Abstract
Duchenne muscular dystrophy (DMD) is the most common, lethal, muscle-wasting disease of childhood. Previous investigations have shown that muscle macrophages may play an important role in promoting the pathology in the mdx mouse model of DMD. In the present study, we investigate the mechanism through which macrophages promote mdx dystrophy and assess whether the phenotype of the macrophages changes between the stage of peak muscle necrosis (4 weeks of age) and muscle regeneration (12 weeks). We find that 4-week-old mdx muscles contain a population of pro-inflammatory, classically activated M1 macrophages that lyse muscle in vitro by NO-mediated mechanisms. Genetic ablation of the iNOS gene in mdx mice also significantly reduces muscle membrane lysis in 4-week-old mdx mice in vivo. However, 4-week mdx muscles also contain a population of alternatively activated, M2a macrophages that express arginase. In vitro assays show that M2a macrophages reduce lysis of muscle cells by M1 macrophages through the competition of arginase in M2a cells with iNOS in M1 cells for their common, enzymatic substrate, arginine. During the transition from the acute peak of mdx pathology to the regenerative stage, expression of IL-4 and IL-10 increases, either of which can deactivate the M1 phenotype and promote activation of a CD163+, M2c phenotype that can increase tissue repair. Our findings further show that IL-10 stimulation of macrophages activates their ability to promote satellite cell proliferation. Deactivation of the M1 phenotype is also associated with a reduced expression of iNOS, IL-6, MCP-1 and IP-10. Thus, these results show that distinct subpopulations of macrophages can promote muscle injury or repair in muscular dystrophy, and that therapeutic interventions that affect the balance between M1 and M2 macrophage populations may influence the course of muscular dystrophy.
INTRODUCTION
Duchenne muscular dystrophy (DMD) results from mutation of dystrophin, a membrane-associated structural protein in striated muscle (1). Loss of functional dystrophin causes weakening of the muscle cell membrane (2), resulting in muscle cell damage and necrosis that lead to muscle wasting and finally to death of the afflicted. However, much of the muscle injury that occurs in dystrophin-deficiency is attributable to secondary damage caused by an immune response to dystrophic muscle, rather than mechanical damage to the weakened muscle per se. The complex response includes a cellular immune response in which cytotoxic T-lymphocytes (CTLs) promote muscle apoptosis by the release of perforin in the dystrophic muscle, and an innate response in which muscle damage is attributable in part to invading neutrophils and mast cells, as well as an increase in muscle cytolysis and muscle fibrosis that are mediated by the release of major basic protein from eosinophils (3–8). Despite the complexity of the inflammatory infiltrate, macrophages are the primary inflammatory cell type involved in dystrophinopathy, and their depletion from mdx mice, a genetic model for DMD, reduces muscle cell necrosis by 80% at early stages of the disease (9). Thus, macrophages play a primary role in the pathogenesis of muscular dystrophy.
The importance of inflammation in promoting dystrophinopathies likely underlies many of the beneficial effects of corticosteroid treatments of DMD. In particular, prednisone and deflazacort are lead compounds in the treatment of DMD patients, and both can reduce muscle damage and slow the progressive weakness (4,10,11). Although early investigators disputed that the beneficial effects of prednisone were attributable to its effects as an immunosuppressant in DMD patients, those conclusions were largely based on the observations that azathioprine worsened the pathology of DMD (12). However, azathioprine is an anti-mitotic drug that does not specifically target inflammatory cells, and its administration would also impair the proliferation of satellite cells that would participate in muscle regeneration. More recently, prednisone has been shown to reduce the numbers of macrophages and other myeloid cell populations in dystrophic muscle and produce a concomitant reduction in muscle membrane lysis, supporting the conclusion that the beneficial effects of prednisone treatment in dystrophinopathy lie in its anti-inflammatory effects, at least in part (13).
Although corticosteroids have beneficial effects in the treatment of DMD and depletion of macrophages from the mdx mouse model of DMD reduces mdx pathology at the early stages of the disease, macrophages are a phenotypically diverse population that can promote tissue repair as well as injury (14–16). According to the macrophage nomenclature that we will use (discussed in 15), M1 macrophages are classically activated macrophages that participate in Th1 immune responses, and are capable of damaging host tissue. M2 macrophages, a more diverse group, participate in Th2 immune responses and can promote tissue repair. M2 macrophages are categorized into three sub-groups: M2a macrophages that are activated by interleukin-4 (IL-4) and IL-13, M2b macrophages that can be activated by immune complexes or toll-like receptors or M2c macrophages that can be activated by IL-10. Following muscle injury, macrophages are capable of promoting either further injury or repair, according to macrophage phenotype. For example, macrophages that are driven in vitro to a pro-inflammatory, M1 phenotype lyse muscle cells through inducible nitric oxide synthase (iNOS)-mediated processes, and macrophages that express CD68, a marker of M1 macrophages, are the first to invade injured muscle following acute injury (17–19). However, a later-invading population of M2 macrophages that express markers of M2a or M2c phenotype, such as CD163, can promote muscle growth and regeneration (18,20). Thus, according to this model of inflammation following acute muscle damage, suppression of macrophage activation or numbers at early stages of muscular dystrophy could reduce muscle damage, as has been previously demonstrated in mdx mice (9). However, suppression at later stages may have a less beneficial effect, or even a detrimental effect, if M2 macrophages that promote tissue repair are present during the subsequent, regenerative phase of mdx muscular dystrophy.
Macrophages that shift from an M1 phenotype to an M2a phenotype experience an increase in arginase expression that is concomitant with their reduction in inducible nitric oxide synthase (iNOS) expression (21,22). The down-regulation of iNOS and elevated expression of arginase reflects a major shift in the metabolism of arginine, which is the substrate for both enzymes. Within 1–2 days of tissue damage in at least some injury models, most arginine at injury sites is converted to citrulline (23), reflecting the activities of early-invading, M1 macrophages in which iNOS converts arginine to citrulline and nitric oxide (NO). However, from 3 to 15 days following tissue damage, most arginine at the injury site is hydrolyzed to ornithine and urea (23), reflecting arginase activity in M2a macrophages. These products of arginine metabolism are physiologically important in influencing the course of tissue injury and repair because an early metabolite, NO, can increase damage to the tissue while metabolites of the subsequent catabolism of arginine by arginase can increase tissue repair (24).
In the present investigation, we test the hypothesis that macrophages in dystrophin-deficient muscles shift from an M1 phenotype to an M2 phenotype during the course of the disease. We characterize the phenotype of macrophages at the acute peak of the pathology of muscular dystrophy, using the mdx mouse model. Similarly, we characterize the phenotype of macrophages that dominates the inflammatory infiltrate during tissue repair and regeneration, and assay for substrate competition between iNOS and arginase expressed by M1 and M2a macrophages. Finally, we test whether NO generated by iNOS in M1 macrophages from mdx mice lyses muscle cells in vitro and in vivo, and test whether M2 macrophages stimulated with IL-10 can promote the proliferation of satellite cells. Collectively, our findings clarify important roles that arginine metabolism by macrophages may play in the pathophysiology of muscular dystrophy, and identify potential consequences of perturbations of arginine levels or macrophage phenotype in muscular dystrophy. Furthermore, our results show that M1 macrophages that are at elevated levels in 4-week-old mdx muscle, increase muscle cell damage, and that IL-10-stimulated, M2c macrophages are capable of promoting satellite cell proliferation.
RESULTS
Dystrophic muscle contains M1 and M2 macrophages
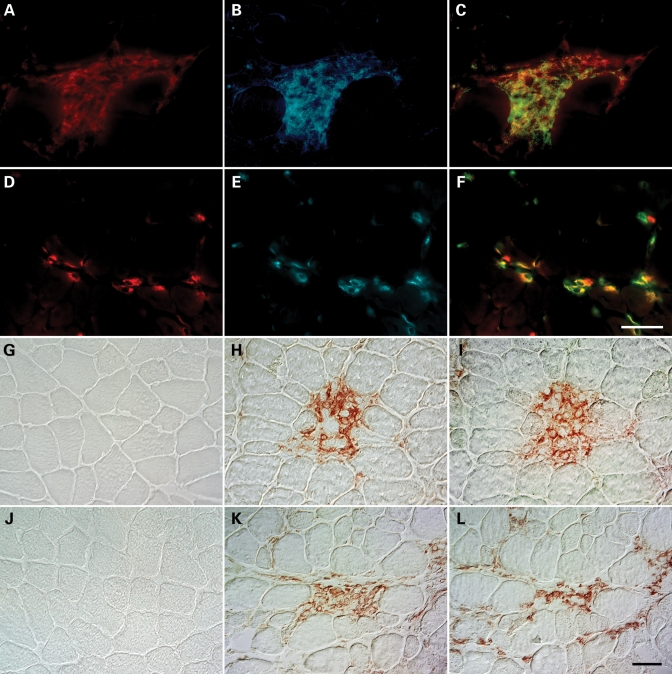
We tested whether M1 and M2 macrophages were present in dystrophic muscle by double-labeling frozen cross-sections of 4-week-old mdx quadriceps with antibodies to F4/80, a pan macrophage marker and markers of M1 (iNOS) or M2 macrophages (CD206). F4/80-expressing cells were abundant in the quadriceps of mdx mice (Fig. 1A and D). Mdx quadriceps also contained necrotic lesions with densely packed iNOS-expressing cells (Fig. 1B), and mononucleated cells that strongly expressed CD206 (Fig. 1E). Superimposed images demonstrate that iNOS and CD206-expressing cells co-expressed F4/80, indicating that M1 and M2 macrophages are present in dystrophic muscle at the acute peak of muscle pathology at 4 weeks of age (Fig. 1C and F).
Figure 1.
Th1 and Th2 responsive macrophages that express markers of M1 or M2 phenotype are present in mdx muscle. Cross-sections of 4-week-old mdx mouse quadriceps were immunolabeled with rat anti-mouse F4/80 to identify macrophage populations (A and D). The expression of iNOS (B) and CD206 (E), markers of classical and alternative activation, respectively, were simultaneously assayed by double immunofluorescence staining to determine the activation state of macrophages. Superimposed images demonstrate that macrophages undergo classical, M1 (C) and alternative, M2 activation (F) in mdx dystrophic muscle. Scale bar: 50 µm. Macrophages in mdx muscle express receptors for IFNγ and IL-4, suggesting that their activation may occur through IFNγ and IL-4-mediated signaling. Cross-sections of 4-week-old C57 (G, J) and mdx (H, I, K and L) quadriceps were immunohistochemically stained to determine the tissue distribution of IFNγR1 and IL-4R. Red reaction products, reflecting IFNγR1 (H) and IL-4R (K) expression, were noted in inflammatory cells present in necrotic lesions of mdx quadriceps, but absent in C57 quadriceps (G, J). Labeling of adjacent cross-sections with antibodies against F4/80 indicated that IFNγR1 and IL-4R-expressing cells were macrophages (I, L). Scale bar: 50 µm.
Activation of dystrophic muscle macrophages is mediated by IFNγ or IL-4 signaling
Interferon-γ (IFNγ) and IL-4 induce activation of M1 or M2a macrophages, respectively, and are expressed in DMD patients and mdx mice (25,26). Therefore, we used an immunohistochemical approach to test whether macrophages in 4-week-old mdx quadriceps express receptors for IFNγ or IL-4. Mononucleated cells in the necrotic lesions of dystrophic muscle expressed IFNγ receptor-1 (IFNγR1) (Fig. 1H) and IL-4 receptor (IL-4R) (Fig. 1K). Furthermore, the immunohistochemical staining of adjacent cross-sections with anti-F4/80 indicated that the majority of cells expressing IFNγR1 or IL-4R within these lesions were macrophages (Fig. 1I and L). Age-matched wild-type controls did not show any immunoreactivity for IFNγRI (Fig. 1G) or IL-4R (Fig. 1J).
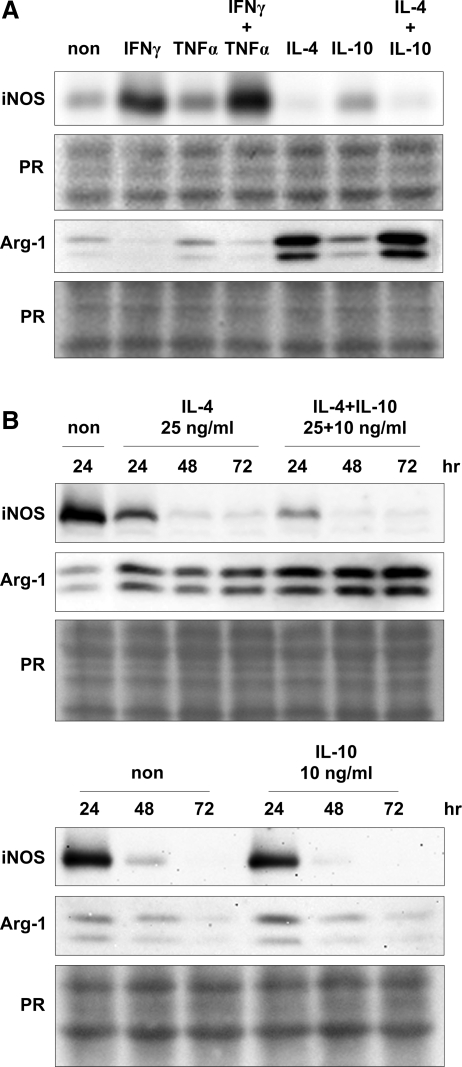
We tested whether cytokine treatment of macrophages purified from 4-week-old mdx hindlimb muscles induced activation of the M1 or M2a phenotype by assaying for changes in the expression of iNOS or arginase-1 protein. Western analysis showed that iNOS is expressed by muscle macrophages (Fig. 2), consistent with the immunofluorescent detection of iNOS-expressing macrophages in vivo (Fig. 1B). IFNγ treatment of mdx muscle macrophages caused an upregulation iNOS protein following 24 h of treatment. TNFα caused a further increase in iNOS expression when used simultaneously with IFNγ to stimulate macrophages (Fig. 2A). In contrast, treatment with IL-4 alone or IL-4 and IL-10 caused a marked reduction in iNOS expression by muscle macrophages, whereas IL-10 alone had a negligible effect after 24 h of stimulation (Fig. 2A). We also performed a time-course stimulation to test whether IL-4 or IL-10 would further decrease iNOS expression with time. IL-4 or IL-10 caused a time-dependent decrease in iNOS expression, while IL-4 and IL-10 treatment had a greater repressive effect on iNOS expression when compared with IL-4- or IL-10-alone treatment (Fig. 2B).
Figure 2.
Macrophages isolated from mdx muscle express iNOS and arginase-1, further supporting the conclusion that M1 and M2a macrophages are present in mdx muscle. Western blot shows that IFNγ induces iNOS expression in mdx muscle macrophages, which is further increased with the addition of TNFα (A, top panel). However, IL-4 reduced the expression of iNOS protein by mdx muscle macrophages (A, top panel). IFNγ reduced arginase-1 expression, while IL-4 or IL-10 increased arginase-1 expressed by mdx muscle macrophages (A, bottom panel). Representative blots from 3 to 4 independent experiments are shown. The treatment of muscle macrophages with IL-4 further decreased iNOS expression with time, and a greater repression was observed when IL-4 was used together with IL-10 (B, top panel). IL-4 treatment of muscle macrophages maximally induced arginase-1 expression by 24 h (B, bottom panel). However, arginase-1 expression was further induced by IL-4 in the presence of IL-10 with levels peaking by 48 h (B, bottom panel).
We observed a reciprocal effect of cytokine treatment when using arginase-1 as indicator of activation of the M2a phenotype. Western blots indicated that muscle macrophages express arginase-1, suggesting that macrophages isolated from 4-week-old hindlimb are a heterogeneous population that includes M1 and M2a macrophages. IFNγ decreased the expression levels of arginase-1, while TNFα had no effect after 24 h of treatment (Fig. 2A). In contrast, IL-4 induced the expression of arginase-1, while IL-10 had a minimal effect (Fig. 2A). However, we noted that IL-10 potentiated IL-4-mediated induction of arginase-1 in a time-dependent manner. While IL-4 or IL-10 treatment did not further induce arginase-1 expression after 24 h, the simultaneous treatment of macrophages with IL-4 and IL-10 caused a further increase of arginase-1 that was evident by 48 h (Fig. 2B). Collectively, these findings indicate that IFNγ and IL-4 signaling contribute to activation of the M1 or M2a phenotype of macrophages in dystrophic muscle.
M1 macrophages lyse muscle cells via an iNOS/NO-dependent mechanism
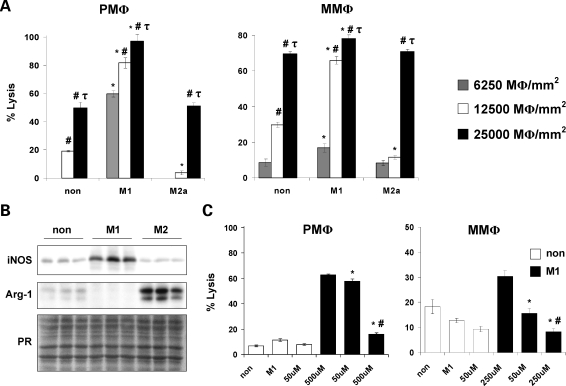
M1 macrophages are a cytotoxic population of macrophages that produce high levels of NO via the induction of iNOS (27). Therefore, we hypothesized that M1 macrophages present in dystrophic muscle contribute to muscle fiber injury via an iNOS/NO-dependent mechanism. Cytotoxicity assays, in which macrophages were cocultured with C2C12 myotubes, demonstrated that there was a concentration-dependent increase in muscle cell lysis by macrophages that were isolated from mdx muscle or the peritoneal space and then stimulated to an M1 or M2a phenotype (Fig. 3A). However, muscle and peritoneal macrophages stimulated to the M1 phenotype were the most cytotoxic at all concentrations tested (Fig. 3A). Consistent with the increased cytotoxic potential displayed by M1 muscle macrophages, we noted a concomitant increase in iNOS protein and down-regulation of arginase-1 expression (Fig. 3B), suggesting that M1 macrophages lyse muscle via an iNOS/NO-dependent mechanism.
Figure 3.
Classically activated macrophages mediate muscle cell lysis via an NO-dependent mechanism. Cytotoxicity assays showed that M1 peritoneal (left panel, PMΦ) and mdx muscle macrophages (right panel, MMΦ) had the highest cytotoxic activity (A). *, P < 0.05 when compared with non-stimulated control at the same concentration. #, P < 0.05 when compared with macrophages cultured at 6250 MΦ/mm2 within the same treatment condition. τ, P < 0.05 when compared with macrophages cultured at 6250 and 12 500 MΦ/mm2 within the same treatment condition. The increase in muscle cells lysis mediated by M1 muscle macrophages paralleled increases in iNOS expression when they were cocultured with myotubes (B). Cytotoxicity assays performed in the presence of L-NAME showed that inhibition of NO synthesis resulted in a dose-dependent decrease in muscle cell lysis mediated by M1 peritoneal and muscle macrophages (C). *, P < 0.05 when compared with M1. #, P<0.05 when compared with M1 treated with 50 µM L-NAME. Non, non-stimulated macrophages; M1, M1 macrophages; M2a, M2a macrophages; PR, nitrocellulose membranes stained with ponceau red to ensure equal loading of total protein. Representative histograms or blots of 2–3 independent experiments are shown.
We tested NO-mediated muscle cell lysis by M1 macrophages by repeating the above-mentioned cytotoxicity assay in the presence of the NOS inhibitor L-NG-Nitroarginine methyl ester (L-NAME) and found that L-NAME inhibited the cytotoxicity mediated by M1 macrophages that were isolated from muscle or the peritoneal space. In experiments using M1 peritoneal macrophages, 500 µM L-NAME reduced cytotoxicity by 75% when compared with non-treated M1 peritoneal macrophages (Fig. 3C). We did not observe a treatment effect in non-stimulated peritoneal macrophages in response to L-NAME. Similarly, 250 µM L-NAME treatment of M1 muscle macrophages resulted in 75% reduction in cytotoxicity when compared with non-treated, M1 muscle macrophages (Fig. 3C). Although the cytotoxicity mediated by non-stimulated muscle macrophages was further reduced when cells were treated with 250 µM L-NAME (50% reduction), the decrease was not quite significant (P = 0.09, Fig. 3C). These results strongly suggest that M1 macrophages mediate muscle cell lysis via an iNOS/NO-dependent mechanism.
Null mutations of iNOS in mdx mice reduce muscle fiber injury
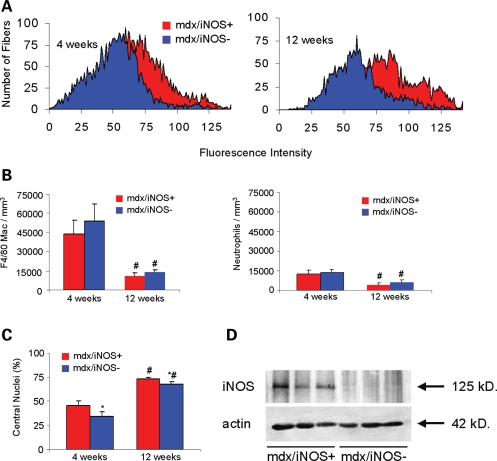
Assays of muscle membrane lysis in soleus muscles of 4 and 12-week-old mdx mice and in mdx mice that were null mutants for iNOS showed that ablation of the iNOS gene in mdx mice reduced muscle fiber damage. Analysis of mean fluorescence intensities of soleus muscle fibers from mdx mice that expressed the wild-type iNOS gene (six mice analyzed) compared with mdx mice that were null mutants for iNOS (nine mice analyzed) after muscle incubation with procion orange showed a significant reduction in intracellular fluorescence in the double-mutant mice (P < 0.05); Fig. 4A), indicating a significant reduction in membrane lysis in iNOS-null mdx mice. Furthermore, immunohistochemical assays measuring the concentration of F4/80+ macrophages in the soleus muscles of iNOS-expressing and iNOS-null mdx mice showed that there was a significant reduction in macrophage density with age (Fig. 4B). However, no difference in macrophage density was observed between iNOS-expressing and iNOS-null mdx mice (Fig. 4B). These results suggest that the observed reduction in muscle fiber injury in the iNOS-null mdx mouse (Fig. 4A) is attributed to a reduction in macrophage cytotoxicity, not macrophage numbers. Similarly, neutrophils numbers were unaffected by the mutation (Fig. 4B). Ablation of iNOS expression in mdx mice also reduced the percentage of muscle fibers in 4 and 12-week-old soleus muscles that were central-nucleated, regenerating fibers (Fig. 4C), which may be a consequence of reduced levels of muscle fiber injury.
Figure 4.
Null mutation of iNOS reduces muscle membrane lysis in mdx muscles. (A) Intracellular fluorescence of procion orange treated muscles was used as an index of muscle fiber membrane damage. The peaks show the aggregate data for all fibers in cross-sections of the entire soleus muscle from all mice in each treatment group. The leftward shift of peaks on the abscissa indicates a decrease in fibers with membrane lesions in the iNOS null/mdx muscles, compared with iNOS expressing mdx. Background fluorescence set at intensity=0 was determined by measuring fluorescence at a region of the section where there was no tissue. (B and C) Null mutation of iNOS did not significantly affect the numbers of macrophages or neutrophils in mdx muscles at 4 or 12 weeks of age. However, ablation of iNOS caused a significant reduction in the percentage of muscle fibers that were regenerative, central-nucleated fibers. Bars=sem. *, P < 0.05 when compared with age-matched, iNOS-expressing mdx. #, P < 0.05 when compared with 4-week-old mice of the same genotype. (D) Western blotting for iNOS confirmed that iNOS was expressed in mdx muscle extracts, and that no iNOS protein expression was detectble in the double-mutant mouse muscles.
M2a macrophages inhibit the cytotoxicity of M1 macrophages via an arginase-1 dependent mechanism
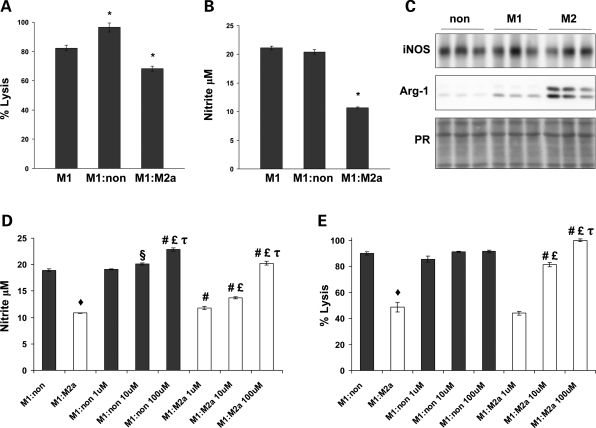
Our in vitro assays of macrophage-mediated cytotoxicity indicate that M2a macrophages promote tissue repair by inhibiting the cytolytic properties of M1 macrophages via an arginase-1 dependent mechanism. Macrophages, isolated from the peritoneal cavities of C57 mice 3 days after Na-caseinate injection, were stimulated with IFNγ or IL-4 to generate M1 and M2a macrophages, respectively. Approximately 90% of cells isolated were F4/80+ (data not shown). We found that cocultures of M1 and M2a macrophages displayed a 30% reduction in muscle cell lysis when compared with M1 macrophages cocultured with non-stimulated macrophages (Fig. 5A). Furthermore, cocultures of M1 and M2a macrophages reduced muscle cell lysis by 17% when compared with M1 macrophage-only cultures (Fig. 5A). In contrast, we observed a 17% increase in the amount of muscle cell lysis in cocultures of M1 and non-stimulated macrophage when compared with M1 macrophage-only cultures (Fig. 5A). In addition, the presence of M2a macrophages in cocultures with M1 macrophages reduced NO levels in the media by 49% (Fig. 5B). Interestingly, the reduction in muscle cell lysis observed in cocultures of M1 and M2a macrophages occurred without any large perturbations in iNOS protein expression (Fig. 5C).
Figure 5.
M2 macrophages inhibit muscle cell lysis by M1 macrophages via an arginase-1 dependent mechanism. Cytotoxicity assays showed that M1 and M2 peritoneal macrophage cocultures resulted in a 17% reduction in myotube lysis when compared with M1 only cultures (A). In contrast, coculturing M1 macrophages with non-stimulated peritoneal macrophages resulted in a 17% increase in myotube lysis when compared with M1 only cultures (A). Measurements of nitrite showed that the reduction of muscle cell lysis observed in M1:M2 cocultures was attributed to reduced nitrite levels (B) and occurred without a reduction in iNOS protein (C). Cytotoxicity assays performed in the presence of BEC showed that inhibition of arginase activity resulted in a dose-dependent increase in nitrite formation (D) and muscle cell lysis (E) in M1:M2 cocultures compared with non-treated macrophage:M2 cocultures. *, P < 0.05 when compared with M1 only cultures. ♦, P < 0.01 when compared with non-treated macrophage:M1 cocultures. §, P < 0.05 when compared with non-treated M1:non-coculture. #, P < 0.01 when compared with same coculture conditions not treated with BEC. £, P < 0.01 when compared with same coculture condition treated with 1 µM BEC. τ, P < 0.05 when compared with same coculture condition treated with 10 µM BEC. Representative histograms of 2–5 independent experiments are shown.
M2a macrophages express high levels of arginase-1, which can compete with iNOS for l-arginine and reduce NO production by iNOS. Therefore, we anticipated that the protective effect mediated by M2a macrophages in cytotoxicity assays was due to their increased expression of arginase-1. Although iNOS expression was not perturbed, a large increase in arginase-1 expression was noted in M1 and M2a cocultures, suggesting that the reduction in NO levels mediated by M2a macrophages may be attributed to substrate competition between iNOS and arginase (Fig. 5C). In addition, we found that arginase inhibition with BEC dose-dependently increased NO levels in cocultures of M1 and M2a macrophages (Fig. 5D). A smaller increase in NO was also observed in cocultures of M1 and non-stimulated macrophages (Fig. 5D). Consistent with the increases in NO production, macrophage-mediated muscle cell lysis was dose-dependently increased in cocultures of M1 and M2a macrophages when arginase activity was inhibited (Fig. 5E). Interestingly, a maximal dose of 100 µM BEC completely inhibited the protective effect of M2a macrophages, achieving cytotoxic levels no different than those of cocultures of M1 and non-stimulated macrophages (Fig. 5D). No significant increase in cytotoxicity caused by the addition of BEC to non-stimulated macrophage and M1 macrophage cocultures was observed at either high macrophage concentrations (12 500 macrophages/mm2; Fig. 5E) or relatively low concentrations of macrophages (6250 macrophages/mm2; data not shown), indicating that BEC does not increase the cytotoxicity of M1 macrophages directly. Collectively, these findings suggest that M2a macrophages are a protective subpopulation that inhibits the cytotoxic activities of M1 macrophages through an arginase-1 dependent mechanism.
Macrophages in mdx muscle shift to an M2c phenotype after 4-weeks of age
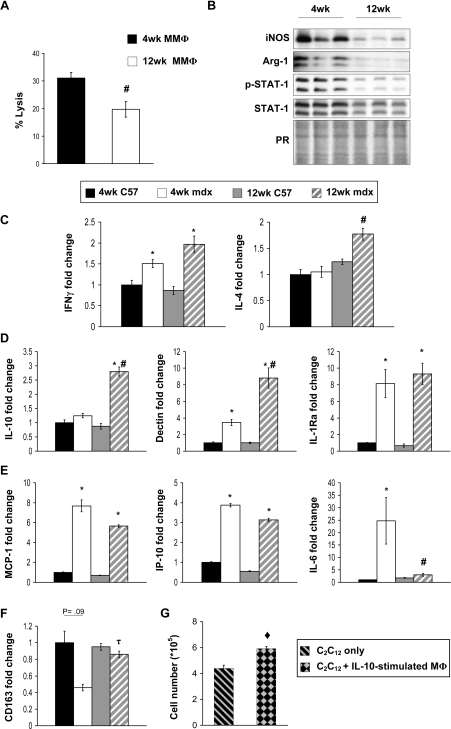
We found using 51Cr cytotoxicity assays that macrophages isolated from 4-week-old mdx muscle were more cytolytic than muscle macrophages isolated at 12 weeks (Fig. 6A). Inducible NOS protein levels were similarly elevated in macrophages from 4-week-old mdx muscles (Fig. 6B). Previous reports indicate that STAT-1 is a key transcription factor in IFNγ-dependent iNOS expression (28). Consistent with elevated levels of iNOS at 4 weeks, we noted an increased expression of STAT-1 at this age, which leads to increased levels of STAT-1 activation (phosphorylated STAT-1; Fig. 6B). These results suggest that the age-associated decrease in muscle cell lysis may reflect a switch in macrophage phenotype, in which there is a reduction in M1 macrophages during muscle regeneration. However, we also observed that there was a similarly large reduction of arginase expression in macrophages isolated from regenerative, 12-week-old muscle compared with 4-week-old muscle (Fig. 6B). This indicates that there was a coinciding shift in macrophages away from the M2a phenotype.
Figure 6.
M1 macrophages in mdx muscle undergo deactivation as the disease progresses from the acute peak of pathology (4-weeks-old) to the regenerative phase of the disease (12 weeks). Cytotoxicity assays showed that macrophages isolated from 4-week-old mdx muscle are more cytolytic in vitro compared with macrophages isolated at 12 weeks (A). A representative of three independent experiments is shown. The greater cytotoxicity of 4-week-old macrophages correlated with higher expression levels of iNOS protein (B). The elevated levels of iNOS at 4 weeks are partly attributed to increased expression of STAT-1, which leads to increased levels of STAT-1 activation (phosphorylated STAT-1, B). Measurements of IFNγ by real-time PCR showed that it was elevated in mdx hamstrings but did not differ significantly between 4 and 12 weeks (C, left panel). Transcript levels of IL-4 were upregulated in 12-week-old mdx hamstrings (C, right panel). Representative histograms of two independent experiments are shown. Consistent with the increased expression of IL-4, genes associated with alternative activation increased in 12-week-old mdx hamstrings relative to 4-week-old mdx hamstrings (D). Measurements of genes associated with classical activation showed a downward trend of expression in 12-week-old mdx hamstrings relative to 4-week-old mdx hamstrings (D, top panel). *, P < 0.05, relative to age-matched control. #, P < 0.05, relative to 4-week-old mdx. τ, P < 0.01, relative to 4-week-old mdx. ♦, P < 0.01, relative to C2C12 cultures grown without macrophages (C2C12 only).
Because IFNγ is necessary for activation of macrophages to the M1 phenotype and IL-4 is necessary for activation of the M2a phenotype, we assayed whether changes in expression of these cytokines coincided with the reduced levels of iNOS and arginase expression that occurred in macrophages during muscle regeneration. Measurements of IFNγ and IL-4 expression by real-time PCR showed that both were upregulated in mdx muscles at 4 weeks of age relative to age-matched controls (Fig. 6C). Interestingly, IFNγ expression did not change significantly between 4-week-old and 12-week-old mdx muscles and the expression of IL-4 increased between 4 and 12 weeks of age in mdx muscles (Fig. 6C). We also noted that the expression of Dectin-1, another gene that is upregulated during M2 activation, was increased in 12-week-old mdx muscles relative to 4-week-old mdx and wild-type controls (Fig. 6D) and the expression of IL-6, which is associated with the M1 phenotype, was decreased in 12-week-old muscles relative to 4-week-old mdx muscles (Fig. 6E). Most importantly, we observed a large increase in IL-10 expression in 12-week-old mdx muscle; as shown in our in vitro studies, IL-10 is sufficient to potentiate the deactivation of the M1 phenotype and the reduction of iNOS expression caused by IL-4, and thereby induce a net shift to the M2 phenotype. Furthermore, the shift to the M2 phenotype at 12-weeks of age in mdx muscle macrophages compared with 4-week-old mdx is specifically attributable to an increase in the proportion of M2c macrophages, shown by the significant elevation in expression of CD163 (Fig. 6F).
IL-10 activated macrophages promote satellite cell proliferation in vitro
We tested whether activation of macrophages with IL-10 prior to their co-culture with C2C12 myoblasts would affect the proliferative capacity of myoblasts, and observed a 26% increase in myoblast proliferation in the presence of IL-10-stimulated macrophages compared with unstimulated macrophages during a 48 h period of co-culture (P < 0.01) (Fig. 6G). Thus, enhancement of myogenic cell proliferation may provide a mechanism through which M2c macrophages may promote muscle repair or regeneration.
DISCUSSION
The results of the present investigation reveal that arginine metabolism by macrophages can play an important role in the pathophysiology of muscular dystrophy. Our findings show that null mutation of iNOS in dystrophin-deficient mice significantly reduces muscle membrane lysis at the acute peak of the disease in vivo, indicating that arginine oxidation by iNOS to form NO promotes injury of dystrophic muscle. Similarly, our in vitro cytotoxicity assays show that muscle macrophages that are collected at the acute peak of pathology lyse muscle fibers through an iNOS-dependent mechanism but that macrophages from regenerative mdx muscles in which iNOS levels have declined are less cytotoxic. However, our results disprove our initial hypothesis that the regenerative stage of mdx pathology would be characterized by increases in macrophages expressing relatively high levels of arginase. Instead, we found that there was also a dramatic decrease in arginase expression during the transition from the acute pathology at 4 weeks of age to the regenerative stage at 12 weeks. Thus, mdx muscles do not show the anticipated shift in arginine metabolism from iNOS to arginase as tissue regeneration occurs; both enzymes are expressed at high levels concurrently at the acute peak of the disease, suggesting that they may compete for substrate.
Substrate competition between iNOS and arginase can play an important role in influencing macrophage lysis of muscle cells. We observed that M1 macrophages in which iNOS expression was induced were cytolytic to muscle cells, but that cytolysis by M1 macrophages was greatly reduced by the additional presence of M2a macrophages. Furthermore, we demonstrated that the protective effect of M2a macrophages was attributable to arginase activity because the reduction in cytolysis did not occur if arginase activity were inhibited. We also observed that M1 and M2 macrophages were both present at high numbers in lesions within muscles of 4-week-old mdx mice, which would make their competition for substrate in vivo feasible. The occurrence of substrate competition between arginase and iNOS is supported by enzyme kinetics, in addition to our empirical findings. Although the Km for mammalian arginases is about 10 mm and the Km for iNOS is about 10 µM, the Vmax for arginase is more than 1000-times greater than NOS (29–33). Thus, the rates of substrate use by the two enzymes would be similar and competition for substrate would occur, especially in injured, inflamed tissue, where arginine concentrations are low.
Collectively, these data indicate that arginase-expressing, M2a macrophages can reduce muscle cell damage caused by M1 macrophages in mdx dystrophy. This finding may be relevant for predicting the consequences of currently employed therapeutic treatments for DMD, in which dietary supplementation with arginine is provided. Arginine-supplemention in the diets of DMD boys has been employed for several years, through dietary supplements such as Juven™ that can provide 14 g of dietary, supplemental arginine per day in some treatment protocols; for a 35 kg boy, this dosage is 400 mg/kg/day. Experimental investigations have shown that injured tissue exhibits substrate competition for arginine between iNOS and arginase (34–36), and that supplemental arginine can increase iNOS activity in vivo (23). Thus, the present investigation suggests that arginine supplementation in inflamed, dystrophin-deficient muscles in which there is substrate competition between iNOS and arginase could promote iNOS-mediated muscle damage. Nevertheless, previous investigators have found that supplemental arginine can also have beneficial effects in treatments of mdx mice. For example, mdx mice that received 400 mg arginine/kg of body weight for 28 days showed fewer muscle fibers into which the extracellular marker dye Evans Blue entered, indicating less membrane damage (37). However, there was no reduction in serum creatine kinase concentration, which also provides a measure of muscle membrane damage, and no reduction in the proportion of regenerative fibers and no improvement in specific force in arginine treated mice (37). Conversely, other investigators reported that 200 mg of arginine/kg for 6 weeks reduced serum creatine kinase and increased force production in mdx mice (38). Importantly, both of these investigations were performed on mice during the regenerative stage of mdx pathology; the present investigation indicates that iNOS expression by macrophages would be low at that stage.
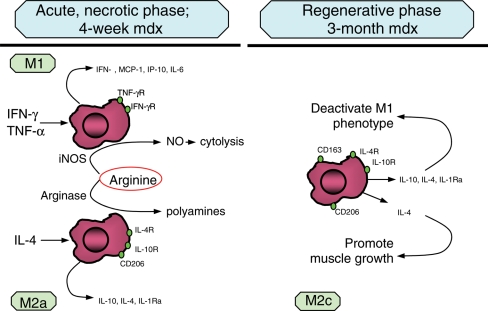
Previous, in vivo studies showed that macrophage phenotype switching occurs in skeletal muscle following acute damage, as macrophage populations change from a phagocytic, M1 population to an M2 population that promotes muscle growth, differentiation and regeneration (18,20,39). However, we find that the shifts in macrophage phenotype in muscular dystrophy are more complex. Although our in vitro findings show that stimulation of mdx muscle macrophages with IFN-γ or IL-4 produces macrophages that have characteristic M1 or M2 phenotypes, the expression of both IFN-γ and IL-4 is elevated concurrently in mdx muscle macrophages at the acute peak of the pathology. Similarly, macrophages that express receptors for IFN-γ or IL-4 are both present in 4-week-old mdx muscle. As mdx dystrophy progresses to the regenerative phase, when previous models would predict a decline in IFN-γ stimulation of M1 macrophages, IFN-γ levels in mdx muscle macrophages remain high. However, expression levels of other proteins or transcripts that are characteristic of the M1 phenotype decline (iNOS, IL-6), showing deactivation of the M1 phenotype, despite the constant level of IFN-γ production. Although IFN-γ expression in mdx muscle macrophages remains elevated into the regenerative stage of the pathology, expression of IL-4 and IL-10 are increased in 12-week-old mdx muscle, which indicates a net shift toward an M2 phenotype. Furthermore, our in vitro findings show that mdx muscle M1 macrophages can be deactivated by IL-4, which may be sufficient to attenuate activation of M1 macrophages, even in the presence of IFN-γ. We also show that IL-4 deactivation of M1 macrophages from mdx muscle is potentiated by co-stimulation with IL-10 in vitro, and that IL-10 expression is greatly increased in mdx regenerative muscle. Thus, our in vivo and in vitro data support a model of mdx muscle inflammation in which the acute peak of the muscle pathology is characterized by M1 macrophages (iNOShigh/IFN-γR+) and M2a macrophages (Arghigh/IL-4R+/CD206+), after which high levels of IL-4 and IL-10 production leads to deactivation of M1 macrophages, and a reduction in muscle membrane damage (Fig. 7). Elevation of IL-10 production could then drive the macrophages toward an M2c phenotype (iNOSlow/Arglow/CD163+/CD206+), that is associated with tissue repair and remodeling (15,16,21,40).
Figure 7.
Summary diagram of the macrophage phenotypes that dominate the acute necrotic phase and the regenerative phase of mdx muscular dystrophy. Muscle in the acute necrotic phase of the disease contains iNOS-expressing, M1 macrophages that are capable of lysing muscle fibers by NO-mediated cytotoxicity, and arginase-expressing M2a macrophages that compete with iNOS for arginine, which can thereby reduce M1 macrophage cytotoxicity. As mdx mice enter the regenerative phase, there is an increase in IL-10 expression that accompanies deactivation of the M1 and M2a phenotypes, reflected in a reduction in iNOS and arginase expression. Macrophages in regenerative muscle resemble an M2c population in which cytotoxicity is low, and the production of anti-inflammatory cytokines (IL-10, IL-1, IL-1Ra) are elevated, but arginase expression is greatly reduced.
The elevated expression in CD163 in 12-week-old mdx muscle compared with 4-week-old mdx muscle is consistent with previous speculations concerning the role of CD163+ macrophages in muscle repair and regeneration following acute injury. CD163+ macrophages begin to increase in numbers in damaged muscle just before the increased expression of proteins that are indicators of muscle repair and regeneration and they are present in highest numbers near regenerative fibers (18,41). Furthermore, our observation that IL-10 promotes the stimulation of muscle cell proliferation by macrophages is consistent with previous reports that have shown that IL-10 is a powerful inducer of CD163 expression (42–44) and that CD163+ peritoneal macrophages release factors in vitro that can promote myoblast proliferation (45). Nevertheless, our observation that CD163 expression is higher in 4-week-old wild-type muscle appears to be inconsistent with the role of CD163+ macrophages in promoting proliferation of myogenic cells. However, previous investigations have demonstrated healthy muscle contains resident populations of apparently quiescent macrophages that express CD163; thus, the relatively lower levels of CD163 expression in 4-week-old mdx muscle may reflect a shift of the resident macrophage population to the M1 or M2a phenotype during the acute peak of mdx pathology.
The change in macrophage phenotypes during the course of mdx dystrophy may underlie the differences in efficacy of glucocorticoid (GC) treatments of DMD boys that vary with the ages at which GC treatments are initiated. DMD patients who begin prednisone treatments at 3–5 years of age retain ambulation significantly longer than DMD patients who begin treatments later in the disease (46–48). Because prednisone is capable of inhibiting macrophage activation and reducing the expression of adhesion molecules that would be necessary for extravasation of macrophages into the dystrophic muscle, this age-influenced effect may reflect the presence of greater numbers of muscle-damaging, M1 macrophages at early stages of the disease (13). GCs can also affect macrophage phenotype, especially by favoring a shift to an M2 phenotype. For example, dexamethazone treatments of macrophages induce their stimulation of IL-4 production and inhibit their stimulation of IFN-γ production by T-cells, which would bias the inflammatory response to an M2 type (49,50). Furthermore, GCs can cause an over 10-fold induction of CD163 expression by macrophages (44) that could lead to a shift to the M2c phenotype that would promote myoblast proliferation, according to the present findings.
Macrophage phenotype switching during the course of muscular dystrophy may play a significant role in the regulation of muscle regeneration by direct effects on muscle cells, as well as influencing muscle death through the deactivation of cytolytic, M1 macrophages. Previous investigators have shown that IL-4 is chemoattractant to myoblasts, and can promote their fusion with existing myotubes, thereby promoting their growth in vitro (51). Muscle fibers in mice that are null mutants for IL-4 or the IL-4 receptor α chain are smaller in diameter than wild-type muscle fibers, which also suggests that IL-4 promotes muscle growth in vivo (51). Furthermore, IL-4 can promote the recruitment of mesenchymal adult stem cells to muscle fibers with which they fuse, which could further promote muscle growth and repair (52). Thus, the elevated production of IL-4 during the regenerative phase of mdx dystrophy may boost the regenerative capacity of the muscle cells.
Although numerous treatment strategies, including genetic, oligonucleotide, and cellular therapies, are currently under development and may be clinically available in the future for DMD (4,53–55), more immediate treatment options may lie in manipulations of the immune response to dystrophic muscle. The findings reported in this study provide evidence of the complex interactions that exist between macrophages during dystrophinopathy and identify macrophages as potential therapeutic targets. However, our data emphasize the importance of targeting M1 macrophages in order to optimize this intervention strategy. In addition to the M1 macrophages themselves, potential targets would include signaling pathways activated by Th1 cytokines that lead to M1 macrophage activation, or signaling molecules downstream of Th1 cytokine stimulation (e.g. JAK-STAT1 and NF-κB pathways). The potential efficacy of this approach was recently demonstrated by the myeloid cell-specific genetic ablation or pharmacological inhibition of the NF-κB pathway in mdx mice that decreased the number of macrophages in dystrophic muscle, reduced the proportion of injured muscle fibers and decreased the expression of proinflammatory cytokines that are produced by M1 macrophages (56). Finally, disrupting the production or reactivity of cytolytic substances by M1 macrophages, such as iNOS-derived NO, may provide an additional option to delay disease progression in muscular dystrophy.
MATERIALS AND METHODS
Animals
C57BL/6, C57BL/10ScSn-Dmdmdx/J and B6.129P2-Nos2tm1Lau/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in pathogen-free vivaria at the University of California, Los Angeles. Mice carrying null mutation for iNOS (B6.129P2-Nos2tm1Lau/J mice) were crossed into the mdx background using a breeding strategy previously described (3). Null mutation of iNOS was confirmed by PCR using an upstream primer that was common for both wild-type and mutant DNA (5′-ACA TGC AGA ATG AGT ACC GG-3’), a wild-type downstream primer (5′-TCA ACA TCT CCT GGT GGA AC-3′) and a downstream primer for the neomycin cassette (5′-AAT ATG CGA AGT GGA CCT CG-3′). Null mutation of the dystrophin gene was confirmed using mdx-amplification-resistant mutation system PCR (57). All animals were handled according to guidelines approved by the Chancellor’s Animal Research committee at the University of California, Los Angeles.
In vivo detection of M1 and M2 macrophages
Muscles from 4-week-old mdx mice were dissected, embedded in OCT compound, and frozen in liquid nitrogen-cooled isopentane and stored at −80°C. Frozen muscle sections of 10 µm thickness were prepared with a Microm 550 cryostat unit and then stored at −20°C. On the day of staining, sections were air-dried, acetone-fixed and blocked with 10% normal horse serum and 0.05% Tween-20 diluted in 50 mm Tris–HCl and 150 mm NaCl buffer. M1 macrophages were identified by incubating sections overnight at 4°C with antibodies against iNOS (Upstate Biotechnology, 1:50). Sections were then washed with phosphate buffered saline (PBS) and then incubated with fluorescein-conjugated anti-rabbit secondary antibody (Vector Laboratories, 1:200) for 1 h at room temperature. Sections were subsequently washed and blocked for another 30 min before probing with anti-F4/80 for 3 h at room temperature. Anti-F4/80 was obtained by ammonium sulfate precipitation from HB-198 hybridoma cultures (ATCC). Sections were washed with PBS and incubated with Texas Red-conjugated anti-rat secondary antibody for 1 h at room temperature (Vector Laboratories, 1:200). M2 macrophages were identified in sections incubated overnight with anti-F4/80 followed by labeling with Texas Red-conjugated anti-rat IgG secondary antibody. Sections were then washed, blocked and incubated with rat anti-CD206 (Serotec, 1:50) for 3 h at room temperature. Sections were subsequently washed and incubated with fluorescein-conjugated anti-rat IgG secondary antibody (Vector Laboratories, 1:200). Sections were washed with PBS and treated with RNase A (Sigma, 0.2 µg/ml) and DAPI diluted 1:100 000 in PBS for 5 min. Following washes with PBS, sections were mounted with Gel Mount (Biomedia) and glass coverslips. Images were captured with a Zeiss Axio Imager M1 microscope and AxioVision LE release 4.4 software.
Immunohistochemistry
Frozen, serial sections of 4-week-old mdx quadriceps were air-dried for 30 min and fixed in ice cold acetone for 10 min. Sections were blocked for 1 h with 3% BSA and 0.05% Tween-20 diluted in 50 mm Tris–HCl pH 7.6 containing 150 mm NaCl. Sections were then probed with antibodies against IFNγR1, IL-4R (R&D systems, 1:50), F4/80 or antibodies to neutrophils (rat anti-Ly6G; Pharmingen) for 3 h at room temperature. Sections were washed with PBS and then probed with biotin-conjugated secondary antibodies (Vector Laboratories, 1:200) for 1 h at room temperature. Sections were subsequently washed with PBS and then incubated for 30 min with Avidin d-conjugated horseradish peroxidase (Vector Laboratories, 1:1000). Positive staining was visualized with the peroxidase substrate, 3-amino-9-ethylcarbazole (Vector Laboratories), yielding a red reaction product.
Peritoneal and muscle macrophage isolation
Four C57BL/6 mice of 3–6 months of age were injected with 1 ml of 12% sodium caseinate in 0.9% sodium chloride. Three days post-injection, mice were euthanized by inhalation of 32% isoflurane. Mice were then immersed briefly in 70% ethanol, after which a midline incision was performed. The peritoneal cavity of incised mice was rinsed with 25 ml of PBS and lavages collected in 150 mm culture plates. Lavages from the four mice were pooled, centrifuged and resuspended with 0.85% ammonium chloride to lyse red blood cells. Peritoneal cells were then centrifuged, resuspended with 15 ml of Dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (complete DMEM) and filtered through a 70 µm cell strainer (BD Bioscience). Single cell suspensions were overlain on 15 ml of Histopaque 1077 (Sigma-Aldrich) and centrifuged at 1000g for 30 min. Cells at the Histopaque and DMEM buffer interface were collected, centrifuged, resuspended in complete DMEM and counted with a hemacytometer.
Muscle macrophages were isolated using a modification of a previously described procedure (9). Hindlimb muscles of 4–6 mice were dissected and pooled in a 10 cm plate containing cold PBS. Muscles were cleared of discernible fat, rinsed with fresh PBS and weighed. Dissected muscles were then minced to a fine pulp with surgical scissors and placed into 50 ml conical tubes, which received 10 ml per gram muscle mass of collagenase Type IV (1.0 mg/ml) in DMEM. Tubes were incubated in a rotary incubator at 37°C for two 45-min periods. After completing the initial 45 min of collagenase treatment, undigested muscle was allowed to settle for 2 min. The resulting cell suspension was aspirated, centrifuged in new 50 ml conical tubes at 850g and resuspended with PBS. The remaining undigested muscle was further digested in fresh collagenase buffer for a second 45-min period, after which the cell suspensions were centrifuged, resuspended and pooled with cells from the first 45-min digestion. Pooled single-cell suspensions were then filtered through a 70 µm cell strainer and subsequently centrifuged at 850g for 5 min. Filtered, single-cell suspensions were then applied to 15 ml of Histopaque 1077 and centrifuged at 1000g for 30 min. Cells were collected from the Histopaque and DMEM interface, washed with complete DMEM and counted.
The efficiency of macrophage purification was assayed by indirect immunofluorescence of 200 000 peritoneal or muscle-derived cells that were cultured on glass coverslips and stained with anti-F4/80 antibody. Cells were visualized by fluorescence microscopy and images recorded with AxioVision LE release 4.4 software. Macrophage purity was expressed as the percentage of F4/80 positive cells divided by the total number of cells counted in a randomly chosen field. Data were expressed as the mean and SEM of five randomly counted fields in three independent experiments.
Ex vivo stimulation of peritoneal and muscle macrophages with inflammatory cytokines
Peritoneal (5.0 × 106 cells/well) or muscle-derived (3.0 × 106 cells/well) macrophages were plated for 3 h in six-well plates with complete DMEM and then washed with PBS to remove cellular debris. Macrophages were stimulated with the following cytokines at 10 ng/ml: IFNγ alone, TNFα alone, IFNγ and TNFα together, IL-4 alone, IL-10 alone or IL-4 and IL-10 together (BD Bioscience). Cells were then incubated at 37°C with 5% CO2 for 24–72 h before lysing with reducing sample buffer containing a protease inhibitor cocktail (Sigma-Aldrich) diluted 1:100. Samples were heated for 3 min at 95°C before colorimetric determination of their protein concentration and stored at −20°C.
Western blot analysis
The expression levels of iNOS and arginase-1 were assayed by western blot analysis. Thirty micrograms of total protein from stimulated macrophages were resolved by SDS–PAGE using 8 or 12% polyacrylamide gels and transferred to nitrocellulose. Equal loading of samples was determined by staining nitrocellulose blots with 0.1% Ponceau S solution (Sigma-Aldrich). Nitrocellulose membranes blocked with 3% milk were probed with rabbit antibodies against mouse iNOS (Upstate Biotechnology, 1:300), human arginase-1 (a gift from Dr. Tomomi Gotoh, Kumamoto University School of Medicine; 1:1000), STAT-1 (Santa Cruz Biotechnology, 1:500) or phospho-STAT-1 (Cell Signaling Technology, 1:500) for 3 h at room temperature with light agitation. Membranes were then washed with PBS containing 0.05% Tween-20 and probed with horseradish peroxidase conjugated-donkey anti-rabbit IgG (Amersham, 1:10 000) for 1 h at room temperature while slowly agitating. Membranes were washed and the expression levels of iNOS or arginase-1 were visualized with chemiluminescent substrate and a fluorochem imaging system (Alpha Innotech).
Macrophage-mediated cytotoxicity assay
Macrophage-mediated cytotoxicity was assessed with a previously reported assay (9,17). Ninety-six-well plates were seeded with 15 000 C2C12 cells per well in complete DMEM and allowed to reach confluency before overnight serum starvation to trigger fusion. Following serum starvation, cells were returned to complete DMEM and allowed to continue differentiation for 3 days prior to coculturing with macrophages. On the second day of C2C12 cell differentiation, mdx muscle macrophages or peritoneal macrophages were isolated as described earlier and stimulated with recombinant murine IFNγ or IL-4 (R&D Systems; 10 ng/ml) in 100 mm, low-attachment dishes (Costar). Following 24 h of stimulation, macrophages were washed with HBSS before coculturing with myotubes to prevent the stimulation of myotubes by IFNγ or IL-4. Macrophages were then treated with 8 ml of 0.05% trypsin-EDTA (Invitrogen) for 5 min to dissociate aggregated cells. Trypsin-treated cells were then given 10 ml of complete DMEM, centrifuged and resuspended with HBSS assay buffer (HBSS containing 0.25% FBS and 400 µM l-arginine).
Prior to coculturing with macrophages, myotubes were pulsed with 0.4% 51Cr in HBSS assay buffer for 2 h. Macrophages were cocultured with 51Cr-pulsed myotubes at concentrations ranging from 6250 to 25 000 macrophages/mm2 in 150 µl of HBSS assay buffer. Following 24 h of coculturing, 75 µl of media were collected from each well and 51Cr release was measured using a Beckman Gamma 5500 liquid scintillation counter. Cytotoxicity was expressed as a percentage of total lysis by setting 0% as 51Cr released spontaneously by myotubes cultured without macrophages. Hundred percent cytotoxicity was set as the 51Cr released into the media by myotubes incubated with 0.1% Triton X-100 in HBSS assay buffer. L-NG-Nitroarginine methyl ester, HCl (L-NAME, Sigma-Aldrich) or (S)-(2-boronoethyl)-l-cysteine, HCL (BEC, Calbiochem) was added at concentrations ranging from 1 to 500 µM at the beginning of macrophage and myotube coculturing to inhibit iNOS or arginase activity, respectively.
Assay for muscle membrane lesions in vivo
Muscle fiber membrane lesions were assessed by assaying for the presence of the extracellular matrix marker dye, procion orange, within muscle fibers. Procion orange was selected as a marker of membrane lesions because it is a vital dye that is not actively transported across cell membranes, instead entering through membrane lesions. After euthanasia, the left soleus of each mouse was dissected and incubated in 0.5% procion orange dye (Sigma) in Kreb’s Ringer solutions for 1 h, followed by three, 5-min rinses in Krebs’ Ringer. The muscles were then rapidly frozen in liquid nitrogen-cooled isopentane. Cross-sections 10-µm thick were taken from the mid-belly of each soleus and viewed by fluorescence microscopy. Intracellular fluorescence intensity caused by dye influx was assayed within an 8-µm diameter, optical sampling circle by flourimetry using a microscope equipped with a digital imaging system (Bioquant, Nashville, TN). Fluorescence intensity was measured for every fiber present in complete cross-sections of entire mid-belly cross-sections of each soleus muscle. Measurements were corrected for background fluorescence measured at a site on the section that contained no tissue. Approximately 760 fibers were assayed in each muscle. Data were expressed as fluorescence intensity in arbitrary units and displayed graphically as the distribution frequency of fiber fluorescence in mdx mice versus iNOS-null mdx mice.
RNA isolation and quantitative PCR
Frozen muscles were homogenized with a mortar and pestle while partially submerged in liquid nitrogen. One milliliter of Trizol (Invitrogen) per 50 mg of tissue was pipetted directly into the mortar and pestle and further homogenized while in liquid nitrogen. The resulting homogenized powder was thawed and transferred to 2 ml centrifuge tubes and RNA extracted according to the manufacturer’s protocol (Invitrogen). RNA from freshly isolated muscle macrophages was isolated with RNeasy spin columns (Qiagen). Muscle and macrophage RNA samples were further cleaned and DNase-treated using RNeasy spin columns according to the manufactures protocol. Five hundred nanograms of total RNA was loaded on a 1.2% agarose gel and RNA quality assessed by determining 28S and 18S ribosomal RNA integrity. Five hundred nanograms of total RNA were reverse transcribed with Super Script Reverse Transcripase II using oligo dTs to prime extension (Invitrogen). The resulting cDNA was used to measure quantitatively the expression of genes involved in classical and alternative activation using SYBR green qPCR master mix according to the manufacturer’s protocol (BioRad). Real-time measurements of gene expression were performed with an iCycler thermocycler system and iQ5 optical system software (BioRad). Primers used to measure gene expression are provided in Table 1.
Table 1.
Real-time-PCR primers
| Gene | Accession | Forward primer | Reverse primer | Amplicon size |
|---|---|---|---|---|
| IL-Ra | NM_031167 | 5′-AAGCCTTCAGAATCTGGGATAC-3′ | 5′-TCATCTCCAGACTTGGCACA-3′ | 193bp |
| IFNγ | NM_008337 | 5′-GACAATCAGGCCATCAGCAAC-3′ | 5′-CGGATGAGCTCATTGAATGCTT-3′ | 161bp |
| IL-6 | NM_031168 | 5′-GAACAACGATGATGCACTTGC-3′ | 5′-CTTCATGTACTCCAGGTAGCTATGGT-3′ | 154bp |
| IL-4 | NM_021283 | 5′-GGATGTGCCAAACGTCCTC-3′ | 5′-GAGTTCTTCTTCAAGCATGGAG-3′ | 126bp |
| Dectin-1 | NM_020008 | 5′-GCCAAACATCGTCTCACC-3′ | 5′-GAATCCATACACAATTGTGCAG-3′ | 165bp |
| IL-10 | NM_010548 | 5′-CAAGGAGCATTTGAATTCCC-3′ | 5′-GGCCTTGTAGACACCTTGGTC-3′ | 157bp |
| MCP-1 | NM_011333 | 5′-GCTCAGCCAGATGCAGTTAAC-3′ | 5′-CTCTCTCTTGAGCTTGGTGAC-3′ | 153bp |
| IP-10 | NM_021274 | 5′-CCTCATCCTGCTGGGTCTG-3′ | 5′-GTGGCAATGATCTCAACACG-3′ | 165bp |
| CD163 | NM_053094 | 5′-GCAAAAACTGGCAGTGGG -3′ | 5′-GTCAAAATCACAGACGGAGC-3′ | 153bp |
| PPIA | NM_008907 | 5′-GCAAATGCTGGACCAAACAC -3′ | 5′-TCACCTTCCCAAAGACCACAT-3′ | 97bp |
| B-actin | NM_007393 | 5′-CAACCGTGAAAAGATGACCC -3′ | 5′-GTAGATGGGCACAGTGTGGG-3′ | 157bp |
Measurement of nitric oxide concentration
Nitric oxide concentration was determined by measuring nitrite levels in supernatants of macrophages and myotubes cocultures using a modification of the Griess reaction (58).
Myoblast growth in cocultures with IL-10 stimulated macrophages
The cell growth of C2C12 myoblasts in coculture with macrophages was determined as previously reported (59). Peritoneal macrophages were seeded at 4.0 × 105 cells/well in six-well plates and stimulated with IL-10 (10 ng/mL) in DMEM containing 0.25% FBS for 24 h. Macrophages were then washed with DMEM twice and C2C12 cells were plated on top of the stimulated macrophages in DMEM containing 5% FBS at the density of 1.0 × 105 cells/well. C2C12 cells were serum starved in DMEM containing 0.1% FBS for 24 h before adding to macrophages. Following 48 h of coculture, C2C12 cells were dissociated with 0.05% trypsin-EDTA, collected and counted. Trypsin treatment did not detach the macrophages.
Statistics
Statistical analysis was performed in InStat version 2.03 (GraphPad Software). For multi-factorial comparisons, we performed a Kruskal–Wallis test to determine statistical significance, followed by a post-hoc non-parametric test (Wilcoxon) to determine significance of differences between two groups. These tests allow the comparison of groups without the assumption of normality in data sets. Significance was accepted at P < 0.05.
FUNDING
This work was supported by grants from the Muscular Dystrophy Association, USA (4031 to J.G.T.) and the National Institutes of Health (R01 AR40343 and R01 AR47721 to J.G.T. and F31 AR054724 to S.A.V.).
ACKNOWLEDGEMENTS
We thank Khoa Pham, Zainab Naji, Anna Avik, and Katherine Wen for their technical assistance.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Hoffman E.P., Brown R.H., Jr, Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Petrof B.J., Shrager J.B., Stedman H.H., Kelly A.M., Sweeney H.L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl Acad. Sci. USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer M.J., Walsh C.M., Dorshkind K.A., Rodriguez E.M., Tidball J.G. Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J. Clin. Invest. 1997;99:2745–2751. doi: 10.1172/JCI119464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tidball J.G., Wehling-Henricks M. Evolving therapeutic strategies for Duchenne muscular dystrophy: targeting downstream events. Pediatr. Res. 2004;56:831–841. doi: 10.1203/01.PDR.0000145578.01985.D0. [DOI] [PubMed] [Google Scholar]
- 5.Hodgetts S., Radley H., Davies M., Grounds M.D. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul. Disord. 2006;16:591–602. doi: 10.1016/j.nmd.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Gorospe J.R., Tharp M., Demitsu T., Hoffman E.P. Dystrophin-deficient myofibers are vulnerable to mast cell granule-induced necrosis. Neuromuscul. Disord. 1994;4:325–333. doi: 10.1016/0960-8966(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 7.Gorospe J.R., Tharp M.D., Hinckley J., Kornegay J.N., Hoffman E.P. A role for mast cells in the progression of Duchenne muscular dystrophy? Correlations in dystrophin-deficient humans, dogs, and mice. J. Neurol. Sci. 1994;122:44–56. doi: 10.1016/0022-510x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 8.Wehling-Henricks M., Sokolow S., Lee J.J., Myung K.H., Villalta S.A., Tidball J.G. Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum. Mol. Genet. 2008;17:2280–2292. doi: 10.1093/hmg/ddn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wehling M., Spencer M.J., Tidball J.G. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell. Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manzur A.Y., Kuntzer T., Pike M., Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst. Rev., CD003725. 2008 doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Moxley R.T., III, Ashwal S., Pandya S., Connolly A., Florence J., Mathews K., Baumbach L., McDonald C., Sussman M., Wade C. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2005;64:13–20. doi: 10.1212/01.WNL.0000148485.00049.B7. [DOI] [PubMed] [Google Scholar]
- 12.Griggs R.C., Moxley R.T., III, Mendell J.R., Fenichel G.M., Brooke M.H., Pestronk A., Miller J.P., Cwik V.A., Pandya S., Robison J., et al. Duchenne dystrophy: randomized, controlled trial of prednisone (18 months) and azathioprine (12 months) Neurology. 1993;43:520–527. doi: 10.1212/wnl.43.3_part_1.520. [DOI] [PubMed] [Google Scholar]
- 13.Wehling-Henricks M., Lee J.J., Tidball J.G. Prednisolone decreases cellular adhesion molecules required for inflammatory cell infiltration in dystrophin-deficient skeletal muscle. Neuromuscul. Disord. 2004;14:483–490. doi: 10.1016/j.nmd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends. Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Gratchev A., Schledzewski K., Guillot P., Goerdt S. Alternatively activated antigen-presenting cells: molecular repertoire, immune regulation, and healing. Skin Pharmacol. Appl. Skin Physiol. 2001;14:272–279. doi: 10.1159/000056357. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen H.X., Tidball J.G. Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J. Physiol. 2003;547:125–132. doi: 10.1113/jphysiol.2002.031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Pierre B.A., Tidball J.G. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J. Appl. Physiol. 1994;77:290–297. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- 19.Krippendorf B.B., Riley D.A. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve. 1993;16:99–108. doi: 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- 20.Tidball J.G., Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J. Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munder M., Eichmann K., Moran J.M., Centeno F., Soler G., Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 22.Munder M., Eichmann K., Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- 23.Albina J.E., Mills C.D., Henry W.L., Jr, Caldwell M.D. Temporal expression of different pathways of 1-arginine metabolism in healing wounds. J. Immunol. 1990;144:3877–3880. [PubMed] [Google Scholar]
- 24.Shi H.P., Fishel R.S., Efron D.T., Williams J.Z., Fishel M.H., Barbul A. Effect of supplemental ornithine on wound healing. J. Surg. Res. 2002;106:299–302. doi: 10.1006/jsre.2002.6471. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg I., Brengman J.M., Engel A.G. Analysis of cytokine expression in muscle in inflammatory myopathies, Duchenne dystrophy, and non-weak controls. J. Neuroimmunol. 1995;63:9–16. doi: 10.1016/0165-5728(95)00122-0. [DOI] [PubMed] [Google Scholar]
- 26.Lagrota-Candido J., Vasconcellos R., Cavalcanti M., Bozza M., Savino W., Quirico-Santos T. Resolution of skeletal muscle inflammation in mdx dystrophic mouse is accompanied by increased immunoglobulin and interferon-gamma production. Int. J. Exp. Pathol. 2002;83:121–132. doi: 10.1046/j.1365-2613.2002.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosser D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 28.Blanchette J., Jaramillo M., Olivier M. Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology. 2003;108:513–522. doi: 10.1046/j.1365-2567.2003.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori M. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J. Nutr. 2007;137:1616S–1620S. doi: 10.1093/jn/137.6.1616S. [DOI] [PubMed] [Google Scholar]
- 30.Griffith O.W., Stuehr D.J. Nitric oxide synthases: properties and catalytic mechanism. Annu. Rev. Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 31.Stuehr D.J., Cho H.J., Kwon N.S., Weise M.F., Nathan C.F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc. Natl Acad. Sci. USA. 1991;88:7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu G., Morris S.M., Jr Arginine metabolism: nitric oxide and beyond. Biochem. J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spolarics Z., Bond J.S. Comparison of biochemical properties of liver arginase from streptozocin-induced diabetic and control mice. Arch. Biochem. Biophys. 1989;274:426–433. doi: 10.1016/0003-9861(89)90455-4. [DOI] [PubMed] [Google Scholar]
- 34.Tenu J.P., Lepoivre M., Moali C., Brollo M., Mansuy D., Boucher J.L. Effects of the new arginase inhibitor N(omega)-hydroxy-nor-L-arginine on NO synthase activity in murine macrophages. Nitric Oxide. 1999;3:427–438. doi: 10.1006/niox.1999.0255. [DOI] [PubMed] [Google Scholar]
- 35.Gotoh T., Mori M. Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells. J. Cell. Biol. 1999;144:427–434. doi: 10.1083/jcb.144.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutschman R., Lang R., Hesse M., Ihle J.N., Wynn T.A., Murray P.J. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J. Immunol. 2001;166:2173–2177. doi: 10.4049/jimmunol.166.4.2173. [DOI] [PubMed] [Google Scholar]
- 37.Barton E.R., Morris L., Kawana M., Bish L.T., Toursel T. Systemic administration of L-arginine benefits mdx skeletal muscle function. Muscle Nerve. 2005;32:751–760. doi: 10.1002/mus.20425. [DOI] [PubMed] [Google Scholar]
- 38.Voisin V., Sebrie C., Matecki S., Yu H., Gillet B., Ramonatxo M., Israel M., De la Porte S. L-arginine improves dystrophic phenotype in mdx mice. Neurobiol. Dis. 2005;20:123–130. doi: 10.1016/j.nbd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R.K., Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Pomares L., Reid D.M., Brown G.D., Taylor P.R., Stillion R.J., Linehan S.A., Zamze S., Gordon S., Wong S.Y. Analysis of mannose receptor regulation by IL-4, IL-10, and proteolytic processing using novel monoclonal antibodies. J. Leukoc. Biol. 2003;73:604–613. doi: 10.1189/jlb.0902450. [DOI] [PubMed] [Google Scholar]
- 41.McLennan I.S. Resident macrophages (ED2- and ED3-positive) do not phagocytose degenerating rat skeletal muscle fibres. Cell Tissue Res. 1993;272:193–196. doi: 10.1007/BF00323586. [DOI] [PubMed] [Google Scholar]
- 42.Buechler C., Ritter M., Orso E., Langmann T., Klucken J., Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 43.Sulahian T.H., Hogger P., Wahner A.E., Wardwell K., Goulding N.J., Sorg C., Droste A., Stehling M., Wallace P.K., Morganelli P.M., et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 44.Schaer D.J., Boretti F.S., Schoedon G., Schaffner A. Induction of the CD163-dependent haemoglobin uptake by macrophages as a novel anti-inflammatory action of glucocorticoids. Br. J. Haematol. 2002;119:239–243. doi: 10.1046/j.1365-2141.2002.03790.x. [DOI] [PubMed] [Google Scholar]
- 45.Massimino M.L., Rapizzi E., Cantini M., Libera L.D., Mazzoleni F., Arslan P., Carraro U. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem. Biophys. Res. Commun. 1997;235:754–759. doi: 10.1006/bbrc.1997.6823. [DOI] [PubMed] [Google Scholar]
- 46.Dubowitz V., Kinali M., Main M., Mercuri E., Muntoni F. Remission of clinical signs in early duchenne muscular dystrophy on intermittent low-dosage prednisolone therapy. Eur. J. Paediatr. Neurol. 2002;6:153–159. doi: 10.1053/ejpn.2002.0583. [DOI] [PubMed] [Google Scholar]
- 47.Kinali M., Mercuri E., Main M., Muntoni F., Dubowitz V. An effective, low-dosage, intermittent schedule of prednisolone in the long-term treatment of early cases of Duchenne dystrophy. Neuromuscul. Disord. 2002;12(Suppl. 1):S169–S174. doi: 10.1016/s0960-8966(02)00097-4. [DOI] [PubMed] [Google Scholar]
- 48.Merlini L., Cicognani A., Malaspina E., Gennari M., Gnudi S., Talim B., Franzoni E. Early prednisone treatment in Duchenne muscular dystrophy. Muscle Nerve. 2003;27:222–227. doi: 10.1002/mus.10319. [DOI] [PubMed] [Google Scholar]
- 49.Blotta M.H., DeKruyff R.H., Umetsu D.T. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J. Immunol. 1997;158:5589–5595. [PubMed] [Google Scholar]
- 50.DeKruyff R.H., Fang Y., Umetsu D.T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J. Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]
- 51.Horsley V., Jansen K.M., Mills S.T., Pavlath G.K. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 52.Schulze M., Belema-Bedada F., Technau A., Braun T. Mesenchymal stem cells are recruited to striated muscle by NFAT/IL-4-mediated cell fusion. Genes Dev. 2005;19:1787–1798. doi: 10.1101/gad.339305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rando T.A. Oligonucleotide-mediated gene therapy for muscular dystrophies. Neuromuscul. Disord. 2002;12(Suppl. 1):S55–S60. doi: 10.1016/s0960-8966(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 54.Odom G.L., Gregorevic P., Chamberlain J.S. Viral-mediated gene therapy for the muscular dystrophies: successes, limitations and recent advances. Biochim. Biophys. Acta. 2007;1772:243–262. doi: 10.1016/j.bbadis.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peault B., Rudnicki M., Torrente Y., Cossu G., Tremblay J.P., Partridge T., Gussoni E., Kunkel L.M., Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 56.Acharyya S., Villalta S.A., Bakkar N., Bupha-Intr T., Janssen P.M., Carathers M., Li Z.W., Beg A.A., Ghosh S., Sahenk Z., et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amalfitano A., Chamberlain J.S. The mdx-amplification-resistant mutation system assay, a simple and rapid polymerase chain reaction-based detection of the mdx allele. Muscle Nerve. 1996;19:1549–1553. doi: 10.1002/(SICI)1097-4598(199612)19:12<1549::AID-MUS4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 58.Amano F., Noda T. Improved detection of nitric oxide radical (NO.) production in an activated macrophage culture with a radical scavenger, carboxy PTIO and Griess reagent. FEBS Lett. 1995;368:425–428. doi: 10.1016/0014-5793(95)00700-j. [DOI] [PubMed] [Google Scholar]
- 59.Chazaud B., Sonnet C., Lafuste P., Bassez G., Rimaniol A.C., Poron F., Authier F.J., Dreyfus P.A., Gherardi R.K. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell. Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]