Abstract
The human anterior cingulate cortex (ACC), which is active during conflict-monitoring tasks, is thought to participate with prefrontal cortices in a distributed network for conscious self-regulation. This hypothesis predicts that conflict-related ACC activation should occur only when the conflicting stimuli are consciously perceived. To dissociate conflict from consciousness, we measured the behavioral and brain imaging correlates of a motor conflict induced by task-irrelevant subliminal or conscious primes. The same task was studied in normal subjects and in patients with schizophrenia in whom the ACC and prefrontal cortex are thought to be dysfunctional. Conscious, but not subliminal, conflict affected anterior cingulate activity in normal subjects. Furthermore, patients with schizophrenia, who exhibited a hypoactivation of the ACC and other frontal, temporal, hippocampal, and striatal sites, showed impaired conscious priming but normal subliminal priming. Those findings suggest that subliminal conflicts are resolved without ACC contribution and that the ACC participates in a distributed conscious control network that is altered in schizophrenia.
Keywords: neuroimaging, priming, consciousness, psychiatry
The anterior cingulate cortex (ACC) is active during a variety of cognitive tasks that involve mental effort (1, 2). Whether a single overarching theory of ACC function can account for this diversity of findings remains a matter of some debate. However, the involvement of the ACC in many tasks can be explained by its role in the detection of conflicting response tendencies (3-7). The conflict-monitoring model has been implemented as a working simulation that accounts for some of the major conditions known to activate the ACC, including flanker interference (3), color-word Stroop interference (5), and error detection (8, 9). Furthermore, it has led to new observations of a dissociation between ACC and prefrontal cortex (PFC) activity: when cognitive control diminishes, PFC decreases, but ACC activity increases together with error rate, presumably due to an increase in conflict (5).
In the present work, we examine whether the ACC is involved in any form of conflict monitoring, or more specifically with conscious monitoring. Posner (10, 11) proposed a broad role for ACC in the conscious self-regulation of behavior, based on its activation during conscious effortful tasks and its reciprocal anatomical connectivity with many distant prefrontal and limbic regions. Dehaene et al. (12-14) further suggested that ACC is an important node in a distributed “conscious neuronal workspace” comprising neurons with long-distance connections that distribute information broadly to many higher cortical and subcortical targets, thus providing a possible neural basis for conscious access and flexible adaptive behavior. Within this distributed network, the ACC may play a particular role in the computation and dissemination of information about anticipated emotions and rewards, which is essential for conscious self-regulation and decision making (12, 15, 16). This view fits with the finding of ACC activation on conscious conflict or error trials. In addition, it can account for several neuroimaging studies that have evidenced increased joint cingulate and prefrontal activity during conscious perception relative to a comparable nonconscious stimulation, often in the absence of obvious response conflicts (17-20).
To examine the relation between ACC and consciousness, we induced motor conflict in the absence of consciousness by relying on a previously studied subliminal priming paradigm (refs. 21-23 and Fig. 1). In this task, subjects are asked to decide whether target numbers are larger or smaller than 5. Unbeknownst to them, the prior subliminal presentation of another masked number for 43 ms affects their performance. Response times are faster when the hidden prime and the visible target are congruent (both larger or both smaller than 5) than when they are incongruent (one larger and the other smaller). By using functional MRI (fMRI) and event-related potentials, the source of this congruity effect was traced to a subliminal lateralized motor preparation induced by the prime, which conflicted with the preparation of the response to the target (21).
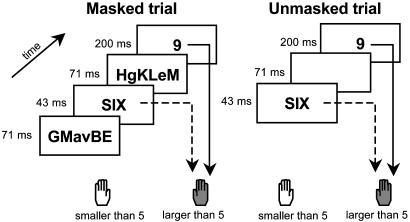
Fig. 1.
Experimental paradigm. Subjects compared a 200-ms target number to a fixed numerical standard. Each target was preceded by a fast presentation of another number that served as a prime. In different blocks, the prime could be masked by random consonant strings (Left), or it could be visible and had to be actively ignored (Right).
In the present work, we adapted this paradigm to measure behavioral and fMRI responses to conscious and subliminal sources of conflict. On different blocks, the prime could be masked and therefore essentially invisible, or it could be unmasked by removing the random letter masks, thus requiring subjects to actively inhibit prime processing (Fig. 1). Based on earlier work, we expected both conditions to lead to measurable conflict effects in response times. However, if ACC activation requires a conscious appraisal of the conflicting stimuli, then only the conscious conflict situation should lead to increased ACC activity, together with extended activity in a distributed cortical network including PFC.
To further probe the relation between conflict, consciousness, and a distributed network including ACC and PFC, we compared the behavioral and fMRI responses in normal controls and in patients with schizophrenia. Converging investigations have revealed that patients with schizophrenia exhibit distributed impairments in ACC, PFC, and other interconnected regions, such as the hippocampus and cerebellum. Anatomically, neuronal density is reduced in ACC and PFC (24), and the amount of reduction in ACC gray matter and underlying white matter correlates with the severity of negative symptoms and executive dysfunction (25-28). Functionally, patients with schizophrenia exhibit reduced ACC and PFC metabolism at rest (29), and altered ACC and PFC activation in conflict tasks (30-32). The functional connectivity between ACC and PFC is also reduced (33).
We reasoned that schizophrenia provides a testing ground for theories of the engagement of ACC and PFC in a distributed network for conscious regulation. Such theories predict that patients with schizophrenia would exhibit a normal subliminal conflict effect and would be impaired only in the conscious monitoring of conflict. This predicted dissociation of subliminal and conscious processing in schizophrenia has not been studied directly in the literature (but see refs. 34-38). Given the evidence for multiple, distributed sites of brain dysfunction in schizophrenia, including ACC and PFC, the finding of preserved subliminal priming would provide strong evidence that ACC and PFC are not involved in automatic conflict resolution, but solely in conscious monitoring.
Methods
Participants. Eighteen neurologically normal subjects (mean age 27.4 yr, range 18-44 yr) and 15 patients with schizophrenia (mean age 28.1 yr, range 19-36 yr), matched in age and in years of education, were tested. All were right-handed males and native speakers of French. Exclusion criteria included alcohol or other drug abuse, depression, neurological disease, or impaired visual acuity. Patients met Diagnostic and Statistical Manual of Mental Disorders-IV criteria for schizophrenia and were recruited from psychiatric departments of the Assistance Publique, Hôpitaux de Paris. They had a chronic course and were stabilized with a moderate dose maintenance neuroleptic treatment. None were receiving antidepressants, lithium, or electro-convulsive therapy at the time of testing. Patients scored 49 ± 18 on the Scale for the Assessment of Negative Symptoms (SANS), and 20 ± 17 on the Scale for the Assessment of Positive Symptoms (SAPS). On the Positive and Negative Syndrome Scale (PANSS), their scores were 23 ± 7 for negative symptoms, 14 ± 6 for positive symptoms, and 35 ± 9 for general psychopathology. Behavioral data were available for all subjects, but, due to motion artifacts and other technical difficulties, fMRI data were analyzed only in 12 controls and 13 patients. All experiments were approved by the French Regional Ethical Committee for Biomedical Research (Hôpital de Bicêtre), and subjects gave written informed consent.
Stimuli. The stimulus set consisted of 64 pairs of prime and target numbers, each consisting of the numbers 1, 4, 6, and 9 written in either Arabic or verbal (spelled-out) format. Subjects were asked to compare each target number with 5, pressing the right-hand key as fast as possible for numbers larger than 5 and the left hand key for numbers smaller than 5. The following factors were manipulated: target notation (Arabic or verbal), target distance (close or far from 5), target size (larger or smaller than 5), response congruity (whether or not the prime and target fell on the same side of 5), and repetition (within the congruent trials, whether or not the prime and target were the same number).
On masked trials, we first presented a randomly chosen uppercase consonant string for 71 ms, the prime for 43 ms, another consonant string for 71 ms, and finally the target for 200 ms. On unmasked trials, the same sequence was used, but the consonant strings were replaced with blank screens (Fig. 1). Before each trial, a warning signal (a rectangle 10° wide and 6° high surrounding the stimulus position) appeared with a lag of one second (training) or two seconds (single-event fMRI).
Procedure. Before fMRI scanning, participants took a behavioral test with masked primes only. This test consisted of 20 training trials followed by 128 experimental trials (4 blocks of 32 trials, plus one initial training trial, which was later discarded). Masked trials were presented at a 3-s rate on a computer screen (70-Hz refresh rate). During fMRI scanning, subjects continued with the masked trials (training, 20 trials; scanning, two blocks of 32 trials plus 1 discarded trial), then were introduced to the unmasked trials (training, at least one block of 20 trials; scanning, two blocks of 32 trials plus 1 discarded trial). Stimuli were presented every 14 s through mirror glasses and an active matrix video projector (70-Hz refresh rate). Stimulus onset was synchronized with the acquisition of the first slice in a series of seven volumes of 18 slices each. We used a gradient-echo echo-planar imaging sequence sensitive to brain oxygen-level-dependent (BOLD) contrast (18 contiguous axial slices, 6-mm thickness, repetition time/echo time = 2,000/60 ms, field of view 24 cm, 64 × 64 matrix, voxel size 3.75 × 3.75 × 6 mm) on a 1.5-Tesla whole body system (Signa, General Electric). High-resolution anatomical images were also acquired by using a 3D fast gradient-echo inversion-preparation sequence (124 contiguous axial slices, 1.2-mm thickness, inversion time = 600 ms, echo time = 2.2 ms, field of view 24 cm, 256 × 192 matrix, voxel size 0.9375 × 0.9375 × 1.2 mm).
After scanning, all but four subjects (three controls, one patient) participated in a forced-choice prime detection task by using two successive blocks of trials. In block 1, subjects were presented with a list of 64 masked trials, 16 trials at each of four prime durations (43, 71, 114, or 200 ms). In block 2, subjects were presented with 16 randomized unmasked trials, using a 43-ms prime duration. In both blocks, the prime was omitted on half the trials, and subjects were asked whether they thought that a prime was present or absent.
fMRI Analysis. Analysis was done with SPM99 software. The first 7 fMRI volumes of each block, corresponding to the first trial, were discarded, leaving 224 volumes in each of four blocks. Images were corrected for subject motion and slice acquisition delays, normalized to Talairach coordinates by using a linear transform calculated on the anatomical images, and smoothed. The signal was modeled as a linear combination, for each subject and each event type, of a standard haemodynamic response function and its temporal derivative, thus allowing for different delays across brain regions. Event types were defined by a combination of the following factors: response hand, prime-target relation (congruent repeated, congruent nonrepeated, or incongruent), notation identity (prime and target in same or different notation), and masking. Random-effect analyses were then performed on those factors (voxel P = 0.01, cluster P < 0.05 corrected).
Behavioral Results
Number Comparison. We first tested whether patients and controls differed on number comparison processes through an analysis of variance (ANOVA) on median response time (RT) with factors of group, target notation, distance, and size, pooling over all masked and unmasked trials and over prescanning and scanning blocks (the results did not differ across those periods). Patients were overall slower than controls [559 vs. 495 ms, F(1,31) = 6.20, P = 0.018]. However, no interaction was found between group and any of the variables known to affect number comparison (ref. 39; see Fig. 2). Both groups were slower with verbal notation than with arabic notation [34-ms notation effect, F(1,31) = 133.87, P < 0.0001]. Both groups were also affected by a similar distance effect [32-ms effect, F(1,31) = 85. 89, P < 0.0001]. Furthermore, as previously reported (39), a triple interaction of notation, distance, and size reflected an effect of word length [slower responses for QUATRE in French, and faster responses for SIX, only in verbal notation; F(1,31) = 6.29, P = 0.018]. No other interactions were significant.
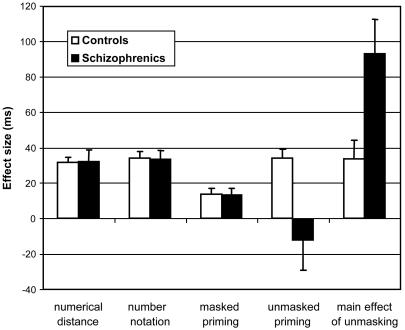
Fig. 2.
Behavioral performance in number comparison in control subjects and in patients with schizophrenia (mean effect size in milliseconds ± SE). Both groups show identical effects of numerical distance, number notation, and subliminal priming. However, they differed in the unmasked priming effect, which required conscious control of interference. Patients were also severely slowed in the unmasked condition compared with the masked condition.
The error rate was slightly and nonsignificantly higher in patients than in controls (4.0% vs. 2.7% errors, P = 0.18). Error analyses showed effects of distance [2.2% effect, F(1,31) = 19.59, P = 0.0001] and notation [1.1% effect, F(1,31) = 4.34, P = 0.046], which did not differ across groups. The only interaction involving group was a small and unexplained group by size interaction [F(1,31) = 4.69, P = 0.038], suggesting that normal subjects made slightly more errors with larger than with smaller targets whereas the converse tended to be true of patients. In summary, despite their overall slower responses, patients showed normal performance on every measure of the number comparison process.
Masked Priming. We then tested the prediction that patients should be unimpaired on measures of masked priming and subliminal motor conflict. This prediction was tested by an ANOVA on median RT from all masked trials, with factors of group, prime-target relation (congruent repeated, congruent nonrepeated, and incongruent), and notation identity (prime and target in same or in different notation). The main effect of prime-target relation was significant overall [F(1,31) = 14.42, P < 0.0001] and in each group (controls, P = 0.0003; patients, P = 0.015; Fig. 2). It could be decomposed into two distinct effects that replicated our earlier results (21-23): response priming and quantity priming. First, responses were faster on congruent nonrepeated trials than on incongruent trials [508 vs. 519 ms, t(31) = 2.95, one-tailed P = 0.003]. Second, within the congruent trials, responses tended to be even faster when the same number was presented as prime and target, than when two different numbers were presented [502 vs. 508 ms; t(31) = 1.83, one-tailed P = 0.038]. Both effects were unaffected by changes in number notation. Crucially, there were no interactions with group. In particular, subliminal response priming was identical and significant within each group (Fig. 2).
Prime Detection. Behavioral data from the prime detection block, where subjects performed a present/absent judgement on the masked primes, were used to compute a prime detection score (d′) for each of the four masked durations and for the unmasked 43-ms condition. An ANOVA with prime presentation (five levels) and group (normal subjects and patients) revealed a progressive improvement with prime duration (43 ms, d′ = 0.56; 71 ms, d′ = 1.15; 114 ms, d′ = 1.39; 200 ms, d′ = 2.18; and 43 ms unmasked, d′ = 2.74). There was also a main effect of group (P = 0.02): the detection of primes was better in controls than in patients, in agreement with previous studies (40, 41). There was no duration by group interaction. Even at the duration of 43 ms, which was used during fMRI, prime perception was better than chance (Z test, P < 0.0001), and higher in normal subjects than in patients (d′ = 0.88 vs. 0.29, P = 0.03).
The d′ values in normal subjects were higher than in our previous experiments (21), perhaps due to the use of a different fMRI setup and video projector. To evaluate whether this small amount of prime visibility could account for the masked priming effect in RTs, we used a regression method developed by Greenwald et al. (42). Using each subject's mean response time to masked incongruent and congruent trials (RTICG and RTCG), we computed an individual priming index I = 100 × (RTICG - RTCG)/(RTICG + RTCG) and correlated it with the individual d′ values for 43-ms masked primes. There was no significant correlation between d′ and the amount of priming (P = 0.75). Crucially, the priming index at the d′ = 0 intercept was significantly positive (I = 5.5%, P = 0.001), suggesting significant priming in the absence of prime detection. We also conducted regression analyses separately for controls and patients, with similar results. Those findings suggest that, although the presence of masked primes could be partially detected with effort, masked priming was independent of prime visibility and identical in both groups.
Comparison of Masked and Unmasked Priming. A final behavioral analysis tested the prediction that patients are impaired in conscious conflict control. An ANOVA on median RT from the fMRI trials (with factors of group, prime-target relation, and masking) revealed a group by masking interaction [F(1,31) = 7.87, P = 0.0086]. In normal subjects, the unmasked condition was slower than the masked condition (34 ms effect, P < 0.005). However, this effect was about three times larger in the patients (93 ms effect, P < 0.0003), indicating a disproportionately greater difficulty in controlling interference from an unmasked prime than from a masked prime.
Furthermore, a triple interaction of group, prime-target relation, and masking [F(2, 62) = 3.76, P = 0.029] indicated an anomalous conscious conflict effect in patients (Fig. 2). As noted above, there was no group difference on masked priming, but analyses restricted to the unmasked priming condition revealed a significant group effect [F(2,28) = 6.17, P = 0.006]. As previously described (21), normal subjects were 34 ms slower when the prime and target were incongruent than when they were congruent [F(1,17) = 51.2, P < 0.0001). Surprisingly, this effect was absent in patients (effect size -12 ms, F < 1), creating a significant interaction with group [F(1,31) = 7.72, P = 0.0092].
Even within the unmasked condition, repetition priming itself was normal. Responses were faster when the prime and target were the same number [59-ms effect; F(1,31) = 25.9, P < 0.0001], especially when they appeared in the same notation (interaction F = 4.00, P = 0.054), but neither of those effects differed between groups (P > 0.20). Together with the normality of the distance effect, notation effect, and masked priming effect, this finding suggests that the cognitive impairment in schizophrenia is rather selectively linked to the anomalous monitoring and regulation of conscious conflicts.
Error rates were low and no significant differences were observed between groups or conditions.
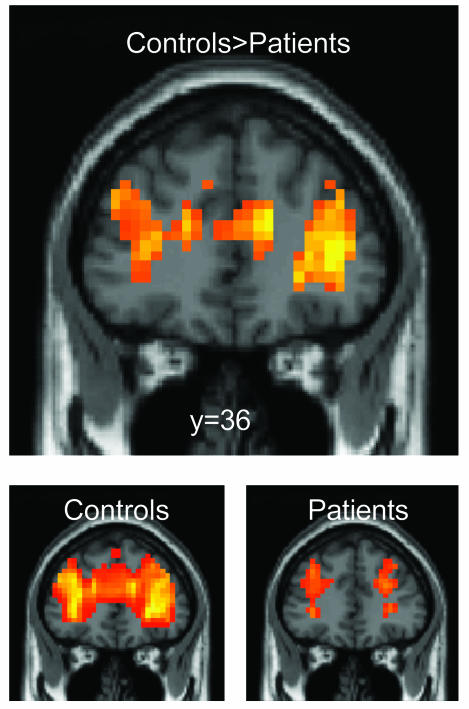
Imaging Results. In both patients and controls, a broad network was activated during task performance relative to the intertrial resting period. The main areas activated by the number comparison task, including bilateral ventral occipito-temporal, intraparietal and central sulci (39), were identically activated in patients and controls. However, several areas showed reduced activation in patients relative to controls, including the bilateral ACC, inferior frontal and middle temporal gyri, right superior frontal and right postcentral gyri, bilateral hippocampus, thalamus, and caudate nuclei (Table 1, which is published as supporting information on the PNAS web site). In particular, there was no detectable task-induced activity in the most anterior sector of the cingulate (y > 28, z < 24) in patients (Fig. 3).
Fig. 3.
Reduced overall activation of the anterior cingulate and frontal cortices, relative to the intertrial resting period, in patients relative to controls.
We first tested with normal subjects the predicted engagement of the ACC in conscious, but not subliminal, control through the interaction of the congruity effect with masking. A large extent of the ACC, particularly in the left hemisphere, showed the predicted effect. There was a greater activation on incongruent trials than on congruent trials with visible primes, but no such difference with masked primes (Fig. 4). In addition to the ACC, conscious but not subliminal conflict also affected activation in a distributed network including the bilateral inferior frontal gyri, precunei, superior and middle temporal gyri, striate cortex, cerebellum, caudate, and putamen, as well as the right central/postcentral region (Table 1).
Fig. 4.
Effect of conscious conflict in the anterior cingulate in controls and in patients. (A Upper) Congruity × visibility interaction in normal subjects, showing greater activation in ACC and other brain regions on incongruent trials than on congruent trials, but only when the prime was unmasked. This effect was not found in patients, thus resulting in a triple-interaction group × congruity × visibility (A Lower). Curves show the mean percent signal change in the left ACC as a function of time (B), revealing a hypoactivation and an absence of conscious conflict effect in the patients.
We then contrasted this pattern with that of patients. The predicted triple interaction of congruity, consciousness, and group was significant in many of the above regions, including the left ACC (Fig. 4 and Table 1). This finding reflected a significant alteration of the responses to conscious conflicts in patients compared with controls. Contrary to normal subjects, patients did not show any region with a significant congruity effect to unmasked trials. The brain oxygen-level-dependent signal in the left ACC showed both a hypoactivation and an absence of conflict effect in patients (Fig. 4).
Discussion
Our experimental results can be summarized as follows: (i) both masked and unmasked primes induced a behavioral conflict effect in normal subjects; (ii) the ACC was sensitive to conflict only when the primes were unmasked; (iii) in patients with schizophrenia, the ACC was hypoactivated and insensitive to conflict; and (iv) patients showed normal behavior in number comparison and masked priming but were disproportionately slower and showed an absence of conflict when the primes were unmasked.
The behavioral results in normal subjects replicate our earlier findings (21-23). Masked numerical primes affect the processing of a subsequent visible target number in two different ways: subjects are faster when the target is the same number as the prime (repetition priming), and when the prime induces the same response as the target (response priming). Although the latter effect is due to a conflict between motor activations induced by the prime and by the target (21), our fMRI results indicate that it does not yield a detectable activation of the ACC. This finding suggests that the ACC is not activated mechanically by any motor conflict but is particularly engaged in the regulation of consciously detected conflicts. This conclusion also fits with previous experiments that have compared brain activity to subliminal and supraliminal auditory tones (17) or written words (19). In those experiments, ACC activation occurred solely in response to the supraliminal stimuli. As in the present experiment, it was accompanied by distributed activation in distant areas, including prefrontal and parietal cortices, consistent with anatomical studies of a widespread prefrontal network involved in the cognitive regulation of behavior (43).
We cannot exclude that a subliminal conflict effect was present in the ACC but could not be detected at a statistically significant level. Note, however, that, behaviorally, the subliminal congruity effect was highly significant (P < 0.0001) and about half the size of the behavioral conscious congruity effect whereas the ACC activation curves showed not even a trend toward a difference between congruent and incongruent masked trials (Fig. 4). We also replicated this absence of a difference between congruent and incongruent trials in the ACC in a reanalysis of our earlier results with the same masked priming paradigm (21). At a minimum, this result indicates a highly nonlinear relation between the size of behavioral conflict effects and the ACC activation, as indicated by a significant congruity × masking interaction. There might be a minimal threshold on the amount of conflict needed to trigger ACC activation. The conscious workspace theory would further predict that this threshold coincides precisely with the threshold for conscious report of the masked primes. In a future experiment, this could be tested by continuously varying prime duration while monitoring prime visibility, behavioral priming, and the ACC conflict effect.
Further support for the hypothesis that the ACC and PFC do not contribute to the management of subliminal conflicts came from the study of subliminal and supraliminal conflicts in patients with schizophrenia. Our fMRI results confirm a major hypoactivation of the ACC in schizophrenia. Consistent with previous functional imaging findings (29-33) and with the well-documented anatomical features of such patients (25-28), activation was also reduced at several other sites, including inferior prefrontal, superior temporal, and subcortical regions. Despite those anomalies, behavior was strictly normal in all aspects of number processing and of subliminal priming. Even within the blocks with conscious primes, repetition priming was unaffected. This dissociation between impaired ACCPFC and intact behavioral forms of priming is consistent with the finding of normal or even enhanced repetition and semantic priming effects in schizophrenia (34, 35). Automatic priming effects are thought to arise within perceptual and semantic posterior regions such as the visual word form system (19, 23) where conflicting primes exert a subliminal competition that induces a measurable delay in response times. Similarly, subliminal response congruity effects are thought to be caused by competition at the motor level where incongruent primes induce a small transient lateralized motor preparation that is subsequently replaced, after a period of response competition, by the target-induced motor activity (21). Both forms of priming, then, seem to be resolved spontaneously within specialized processors without the need for global executive control, explaining that they do not cause ACCPFC activation and are unaffected by ACC-PFC dysfunction in schizophrenia.
As predicted, the only behavioral impairments in schizophrenia were found in the management of conscious conflict induced by the unmasked primes. First, the patients were disproportionately slow in blocks with unmasked primes, suggesting that their pathology interferes with the filtering-out of the irrelevant primes and/or the management of the interference that they induce. Indeed, our study comprised mostly stabilized patients with marked negative symptoms, who are known to often exhibit a general slowness in higher cognitive tasks (44). Second, surprisingly, our patients showed a significantly smaller conscious conflict effect than control subjects. In fMRI, this finding was accompanied by a generalized hypoactivation of anterior ACC and PFC (Fig. 4) and by an absence of a differential activation on conscious congruent vs. incongruent trials. A similar lack of mobilization of ACC and PFC in schizophrenia, in proportion to the executive demands of the task, was reported in other paradigms, such as random number generation or the Stroop task (30-32).
Our behavioral result departs from the classical finding that schizophrenia is associated with a greater number of errors in Stroop interference trials (31), although not always with a larger interference effect in response times (45, 46). One possibility is that the patients were globally slower, thus allowing for a spontaneous dissipation of the prime-induced motor interference effects and its replacement by target-induced activation. Another possibility is that unmasking the primes created new unforeseen sources of interference, including a tendency to compare the target number with the prime rather than with the memorized number 5. Such an additional interference effect may have overridden the motor congruity effect, resulting in a paradoxical reduction of this effect in the patients' behavioral results.
The observed dissociation between preserved automatic subliminal processing and impaired conscious control fits with several similar observations of a selective failure of conscious appraisal in schizophrenia. For instance, patients with schizophrenia experience a reduced perception of masked stimuli (40, 41), as replicated in our d′ measurements. Interestingly, only backward masking is impaired whereas forward masking, which is thought to be based on retinal and cortical bottom-up mechanisms, remains intact (41). Patients with schizophrenia also exhibit a dissociation between impaired explicit recollection and normal implicit memory (36-38). Together with evidence for impaired awareness of self-generated action (47), those results converge to suggest that a core deficit in schizophrenia concerns a distributed cortical conscious monitoring system that involves the ACC as a crucial node (48, 49).
Supplementary Material
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACC, anterior cingulate cortex; PFC, prefrontal cortex; RT, response time; fMRI, functional MRI.
References
- 1.Paus, T., Koski, L., Caramanos, Z. & Westbury, C. (1998) NeuroReport 9, R37-R47. [DOI] [PubMed] [Google Scholar]
- 2.Bush, G., Luu, P. & Posner, M. I. (2000) Trends Cogn. Sci. 4, 215-222. [DOI] [PubMed] [Google Scholar]
- 3.Botvinick, M., Nystrom, L. E., Fissell, K., Carter, C. S. & Cohen, J. D. (1999) Nature 402, 179-181. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, J. D., Botvinick, M. & Carter, C. S. (2000) Nat. Neurosci. 3, 421-423. [DOI] [PubMed] [Google Scholar]
- 5.Carter, C. S., Macdonald, A. M., Botvinick, M., Ross, L. L., Stenger, V. A., Noll, D. & Cohen, J. D. (2000) Proc. Natl. Acad. Sci. USA 97, 1944-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald, A. W., 3rd, Cohen, J. D., Stenger, V. A. & Carter, C. S. (2000) Science 288, 1835-1838. [DOI] [PubMed] [Google Scholar]
- 7.Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S. & Cohen, J. D. (2001) Psychol. Rev. 108, 624-652. [DOI] [PubMed] [Google Scholar]
- 8.Dehaene, S., Posner, M. I. & Tucker, D. M. (1994) Psychol. Sci. 5, 303-305. [Google Scholar]
- 9.Carter, C. S., Braver, T. S., Barch, D., Botvinick, M. M., Noll, D. & Cohen, J. D. (1998) Science 280, 747-749. [DOI] [PubMed] [Google Scholar]
- 10.Posner, M. I. & Rothbart, M. K. (1998) Philos. Trans. R. Soc. London B Biol. Sci. 353, 1915-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posner, M. I. (1994) Proc. Natl. Acad. Sci. USA 91, 7398-7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehaene, S., Kerszberg, M. & Changeux, J. P. (1998) Proc. Natl. Acad. Sci. USA 95, 14529-14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dehaene, S. & Naccache, L. (2001) Cognition 79, 1-37. [DOI] [PubMed] [Google Scholar]
- 14.Dehaene, S., Sergent, C. & Changeux, J. P. (2003) Proc. Natl. Acad. Sci. USA 100, 8520-8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bush, G., Vogt, B. A., Holmes, J., Dale, A. M., Greve, D., Jenike, M. A. & Rosen, B. R. (2002) Proc. Natl. Acad. Sci. USA 99, 523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holroyd, C. B. & Coles, M. G. (2002) Psychol. Rev. 109, 679-709. [DOI] [PubMed] [Google Scholar]
- 17.Stephan, K. M., Thaut, M. H., Wunderlich, G., Schicks, W., Tian, B., Tellmann, L., Schmitz, T., Herzog, H., McIntosh, G. C., Seitz, R. J. & Homberg, V. (2002) NeuroImage 15, 345-352. [DOI] [PubMed] [Google Scholar]
- 18.Laureys, S., Faymonville, M. E., Luxen, A., Lamy, M., Franck, G. & Maquet, P. (2000) Lancet 355, 1790-1791. [DOI] [PubMed] [Google Scholar]
- 19.Dehaene, S., Naccache, L., Cohen, L., Le Bihan, D., Mangin, J. F., Poline, J. B. & Rivičre, D. (2001) Nat. Neurosci. 4, 752-758. [DOI] [PubMed] [Google Scholar]
- 20.Portas, C. M., Krakow, K., Allen, P., Josephs, O., Armony, J. L. & Frith, C. D. (2000) Neuron 28, 991-999. [DOI] [PubMed] [Google Scholar]
- 21.Dehaene, S., Naccache, L., Le Clec'H, G., Koechlin, E., Mueller, M., Dehaene-Lambertz, G., van de Moortele, P. F. & Le Bihan, D. (1998) Nature 395, 597-600. [DOI] [PubMed] [Google Scholar]
- 22.Naccache, L. & Dehaene, S. (2001) Cognition 80, 215-229. [DOI] [PubMed] [Google Scholar]
- 23.Naccache, L. & Dehaene, S. (2001) Cereb. Cortex 11, 966-974. [DOI] [PubMed] [Google Scholar]
- 24.Benes, F. M., Vincent, S. L. & Todtenkopf, M. (2001) Biol. Psychiatry 50, 395-406. [DOI] [PubMed] [Google Scholar]
- 25.Szeszko, P. R., Bilder, R. M., Lencz, T., Ashtari, M., Goldman, R. S., Reiter, G., Wu, H. & Lieberman, J. A. (2000) Schizophr. Res. 43, 97-108. [DOI] [PubMed] [Google Scholar]
- 26.Sigmundsson, T., Suckling, J., Maier, M., Williams, S., Bullmore, E., Greenwood, K., Fukuda, R., Ron, M. & Toone, B. (2001) Am. J. Psychiatry 158, 234-243. [DOI] [PubMed] [Google Scholar]
- 27.Paillere-Martinot, M., Caclin, A., Artiges, E., Poline, J., Joliot, M., Mallet, L., Recasens, C., Attar-Levy, D. & Martinot, J. (2001) Schizophr. Res. 50, 19-26. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki, M., Nohara, S., Hagino, H., Kurokawa, K., Yotsutsuji, T., Kawasaki, Y., Takahashi, T., Matsui, M., Watanabe, N., Seto, H. & Kurachi, M. (2002) Schizophr. Res. 55, 41-54. [DOI] [PubMed] [Google Scholar]
- 29.Andreasen, N. C., O'Leary, D. S., Flaum, M., Nopoulos, P., Watkins, G. L., Boles Ponto, L. L. & Hichwa, R. D. (1997) Lancet 349, 1730-1734. [DOI] [PubMed] [Google Scholar]
- 30.Artiges, E., Salame, P., Recasens, C., Poline, J. B., Attar-Levy, D., De La Raillere, A., Paillere-Martinot, M. L., Danion, J. M. & Martinot, J. L. (2000) Am. J. Psychiatry 157, 1517-1519. [DOI] [PubMed] [Google Scholar]
- 31.Carter, C. S., Mintun, M., Nichols, T. & Cohen, J. D. (1997) Am. J. Psychiatry 154, 1670-1675. [DOI] [PubMed] [Google Scholar]
- 32.Carter, C. S., MacDonald, A. W., 3rd, Ross, L. L. & Stenger, V. A. (2001) Am. J. Psychiatry 158, 1423-1428. [DOI] [PubMed] [Google Scholar]
- 33.Meyer-Lindenberg, A., Poline, J. B., Kohn, P. D., Holt, J. L., Egan, M. F., Weinberger, D. R. & Berman, K. F. (2001) Am. J. Psychiatry 158, 1809-1817. [DOI] [PubMed] [Google Scholar]
- 34.Minzenberg, M. J., Ober, B. A. & Vinogradov, S. (2002) J. Int. Neuropsychol. Soc. 8, 699-720. [DOI] [PubMed] [Google Scholar]
- 35.Hoschel, K. & Irle, E. (2001) Schizophr. Bull. 27, 317-327. [DOI] [PubMed] [Google Scholar]
- 36.Huron, C., Danion, J. M., Giacomoni, F., Grange, D., Robert, P. & Rizzo, L. (1995) Am. J. Psychiatry 152, 1737-1742. [DOI] [PubMed] [Google Scholar]
- 37.Kazes, M., Berthet, L., Danion, J. M., Amado, I., Willard, D., Robert, P. & Poirier, M. F. (1999) Neuropsychology 13, 54-61. [DOI] [PubMed] [Google Scholar]
- 38.Danion, J. M., Meulemans, T., Kauffmann-Muller, F. & Vermaat, H. (2001) Am. J. Psychiatry 158, 944-948. [DOI] [PubMed] [Google Scholar]
- 39.Dehaene, S. (1996) J. Cognit. Neurosci. 8, 47-68. [DOI] [PubMed] [Google Scholar]
- 40.Green, M. F., Nuechterlein, K. H., Breitmeyer, B. & Mintz, J. (1999) Am. J. Psychiatry 156, 1367-1373. [DOI] [PubMed] [Google Scholar]
- 41.Saccuzzo, D. S., Cadenhead, K. S. & Braff, D. L. (1996) Am. J. Psychiatry 153, 1564-1570. [DOI] [PubMed] [Google Scholar]
- 42.Greenwald, A. G., Draine, S. C. & Abrams, R. L. (1996) Science 273, 1699-1702. [DOI] [PubMed] [Google Scholar]
- 43.Goldman-Rakic, P. S. (1988) Annu. Rev. Neurosci. 11, 137-156. [DOI] [PubMed] [Google Scholar]
- 44.Salame, P., Danion, J. M., Peretti, S. & Cuervo, C. (1998) Schizophr. Res. 30, 11-29. [DOI] [PubMed] [Google Scholar]
- 45.Chen, E. Y., Wong, A. W., Chen, R. Y. & Au, J. W. (2001) Schizophr. Res. 48, 29-44. [DOI] [PubMed] [Google Scholar]
- 46.Barch, D. M., Carter, C. S., Hachten, P. C., Usher, M. & Cohen, J. D. (1999) Schizophr. Bull. 25, 749-762. [DOI] [PubMed] [Google Scholar]
- 47.Franck, N., Farrer, C., Georgieff, N., Marie-Cardine, M., Dalery, J., d'Amato, T. & Jeannerod, M. (2001) Am. J. Psychiatry 158, 454-459. [DOI] [PubMed] [Google Scholar]
- 48.Frith, C. D., Blakemore, S. & Wolpert, D. M. (2000) Brain Res. Brain Res. Rev. 31, 357-363. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher, P., McKenna, P. J., Friston, K. J., Frith, C. D. & Dolan, R. J. (1999) NeuroImage 9, 337-342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.