Abstract
Human motor skills can be acquired by observation without the benefit of immediate physical practice. The current study tested if physical rehearsal and observational learning share common neural substrates within an action observation network (AON) including premotor and inferior parietal regions, that is, areas activated both for execution and observation of similar actions. Participants trained for 5 days on dance sequences set to music videos. Each day they physically rehearsed one set of dance sequences (“danced”), and passively watched a different set of sequences (“watched”). Functional magnetic resonance imaging was obtained prior to and immediately following the 5 days of training. After training, a subset of the AON showed a degree of common activity for observational and physical learning. Activity in these premotor and parietal regions was sustained during observation of sequences that were danced or watched, but declined for unfamiliar sequences relative to the pretraining scan session. These imaging data demonstrate the emergence of action resonance processes in the human brain based on observational learning without physical practice and identify commonalities in the neural substrates for physical and observational learning.
Keywords: dance, mirror neuron system, motor learning, parietal, premotor
Introduction
Many avenues exist for learning new skills. For example, learning to flamenco dance could be achieved by following a verbal description of where, when, and how to move through space. Alternatively, a dance sequence could be learned by following step patterns traced on the floor, by trial and error, or by following a dancer who knows the movements and performing alongside this individual. Behavioral research on action learning suggests that the final option, simultaneously observing and reproducing the correct pattern of movements, results in the quickest and most accurate learning (e.g., Sheffield 1961; Schmidt 1975; Bandura 1977, 1986; Blandin et al. 1999; Blandin and Proteau 2000; Badets et al. 2006). Nevertheless, the ability to improve by observation alone, without concurrent practice, is a powerful capacity of humans (Mattar and Gribble 2005; Torriero et al. 2007). The current study investigated the contribution of passive observation to acquiring novel movement sequences. Using functional neuroimaging, we aimed to characterize the neural underpinnings of observational learning with and without the added benefit of physical practice.
Early behavioral investigations of observational learning by Sheffield (1961) led to the proposal that observation of a motor sequence improved learning by means of providing a “perceptual blueprint,” or a standard of reference for how the task should be performed. Behavioral studies comparing observational and physical learning supported the value of a perceptual blueprint (Zelaznik and Spring 1976; Doody et al. 1985; Carroll and Bandura 1990; Lee et al. 1990; Blandin and Proteau 2000). Although the bulk of observational learning research has focused on learning from an expert human model (see Hodges et al. 2007, for a review), the use of a human actor performing the target behavior is not a requirement for forming a perceptual blueprint. A more inclusive conceptualization of observational learning encompasses encoding any instruction, whether physical or symbolic, that can provide a sufficient model of the to-be-performed actions (e.g., Cisek and Kalaska 2004). The key distinction of what defines observational learning is not the type of instruction; rather, it is defined by the subject not performing concurrent physical practice at the time instructions are provided.
Evidence from recent nonhuman primate neurophysiology as well as psychophysical and electromyographic (EMG) work with humans suggests that physical and observational learning might share common neural substrates (Cisek and Kalaska 2004; Mattar and Gribble 2005). Cisek and Kalaska recorded extracellular spike activity from single neurons within the dorsal premotor cortex (PMd) of monkeys while they observed the sensory events associated with accurate performance of a saccadic motor task. They reported that when the monkeys passively observed a symbolic representation of accurate task performance, PMd neurons responded in a similar manner as when the monkeys physically performed the task. These authors suggest that such findings are congruent with the notion of mental rehearsal at the single neuron level.
In a study using psychophysical and EMG measures with human participants, Mattar and Gribble (2005) demonstrated that participants' learning of a novel, complex motor task was facilitated if they previously observed another individual learning to perform that same task, compared with watching another individual perform the task without learning or learning to perform a different task. This established the specificity of a perceptual blueprint formed during the observational period on subsequent physical practice. What emerges from these studies and others (Barzouka et al. 2007; Bouquet et al. 2007) is the hypothesis that observation of movement sequences and physical rehearsal share common cognitive mechanisms and training of this circuit by either means improves novel motor skill learning.
The studies presented above provide a foundation for exploring areas of overlap and divergence between observational and physical learning in the human brain. However, it is difficult to determine from behavioral procedures alone the degree of correspondence between cognitive and neural processes serving these 2 types of learning. Functional neuroimaging enables us to explore this question further by determining whether observational and physical learning modify similar or distinct neural regions. In the current study, we investigate this hypothesized overlap of cognitive and neural mechanisms for observational and physical learning through concomitant use of behavioral and neuroimaging procedures. We hypothesized that observational and physical learning would engage comparable cognitive and neural processes. This would be demonstrated by performance improvements on a behavioral task and similar patterns of brain activity for both types of learning. In particular, it is hypothesized that physical rehearsal and observational learning both modulate activity in areas known to show corresponding activity for execution and observation of similar actions. Conversely, the emergence of different areas of neural activity for physical and observational learning would imply that distinct cognitive processes underlie these 2 types of learning.
We investigated observational learning by training novice dancers to perform complex dance sequences while manipulating the presence or absence of concurrent physical practice. Due to the complexity and unfeasibility of having participants actually perform dance sequences in the scanner (but see Brown et al. 2006), we instead chose to train participants to perform the movement sequences with videos outside the scanner, and asked them to observe the training videos during the scanning sessions.
A growing body of evidence indicates that action observation during functional magnetic resonance imaging (fMRI) can be used as a surrogate marker for studying the neural systems involved in physical skill. Numerous studies have demonstrated that action observation paradigms can be used to characterize substrates for action understanding and action learning (e.g., Decety and Grèzes 1999; Brass et al. 2000; Buccino et al. 2001; Grèzes and Decety 2001; Rizzolatti and Craighero 2004; Frey and Gerry 2006). These experiments identify a distinct set of brain regions that are active both when observing and when performing actions, referred to as the “mirror neuron system” (MNS) or, more broadly, the “action observation network” (AON). The AON encompasses a broader network of neural regions involved in visual analysis of action as well as areas involved in visuomotor and sequence learning (e.g., Jenkins et al. 1994; Sakai et al. 2002; Grèzes et al. 2003). In the current paper emphasis is given to the AON over the MNS because this term is more general and encompasses all of the brain regions involved in action observation processes, rather than those exclusively engaged for observation and execution (e.g., inferior parietal and premotor cortices). In the present study, we define the AON empirically by comparing observation of movement sequences to a resting baseline before participants have any training experience with the stimuli.
Past imaging studies have demonstrated the feasibility of using dance learning and observation as a paradigm for investigating the properties of the AON (Calvo-Merino et al. 2005; Calvo-Merino et al. 2006; Cross et al. 2006). The experimental work with dancers most relevant to the present investigation is a study by Calvo-Merino and colleagues that examined the influence of visual compared with motor experience on AON activity during action observation (Calvo-Merino et al. 2006). In order to parse visual familiarity from physical experience, expert men and women ballet dancers observed videos of movements learned only by their sex, only by the opposite sex, or moves that are performed by all dancers. The motivation behind this procedure was to determine whether equally robust action resonance processes may be elicited by observation of movements that are equally visually familiar, because men and women dancers train together, but unequal in terms of physical experience. The authors reported that when effects of visual familiarity are controlled for (i.e., when dancers watched moves from their own movement repertoire, compared with moves that they frequently saw, but never physically performed), evidence for action resonance based on pure motor experience was found in inferior parietal, premotor, and cerebellar cortices. The authors concluded that actual physical experience is a necessary prerequisite for robust activation in these areas of the AON. Despite this interpretation, participants in this study observed only movements that were familiar (either physically or visually). Therefore, it is not possible to draw the strong conclusion that physical experience is necessary to build an action representation—only that physical experience results in relatively greater recruitment of the AON than visual experience alone.
Three prior dance studies (Calvo-Merino et al. 2005, 2006, Cross et al. 2006) provide robust evidence for changes within the AON with the presence (or emergence) of action embodiment after subjects gain physical experience, as evidenced by performance competency. The current study builds upon this foundation by addressing whether observational learning alone can lead to response changes within the AON, thus broadening the capacity of the AON to include learning from observation. To that end, we assess how training via physical and observational measures influences AON activity. Critically, the present study includes 2 control conditions: a pretraining fMRI scan using the same stimuli as the post-training scan, and a set of unfamiliar movement sequences never viewed during training that were only viewed during scanning sessions. These controls enable us to assess general training effects and the specificity of these effects to familiar dance sequences, respectively. We predict that both physical and observational training will lead to increased activity within the AON, as evidenced by increased activity within these regions after a week of training.
Materials and Methods
Participants
Seventeen physically and neurologically healthy adults were recruited from the Dartmouth College undergraduate and graduate student community to participate in 1 week of dance training and 2 scans (1 pretraining scan and 1 post-training scan—illustrated in Fig. 1). All were monetarily compensated for their involvement. The local ethics committee approved all components of this study. Participants had no significant prior experience playing dance video games. One participant was excluded from analyses due to an aberrant learning trajectory for behavioral training (>2 standard deviations different from the group mean performance score). The remaining 16 participants (10 female) ranged in age from 20 to 32 years (mean age = 25 years). All participants were strongly right handed as measured by the Edinburgh Handedness Inventory (Oldfield 1971).
Figure 1.
Schematic depiction of the 4 phases of this study, chronologically arranged. All participants completed 2 fMRI sessions, the 5 consecutive days of behavioral training, and the surprise behavioral retest.
Stimuli and Apparatus
Stimuli were 21 upbeat techno-dance songs with no lyrics (120–180 beats per minute [BPM]; mean = 149.6 BPM). Unknown songs were chosen (and verified as “unknown” by postexperiment interviews) so that participants would have no prior auditory or dance experience with any portion of the stimuli. Songs were edited into 30-s segments and individualized files comprising dance steps synchronized to the music were created using the Dancing Monkeys MATLAB script (O'Keefe 2003). This script analyzes each song to determine where and when the steps should occur in order to be tightly synchronized with the music on whole, half and quarter beats. Each 30-s segment was then triplicated, resulting in 21 stimuli, each 1 min 30 s in length, and each containing 3 identical repeats of a song and step-sequence pairing.
Songs were then paired with visual cues instructing the participant how to dance to that track. A scrolling display of arrows moving upwards across the screen cued each move, and the participant had to make the indicated step when the arrows reached the top of the screen (Fig. 2A). The symbolic arrow sequences were generated for all 21 tracks. In addition, for 11 of these video stimuli, the instruction stimulus consisted of a video of an expert dancer accurately performing the steps with the arrows superimposed over it, providing both human and symbolic cues (Fig. 2, upper row).
Figure 2.
(A) Representation of the 3 (training experience: danced, watched, or untrained) by 2 (action cue: person with arrows, or just arrows) study design. (B) Layout of training apparatus. On a dance pad connected to a computer, participants step on arrows arranged in the 4 cardinal directions (front, back, right, and left) in time with arrow cues on the screen.
Each participant physically trained on 3 human tracks and 3 arrow tracks (henceforth referred to as the “danced” condition), for a total of 6 different dance sequences, every day for 5 days. Participants also passively observed an additional 6 different tracks (3 humans and 3 arrows) on the same schedule (the “watched” condition). Six of the remaining tracks were presented only during fMRI scanning and composed the “untrained” condition. The final 3 tracks were never seen at any point during scanning or training, and were used as entirely novel stimuli in the surprise retest portion of the study. This design is illustrated in Figure 1.
Study Design
The experimental conditions fall into a 3 (training experience: danced, watched or untrained) by 2 (cue type: symbolic arrows plus human model, symbolic arrows without human model) factorial design, illustrated in Figure 2A. In the present study, we compare the danced, watched and untrained conditions collapsed across cue type (results evaluating differences between the human model and symbolic cues conditions will be reported elsewhere; Cross et al. unpublished data). During scanning, subjects viewed the 6 trained sequences that composed the “danced” condition, the 6 trained sequences that composed the “watched” condition, and the 6 untrained sequences that composed the “untrained” condition. Note, however, that during the first week of imaging, all 18 sequences that were observed during scanning were novel to the participants.
Neuroimaging
Neuroimaging Procedure
A block design fMRI procedure was used to localize the putative AON in the particular group of subjects for this study and in response to the particular stimuli we used (week 1, pretraining scan) and to identify neural responses to dance sequences that were physically trained, passively observed, or unfamiliar (week 2, post-training scan). During functional imaging, participants watched and listened to the same 18 StepMania dance sequences they were about to train on for a week (pretraining scan) or which they had viewed and practiced during the week of training (post-training scan). Instructions were to simply observe the videos and to keep as still as possible. In order to rule out the potential of action network activation as a consequence of participants tapping their feet or moving their bodies in the scanner along with the videos, participants were video recorded during both scanning sessions and the videos were evaluated offline for any foot or body movement. All participants were able to remain still within the normally acceptable range throughout each scan. Each 30-s video was followed by 30 s of fixation. Video order was counterbalanced across conditions (danced, watched, and untrained), participants, and scanning sessions. Participants saw the same 18 videos in both the week 1 pretraining session and the week 2 post-training scanning session. Following the second scanning session, all participants were asked whether they engaged in any kind of mental imagery during either scanning session. Each participant reported engaging in mental imagery during the second scanning session only, and many of them reported being especially eager to dance along with the sequences that they had physically rehearsed.
The experiment was carried out in a 3T Philips Intera Achieva scanner using an 8 channel phased array coil and 30 slices per time repetition (TR) (3.5-mm thickness, 0.5 mm gap); TR: 1988 ms; time echo (TE): 35 ms; flip angle: 90°; field of view: 24 cm; matrix 80 × 80. For each of 3 functional runs, the first 2 brain volumes were discarded, and the following 181 volumes were collected and stored.
Neuroimaging Analyses
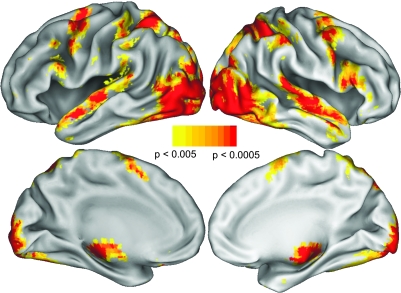
The neuroimaging analyses were designed to achieve 3 objectives. First, we wanted to determine the precise neural regions that composed a subject- and task-specific AON for this study. Within a mask of the regions identified from this contrast, we then sought to identify neural regions that are the same between the danced and watched conditions, and also neural regions that differ between the danced and watched conditions. To remove sources of noise and artifact, functional data were realigned, unwarped and normalized to the MNI template with a resolution of 3 × 3 × 3 mm in SPM2. Following this, 8 mm smoothing was applied to the images. A design matrix was fitted for each subject, with each type of video modeled as a boxcar function convolved with the standard hemodynamic response function. Covariates of noninterest (a session mean, a linear trend, and 6 movement parameters derived from realignment corrections) were included in the design. SPM2 was used to compute parameter estimates (beta) and contrast images (containing weighted parameter estimates) for each comparison at each voxel. To achieve our first imaging objective, we identified brain regions within the AON in an unbiased manner through a contrast that evaluated all video observation conditions relative to baseline for the week 1 (pretraining) scan. This contrast was used as a mask for all remaining contrasts reported in this paper at the P < 0.005, k = 5 level false discovery rate (FDR) corrected, and is illustrated Figure 3.
Figure 3.
Neural regions active in the contrast comparing all conditions (videos to be danced, watched and untrained) > baseline for the pretraining scan (week 1). This contrast was made to determine, in an unbiased, subject- and task-specific manner, which regions were to be included in the mask of the AON.
In order to identify neural regions that are similar between physical and observational learning, we calculated 2 independent sets of contrast images: one for the main effect of physical training (danced > untrained) and another for the main effect of observational training (watched > untrained). Both contrasts evaluated imaging data from the post-training (week 2) scan session only. Contrast images for all participants across all main effects were taken to the second level for random effects analysis. We report regions that survive the masking procedure discussed above and a voxelwise threshold of P < 0.005 uncorrected and a cluster size of 5 voxels for each contrast.
To further explore areas of overlap between the danced and watched conditions, region of interest (ROI) analyses were performed on regions that emerged from a conjunction analysis for the danced and watched conditions. The conjunction analysis selected regions where both the danced > untrained and watched > untrained contrasts passed the P < 0.005 and k = 5 voxels threshold. This was done to evaluate the effects of training among those regions in the AON that showed the greatest degree of overlap between these 2 contrasts. Beta-estimates were extracted from a sphere with a 3 mm radius centered on the peak voxel from 2 ROIs for all 3 training conditions. The beta-estimates were then evaluated with a repeated-measures ANOVA to investigate differences between the individual training conditions.
The next analysis we performed was a correlation between training benefits afforded by 5 consecutive days of physical practice (calculated as day 5 dance score—day 1 dance score) and activation within the 2 ROIs identified by the conjunction analysis detailed above. The objective of this analysis was to determine if a strong relationship existed between individual rates of learning and bold signal when observing the trained sequences, and how this relationship might compare for physical and observational learning. Across participants, improvement on the danced sequences was correlated with the post-training danced > untrained contrast and improvement on the watched sequences was correlated with the post-training watched > untrained contrast. In order to determine differences in neural activity between the danced and watched conditions, we also evaluated the direct contrasts of danced > watched and watched > danced at the P < 0.001 uncorrected and k = 5 voxels threshold. For visualization purposes, the t-image for the AON mask is displayed on partially inflated cortical surfaces using the PALS data set and Caret visualization tools (Fig. 3; http://brainmap.wustl.edu/caret). To most clearly illustrate the conjunction analysis, the t-images from the danced > untrained and watched > untrained contrasts are visualized on a rendered cortical surface of a standard brain from the Montreal Neurological Institute (MNI) (Fig. 5). The surviving activated voxels from the ROI analyses and the direct contrasts between the danced and watched conditions are displayed on a high-resolution structural MRI scan of a standard brain (Figs 6 and 8; MNI).
Figure 5.
Direct comparison of dance training contrast (danced > untrained; in red) and passive observation contrast (watched > untrained; in blue), rendered on the same average brain images to facilitate comparison. Voxels of overlap between the 2 contrasts are visible in yellow.
Figure 6.
ROI analyses examining the main effect of training, t = 2.95, P < 0.005, k = 5 voxels; masked by contrast: week 1 task > baseline (P < 0.005, FDR). Activations are rendered on a standard brain from the MNI.
Figure 8.
Contrast comparing danced > watched activity, P < 0.001, uncorrected; k = 5 voxels; masked by contrast: week 1 task > baseline (P < 0.005, FDR).
Behavioral Training and Evaluation
Behavioral Procedure
StepMania (www.stepmania.com), a freeware program similar to the popular video game Dance Dance Revolution (Konami Digital Entertainment, Inc., Redwood City, CA), was used for step file modification, training, and response recording outside of the scanner. Participants performed dance training and post-test evaluations on a 3′ × 3′ dance pad connected by USB to a desktop computer (Fig. 2B). Electronic sensors in the dance pad detected position and timing information that was then used to provide participants with real-time visual feedback. If participants struck the correct foot position within ±45 ms of the arrow reaching its target at the top of the computer screen, they received feedback saying “Perfect.” If they stepped on the correct arrow between 46 and 90 ms after the arrow appeared in position on the screen, the feedback read “Great,” between 91 and 135 ms, feedback read “Good,” and between 136 and 180 ms, they received feedback that read “Boo.” If participants stepped on the wrong arrow or if they stepped on the correct arrow after 181 ms had elapsed, the feedback read “Miss.” The goal of each dance was to earn as many “Perfect” marks and as few “Miss” marks as possible. StepMania recorded participants' performance, and presented participants with a summary score and letter grade corresponding to performance after each song.
Each dance sequence comprised 90 steps. A final numeric score was calculated by assigning 2 points for every “Perfect” step, 1 point for every “Great” step, 0 points for every “Good” step, −1 point for every “Boo” step, and −2 points for every “Miss” step. The maximum possible score for each sequence (if the entire dance was performed perfectly) was 180 points. Additionally, it was possible for 2 arrow cues to appear simultaneously (e.g., a left and a right arrow), in which case the participant would have to jump and land on both cues at the same time in order to achieve a correct response. In each dance sequence there were 6 such jumps. Controlling for the number of steps and jumps in each sequence and the tempo of each song (described above) ensured that the range of difficulty was matched across conditions. Between the 2 fMRI sessions, participants spent 5 days learning to perform the steps associated with 6 songs. Song assignments for each condition were randomized across participants, and the order in which participants watched or danced each song was counterbalanced across days.
Behavioral Retest Procedure
Following the second and final scanning session, participants were asked to return to the lab for a surprise follow-up test that involved dancing a total of 12 StepMania songs. In order to keep the cognitive and physical requirements of this follow-up task similar to what was required during the week of training, participants danced only 3 of the 6 songs they trained to dance, 3 of the 6 songs they watched during training, and 3 of the 6 songs that were in their untrained group of stimuli. The 3 sequences from each of these groups were randomly selected for each participant. In addition, participants were asked to perform 3 entirely novel sequences (i.e., participants had never before these sequences before during scanning or training). Participants were asked to perform sequences from both the untrained and novel conditions so we could determine whether they had learned any parts of the untrained sequences they observed twice in the scanner, compared with sequences that were truly novel. The overall objective of this behavioral retest measure was to evaluate whether passive observation for an equivalent amount of time as active physical rehearsal influenced performance ability compared with performing untrained or novel songs.
Results
Behavioral Training Results
Participants performance was measured by their ability to step on the indicated dance pad positions precisely in time with the arrows and music. Performance on the rehearsed dance sequences improved across days, F1.72,25.86 = 58.25, P < 0.0001 (Fig. 4A). This indicates that participants were learning to embody the dance sequences effectively through physical practice during the week of training.
Figure 4.
Mean dance performance across training day (A) and dance retest performance (B). Participants' performance improved as the days progressed, and retest performance was best for the dance sequences that were physically rehearsed the week prior. Error bars represent standard error of the mean. Maximum possible score is 180 points, corresponding to perfectly timed steps for each training sequence.
Behavioral Retest Results
For the postscanning dance retest, scored in the same manner as the dance training procedure, results from a repeated-measures ANOVA on the 4 training conditions (danced, watched, untrained and novel) demonstrated a main effect of prior training experience on performance, F3,45 = 4.72, P = 0.006 (Fig. 4B). Pairwise comparisons revealed significant differences between danced and untrained sequences (P < 0.001) and between danced and novel sequences (P < 0.001), but not danced and watched (P = 0.143) or watched and untrained or novel sequences (P values = 0.249 and 0.321, respectively). Because performance was so similar between the untrained and novel sequences, we focus the discussion of postscan dancing data only on differences between stimuli that were danced, watched, and untrained. Results from a repeated-measures ANOVA on just these 3 conditions still revealed a main effect of training experience, F1.5,21.27 = 4.91, P = 0.027, and this effect is best captured with a linear test of the within-subjects contrast, F1,15 = 26.56, P < 0.0001. Together, this demonstrates a pattern of monotonic descent, with participants performing the best on sequences they danced, an intermediate level on those they watched, and the poorest on untrained sequences.
Imaging Results
As discussed above, there were 3 objectives to the imaging analyses. The first was to determine which neural regions are active when participants observe music videos of dance sequences before they ever attempt to perform those sequences. This contrast, evaluated as task > fixation baseline for the week 1, pretraining scan, revealed broad activation in a network that included areas classically associated with action observation (including parietal, premotor, supplementary motor, and superior temporal areas), and other areas that are associated with visual analysis, including primary through higher level visual cortices. This contrast is illustrated in Figure 3 and was used as a mask for the analyses described below.
Similarities between Physical and Observational Learning
The next set of imaging analyses focused on using data from the post-training session to identify brain regions that demonstrated a significant main effect of dance training and those that demonstrated a significant main effect of observational learning, calculated within the AON mask. Analyses were performed comparing the relative blood oxygenation level–dependent (BOLD) responses while participants watched and listened to the set of videos in the danced or watched conditions compared with the untrained condition. A direct contrast revealed a significant effect of physical training (danced > untrained; P < 0.005, uncorrected), collapsed across cue type, in several areas of the AON (masking procedure described above), including right premotor and primary motor areas and several regions of right prefrontal cortex (red colors of Fig. 5, and Table 1). With a separate set of analyses we sought to determine whether any of the brain regions within the AON mask demonstrated a greater response to videos in the watched condition relative to the untrained condition. Figure 5 illustrates the main effect of this type of learning (watched > untrained; blue colors; P < 0.005, uncorrected) rendered on the same brain image as the effects of physical training. As this figure illustrates, there was a high degree of correspondence between neural regions activated by observational training (watched condition) and by physical learning (danced condition).
Table 1.
| Region | BA | MNI coordinates |
Functional name | t-value | P value | ||
| x | y | z | |||||
| Effects of physical training: danced > untrained (post-training) | |||||||
| R precentral gyrus | 4 | 48 | 0 | 63 | M1 | 5.08 | <0.001 |
| R middle frontal gyrus | 9 | 39 | 12 | 33 | MFC/DLPFC | 4.86 | <0.001 |
| R inferior frontal gyrus | 44/45 | 36 | 24 | −6 | IFG | 4.31 | <0.001 |
| R middle temporal gyrus | 21 | 51 | −24 | −6 | MTG | 4.31 | <0.001 |
| R precentral gyrus | 6 | 39 | −3 | 51 | Premotor cortex** | 4.28 | <0.001 |
| R inferior frontal gyrus | 45 | 51 | 33 | 9 | IFG | 3.89 | 0.001 |
| R anterior mid. frontal gyrus | 10 | 45 | 57 | 3 | DLPFC | 3.76 | 0.001 |
| L anterior cerebellum | −3 | −39 | −42 | 3.58 | 0.001 | ||
| L inferior frontal gyrus | 45/46 | −54 | 33 | 21 | IFG | 3.56 | 0.001 |
| R inferior frontal gyrus | 44 | 48 | 12 | 18 | IFG | 3.20 | 0.003 |
| L IPL | 40 | −33 | −51 | 39 | IPL ** | 3.19 | 0.003 |
| R premotor cortex | 57 | 9 | 48 | PMv | 3.15 | 0.003 | |
| Effects of passive observation: watched > untrained (post-training) | |||||||
| L putamen | −12 | 0 | −3 | 5.07 | <0.001 | ||
| R inferior frontal gyrus | 45 | 33 | 27 | −6 | IFG | 4.96 | <0.001 |
| L anterior inf. frontal gyrus | 10 | −51 | 42 | −3 | IFG | 4.92 | <0.001 |
| R middle frontal gyrus | 10 | 42 | 57 | 9 | DLPFC | 4.18 | <0.001 |
| R precentral gyrus | 6 | 36 | 6 | 51 | Premotor cortex** | 4.14 | <0.001 |
| L temporal pole | 38 | −30 | 33 | −33 | 4.01 | 0.001 | |
| L pars opercularis | 44 | −42 | 12 | 27 | 3.73 | 0.001 | |
| L intraparietal sulcus | 40 | −36 | −51 | 36 | IPL** | 3.67 | 0.001 |
| R posterior cerebellum | 9 | −81 | −30 | 3.63 | 0.001 | ||
| R inferior frontal gyrus | 47 | 42 | 39 | −27 | VLPFC | 3.55 | 0.001 |
| L intraparietal sulcus | 7/40 | −36 | −63 | 51 | IPS/IPL | 3.43 | 0.002 |
| R inferior frontal gyrus | 45 | 51 | 24 | 18 | IFG | 3.30 | 0.002 |
| Effects of physical compared with observational training: danced > watched (post-training) | |||||||
| R middle frontal gyrus | 6 | 54 | 6 | 48 | Premotor cortex—PMd | 3.91 | 0.001 |
| R precentral gyrus | 6 | 51 | −3 | 51 | Premotor cortex—PMd | 3.56 | 0.001 |
Note: Significance at all sites for each contrast was tested by a one-sample t-test on beta values averaged over each voxel in the cluster, P < 0.005, uncorrected. Coordinates are from the MNI template and use the same orientation and origin as found in the Talairach and Tournoux (1988) atlas. BA: Brodmann's area; R: right; L: left; IPS: intraparietal sulcus; M1: primary motor cortex; MFG: middle frontal gyrus; IFG: inferior frontal gyrus; MTG: middle temporal gyrus; VLPFC: ventrolateral prefrontal cortex. The double asterix (**) denotes the peaks of the clusters examined in the ROI analyses of the overlapping activations from the danced and watched conditions (Fig. 6).
A conjunction analysis performed on the t-maps of these 2 contrasts in MATLAB enabled us to determine precisely which voxels overlapped between the danced > untrained and watched > untrained contrasts, using the logical and construction. The 2 regions that demonstrated the largest areas of overlap were the right premotor cortex (centered on the MNI coordinates; x = 39, y = 3, z = 43) and left inferior parietal lobule (IPL) (x = −35, y = −50, z = 39). The extent of overlap between the danced and watched contrasts was 324 mm3 in the premotor region, and 162 mm3 in the inferior parietal region. Parameter estimates were extracted from these 2 regions of overlap for the post-training scanning session, and are illustrated in the lower plots of in Figure 6. Results from a repeated-measures ANOVA for the premotor parameter estimates revealed a main effect of training, F2,30 = 6.827, P = 0.004, whereas pairwise comparisons between the training conditions revealed a significant difference between the danced and untrained conditions (P = 0.011) and the watched and untrained conditions (P = 0.001), but not between the danced and watched conditions (P = 0.667). The same ANOVA performed on the parameter estimates for the IPL region also demonstrated a significant effect of training experience, F2,30 = 6.80, P = 0.004, and here as well, the pairwise comparisons revealed significant differences between the danced and untrained (P = 0.008) and watched and untrained conditions (P = 0.009), but no differences between the danced and watched conditions (P = 0.252). These results illustrate that these 2 regions in particular did not differentiate between videos that were danced or watched, but certainly responded more strongly to videos that had been trained in some manner compared with videos that were untrained and only observed in the scanner.
Correlations between Training Benefits, Physical, and Observational Learning
The next set of analyses quantified the relationship between the benefits of physical practice and activity levels within the left IPL and the right premotor ROIs. When participants' training benefit scores were correlated with their individual parameter estimates from the IPL ROI, no relationship emerged between these variables (R = 0.0006). However, when participants training benefit scores were correlated with activity level in the right premotor region, a significant negative correlation emerged, R = −0.60, P = 0.015. This suggests that participants who improved the most across the week of training had the least amount of activation in this region of right premotor cortex (Fig. 7). To characterize this further, we ran one additional correlation on these data comparing day 5 scores (participants' highest scores) with bold signal in this premotor area. Interestingly, this exploratory analysis revealed a significant positive correlation, R = 0.624, P = 0.01, which suggests that participants who danced the sequences the best had the most activity in this premotor region when viewing these sequences after training. Taken together, these 2 correlations show that participants who were the best overall performers did not improve as much across the week of physical rehearsal. The correlations with BOLD activity in right premotor cortex capture the competency and learning effects, supporting a role for this area in learning. When we correlated the learning scores from the watched condition, calculated as: (performance on the sequences that were watched from the surprise dance retest) − (day 1 of training dance scores) with the magnitude of activation for the watched > untrained stimuli, no significant effect appeared for either the IPL or premotor site (all R values < 0.03). This suggests that the significant relationship we found between premotor activity and performance change is specific to physical rehearsal and embodiment.
Figure 7.
Correlation between improvement in dance score across week (training gain) and percent signal change in the danced compared with the untrained conditions for the post-training scanning session for the premotor ROI illustrated in Figure 6.
Differences between Physical and Observational Learning
A third set of imaging analyses directly evaluated patterns of activity between the danced and the watched conditions, significant at the P < 0.001 uncorrected, k = 5 voxels threshold. From the danced > watched comparison, greater activity was observed in the right precentral gyrus and right middle frontal gyrus (Fig. 8, Table 1). The inverse contrast, watched > danced, did not reveal any regions within our AON mask that survived the same thresholds. This pattern of findings is consistent with the notion that physical practice engages select components of the AON above and beyond passive observation.
Discussion
The primary aim of the present study was to evaluate similarities and differences in neural activity and behavioral performance induced by physical training compared with passive observation. Our data demonstrate robust activity within the AON, specifically premotor and inferior parietal regions, when observing movement sequences that have either been physically rehearsed or passively observed across 1 week of training, relative to observing untrained sequences. These results provide empirical evidence to link the rich history of behavioral investigations of observational learning with neuroimaging work, and they also inform our understanding of the sensitivity of the AON to different types of training. In particular, our behavioral data provide evidence that passive observation of complex movement sequences is associated with better performance on subsequent testing compared with untrained sequences, and observation of these sequences recruits similar patterns of neural activity as sequences learned by physical rehearsal.
The Benefits of Observation on Action Performance
The present findings advance the behavioral literature on observational learning by providing evidence that passive observational learning and active physical practice engage several neural regions in a similar manner in response to motion cues. This finding addresses Blandin and Proteau's (2000) suggestion that observational and physical practice engage similar neural processes through demonstration that observing sequences experienced previously through physical training or watching recruits similar neural substrates. Our results extend prior behavioral investigations on participants' ability to learn movement sequences from observation (Heyes and Foster 2002; Vinter and Perruchet 2002; Kelly et al. 2003; Bird et al. 2005) by demonstrating that beyond increasing familiarity with the movement sequences, other elements of the task are learned from observation as well, such as the timing of the sequences.
It is especially remarkable that similar patterns of neural activity emerge within several key areas of the AON when participants observe sequences from the danced and watched conditions because participants were never instructed to intentionally learn the observed sequences for later performance. This finding is in accord with what Mattar and Gribble (2005) report from their behavioral and EMG study on observational learning. One of their experimental conditions directly examined the role of conscious strategies in observational learning by requesting participants to perform an arithmetic task that also taxed working memory while observing videos of another individual performing the task. Mattar and Gribble report that participants' performance benefited from the observation task, even when attentional and cognitive systems were engaged in the arithmetic task. We believe that our finding of an intermediate level of performance for observed dance sequences provides further evidence that observational learning can occur even when attention is not focused on learning from observing. On the other hand, we do not disagree with Hodges et al. (2007) that observers' knowledge of the task and intentions to accurately reproduce the observed movement does significantly influence the benefits drawn from observation. Along these lines, we would predict better performance on our behavioral retest task had we told participants to study the sequences for later performance. Future studies could easily manipulate participants' intention to learn from observation to decisively address this question.
In addition, we observed that activity in the premotor ROI during the post-training scanning session was negatively correlated with the degree of performance benefit achieved across the week of training (but positively correlated with participants' day 5 dance performance scores). This same relationship between BOLD signal and physical performance was not present when participants observed the videos from the watched condition. This suggests 2 important points. First, during the post-training scan, we see that the amount of right premotor cortex activity in participants who gained a lot from training, while increased from pretraining levels, still is not as great as it is in those participants who are better dancers on the last day of training. It is important to note that participants who were the least proficient at performing the sequences during the first day of training improved greatly across training, but they still did not achieve dance scores that were as high as those participants who started off with higher scores and whose scores improved, but to a lesser degree. In other words, we see that activity in this region of the premotor cortex captures both competency and learning related aspects of the dancing skill. Secondly, our findings suggest this relationship is specific to physical learning, and does not generalize to observational learning. Activity in the IPL, our other ROI, did not show any relationship between signal intensity and performance for either the danced or watched conditions. Thus, our data imply that the right premotor cortex is specifically involved in action embodiment achieved by physical rehearsal. Moreover, this area is driven more strongly in the most proficient dancers, a finding complimentary to previous work in our laboratory performed with expert contemporary dancers (Cross et al. 2006).
Observation and the AON
Although it is by now well established that a strong degree of correspondence exists between neural activity when performing or observing an action (Grèzes and Decety 2001; Rizzolatti and Craighero 2004), this study provides evidence for the construction of similar neural representations within the AON for physically rehearsed and passively observed movement sequences. One hypothesis about the core purpose of the AON is that it exists to facilitate new motor learning through imitation (Meltzoff 2002; Meltzoff and Prinz 2002). Although the evidence in support of this hypothesis is abundant, the present findings suggest that this system is not simply hardwired for immediate imitation, but it is highly sensitive to the longer-term effects of physical and perceptual experience as well.
This idea is further supported by 2 recent studies, one employing fMRI (Bird et al. unpublished data) and the other a transcranial magnetic stimulation (TMS) study (Catmur et al. 2007; Bird et al. unpublished data). In both studies, the authors endeavored to manipulate responses within the AON based on congruent and incongruent training procedures, such as teaching participants to move the foot when observing a hand moving. Using between-groups designs, both studies found that participants who had trained in the incongruent response group had “reversed” responses within the AON, such that areas more active during execution of hand than foot movements were now less active when participants observed hand movements (compared with foot movements). The authors concluded that this system is not hardwired, but rather quite flexible based on perceptual experience and motor experience (Catmur et al. 2007; Bird et al. unpublished data). The present findings are consistent with these 2 previous studies in that neural activity while observing dance sequences that were physically trained or passively observed is greater than activity when watching the untrained sequences after training across several regions of the AON. This suggests that components of the AON do not generalize completely across cues for new motor skills, and that actual experience shapes the response of this system when presented with familiar action sequences.
Although we might have predicted increases in activity within more areas of the AON following a week of training, the present pattern of results is still consistent with the interpretation that the AON is sensitive both to observational and physical training. In particular, the effect of repetition suppression is well-documented, in which familiar stimuli elicit less brain activation upon repeated presentations than they do when initially presented (Wiggs and Martin 1998; Henson and Rugg 2003; Wig et al. 2005). Given this common phenomenon, it could have been predicted that during the post-training scan the untrained sequences would elicit the most activation of any condition due to their relative novelty as compared with the other 2 conditions. Instead, the finding that this control condition showed a substantial decrease in activity from pretraining to post-training scans likely reflects the level of general familiarity with the paradigm context, including the look of the display, the timbre of the music, and the expectation of arrows and movement on the screen. Therefore, the result that the highly familiar danced and watched stimuli elicited a greater response in the AON relative to an unfamiliar and untrained dance and music sequence is compelling evidence that this network is preferentially tuned to embodied action cues.
It is of interest to note that we did not see activation in the SMA region of our AON mask in the post-training analyses. We did expect to see activity here after training, because this is a region associated with action embodiment and preparation (e.g., Grèzes and Decety 2001). Indeed, when we performed a whole-brain exploratory analysis on the danced > untrained and watched > untrained contrasts from week 2 (also at the P < 0.005, k = 5 levels), we did see activity in SMA/pre-SMA in a region just anterior to that covered by our mask. It is important to recall that the mask was created by a video > fixation baseline comparison from the pretraining scan session, indicating regions that are responsive specifically to action observation apart from any personal experience with the movements. That a region of SMA becomes active for the danced and watched conditions relative to the unfamiliar control only after training would appear to indicate that this region responds specifically to experience with the depicted movement sequence. This brain activity, as well as the modulation of activity in the masked regions of the AON discussed above, may be related to the subjective experience of mental imagery in which participants reported engaging during the familiar sequences in the post-training session.
Beyond the findings reported in the core regions of the AON, we see 2 other noteworthy regions with complimentary patterns of neural activity in the danced > untrained and watched > untrained contrasts. These 2 regions are located in the dorsolateral prefrontal cortex (DLPFC) and the cerebellum. The finding of similar patterns of neural activity in the DLPFC for observational and physical learning is in accord with recent TMS data by Torriero and colleagues that implicates this region and the left lateral cerebellum as critically involved in observational learning (Torriero et al. 2007). These authors discuss how these 2 regions work together to facilitate observational learning, with the cerebellum assisting with procedural learning of the motor sequence elements while the DLPFC enables flexible recall and adaptation of previously learned movement sequences. Interestingly, the cerebellum was implicated in both observational and physical learning in the present study as well, but distinct regions were activated according to the 2 kinds of experience. To return to Figure 5, physical learning was associated with greater activity in the left/midline anterior cerebellum, whereas observational learning was associated with activation in the right posterior cerebellum. The former site overlaps strongly with cerebellar cortex that is activated for a broad range of sensorimotor tasks and may reflect simulation within motor related circuits (e.g., Grafton et al. 2008). In contrast, the posterior cerebellar activation may represent simulation by other circuits including the DLPFC. This would support the idea that physical rehearsal leads to simulation in circuits more closely associated with action execution.
Further support for the role of DLPFC in observational learning comes from a study on imitation learning (in this case, learning to play guitar chords from watching a model) by Buccino et al. (2004). These authors reported activations in a similar region of prefrontal cortex when participants observed a guitar chord to imitate it or simply executed a chord of choice. Buccino et al. suggest that these similar patterns of activity in this region of the prefrontal cortex during observation and physical execution are likely driven by the selection of movement sequences necessary for task performance. Such an account of DLPFC activation is in accord with the present task and our pattern of findings that implicate this region's involvement in both physical and observational learning.
In terms of what our results contribute to the extant literature on dance representation in the brain, these latest findings are in agreement with prior dance neuroimaging research from this lab (Cross et al. 2006) and others (Calvo-Merino et al. 2005; Brown et al. 2006), suggesting that the AON, particularly parietal and premotor regions, is modulated by experience. However, an interesting distinction emerges between our data and recent findings reported by Calvo-Merino et al. (2006) on observational compared with physical experience. In their study, they report quantifiably stronger responses within inferior parietal and premotor areas when dancers observed movements they physically rehearsed compared with movements that were observed only. They conclude that pure motor expertise is represented in these neural regions, and that visual expertise or observation alone is not sufficient to drive activity in these areas. However, when studying dancers who have trained over many years as part of their careers, it is difficult to determine whether observational and physical experience were actually equal.
The present study provides a more exact control of observational and physical experience for several reasons. First, by including 2 extra control conditions (the use of a pretraining scan and an untrained condition separate from the danced and watched conditions), we are able to gather more precise information about participants' experience with stimuli observed in the scanner, such as which regions respond to the stimuli before participants have even attempted to dance one step. Our results also provide an interesting counterpoint to those of Calvo-Merino et al.'s (2006) through our demonstration that precisely equivalent time on task for physical rehearsal and passive observation can result in activation of similar neural substrates (Figs 5 and 6). However, the pattern of results illustrated by the direct contrasts between the danced and watched conditions is mostly in agreement with what Calvo-Merino et al. (2006) report. Similar to these researchers, we found greater activity in 2 regions of right premotor cortex, roughly corresponding to the PMd. A plausible interpretation of this pattern of activity is that this region is responsible for linking the specific motor sequences to the visual signals, which physical rehearsal enables. Such an interpretation is in agreement with data from single-unit recordings within monkey PMd and ventral premotor cortex (PMv) (Hoshi and Tanji 2006). We believe that the fact that we did not find nearly as broad a network as that reported by Calvo-Merino et al. (2006) in our direct comparison between the danced and untrained conditions suggests that a more rigidly controlled observational experience might lead to patterns of activity that are more similar between the physically and observationally experienced actions.
Potential Limitations
One potential limitation that must be considered in light of the present results is due to the nature of employing a within-subjects design, where all participants physically rehearsed several sequences, passively observed a different set of sequences, and had no training experience with a third set of sequences. A valid criticism of using a within-subjects design to study observational learning is that, in such an experimental set up, observational learning does not occur in a purely observational context. In other words, observational learning is not strictly compartmentalized, and could be ameliorated based on practice of similar sequences during each training day. It could be the case that certain parts of the StepMania task generalized well from the danced condition to the watched and untrained conditions, and it is certainly true that participants were more familiar with the sequences they performed or observed each day compared with those they did not. Classic behavioral studies of observational learning avoid this confound by employing between-groups experimental designs so that the observational learning group does not benefit from any practice of a similar task (e.g., Blandin and Proteau 2000).
Keeping in mind the limitations of a within-subjects experimental design, we are still able to address the possibility that any observational learning effects are simply due to generalization or increased familiarity with the task parameters across conditions. If it were the case that the task parameters learned in the danced condition generalized uniformly to the watched and the untrained conditions, we would predict that neural areas associated with action embodiment (AON areas) would show similar patterns of activity across training conditions not only during the pretraining (week 1) scan, but also during the post-training (week 2) scan. In other words, we would not expect to see differentiation between the 3 training conditions after the week of training if task parameters learned from the danced sequences generalized to both the watched and untrained conditions. Revisiting the parameter estimates plotted in Figure 6, we see that this is not the case. What these plots reveal is a relative decrease in activity in the untrained condition between pre- and post-training scans in this baseline control task, potentially reflecting long-term repetition suppression effects (Vuilleumier et al. 2005; Meister et al. 2007). In contrast, the other 2 conditions do not reflect a relative decrease in activity in these particular ROIs between pre- and post-training scans, a finding that is possibly indicative of action embodiment or learning that is exclusive to the danced and watched conditions.
The post-training behavioral retest data further address the potential confound of generalization. For the behavioral retest task, participants were asked to dance sequences from 3 distinct conditions for which they had no physical training: the watched, untrained, and novel conditions (Fig. 4B). The behavioral data show a pattern of monotonic descent that tracks with predicted embodiment, where physically trained sequences are performed better than watched sequences, which are performed better than untrained or novel sequences. This suggests that if generalization of task parameters did occur in our manipulation, it did so only at a basic level (such as left arrow means step left), and consequently did not help participants to perform all sequences equally well after 1 week of physical rehearsal and observation.
Our data enable us to further examine the degree to which generalization of task parameters occurred. Participants' performance on the “untrained” and “novel” dance sequences (Fig. 4B) compared with their performance on the first day of training (“day 1” bar of Fig. 4A) clearly indicates that they performed the untrained and novel sequences better after 5 days of dance training than they performed the danced sequences after the first day of training. Therefore, although it can be argued that all learning generalizes to a certain degree, a notable feature of the present findings is the commonality of neural areas active for danced and watched movement sequences. What is common between these 2 conditions is the retrieval of sequential knowledge, timing, and physical anticipation of upcoming postural/positional requirements.
Another possible limitation could be that participants paid more attention to those sequences with which they were familiar while being scanned, and such differences in attention could be partially driving our results. We do acknowledge that the larger activation in the premotor and parietal areas for the danced and watched conditions, compared with the untrained condition, might be at least partially driven by effects of increased familiarity with these stimuli. However, we believe this to be a less plausible explanation of the present results, because the localizations reported here are not consistent with attention as typically defined (e.g., Fan et al. 2005; Ikkai and Curtis 2007; Slagter et al. 2007). In more detailed response to this issue, it should also be noted that abundant support exists in the literature to suggest that AON embodiment only occurs if there has been physical rehearsal (e.g., Calvo-Merino et al. 2005, 2006; Brown et al. 2006; Cross et al. 2006). Revisiting the contrast directly comparing danced to watched sequences, we see evidence of this in our task as well, in that physical rehearsal resulted in greater activation of 2 regions within the PMd when participants observed sequences they had extensively physically practiced. This suggests that physical and observational experience were not manifest in an identical manner, and that equal familiarity with the sequences did not result in equivalent patterns of neural activity while observing the sequences from these 2 conditions.
Conclusions
In sum, this paper demonstrates that the several of the neural regions composing the AON respond to both observational and physical learning. Moreover, it is possible to achieve new action learning from passive observation, without instructing participants to learn the movements they are watching. Future studies could attempt to address the limitation introduced by using a within-subjects design by combining methodologies from the present study and the Calvo-Merino et al. (2006) study to examine de novo observational learning in different groups of participants. Future work could also endeavor to determine how manipulating different parameters of the observational condition (such as instructions, watching another subject perform live compared with watching a video of performance, or time between observation and test of observational learning) influences how observational learning is represented in the brain and how it influences behavior. In addition, the present work could serve as a point of departure for exploration of observational learning-based techniques for rehabilitation from neurological or physical injury (e.g., Johnson-Frey 2004; Celnik et al. 2006).
Funding
Dana Foundation grant; US Department of Health and Human Services public health service grant (NS33504) to S.T.G.; and National Institutes of Health national research service award (F31-NS056720) to E.S.C.
Acknowledgments
We would like to thank B. Russ for helpful comments on an earlier draft of this manuscript, George Wolford for his excellent statistical advice, and 3 anonymous reviewers for their insightful suggestions. Conflict of Interest: None declared.
References
- Badets A, Blandin Y, Shea CH. Intention in motor learning through observation. Q J Exp Psychol. 2006;59:377–386. doi: 10.1080/02724980443000773. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social learning theory. Englewood Cliffs (NJ): Prentice-Hall; 1977. [Google Scholar]
- Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs (NJ): Prentice-Hall; 1986. [Google Scholar]
- Barzouka K, Bergeles N, Hatziharistos D. Effect of simultaneous model observation and self-modeling of volleyball skill acquisition. Percept Mot Skills. 2007;104:32–42. doi: 10.2466/pms.104.1.32-42. [DOI] [PubMed] [Google Scholar]
- Bird G, Osman M, Saggerson A, Heyes C. Sequence learning by action, observation and action observation. Br J Psychol. 2005;96:371–388. doi: 10.1348/000712605X47440. [DOI] [PubMed] [Google Scholar]
- Blandin Y, Lhuisset L, Proteau L. Cognitive processes underlying observational learning of motor skills. Q J Exp Psychol Hum Exp Psychol. 1999;52A:957–979. [Google Scholar]
- Blandin Y, Proteau L. On the cognitive basis of observational learning: development of mechanisms for the detection and correction of errors. Q J Exp Psychol A. 2000;53:846–867. doi: 10.1080/713755917. [DOI] [PubMed] [Google Scholar]
- Bouquet CA, Gaurier V, Shipley T, Toussaint L, Blandin Y. Influence of the perception of biological or non-biological motion on movement execution. J Sports Sci. 2007;25:519–530. doi: 10.1080/02640410600946803. [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Wohlschlager A, Prinz W. Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cogn. 2000;44:124–143. doi: 10.1006/brcg.2000.1225. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Parsons LM. The neural basis of human dance. Cereb Cortex. 2006;16:1157–1167. doi: 10.1093/cercor/bhj057. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P. Action observation and acquired motor skills: an FMRI study with expert dancers. Cereb Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol. 2006;16:1905–1910. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Carroll WR, Bandura A. Representational guidance of action production in observational learning: a causal analysis. J Mot Behav. 1990;22:85–97. doi: 10.1080/00222895.1990.10735503. [DOI] [PubMed] [Google Scholar]
- Catmur C, Walsh V, Heyes C. Sensorimotor learning configures the human mirror system. Curr Biol. 2007;17:1527–1531. doi: 10.1016/j.cub.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Celnik P, Stefan K, Hummel F, Duque J, Classen J, Cohen LG. Encoding a motor memory in the older adult by action observation. Neuroimage. 2006;29:677–684. doi: 10.1016/j.neuroimage.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of mental rehearsal in dorsal premotor cortex. Nature. 2004;431:993–996. doi: 10.1038/nature03005. [DOI] [PubMed] [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST. Building a motor simulation de novo: Observation of dance by dancers. Neuroimage. 2006;31:1257–1267. doi: 10.1016/j.neuroimage.2006.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Grèzes J. Neural mechanisms subserving the perception of human actions. Trends Cogn Sci. 1999;3:172–178. doi: 10.1016/s1364-6613(99)01312-1. [DOI] [PubMed] [Google Scholar]
- Doody SG, Bird AM, Ross D. The effect of auditory and visual models on acquisition of a timing task. Hum Mov Sci. 1985;4:271–281. [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Frey SH, Gerry VE. Modulation of neural activity during observational learning of actions and their sequential orders. J Neurosci. 2006;26:13194–13201. doi: 10.1523/JNEUROSCI.3914-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Schmitt PS, Van Horn J, Diedrichsen J. Neural substrates of visuomotor learning based on improved feedback control and prediction. Neuroimage. 2008;29:1383–1395. doi: 10.1016/j.neuroimage.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Mapp. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Armony JL, Rowe J, Passingham RE. Activations related to “mirror” and “canonical” neurones in the human brain: an fMRI study. Neuroimage. 2003;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Heyes CM, Foster CL. Motor learning by observation: evidence from a serial reaction time task. Q J Exp Psychol A. 2002;55:593–607. doi: 10.1080/02724980143000389. [DOI] [PubMed] [Google Scholar]
- Hodges NJ, Williams AM, Hayes SJ, Breslin G. What is modeled during observational learning? J Sports Sci. 2007;25:531–545. doi: 10.1080/02640410600946860. [DOI] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. Cortical activity time locked to the shift and maintenance of spatial attention. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm171. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: a study with positron emission tomography. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH. Stimulation through simulation? Motor imagery and functional reorganization in hemiplegic stroke patients. Brain Cogn. 2004;55:328–331. doi: 10.1016/j.bandc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Kelly SW, Burton AM, Riedel B, Lynch E. Sequence learning by action and observation: evidence for separate mechanisms. Br J Psychol. 2003;94:355–372. doi: 10.1348/000712603767876271. [DOI] [PubMed] [Google Scholar]
- Lee TD, White MA, Carnahan H. On the role of knowledge of results in motor learning: exploring the guidance hypothesis. J Mot Behav. 1990;22:191–208. doi: 10.1080/00222895.1990.10735510. [DOI] [PubMed] [Google Scholar]
- Mattar AA, Gribble PL. Motor learning by observing. Neuron. 2005;46:153–160. doi: 10.1016/j.neuron.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Meister IG, Buelte D, Sparing R, Boroojerdi B. A repetition suppression effect lasting several days within the semantic network. Exp Brain Res. 2007;183:371–376. doi: 10.1007/s00221-007-1051-8. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. Elements of a developmental theory of imitation. In: Meltzoff AN, Prinz W, editors. The imitative mind: development, evolution, and brain bases. Cambridge (UK): Cambridge University Press; 2002. pp. 19–41. [Google Scholar]
- Meltzoff AN, Prinz W, editors. The imitative mind: development, evolution, and brain bases. Cambridge (UK): Cambridge University Press; 2002. [Google Scholar]
- O'Keefe K. Computer engineering. London: Imperial College; 2003. Automated analysis of music for creation of dance tracks; p. 320. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ramnani N, Passingham RE. Learning of sequences of finger movements and timing: frontal lobe and action-oriented representation. J Neurophysiol. 2002;88:2035–2046. doi: 10.1152/jn.2002.88.4.2035. [DOI] [PubMed] [Google Scholar]
- Schmidt RA. A schema theory of discrete motor skill learning. Psychol Rev. 1975;82:225–260. [Google Scholar]
- Sheffield FD. Theoretical consideration in the learning of complex sequential task from demonstration and practice. In: Lumsdaine AA, editor. Student response in programmed instruction. Washington (DC): National Academy of Sciences—National Research Council; 1961. [Google Scholar]
- Slagter HA, Giesbrecht B, Kok A, Weissman DH, Kenemans JL, Woldorff MG, Mangun GR. fMRI evidence for both generalized and specialized components of attentional control. Brain Res. 2007;1177:90–102. doi: 10.1016/j.brainres.2007.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. The what and how of observational learning. J Cogn Neurosci. 2007;19:1656–1663. doi: 10.1162/jocn.2007.19.10.1656. [DOI] [PubMed] [Google Scholar]
- Vinter A, Perruchet P. Implicit motor learning through observational training in adults and children. Mem Cognit. 2002;30:256–261. doi: 10.3758/bf03195286. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S, Duhoux S, Dolan RJ, Driver J. Selective attention modulates neural substrates of repetition priming and “implicit” visual memory: suppressions and enhancements revealed by FMRI. J Cogn Neurosci. 2005;17:1245–1260. doi: 10.1162/0898929055002409. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat Neurosci. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Zelaznik H, Spring J. Feedback in response recognition and production. J Mot Behav. 1976;8:309–312. doi: 10.1080/00222895.1976.10735087. [DOI] [PubMed] [Google Scholar]