Abstract
Cognitive studies show that both younger and older adults can increase their memory performance after training in using a visuospatial mnemonic, although age-related memory deficits tend to be magnified rather than reduced after training. Little is known about the changes in functional brain activity that accompany training-induced memory enhancement, and whether age-related activity changes are associated with the size of training-related gains. Here, we demonstrate that younger adults show increased activity during memory encoding in occipito-parietal and frontal brain regions after learning the mnemonic. Older adults did not show increased frontal activity, and only those elderly persons who benefited from the mnemonic showed increased occipito-parietal activity. These findings suggest that age-related differences in cognitive reserve capacity may reflect both a frontal processing deficiency and a posterior production deficiency.
The existence of age-related deficits in episodic memory functioning are well documented (1). Given the impact of such deficits, much research has been directed at examining possible means of enhancing memory performance in older adults by various forms of cognitive support (2). One approach that has received considerable attention involves estimation of latent cognitive potential, or cognitive reserve capacity, in older age (3). In one variant of this approach, younger and older participants are given training in using a classical mnemonic, the method of loci (4), to memorize and retrieve words. This method involves learning to visualize a series of mental landmarks (e.g., places along one's route to work). After acquisition of the landmarks, the to-be-remembered information is linked to the various loci at the time of encoding. At test, the landmarks are mentally revisited in serial order, and the information associated with each locus is retrieved.
Serial recall is substantially enhanced by the loci mnemonic for both younger and older adults (5, 6), demonstrating cognitive reserve capacity in aging (i.e., cognitive reserve capacity is defined as the ability to enhance one's memory performance after learning a mnemonic). However, the most striking aspect of previous findings is that age differences in memory performance are magnified rather than reduced after training (7). This pattern of results suggests an age-related decrease in cognitive reserve capacity. Little is known about the basis for this phenomenon. Baltes and Kliegl (7) hypothesized that older adults may have difficulty in forming novel relations between the landmarks and the to-be-remembered information (i.e., a difficulty in using rather than acquiring the mnemonic), and they proposed neurobiological constraints as a determinant of this deficit. However, no direct evidence for this account has been provided. Here, we present the results from an age-comparative positron emission tomography (PET) study of the neural underpinnings of acquisition and use of the loci method.
Methods
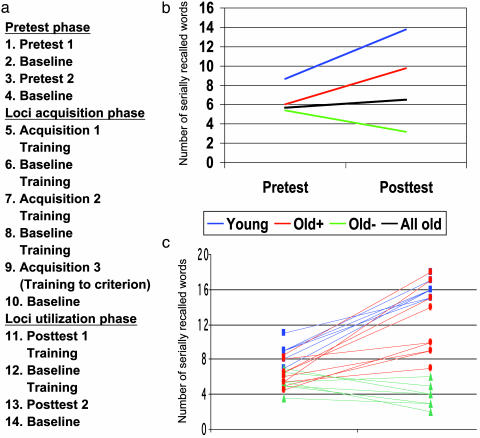
Tasks and Procedure. The whole experiment, including a pretest phase, a loci acquisition phase, and a loci utilization posttest phase, as outlined in Fig. 1a, was carried out while the participants were placed in the scanner (data on background variables were collected before scanning). In pretest 1-2 the participants were instructed to encode 18 words in the order they were presented. They were scanned during encoding and immediately after encoding/scanning they were instructed to orally recall the encoded words (serial recall). In loci acquisition 1-3 participants were instructed to memorize a list of 18 words denoting locations in the order they were presented (the loci list). They were scanned during location encoding, and immediately after encoding/scanning they were instructed to orally recall the locations (serial recall of locations). In between each scan in the acquisition phase, the participants were presented the loci list and instructed to try to memorize the locations. Hence, after loci acquisition 3, the loci list had been presented seven times. At that point, if a participant was unable to recall the list of locations in correct serial order in two consecutive runs with a maximum of one error, additional training was given until the criterion was reached. Only one participant needed additional training. Finally, in posttest 1-2 the participants were instructed to use the loci that they learned in the acquisition phase to encode 18 words in serial order (loci utilization). They were scanned during encoding, and immediately after encoding/scanning they were instructed to orally recall the words (serial recall). In between scans in the utilization phase, participants practiced using the loci method by using the same word for all locations (e.g., the participants were instructed to imagine placing a ball in each of the loci and then to retrieve the ball from each loci while walking through the imagined home). Seven baseline tasks were interspersed throughout the scanning protocol. During these tasks, participants were to covertly count the number of abstract words in a list of 18 words. In all 14 scans, each word was presented for 5,000 ms with no delay between words.
Fig. 1.
(a) Experimental protocol. (b) Group differences in memory performance at pretest and posttest. A magnification of age differences in memory performance after training was indicated by a significant interaction in a group (young vs. old) by test (pre vs. post) ANOVA [F(1,21) = 6.02, P = 0.02]. (c) Individual differences in training-related changes. All young and the facilitated old increased their posttest performance relative to pretest, whereas the unimproved old showed a performance decrease or remained at the same level.
Word Lists. Three types of word lists with words varying between five and eight letters in length were prepared. The words were presented in black lowercase letters on a white computer screen. For pretest and posttest, four lists of 18 concrete words were used. The lists were equal with regard to mean word frequency, according to established Swedish norms (8). The lists were randomly assigned to be either pretest or posttest lists for each participant. For loci acquisition, a list of 18 words representing locations in a home was used (e.g., bed, cupboard, sofa). The locations and their presentation order were the same for each participant. For the baseline task we used seven lists of 18 words, 3-6 abstract words per list, and the rest concrete words. The presentation order of baseline lists was randomized across participants.
Participants. Eight young and 16 older volunteers gave informed consent and were paid to participate in the study. All reported to be right-handed, with no neurological, psychiatric, head trauma, or hypertension history. The study was screened and approved by the Ethics and Radiation Safety Committees at the Karolinska Hospital.
Image Acquisition and Data Analysis. Each subject underwent 14 measurements of regional cerebral blood flow with a 3D ECAT Exact HR PET scanner (Siemens CTI, Knoxville, TN) and bolus injections of [O15] water (11 mCi per scan). The PET scanner was used in 3D sampling mode, producing 60-s tracer uptake images. The different conditions were initiated at the time of tracer injection, and scanning started automatically when the brain radioactivity exceeded a predetermined level of radioactivity above background. Scatter correction was made, and a 2D transmission scan was used for attenuation correction.
PET data were realigned, anatomically normalized to a common stereotactic template, smoothed (14 mm full width at half maximum), and proportionally scaled to account for global confounders. In the general linear framework of SPM99 (www.fil.ion.ucl.ac.uk/spm), the data were characterized as a multigroup study with three groups of unequal size (one older subject had to be removed because of motion artefacts and the last six scans of another older subject were not acquired because of technical problems). We used interaction contrasts to control for unspecific time effects as described (9). Thus, the results were based on contrasts between the various experimental conditions (pretest, acquisition, use) and their subsequent baseline scan. Resulting statistical parametric mappings were thresholded at P < 0.05 (corrected for nonindependent comparisons by using the false discovery rate; ref. 10). Group differences are reported at P < 0.001 (uncorrected), unless otherwise indicated in the text. All significant activations are reported.
In the recall tests (pretest and posttest), the participants had to recall the correct words in (relative) order. For example, if words were encoded as (A, B, C, D) and recalled in that order the score was 4. If a word was omitted but the relative order of the remaining words preserved (A, C, D), the score was 3. Occasionally, a participant “went back” and retrieved a word that was associated with a location that already had been passed. Such items are included in the reported data. However, typically, if an item was recalled, it was recalled in the correct relative order. The pattern of results remained the same if the data were scored according to absolute item recall rather than relative serial recall (the correlation between item and serial recall was >0.9 at posttest).
Results
Memory Performance. All younger and older subjects were able to acquire the loci method, i.e., to learn all locations to the criterion level (see Methods). In line with earlier findings, a comparison of the younger subjects with all older subjects showed that age differences in memory performance were magnified at posttest compared with pretest (Fig. 1b). Analyses at the individual level showed that the magnified age difference was largely the result of a subset of older adults not benefiting from the mnemonic (Old, Fig. 1 b and c). Specifically, eight of the older participants showed no increase in memory performance at posttest compared with pretest. Rather, several showed decreased performance. This group will be referred to as the unimproved old. The remaining eight older participants (Old+, Fig. 1 b and c) showed an increase in posttest performance compared with pretest performance. The magnitude of the increase varied between individuals but all showed facilitation and will be referred to as the facilitated old. The unimproved old were comparable to the facilitated old with regard to pretest performance as well as several background variables (Table 1). Consistent with previous findings (7), the posttest performance for the young group was significantly higher than that for the facilitated old.
Table 1. Subject characteristics and test performance.
| Young
|
Facilitated old
|
Unimproved old
|
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age | 25.75 | 2.60 | 69.75 | 2.96 | 67.75 | 1.98 |
| Female/male | 3/5 | 8/0 | 2/6 | |||
| Education, yr | 15.37 | 2.28 | 12.50 | 4.70 | 13.12 | 4.32 |
| Pretest | 8.69 | 1.16 | 6.00 | 1.22 | 5.44 | 1.05 |
| Posttest | 13.81 | 3.01 | 9.75 | 4.42 | 3.12 | 1.03 |
| MMSE | 29.12 | 0.83 | 28.62 | 0.74 | 28.25 | 0.71 |
| SRB | 22.87 | 2.42 | 23.00 | 2.88 | 23.37 | 2.82 |
| Digit symbol* | 63.50 | 6.26 | 41.50 | 7.84 | 46.62 | 10.90 |
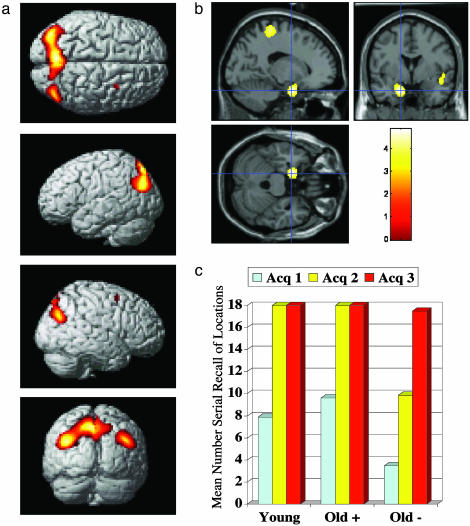
Brain Activity During Acquisition of the Loci Structure. To identify neural correlates of visualization and trying to memorize the various locations (i.e., acquiring the loci structure), all acquisition conditions were compared with their baseline conditions across age groups. Increased activity was observed in the bilateral parietal cortex and medial parietal cortex (Fig. 2a). We then tested for learning-related changes by contrasting late acquisition with early acquisition (acquisition 3 vs. 1). Learning-related changes involved the left hippocampal region (Fig. 2b), where activity increased across acquisition trials.
Fig. 2.
(a) Brain regions showing increased activity during loci acquisition relative to baseline. Significant differences were observed in the lateral and medial parietal cortex (-38, -78, 30; -8, -72, 52; 44, -74, 32) and the right dorsal frontal cortex (32, 6, 58). Activations are shown on the statistical parametric mapping cortical rendering template. (b) Regions where brain activity increased as a function of loci acquisition (late > early). Significant learning-related changes were observed near the left hippocampus (-16, 0, -26). Activations are shown on the Montreal Neurological Institute structural template. (c) Rate of acquisition of the loci structure as a function of group.
Although all subjects did acquire the location structure, there was a tendency to slower learning in the unimproved old group (Fig. 2c). We therefore tested for differences in learning-related changes of brain activity (i.e., acquisition 3 vs. 1), contrasting the young and facilitated old adults with the unimproved old adults. No significant differences were found.
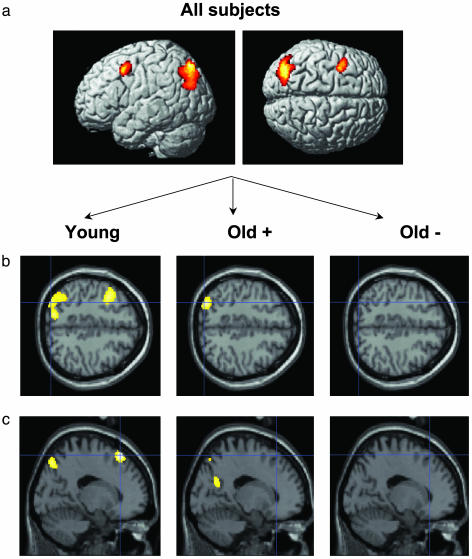
Brain Activity During Use of the Loci Mnemonic. To identify brain regions where activity increased when the loci mnemonic was used, the posttest condition was contrasted with the pretest condition. First, this was done across all subjects. The results showed that encoding was associated with increased activity in the left occipito-parietal cortex and left dorsolateral prefrontal cortex (Fig. 3a).
Fig. 3.
(a) Brain regions showing increased activity during loci use relative to pretest. Significant differences were observed in the left occipito-parietal cortex (-28, -78, 48; -42, -76, 28) and the left dorsal frontal cortex (-38, 6, 48). (b) Group differences in the comparison of loci use with pretest. The young (-30, -82, 46; -14, -76, 48; -32, -88, 36) and the facilitated old (-38, -80, 32; -32, -76, 38; -28, -78, 46), but not the unimproved old, activated the left occipito-parietal cortex. (c) Age differences in the comparison of loci use with pretest. The young but not the old subjects activated the left dorsal frontal cortex (-16, 28, 58).
Next, to determine group differences, posttest vs. pretest contrasts were made at the group level (older adults were divided into facilitated and unimproved). Left occipito-parietal activation was present for the young and facilitated old (Fig. 3b). This result indicates that successful use of the mnemonic was associated with occipito-parietal activity, and a direct comparison of those who improved (young + facilitated old) with the unimproved old revealed left occipito-parietal activation (x, y, z = -34, -90, 30). The analyses further showed that left frontal activation was specific to the young subjects (Fig. 3c). A direct comparison of the young group against all older subjects revealed increased left frontal activation (x, y, z = -18, 30, 60). Importantly, differential left frontal activation was also found when the young-old comparison was based only on the facilitated old (x, y, z = -16, 32, 58; P = 0.002). The possibility remained that the pretest taxed more cognitive resources and therefore was associated with relative greater frontal activity for older compared with younger adults. If true, this could underlie the age-related difference in frontal activity in the posttest-pretest contrast. To address this concern we contrasted pretest with baseline and tested whether the facilitated older adults showed higher frontal activity than the younger adults. No significant differences (at P < 0.001 uncorrected) were observed.
A final set of analyses contrasted loci use directly against baseline, focusing on differences between those who did and did not improve from the loci mnemonic. As in the comparison of loci use with pretest, relative to the unimproved old, both the young and facilitated old showed increased activity in the left occipito-parietal cortex (x, y, z = -32, -90, 32). In addition, the young and the facilitated old showed increased activity in the left retrosplenial cortex (x, y, z = -14, -56, 18). Retrosplenial activation was not seen in the contrast between loci use and pretest, but a directed search revealed increased activation among the young and facilitated old relative to the unimproved old in this contrast as well (x, y, z = -14, -54, 18; P < 0.05). Thus, compared with both pretest and baseline, successful loci use was associated with increased activity in the left occipito-parietal and retrosplenial cortex.
Discussion
Parietal activation was salient during location learning, which is consistent with previous studies of encoding of spatial information (11). In addition, activity in the left hippocampal region increased as a function of learning. Hippocampal activity has been related to binding processes (12) and could have reflected gradual linkage of the various locations into a coherent sequence and/or retrieval of previously learned locations. All participants learned the locations, and we found no significant group differences in activity associated with learning of the locations. Thus, although it cannot be ruled out that acquisition-related factors contributed, our findings converge with previous observations (5-7) in suggesting that the unimproved old had difficulty in using rather than acquiring the mnemonic.
Increased activity was observed in the left dorsal frontal cortex when the loci structure was used for word encoding (i.e., posttest vs. pretest). Importantly, the group-specific comparisons showed that the frontal activity increase was specific to the young group. Task-relevant processes that have been linked to the dorsal frontal cortex include feature binding (13), creation of an organizational structure (14), generation of images based on words (15), and integration of information in working memory (16). The lack of differential frontal activation at posttest indicates that such processing was impaired for the older adults, possibly because of age-related changes in basic processing capacity. Working-memory decline in older age has been linked to reduced dorsal frontal activity (17), and age-related deficits in mental imagery tasks have been related to shrinkage of the prefrontal cortex and decline in working memory (18). Another basic processing resource is speed of mental information processing, and consistent with numerous previous studies (19) we observed pronounced age differences in a test of processing speed. There is independent evidence that processing speed is related to plasticity in the loci method (20). Here, the relative magnitude of the prepost increase in memory performance was only marginally greater for the young adults than for the facilitated old, but the young adults recalled on average an additional 5.12 words after learning the loci method, whereas the corresponding number for the facilitated old was only 3.75 words. Hence, a major origin of the observed performance differences may be age-related changes in basic processing capacity that at least partly are of a frontal origin.
The younger adults and the facilitated old showed increased activity in the occipito-parietal cortex. This finding suggests that this is a critical region for successful task completion. The peak activation fell in dorsal BA 19, which is typically activated during visual imagery, especially when there is a spatial task component (11), and activity in this area correlates with quantitative (21) and qualitative (22) measures of the ability to use visual imagery. In addition, the young and facilitated old showed increased activity in the left retrosplenial cortex. A recent study reported evidence that superior memorizers spontaneously used the loci method for encoding (23), and one region where the superior memorizers showed increased activity was the left retrosplenial cortex. It was suggested that retrosplenial activation, along with other activations, reflected the use of a route strategy. Thus, occipito-parietal and retrosplenial activation may reflect spatial imagery processes that are recruited during use of the loci mnemonic.
The lower posterior brain activity in the unimproved old indicates that they did not engage in task-appropriate processing. Importantly, this was not a low-performing group in general (Table 1). Rather, except for the gender distribution, this group was comparable to the facilitated old on several variables that have been linked to cognitive reserve capacity in old age, including the Mini Mental State Examination (24), digit symbol substitution (5), and calendar age (24). In addition, the two older groups had comparable levels of education and vocabulary. Similarly, in a previous study on the loci method it was found that some older adults did not use the method at all at posttest (20), but these older participants did not differ from the other old users with respect to various measures of cognitive performance. The similar cognitive profiles of the two older groups suggest that the unimproved old did not lack the neural resources to use the loci mnemonic. Instead, as has been suggested in the context of other forms of training (25), it is conceivable that they simply did not use the loci mnemonic for word encoding. This could be caused by a perseverance effect, where those who believe that their own strategies are more effective than new strategies are less apt to shift strategy (20). Informal postexperimental questioning indicated that an additional reason for not engaging in task-relevant processing was that several of the unimproved old found it difficult to associate some of the loci and the to-be-remembered words, especially under the prevailing time-limited conditions. This observation is in line with findings that some older adults have difficulties forming bizarre or unnatural images (26).
The present pattern of results is interesting to consider in light of prominent theoretical conceptions in the cognitive aging literature. A recurrent debate in that literature has concerned whether age-related memory deficits reflect limitations in the basic resources available for task-relevant cognitive processing (19, 27) or failure to engage in appropriate cognitive operations during remembering (25, 28). We have argued that the age-related reduction in frontal activity likely reflects diminished processing resources, whereas the reduction in posterior activity seen in the group of unimproved old was a result of not engaging in task-relevant processing. This argument is consistent with demonstrations of pronounced structural changes in the frontal cortex along with small age-related structural changes in posterior cortical regions (29). Thus, our findings indicate that age-related reductions in cognitive reserve capacity have two bases: a failure of some elderly persons to engage in task-relevant processing (a posterior production deficiency) and a general age-related deficit in basic cognitive resources (a frontal processing deficiency).
Acknowledgments
We gratefully acknowledge support from the PET unit at the Karolinska Hospital. This work was supported by Swedish Research Council Grant 8246, the Family Hedlund Foundation, and the Karolinska Institutet.
Abbreviation: PET, positron emission tomography.
References
- 1.Craik, F. I. M. & Salthouse, T. A., eds. (1999) The Handbook of Aging and Cognition (Erlbaum, Mahwah, NJ), Vol. 2.
- 2.Glisky, E. L. & Glisky, M. L. (1999) in Cognitive Neurorehabilitation, eds. Stuss, D. T., Winocur, G. & Robertson, I. H. (Cambridge Univ. Press, Cambridge, U.K.), pp. 347-361.
- 3.Baltes, P. B. (1987) Dev. Psychol. 23, 611-626. [Google Scholar]
- 4.Bower, G. H. (1970) Am. Sci. 58, 496-510. [Google Scholar]
- 5.Kliegl, R., Smith, J. & Baltes, P. B. (1989) Dev. Psychol. 25, 247-256. [Google Scholar]
- 6.Kliegl, R., Smith, J. & Baltes, P. B. (1990) Dev. Psychol. 26, 894-904. [Google Scholar]
- 7.Baltes, P. B. & Kliegl, R. (1992) Dev. Psychol. 28, 121-125. [Google Scholar]
- 8.Molander, B. (1989) Imagery, Visual, and Tactual Dimensions of Imagery and Meaningfulness: Swedish Norms for 858 Nouns (Umeå University, Umeå, Sweden).
- 9.Petersson, K. M., Elfgren, C. & Ingvar, M. (1999) Hum. Brain Mapp. 7, 234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genovese, C. R., Lazar, N. A. & Nichols, T. (2002) NeuroImage 4, 870-878. [DOI] [PubMed] [Google Scholar]
- 11.Cabeza, R. & Nyberg, L. (2000) J. Cognit. Neurosci. 12, 1-47. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, N. J., Ryan, J., Hunt, C., Romine, L., Wszalek, T. & Nash, C. (1999) Hippocampus 9, 83-98. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell, K. J., Johnson, M. K., Raye, C. L. & D'Esposito, M. (2000) Cognit. Brain Res. 10, 197-206. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher, P. C., Shallice, T. & Dolan, R. J. (1998) Brain 121, 1239-1248. [DOI] [PubMed] [Google Scholar]
- 15.Mellet, E., Tzourio, N., Denis, M. & Mazoyer, B. (1998) NeuroReport 9, 3509-3514. [DOI] [PubMed] [Google Scholar]
- 16.Prabhakaran, V., Narayanan, K., Zhao, Z. & Gabrieli, J. D. E. (2000) Nat. Neurosci. 3, 85-90. [DOI] [PubMed] [Google Scholar]
- 17.Rypma, B. & D'Esposito, M. (2000) Nat. Neurosci. 3, 509-515. [DOI] [PubMed] [Google Scholar]
- 18.Raz, N., Briggs, S. D., Marks, W. & Acker, J. D. (1999) Psychol. Aging 14, 436-444. [DOI] [PubMed] [Google Scholar]
- 19.Salthouse, T. A. (1996) Psychol. Rev. 103, 403-428. [DOI] [PubMed] [Google Scholar]
- 20.Verhaeghen, P. & Marcoen, A. (1996) Psychol. Aging 11, 164-178. [DOI] [PubMed] [Google Scholar]
- 21.Kosslyn, S. M., Thompson, W. L., Kim, I. J., Rauch, S. L. & Alpert, N. M. (1996) J. Cognit. Neurosci. 8, 78-82. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg, G., Podreka, I., Steiner, M., Willmes, K., Suess, E. & Deecke, L. (1989) Neuropsychologia 27, 641-664. [DOI] [PubMed] [Google Scholar]
- 23.Maguire, E. A., Valentine, E. R., Wilding, J. M. & Kapur, N. (2003) Nat. Neurosci. 6, 90-95. [DOI] [PubMed] [Google Scholar]
- 24.Yesevage, J. A., Sheikh, J. I., Friedman, L. & Tanke, E. (1990) Psychol. Aging 5, 133-137. [DOI] [PubMed] [Google Scholar]
- 25.Rogers, W. A., Hertzog, C. & Fisk, A. D. (2000) J. Exp. Psychol. Learn. Mem. Cognit. 26, 359-394. [DOI] [PubMed] [Google Scholar]
- 26.Poon, L. W. & Walsh-Sweeney, L. (1981) Exp. Aging Res. 7, 65-70. [DOI] [PubMed] [Google Scholar]
- 27.Verhaeghen, P. & Salthouse, T. A. (1997) Psychol. Bull. 122, 231-249. [DOI] [PubMed] [Google Scholar]
- 28.Dunlosky, J. & Hertzog, C. (2001) Mem. Cognit. 29, 247-253. [DOI] [PubMed] [Google Scholar]
- 29.Raz, N. (1999) in The Handbook of Aging and Cognition, eds. Craik, F. I. M. & Salthouse, T. A. (Erlbaum, Mahwah, NJ), Vol. 2, pp. 1-90. [Google Scholar]
- 30.Folstein, M. F., Folstein, S. E. & McHugh, P. R. (1975) J. Psychiatr. Res. 12, 189-198. [DOI] [PubMed] [Google Scholar]
- 31.Dureman, I. (1960) SRB:1 (Psyhologifo̊rlaget, Stockholm).
- 32.Wechsler, D. (1981) Wechsler Adult Intelligence Scale: Revised (Psychological Corp., New York).