Abstract
Eye and head movements are coordinated during head-free pursuit. To examine whether pursuit neurons in frontal eye fields (FEF) carry gaze-pursuit commands that drive both eye-pursuit and head-pursuit, monkeys whose heads were free to rotate about a vertical axis were trained to pursue a juice feeder with their head and a target with their eyes. Initially the feeder and target moved synchronously with the same visual angle. FEF neurons responding to this gaze-pursuit were tested for eye-pursuit of target motion while the feeder was stationary and for head-pursuit while the target was stationary. The majority of pursuit neurons exhibited modulation during head-pursuit, but their preferred directions during eye-pursuit and head-pursuit were different. Although peak modulation occurred during head movements, the onset of discharge usually was not aligned with the head movement onset. The minority of neurons whose discharge onset was so aligned discharged after the head movement onset. These results do not support the idea that the head-pursuit–related modulation reflects head-pursuit commands. Furthermore, modulation similar to that during head-pursuit was obtained by passive head rotation on stationary trunk. Our results suggest that FEF pursuit neurons issue gaze or eye movement commands during gaze-pursuit and that the head-pursuit–related modulation primarily reflects reafferent signals resulting from head movements.
Keywords: frontal eye fields, gaze, head-free pursuit, reafferent, smooth-pursuit, vestibular
Introduction
Smooth-pursuit eye movements are essential to obtain accurate visual information about a slowly moving object and are made in response to visual information about the velocity of slip of the object's image on the retina. In daily life where the head is free to move, eye and head movements are coordinated during pursuit of a slowly moving visual target (i.e. gaze-pursuit, gaze = eye in space, see Leigh and Zee 2006 for a review). Eye and head coordination is well known during saccadic gaze shifts, although the responsible neural mechanisms still remain controversial. There are 2 competing hypotheses (for a review, see Chen 2006); 1) the brain issues an integrated gaze command that is then decomposed into separate eye and head commands; and 2) the brain issues independent eye and head commands at all levels. For gaze-pursuit also, Lanman et al. (1978) suggested that the brain issues gaze-pursuit commands driving both eye and head movements and further that the eye-pursuit command is combined with vestibular feedback from head movement to compensate for variations in the amount of head movement (also Miles and Lisberger 1981). Whereas independent commands have also been suggested (Belton and McCrea 1999, 2000a, 2000b) for gaze-velocity Purkinje cells in the cerebellar floccular region (Miles and Fuller 1975; Lisberger and Fuchs 1978).
Saccade neurons in the frontal eye fields (FEF) are thought to signal both rapid eye movement commands and rapid head movement commands for head-free gaze shifts (Tu and Keating 2000; Knight and Fuchs 2006; however, Chen 2006). The caudal part of the FEF in the fundus of the arcuate sulcus has been known to contain neurons that discharge in relation to smooth-pursuit (pursuit neurons) in head-fixed monkeys and these neurons are thought to issue a pursuit command (MacAvoy et al. 1991; Gottlieb et al. 1993, 1994; Tian and Lynch 1996; Tanaka and Fukushima 1998; Fukushima et al. 2000; Fukushima, Yamanobe, Shinmei, Fukushima 2002; Fukushima, Yamanobe, Shinmei, Fukushima, Kurkin, et al. 2002; Tanaka and Lisberger 2002).
The question we address in the present study is whether FEF pursuit neurons carry gaze-pursuit commands that drive both eye-pursuit and head-pursuit during head-free gaze-pursuit. Detailed information about FEF pursuit signals is necessary to elucidate their role in head-free, gaze-pursuit. However, FEF pursuit neurons have never been tested during head-free gaze-pursuit. Therefore, it is unknown whether they carry gaze-pursuit commands that drive both eye-pursuit and head-pursuit during gaze-pursuit or if they carry signals that specifically command either eye or head-pursuit. To help answer this question, we examined discharge characteristics of FEF pursuit neurons during head-pursuit of a reward-feeder in macaques whose head was free to rotate about a vertical axis on the stationary trunk.
Some of the results were presented in preliminary form (Fukushima et al. 2005).
Materials and Methods
General Procedures
A total of 4 monkeys (Macaca fuscata: B, H, Si, and Sh, 3.4–5.0 kg) were used. All procedures were performed in strict compliance with the guidelines for the Care and Use of Animals of National Institutes of Health. Specific protocols were approved by the Animal Care and Use Committee of Hokkaido University School of Medicine. Methods for animal preparation, training, recording, and data analysis were similar to previous studies (Fukushima et al. 2000; Akao et al. 2005; Kasahara et al. 2006), except for head-free pursuit and are summarized here briefly.
Each monkey was sedated with ketamine hydrochloride (5 mg/kg, i.m.), and then anesthetized with pentobarbital sodium (25 mg/kg, i.p.). Under aseptic conditions, a head holder was installed to restrain the head in the stereotaxic plane to compare neuronal activity during head-free and head-fixed conditions. Vertical and horizontal components of eye movements were recorded using the scleral search coil method (Fuchs and Robinson 1966; Judge et al. 1980). A 0.2° laser spot was presented on the tangent screen 75 cm in front of the monkey's eye in an otherwise dark enclosure. Eye position signals were calibrated by requiring the monkeys to fixate stationary targets at known positions or to pursue a slowly moving target with known excursion while the monkeys’ head was stabilized to the chair. Reward circuits compared target position signals with the monkeys’ gaze position signals (Fig. 1). If the monkeys’ gaze was within an error window of ±1° for 0.5–1 s, a drop of apple juice was automatically delivered to the animal.
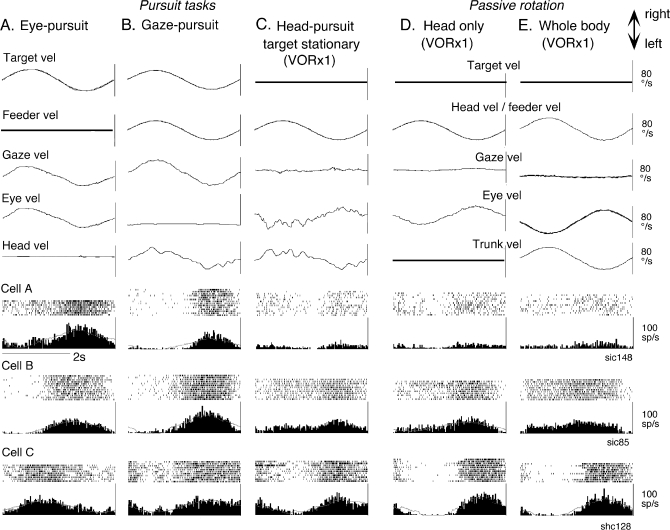
Figure 1.
Representative discharge modulation of FEF pursuit neurons during different task conditions. Responses of 3 representative neurons (cells A–C) are shown during eye-pursuit (A), gaze-pursuit (B), head-pursuit (target stationary in space, C), passive head rotation on the stationary trunk (D) and passive whole-body rotation (E). In (D) and (E), a target was stationary in space. Five analog traces (top to bottom) in (A–C) are averaged traces for target-velocity (vel), feeder velocity, gaze-velocity, eye-velocity, and head-velocity. Analog traces in (D) and (E) are as indicated. Two traces for cell responses in (A–E) are raster and histograms of cell discharge.
The monkeys’ body movement was restricted back and front by polystyrene foam in the primate chair so that they were unable to move their trunk and sat inside the chair with their back positioned near the earth vertical axis (Kasahara et al. 2006). To allow the monkeys to make head-free, gaze-pursuit, the head holders were fixed to a mechanical device similar to the one used by Belton and McCrea (1999, 2000b). This device allowed only horizontal head rotation along the vertical axis that was coincident with the axis of turntable rotation and passed through the intersection of the mid-sagittal and interaural planes and within 5 mm of the C1–C2 axis (Belton and McCrea 2000b). The monkey's head rotated inside the magnetic fields so that eye coil signals provided gaze (eye in space) signals. Horizontal head rotation was recorded by a potentiometer attached to the shaft of the vertical axis. Eye movement in the orbit was calculated as the difference between gaze movement and head movement. The primate chair was positioned on the turntable, and the chair was either firmly fixed to the turntable or fixed in space by a mechanical device irrespective of turntable rotation (Fig. 1).
Recording Procedures and Behavioral Paradigms
Extracellular recordings were made in the left periarcuate sulcus region to locate neurons that responded during head-free pursuit of a visual target and juice feeder in the horizontal plane (gaze-pursuit task—see below). To induce head-pursuit, a feeder for juice-reward was moved sinusoidally at 0.3 Hz through ±15° of visual angle. The juice feeder was positioned close to the monkey's mouth but not touching it. The monkey drank juice by sticking its tongue out to access the feeder. During head-free pursuit, we used 3 tasks. In the task used while searching for neurons, the target and juice feeder moved together sinusoidally with the same phase, amplitude and direction (at 0.3 Hz, ±15°) and the monkey tracked the feeder by moving the head and the spot with the eyes which required canceling the vestibulo-ocular reflex (VOR) (Fig. 1B). We call this task gaze-pursuit. Once we encountered an isolated neuron that responded during this search task as judged visually on the computer monitor and on the audio monitor, the monkey was required to track the spot by smooth-pursuit while the juice feeder was stationary (eye-pursuit, Fig. 1A). The monkey was also required to track the feeder by moving the head while the spot remained stationary in space (Fig. 1C). The monkey could detect feeder motion by its peripheral vision while fixating a stationary spot and also by tactile stimuli to its tongue induced by feeder motion. We call this task head-pursuit (target stationary in space). This condition was achieved with perfect VOR (i.e. VOR x1) that held gaze steady in space while the head rotated on the stationary trunk (Fig. 1C). During eye-pursuit while the feeder was stationary, our monkeys occasionally made small head movements that were mostly small changes in head position but that were not synchronized with eye movements.
To determine whether discharge modulation of FEF pursuit neurons preceded the onset of head movements during head-pursuit (target stationary in space), we moved the juice feeder with a ramp trajectory (at 20°/s, ±10°) and random intertrial intervals (1–3.5 s) in 2 monkeys (Si, Sh). For comparison, the spot was also moved with or without the feeder in the same ramp trajectory to test discharge modulation during gaze-pursuit and eye-pursuit, respectively. To assure head movements during gaze-pursuit and head-pursuit (target stationary in space), the reward circuits compared feeder position signals with the monkey's head position signals with a wider error window (± 3°). A total of 52 neurons that responded to sinusoidal head-pursuit were also tested for ramp head-pursuit.
As described in the Results, head-pursuit–related modulation does not seem to reflect head-pursuit commands. To examine whether the modulation during head-pursuit reflected reafferent signals from head movements, in the 2 monkeys (Si, Sh) we examined discharge modulation during 2 additional conditions involving passive rotations driven by our motorized turntable. In the head-only condition, the motor rotated the head only while the monkey fixated a stationary spot in space. The primate chair was stabilized in space by a mechanical lock during turntable rotation, thus allowing only the head to be passively rotated by the turntable. In the second condition (whole-body rotation), the turntable rotated the trunk and body together.
In both conditions, the juice feeder was moved together with the head and the target stayed stationary in space straight ahead of the monkey's eyes, requiring perfect VOR (VOR x1) at 0.3 Hz (±10°). A single horizontal motor was used to apply passive rotation in these task conditions so that the same horizontal rotation was applied along an identical vertical axis. For comparison, the feeder was also moved at the same magnitude (0.3 Hz, ±10°) to test the modulation during head-pursuit (target stationary in space, cf., Fig. 5).
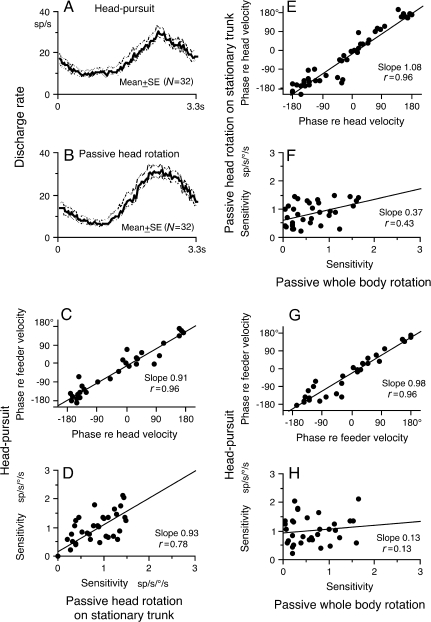
Figure 5.
Correlation of discharge modulation during head-pursuit, passive head rotation on the stationary trunk and passive whole-body rotation. (A and B) Population response (mean ± SE) of 32 FEF pursuit neurons during head-pursuit (A) and passive head rotation on the stationary trunk (B). (C and D) Plot response phase shifts (relative to stimulus velocity) and sensitivity of FEF pursuit neurons during head-pursuit (target stationary in space) against those during passive head rotation on the stationary trunk with linear regressions. (E and F) Plot response phase shifts (relative to stimulus velocity) and sensitivity of FEF pursuit neurons during passive head rotation on the stationary trunk (target stationary in space) against those during passive whole-body rotation with linear regressions. (G and H) Plot response phase shifts (relative to stimulus velocity) and sensitivity of FEF pursuit neurons during head-pursuit (target stationary in space) against those during passive whole-body rotation with linear regressions. (D and H) Sensitivity during head-pursuit was corrected for lower head-velocity gains present while recording discharge of individual neurons. See text for further explanation.
Because the target was presented 75 cm in front of the monkey and the eyes were positioned about 2.5 cm in front of the axis for head rotation during head-pursuit and passive head and whole-body rotation (target stationary in space, see above), the VOR x1 gain during these task conditions should be 1.03, instead of 1.0, resulting in gaze-velocity gain of 0.03 (see Collewijn et al. 1982, for details). To minimize gaze-velocity related discharge modulation during active or passive head movement, we also tested these task conditions without a target in complete darkness.
Data Analysis
Eye, target and chair position signals and their derivatives were low-pass filtered (250 Hz) and digitized at 500 Hz. Neural discharge was discriminated, detected at 100 kHz, and stored in temporal register with analog signals. Traces with poor performance were rejected and only those traces in which monkeys performed the tasks were accepted for analysis (see below and Results). Saccades were marked with a cursor on eye-velocity traces and removed using an interactive computer program as described previously (Singh et al. 1981; Fukushima et al. 2000).
Head-free pursuit contained periods where the head moved faster than the target, which contributed to calculation of head-velocity gains. Previous studies in our laboratory used a similar head-pursuit task in 2 monkeys (B, H, which were also used in the present study). Our analysis in those studies revealed that head-velocity gains with and without removing periods of fast head-velocity were similar (Kasahara et al. 2006). Therefore, in the present study, we used head-velocity traces without removing fast head movements prior to analysis.
Sinusoidal Stimulus Presentation
All traces were aligned with stimulus velocity to average movement responses across 10–30 cycles, and raster and histograms of neuronal responses were constructed. Eye-, head- and gaze-velocities were calculated by fitting them with a sine function using a least-square method after deleting saccades. Gains of eye-, head- and gaze-velocities were calculated as the peak amplitude of the fundamental component divided by the peak amplitude of the stimulus velocity. To quantify neuronal responses, each cycle was divided into 128 equal bins. A sine function was fitted to averaged velocities and cycle histograms of cell discharge, excluding the bins with zero spikes, by means of a least-squared error algorithm. Signal-to-noise ratio of the response (S/N) was defined as the ratio of amplitude of the fitted fundamental frequency component to the root mean square amplitude of the third through eighth harmonics. Harmonic distortion (HD) was defined as the ratio of the amplitude of the second harmonic to that of the fundamental according to Wilson et al. (1984). Responses with HD ≤ 50% or S/N ≥ 1.0 were accepted for further analysis. The HD of the chair and juice feeder was very small, typically 0.005-0.007.
Amplitude of discharge modulation was calculated as the peak amplitude of the fundamental component fitted to the cycle histograms. Phase shifts were measured between the peak of the fundamental component of the response and the peak contralateral stimulus velocity during target motion, feeder movement, passive head rotation, and passive whole-body rotation. Sensitivity was calculated as the peak amplitude of the fundamental component divided by the stimulus velocity. As the stimulus velocity, feeder velocity was used for gaze-pursuit and head-pursuit (target stationary in space) and visual target-velocity was used for eye-pursuit. Head-velocity was used as the stimulus velocity during passive head rotation on the stationary trunk and passive whole-body rotation.
To compare neuronal discharge during different tasks, we calculated correlation coefficients between 2 tasks. We defined significant differences as those having a P value < 0.05.
Response to Ramp Stimulus Motion
To examine the latency of neuronal discharge during head-free pursuit in response to ramp motion of the feeder and/or target spot, we first aligned 20–40 trials on the stimulus onset. Because discharge may have been affected by saccades, we then omitted all traces in which saccades appeared within ∼100 ms of the stimulus onset. Traces in which head-pursuit appeared before stimulus onset (0–100 ms) were deleted to minimize predictive performance. Prestimulus baseline values (mean and standard deviations, SD) for neural discharge and eye-, gaze, and/or head-velocity were calculated from the 200 ms interval immediately prior to stimulus onset. Onset of the neuronal response to the stimulus motion was defined as the time at which the mean discharge rate in the histogram exceeded 2SD of the control value (Akao et al. 2005). Latencies of eye-, gaze, and/or head-velocity responses were analyzed similarly. Neuronal discharge during head-pursuit was also aligned with the onset of head movements to examine whether the onset of discharge histograms was better aligned with head movement onset or onset of feeder motion (see Results).
Histological Procedures
The recording sites were marked by electrolytic lesions made by passing current through the recording electrodes. Two monkeys (B, H) were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused with physiological saline followed by 3.5% formalin. After histological fixation, coronal sections were cut at 100-μm thickness on a freezing microtome. The sections were stained for cell bodies and fibers, and the locations of recording sites were verified as previously described (Tanaka and Fukushima 1998; Fukushima et al. 2000; Fukushima, Yamanobe, Shinmei, Fukushima 2002; Fukushima, Yamanobe, Shinmei, Fukushima, Kurkin, et al. 2002). Two other monkeys are still being used.
Results
We examined discharge characteristics of a total of 134 neurons that responded during gaze-pursuit. In this condition, the monkeys tracked the juice feeder with the head and spot with the eyes while canceling the VOR (Fig. 1B, see Materials and Methods). These neurons were tested for eye-pursuit of spot motion while the feeder was stationary in space (Fig. 1A) and for head-pursuit of feeder motion while the target was stationary in space (Fig. 1C). Table 1 summarizes overall mean (±SD) gains for gaze-velocity, head-velocity, and eye-velocity during the 3 tasks. During gaze-pursuit and head-pursuit (target stationary in space), the monkeys performed the tasks mostly by moving the head (mean head-velocity gains 0.93 and 0.82, respectively). Head-velocity gain during eye-pursuit was less than 0.1. As requested by the tasks, gaze-velocity gain was near 1.0 (0.92, 0.94) during gaze-pursuit and eye-pursuit and near zero during head-pursuit (target stationary in space) (Table 1). Mean (±SD) phase shifts of gaze-velocity during gaze-pursuit and eye-pursuit were 4 (±1) and 2 (±1) deg lag (re-stimulus velocity), respectively. Mean (±SD) phase shifts of head-velocity during gaze-pursuit and head-pursuit (target stationary in space) were 0 (±6) and 1 (±3) deg lag, respectively. Thus, our monkeys performed gaze-pursuit and head-pursuit (target stationary in space) using head movements that were similar to the eye movements observed during eye-pursuit.
Table 1.
Mean (±SD) gains for gaze-velocity, head-velocity, and eye-velocity (re-stimulus velocity at 0.3 Hz, ±15°) during the 3 tasks
| Task conditions | Gaze-velocity gain | Head-velocity gain | Eye-velocity gain |
| Gaze-pursuit | 0.92 (±0.10) | 0.93 (±0.13) | |
| Eye-pursuit | 0.94 (±0.04) | 0.06 (±0.10) | 0.89 (±0.08) |
| Head-pursuit | 0.06 (±0.02) | 0.82 (±0.11) |
Note: As the stimulus velocity, feeder velocity was used for gaze-pursuit and head-pursuit (target stationary in space) and target-velocity was used for eye-pursuit. Values were omitted for eye-velocity gain in rows 1 and 3, because in these conditions eye-velocity gain should be calculated relative to head-velocity but not feeder velocity.
Discharge of FEF Neurons during the Three Task Conditions
If the neural modulation observed during head-pursuit simply reflected a command that drives both eye and head-pursuit, preferred directions for eye-pursuit and head-pursuit should be similar. FEF neurons that responded during gaze-pursuit exhibited a wide range of modulation, from weak to robust, during head-pursuit (target stationary in space). Figure 1A–C illustrates discharge of 3 representative neurons (cells A–C) during the 3 task conditions. Cell A discharged during gaze-pursuit and eye-pursuit with similar preferred directions and similar response magnitudes, but it discharged minimally during head-pursuit (target stationary in space) when gaze was nearly stationary. Cell B discharged during gaze-pursuit and eye-pursuit with similar preferred directions and it also discharged clearly during head-pursuit (target stationary in space) when gaze was stationary, although the response magnitude during head-pursuit (target stationary in space) was smaller than that during gaze-pursuit (Fig. 1B,C). In contrast, modulation of cell C during head-pursuit (target stationary in space) was strong, and preferred directions during eye-pursuit and head-pursuit (target stationary in space) were clearly opposite to each other (Fig. 1A,C). Thus, this neuron discharged during rightward eye-velocity and leftward head-velocity.
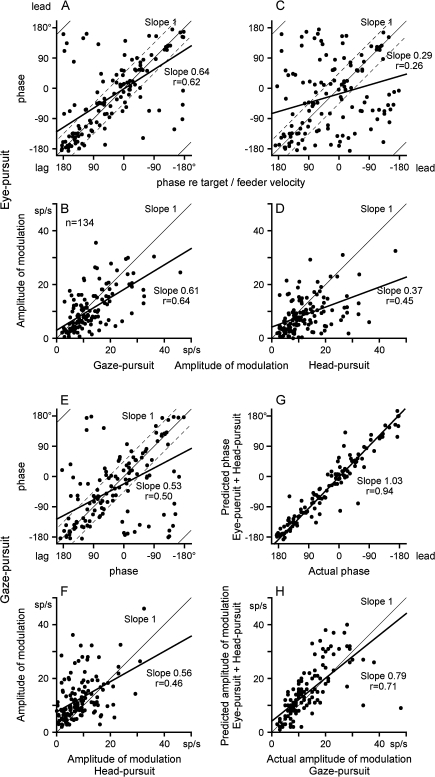
To illustrate the ranges of discharge modulation during the 3 tasks, Figure 2A–F compares discharge modulation of all neurons examined that exhibited modulation during gaze-pursuit (n = 134). When the response phase and amplitude of modulation during eye-pursuit were plotted against those during gaze-pursuit (Fig. 2A,B), the majority showed similar responses (i.e. point near the slope one thin line in A and B). Because we tested eye-pursuit and head-pursuit only in the horizontal plane, the response phase provides rough estimate of the preferred direction of each neuron. In Figure 2A, response phases of the majority of tested neurons (101/134 = 75%) were scattered within ±45° of the slope one line (thin and 2 dashed lines), indicating that their preferred directions were similar. Mean (±SD) amplitudes of modulation during eye-pursuit and gaze-pursuit were 11.2 (±7.5) and 12.4 (±8.0) sp/s, respectively, indicating that they were also similar. Correlation coefficients for the linear regressions in Figure 2A and B were 0.62 and 0.64, respectively. These results indicate that modulation of the population of FEF neurons during eye-pursuit was significantly correlated with modulation during gaze-pursuit. This is expected because pursuit signals of FEF pursuit neurons contribute during gaze-pursuit that requires cancellation of the VOR induced by head movement (Akao, Saito, et al. 2007).
Figure 2.
Comparison of discharge modulation during eye-pursuit, gaze-pursuit and head-pursuit. Response phase shifts (relative to target/feeder velocity) and amplitude of modulation of FEF pursuit neuron discharge are plotted during eye-pursuit, gaze-pursuit and head-pursuit (target stationary in space). (A and B) Plot response phase and amplitude of modulation during eye-pursuit against those during gaze-pursuit with linear regressions (thick lines). (C and D) Plot response phase and amplitude of modulation during eye-pursuit against those during head-pursuit (target stationary in space) with linear regressions (thick lines). (E and F) Plot response phase and amplitude of modulation during gaze-pursuit against those during head-pursuit (target stationary in space) with linear regressions (thick lines). (G and H) Plot predicted phase and amplitude of modulation that were calculated by linear addition of modulation during eye-pursuit and head-pursuit against actual modulation during gaze-pursuit. Thin lines in (A–F) and (H) are slope one lines. In (A), (C), and (E), ±45° ranges are indicated by dashed lines. See text for further explanation.
Figure 2C,D compares response phase and amplitude of modulation during eye-pursuit and those during head-pursuit (target stationary in space). Although the modulation during eye-pursuit and head-pursuit exhibited some correlation, the correlation coefficients between the 2 were much lower (r = 0.26 and 0.33, Fig. 2C,D) compared with the correlations between eye-pursuit and gaze-pursuit (r = 0.62 and 0.64, Fig. 2A,B). Response phases of the majority of tested neurons (76/134 = 57%) were scattered more than ±45° from the slope one line (thin and 2 dashed lines, Fig. 2C), suggesting that their preferred directions during eye-pursuit and head-pursuit (target stationary in space) were different. Most neurons exhibited modulation during head-pursuit (target stationary in space) even though gaze was stationary (Fig. 2D). Mean (±SD) amplitude of modulation during head-pursuit (target stationary in space) was 8.8 (±6.5) sp/s.
For comparison, Figure 2E,F plots response phase and amplitude of modulation during gaze-pursuit against those during head-pursuit (target stationary in space). The 2 were significantly correlated not only in phase (r = 0.50, Fig. 2E) but also in amplitude of modulation (r = 0.46, Fig. 2F), suggesting contribution of a common factor (i.e. head movements) in the discharge modulation in the 2 conditions (see below).
If discharge of FEF pursuit neurons during head-pursuit (target stationary in space) does not represent a command to move the head that is also present during gaze-pursuit, it is possible that eye-pursuit signals and head-pursuit signals are separate in origin. Figure 2G,H supports this possibility. Phase and amplitude of modulation during gaze-pursuit were predicted by addition of eye-pursuit modulation and head-pursuit modulation. Predicted phase and amplitude of modulation were plotted against actual modulation in Figure 2G,H. The 2 were significantly positively correlated (r = 0.94 and 0.71) with the slopes close to one (1.01 and 0.79).
Head-Pursuit–Related Modulation of FEF Pursuit Neurons during Ramp Feeder Motion
If the head-pursuit (target stationary in space)-related modulation of FEF pursuit neurons reflected a command that drives both eye- and head-pursuit, their discharge not only should show similar preferred directions during eye-pursuit and head-pursuit, but should also precede the onset of head movements. We examined discharge of 52 neurons during head-pursuit (target stationary in space) of ramp feeder motion with random intertrial intervals (see Materials and Methods). Of these, 24 neurons exhibited the same preferred directions during eye-pursuit and head-pursuit. Of the remaining 28 neurons, the majority (n = 24) exhibited opposite preferred directions during eye-pursuit and head-pursuit; and the remaining 4 neurons did not exhibit any clear modulation during head-pursuit irrespective of the directions.
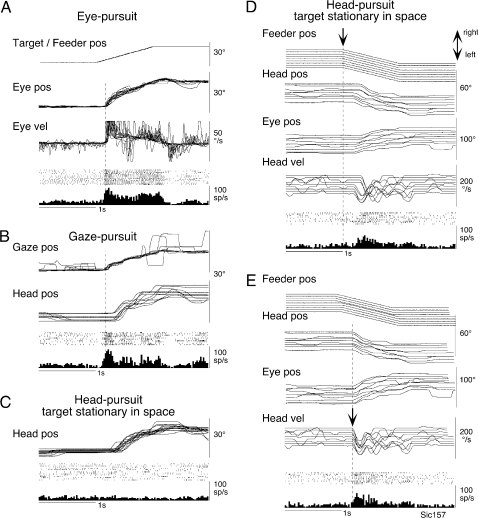
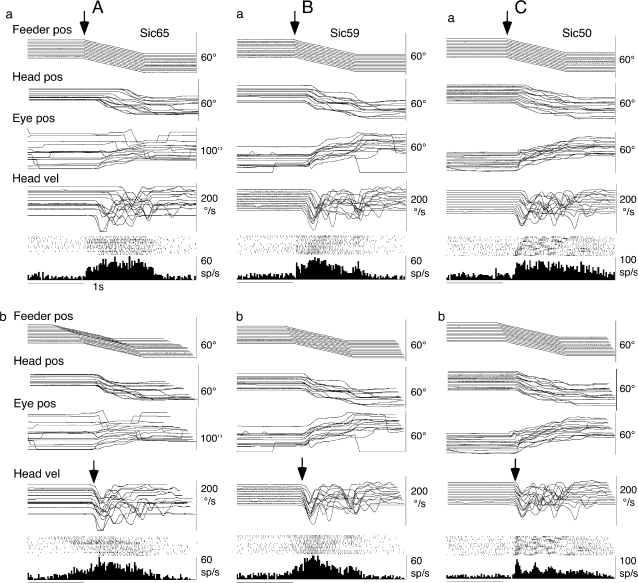
Figure 3 illustrates behavior of a neuron that had opposite preferred directions during eye-pursuit (Fig. 3A) and during head-pursuit (target stationary in space) (Fig. 3C,D). It was activated during eye-pursuit and gaze-pursuit to the right and its discharge began before the onset of eye or gaze motion (Fig. 3A,B, dashed lines). During head-pursuit (target stationary in space), it was not activated during rightward pursuit (Fig. 3C) but rather during leftward pursuit (Fig. 3D,E). Figure 3D,E compares discharge of this neuron when discharge was aligned with the onset of feeder motion (Fig. 3D) and when discharge was aligned with the onset of head movements (Fig. 3E). The onset of discharge histograms was aligned better with the onset of head movements, but the discharge lagged the onset of head movements by 20 ms. Similar responses were observed in 4 other neurons that exhibited opposite preferred directions during eye-pursuit and head-pursuit.
Figure 3.
Head-pursuit–related modulation of a representative FEF pursuit neuron. (A–C) Discharge modulation aligned with the onset of rightward target and/or feeder motion during eye-pursuit, gaze-pursuit, and head-pursuit (target stationary in space), respectively. Analog traces were superimposed. Traces in (D) and (E) were aligned with the onset of leftward feeder motion (D, arrow) and with the onset of leftward head movements (E, arrow) during head-pursuit (target stationary in space). Pos and vel indicate position and velocity, respectively.
Examples of the 24 neurons that discharged during head-pursuit with the same preferred directions during eye-pursuit and gaze-pursuit are shown in Figure 4. This figure compares discharge of 3 neurons during head-pursuit (target stationary in space) when discharge was aligned with the onset of feeder motion (Fig. 4Aa, Ba, Ca) and when discharge was aligned with the onset of head movements (Fig. 4Ab, Bb, Cb). Discharge of the 2 neurons illustrated in Figure 4A,B was clearly aligned with the onset of feeder motion with latencies of 53 and 44 ms, respectively. When the traces were aligned with the onset of head movements, the onset of neuronal discharge in the histogram was not well synchronized (Fig. 4Ab, Bb). This type of behavior was observed in most of the neurons with the same preferred directions during eye-pursuit and gaze-pursuit (21/24 = 88%). Latencies to the onset of feeder motion ranged from 19 to 270 ms with the mean being 99 ms. In the remaining 3 neurons, the onset of the discharge was synchronized with the onset of head movements. However, as shown in Figure 4Cb, discharge modulation of these neurons occurred 20, 32, and 80 ms after the onset of head movements.
Figure 4.
Head-pursuit–related discharge modulation of representative FEF pursuit neurons. (A–C) Three different neurons during head-pursuit (target stationary in space). Traces were aligned with the onset of feeder motion (a, arrow) and the onset of head movements (b, arrow). Other conventions are as in Figure 3.
None of the 52 tested neurons exhibited discharge modulation that preceded and was aligned with the onset of head movement. Six of the 52 neurons exhibited an initial weak burst in the histogram aligned with the onset of feeder motion (Fig. 4Ba), but these bursts did not align well with head movement onset (Fig. 4Bb). These results suggest a contribution of feeder motion–related discharge modulation (most probably tactile response) before the onset of head movements (see Discussion).
In all pursuit neurons tested (n = 52), peak modulation occurred during head movements, and 2 thirds of them exhibited peak discharge during peak head-velocity when aligned with the onset of head movements (Figs 3E, 4Cb). These results suggest an additional contribution of head movement-related discharge modulation after the onset of head movements (see below).
Comparison of Head-Pursuit and Passive Head Rotation on Stationary Trunk
Our results (Figs 2C, 3, 4) do not support the notion that the head-pursuit–related discharge modulation reflects motor commands involved in the initiation of head-pursuit. Because in the majority of neurons tested, peak modulation occurred during head movements as described above, we examined whether the modulation during head-pursuit reflects reafferent signals resulting from head movements. We compared discharge modulation of 32 FEF pursuit neurons during sinusoidal head-pursuit and passive head rotation on the stationary trunk in 2 monkeys (Sh, Si). In both conditions, the monkeys fixated an earth-stationary spot straight ahead of the monkeys’ eyes to minimize smooth-pursuit–related discharge modulation (see Materials and Methods). Figure 1D illustrates discharge of the 3 neurons (cells A–C). Their modulation during active head-pursuit (target stationary in space, Fig. 1C) and passive head rotation on the stationary trunk (Fig. 1D) was basically similar.
Figure 5A,B shows the population response (mean ± SE) of the 32 neurons during head-pursuit (target stationary in space) (Fig. 5A) and passive head-only rotation (target stationary in space) (Fig. 5B). Discharge modulation was aligned with the sinusoidal stimulus so that the increased modulation occurred during the later half of the cycle. Although the modulation during head-pursuit was slightly smaller than that during passive head-only rotation, this could be due to the fact that these 2 monkeys exhibited head-velocity gains lower than 1.0 while we were recording activity of these neurons during active head-pursuit. To compare discharge modulation of the 32 neurons during the 2 task conditions, Figure 5C,D plots response phase and sensitivity (re-stimulus velocity, see Materials and Methods) of discharge modulation of each neuron during head-pursuit against those during passive head rotation on the stationary trunk. Sensitivity during head-pursuit was corrected for lower head-velocity gains present while recording from individual neurons. After this correction, the sensitivities of neural modulation during head-pursuit and passive head-only rotation were significantly correlated (correlation coefficients r = 0.96 and 0.78) with slopes close to 1 (0.91 and 0.93, Fig. 5C,D). This suggests involvement of a common factor in discharge modulation during the 2 task conditions.
Because the majority of FEF pursuit neurons respond to vestibular inputs induced by passive whole-body rotation (Fukushima et al. 2000; Akao, Saito, et al. 2007), it is possible that the significant correlation between the 2 (Fig. 5C,D) was due to common vestibular inputs induced by head movements. Indeed, the cells illustrated in Figure 1 exhibited basically similar modulation during head-pursuit (Fig. 1C) and passive whole-body rotation (Fig. 1E), although in cell B there was a difference in response phase between passive head rotation on the stationary trunk (Fig. 1D) and passive whole-body rotation (Fig. 1E).
Figure 5E,F plots response phase and sensitivity of discharge modulation of the 32 neurons during passive head-only rotation against those during passive whole-body rotation. Although significant (Fig. 5E,F), the correlation coefficient in this sensitivity plot was lower than that in the plot between head-pursuit and passive head-only rotation (r = 0.96 and 0.43, Fig. 5E, F vs. r = 0.96 and 0.78, Fig. 5C,D). To further examine how vestibular inputs contributed to the modulation during head-pursuit, Figure 5G,H plots phase and sensitivity of discharge modulation of the 32 neurons during head-pursuit against phase and sensitivity during passive whole-body rotation. Although the phases showed high correlation (Fig. 5G, r = 0.96), there was no significant correlation between the sensitivities despite the fact that vestibular inputs induced clear modulation (r = 0.13, Fig. 5H). These results suggest that reafferent inputs other than vestibular also contributed to discharge modulation during passive head rotation on the stationary trunk and most probably during head-pursuit (target stationary in space) as well (see Discussion).
Discharge of FEF Neurons during Head Movement in Complete Darkness
Our use of a tangent screen with the target distance of 75 cm resulted in a mean gaze-velocity gain during head-pursuit (target stationary in space) of 0.06 (±0.02 SD, Table 1). This was due to the fact that the axis for head rotation during head-pursuit and also during passive head and whole-body rotation (target stationary in space, Fig. 1C–E, VOR ×1) was shifted from the axis for eye rotation (see Methods; Collewijn et al. 1982). This residual gaze-velocity component must have been induced by smooth-pursuit and it may have contributed to the discharge modulation (Fig. 1C–E). To minimize smooth-pursuit–related discharge modulation during active or passive sinusoidal head movement, we tested head-pursuit and passive head and whole-body rotation without a target in complete darkness. Representative discharge is illustrated in Fig. 6 for the same neuron shown as cell B in Figure 1. Basically similar, but slightly weaker, modulation was observed in complete darkness during head-pursuit (Fig. 1C vs. Fig. 6A) and passive head-only rotation (Fig. 1D vs. Fig. 6B) and passive whole-body rotation (Fig. 1E vs. Fig. 6C).
Figure 6.
Discharge of a single neuron during head movement in complete darkness. This neuron is the same neuron shown in Figure 1 (cell B). All traces were superimposed during head-pursuit (A), passive head-only rotation (B) and passive whole-body rotation (C) without a target stationary in space. Head-velocity (vel) and eye-velocity were clipped.
We compared discharge modulation in 56 FEF pursuit neurons during sinusoidal head-pursuit with and without a target in complete darkness. Mean (±SD) sensitivities (re-feeder velocity) of modulation were 0.49 (±0.33) and 0.38 (±0.30) sp/s/°/s, respectively. Passive whole-body rotation was tested in 63 neurons with and without a target. Mean (±SD) sensitivities (re-head-velocity) of modulation were 0.74 (±0.45) and 0.62 (±0.43) sp/s/°/s, respectively. Passive head-only rotation was also tested in 18 neurons with and without a target. Mean (±SD) sensitivities (re-head-velocity) of modulation were 0.82 (±0.39) and 0.66 (±0.43) sp/s, respectively. These results indicate that head rotation in complete darkness still induced clear discharge modulation of FEF pursuit neurons, suggesting that the residual gaze-velocity components alone cannot explain the modulation during head-pursuit and passive head and whole-body rotation (target stationary in space).
Recording Locations
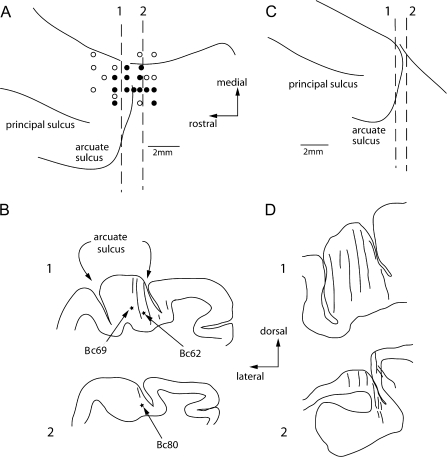
Figure 7 illustrates representative recording locations in 2 monkeys (A–B, C–D). Recordings were performed mostly in the fundus and posterior bank of the arcuate sulcus as in previous studies (MacAvoy et al. 1991; Gottlieb et al. 1993; Tanaka and Fukushima 1998; Fukushima et al. 2000; Fukushima, Yamanobe, Shinmei, Fukushima 2002; Fukushima, Yamanobe, Shinmei, Fukushima, Kurkin, et al. 2002). Neurons that modulated during gaze-pursuit and eye-pursuit (Fig. 1) were recorded mostly in the fundus of the arcuate sulcus (Fig. 7B1, D1, D2). Neurons that responded similarly during gaze-pursuit and head-pursuit (target stationary in space) but that were not modulated during eye-pursuit were recorded in the posterior bank of the arcuate sulcus (Fig. 7B2). In the present study such neurons were in the minority (10/134 = 7%), and were recorded in the recording tracks indicated by the dashed line 2 and caudally (Fig. 7A). Because these neurons did not respond to eye-pursuit, we did not search for pursuit neurons further caudally.
Figure 7.
Recording locations. (A and C) Top views of the left frontal cortex of 2 monkeys. Filled circles and open circles indicate locations of responsive neurons and unresponsive neurons during the pursuit tasks, respectively. (B and D) Transverse sections (1, 2) of representative recording tracks indicated in A (1, 2) and C (1, 2), respectively. Responding neurons were recorded mostly in the fundus of the arcuate sulcus in B1, D1, and D2. B2 is a section through the posterior bank. Two asterisks in A1 are locations of neurons that responded like those shown in Figure 1 (cells A and B). The asterisk in A2 is location of a neuron that exhibited modulation during gaze-pursuit and head-pursuit (target stationary in space) but not during eye-pursuit.
Discussion
Head Movement Commands in the Caudal Part of the FEF
This study examined the behavior of pursuit neurons in the caudal FEF when monkeys were free to move their heads to pursue visual and tactile targets. FEF pursuit neurons are known to carry command-related signals that precede the onset of eye or gaze tracking (MacAvoy et al. 1991; Gottlieb et al. 1993, 1994; Tanaka and Fukushima 1998; Fukushima et al. 2000; Fukushima, Yamanobe, Shinmei, Fukushima 2002; Fukushima, Yamanobe, Shinmei, Fukushima, Kurkin, et al. 2002; Tanaka and Lisberger 2002; Akao et al. 2005; Akao, Saito, et al. 2007). The first question here was whether FEF pursuit neurons also carry command signals that precede the onset of head tracking movements made in the absence of gaze-pursuit. Our data do not provide evidence for the presence of such head-specific commands.
The major findings are the following; 1) the majority of FEF pursuit neurons exhibited modulation during head-pursuit (target stationary in space, Fig. 1C) even without an earth-stationary target (Fig. 6A), but their preferred directions during eye-pursuit and head-pursuit were different (Figs 2C, 3A,D). 2) Discharge modulation during gaze-pursuit was well predicted by linear addition of modulation during eye-pursuit and head-pursuit (Fig. 2G,H), suggesting separate origins for the discharge modulation during eye-pursuit and head-pursuit. 3) Although peak modulation occurred during head movements, discharge onset of the majority of neurons was not aligned with the onset of head movements during head-pursuit. The discharge onset of the minority of FEF pursuit neurons that was aligned well with the onset of head movements did not precede the onset of head movements (Fig. 4). We did observe cases where a neuron began to discharge prior to movement of the head but these responses were better time-locked to onset of the feeder motion than to onset of head tracking. This suggests a contribution of somato-sensory responses induced by feeder motion to the initial discharge. The initial weak burst in Fig. 4Ba is consistent with this interpretation. The latencies to the onset of feeder motion (40–50 ms, Fig. 4A,B; shortest 19 ms) are also consistent with the latencies reported for frontal cortical neurons to tactile stimuli (Lemon 1981; Kurata and Tanji 1985). We conclude that the responses we observed prior to onset of head motion were likely related to sensory stimuli generated by the feeder's motion.
It must be noted that our results only argue against the presence of head tracking commands when gaze was intentionally held steady. They do not preclude the possibility that the gaze-tracking commands exhibited by FEF pursuit neurons might drive both eye and head components of the gaze-pursuit downstream of the FEF.
Does Feedback Related to Movement of the Head on the Trunk Account for the Behavior of FEF Pursuit Neurons?
If discharge of FEF pursuit neurons during head-pursuit (target stationary in space) does not represent a command to move the head, is it instead generated by feedback produced by the head movement? This seems likely because 1) the latency of the onset of discharge that was aligned well with the onset of head movements (≥20 ms) is consistent with the latency of vestibular responses induced by passive whole-body step rotation (Akao, Saito, et al. 2007), and 2) discharge modulation similar to that during head-pursuit was also obtained during passive head rotation on the stationary trunk (Fig. 5A–D).
To obtain the data shown in Figure 5C,D, we examined responses of the same FEF pursuit neurons during head-pursuit (target stationary in space) and during passive head rotations with the same amplitude and frequency as the feeder motion. When we took into account the actual magnitude of head-velocity during the head-pursuit movements, which were sometimes smaller than the feeder velocity, our data suggested that most if not all of the discharge of these neurons during head-pursuit could be replicated by passively driving the head (Fig. 5C,D). As shown in Figure 5A,B, the ensemble average of the responses to passive head rotation closely resembled the ensemble average of the responses during head-pursuit. After correcting for the magnitude of actual head movements relative to the feeder (and hence passive head) movements, the neuron-by-neuron correlation analysis presented in Figure 5C,D indicated that passive head rotation could account for the discharge observed during head-pursuit.
Is the Feedback Primarily due to Sensors Measuring the Movement of the Head in Space?
Previous studies have shown that FEF pursuit neurons receive vestibular signals related to head rotation in space (Fukushima et al. 2000; Akao, Saito, et al. 2007). The present results also confirm this result (Fig. 1E vs. Fig. 6C). To determine the extent to which such signals account for the responses we observed during passive rotation of the head on the stationary trunk, we measured the responses of the same neurons to passive whole-body rotation, which presents the same vestibular stimulus as does passive head rotation but without twisting of the neck (Fig. 5E,F). The correlation of neuron responses to passive head-on-trunk rotation and whole-body rotation was not as strong as one would expect if both were produced by the same (i.e. vestibular) receptors (Fig. 5F). Furthermore, although vestibular inputs resulted in clear modulation, our results indicate that there was no significant correlation in the magnitudes of discharge modulation of individual neurons during head-pursuit and passive whole-body rotation (Fig. 5H).
To further confirm the reliability of these correlations (Fig. 5C–H), we calculated confidence levels for Pearson's correlation coefficients using the bootstrap method (10 000 regressions on randomly drawn realizations of the data set) (Press et al. 1996; also Efron 1987). The results are summarized in Table 2. Narrow and non-overlapping confident intervals for correlation coefficients suggest that differences in correlation observed in Figure 5D,F,H cannot be explained by inter-neuronal variability alone. Therefore, the logical conclusion is that some factor related to twisting of the neck altered the responses to passive head-on-trunk rotation making them different from responses of the same neurons to whole-body rotation (Fig. 5F and H vs. Fig. 5D).
Table 2.
Bootstrap mean± SD, median and 95% confidence intervals for correlation coefficients estimated among the 3 tested conditions
| Sensitivity | Correlation coefficient | Bootstrap (mean ± SD) | Bootstrap median and 95% confident interval | Two sided t-test for nonzero correlation |
| Head-pursuit versus passive head-only rotation | 0.7810 | 0.7770 ± 0.0647 | (0.7385, 0.7833, 0.8227) | P < 0.0001 |
| Head-pursuit versus passive whole-body rotation | 0.4361 | 0.4288 ± 0.1463 | (0.3352, 0.4403, 0.5319) | P < 0.0001 |
| Passive head-only rotation versus passive whole-body rotation | 0.1286 | 0.1194 ± 0.1904 | (0.0121, 0.1229, 0.2522) | P < 0.5390 |
Note: The same population of 32 FEF pursuit neurons was used to calculate Pearson correlation coefficient for each pair of conditions. The correlation coefficient was calculated for 10 000 independent bootstrap samples each consisting of n = 32 paired data points drawn randomly with replacement from the analyzed data set. See text for further explanation.
Neurons in other brain regions are known to respond to rotation of the neck (Wilson et al. 1984; Gdowski and McCrea 2000). We are currently investigating the possibility that such responses are present in the frontal pursuit area. Preliminary studies indicate that many FEF pursuit neurons modulate their discharge during passive trunk rotation against the stationary head (Fukushima et al. 2007). This supports the possibility that neck proprioceptive inputs account for the divergence between responses to passive head rotation on the stationary trunk and head-pursuit (target stationary in space) observed in this study (Fig. 5D vs. Fig. 5H). A more complete analysis is the subject of a paper in preparation.
Role of Pursuit Neurons in the Caudal FEF in Head-Free Gaze-Pursuit
In daily life where the head is free to move, eye and head movements are coordinated during tracking of a moving visual target. As described in the Introduction, there have been 2 competing hypotheses for the neural mechanisms of the eye-head coordination; one proposes an integrated gaze command driving both eye and head movements, and the other proposes independent eye and head commands together with different coordinating mechanisms such as vestibular feedback (for reviews, see Chen 2006; Leigh and Zee 2006).
Single neuron- and electrical stimulation- studies have suggested involvement of the FEF in saccadic gaze shifts that consist of rapid eye and head movements (Bizzi and Schiller 1970; Tu and Keating 2000; Knight and Fuchs 2007; see however, Chen 2006). In these studies, the head movements elicited by electrical stimulation of the FEF are directed to the side contralateral to the stimulus, consistent with preferred directions of FEF saccade neurons. Lesion studies (van der Steen et al. 1986; Keating et al. 1997) also suggest the involvement of the FEF in coordination of rapid gaze shifts that are composed of head and eye movements (also Guitton and Mandl 1978 in cats).
It is well known that, unlike FEF saccade neurons, the preferred directions of individual pursuit neurons in the caudal FEF are distributed evenly for all directions (MacAvoy et al. 1991; Gottlieb et al. 1993, 1994; Tanaka and Fukushima 1998; Fukushima et al. 2000). Our conclusion based on the present results that FEF pursuit neurons do not carry head-pursuit commands could therefore reflect a difference in the way the FEF is involved in generating rapid saccadic gaze shift on one hand (Tu and Keating 2000; Knight and Fuchs 2007) and slow gaze-pursuit on the other.
This difference may reflect the uniqueness of the pursuit system that is used for precise control of smooth eye movements to maintain a target image on the fovea during movement in order to facilitate continuous processing of visual information about the moving target. The pursuit system must work during head and/or whole-body rotation. Head motion signals during active head movements could be obtained using head movement commands. However, unlike the oculomotor system, the head motor system must deal with a wide variety of external loads. As a result, during eye-head coordination, the head movement trajectory exhibits a wide variability (see Leigh and Zee 2006 for review), suggesting that head command signals alone do not provide the accurate measure of head movement that is needed for maintaining target image on the high acuity fovea during eye-head tracking. Actual head motion could be precisely detected by vestibular and neck proprioceptive inputs as discussed above. The present results suggest that FEF pursuit neurons issue gaze-pursuit commands that do not include commands to move the head independent of gaze and that the head-pursuit–related modulation reflects primarily reafferent signals resulting from head movements. Thus, with regard to the above 2 competing hypothesis on the neural mechanisms of eye-head coordination, our results support an eye or gaze command at the level of the caudal FEF (Fig. 1, cell A and Fig. 2A,B) together with afferent feedback mechanisms resulting from the head movement component of gaze shifts (Fig. 5C,D, also Akao, Kumakura, et al. 2007; Akao, Saito, et al. 2007; Fukushima et al. 2007).
However, we must note that our paradigm examined neuronal discharge during head movements in the absence of gaze shifts. Parallel studies have not been attempted in the saccadic portion of the FEF. It may be that there, too, discharge accompanying gaze shifts (saccades in this case) can drive both eye or head movements downstream but that such commands are only generated when the behavior is intended to shift gaze. Recently, Walton et al. (2007) have shown that 26% of classic gaze-related burst neurons in the superior colliculus exhibited significant changes in average firing rate in association with head movements in the absence of gaze shifts, suggesting that the superior colliculus plays a role in the control of head movements independent of gaze shifts.
In head-restrained monkeys, not only gaze-velocity signals but also eye-velocity (or eye/head-velocity) signals are represented during passive whole-body rotation in many brain areas related to smooth-pursuit including the caudal FEF (see Leigh and Zee 2006 for a review; also Lisberger and Fuchs 1978; Miles et al. 1980; Belton and McCrea 1999, 2000a, 2000b; Fukushima et al. 1999, 2000; Shinmei et al. 2002). In particular, the gaze-velocity related discharge modulation of FEF pursuit neurons was predicted by the linear sum of vestibular and smooth-pursuit–related modulation (Akao, Saito, et al. 2007). Pursuit neurons in the caudal FEF in head-free pursuit in the present study also exhibited a wide range of modulation from a gaze-velocity type (Fig. 1, cell A, Fig. 3) to eye/head-velocity type (Fig. 1, cell C). The present results suggest that both groups of FEF pursuit neurons issue primarily eye- or gaze-pursuit commands during gaze-pursuit and that the head-pursuit–related modulation reflects primarily reafferent signals resulting from head movements. To further elucidate how these signals are integrated for the precise control of pursuit eye movements during head movements, future studies must characterize neck proprioceptive responses of FEF pursuit neurons. Experiments are now in progress.
Funding
Grant-in-Aid for Scientific Research on Priority Areas (system study on higher-order brain functions) (17022001) and (18300130) from MEXT of Japan.
Acknowledgments
We thank Dr C.R.S. Kaneko for his valuable comments on the early version of the manuscript. Mr Hiroshi Saito participated in some experiments. Conflict of Interest: None declared.
References
- Akao T, Kumakura Y, Kurkin S, Fukushima J, Fukushima K. Directional asymmetry in vertical smooth-pursuit and cancellation of the vertical vestibulo-ocular reflex in juvenile monkeys. Exp Brain Res. 2007;182:469–478. doi: 10.1007/s00221-007-1005-1. [DOI] [PubMed] [Google Scholar]
- Akao T, Kurkin S, Fukushima J, Fukushima K. Visual and vergence eye movement related responses of pursuit neurons in the caudal frontal eye fields to motion-in-depth stimuli. Exp Brain Res. 2005;164:92–10. doi: 10.1007/s00221-004-2213-6. [DOI] [PubMed] [Google Scholar]
- Akao T, Saito H, Fukushima J, Kurkin S, Fukushima K. Latency of vestibular responses of pursuit neurons in the caudal frontal eye fields to whole body rotation. Exp Brain Res. 2007;177:400–410. doi: 10.1007/s00221-006-0682-5. [DOI] [PubMed] [Google Scholar]
- Belton T, McCrea RA. Contribution of the cerebellar flocculus to gaze control during active head movements. J Neurophysiol. 1999;81:3105–3109. doi: 10.1152/jn.1999.81.6.3105. [DOI] [PubMed] [Google Scholar]
- Belton T, McCrea RA. Role of the cerebellar flocculus region in cancellation of the VOR during passive whole body rotation. J Neurophysiol. 2000a;84:1599–1613. doi: 10.1152/jn.2000.84.3.1599. [DOI] [PubMed] [Google Scholar]
- Belton T, McCrea RA. Role of the cerebellar flocculus region in the coordination of eye and head movements during gaze pursuit. J Neurophysiol. 2000b;84:1614–1626. doi: 10.1152/jn.2000.84.3.1614. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Schiller PH. Single unit activity in the frontal eye fields of unanesthetized monkeys during eye and head movement. Exp Brain Res. 1970;10:151–158. doi: 10.1007/BF00234728. [DOI] [PubMed] [Google Scholar]
- Chen LL. Head movements evoked by electrical stimulation in the frontal eye field of the monkey: evidence for independent eye and head control. J Neurophysiol. 2006;95:3528–3542. doi: 10.1152/jn.01320.2005. [DOI] [PubMed] [Google Scholar]
- Collewijn H, Conjin P, Tamminga EP. Eye-head coordination in man during the pursuit of moving targets. In: Lennerstrand G, Zee DS, Keller EL, editors. Functional basis of ocular motility disorders. Oxford (United Kingdom): Pergamon Press; 1982. pp. 369–385. [Google Scholar]
- Efron B. Better bootstrap confidence intervals. J Am Statist Assoc. 1987;82:171–185. [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movements chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Akao T, Saito H, Kurkin S, Fukushima J, Barry W Peterson. Neck proprioceptive signals in pursuit neurons in the frontal eye fields (FEF) of monkeys. Program No. 398.5. Abstract Viewer/Itinerary Planner. San Diego (CA): Society for Neuroscience; 2007. Washington. [Google Scholar]
- Fukushima K, Fukushima J, Kaneko CRS, Fuchs AF. Vertical Purkinje cells of the monkey floccular lobe: simple-spike activity during pursuit and passive whole body rotation. J Neurophysiol. 1999;82:787–803. doi: 10.1152/jn.1999.82.2.787. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Kasashara S, Akao T, Kurkin S. Discharge of pursuit neurons in frontal eye fields during active head movements. Program No. 166.6. Abstract Viewer/Itinerary Planner. Washington (DC): Society for Neuroscience; 2005. Washington. [Google Scholar]
- Fukushima K, Sato T, Fukushima J, Shinmei Y, Kaneko CRS. Activity of smooth pursuit-related neurons in the monkey periarcuate cortex during pursuit and passive whole body rotation. J Neurophysiol. 2000;83:563–587. doi: 10.1152/jn.2000.83.1.563. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Yamanobe T, Shinmei Y, Fukushima J. Predictive responses of peri-arcuate pursuit neurons to visual target motion. Exp Brain Res. 2002;145:104–120. doi: 10.1007/s00221-002-1088-7. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Yamanobe T, Shinmei Y, Fukushima J, Kurkin S, Peterson BW. Coding of smooth eye movements in three-dimensional space by frontal cortex. Nature. 2002;419:157–162. doi: 10.1038/nature00953. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, McCrea RA. Neck proprioceptive inputs to primate vestibular nuclear neurons. Exp Brain Res. 2000;135:511–526. doi: 10.1007/s002210000542. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, MacAvoy MG, Bruce CJ. Neural responses related to smooth pursuit eye movements and their correspondence with electrically elicited slow eye movements in the primate frontal eye field. J Neurophysiol. 1994;72:1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- Guitton D, Mandl G. Frontal ‘oculomotor’ area in alert cat. I. Eye movements and neck activity evoked by stimulation. Brain Res. 1978;149:295–312. doi: 10.1016/0006-8993(78)90477-8. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kasahara S, Akao T, Fukushima J, Kurkin S, Fukushima K. Further evidence for selective difficulty of upward eye pursuit in young monkeys: effects of optokinetic stimulation, static roll tilt, and active head movements. Exp Brain Res. 2006;171:306–321. doi: 10.1007/s00221-005-0278-5. [DOI] [PubMed] [Google Scholar]
- Keating EG, Chopra S, Tu T. Peri-arcuate lesions alter head and eye components of gaze shifts. Soc Neurosci Abstr. 1997;23:474. [Google Scholar]
- Knight TA, Fuchs AF. Contribution of the frontal eye field to gaze shifts in the head-unrestrained monkey: effects of microstimulation. J Neurophysiol. 2007;97:618–634. doi: 10.1152/jn.00256.2006. [DOI] [PubMed] [Google Scholar]
- Kurata K, Tanji J. Contrasting neuronal activity in supplementary and precentral motor cortex of monkeys 2: Responses to movement triggering vs. nontriggering sensory signals. J Neurophysiol. 1985;53:142–152. doi: 10.1152/jn.1985.53.1.142. [DOI] [PubMed] [Google Scholar]
- Lanman J, Bizzi E, Allum J. The coordination of eye and head movement during smooth pursuit. Brain Res. 1978;153:39–53. doi: 10.1016/0006-8993(78)91127-7. [DOI] [PubMed] [Google Scholar]
- Leigh R, Zee DS. The neurology of eye movements. 4th ed. New York: Oxford University Press; 2006. [Google Scholar]
- Lemon RN. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. J Physiol. 1981;311:497–519. doi: 10.1113/jphysiol.1981.sp013601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger S, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex 1: Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978;41:733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- Miles FA, Fuller DJ, Braitman DJ, Dow BM. Long-term adaptive changes in primate vestibuloocular reflex 3: Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol. 1980;43:1437–1476. doi: 10.1152/jn.1980.43.5.1437. [DOI] [PubMed] [Google Scholar]
- Miles FA, Fuller JH. Visual tracking and the primate flocculus. Science. 1975;189:1000–1003. doi: 10.1126/science.1083068. [DOI] [PubMed] [Google Scholar]
- Miles FA, Lisberger SG. The “error” signals subserving adaptive gain control in the primate vestibulo-ocular reflex. Ann NY Acad Sci. 1981;374:513–525. doi: 10.1111/j.1749-6632.1981.tb30896.x. [DOI] [PubMed] [Google Scholar]
- Press WH, Teukolsky SA, Vettering WT, Flannery BP. Numerical recipes in C. 2nd ed. Cambridge (MA): Cambridge University Press; 1996. [Google Scholar]
- Shinmei Y, Yamanobe T, Fukushima J, Fukushima K. Purkinje cells of the cerebellar dorsal vermis in the monkey: simple-spike activity during pursuit and passive whole body rotation. J Neurophysiol. 2002;87:1836–1849. doi: 10.1152/jn.00150.2001. [DOI] [PubMed] [Google Scholar]
- Singh A, Thau GE, Raphan T, Cohen B. Detection of saccades by a maximum likelihood ratio criterion. Proc 34th Annu Conf Eng Biol (Houston, TX). 1981:136. [Google Scholar]
- Tanaka K, Fukushima K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J Neurophysiol. 1998;80:28–47. doi: 10.1152/jn.1998.80.1.28. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. I. Basic response properties to retinal image motion and position. J Neurophysiol. 2002;87:2684–2699. doi: 10.1152/jn.2002.87.6.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Lynch JC. Functionally defined smooth and saccadic eye movement subregions in the frontal eye field of Cebus monkeys. J Neurophysiol. 1996;76:2740–2771. doi: 10.1152/jn.1996.76.4.2740. [DOI] [PubMed] [Google Scholar]
- Tu TA, Keating G. Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J Neurophysiol. 2000;84:1103–1106. doi: 10.1152/jn.2000.84.2.1103. [DOI] [PubMed] [Google Scholar]
- van der Steen J, Russel IS, James GO. Effects of unilateral frontal eye-field lesions on eye-head coordination in monkey. J Neurophysiol. 1986;55:696–714. doi: 10.1152/jn.1986.55.4.696. [DOI] [PubMed] [Google Scholar]
- Walton MMG, Bechara B, Gandhi NJ. Role of the primate superior colliculus in the control of head movements. J Neurophysiol. 2007;98:2022–2037. doi: 10.1152/jn.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VJ, Ezure K, Timerick SJB. Tonic neck reflex of the decerebrate cat: response of spinal interneurons to natural stimulation of neck and vestibular receptors. J Neurophysiol. 1984;51:567–577. doi: 10.1152/jn.1984.51.3.567. [DOI] [PubMed] [Google Scholar]