Abstract
Sentence comprehension (SC) studies in typical and impaired readers suggest that reading for meaning involves more extensive brain activation than reading isolated words. Thus far, no reading disability/dyslexia (RD) studies have directly controlled for the word recognition (WR) components of SC tasks, which is central for understanding comprehension processes beyond WR. This experiment compared SC to WR in 29, 9–14 year olds (15 typical and 14 impaired readers). The SC-WR contrast for each group showed activation in left inferior frontal and extrastriate regions, but the RD group showed significantly more activation than Controls in areas associated with linguistic processing (left middle/superior temporal gyri), and attention and response selection (bilateral insula, right cingulate gyrus, right superior frontal gyrus, and right parietal lobe). Further analyses revealed this overactivation was driven by the RD group's response to incongruous sentences. Correlations with out-of-scanner measures showed that better word- and text-level reading fluency was associated with greater left occipitotemporal activation, whereas worse performance on WR, fluency, and comprehension (reading and oral) were associated with greater right hemisphere activation in a variety of areas, including supramarginal and superior temporal gyri. Results provide initial foundations for understanding the neurobiological correlates of higher-level processes associated with reading comprehension.
Keywords: dyslexia, neuroimaging, reading disabilities, sentence comprehension
Introduction
Studies over the past few decades have shown that one of the defining characteristics of adults and children with reading disabilities (RD or dyslexia) is an inability to recognize and decode words accurately and efficiently, which impedes reading comprehension (Adams 1990; Lyon 1995; Torgesen 2000). As such, there has been significant interest in understanding the neural processes associated with reading single words in individuals with RD. Functional neuroimaging studies have shown that when reading real or nonwords, skilled readers show brain activation in left hemisphere regions, including the inferior frontal gyrus, temporoparietal, and occipitotemporal areas. In contrast, individuals with RD tend to show activation in the right hemisphere, versus left hemisphere, posterior regions (Pugh et al. 2000; Simos et al. 2000; Shaywitz et al. 2002, 2004; Eden et al. 2004); furthermore, overactivation of the left inferior frontal gyrus has been associated with dyslexia (Shaywitz et al. 1998, 2002; Brunswick et al. 1999).
As the understanding of the word recognition (WR)/decoding aspects of RD has increased, there has been a growing interest in other components critical to comprehending text, especially for impaired readers (McCardle et al. 2001; Leach et al. 2003; Nation and Snowling 2004; Cutting and Scarborough 2006). Reading is a multifaceted process, the ultimate goal of which is to comprehend multiple sentences and paragraphs. In addition to WR/decoding, other processes are increasingly important for comprehension, such as syntax, vocabulary, short-term/working memory, and reading strategies (Swanson and Trahan 1996; Biancarosa and Snow 2004; Materek and Cutting, unpublished data). In dyslexia, although WR is the main impediment, many behavioral studies have shown that it is not the only limiting factor for comprehension. In fact, many individuals with RD also show difficulty with higher-level language processes (and often have oral language disorders), which are thought to contribute to comprehending connected text, in addition to their word-level difficulties (Catts et al. 1999, 2003; Leach et al. 2003; Nation and Snowling 2004; Materek and Cutting, unpublished data). Here we offer functional magnetic resonance imaging (fMRI) data of variations in cortical activation during sentence comprehension (SC) tasks between older children with dyslexia versus typically developing readers of the same age.
Although comprehending connected text, particularly sentences, is a critical component of reading, to date most functional neuroimaging SC studies have been conducted with skilled readers (Booth et al. 1999; Meyer et al. 2000; Ni et al. 2000; Caplan et al. 2001; Ferstl and von Cramon 2001; Keller et al. 2001, 2003; Grossman et al. 2002; Hashimoto and Sakai 2002; Friederici et al. 2003; Ben-Shachar et al. 2004; Capek et al. 2004; Cooke et al 2006; Cutting et al. 2006; Maess et al. 2006; Jobard et al. 2007; Mason and Just 2007). In general, findings from these studies have revealed that SC is associated with patterns of activation that are similar to those involved with processing isolated words; however, the activation is more widespread, with bilateral activation of the inferior frontal gyrus (L > R) and the posterior superior and middle temporal gyri (Meyer et al. 2000; Caplan et al. 2001; Ferstl and von Cramon 2001; Keller et al. 2001; Grossman et al. 2002; Friederici et al. 2003; Cooke et al. 2006; Cutting et al. 2006; Jobard et al. 2007). More specifically, studies that have directly contrasted SC to isolated words in the same experiment (Bottini et al. 1994; Stowe et al. 1994, 1998, 1999; Jobard et al. 2007) have found that SC elicits more activation than isolated words, especially in left middle and superior temporal gyri, as well as right hemisphere; greater bilateral temporal poles as well as left frontal and parietal lobe activation for sentences versus words has also been reported (Bottini et al. 1994; Stowe et al. 1999). It is important to mention that in addition to contrasting SC and isolated words, many SC studies have focused on isolating regions associated with specific components of SC, such as syntax, semantics, and/or pragmatics (e.g., Dapretto and Bookheimer 1999; Ni et al. 2000; Caplan et al. 2001; Keller et al. 2001; Grossman et al. 2002; Shankweiler et al. forthcoming). These studies suggest that different left hemisphere networks subserve different components of SC and that, furthermore, interaction between syntax and semantic processes may together modulate different brain regions (e.g., Keller et al. 2001). Nevertheless, it is important to note that it is clear from a number of studies that there is a large measure of overlap in cortical responses, even when sentence tasks have stimuli designed to tap specific aspects of processing (e.g., see Ni et al. 2000; Cooke et al. 2001; Keller et al. 2001).
Compared with the literature available for skilled readers, the functional neuroimaging literature on SC in individuals with RD is relatively limited. However, studies have shown that individuals with WR/decoding difficulties show abnormal patterns of activation during SC tasks (Rumsey et al. 1994; Helenius et al. 1999; Breznitz and Leikin 2000, 2001; Seki et al. 2001; Leikin 2002; Kronbichler et al. 2006; Sabisch et al. 2006; Meyler et al. 2007). Of particular relevance is that these abnormalities in RD appear to be present in higher-level language comprehension independent of WR, as differences have been found between dyslexic and Control groups not only when reading sentences, but also while listening to sentences (Rumsey et al. 1994; Leikin 2002; Sabisch et al. 2006). For example, in a positron emission tomography study, Rumsey et al. (1994) compared regional cerebral blood flow in adult skilled and poor readers listening to pairs of sentences that, “Differed in grammatic construction (e.g., “A free hamburger comes with a coke. The hamburger comes with a free coke.”); participants pressed a button only if both sentences had the same meaning. This study showed that although the syntactic processing of auditorally presented sentences was generally the same between groups, activating temporal and inferior frontal gyrus sites (L > R), dyslexics showed some abnormalities, including reversed asymmetry in the temporoparietal region (R > L) and increased right anterior frontal activation as compared with Controls. Additionally, more left lateralized temporal/IFG activation was associated with better performance. More recently Meyler et al. (2007) did an fMRI study that varied sentences on semantic sensibility (nonsense vs. sensible) and syntactic complexity (active vs. passive) in a block design with each condition contrasted against a baseline of fixation on a plus (+) sign in the center of the screen; they found, in third and fifth graders, that lower reading ability was associated with decreased activation in left middle temporal gyrus, right inferior parietal lobule, and left postcentral gyrus. Although neuroimaging studies comparing sentence processing in RD and Control groups in the English language are quite limited, there are more studies in other languages, which have also revealed differences between dyslexics and Controls in processing sentences (Helenius et al. 1999; Breznitz and Leikin 2000, 2001; Seki et al. 2001; Karni et al. 2005; Kronbichler et al. 2006; Sabisch et al. 2006). However, results have varied and no clear patterns of difference have emerged; this may be, in part, due to differences in task design. In summary, it is apparent that individuals with dyslexia exhibit abnormalities in sentence processing, and that these differences cannot be fully attributed to WR; however, unlike neuroimaging studies involving isolated words, clear patterns of abnormalities have not emerged.
A consideration in understanding abnormalities in lower and higher-level linguistic processing in dyslexia is being able to understand patterns of abnormal activation that can be attributed to comprehension-specific processes. Without controlling for the activation associated with processing individual words, it is difficult to know whether there are specific comprehension abnormalities independent of/in addition to word-level abnormalities in individuals with WR or decoding difficulties. Understanding if (and how) these higher-level processes might be affected in RD is potentially important for pinpointing deficits in comprehension-specific processes.
In an effort to explore the contributions of different component processes to the complex task of SC in children with RD as compared with Controls, we designed an fMRI experiment that compared the neural correlates of SC with isolated WR; this is an especially important area of investigation as to our knowledge, no other fMRI study has examined sentence processing while controlling for WR in impaired readers. We designed tasks that tapped processes inherent to comprehending a written sentence, and that are particularly central in individuals with RD, that is, WR. In particular, we intended to examine regions of activation present during SC as contrasted to the activation attributable to WR; importantly, we aimed to understand patterns of activation associated with SC relative to each individual person's processing of words. In applying this paradigm to children with word-level RD as compared with skilled readers, we hoped to 1) understand more about the neurological correlates of comprehending connected text beyond word-level processing 2) examine differences between children with RD versus Controls with a specific focus on examining whether the RD group showed abnormalities in brain activation, beyond those associated with single words, and 3) examine the correlates of good and poor SC by determining the associations between behavioral (out-of-scanner) measures of basic reading, fluency and oral language, to neurobiological response to SC.
Materials and Methods
Participants
Twenty-nine children, ages 9–14 years, were recruited for the study: 14 with a history of RD and 15 Controls. Participants met criteria for reading difficulty if they had a standard score at or below the 25th percentile on either the Word Identification (Word ID) or the Word Attack (WA) subtest of the Woodcock Johnson Reading Mastery Test/Normative Update (WRMT-R/NU) and on the average of both subtests. Additionally, participants met criteria to be a Control by having a standard score at or above the 40th percentile on both Word ID and WA subtests. Twelve participants met research criteria for attention-deficit/hyperactivity disorder (6 Controls, 6 RD) (participants needed to meet 2 out of 3 of the following criteria: 1) Attention Deficit Hyperactivity Disorder Rating Scale-IV (DuPaul et al. 1998)—6/9 (or ≥94th percentile) on either Inattentive or Hyperactivity Index; 2) Behavior Assessment System for Children—≥65 (T-score) on either Hyperactivity or Attention Problems Index; and/or 3) Behavioral Rating Inventory of Executive Function—≥65 (T-score) on Global Executive Composite). All participants had a full scale IQ above 80, no history of major psychiatric illness, no traumatic brain injury/epilepsy, and no contraindication to the MRI. Written assent/consent was obtained from each participant at the start of the study in accordance with the Johns Hopkins Medical Institutional Review Board.
As part of a larger study, each participant was given a series of standardized tests, including the WA, Word ID, and Passage Comprehension subtests from the WRMT-R/NU, the Test of Word Reading Efficiency (TOWRE), the Gray Oral Reading Test—4 (GORT-4), the subtests needed to derive receptive and expressive language composite scores from the Clinical Evaluation of Language Fundamentals-3 (CELF-3), and the Word Reading, Reading Comprehension, and Listening Comprehension subtests of the Wechsler Individual Achievement Test-2 (WIAT-2). To control for type I error, the 2 groups’ performance on the battery of behavioral measures listed in Table 1 was compared using a Multivariate Analysis of Variance.
Table 1.
Test battery scores for each participant
| (a) Participants by BRIEF scores and IQ scores | |||||||||
| Participants |
BRIEF |
WISCa |
|||||||
| Group | Age | Sex | ADHD | Behavioral Regulation Index | Metacognition Index | Global Executive Composite | FSIQ | Block design | Vocabulary |
| Control | 10.11 | Female | No | 50 | 53 | 52 | 113 | 11 | 9 |
| Control | 10.6 | Female | No | 36 | 37 | 36 | 115 | 10 | 16 |
| Control | 10.7 | Female | No | 39 | 40 | 39 | 127 | 14 | 15 |
| Control | 11.5 | Female | No | 120 | 15 | 12 | |||
| Control | 11.8 | Female | Yes | 71 | 64 | 68 | 112 | 14 | 16 |
| Control | 12.1 | Female | Yes | 46 | 66 | 59 | 108 | 13 | 17 |
| Control | 12.1 | Female | Yes | 55 | 65 | 62 | 103 | 6 | 12 |
| Control | 13 | Female | Ambig | 49 | 63 | 59 | 90 | 2 | 11 |
| Control | 14.8 | Female | No | 44 | 42 | 42 | 104 | 10 | 13 |
| Control | 10.1 | Male | No | 36 | 58 | 49 | 120 | 15 | 14 |
| Control | 10.4 | Male | No | 54 | 46 | 48 | 135 | 19 | 16 |
| Control | 10.5 | Male | No | 49 | 37 | 41 | |||
| Control | 13.1 | Male | No | 54 | 59 | 58 | 109 | 13 | 13 |
| Control | 13.1 | Male | Ambig | 54 | 64 | 62 | 101 | 10 | 12 |
| Control | 13.2 | Male | Ambig | 40 | 59 | 53 | 123 | 8 | 16 |
| Mean | 11.81 | 48.36 | 53.79 | 52.00 | 113.15 | 11.31 | 13.77 | ||
| SD | 1.42 | 9.61 | 11.29 | 10.18 | 12.12 | 4.42 | 2.45 | ||
| RD | 10 | Female | No | 49 | 54 | 52 | 87 | 4 | 10 |
| RD | 11.11 | Female | No | 44 | 48 | 46 | 102 | 14 | 11 |
| RD | 11.5 | Female | No | 42 | 55 | 50 | 95 | 5 | 8 |
| RD | 11.7 | Female | Yes | 77 | 80 | 81 | 113 | 13 | 12 |
| RD | 12.5 | Female | No | 54 | 58 | 64 | 108 | 11 | 11 |
| RD | 13.8 | Female | Yes | 89 | 85 | 89 | 121 | 14 | 11 |
| RD | 14.7 | Female | No | 49 | 65 | 59 | 106 | 15 | 10 |
| RD | 9.1 | Male | Yes | 90 | 9 | 7 | |||
| RD | 10.2 | Male | Ambig | 38 | 48 | 44 | 84 | 10 | 8 |
| RD | 10.2 | Male | Yes | 58 | 79 | 72 | 88 | 6 | 11 |
| RD | 11.1 | Male | No | 61 | 42 | 48 | 111 | 11 | 18 |
| RD | 11.1 | Male | No | 54 | 50 | 52 | 88 | 5 | 10 |
| RD | 12.7 | Male | No | 54 | 53 | 54 | 104 | 10 | 9 |
| RD | 13.9 | Male | Yes | 79 | 73 | 77 | 103 | 10 | 12 |
| Mean | 11.69 | 57.54 | 60.77 | 60.62 | 100.00 | 9.79 | 10.57 | ||
| SD | 1.64 | 16.04 | 13.61 | 14.91 | 11.43 | 3.62 | 2.62 | ||
| (b) Participants by word-level and text-level accuracy scores | ||||||||
| Participants |
WIAT-2 |
WRMT-R/NU |
GORT-4 |
|||||
| Group | Age | Sex | ADHD | Word reading | Word ID | WA | Avg of WID and WA | Accuracy |
| Control | 10.11 | Female | No | 103 | 92 | 92 | 92 | 10 |
| Control | 10.6 | Female | No | 107 | 107 | 101 | 104 | 12 |
| Control | 10.7 | Female | No | 120 | 113 | 114 | 113.5 | 12 |
| Control | 11.5 | Female | No | 110 | 107 | 99 | 103 | 8 |
| Control | 11.8 | Female | Yes | 118 | 114 | 104 | 109 | 9 |
| Control | 12.1 | Female | Yes | 112 | 110 | 103 | 106.5 | 11 |
| Control | 12.1 | Female | Yes | 114 | 103 | 112 | 107.5 | 9 |
| Control | 13 | Female | Ambig | 98 | 100 | 95 | 97.5 | 8 |
| Control | 14.8 | Female | No | 114 | 102 | 101 | 101.5 | 14 |
| Control | 10.1 | Male | No | 126 | 123 | 126 | 124.5 | 17 |
| Control | 10.4 | Male | No | 118 | 112 | 109 | 110.5 | 10 |
| Control | 10.5 | Male | No | 133 | 125 | 125 | 125 | 20 |
| Control | 13.1 | Male | No | 105 | 100 | 97 | 98.5 | 7 |
| Control | 13.1 | Male | Ambig | 99 | 95 | 100 | 97.5 | 7 |
| Control | 13.2 | Male | Ambig | 111 | 100 | 97 | 98.5 | 9 |
| Mean | 11.81 | 112.53 | 106.87 | 105.00 | 105.93 | 10.87 | ||
| SD | 1.42 | 9.64 | 9.47 | 10.29 | 9.55 | 3.70 | ||
| RD | 10 | Female | No | 77 | 77 | 82 | 79.5 | 4 |
| RD | 11.11 | Female | No | 73 | 82 | 82 | 82 | 2 |
| RD | 11.5 | Female | No | 86 | 94 | 91 | 92.5 | 5 |
| RD | 11.7 | Female | Yes | 82 | 92 | 99 | 95.5 | 7 |
| RD | 12.5 | Female | No | 85 | 89 | 92 | 90.5 | 8 |
| RD | 13.8 | Female | Yes | 88 | 93 | 86 | 89.5 | 7 |
| RD | 14.7 | Female | No | 83 | 83 | 87 | 85 | 8 |
| RD | 9.1 | Male | Yes | 89 | 87 | 87 | 9 | |
| RD | 10.2 | Male | Ambig | 92 | 93 | 88 | 90.5 | 3 |
| RD | 10.2 | Male | Yes | 73 | 75 | 69 | 72 | 1 |
| RD | 11.1 | Male | No | 93 | 86 | 84 | 85 | 5 |
| RD | 11.1 | Male | No | 83 | 86 | 86 | 86 | 3 |
| RD | 12.7 | Male | No | 83 | 84 | 89 | 86.5 | 4 |
| RD | 13.9 | Male | Yes | 86 | 88 | 85 | 86.5 | 2 |
| Mean | 11.69 | 83.79 | 86.67 | 86.21 | 86.29 | 4.86 | ||
| SD | 1.64 | 6.15 | 6.18 | 6.62 | 5.83 | 2.57 | ||
| (c) Participants by word-level and text-level fluency scores | |||||||
| Participants |
TOWRE |
GORT-4 |
|||||
| Group | Age | Sex | ADHD | SiteWrdEff | PhonDecode | Overall | Rate |
| Control | 10.11 | Female | No | 96 | 84 | 88 | 11 |
| Control | 10.6 | Female | No | 95 | 90 | 91 | 11 |
| Control | 10.7 | Female | No | 115 | 113 | 117 | 15 |
| Control | 11.5 | Female | No | 102 | 102 | 102 | 11 |
| Control | 11.8 | Female | Yes | 97 | 105 | 101 | 11 |
| Control | 12.1 | Female | Yes | 97 | 108 | 103 | 11 |
| Control | 12.1 | Female | Yes | 101 | 105 | 104 | 8 |
| Control | 13 | Female | Ambig | 91 | 91 | 89 | 8 |
| Control | 14.8 | Female | No | 88 | 107 | 97 | 12 |
| Control | 10.1 | Male | No | 107 | 113 | 112 | 15 |
| Control | 10.4 | Male | No | 110 | 107 | 110 | 13 |
| Control | 10.5 | Male | No | 117 | 134 | 131 | 19 |
| Control | 13.1 | Male | No | 98 | 100 | 99 | 9 |
| Control | 13.1 | Male | Ambig | 101 | 93 | 96 | 9 |
| Control | 13.2 | Male | Ambig | 99 | 94 | 96 | 11 |
| Mean | 11.81 | 100.93 | 103.07 | 102.40 | 11.60 | ||
| SD | 1.42 | 8.22 | 12.22 | 11.42 | 2.95 | ||
| RD | 10 | Female | No | 84 | 83 | 80 | 5 |
| RD | 11.11 | Female | No | 85 | 79 | 78 | 5 |
| RD | 11.5 | Female | No | 94 | 80 | 84 | 9 |
| RD | 11.7 | Female | Yes | 62 | 73 | 61 | 4 |
| RD | 12.5 | Female | No | 85 | 72 | 74 | 6 |
| RD | 13.8 | Female | Yes | 89 | 92 | 89 | 8 |
| RD | 14.7 | Female | No | 95 | 75 | 82 | 8 |
| RD | 9.1 | Male | Yes | 7 | |||
| RD | 10.2 | Male | Ambig | 91 | 84 | 85 | 7 |
| RD | 10.2 | Male | Yes | 81 | 59 | 64 | 3 |
| RD | 11.1 | Male | No | 94 | 81 | 85 | 8 |
| RD | 11.1 | Male | No | 91 | 82 | 84 | 5 |
| RD | 12.7 | Male | No | 92 | 85 | 86 | 7 |
| RD | 13.9 | Male | Yes | 91 | 83 | 84 | 7 |
| Mean | 11.69 | 87.42 | 79.08 | 79.83 | 6.36 | ||
| SD | 1.64 | 9.10 | 8.40 | 8.90 | 1.74 | ||
| (d) Participants by oral language scores | ||||||
| Participants |
CELF-3 |
WIAT-2 |
||||
| Group | Age | Sex | ADHD | Receptive | Expressive | Listening comp. |
| Control | 10.11 | Female | No | 104 | 90 | 102 |
| Control | 10.6 | Female | No | 108 | 114 | 94 |
| Control | 10.7 | Female | No | 122 | 125 | 113 |
| Control | 11.5 | Female | No | 100 | 106 | 126 |
| Control | 11.8 | Female | Yes | 100 | 112 | 108 |
| Control | 12.1 | Female | Yes | 78 | 94 | 104 |
| Control | 12.1 | Female | Yes | 116 | 104 | 101 |
| Control | 13 | Female | Ambig | 94 | 90 | 103 |
| Control | 14.8 | Female | No | 120 | 110 | 104 |
| Control | 10.1 | Male | No | 114 | 118 | 118 |
| Control | 10.4 | Male | No | 112 | 98 | 122 |
| Control | 10.5 | Male | No | 128 | 102 | 134 |
| Control | 13.1 | Male | No | 118 | 94 | 121 |
| Control | 13.1 | Male | Ambig | 100 | 98 | 107 |
| Control | 13.2 | Male | Ambig | 100 | 106 | 124 |
| Mean | 11.81 | 107.60 | 104.07 | 112.07 | ||
| SD | 1.42 | 12.88 | 10.40 | 11.45 | ||
| RD | 10 | Female | No | 96 | 112 | 99 |
| RD | 11.11 | Female | No | |||
| RD | 11.5 | Female | No | 100 | 116 | 114 |
| RD | 11.7 | Female | Yes | 120 | 112 | 104 |
| RD | 12.5 | Female | No | 114 | 104 | 96 |
| RD | 13.8 | Female | Yes | 94 | 94 | 100 |
| RD | 14.7 | Female | No | 104 | 86 | 85 |
| RD | 9.1 | Male | Yes | 69 | 84 | 101 |
| RD | 10.2 | Male | Ambig | 75 | 80 | 102 |
| RD | 10.2 | Male | Yes | 78 | 88 | 106 |
| RD | 11.1 | Male | No | |||
| RD | 11.1 | Male | No | 69 | 84 | 87 |
| RD | 12.7 | Male | No | 90 | 94 | 96 |
| RD | 13.9 | Male | Yes | 78 | 102 | |
| Mean | 11.69 | 87.70 | 94.00 | 101.00 | ||
| SD | 1.64 | 16.85 | 12.33 | 8.33 | ||
| (e) Participants by reading comprehension scores | ||||||
| Participants |
GORT-4 |
WIAT-2 |
WRMT-R/NU |
|||
| Group | Age | Sex | ADHD | Comprehension | Reading comp. | Passage comp. |
| Control | 10.11 | Female | No | 11 | 101 | 108 |
| Control | 10.6 | Female | No | 14 | 114 | 112 |
| Control | 10.7 | Female | No | 8 | 107 | 114 |
| Control | 11.5 | Female | No | 12 | 119 | 111 |
| Control | 11.8 | Female | Yes | 14 | 104 | 123 |
| Control | 12.1 | Female | Yes | 15 | 118 | 111 |
| Control | 12.1 | Female | Yes | 5 | 108 | 116 |
| Control | 13 | Female | Ambig | 8 | 104 | 98 |
| Control | 14.8 | Female | No | 12 | 124 | 108 |
| Control | 10.1 | Male | No | 13 | 107 | 127 |
| Control | 10.4 | Male | No | 15 | 102 | 116 |
| Control | 10.5 | Male | No | 20 | 127 | 126 |
| Control | 13.1 | Male | No | 14 | 118 | 103 |
| Control | 13.1 | Male | Ambig | 13 | 106 | 93 |
| Control | 13.2 | Male | Ambig | 12 | 120 | 117 |
| Mean | 11.81 | 12.40 | 111.93 | 112.20 | ||
| SD | 1.42 | 3.54 | 8.49 | 9.54 | ||
| RD | 10 | Female | No | 13 | 89 | 83 |
| RD | 11.11 | Female | No | 9 | 84 | |
| RD | 11.5 | Female | No | 12 | 118 | 92 |
| RD | 11.7 | Female | Yes | 9 | 96 | 90 |
| RD | 12.5 | Female | No | 11 | 90 | 100 |
| RD | 13.8 | Female | Yes | 8 | 97 | 93 |
| RD | 14.7 | Female | No | 7 | 105 | 91 |
| RD | 9.1 | Male | Yes | 8 | 91 | |
| RD | 10.2 | Male | Ambig | 8 | 91 | 93 |
| RD | 10.2 | Male | Yes | 6 | 98 | 73 |
| RD | 11.1 | Male | No | 19 | 123 | |
| RD | 11.1 | Male | No | 11 | 88 | 88 |
| RD | 12.7 | Male | No | 10 | 110 | 93 |
| RD | 13.9 | Male | Yes | 8 | 100 | |
| Mean | 11.69 | 9.93 | 100.75 | 93.25 | ||
| SD | 1.64 | 3.27 | 9.53 | 11.82 | ||
Children received either the third or fourth editions of the Wechsler Intelligence Scale for Children (WISC).
Functional Paradigms
To examine the neurological correlates of SC, participants completed a SC task that alternated with a WR task. The paradigm included 3 runs that contained 6 blocks of each of the 2 tasks, yielding 18 total blocks per task. Participants viewed the paradigms via an LCD projector on a rear projection screen at the head of the scanner via a 45° angled mirror affixed to the MRI head coil. E-Prime (Psychology Software Tools, Pittsburgh, PA) was used to present the task and record the timing of both stimulus presentations and participant responses. Participants responded by pressing a button with either their right index finger or their right middle finger via a button box held in their right hand.
The SC task consisted of a sequence of 6 words that formed a sentence. Participants decided whether the sentences were meaningful or not meaningful. Each sentence that was nonmeaningful contained both semantic and syntactic errors (e.g., a nonmeaningful sentence, “Clocks ticks with gigantic fuzzy chimes.” as compared with a meaningful sentence, “ The people lived happily ever after”). Stimuli were presented word-by-word (2000 ms presentation with an interstimulus interval of 1000 ms) and participants pushed a button with their right index finger every time a word appeared. At the end of the 6 words, participants viewed a “Decide” screen at which time they were to press the button with their right index finger if the sentence was meaningful and with their right middle finger if the sentence was nonmeaningful (3000-ms decision screen). Fifty percent were meaningful. All sentences were composed of words with an overall mean frequency of 62 and higher (Carroll et al. 1971), mean length of 30 letters, and mean number of 9 syllables.
The WR task consisted of having participants view a string of 6 words (all nouns). For each WR block, participants pressed the button with their right index finger every time they saw a word for the first time; however, if they had seen a word previously, they pressed a button with their right middle finger. We chose this design for our control task not only to account for the WR aspect of SC, but also to account for the maintenance/memory demands inherent in employing a word-by-word presentation for the SC task. (As the maintenance component intrinsic to any SC task was likely enhanced by a word-by-word presentation of sentences; Potter 1999.) Additionally, because WR has been identified as a fundamental area of deficiency in RD, our task was specifically designed to examine WR that is distinct from word comprehension as discussed by Sinatra and Royer (1993). Approximately, 56% of the blocks consisted of a word repeated once (e.g., “ten same cut ten may grew”), 22% of the blocks consisted of a word repeated twice (e.g., “like took been like can like”), and 22% of the blocks consisted of no repeated words (e.g., “find told buy big out best”). The stimuli were matched to the SC task on overall word frequency, length, and number of syllables. Word presentation rates were also the same as the SC task, although there was not a decision screen at the end of each block.
Scan Procedure
Scanning was carried out in a 1.5 Tesla ACS-NT Powertrack 6000 MRI scanner (Philips Medical Systems, Andover, MA.) using body coil transmission and quadrature end-capped head coil reception. Single shot echo planar images were coronally acquired with a 40-ms echo time, a 2.6-s repetition, 64 × 64 acquisition matrix, 230-mm field of view with 41 volumes consisting of 4.0-mm slices and a 0.5-mm gap, yielding a nominal acquisition voxel size of 3.579 × 3.579 × 4.5 (to provide whole brain coverage).
Image Processing and Data Analysis
Post acquisition image processing and analysis was carried out using SPM2 (http://ww.fil.ion.ucl.ac.uk/spm/) on Matlab 6.1 (Mathworks, Inc., Natick, MA). Images obtained from the scanner were converted to Analyze format, time corrected to adjust for within volume time of acquisition differences (Calhoun et al. 2000), and then realigned. Prior to estimation, the data were spatially normalized to Montreal Neurological Institute (MNI)–labeled space (Evans et al. 1993), resampled into (2 mm3) voxels, and smoothed using an (8 mm3) Gaussian kernel (Friston et al. 1995). Task associated brain activation was assessed using a block design and statistical parametric maps were created corresponding with the time courses for the following contrast: SC greater than WR (SC-WR). The primary analysis was to examine overall patterns of activation for each group and between groups. To examine each group's patterns of activation, the contrast images were entered into a random effects analysis, and a 2-sample t-test was conducted to look at differences in activation patterns between the groups. A secondary analysis was then done to determine potential differences between meaningful versus nonmeaningful sentences; for these analyses, we contrasted each sentence type for each group. Finally, to assess the impact of other dimensions of reading besides the dichotomous division into RD and Controls based on decoding (WA) and WR (Word ID) scores, we examined the patterns of activation correlated (in a continuous fashion) with performance on word-level measures (i.e., Word ID and TOWRE), text-level measures (i.e., GORT-4 Rate and Accuracy and WRMT-R/NU Passage Comprehension) and oral language measures (i.e., CELF-3 Receptive and Expressive scores and WIAT-2 Listening Comprehension). Using simple regression analyses, participant scores on these tests and subtests were correlated with the statistical parametric contrast maps generated from the functional data.
All data are reported at an uncorrected P < 0.001 with an extent threshold of 78 voxels, which is equivalent to a false positive rate of <0.05 over the whole brain based on Monte Carlo simulations run using AlphaSim (NIMH, Bethesda, MD; http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). The location of voxels significantly associated with the tasks was summarized by their local maxima, separated by at least 4 mm. The maxima coordinates were converted from MNI to Talaraich coordinate space using the formulas provided by Matthew Brett (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) and then assigned neuroanatomic and cytoarchitectonic labels using the Talaraich Daemon (http://ric.uthscsa.edu/projects/tdc/).
Results
Behavioral Results
Demographic characteristics are shown in Table 1. There was no significant difference in age between groups. The Control and RD groups performed similarly on a proxy of Performance IQ, Block Design. There were, however, significant differences between the groups in Full Scale Intelligence Quotient and Vocabulary; as is generally found in individuals with RD (Foorman et al. 1996), their verbal scores were lower than the Control group, and, consequently, their FSIQ was too. In addition, all of the reading measures showed significant differences between groups. In addition to the reading measures, as commonly observed in RD, the RD group performed lower on oral language measures. Specifically, the RD group showed significant differences from the Control group for the CELF-3 Receptive language composite and the WIAT-2 Listening Comprehension subtest. Furthermore, in the RD group, 58% had scores of ≤90 on either or both CELF-3 composite scores, whereas the same was true for only 20% of the Control group, which was significantly different (chi-square = 4.28, P = 0.04).
In-scanner behavioral performance as measured by mean response time and task accuracy was recorded separately for 3 conditions: 1) the SC simple button-press response to each single word stimulus; 2) the WR decision regarding “repeated versus not repeated” for each single word stimulus; and 3) the SC decision regarding “meaningful versus nonmeaninfgul” after each 6 word sentence block. For the SC simple button-press response, both groups had the same mean accuracy (Controls = 96.0 ± 7.7, RDs = 96.6 ± 4.2, P < 0.83) and mean reaction time (Controls = 784.8 ± 217.4, RDs = 777.9 ± 131.6, P < 0.92). Additionally for the WR decision, both groups demonstrated the same mean accuracy (Controls = 93.1 ± 8.8, RDs = 90.7 ± 6.9, P < 0.42) and mean reaction time (Controls = 865.42 ± 225.4, RDs = 835.34 ± 133.11, P < 0.42). However, during the SC decision portion of the task (correctly identifying whether the 18 sentences were meaningful or not), Controls (87.2 ± 9.2) were significantly more accurate (P < 0.001) than RDs (65.9 ± 21.6) although there remained no difference in reaction time (Controls = 980.4 ± 254.9, RDs = 1108.9 ± 294.3, P < 0.24). Because of this significant difference in accuracy for the sentence judgment, we modeled the fMRI results both with and without those participants in the RD group that performed poorly; because the same activation patterns were present with and without these participants, we did not discard these participants’ data. Similarly, fMRI contrasts covarying for sentence accuracy were nearly identical to those without this covariable (see Table 2).
Table 2.
Regions of significant activation for the rd and control groups separately and group comparisons
| Cluster size | Region included | BA | Side | Coordinates |
Z value | ||
| x | y | z | |||||
| Controls: SC > WR | |||||||
| 2242 | Lingual gyrus | R | 14 | −80 | −4 | 5.50 | |
| Cuneus | R | 8 | −74 | 6 | 4.77 | ||
| Fusiform gyrus | R | 26 | −59 | −7 | 4.17 | ||
| Cerebellum | R | 26 | −67 | −15 | 4.22 | ||
| 248 | Middle occipital gyrus | 19 | R | 34 | −83 | 19 | 4.99 |
| 274 | Inferior frontal gyrus | 47 | L | −53 | 25 | −6 | 4.68 |
| 115 | Superior frontal gyrus | L | −4 | 15 | 62 | 4.02 | |
| RDs: SC > WR | |||||||
| 730 | Superior temporal gyrus | 38 | L | −54 | 5 | −10 | 4.63 |
| Inferior frontal gyrus | L | −53 | 29 | −8 | 4.21 | ||
| 85 | Cerebellum | L | −22 | −81 | −33 | 4.51 | |
| 330 | Lingual gyrus | R | 24 | −82 | −3 | 4.25 | |
| 89 | Superior frontal gyrus | R | 6 | 11 | 58 | 4.16 | |
| 119 | Inferior frontal gyrus | 47 | R | 53 | 31 | 0 | 4.05 |
| 171 | Middle temporal gyrus | 21 | L | −46 | −29 | −4 | 3.92 |
| 198 | Cuneus | R | 6 | −75 | 6 | 3.79 | |
| Lingual gyrus | R | 18 | −68 | 2 | 3.34 | ||
| 94 | Superior temporal gyrus | 22 | L | −63 | −49 | 21 | 3.62 |
| Controls: WR > SC | |||||||
| 2548 | Anterior cingluate | L | −6 | 19 | 25 | 4.68 | |
| Cingulate gyrus | R | 6 | 19 | 27 | 4.65 | ||
| 1373 | Superior temporal gyrus | L | −48 | −18 | −2 | 4.52 | |
| Precentral gyrus | 6 | L | −46 | −9 | 6 | 4.49 | |
| Insula | L | −42 | 2 | 11 | 4.30 | ||
| 1045 | Inferior parietal lobe | R | 46 | −32 | 29 | 4.45 | |
| 446 | Cerebellum | L | −14 | −61 | −19 | 4.44 | |
| 177 | Pons | R | 14 | −24 | −22 | 4.43 | |
| 587 | Subgyral temporal | R | 40 | −9 | −15 | 4.08 | |
| Insula | R | 40 | −8 | −5 | 3.81 | ||
| 101 | Superior temporal gyrus | 22 | R | 61 | −10 | 0 | 4.26 |
| Precentral gyrus | 43 | R | 57 | −7 | 10 | 3.25 | |
| 331 | Medial frontal gyrus | 10 | R | 8 | 47 | 9 | 4.12 |
| Anterior cingulate | R | 8 | 41 | −2 | 3.57 | ||
| 222 | Middle frontal gyrus | 8 | R | 30 | 20 | 47 | 4.08 |
| 182 | Middle frontal gyrus | L | −32 | 31 | 28 | 3.96 | |
| 235 | Inferior parietal lobe | 40 | L | −42 | −56 | 40 | 3.92 |
| Supramarginal gyrus | L | −50 | −49 | 37 | 3.67 | ||
| 215 | Superior frontal gyrus | 10 | R | 24 | 44 | 22 | 3.71 |
| Middle frontal gyrus | R | 34 | 36 | 29 | 3.54 | ||
| 80 | Thalamus | L | −14 | −29 | 11 | 3.66 | |
| 151 | Middle temporal gyrus | 21 | R | 65 | −12 | −11 | 3.60 |
| Fusiform gyrus | 20 | R | 57 | −17 | −23 | 3.58 | |
| RDs > Controls for SC > WRa (or Controls > RDs for WR > SC) | |||||||
| 373 | Cerebellum | L | −16 | −57 | −19 | 4.41 | |
| 137 | Inferior parietal lobe | 40 | R | 61 | −39 | 30 | 4.31 |
| 1351 | Insula | 13 | L | −42 | 1 | 11 | 4.27 |
| Superior temporal gyrus | 38 | L | −44 | 9 | −7 | 3.82 | |
| 247 | Insula | R | 40 | −6 | −6 | 4.15 | |
| Subgyral temporal | R | 44 | −12 | −16 | 3.47 | ||
| 97 | Cuneus | L | −20 | −74 | 30 | 4.14 | |
| 129 | Middle temporal gyrus | L | −50 | −16 | −4 | 4.09 | |
| Superior temporal gyrus | L | −51 | −8 | −5 | 3.35 | ||
| 84 | Temporal lobe/uncus | L | −28 | 0 | −32 | 4.06 | |
| 330 | Cingulate gyrus | R | 12 | 12 | 41 | 3.67 | |
| Superior frontal gyrus | R | 18 | 18 | 43 | 3.54 | ||
| 78 | Claustrum | R | 26 | 17 | −3 | 3.62 | |
Covarying for CELF-3 receptive scores yielded significant activation in left cerebellum for RD's greater than Controls. Covarying for CELF-3 expressive scores yielded very similar left hemisphere activations (STG, insula and cerebellum) to those found without co-varying but the previously found right hemisphere activation did not persist. Covarying for accuracy on the SC decision yielded almost identical results to those found without covarying, including left cerebellum, left STG, left insula, left temporal lobe/uncus, right IPL right cingulate gyrus, and right SFG.
fMRI Results
SC versus WR Contrasts
(Note: To insure our results were not overly confounded with the presence of ADHD, we conducted analyses of covariance [ANCOVAs] covarying for various BRIEF scores (Global Composite, Metacognition Index, as well as Behavioral Index). The results of the ANCOVA analyses are not presented because they revealed almost identical findings to those analyses without covariates.)
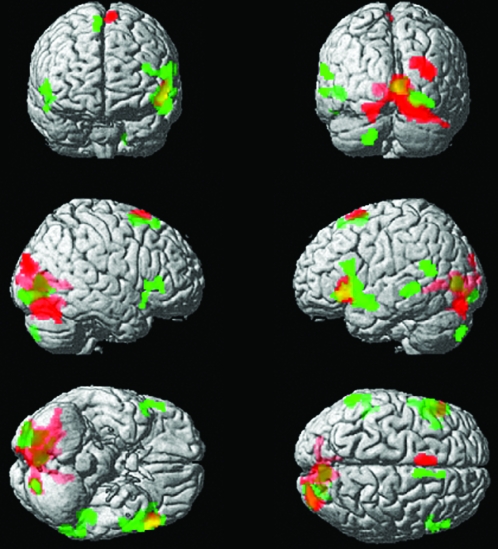
Control group results.
For Controls, the SC greater than WR contrast (SC-WR) showed activation in areas typically associated with both word-level and sentence-level reading tasks, including Brodmann Area (BA) 47 of the left inferior frontal gyrus, left superior frontal gyrus and extrastriate regions (Fig. 1). The opposite contrast (WR-SC) showed activation in areas more typically associated with isolated WR, including a left insula/superior temporal gyrus region and left supramarginal gyrus.
Figure 1.
Areas of activation associated with SC processing after controlling for WR. Areas in red represent activation for Controls; areas in green represent RDs (overlapping areas are in yellow).
RD group results.
For the RD group, the SC-WR contrast closely resembled that of Controls; however, the RD group lacked the left superior frontal gyrus activation seen in the Control group and instead showed additional activation in left language-related regions including Inferior Frontal Gyrus (IFG), Middle Temporal Gyrus (MTG) and Superior Temporal Gyrus (STG).
Between-group results.
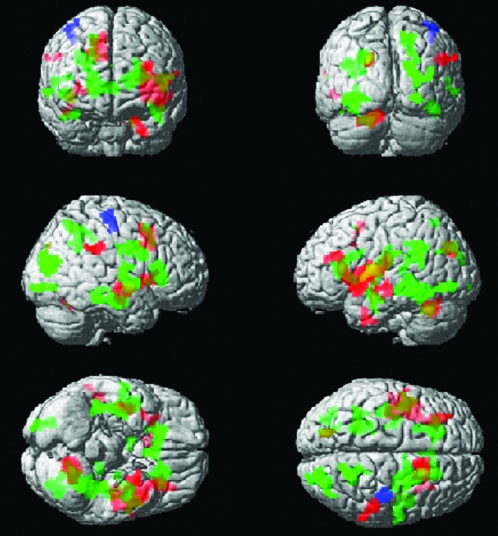
There was no significant difference between the mean SC-WR images for RD's and Controls; however, the opposite contrast (WR-SC) did show RD greater than Control activation in left MTG, STG, and insula, and right temporal and inferior parietal lobe regions (see Fig. 2).
Figure 2.
All areas of activation represent Group × Task interactions. Areas in red represent activation associated with RD greater than Control for SC greater than WR. Areas of activation in green are associated with RD greater than Control for nonmeaningful sentences greater than WR. Lastly, the area in blue is associated with RD greater than Control for meaningful sentences greater than WR.
Meaningful versus Nonmeaningful Sentence Contrasts
Control group results.
The contrast of meaningful SC greater than nonmeaningful SC (MSC-NMSC) revealed very little significant activation. For the opposite contrast (NMSC-MSC) we found significant activation in the Control group in left IFG (BA 44/45) and left STG.
RD group results.
Neither contrast (MSC-NMSC and MSC-NMSC) revealed significant activation among the RD group.
Between-group results.
Results of 2-sample t-tests revealed significant differences between groups only for the MSC-NMSC contrast; these differences were confined to right pre/postcentral gyrus (see Table 3). T-tests for between-group differences on the opposite contrast (NMSC-MSC), showed greater Control group activation in the left inferior frontal gyrus (BA 45/47) and bilateral extrastriate regions.
Table 3.
Regions of significant activation for different sentence types (meaningful and nonmeaningful) versus repeat random effects analysis and between-group contrasts
| Region included | BA | Side | Coordinates |
||
| x | y | z | |||
| Controls: meaningful sentences > WR | |||||
| Lingual gyrus | 18 | L | −8 | −80 | −4 |
| Lingual gyrus | 18 | R | 16 | −76 | −5 |
| Cuneus | R | 26 | −86 | 26 | |
| Middle occipital gyrus | R | 36 | −85 | 17 | |
| RDs: Meaningful sentences > WR | |||||
| Superior occipital gyrus | 19 | R | 36 | −84 | 32 |
| Middle occipital gyrus | R | 16 | −90 | 17 | |
| Lingual gyrus | R | 10 | −80 | −4 | |
| Cuneus | R | 6 | −77 | 9 | |
| Middle temporal gyrus | R | 55 | −67 | 20 | |
| Middle temporal gyrus | 22 | L | −65 | −39 | 4 |
| Inferior frontal gyrus | 47 | L | −53 | 27 | −8 |
| Middle frontal gyrus | 6 | L | −40 | −1 | 57 |
| RDs > Controls for meaningful sentences > WR | |||||
| Postcentral gyrus | 3 | R | 44 | −20 | 56 |
| Controls: nonmeaningful sentences > WR | |||||
| Lingual gyrus | R | 12 | −80 | −4 | |
| Lingual gyrus | L | −22 | −84 | −9 | |
| Cuneus | 19 | R | 10 | −74 | 6 |
| Cerebellum | R | 16 | −79 | −16 | |
| Cerebellum | L | −18 | −82 | −18 | |
| Middle occipital gyrus | R | 32 | −88 | 17 | |
| Inferior frontal gyrus | 45/47 | L | −53 | 25 | −6 |
| Superior frontal gyrus | L | −6 | 13 | 62 | |
| RDs: nonmeaningful sentences > WR | |||||
| Cuneus | 17/7 | L | −20 | −93 | −2 |
| Inferior occipital gyrus | L | −38 | −68 | −3 | |
| Middle occipital gyrus | 19 | L | −38 | −87 | 15 |
| Precuneus | R | 26 | −78 | 26 | |
| Lingual gyrus | 18 | R | 20 | −72 | −1 |
| Inferior frontal gyrus | 45/46 | R | 57 | 34 | 13 |
| Middle temporal gyrus | 21 | L | −51 | −1 | −15 |
| Superior temporal gyrus | L | −53 | 7 | −10 | |
| RDs > Controls for nonmeaningful sentences > WR | |||||
| Subgyral parietal | L | −26 | −45 | 32 | |
| Subgyral temporal | R | 44 | −34 | −13 | |
| Subgyral temporal | L | −38 | −68 | −2 | |
| Thalamus | L | −14 | −30 | 13 | |
| Cerebellum | L | −20 | −57 | −19 | |
| Insula | 13 | R | 42 | −2 | −3 |
| Insula | L | −32 | −9 | 15 | |
| Superior frontal gyrus | R | 20 | 14 | 45 | |
| Precentral gyrus | 6 | L | −55 | −3 | 13 |
| Precentral gyrus | R | 53 | −9 | 10 | |
| Postcentral gyrus | L | −42 | −20 | 25 | |
| Cingulate gyrus | R | 14 | 1 | 29 | |
| Middle temporal gyrus | R | 40 | −69 | 20 | |
| Superior temporal gyrus | 21 | R | 61 | −6 | −1 |
| Parahippocampal gyrus | 19 | L | −34 | −45 | −6 |
| Middle occipital gyrus | R | 30 | −73 | 17 | |
| Precuneus | L | −20 | −68 | 33 | |
| Precuneus | 7 | R | 14 | −59 | 55 |
| Cuneus | L | −18 | −76 | 33 | |
| Controls: meaningful sentences > nonmeaningful sentences | |||||
| Cingulate gyrus | 31 | R | 10 | −41 | 35 |
| Postcentral gyrus | 3 | L | −55 | −23 | 38 |
| RDs: meaningful sentences > nonmeaningful sentences | |||||
| Superior frontal gyrus | R | 8 | 1 | 64 | |
| RDs > Controls for meaningful sentences > nonmeaningful sentences | |||||
| Precentral gyrus | R | 36 | −26 | 64 | |
| Postcentral gyrus | 3 | R | 44 | −21 | 54 |
| Controls: nonmeaningful sentences > meaningful sentences | |||||
| Inferior frontal gyrus | 44/45 | L | −57 | 18 | 5 |
| Superior temporal gyrus | L | −48 | 17 | −9 | |
Meaningful Sentences versus WR Contrasts
Control group results.
For the Control group, the MSC-WR contrast showed activation in bilateral extrastriate areas.
RD group results.
For the RD group, the MSC-WR contrast showed activation in left inferior frontal gyrus (BA 47), bilateral middle temporal gyrus, and right extrastriate regions. The RD group showed activation in right inferior frontal gyrus (45/46), left middle and superior temporal gyri, and bilateral extrastriate.
Between-group results.
The results of the 2-sample t-tests on both contrast (MSC-WR and WR-MSC) showed no significant Control group activation greater than RDs. However, the RD group did show greater activation on the MSC-WR contrast in a small cluster of activation in the right postcentral gyrus. Additionally, on the opposite contrast (WR-MSC) the RD group showed extensive activation compared with Controls in many areas, including right superior temporal gyrus, left subgyral temporal (extending into middle and superior temporal gyri), right superior frontal gyrus, left insula, and bilateral extrastriate regions (see Table 3).
fMRI Correlations with Behavioral Measures
Results of the regression analyses showed a number of significant correlations with behavioral measures, and distinct differential patterns of activation associated with different components of reading/language. Findings from all of the correlations between the clusters of activation and behavioral measures were too extensive to list exhaustively; however, we describe the patterns of significant findings below. All areas of activation discussed were correlated at P < 0.05, corrected for multiple comparisons. Behavioral tests included in correlation analyses were broken down into: word- and text-level accuracy measures (i.e., WRMT-R/NU Word ID and WA, WIAT-2 Word Reading, and the GORT-4 Accuracy), word- and text-level fluency measures (the TOWRE composite and GORT-4 Rate); oral language measures (Listening Comprehension and Expressive/Receptive subtests from CELF-3); and reading comprehension measures (GORT-4 Comprehension, WIAT-2 Reading Comprehension, and WRMT-R/NU Passage Comprehension). Both positive and negative correlations were conducted for each of the accuracy, fluency, oral language, and reading comprehension tests; below we report results of significant correlations only.
Accuracy Measures
The WA showed significant positive correlations with right middle and superior frontal gyri, whereas Word ID was positively correlated with left medial frontal gyrus activation. Negative correlations with bilateral insula (BA 13), left cerebellum, and right supramarginal gyrus (BA 40) were present with all accuracy measures (Word Reading, WA, Word ID, GORT-4 Accuracy). On further subdivision of the accuracy measures, analyses revealed real word measures (Word Reading, Word ID, GORT-4 Accuracy) were negatively correlated to right superior temporal lobe activation, whereas the nonsense word measure (WA) was negatively correlated to right inferior temporal lobe.
Fluency Measures
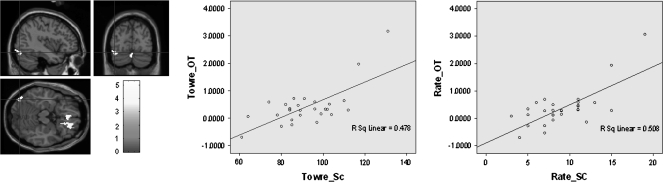
On those word-level tests measuring fluent word- and text-level abilities (i.e., TOWRE and GORT-4 Rate, respectively) there were significant positive correlations to a region within a left occipitotemporal area near the visual word form area (see Fig. 3). For word-level fluency measures (GORT-4 Rate, TOWRE), negative correlations were seen in right superior temporal gyrus as well as left insula and left cerebellum.
Figure 3.
On the left side of the figure is a graphical representation of the occipitotemporal activation associated with a significant positive correlation for both the TOWRE (middle graph) and GORT-4 rate scores (right graph).
Oral Language Measures
The Listening Comprehension subtest from the WIAT-2 showed significant negative correlation with right parietal lobe (BA 7), inferior temporal gyrus, and several left hemisphere frontal regions (inferior, middle, superior, and medial frontal gyri). The CELF-3 Receptive subtest was negatively correlated with right supramarginal gyrus (BA 40) and the CELF-3 Expressive subtest was negatively correlated with left cerebellum.
Reading Comprehension Measures
The only reading comprehension measure that showed significant correlation was WRMT-R/NU Passage Comprehension; it was significantly negatively correlated with bilateral insula, right temporal lobe and supramarginal gyrus (BA 40, and left hemisphere regions including inferior frontal gyrus (BA 44/45), superior temporal gyrus (BA 38), and cerebellum.
Discussion
Overall, our findings revealed activation patterns that are commonly associated with SC, particularly when controlling for single word reading. They are also consistent with previous findings of more extensive activation during reading tasks among impaired readers as compared with Controls. In this task, which controlled for the dyslexic group's weaknesses in WR, both groups showed activation in a number of areas previously found to be associated with linguistic processing, including left inferior frontal gyrus and extrastriate regions (Démonet et al. 2005). When the 2 groups were compared, the RD group showed more activation than the Control group in several areas most prominently areas associated with linguistic processing (left middle and superior temporal gyri) as well as attention and response selection (bilateral insula, right cingulate gyrus, right superior frontal gyrus, and right parietal lobe; Braver et al. 2001; Downar et al. 2001; Hahn et al. 2006).
Brain–behavior correlations revealed that better fluency, both at the word and text level, were related to greater intensity of activation within a left occipitotemporal region, near the area commonly referred to as the visual word form area (although lower than is typically found). This region has been found to be associated with fluency of WR (Pugh et al. 2000; Shaywitz et al. 2004) and these findings were incredibly consistent across reading measures with the same location of peak area of activation (−44, −76, −11). Many behavioral studies have found that fluency has a direct relationship to comprehension, with greater fluency yielding better comprehension (Rasinski 1990; Swanson and Trahan 1996; Rupley et al. 1998); therefore, it is not surprising that we would find a positive correlation between an area commonly found to be associated with fluency (occipitotemporal region) and fluency measures for a comprehension task. This pattern of correlations supports the concept that fluency is an additional important component to comprehension, beyond WR accuracy (especially because we did not find significant correlations with out-of-scanner WR accuracy measures in this region). Poorer performance on word-level (accuracy and fluency) measures was associated with increased activation in bilateral insula, right inferior parietal lobe (supramarginal gyrus), and right temporal lobe (superior temporal gyrus for real words; inferior temporal gyrus for nonsense words). Lower out-of-scanner oral language and reading comprehension abilities were associated with greater right hemisphere activation, particularly in the parietal lobe. More specifically, right supramarginal gyrus activation (BA 40) was negatively correlated with reading comprehension and receptive language, and a listening comprehension task was negatively correlated with right superior parietal lobe (BA 7). These findings are not surprising as individuals with lower comprehension abilities may tend to rely more upon the right hemisphere more than skilled comprehenders, particularly for visualization strategies. In general, the correlation patterns revealed a network of increased activation in bilateral insula, right superior temporal gyrus, right inferior parietal lobe, as well as left cerebellum that was consistently associated with poorer performance on reading and language measures; it may be of interest for future investigations to explore the relationships/connections between these regions in relation to reading ability.
Although many of our findings corroborated our expectations, other findings were unanticipated. Most specifically, the RD group showing greater response than Controls in left middle and superior temporal gyri, which are areas typically associated with language comprehension. There are several possible explanations for this finding including 1) greater activations of Controls during WR than RDs, which would yield overall greater activation when subtracting WR from SC and/or 2) that children with RD do not comprehend sentences in the same manner as Controls do; specifically, that the RD group differentially responds to meaningful versus nonmeaningful sentences as compared with Controls.
The explanation that perhaps the greater activation for the RD group is simply a reflection of their decreased activation during the WR task is plausible. Therefore, it is reasonable to conclude that less activation for words in the RD group could be the basis for our findings. However, our other more fine-grained analyses that divided our sentences into MSC and NMSC conditions suggested a more complex picture. Specifically, when accounting for activation due to WR, the RD and Control groups both appeared to process meaningful sentences (on the MSC-WR contrast) similarly. In contrast, when accounting for WR, nonmeaningful sentences (on the NMSC-WR contrast) were processed differently by the RD than the Control group; namely, the RD group showed additional areas of activation that were similar to those revealed on the more general SC-WR contrast. Thus, findings suggest that the RD group's response to nonmeaningful sentences was a substantial contributor to their greater activation in the SC-WR contrast, rather than exclusively a deactivation response to WR (especially because both contrasts used the same WR stimuli). Nevertheless, we cannot entirely rule out the possibility that some of the differences, particularly those in left temporal lobe, for which there was definite overlap between the SC-WR and NMSC-WR contrasts but not complete correspondence, was driven by in part deactivation in the RD group to word stimuli.
Given that findings indicate that the RD group showed atypical patterns of activation on this higher-level linguistic processing task (SC) and that this does not appear to be solely accounted for by WR, it is important to consider the functional meaning of our results. Our findings suggest that more effortful processing was needed for the RD group to comprehend sentences. Indeed, they recruited regions associated with attention and response selection much more than Controls, particularly to detect sentences with errors. Results also indicated that the RD group tended to show greater recruitment of linguistic regions that have been associated with semantic and syntactic networks (left middle and superior temporal gyri; Friederici et al. 2003); these differences appeared to be more specifically related to the processing of nonmeaningful sentences. Overall, the RD group tended to show much more diffuse patterns of activation than Controls for all of the sentence contrasts (SC-WR, MSC-WR, NMSC-WR).
In contrast to the RD group, it appeared that Controls were more easily able to comprehend sentences as indicated by much less diffusivity; they also showed neurobiological responses that suggested more distinction between the 2 types of sentences. In particular, the examination of the activation patterns for the Controls for the various conditions indicated that they used a tightly coordinated, much less diffuse network of areas associated with language processing on all 3 contrasts (SC-WR, MSC-WR, NMSC-WR). They drew upon occipital lobe and left inferior frontal gyrus for SC-WR and NMSC-WR with no additional activation in left middle and superior temporal gyri—areas that are almost universally activated in linguistic tasks. This suggests that the linguistic processing that drew upon these regions, ostensibly to understand the sentences, was accounted for by the response to WR. This is also supported by the fact that the opposite contrast of WR-SC revealed left middle and superior temporal gyri activation. Their MSC-WR processing yielded no left inferior frontal gyrus activation, with activation only in occipital lobes, suggesting that when the sentence made sense, there was even a closer coupling between processing of words and sentences. Indeed, behavioral studies show that younger skilled readers often show a close linkage between their word and comprehension skills as compared with older skilled readers, in whom these skills tend to diverge more (Vellutino et al. 1994; Catts et al. 2003). Additionally, the Control group's response to “oddball” sentences was more traditional, as seen by their response to nonmeaningful sentences. Although our significant differences between groups for the MSC-NMSC were confined to right precentral and right postcentral gyri, it is worth noting that the Controls showed the often reported left inferior frontal gyrus response to nonmeaningful sentences that included syntactic violations (Ni et al. 2000). In contrast, the RDs showed no such neurobiological distinction in activation between these 2 sentence types. Additionally, although somewhat difficult to interpret, the findings of pre- and postcentral gyri differences between groups may indicate a differential role for primary sensory–motor maps in detecting semantic and syntactic foils in the sentences. In sum, it appears that our RD group's deficits in higher-level language processing found on behavioral testing (Table 1) were also reflected, at least in part, by their neurobiological response to comprehending sentences; the exact nature of these differences, that is, whether they are due to a confined deficit such as vocabulary weakness or in some larger set of integrative skills is an important area for further study.
Although our study has much to offer in terms of insights about higher-level language processing in children who are skilled and poor readers, future investigations will need to incorporate multiple modalities (auditory and visual) in order to more fully pinpoint word-level versus comprehension-specific processes in a range of reading skill. To this end, a recent study by Shankweiler et al. (forthcoming) found left inferior frontal region as a common site between auditory and visual presentation of sentences; however, this region also showed a skill level of reader by modality interaction. Future investigations incorporating these types of across modality analyses will be helpful to uncovering more about comprehension processes, which would be particularly enhanced by careful matching of cognitive skill between groups. In addition, it would be helpful to further explore the relationship between WR accuracy, fluency, and comprehension and occipitotemporal activation. Inclusion of individuals who are specifically comprehension impaired (i.e., do not have word-level difficulties), in addition to individuals with more “typical” RD (i.e., dyslexics) and to skilled readers would add further insights into the comprehension process. Furthermore, utilizing different kinds of sentences (and different modalities) with the different reader profiles may allow for a better understanding of the nature of other aspects impacting comprehension (i.e., inferencing, working memory, syntax/semantics, etc.).
Another important aspect of future investigations will be to incorporate baseline fixation into their SC paradigms. Certainly it could be argued that baseline fixation, which our design did not include, could have helped disentangle the issue whether deactivation of words for the RD group was driving the SC activation differences. However, even though it will be important for future investigations to incorporate baseline, it should be pointed out that the primary focus of this study was in understanding SC after accounting for each individual's processing of words (regardless of whether that resulted in activation or deactivation), such that comparing to a baseline fixation was not necessarily a fundamental component of our question.
In sum, our study reports patterns of activation associated with SC (contrasted against WR) in skilled readers. Additionally, we found differences in activation patterns associated with sentence processing in children with RD. This study is an important contribution to beginning to understand how higher-level language processing impacts reading comprehension, especially for disabled readers. Our design of accounting for activation due to WR specifically allowed for examination of this issue in a manner not previously utilized in the fMRI literature, both for children who are skilled readers as well as those with RD.
Funding
Mental Wellness Foundation; Johns Hopkins School of Medicine General Clinical Research Center (National Institutes of Health [M01-RR00052]); National Institutes of Health (R01 HD044073); and F.M. Kirby Research Center.
Acknowledgments
We are grateful for Dana Carmichael's contribution to manuscript preparation. Conflict of Interest: none declared.
References
- Adams MJ. Beginning to read: thinking and learning about print. Cambridge (MA): Massachusetts Institute of Technology Press; 1990. [Google Scholar]
- Ben-Shachar M, Palti D, Grodzinsky Y. Neural correlates of syntactic movement: converging evidence from two fMRI experiments. Neuroimage. 2004;21:1320–1336. doi: 10.1016/j.neuroimage.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Biancarosa G, Snow CE. Reading next—a vision for action and research in middle and high school literacy: a report from Carnegie Corporation of New York. Washington (DC): Alliance for Excellent Education; 2004. [Google Scholar]
- Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic J, Feldman HM. Functional organization of activation patterns in children: whole brain fMRI imaging during three different cognitive tasks. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:669–682. doi: 10.1016/s0278-5846(99)00025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RS, Frith CD. The role of the right hemisphere in the interpretation of figurative aspects of language: a positron emission tomography activation study. Brain. 1994;117:1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition, and errors. Cereb Cortex. 2001;11(9):825–36. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Breznitz Z, Leikin M. Syntactic processing of Hebrew sentence in normal and dyslexic readers: electrophysiological evidence. J Genet Psychol. 2000;161(3):359–380. doi: 10.1080/00221320009596718. [DOI] [PubMed] [Google Scholar]
- Breznitz Z, Leikin M. Effects of accelerated reading rate on processing words’ syntactic functions by normal and dyslexic readers: event related potentials evidence. J Genet Psychol. 2001;162(3):276–296. doi: 10.1080/00221320109597484. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke's Wortschatz? Brain. 1999;122:1901–17. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Calhoun V, Adali T, Kraut M, Pearlson G. A weighted-least squares algorithm for estimation and visualization of relative latencies in event-related functional MRI. Magn Reson Med. 2000;44:947–954. doi: 10.1002/1522-2594(200012)44:6<947::aid-mrm17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Capek CM, Bavelier D, Corina D, Newman AJ, Jezzard P, Neville HJ. The cortical organization of audio-visual sentence comprehension: an fMRI study at 4 Tesla. Cogn Brain Res. 2004;20:111–119. doi: 10.1016/j.cogbrainres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Caplan D, Vijayan S, Kuperberh G, West C, Waters G, Greve D, Dale AM. Vascular responses to syntactic processing: event-related fMRI study of relative causes. Hum Brain Mapp. 2001;15:26–38. doi: 10.1002/hbm.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JB, Davies P, Richman B. The American heritage word frequency book. Boston: Houghton Mifflin; 1971. [Google Scholar]
- Catts HW, Fey ME, Zhang X, Tomblin JB. Language basis of reading and reading disabilities: evidence from a longitudinal investigation. Sci Stud Read. 1999;3:331–361. [Google Scholar]
- Catts HW, Hogan TP, Adlof SM, Barth AE. The simple view of reading changes over time. Boulder (CO): Society for Scientific Study of Reading; 2003. [Google Scholar]
- Cooke A, Grossman M, DeVita C, Gonzalez-Atavales J, Moore P, Chen W, Gee J, Detre J. Large-scale neural network for sentence processing. Brain Lang. 2006;96:14–36. doi: 10.1016/j.bandl.2005.07.072. [DOI] [PubMed] [Google Scholar]
- Cooke A. Working Party of the Division of Educational and Child Psychology of the British Psychological Society: Critical response to dyslexia, literacy and psychological assessment. Dyslexia. 2001;7(1):47–52. doi: 10.1002/dys.181. [DOI] [PubMed] [Google Scholar]
- Cutting LE, Clements AM, Courtney S, Rimrodt SR, Schafer JGB, Wilkins J, Pekar JJ, Pugh KR. Differential components of sentence comprehension: beyond single word reading and memory. Neuroimage. 2006;29(2):429–438. doi: 10.1016/j.neuroimage.2005.07.057. [DOI] [PubMed] [Google Scholar]
- Cutting LE, Scarborough HS. Prediction of reading comprehension: relative contributions of word recognition, language proficiency, and other cognitive skills can depend on how comprehension is measured. Sci Stud Read. 2006;10(3):277–299. [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24(2):427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiol Rev. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage. 2001;14(6):1256–67. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV. New York: Guilford Press; 1998. [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NA, Agnew JA, Flowers DL. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44:411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Evans A, Collins D, Mills S, Brown E, Kelly R, Peters T. Vol. 3. Proceedings of the IEEE-Nuclear Science Symposium and Medical Imaging Conference Report; 1993 Oct 31-Nov 6. San Fransisco (CA): Institute of Electrical and Electronics Engineers; 1993. 3D statistical neuroanatomical models from 305 MRI volumes; pp. 1813–1817. [Google Scholar]
- Ferstl EC, von Cramon DY. The role of coherence and cohesion in test comprehension: an event-related fMRI study. Cogn Brain Res. 2001;11:325–340. doi: 10.1016/s0926-6410(01)00007-6. [DOI] [PubMed] [Google Scholar]
- Foorman BR, Francis DJ, Fletcher JM, Lynn A. Relation of phonological and orthographic processing to early reading: comparing two approaches to regression-based, reading-level-match designs. J Educ Psychol. 1996;88(4):639–652. [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, Gee J. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein EA. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage. 2006;32(2):842–53. doi: 10.1016/j.neuroimage.2006.04.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Sakai KL. Specialization in the left prefrontal cortex for sentence comprehension. Neuron. 2002;35:589–597. doi: 10.1016/s0896-6273(02)00788-2. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF. Semantic cortical activation in dyslexic readers. J Cogn Neurosci. 1999;11(5):535–550. doi: 10.1162/089892999563599. [DOI] [PubMed] [Google Scholar]
- Jobard G, Vigneau M, Mazoyer B, Tzourio-Mazoyer N. Impact of modality and linguistic complexity during reading and listening tasks. Neuroimage. 2007;34:784–800. doi: 10.1016/j.neuroimage.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Karni A, Morocz IA, Bitan T, Shaul S, Kushnir T, Breznitz Z. An fMRI study of the differential effects of word presentation rates (reading acceleration) on dyslexic readers’ brain activity patterns. J Neurolinguist. 2005;18:197–219. [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: an fMRI examination of syntactic and lexical processing. Cereb Cortex. 2001;11:223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA. Brain imaging of tongue-twister sentence comprehension: twisting the tongue and the brain. Brain Lang. 2003;84:189–203. doi: 10.1016/s0093-934x(02)00506-0. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia. 2006;44:1822–1832. doi: 10.1016/j.neuropsychologia.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Leach JM, Scarborough HS, Rescorla L. Late-emerging reading disabilities. J Educ Psychol. 2003;95:211–224. [Google Scholar]
- Leikin M. Processing syntactic functions of words in normal and dyslexic readers. J Psycholinguist Res. 2002;31(2):145–163. doi: 10.1023/a:1014926900931. [DOI] [PubMed] [Google Scholar]
- Lyon GR. Toward a definition of dyslexia. Ann Dyslexia. 1995;45:3–27. doi: 10.1007/BF02648210. [DOI] [PubMed] [Google Scholar]
- Maess B, Herrmann CS, Hahne A, Nakamura A, Friederici AD. Localizing the distributed language network responsible for the N400 measured by MEG during auditory sentence processing. Brain Res. 2006;1096:163–172. doi: 10.1016/j.brainres.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA. Lexical ambiguity in sentence comprehension. Brain Res. 2007;1146:115–127. doi: 10.1016/j.brainres.2007.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCardle P, Scarborough HS, Catts HW. Predicting, explaining, and preventing children's reading difficulties. Learn Disabil Res Pract. 2001;16:230–239. [Google Scholar]
- Meyer M, Friederici AD, von Cramon DY. Neurocognition of auditory sentence comprehension: event related fMRI reveals sensitivity to syntactic violations and task demands. Cogn Brain Res. 2000;9:19–33. doi: 10.1016/s0926-6410(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield-Gabrieli S, Gabrieli JDE, Just MA. Brain activation during sentence comprehension among good and poor readers. Cereb Cortex. 2007;17(12):2780–2787. doi: 10.1093/cercor/bhm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation K, Snowling MJ. Beyond phonological skills: broader language skills contribute to the development of reading. J Res Read. 2004;27:342–356. [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, Shaywitz BA, Gore JC, Shankweiler D. An event-related neuroimaging study distinguishing form and content in sentence processing. J Cogn Neurosci. 2000;12:120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Potter MC. Understanding sentences and scenes: the role of conceptual short-term memory. In: Coltheart V, editor. Fleeting memories: cognition of brief visual stimuli. Cambridge (MA): Massachusetts Institute of Technology Press; 1999. pp. 13–46. [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Mental retardation and developmental disabilities research reviews. Pediatr Neuroimaging. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [special issue] [DOI] [PubMed] [Google Scholar]
- Rasinski TV. Investigating measures of reading fluency. Educ Res Quart. 1990;14(3):37–44. [Google Scholar]
- Rumsey JM, Zametkin AJ, Andreason P, Hanahan AP, Hamburger SD, Aquino T, King AC, Pikus A, Cohen RM. Normal activation of frontotemporal language cortex in dyslexia, as measured with oxygen 15 positron emission tomography. Arch Neurol. 1994;51:27–38. doi: 10.1001/archneur.1994.00540130037011. [DOI] [PubMed] [Google Scholar]
- Rupley WH, Willson VL, Nichols WD. Exploration of the developmental components contributing to elementary school children's reading comprehension. Sci Stud Read. 1998;2:143–158. [Google Scholar]
- Sabisch B, Hahne A, Glass E, von Suchodoletz W, Friederici AD. Auditory language comprehension in children with developmental dyslexia: evidence from event-related brain potentials. J Cogn Neurosci. 2006;18(10):1676–1695. doi: 10.1162/jocn.2006.18.10.1676. [DOI] [PubMed] [Google Scholar]
- Seki A, Koeda T, Sugihara S, Kamba M, Hirata Y, Ogawa T, Takeshita K. A functional magnetic resonance imaging study during sentence reading in Japanese dyslexic children. Brain Dev. 2001;23:312–316. doi: 10.1016/s0387-7604(01)00228-5. [DOI] [PubMed] [Google Scholar]
- Shankweiler D, Mencl WE, Braze D, Tabor W, Pugh KR Forthcoming. Reading Differences and brain: cortical integration of speech and print in sentence processing varies with reader skill. Dev Neuropsychol. doi: 10.1080/87565640802418688. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz B, Pugh K, Fulbright R, Constable R, Mencl W, Shankweiler D, Liberman I, Skudlarski P, Fletcher J, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WR, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Holahan JM, Marchione KE, et al. Development of left occipito-temporal systems for skilled reading in children after a phonologically based intervention. Biol Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Bergman E, Papanicolaou AC. Cerebral mechanisms involved in word reading in dyslexic children: a magnetic source imaging approach. Cereb Cortex. 2000;10:809–816. doi: 10.1093/cercor/10.8.809. [DOI] [PubMed] [Google Scholar]
- Sinatra GM, Royer JM. Development of cognitive component skills that support skilled reading. J Educ Psychol. 1993;85(3):509–519. [Google Scholar]
- Stowe LA, Broere CAJ, Paans AMJ, Wijers AA, Mulder G, Vaalburg W, Zwarts F. Localizing components of a complex task: sentence processing and working memory. Neuroreport. 1998;9:2995–2999. doi: 10.1097/00001756-199809140-00014. [DOI] [PubMed] [Google Scholar]
- Stowe LA, Paans AM, Wijers AA, Zwarts F, Mulder G, Vaalburg W. Sentence comprehension and word repetition: a positron emission tomography investigation. Psychophysiology. 1999;36:786–801. [PubMed] [Google Scholar]
- Stowe LA, Wijers AA, Willemsen ATM, Reuland E, Vaalburg W. Positron emission tomography [150] H2O study of language processing—effects of sentence context and repetition. J Nucl Med. 1994;35(5):P200. [Google Scholar]
- Swanson HL, Trahan M. Learning disabled and average readers’ working memory and comprehension: does metacognition play a role. Br J Educ Psychol. 1996;66:333–55. doi: 10.1111/j.2044-8279.1996.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Torgesen JK. Individual differences in response to early interventions in reading: the lingering problem of treatment resisters. Learn Disabil Res Pract. 2000;15:55–64. [Google Scholar]
- Vellutino FR, Scanlon DM, Tanzman MS. Components of reading ability: issues and problems in operationalizing word identification, phonological coding, and orthographic coding. In: Lyon GR, editor. Frames of reference for the assessment of learning disabilities: new views on measurement issues. Baltimore (MD): Paul H Brookes; 1994. pp. 279–332. [Google Scholar]