional extinction (Oliveri et al. 1999). In the present study, it is plausible that TMS applied unilaterally over the PPC triggered excitatory connections to the visual cortex, preactivating the early visual areas and thus lowering phosphene thresholds. When TMS is applied bilaterally, each PPC receives inhibitory input from the contralateral hemisphere, preventing it from exciting early visual cortex. In other words, inhibitory interactions between the left and right PPC prevents either of them from enhancing the activity levels of the occipital cortex.
In summary, our findings demonstrate directly that the PPC modulates the responses of the visual cortex, that this modulation is subject to interhemispheric competition and that this modulation is consistent with proposed role for the PPC in top-down control of attention.
Funding
Medical Research Council program grant to V.W.; Wellcome Trust award (WT080568MA) to N.L., and University College London Graduate School Research Scholarship to J.S.
Acknowledgments
Conflict of Interest: None declared.
References
- Beck DM, Muggleton N, Walsh V, Lavie N. Right parietal cortex plays a critical role in change blindness. Cereb Cortex. 2006;16:712–717. doi: 10.1093/cercor/bhj017. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Suresh Krishna B, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci. 2005;24:1833–1838. doi: 10.1523/JNEUROSCI.5007-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana G, Cowey A, Casco C, Oudsen I, Walsh V. Left frontal eye field remembers “where” but not “what. Neuropsychologia. 2007;45:2340–2345. doi: 10.1016/j.neuropsychologia.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cowey A, Latto RM. Effects of frontal eye-field ablation on visual fields and on fixation in rhesus monkeys. Brain Res. 1971;31:375–376. doi: 10.1016/0006-8993(71)90209-5. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field: effects on visual perception and attention. J Cogn Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hung J, Driver J, Walsh V. Visual selection and posterior parietal cortex: effects of repetitive transcranial magnetic stimulation on partial report analyzed by Bundesen's theory of visual attention. J Neurosci. 2005;25:9602–9612. doi: 10.1523/JNEUROSCI.0879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Adv Neurol. 1977;18:41–49. [PubMed] [Google Scholar]
- Kinsbourne M. Mechanism of neglect. Neuropsychol Rehabil. 1994;4:151–153. [Google Scholar]
- Latto R, Cowey A. Visual field defects after frontal eye-field lesions in monkeys. Brain Res. 1971;30:1–24. doi: 10.1016/0006-8993(71)90002-3. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulated contributions to the mental representation and attentional targeting of salient extrapersonal events. Phil Trans R Soc Lond. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Postma P, Moutsopoulou K, Nimmo-Smith I, Marcel A, Walsh V. TMS over right posterior parietal cortex induces neglect in a scene-based frame of reference. Neuropsychologia. 2006;44(7):1222–1229. doi: 10.1016/j.neuropsychologia.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Traversa R, Cicinelli P, Filippi MM, Pasqualetti P, Tomaiuolo F, Caltagirone C. Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain. 1999;122:1731–1739. doi: 10.1093/brain/122.9.1731. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Muggleton NG, Cowey A, Walsh V. Timing of target discrimination in human frontal eye field. J Cogn Neurosci. 2004;16:1060–1067. doi: 10.1162/0898929041502634. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Walsh V. Trickle-down theories of vision. Curr Biol. 2006;16:R206–R209. doi: 10.1016/j.cub.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Raiguel SE, Lagae L, Gulyas B, Orban GA. Response latencies of visual cells in macaque areas V1, V2, and V5. Brain Res. 1989;493:155–159. doi: 10.1016/0006-8993(89)91010-x. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Marshall JC. Unilateral neglect: clinical and experimental studies. Hove (UK): Psychology Press; 1993. [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS fMRI. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm128. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci. 2001;4:656–661. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates the sensitivity of the extrastriate visual cortex. J Neurophysiol. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- Taylor PCJ, Nobre AC, Rushworth MFS. FEF TMS affects visual cortical activity. Cereb Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- Turatto M, Sandrini M, Miniussi C. The role of the right dorsolateral prefrontal cortex in visual change awareness. Neuroreport. 2004;15:2549–2552. doi: 10.1097/00001756-200411150-00024. [DOI] [PubMed] [Google Scholar]
- Tyrell RA, Owens DA. A rapid technique to assess the resting states of the eye and other threshold phenomena: the modified binary search (MOBS) Behav Res Methods Instrum Comput. 1988;20:137–141. [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial magnetic stimulation: a neurochronometrics of mind. 1st ed. Boston (MA): Massachusetts Institute of Technology Press; 2003. [Google Scholar]