Abstract
Angelman syndrome is a severe neurodevelopmental disorder mostly caused by loss-of-function mutations in the maternal allele of UBE3A, a gene that encodes an E3 ubiquitin ligase. Drosophila UBE3A (dUBE3A) is highly homologous to human UBE3A (hUBE3A) at the amino acid sequence level, suggesting their functional conservation. We generated dUBE3A-null mutant fly lines and found that dUBE3A is not essential for viability. However, loss of dUBE3A activity reduced dendritic branching of sensory neurons in the peripheral nervous system and slowed the growth of terminal dendritic fine processes. Several lines of evidence indicated that dUBE3A regulates dendritic morphogenesis in a cell autonomous manner. Moreover, overexpression of dUBE3A also decreased dendritic branching, suggesting that the proper level of dUBE3A is critically important for the normal dendritic patterning. These findings suggest that dendritic pathology may contribute to neurological deficits in patients with Angelman syndrome.
INTRODUCTION
In many neurons, dendritic branches account for >90% of the neuronal surface that receives synaptic input from other neurons. Therefore, the proper formation of dendritic branching patterns is critically important for neuronal function and connectivity. Despite considerable progress in understanding the molecular and genetic pathways that control different aspects of dendritic morphogenesis (1–4), little is known about how abnormalities in dendritic formation contribute to neurodevelopmental disorders.
Angelman syndrome (AS) is a severe developmental brain disorder characterized by mental retardation, seizures, abnormal gait, frequent laughter and other abnormalities (5). At least four genomic abnormalities cause AS (6,7). Most patients have de novo deletions of about 4 Mb on maternal chromosome 15q11–q13. Another genomic abnormality, paternal uniparental disomy on chromosome 15, results in two copies of paternal chromosomes but no maternal copy. A third abnormality, found in a small group of patients, is caused by paternal methylation of both paternal and maternal copies of chromosome 15q11–q13, resulting in loss of expression of the maternal copy. The fourth abnormality is loss-of-function point mutations in UBE3A, which has been found in some AS patients with classical symptoms. These genetic evidence indicate that UBE3A is primarily responsible for many if not all cases of AS (8,9).
UBE3A encodes E6-AP ubiquitin ligase, a binding partner of the E6 protein of human papilloma virus that promotes the degradation of the p53 oncoprotein (10,11). UBE3A is imprinted in the brain, especially in the hippocampus and cerebellum (12–14). Homozygous UBE3A knockout mice are growth-retarded and have reduced survival on different genetic backgrounds (15). Mice with maternal deficiency of UBE3A and a normal copy of the paternal gene have defects in context-dependent learning and long-term potentiation (15). Interestingly, the molecular and cellular deficits of an AS mouse model can be rescued by reducing inhibitory phosphorylation of αCAMKII (16). Loss of maternal UBE3A appears to reduce spine density and length on hippocampal neurons (17). However, it is unclear whether the formation of terminal fine dendritic branches is affected.
Drosophila has been used successfully over the years as an excellent model system to understand the genes and molecular pathways that are misregulated in human disease conditions (18,19). In the Drosophila genome, one gene, CG6190, encodes a protein highly homologous to human UBE3A (hUBE3A). Drosophila UBE3A (dUBE3A) contains eight exons that encode a protein with 973 amino acids. The C-terminal 350-amino acid HECT domains of dUBE3A and hUBE3A share 62% identity, suggesting their functional conservation (20). In this study, we generated mutant flies lacking dUBE3A and investigated its in vivo function in dendritic morphogenesis, especially the formation of terminal fine dendritic branches.
RESULTS
Generation of dUBE3A mutant flies
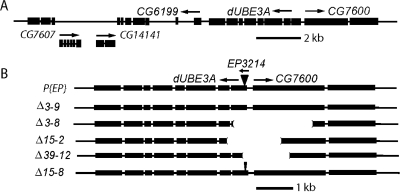
To examine the phenotypes of dUBE3A loss-of-function mutant alleles, we generated fly lines in which dUBE3A was not expressed. dUBE3A is located on chromosome 3L and flanked by two previously uncharacterized genes, CG6199, which encodes a protein homologous to procollagen-lysine 2-oxoglutarate 5-dioxygenase, and CG7600, which encodes a conserved protein of unknown function (Fig. 1A). From the Bloomington Stock Center, we obtained a fly line (EP3214) containing an EP element in the first exon of dUBE3A, 293 nt upstream of the ATG start codon (Fig. 1B). This P-element insertion did not significantly reduce dUBE3A expression (data not shown).
Figure 1.
Generation of dUBE3A mutant alleles. (A) Genomic organization of the dUBE3A locus. dUBE3A contains eight exons and is located on the chromosome 3L and flanked by genes CG6199 and CG7600. Arrows indicate the direction of transcription. (B) Schematic of dUBE3A mutant alleles generated with the P-element local hop-out approach. An EP element is inserted in the first exon of dUBE3A. Excision of this P-element resulted in the generation of deletion mutant alleles of dUBE3A. The size of each deletion was determined by the sequencing of PCR fragments covering the deletion sites. In line Δ15-8, a 1156 bp fragment from the P-element remained in the first exon of dUBE3A, creating an insertional mutant allele.
Our phenotypic analysis relies heavily on the UAS-Gal4 system (21), and the presence of Gal4 drivers will likely lead to the overexpression of dUBE3A through the UAS elements in the EP sequence (Fig. 1B). Therefore, the EP line itself is not suitable for our genetic analysis. To generate loss of function mutant alleles, we used a P-element local hop-out mutagenesis approach. First, the EP3214 line was outcrossed with w1118 flies for several generations to eliminate the background lethal mutation(s) on chromosome 3. Isogenic adult flies homozygous for EP3214 were viable and fertile and were mated with flies expressing transposase. In most cases, EP3214 was excised without any damage to the genomic region (e.g. line Δ3–9) (Fig. 1B). In a few instances, genomic deletions occurred near the insertion site of the EP element, generating potential dUBE3A-null alleles (Fig. 1B). PCR analysis confirmed genomic deletions in the first or second exon of dUBE3A and the first exon of CG7600 in some mutant lines (e.g. line Δ3–8). Fortuitously, in line Δ15–8, a 1.1 kb DNA fragment from the EP element was left behind in the genome after the hop-out (Fig. 1B), creating a new insertional mutant allele.
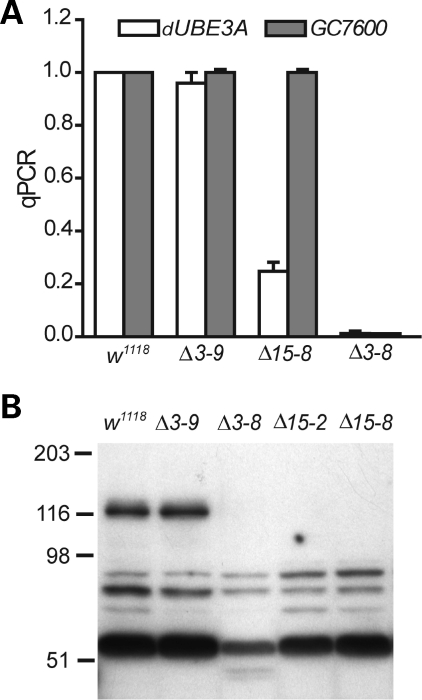
Adult flies that were homozygous for all the deletion mutations or the 1.1 kb insertion were viable. To confirm the generation of dUBE3A-null alleles, we performed two experiments. First, we analyzed total RNA from homozygous mutant flies by quantitative PCR. As expected, the level of dUBE3A mRNA was normal in line Δ3–9, dramatically reduced in line Δ15–8 and absent in line Δ3–8 (Fig. 2A). Earlier PCR analysis on the genomic DNA indicated that all the deletion mutants also affected CG7600 (Fig. 1B). Indeed, CG7600 mRNA was undetectable in line Δ3–8 (Fig. 2A). However, the 1.1 kb insertion in the first exon of dUBE3A specifically reduced the expression of dUBE3A mRNA without affecting the expression of CG7600 mRNA (Fig. 2A), providing a useful strong dUBE3A loss of function allele for further phenotypic analysis. Second, we generated rabbit polyclonal antibodies against the first 300 amino acids of dUBE3A and confirmed, by western blot analysis, the absence of the dUBE3A protein in several mutants, including Δ3–8 and Δ15–8 (Fig. 2B).
Figure 2.
Absence of dUBE3A expression in the mutant alleles. (A) Quantitative PCR (qPCR) analysis of relative mRNA levels of dUBE3A and CG7600 in adult flies. Values are mean ± SEM. (B) Expression levels of dUBE3A protein in fly head extracts. Fly heads were used for western blot analysis because smaller dUBE3A fragments, presumably due to non-specific degradation, were observed when protein extracts from whole flies were used (not shown). This polyclonal antibody recognized several non-specific bands on western blot. However, the dUBE3A band of the predicted molecular weight was absent.
Reduced dendritic growth and branching in dUBE3A mutant larvae
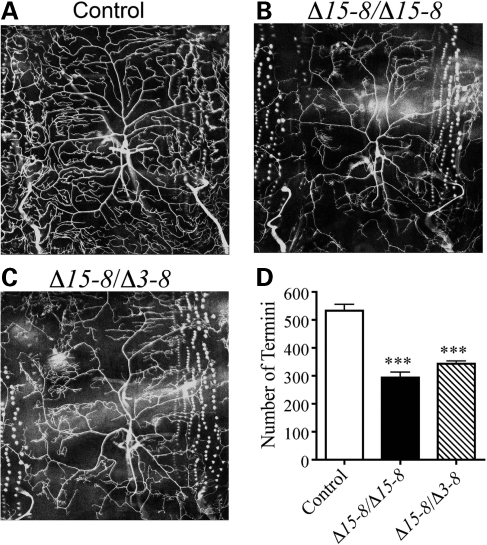
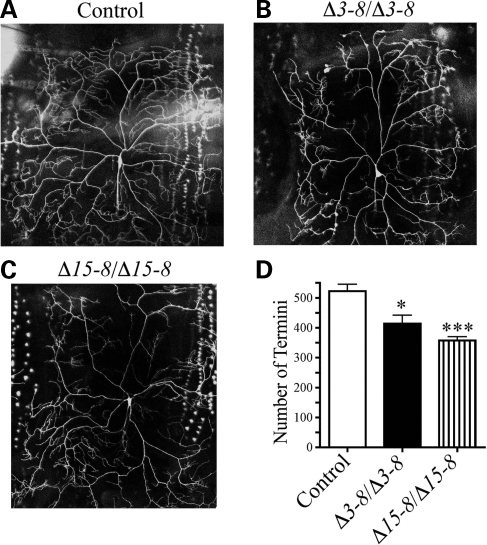
To examine the role of dUBE3A in dendritic morphogenesis, we used dendritic arborization (DA) neurons in the Drosophila peripheral nervous system as an in vivo assay system. Drosophila sensory neurons have been used to dissect the genetic pathways that control dendritic morphogenesis (4). Among these neurons, ddaC in the dorsal cluster and similar neurons in other clusters in the same segment of Drosophila third instar larvae elaborate extensive dendritic arbors that cover the whole body wall. We used Gal4477 to label ddaC neurons with GFP in control and dUBE3A mutant larvae and quantified the number of terminal branches as described (22). For control larvae, we used either w1118 or precise hop out lines (such as Δ3–9) that did not show a difference in dendritic branching of ddaC neurons. In contrast, in dUBE3AΔ15–8 homozygous mutant third instar larvae, the number of terminal branches was significantly reduced (309.9 ± 17.8, n = 13 versus 533.0 ± 22.6, n = 11, P < 0.001) (Fig. 3B and D), although the overall branching patterns of ddaC neurons in the A3 segment, especially the primary and secondary branches, remained the same as in wild-type larvae (Fig. 3A and B).
Figure 3.
Dendritic phenotypes of ddaC neurons in dUBE3A mutant third instar larvae. GFP expression in ddaC neurons was under the control of Gal4477. (A) Gal4477, UAS-mCD8-GFP/+; +/+ larvae were used as controls. (B and C) Dendritic phenotypes were analyzed in Gal4477, UAS-mCD8-GFP/+; dUBE3AΔ15-8/dUBE3AΔ15-8 (B) and Gal4477, UAS-mCD8-GFP/+; dUBE3AΔ15-8/dUBE3AΔ3-8 (C) larvae at the third instar stage. (D) Quantification of the number of dendritic termini of ddaC neurons for genotypes in (A–C). Values are mean ± SEM. ***P < 0.001.
To further confirm this phenotype, we examined dUBE3A mutant larvae transheterozygous with Δ3–8 and Δ15–8 alleles that revealed a similar dendritic phenotype (Fig. 3C and D). Moreover, in wild-type larvae, the terminal dendritic branches of ddaC neurons extended to target the body wall receptive field more or less evenly (Fig. 3A). However, these branches were much shorter in homozygous dUBE3AΔ15–8 mutants and transheterozygous dUBE3AΔ15–8/dUBE3AΔ3–8, and patches of the target field were devoid of dendrites (Fig. 3B and C). Thus, dUBE3A is required for the proper growth and branching of terminal dendrites of ddaC neurons.
dUBE3A regulates dendritic morphogenesis in a cell autonomous manner
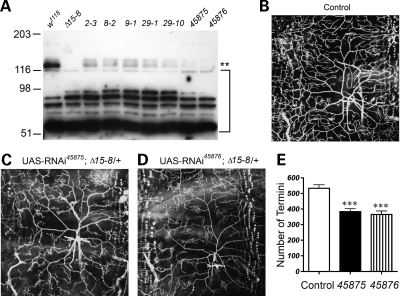
To determine whether the dendritic phenotype in dUBE3A mutant larvae arose through effects of dUBE3A in neurons or through a non-cell-autonomous mechanism, we used RNAi to knock down dUBE3A expression only in ddaC neurons. First, we tested the effectiveness of different UAS-dUBE3A RNAi lines generated in our laboratory or obtained from the Vienna Drosophila RNAi Center (VDRC). The RNAi constructs were expressed in all cell types under the control of tubulin-Gal4, and proteins from adult fly heads were analyzed by western blot. UAS-dUBE3A RNAi (VDRC45875) and UAS-dUBE3A RNAi (VDRC45876) downregulated dUBE3A expression more effectively than several UAS-dUBE3A RNAi lines we generated (Fig. 4A). Polyclonal antibody against recombinant dUBE3A recognized two bands on western blot, both of which were absent in homozygous dUBE3AΔ15–8 mutants and dramatically downregulated in flies expressing UAS-dUBE3A RNAi (VDRC45876), raising the possibility that the top band may be a result of post-translational modification. The biological significance of this modification remains to be determined.
Figure 4.
Genetic analysis of dUBE3A function in dendritic morphogenesis using the RNAi approach. (A) Western blot analysis of dUBE3A expression in extracts of heads from adult control flies, dUBE3AΔ15-8 homozygous mutants and flies expressing different UAS-RNAi constructs under the control of tubulin-Gal4. UAS-RNAi lines 2-3, 8-2, 9-1, 29-1 and 29-10 were generated in our laboratory; 45875 and 45876 were from the VDRC. The bracket indicates the non-specific bands recognized by the dUBE3A polyclonal antibody. Asterisks indicate dUBE3A; the top band is likely a post-translationally modified form. (B) A wild-type ddaC neuron in the A3 segment of a third instar larva. The number of dendritic ends of ddaC neurons in dUBE3AΔ15-8/+ is slightly reduced (See Fig. 7). (C) A ddaC neuron expressing UAS-dUBE3A RNAi (VDRC45875) in the dUBE3AΔ15-8/+ background. (D) A ddaC neuron expressing UAS-dUBE3A RNAi (VDRC45876) in the dUBE3AΔ15-8/+ background. (E) Quantification of dendritic ends from ddaC neurons in the A3 segment from larvae in panels (B–D). Values are mean ± SEM. ***P < 0.001.
To examine the developmental consequence of loss of dUBE3A activity, we targeted the expression of UAS-dUBE3A RNAi (VDRC45875) and UAS-dUBE3A RNAi (VDRC45876) to ddaC neurons using Gal4477. To enhance the effects of RNAi, the RNAi constructs were expressed in the dUBE3AΔ15–8/+ background. The resulting dendritic growth and branching phenotype were highly similar to that in dUBE3A mutant larvae. For instance, there were fewer terminal dendritic branches (VDRC45875, 362.1 ± 23.3, n = 9 versus control 524.3 ± 22.6, n = 11, P < 0.001; VDRC45876, 383.7 ± 18.4, n = 10 versus control 524.3 ± 22.6, n = 11, P < 0.001) (Fig. 4B–E).
To further test the cell autonomous function of dUBE3A, we performed mosaic analysis with a repressible cell marker (MARCM) (23). This technique allows GFP labeling of single ddaC neurons with defined genetic mutations in an otherwise wild-type larva. dUBE3A mutant ddaC neurons labeled by MARCM also exhibited a dendritic phenotype similar to that in mutant larva and in ddaC neurons expressing RNAi constructs (Fig. 5), further demonstrating that dUBE3A functions in a cell autonomous manner to control dendritic growth and branching. When GFP-tagged dUBE3A was expressed in ddaC neurons, the GFP signal was present throughout the dendritic trees (data not shown). This finding raises the possibility that dUBE3A may regulate the abundance of its substrates locally to control the formation of terminal dendritic branches.
Figure 5.
MARCM analysis of the cell autonomous function of dUBE3A in the dendritic morphogenesis of ddaC neurons. (A) A control ddaC neuron in the A3 segment of a third instar larva. (B) A dUBE3AΔ3-8 homozygous mutant ddaC neuron in the A3 segment of a third instar larva. (C) A dUBE3AΔ15-8 mutant ddaC neuron. (D) Quantification of the number of terminal dendritic branches from ddaC neurons with the genotypes of (A–C). Values are mean ± SEM. *P < 0.05; ***P < 0.001.
dUBE3A is required for the proper dendritic formation of other neurons
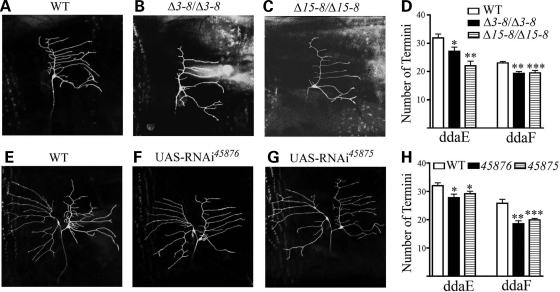
To extend our phenotypic analysis to other neurons in Drosophila larvae, we examined ddaE and ddaF neurons in the dorsal cluster. These sensory neurons extend smooth dendritic branches over a much smaller dendritic field than ddaC neurons (24,25). MARCM analysis revealed that dUBE3A is also required cell-autonomously for proper branching of ddaE and ddaF neurons: the number of dendritic branches was decreased in ddaE neurons that were homozygous for the dUBE3AΔ3–8 (27.1 ± 1.5, n = 7, versus 31.8 ± 2.1, n = 13, P < 0.05) (Fig. 6B and D) or dUBE3AΔ15–8 allele (22.0 ± 1.7, n = 8, versus 31.8 ± 2.1, n = 13, P < 0.01) (Fig. 6C and D). MARCM-labeled ddaF neurons exhibited a similar phenotype (Fig. 6D). The expression of UAS-dUBE3A RNAi driven by Gal4221 decreased the number of dendritic branches in both ddaE neurons (VDRC45876, 27.8 ± 1.2, n = 20 versus control, 32.0 ± 1.0, n = 10, P < 0.05; VDRC45875, 29.2 ± 0.9, n = 9 versus control 32.0 ± 1.0, n = 10, P < 0.05) (Fig. 6E–H). In ddaF neurons, a similar phenotype was observed (VDRC45876, 18.6 ± 1.0, n = 20 versus control, 25.8 ± 1.3, n = 20, P < 0.01; VDRC45875, 19.9 ± 0.5, n = 9 versus control 25.8 ± 1.3, n = 20, P < 0.001) (Fig. 6E–H). These results indicate that dUBE3A is required cell-autonomously for the proper branching of different neurons in the Drosophila PNS.
Figure 6.
Cell autonomous effects of dUBE3A on dendritic branching in ddaE and ddaF neurons. (A) A control ddaE neuron labeled by GFP. (B) A ddaE neuron homozygous for the dUBE3AΔ3-8 allele. (C) A ddaE neuron homozygous for the dUBE3AΔ15-8 allele. The GFP-labeled ddaE neurons in (A–C) were generated by the MARCM technique. (D) Quantification of the number of dendritic branches of ddaE and ddaF neurons of different genotypes. (E) A wild-type ddaE and a wild-type ddaF neuron labeled by GFP under the control of Gal4221. (F) A ddaE and a ddaF neuron expressing UAS-dUBE3A RNAi (VDRC45876) driven by Gal4221. (G) A ddaE and a ddaF neuron expressing UAS-dUBE3A RNAi (VDRC45875) driven by Gal4221. (H) Quantification of the number of dendritic branches of ddaE and ddaF neurons expressing GFP and RNAi constructs. Values are mean ± SEM. *P < 0.05; ***P < 0.001.
Genetic interaction between dUBE3A and eff that encodes an E2 ligase
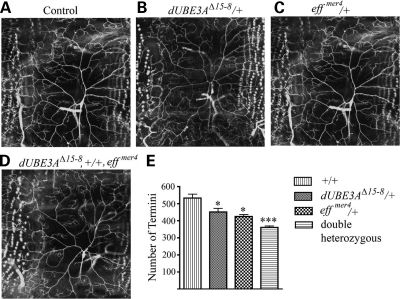
ddaC neurons in dUBE3A mutants fail to completely prune their dendrites during early metamorphosis (unpublished data). A similar pruning defect is found in eff mutants (26), raising the possibility that eff, the gene encoding the E2 ubiquitin-conjugating enzyme UbcD1, may function in the same genetic pathway. To test whether such a genetic interaction occurs in dendritic morphogenesis during larval development, we examined the dendritic branching phenotype in dUBE3A and eff transheterozygous larvae. Loss of one copy of dUBE3A and one copy of eff had a more severe effect in reducing the number of terminal dendritic branches of ddaC neurons than that in dUBE3A or eff heterozygous larvae alone (Fig. 7). For this experiment, we used two different eff alleles (effmer4 and effs1782) and obtained similar results. Thus, UbcD1 and dUBE3A may act in concert to regulate the levels of their target proteins, which in turn may influence dendritic growth and branching.
Figure 7.
Genetic interaction between dUBE3A and eff in dendritic morphogenesis. (A) A control ddaC neuron in the A3 segment of a third instar larva. (B) A ddaC neuron in a heterozygous dUBE3AΔ15-8/+ third instar larva. (C) A ddaC neuron in a heterozygous eff/+ third instar larva. (D) A ddaC neuron in a transheterozygous dUBE3A/eff third instar larva. (E) Quantification of the number of dendritic termini of ddaC neurons in different genetic backgrounds. Values are mean ± SEM. *P < 0.05; ***P < 0.001.
The proper level of dUBE3A is critical for neuronal development
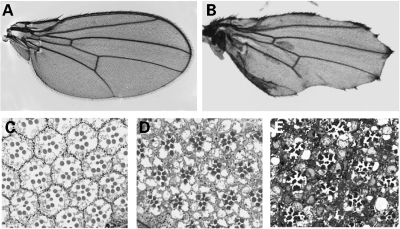
To further understand the function of dUBE3A, we also cloned full length of dUBE3A and generated different UAS-dUBE3A transgenic fly lines. We overexpressed dUBE3A in different tissues using the UAS-Gal4 system and examined the developmental defects. The expression of dUBE3A in the Drosophila embryos by the tubulin-Gal4 led to a lethal phenotype at embryonic and early larval stages. The expression of dUBE3A in the developing nervous system by the Elav-Gal4, which is on the X chromosome, caused male lethality. We also expressed dUBE3A in the wing discs using the vg-Gal4 and observed a dramatic loss of wing margins (Fig. 8B). The expression of dUBE3A in the eye by the GMR-Gal4 caused a rough-eye phenotype and a significant disorganization of omatidia in 1-day-old flies (Fig. 8D), and degeneration of photoreceptors worsens over time (Fig. 8E). These findings suggest that increased expression of dUBE3A can cause severe developmental defects. During the review process of our manuscript, Wu et al. (27) reported the generation of dUBE3A mutant flies and the overexpression phenotypes in the wing and the eye that are very similar to our findings presented here.
Figure 8.
dUBE3A overexpression phenotypes in the wing and the eye. (A) A wild-type wing from a 3-day-old vg-Gal4 fly. (B) A wing expressing dUBE3A under the control of the vg-Gal4. (C) A section of an eye from a 3-day-old GMR-Gal4 fly showing the regular arrangement of omatidia. (D) A section of an eye from a 1-day-old fly expressing dUBE3A under the control of GMR-Gal4. (E) A section of an eye from a 7-day-old fly expressing dUBE3A by the GMR-Gal4 showing extensive retinal degeneration.
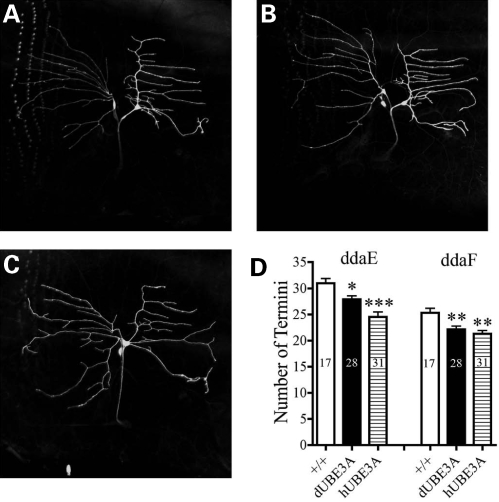
To examine the effects of dUBE3A expression on neuronal development, we targeted UAS-dUBE3A to a subset of DA sensory neurons using the Gal4221 (Fig. 9A). Interestingly, the expression of dUBE3A decreased dendritic branching of ddaE and ddaF neurons (Fig. 9B), a phenotype similar to that caused by loss of dUBE3A activity. This finding suggests that the proper level of dUBE3A is critically important for the normal differentiation of neurons. We also expressed hUBE3A (20) in a subset of fly sensory neurons and observed a similar phenotype (Fig. 9C), suggesting a functional conservation in dendritic patterning.
Figure 9.
The effects of ectopic expression of dUBE3A and hUBE3A on dendritic branching of a subset of Drosophila sensory neurons. (A) Wild-type ddae and ddaF neurons are labeled by GFP under the control of the Gal4221. (B) A representative image of ddaE and ddaF neurons that express dUBE3A. (C) A representative image of ddaE and ddaF neurons that express hUBE3A. (D) Quantifications of the number of dendirtic ends of ddaE and ddaF neurons with different genetic backgrounds as in (A–C). Values are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. The sample numbers for each genotype are listed in the middle of each column.
DISCUSSION
Since the mental retardation disorder AS is primarily caused by loss of the UBE3A protein product, it is of great importance to understand the normal function of UBE3A in neuronal development and synaptic plasticity. In this study, we used the P-element local hop-out approach to generate dUBE3A-null mutant fly lines. Although UBE3A knockout mice often die shortly after birth (15), dUBE3A mutant flies were viable to adulthood. The EP3214 line we obtained from the Bloomington Stock Center was homozygous lethal, but the lethality was probably not due to the EP insertion in the dUBE3A locus itself, as we could segregate the EP insertion from the background lethal mutation(s) after outcrosses with wild-type flies.
To understand the role of dUBE3A in neuronal development, we focused on the dendritic morphogenesis of DA neurons in the Drosophila PNS. One of the advantages of using DA neurons for phenotypic analysis is the ease of visualizing their dendrites in living animals. The dendritic trees of DA neurons are sandwiched between the epidermis and the body muscle wall (28) and are essentially two-dimensional. Thus, the number of dendritic branches, especially their terminal fine processes, can be easily quantified at high resolution. Using this system, we found that loss of dUBE3A reduced the formation of terminal dendritic branches. This finding is consistent with the notion that dendritic pathology contributes to the pathogenesis of AS, as shown by the reduced length and density of dendritic spines in cerebellar, cortical and hippocampal neurons and by the relatively normal appearance of dendritic trees stained with calbindin and examined by light microscopy (17). In dUBE3A mutant larvae, the major dendritic branches of ddaC neurons also appeared to be normal. Our finding that the development of terminal fine dendritic processes was affected by dUBE3A in Drosophila raises the possibility that this defect contributes to the neurological deficits in AS patients and mouse models. It is interesting to note that the overexpression of dUBE3A also decreased dendritic branching, which may have some implications for some forms of autism in which the genomic region containing UBE3A is duplicated (29). During the review process of this manuscript, Wu et al. reported independently the generation of dUBE3A mutant flies and their behavior phenotypes (27). It is plausible that dendritic developmental defects of CNS neurons in dUBE3A mutants may underlie, at least in part, the behavioral abnormalities of these flies.
Another advantage of the Drosophila model system is the ability to examine the cell autonomous functions of a gene of interest (23). Our genetic analyses provide strong evidence that dUBE3A influences dendritic morphogenesis in a cell autonomous manner. Dendritic pathology has been implicated in fragile X syndrome, Rett syndrome and autism (30–34). Although the genes mutated in these neurodevelopmental disorders are different, including FMR1, an RNA-binding protein, MeCP2, a transcription regulator, and UBE3A, an E3 ubiquitin ligase, their downstream targets may participate in the same genetic pathways that regulate the formation of dendritic branches and dendritic spines. Loss of MECP2 activity leads to a significant reduction in UBE3A expression in human brains (35). This finding may help explain the decreased dendritic branching and synaptogenesis caused by MECP2 deficiency (36–39). However, MECP2 knockout mice show normal levels of UBE3A (40); therefore, it seems that there is no direct genetic link between MECP2 and UBE3A.
The substrates that are regulated by UBE3A to mediate its effects on dendritic development are still largely unknown. The Rho-GEF Pebble was identified as a candidate target for UBE3A in both flies and mice (20). However, the expression of Pebble in Drosophila sensory neurons seemed to increase dendritic branching complexity (unpublished data). Therefore, it is unlikely that the potential increase in Pebble expression could account for the decreased dendritic branching in dUBE3A mutant flies even though Pebble may well contribute to other cellular processes affected by dUBE3A. It is conceivable that dUBE3A may have many substrates, and elevated expression of some of them increases dendritic branching while others decrease dendritic branching. The availability of dUBE3A-null mutant flies and powerful genetic tools in this model system will facilitate the identification of UBE3A substrates that mediate its effect on a specific developmental process and may provide further insights into the molecular pathogenesis of AS.
MATERIALS AND METHODS
Fly strains and genetics
All flies were raised on standard food medium and kept at 25°C. Line EP3214 was from the Bloomington Stock Center and was homozygous lethal. To separate the background lethal mutation(s) from the EP insertion, we outcrossed EP3214 with w1118 for several generations and obtained adult viable lines homozygous for the EP insertion. The standard procedure for P-element hop-out was followed to generate genomic deletions in the dUBE3A locus.
Gal4221 was used to label ddaE and ddaF neurons with GFP and drive the expression of UAS-dUBE3A RNAi constructs. To visualize ddaC neurons in third instar larvae, Gal4477, UAS-mCD8-GFP/Gal4477, UAS-mCD8-GFP; TM3, Ser/TM6, Tb flies were crossed with dUBE3AΔ15–8/TM3 or dUBE3AΔ3–8/TM3 flies to generate Gal4477, UAS-mCD8-GFP/+; dUBE3AΔ15–8/dUBE3AΔ15–8 or Gal4477, UAS-mCD8-GFP/+; dUBE3AΔ15–8/dUBE3AΔ3–8 third instar larvae for phenotypic analysis. For RNAi expression, Gal4477, UAS-mCD8-GFP/Gal4477, UAS-mCD8-GFP; TM3, Ser/TM6, Tb flies were crossed with UAS-RNAi lines from the VDRC to obtain Gal4477, UAS-mCD8-GFP/+; UAS-RNAi/TM6, Tb flies, which were crossed with dUBE3AΔ15–8/dUBE3AΔ15–8 flies to select Gal4477, UAS-mCD8-GFP/+; UAS-RNAi, +/+, dUBE3AΔ15–8 third instar larvae for phenotypic analysis. Similar genetic crosses were performed to obtain dUBE3A, eff double-heterozygous third instar larvae. The effmer4 allele was obtained from the Bloomington Stock Center. UAS-hUBE3A line was a gift of E.B. (20). For expression in the eye, we used the GMR-Gal4. For expression in the wing, we used the vg-Gal4. Both lines were obtained from the Bloomington Stock Center.
MARCM analysis of DA neurons in the Drosophila PNS was performed as described (22). Briefly, the dUBE3AΔ15–8 or dUBE3AΔ3–8 mutations were recombined onto the chromosome containing FRT2A. dUBE3AΔ15–8, FRT2A/TM3 or dUBE3AΔ3–8, FRT2A/TM3 male flies were crossed with Gal4C155, UAS-mCD8-GFP, hs-FLP1/FM7 virgin flies. Then, Gal4C155, UAS-mCD8-GFP, hs-FLP1; dUBE3AΔ15–8, FRT2A/+ male flies were crossed with Gal4C155, UAS-mCD8-GFP, hs-FLP1; tubP-Gal80, FRT2A/TM6, Tb virgin flies. Embryos were collected on grape agar plates for 3 h at 25°C, aged for 3 h and heat-shocked in a 37°C water bath for 40 min to induce mitotic recombination. The embryos were allowed to develop for 3–4 days in a moisture chamber at 25°C. Third instar larvae containing a single mCD8-GFP-labeled dorsal cluster PNS neuron were selected under a Nikon fluorescence dissection microscope. A Nikon confocal microscope (D-Eclipse C1) was used to collect fluorescence images of dendritic morphology of single DA neurons. Dendritic termini were quantified as described (22). The data were analyzed by t-test.
Generation of dUBE3A antibody and RNAi lines
Rabbit anti-dUBE3A antibody was generated against the first N-terminal 300 amino acids. For protein expression, DNA fragment of dUBE3A was amplified by PCR using specific primers (sense primer 5′-ggaattcatgaacggtggcgggggtggc-3′; antisense primer 5′-ccgctcgagttaatcatcgtcctcttcttc-3′) and subcloned into EcoRI and XhoI sites of the pGEX4T1 vector. The GST-dUBE3A protein was purified with the GST-Fusion Protein Purification Kit (Pierce Biotechnology, Rockford, IL, USA) and injected into rabbits for antiserum production (Covance, Princeton, NJ, USA). Anti-dUBE3A antibody was purified from antiserum with the IgG Purification Kit (Pierce Biotechnology).
To generate two different UAS-dUBE3A RNAi constructs, each DNA fragment was amplified by PCR using specific primers (construct 1: sense primer 5′-atctcgagcaaatcctgtcgtaactgtc-3′ and antisense primer: 5′-catctagagcagcctaatcaagcgatac-3′; construct 2: sense primer 5′-tactcgagctgatcctcggcgaatttg-3′ and antisense primer 5′-gctctagagtcaaatacgagctataacgg-3′) and inserted into the XhoI and XbaI sites of the pUAST vector. To generate UAS-dUBE3A constructs, full-length dUBE3A-coding region was amplified by PCR. These constructs were used to make transgenic flies.
qRT-PCR analysis
Total RNA was extracted from homozygous adult male flies and purified with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The purified RNA was used as template to generate cDNA with TaqMan reverse transcription reagent (Applied Biosystems, Foster City, CA, USA). cDNA was used as template for qRT-PCR in a final volume of 25 μl. A standard curve was run in each PCR reaction. Individual values were normalized with the value of the gene encoding the ribosomal protein RP-49. Owing to the close proximity of the neighboring gene, CG7600, primers were also designed to detect CG7600 transcripts. All reactions were performed three times. Relative mRNA expression was calculated using the standard curve method and the Delta–Delta Ct method.
Western blot analysis
Adult flies were frozen in a dry ice/ethanol bath and vortexed to remove heads. Heads were homogenized in RIPA buffer (0.137 M NaCl, 20 mm Tris–HCl, pH 8.0, 10% glycerol, 1% NP-40, 0.1% SDS, 0.1% sodium deoxycholate) with 1 mm dithiothreitol and HALT Protease Inhibitor Cocktail (Pierce Biotechnology) and centrifuged to isolate total proteins. Protein concentrations were determined with the Bradford reagent (Bio-Rad, Hercules, CA, USA).
For western blot analysis, ∼40 µg protein was combined with 2× SDS-loading buffer, resolved on an 8% SDS–PAGE gel and transferred to polyvinylidine fluoride membranes. After blocking of non-specific reactions in 5% non-fat milk in 0.1% TBST (25 mm Tris–HCl, 137 mm NaCl, 3 mm KCl, pH 7.4, and 0.1% Tween-20), membranes were incubated in anti-dUBE3A (1:1000) for 2 h at room temperature. After washing with TBST, membrane blots were incubated in anti-rabbit-HRP secondary antibody (Jackson ImmunoResearch, San Jose, CA, USA; 1:20 000) for 1 h at room temperature and detected with Supersignal West Pico (Pierce Biotechnology).
FUNDING
This study was supported by the Angelman Syndrome Foundation and the National Institutes of Health (F.-B.G.) (MH079198 and HD044752).
ACKNOWLEDGEMENTS
We thank J. Fischer, E. Bier, the Bloomington Stock Center and the Vienna Drosophila RNAi Center for fly lines, T. Ahmad for helping with the fly eye sectioning, S. Ordway for editorial assistance, and laboratory members for discussions and comments during the course of this work.
Conflict of Interest statement: None declared.
REFERENCES
- 1.Komiyama T., Luo L. Development of wiring specificity in the olfactory system. Curr. Opin. Neurobiol. 2006;16:67–73. doi: 10.1016/j.conb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Tada T., Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr. Opin. Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Parrish J.Z., Emoto K., Kim M.D., Jan Y.N. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu. Rev. Neurosci. 2007;30:399–423. doi: 10.1146/annurev.neuro.29.051605.112907. [DOI] [PubMed] [Google Scholar]
- 4.Gao F.-B. Molecular and cellular mechanisms of dendritic morphogenesis. Curr. Opin. Neurobiol. 2007;17:525–532. doi: 10.1016/j.conb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robb S.A., Pohl K.R.E., Wilson B.J., Brett E.M. The ‘happy puppet’ syndrome of Angelman: review of the clinical features. Arch. Dis. Child. 1989;64:83–86. doi: 10.1136/adc.64.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Y.H., Beaudet A.L. Human disorders of ubiquitination and proteasomal degradation. Curr. Opin. Pediatr. 2004;76:419–426. doi: 10.1097/01.mop.0000133634.79661.cd. [DOI] [PubMed] [Google Scholar]
- 7.Lalande M., Calciano M.A. Molecular epigenetics of Angelman syndrome. Cell. Mol. Life Sci. 2007;64:947–960. doi: 10.1007/s00018-007-6460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishino T., Lalande M., Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat. Genet. 1997;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura T., Sutcliffe J.S., Fang P., Galjaard R.J., Jiang Y.H., Benton C.S., Rommens J.M., Beaudet A.L. De novo truncating mutations in E6-AP ubiquitin–protein ligase gene (UBE3A) in Angelman syndrome. Nat. Genet. 1997;75:74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- 10.Huibregtse J.M., Scheffner M., Howley P.M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huibregtse J.M., Scheffner M., Howley P.M. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol. Cell Biol. 1993;73:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albrecht U., Sutcliffe J.S., Cattanach B.M., Beechey C.V., Armstrong D., Eichele G., Beaudet A.L. Imprinted expression of the murine Angelman syndrome gene, UbeSa, in hippocampal and Purkinje neurons. Nat. Genet. 1997;77:75–78. doi: 10.1038/ng0997-75. [DOI] [PubMed] [Google Scholar]
- 13.Rougeulle C., Glatt H., Lalande M. The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nat. Genet. 1997;77:14–15. doi: 10.1038/ng0997-14. [DOI] [PubMed] [Google Scholar]
- 14.Vu T.H., Hoffman A.R. Imprinting of the Angelman syndrome gene, UBE3A, is restricted to brain. Nat. Genet. 1997;77:12–13. doi: 10.1038/ng0997-12. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y.H., Armstrong D., Albrecht U., Atkins C.M., Noebels J.L., Eichele G., Sweatt J.D., Beaudet A.L. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;27:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 16.van Woerden G.M., Harris K.D., Hojjati M.R., Gustin R.M., Qiu S., de Avila Freire R., Jiang Y.H., Elgersma Y., Weeber E.J. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nat. Neurosci. 2007;10:280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- 17.Dindot S.V., Antalffy B.A., Bhattacharjee M.B., Beaudet A.L. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum. Mol. Genet. 2008;17:111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- 18.Bilen J., Bonini N.M. Drosophila as a model for human neuro-degenerative disease. Annu. Rev. Genet. 2005;39:153–171. doi: 10.1146/annurev.genet.39.110304.095804. [DOI] [PubMed] [Google Scholar]
- 19.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 20.Reiter L.T., Seagroves T.N., Bowers M., Bier E. Expression of the Rho-GEF Pbl/ECT2 is regulated by the UBE3A E3 ubiquitin ligase. Hum. Mol. Genet. 2006;15:2825–2835. doi: 10.1093/hmg/ddl225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 22.Li W., Wang F., Menut L., Gao F.-B. BTB/POZ-zinc finger protein abrupt regulates dendritic branching in a neuronal subtype-specific and dosage-dependent manner. Neuron. 2004;43:823–834. doi: 10.1016/j.neuron.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 23.Lee T., Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 24.Sweeney N.T., Li W., Gao F.B. Genetic manipulation of single neurons in vivo reveals specific roles of Flamingo in neuronal morphogenesis. Dev. Biol. 2002;247:76–88. doi: 10.1006/dbio.2002.0702. [DOI] [PubMed] [Google Scholar]
- 25.Grueber W.B., Jan L.Y., Jan Y.N. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 26.Kuo C.T., Zhu S., Younger S., Jan L.Y., Jan Y.N. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y., Bolduc F.V., Bell K., Tully T., Fang Y., Sehgal A., Fischer J.A. A Drosophila model for Angelman syndrome. Proc. Natl Acad. Sci. USA. 2008;105:12399–12404. doi: 10.1073/pnas.0805291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodmer R., Carretto R., Jan Y.N. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989;3:21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 29.Koochek M., Harvard C., Hildebrand M.J., Van Allen M., Wingert H., Mickelson E., Holden J.J., Rajcan-Separovic E., Lewis M.E. 15q duplication associated with autism in a multiplex family with a familial cryptic translocation t(14;15)(q11.2;q13.3) detected using array-CGH. Clin. Genet. 2006;69:124–134. doi: 10.1111/j.1399-0004.2005.00560.x. [DOI] [PubMed] [Google Scholar]
- 30.Comery T.A., Harris J.B., Willems P.J., Oostra B.A., Irwin S.A., Weiler I.J., Greenough W.T. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc. Natl Acad. Sci. USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belichenko P.V., Oldfors A., Hagberg B., Dahlström A. Rett syndrome: 3-D confocal microscopy of cortical pyramidal dendrites and afferents. Neuroreport. 1994;5:1509–1513. [PubMed] [Google Scholar]
- 32.Raymond G.V., Bauman M.L., Kemper T.L. Hippocampus in autism: a Golgi analysis. Acta Neuropathol. 1996;91:117–119. doi: 10.1007/s004010050401. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann W.E., Moser H.W. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 34.Zoghbi H.Y. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- 35.Samaco R.C., Hogart A., LaSalle J.M. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum. Mol. Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matarazzo V., Cohen D., Palmer A.M., Simpson P.J., Khokhar B., Pan S.J., Ronnett G.V. The transcriptional repressor Mecp2 regulates terminal neuronal differentiation. Mol. Cell. Neurosci. 2004;27:44–58. doi: 10.1016/j.mcn.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Jugloff D.G., Jung B.P., Purushotham D., Logan R., Eubanks J.H. Increased dendritic complexity and axonal length in cultured mouse cortical neurons overexpressing methyl-CpG-binding protein MeCP2. Neurobiol. Dis. 2005;19:18–27. doi: 10.1016/j.nbd.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Z., Hong E.J., Cohen S., Zhao W.N., Ho H.Y., Schmidt L., Chen W.G., Lin Y., Savner E., Griffith E.C., et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smrt R.D., Eaves-Egenes J., Barkho B.Z., Santistevan N.J., Zhao C., Aimone J.B., Gage F.H., Zhao X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol. Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordan C., Francke U. Ube3a expression is not altered in Mecp2 mutant mice. Hum. Mol. Genet. 2006;15:2210–2215. doi: 10.1093/hmg/ddl146. [DOI] [PubMed] [Google Scholar]