Abstract
Using diffusion tensor imaging and tractography to detail the patterns of interhemispheric connectivity and to determine the length of the connections, and formulae based on histological results to estimate degree of connectivity, we show that connection length is negatively correlated with degree of connectivity in the normal adult brain. The degree of interhemispheric connectivity—the ratio of interhemispheric connections to total corticocortical projection neurons—was estimated for each of 5 subregions of the corpus callosum in 22 normal males between 20 and 45 years of age (mean 31.68; standard deviation 8.75), and the average length of the longest tracts passing through each point of each subregion was calculated. Regression analyses were used to assess the relation between connection length and the degree of connectivity. Connection length was negatively correlated with degree of connectivity in all 5 subregions, and the regression was significant in 4 of the 5, with an average r2 of 0.255. This is contrasted with previous analyses of the relation between brain size and connectivity, and connection length is shown to be a superior predictor. The results support the hypothesis that cortical networks are optimized to reduce conduction delays and cellular costs.
Keywords: brain scaling, connection length, degree of connectivity, optimal wiring, relative size
Introduction
Variation in brain size, both across species and across individuals within a species, is associated with variation in the organization of both gray and white matter. Increases in white matter volume outpace increases in gray matter volume (Frahm et al. 1982; Rilling and Insel 1999b; Schlenska 1974; Zhang and Sejnowski 2000), but increases in gray matter volume outpace increases in the size of the major white matter bundles that interconnect the cerebral hemispheres (Jäncke et al. 1997; Rilling and Insel 1999a). The disproportionate increase in white matter volume has been interpreted as a consequence of the high degree of connectivity within the cortex (Allman 2000; Frahm et al. 1982; Zhang and Sejnowski 2000). The hyposcaling of the commissural white matter bundles likely reflects decreases in the degree of interhemispheric connectivity—the ratio of interhemispheric connections to total corticocortical projection neurons—with increases in brain size and has been hypothesized to be due to the increased conduction delays and cellular costs associated with these fibers (Ringo 1991; Ringo et al. 1994), which tend to be longer in larger brains (Braitenberg 2001). The conduction delay associated with either a myelinated or an unmyelinated axon is primarily a function of its diameter and length (Waxman 1977). The conduction delay for long-distance connections must therefore increase with increases in brain size, unless there is a proportional increase in axon diameter—which appears not to be the case (Aboitiz, Scheibel, Fisher, et al. 1992; Jerison 1991; Olivares et al. 2001; Schüz and Preissl 1996)—and the cellular costs for such connections will increase regardless (Karbowski, 2007). This hypothesis thus predicts a negative correlation between connection length and degree of connectivity.
Neuroanatomy research to date has only indirectly tested this prediction. The use of magnetic resonance morphometry has limited these studies to measures such as brain volume and cortical surface area; and differences in brain shape would suggest that these measures are not particularly good indices of the length of the long-distance connections. This paper reports on a study that uses diffusion tensor imaging (DTI) to more directly measure the predicted relation between the length of the long-distance connections that traverse the corpus callosum and the degree of interhemispheric connectivity.
Methods
Subjects
A total of 22 normal healthy males ranging between 20 and 45 years of age (mean 31.68; standard deviation [SD] 8.75) participated in the study. All subjects gave informed consent, and the study was approved by the ethics committee at the University of California, San Diego (UCSD).
Imaging and Image Processing
All subjects were scanned at the UCSD Center for functional magnetic resonance imaging (fMRI) on a GE Signa EXCITE 3.0T short bore scanner with an 8-channel array head coil. Four types of images were acquired from each subject: 1) one set of 3-dimensional T1-weighted images (fast gradient echo, Spoiled Gradient Recalled; echo time [TE] = 3.1 ms; flip angle = 12°; number of excitations [NEX] = 1; field of view [FOV] = 25cm; matrix = 256 × 256); 2) 2 sets of T2-weighted images (dual spin-echo, EPI; time repetition [TR] = 15 s; TE = 89 ms; 45 axial slices; NEX = 2; FOV = 22 cm; matrix = 128 × 128; resolution = 1.875 × 1.875 × 3 mm; 3 mm interleaved contiguous slices); 3) 2 sets of diffusion-weighted images isotropically distributed along 15 directions (dual spin-echo, echo planar imaging [EPI]; TR = 15 s; TE = 89 ms; 45 axial slices; NEX = 2; FOV = 22 cm; matrix = 128 × 128; resolution = 1.875 × 1.875 × 3 mm; 3 mm interleaved contiguous slices; b value = 1400 s/mm2); and 4) fieldmaps matched to the diffusion-weighted images.
Note that 2 sets of diffusion-weighted images were acquired, each with a NEX of 2; thus each image was acquired 4 times. Likewise for the T2-weighted images, which were acquired together with the diffusion-weighted images.
Fieldmaps were acquired before the first diffusion-weighted images were acquired, and, in cases where there was between scan motion, an additional set of fieldmaps was acquired after the second.
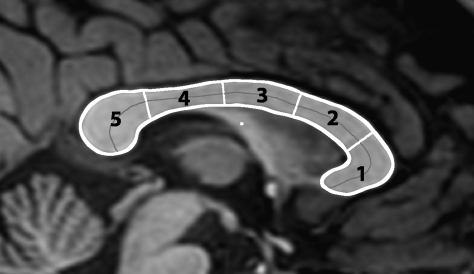
The T1-weighted images were converted to AFNI (Cox 1996)—an open source environment for processing and displaying MRI data—and the resulting volume was anterior commissure-posterior commissure (AC-PC) aligned. The boundary and divisions of the corpus callosum were then identified on the midsagittal slice of the AC-PC–aligned T1-weighted images using a semiautomated procedure created for this project. The boundary and divisions of the corpus callosum were determined as follows. A point manually inserted at the boundary of the callosum was used to seed an intensity-based floodfill of the callosum; additional points were used, in cases where the division between the callosum and fornix was unclear, to identify the boundary of the callosum in that region. The outline of the resulting area was then used as a starting point for an implementation of the active contour algorithm that smoothed the boundary and moved it to the center of the gradient at the edge of the callosum. The resulting boundary was then divided into 5 regions via Clarke's method (Clarke et al. 1989)—that is, the midline of the callosum was computed and divided into 5 equal length segments, and the shortest length lines that cut the callosum at the points defined by these segments were the regional boundaries. Figure 1 illustrates the subregions arrived at via this procedure.
Figure 1.
The divisions of the corpus callosum.
Four-dimensional volumes were created from both sets of diffusion-weighted images; software developed by the UCSD Center for fMRI was used to correct the diffusion-weighted images, using the fieldmaps, of distortions caused by inhomogeneities in the magnetic field and to correct for within-scan motion. Using the 3D Slicer DTMRI module (an open source development project begun at the Massachusetts Institute of Technology Artificial Intelligence Laboratory and the Surgical Planning Laboratory at Brigham and Women's Hospital), the two 4-dimensional diffusion-weighted volumes were then converted to diffusion tensor volumes, coregistered using nonlinear tensor-to-tensor registration (Park et al. 2003), and the resulting tensors averaged across volumes. The average diffusion tensor volume was then coregistered with the T1-weighted volume by generating a fractional anisotropy volume from the tensors, coregistering the fractional anisotropy volume to the T1-weighted volume, and applying that transform to the tensor volume.
The nonlinear transformation used to register the 2 tensor volumes was also used to register the corresponding T2-weighted volumes. These 2 T2-weighted volumes were then averaged and together with the T1-weighted volume were processed with freesurfer (BioMedical Imaging , Charlestown, MA, and CorTech Labs, La Jolla, CA) to obtain a segmentation. This segmentation provided the cortical gray matter measures used in the analyses and allowed for the creation of a seed region for fiber tract generation.
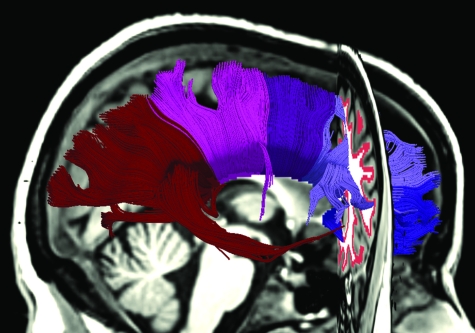
Tracts were seeded along the edge of the white matter where the white matter was bounded by cortical gray matter. This was achieved by dilating a mask of the cortical gray matter and retaining the areas that overlapped with the white matter. Tracts were then generated from all voxels within this seed region. Tracts were generated using a modified version of 3D Slicer, which allowed tracts to be terminated at the midsagittal point of the corpus callosum and to be filtered out if they terminated elsewhere. Tracts were further constrained by a radius of curvature limit of 1 mm and a fractional anisotropy threshold of 0.15. The volume in which the subregions of the callosum had been labeled was then used to identify the set of fiber tracts that passed through each subregion of the callosum. Figure 2 shows the set of fiber tracts produced by this method.
Figure 2.
The set of tracts that pass through the 5 subregions of the corpus callosum. The seed region for the tracts is visible in the coronal plane.
Note that subregion 5 of the callosum consists of both tracts that originate in occipital and parietal cortices and also a smaller number of tracts that originate in temporal cortex. Temporal cortex poses particular difficulties for tractography. Fibers descending from the splenium cross through thalamocortical connections and intermingle with noncallosal fibers in the inferior longitudinal fasciculus. Crossing fibers result in a reduced fractional anisotropy value, which if severe will result in the early termination of any tracts in that area. Fibers that run parallel to one another in close proximity—kissing fibers—cannot be discerned and may result in false tracts. In some subjects, low fractional anisotropy values due to crossing fibers resulted in very few tracts descending from the splenium into the temporal lobe; in others, kissing fibers resulted in a large number of apparently false tracts that originated in anterior regions of temporal cortex. Tractography was thus unreliable, and temporal cortex was excluded from the analysis. The estimate of the number of fibers passing through subregion 5 of the callosum was based on the percentage of subregion 5 that contained fibers that originated from other areas of cortex.
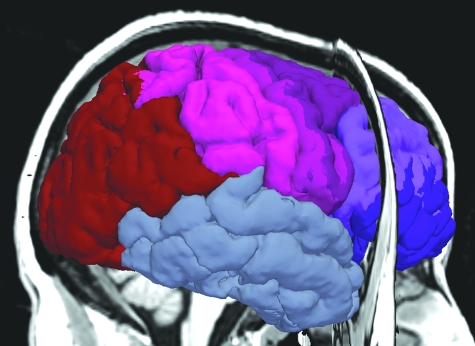
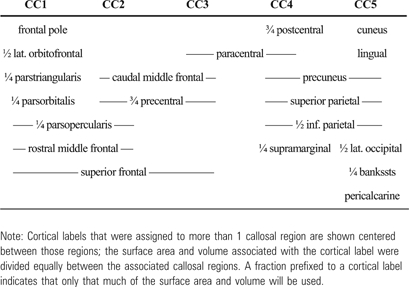
The automatic parcellation of the cortex provided by freesurfer was used to divide the cortex into areas corresponding to the callosal subregions. A weighted assignment of cortical labels to callosal subregions was made. Cortical labels were assigned to callosal subregions with weights that reflected their contribution. Their contribution to a given subregion was defined in terms of the fraction of interhemispheric connections that originate in the area and the fraction of such connections that pass through the subregion. Because the vast majority of the fibers that traverse the callosum connect close to the midline, weighted assignments of medial cortical areas to callosal subregions summed to 1.0; lateral areas were assumed to produce, at most, one quarter as many interhemispheric connections, and thus weighted assignments of these areas to callosal subregions summed to 0.25; weighted assignments to callosal subregions of areas between summed to 0.5. Cortical labels for which, consistently across subjects, all tracts that originated in that cortical area terminated in the same subregion of the callosum contributed their full weight to that callosal subregion. Cortical labels for which some tracts that originated in that cortical area terminated in one callosal subregion, and some in another, contributed the fraction of their weight to each of the associated subregions that reflected the division of their contribution of connections. These cortical divisions are depicted in Figure 3 and detailed in Table 1.
Figure 3.
The cortical divisions corresponding to the 5 subregions of the corpus callosum.
Table 1.
The cortical divisions
 |
Data Analysis
The hypothesized relationship is a negative correlation between connection length and degree of connectivity. This prediction was tested with regression analyses for each subregion of the callosum. The degree of connectivity was calculated as the ratio of an estimate of the number of interhemispheric connections in each subregion of the callosum to an estimate of total corticocortical projection neurons in the cortical areas connected by the subregion; connection length was estimated as the average length of the longest 10% of the fibers passing through each subregion. Note that this definition of degree of connectivity should not be confused with probabilistic definitions of connectivity, for example, the probability of a direct connection between 2 neurons (Karbowski 2001, 2003) or the probability of a direct connection between 2 areas (Changizi and Shimojo 2005; Karbowski 2003).
The midsagittal area of the corpus callosum has been shown to be a reasonable index of the number of interhemispheric connections (Aboitiz, Scheibel, Fisher et al. 1992; Aboitiz, Scheibel, and Zaidel 1992). Studies of axon diameter in the corpus callosum have found that the diameter of the largest approximately 0.1% of the fibers increases with brain size but that other populations of fibers do not vary (Aboitiz, Scheibel and Zaidel 1992; Jerison 1991; Olivares et al. 2001; Schüz and Preissl 1996). The callosum consists of approximately 190 million fibers (Tomasch 1954). Thus, the number of interhemispheric connections in each region can be estimated to be approximately 190 million times the ratio of the area of that region to the across-subject average total area of the callosum. This estimate, however, must be corrected for the effects of age; the callosum continues to grow, at least in posterior regions, throughout the third decade of life (Pujol et al. 1993), but this growth represents primarily an increase in myelination, rather than an increase in the number of axons that comprise it. The area measures of each subregion are thus adjusted for age effects, and the adjusted measures are used in the estimate of the number of axons that pass through that subregion.
The number of corticocortical projection neurons can also be estimated with reasonable accuracy from gender, age, cortical volume, and cortical surface area. Across mammals, cortical thickness increases as the 1/9 power of gray matter volume (Hofman 1985, 1988, 1989), but neuron density decreases as the −1/3 power of gray matter (Prothero 1997; Rockel et al. 1980; Tower 1954). Individual variation in humans does not differ greatly from this pattern, but gender and age effects must be taken into account. Neocortical neuron number can be predicted with a 95% tolerance limit of ±24% based on gender, age, gray matter volume, and cortical surface area (Pakkenberg and Gundersen 1997). The formula is as follows:
This formula was used to estimate the number of projection neurons associated with each of the divisions of cortex, as defined above, assuming that projection neurons scale with the total number of neurons. The measures of cortical gray matter volume and cortical surface area for each cortical area were provided by freesurfer.
The length of the interhemispheric connections was estimated from the tracts emanating from the gray matter and terminating at the midsagittal point of the callosum. The measure of length for each region of the callosum was an average of the lengths associated with each voxel of that region on the midsagittal slice; the length associated with each voxel was calculated as the average of the longest 10% of the fibers terminating at that voxel.
Results
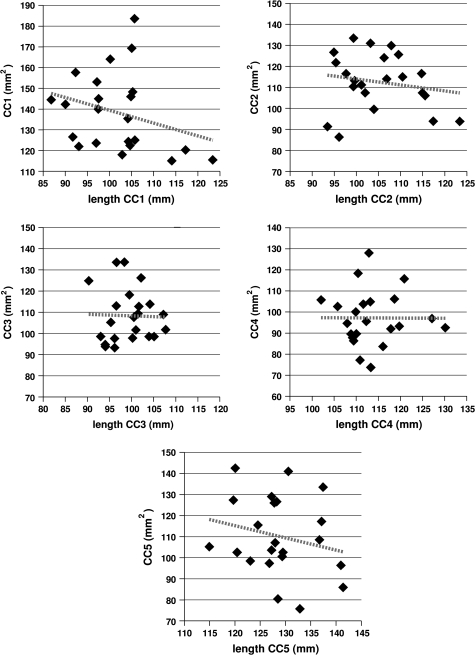
Regressing the cross-sectional area of each subregion of the callosum against the average length of the connections in that subregion yielded no significant relationships but showed a tendency for increased connection length to be associated with decreasing callosal area. This is shown in Figure 4. Note that all subregions show a negative correlation between connection length and callosal area. The significance and r2 values are given in Table 2.
Figure 4.
The relation between the cross-sectional area for a given subregion of the callosum and the average length of the connections passing through that subregion. The dashed lines show that the correlation is negative—callosum size decreases as connection length increases—in all subregions, though none of the relationships are significant at P ≤ .05. The significance and r2 values are given in Table 2.
Table 2.
The significance and r2 values for the regression analyses, for each of the 5 subregions of the callosum
| CC1 |
CC2 |
CC3 |
CC4 |
CC5 |
||||||
| Significance | r2 | Significance | r2 | Significance | r2 | Significance | r2 | Significance | r2 | |
| CC vs length | 0.194 | 0.083 | 0.440 | 0.030 | 0.903 | 0.001 | 0.982 | 0.000 | 0.640 | 0.011 |
| Connect vs length | 0.008 | 0.304 | 0.002 | 0.390 | 0.015 | 0.263 | 0.125 | 0.114 | 0.036 | 0.203 |
Note: The top row provides the results for regressions of the cross-sectional area of subregion i of the callosum on the average length of the connections passing through subregion i. These values correspond to the regression plots in Figure 4. The bottom row provides the results for regressions of the degree of connectivity—abbreviated here as connect—for subregion i against the average length of the connections in subregion i. These values correspond to the regression plots in Figure 5.
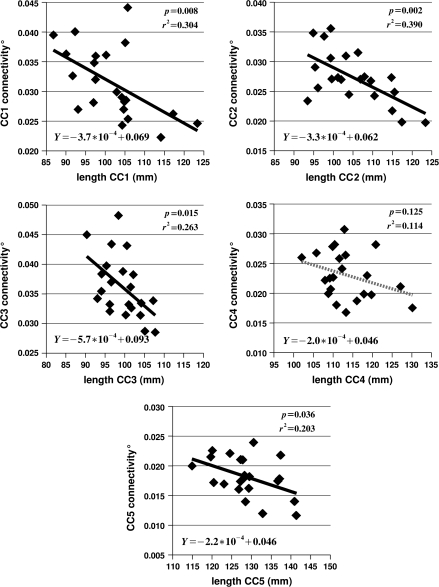
Regressing the degree of connectivity for subregion i of the callosum against the average length of the connections passing through subregion i yielded significant relationships for 4 of the 5 subregions—all but area 4, roughly the isthmus—with an average r2 value of 0.255. The results are shown in Figure 5, with the significance and r2 values, as well as the equations for the regression lines. The significance and r2 values are also given in Table 2.
Figure 5.
The relation between degree of connectivity for subregion i of the callosum—indicated as CCi connectivity—and the average length of the connections passing through region i. The black regression lines in subregions 1, 2, 3, and 5 indicate that the relationship is significant at P ≤ .05. The equation for each regression line is given in the lower left corner of each plot, and the significance and r2 values are presented in the upper right corner.
It should be remembered that the values shown in Figure 5, though plausible—a few percent—are crude estimates based on the assumption that the number of projection neurons scale with the total number of neurons and that there is less interhemispheric connectivity in lateral regions of cortex. But it is the relative values that are of real interest, and the relative values should be roughly correct.
Discussion
Using DTI and tractography to detail the patterns of interhemispheric connectivity and to determine the length of the connections, and formulae based on histological results to estimate degree of connectivity, we have shown that, for interhemispheric connectivity, connection length is negatively correlated with degree of connectivity in the normal adult male brain. Significant relationships were found in 4 of 5 subregions of the callosum—the exception being the isthmus—with an average r2 value of 0.255 over the 5 regions; thus, on average, connection length accounted for about 25.5% of the variance in degree of connectivity. The results concur with the hypothesis that, due to the increased conduction delays and cellular costs associated with the long-distance connections, larger brains should have relatively less long-distance connectivity (Ringo 1991; Ringo et al. 1994). The impact of connection length on intrahemispheric connectivity, and on area-to-area connectivity, remains to be tested.
Comparison with previous research relating measures of brain size to interhemispheric connectivity (Jäncke et al. 1997; Rauch and Jinkins 1994) shows that connection length is a considerably better predictor. Rauch and Jinkins (1994) studied a group of adults between 20 and 87 years of age (mean 36 y, SD not reported). T1-weighted images were used to measure the callosum and to estimate brain size. The callosum was traced on the midsagittal slice; the cerebral outline was traced on the axial and sagittal slices for which cerebral area was greatest, and the average of these 2 areas was taken as the measure of brain size. A statistically significant positive correlation was found between callosal size and brain size, with an r2 of 0.211. But the values for callosal size and brain size were not adjusted for age, though a positive correlation with age was shown in both cases. Also, though it is primarily the posterior of the callosum that continues to grow until the fourth decade of life (Pujol et al. 1993), because the callosum was not subdivided, the overall correlation is confounded by the age effect. It is nonetheless noteworthy that the reported correlation was positive. Our data show a negative, though nonsignificant, correlation between the cross-sectional area of each of the regions of the callosum and the length of the tracts that pass through those regions. The measure of brain size used by Rauch and Jinkins (1994), however, is known to correlate poorly with brain volume (de Lacoste et al. 1990) and presumably also correlates poorly with the average length of the interhemispheric connections.
Jäncke et al. (1997) studied a similar age group (range 18–45; mean 25.7; SD 4.7) to the group reported on here, but with both males and females. Using T1-weighted images, they divided the callosum into 4 regions—the anterior third, the middle third, the posterior fifth, and the remainder—and regressed the cross-sectional area of each subregion against forebrain volume. They report a significant positive correlation in all 4 regions, with an r2 reported separately for men in the middle third and splenium—0.07 and 0.08, respectively——and an r2 for men and women together in the anterior third and the isthmus—0.23 and 0.15, respectively. Again, however, there was no control for age effects. The same analysis with our data, but with age-adjusted measures of corpus callosum subregion area and forebrain volume, and with the callosum divided into 5 subregions, rather than 4, yielded a significant relationship only in subregion 3—and no marginal relationships—and r2 values of 0.002, 0.055, 0.161, 0.000, and 0.078 in subregions 1–5, respectively. That it is subregion 3 that shows a relation with forebrain volume is interesting; across subjects, subregion 3 has the shortest tracts and shows the least variation in connection length. But forebrain volume only accounts for 16.1% of the variation in cross-sectional area in subregion 3, and an average of 3.4% of the variation in the other 4 subregions.
In contrast, as shown in Table 2, regressing the estimate of the degree of interhemispheric connectivity associated with subregion i of the callosum against the length of the interhemispheric connections passing through subregion i yields significant results in subregions 1, 2, 3, and 5, with r2 values of 0.304, 0.390, 0.263, and 0.203, respectively. The lack of significance in subregion 4 might be due to measurement problems associated with the fornix. The fibers of the fornix leave the hippocampus at the level of the splenium and run in a rostromedial direction until they reach the midline under subregion 4 and curve downward to the mamillary bodies. Under subregion 4, the fornix can distort the tensors that determine the path of the tracts that enter the callosum and so introduce inaccuracies in the estimate of the length of the tracts passing through subregion 4. The fornix also confuses the task of identifying the ventral boundary of subregion 4; and any measurement errors are likely amplified due to the relative narrowness of the callosum at that point. The lack of significance in subregion 4 might also be due to an increase in the proportion of very large diameter fibers interconnecting the primary sensory areas of the 2 hemispheres. In cross-species analyses, such an association with brain size has been reported (Olivares et al. 2001; Schüz and Preissl 1996). The connections between sensory areas pass through subregion 4 (Hofer and Frahm 2006). In any case, including subregion 4, an average of 25.5% of the variability in degree of interhemispheric connectivity associated with each subregion is accounted for by the length of the interhemispheric connections.
Moreover, and most relevant to the hypothesis being tested here, as shown in Figure 5, degree of interhemispheric connectivity is negatively correlated with the length of the interhemispheric connections. The strength of this relationship is even apparent in Figure 4, which shows the regressions of the cross-sectional area of subregion i on the length of the connections that pass through subregion i. The hypothesis of Ringo et al. (1994) predicts that longer connections should be associated with a lesser degree of connectivity but not necessarily with absolutely smaller callosa. The importance of utilizing meaningful relative measures has been demonstrated elsewhere (Jungers et al. 1995; Smith 2005). Nonetheless, Figure 4 shows a tendency for longer connections to be associated with absolutely smaller callosa. Taking into account which portion of cortex is connected via each subregion of the callosum, and estimating the degree of connectivity from this and the histological results of Pakkenberg and Gundersen (1997), substantially reduces the variability seen in Figure 4 and strengthens the relationship.
Still more of the variation in the degree of connectivity might be accounted for if developmental data were available. The shape of the growth trajectory during early development may have a substantial impact on connectivity (Lewis and Elman 2008), and brain growth rates can vary considerably during development.
The impact of connection length on the degree of connectivity throughout development may, in fact, be an important part of an account of structural and functional abnormalities in developmental disorders and possibly of an account of the behavioral phenotypes. Children with autism spectrum disorder, for example, undergo a period of brain overgrowth during the first years of life (Aylward et al. 2002; Courchesne et al. 2003; Courchesne et al. 2001; Fombonne et al. 1999; Hazlett et al. 2005; Lainhart et al. 1997; Sparks et al. 2002) and subsequently show structural and functional underconnectivity (Belmonte et al. 2004; Egaas et al. 1995; Herbert 2005; Just et al. 2004, 2007; Murias et al. 2007). Computational modeling has shown that the increased conduction delays presumably accompanying the early brain overgrowth may account for these findings, with increased conduction delays leading to decreased functional connectivity and decreased functional connectivity subsequently leading to decreased structural connectivity (Lewis and Elman 2008), and in vivo magnetic resonance imaging studies of children with autism have related brain size to the relative size of the corpus callosum (Lewis and Courchesne 2004; Lewis et al. 2003, 2004) and to changes in the relative size of the callosum (Lewis et al. 2005). Abnormalities in brain size are pervasive in developmental disorders, and thus, the hypothesized effect of connection length on brain organization might have considerable explanatory value.
Additionally, white matter continues to change throughout life (Courchesne et al. 2000; Ge et al. 2002), and so during aging in such developmental disorders, in which macrocephaly may persist into adulthood (Lainhart et al. 1997), as well as in normal aging, connection length is expected to show a negative correlation with changes in the degree of connectivity.
Brain size also appears to explain differences in interhemispheric connectivity between genders (Jäncke et al. 1997; Jäncke and Steinmetz 1998; Luders et al. 2006), as well as across species (Rilling and Insel 1999a). On average, males have larger brains than females (Pakkenberg and Gundersen 1997) and thus longer interhemispheric connections. The results here suggest that connection length is the relevant aspect of brain size that lies behind the differences in interhemispheric connectivity. Further research is needed, however, to confirm this; in order to eliminate this potential confound, our subject population was limited to males.
Brain size is also positively correlated with the degree of sulcul convolution (Im et al. 2008), and sulcal folding has been hypothesized to result from the mechanical forces associated with connectivity, with greater local connectivity driving a greater degree of sulcul convolution (Van Essen 1997). Retrograde tract–tracing experiments in adult rhesus monkeys has supported this link between connectivity and cortical folding (Hilgetag and Barbas 2006). Thus, the relation between connection length and degree of connectivity may also explain patterns of sulcal folding—in humans, and in general.
Connection length is, of course, not the only factor that might determine the degree of long-distance connectivity. There is evidence that experience, environment, and genetics play a substantial role (Lee et al. 2003; Öztürk et al. 2002; Pfefferbaum et al. 2000; Scamvougeras et al. 2003). But connection length appears to be a more important factor than previously suggested. Even with the limited amount of variation in brain size and connection length in our sample, connection length accounted for about 25% of the variance in degree of connectivity. A sample with substantially more variation in brain size—for example, a cross-species sample—would, we predict, show a considerably stronger relationship.
Several caveats, however, must be considered. The results reported here rely on the assumption that the number of corticocortical projection neurons scales with the total number of neurons in cortex—similar to the assumption of Zhang and Sejnowski (2000) that the number of projection neurons scales with surface area. Additional histological studies will be required to determine if this is correct. An apparent decrease in the degree of interhemispheric connectivity would also be seen if the number of corticocortical projection neurons did not scale with the total number of neurons. But the fact that increases in white matter volume outpace increases in gray matter volume (Frahm et al. 1982; Rilling and Insel 1999b; Schlenska 1974; Zhang and Sejnowski 2000) suggests that this is not the case. The impact of the increase in diameter of the largest fibers of the callosum is also unclear. If these large-diameter fibers connect to a large number of neurons, considerable compensation might be achieved through these increases; and if the branching patterns of callosal neurons depend on brain size, degree of connectivity estimates must be adjusted in accord. Also, crossing fibers interfere with tractrography, and so with measures of the length of the interhemispheric connections. As mentioned, this is particularly a problem in the temporal lobe, where fibers descending from the splenium travel parallel to noncallosal fibers in the inferior longitudinal fasciculus; thus, the temporal lobe was eliminated from consideration. But there are also problems with tractrography elsewhere. Callosal fibers projecting to, or coming from, lateral cortical areas cross with thalamocortical connections. This will produce low fractional anisotropy values, and so callosal tracts from lateral cortex may be truncated and discarded. To the extent that such tracts are among the longest that comprise a subregion of the callosum, and are substantially different in length from tracts for which tractography succeeds, this will introduce inaccuracies in the length measurements. Callosal fibers projecting to more medial areas of cortex in frontal, parietal, and occipital lobes may become confused with thalamocortical connections, as well. But the longest callosal fibers and the longest thalamocortical fibers will both originate or terminate at the outermost point of a gyrus. And finally, in contrast to the hypothesis explored here, decreases in interhemispheric connectivity—and long-distance connectivity, generally—might force a compensatory increase in more local connectivity, and this may give rise to the increased brain size. Longitudinal studies will be critical to determining the direction of causation.
But the estimates of degree of connectivity are presumably a considerable improvement over approaches that ignored the histological results and the tractography-based estimates of length an improvement over volumetric measures. Further, the results here concur with a growing body of evidence that metabolic costs and processing efficiency constrain the way the cortex is organized (Achard and Bullmore 2007; Bassett and Bullmore 2006; Changizi 2001, 2005; Changizi and Shimojo 2005; Chklovskii and Koulakov 2000; Chklovskii et al. 2002; Harrison et al. 2002; He et al. 2007; Kaiser and Hilgetag 2004; Karbowski 2001, 2003; Ringo 1991; Ringo et al. 1994; Schüz and Miller 2002; Sporns and Honey 2006; Sporns and Zwi 2004; Watts and Strogatz 1998) and support theoretical notions of how cortical organization should scale (Changizi 2001, 2005; Changizi and Shimojo 2005; Kaas 2000; Karbowski 2001, 2003; Ringo 1991; Ringo et al. 1994).
Funding
National Institutes of Health/National Institute on Aging RO1 AG18030 to J.T.
Acknowledgments
Conflict of Interest: None declared.
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Zaidel E. Morphometry of the Sylvian fissure and the corpus callosum, with emphasis on sex differences. Brain. 1992;115:1521–1541. doi: 10.1093/brain/115.5.1521. [DOI] [PubMed] [Google Scholar]
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PloS Comp Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM. Evolving brains. New York: W.H. Freeman & Co; 2000. [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg V. Brain size and number of neurons: an exercise in synthetic neuroanatomy. J Comput Neurosci. 2001;10:71–77. doi: 10.1023/a:1008920127052. [DOI] [PubMed] [Google Scholar]
- Changizi MA. Principles underlying mammalian neocortical scaling. Biol Cybern. 2001;84:207–215. doi: 10.1007/s004220000205. [DOI] [PubMed] [Google Scholar]
- Changizi MA. Evolution of nervous systems. 2005. Scaling the brain and its connections. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- Changizi MA, Shimojo S. Parcellation and area-area connectivity as a function of neocortex size. Brain Behav Evol. 2005;66:88–98. doi: 10.1159/000085942. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Koulakov AA. A wire length minimization approach to ocular dominance patterns in mammalian visual cortex. Physica A. 2000;284:318–334. doi: 10.1016/s0896-6273(01)00223-9. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Schikorski T, Stevens CF. Wiring optimization in cortical circuits. Neuron. 2002;34:341–347. doi: 10.1016/s0896-6273(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Clarke S, Kraftsik R, van der Loos H, Innocenti GM. Forms and measures of adult and developing human corpus callosum. J Neuropathol Exp Neurol. 1989;280:213–230. doi: 10.1002/cne.902800205. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Neuroradiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- de Lacoste MC, Adesanya T, Woodward DJ. Measures of gender differences in the human brain and their relationship to brain weight. Biol Psychiatry. 1990;28:931–942. doi: 10.1016/0006-3223(90)90059-b. [DOI] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Roge B, Claverie J, County S, Fremolle J. Microcephaly and macrocephaly in autism. J Autism Dev Disord. 1999;29:113–119. doi: 10.1023/a:1023036509476. [DOI] [PubMed] [Google Scholar]
- Frahm HD, Stephan H, Stephan M. Comparison of brain structure volumes in insectivora and primates. I. Neocortex. J Hirnforsch. 1982;23:375–389. [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Harrison KH, Hof PR, Wang SSH. Scaling laws in the mammalian neocortex: does form provide clues to function? J Neurocytol. 2002;31:289–298. doi: 10.1023/a:1024178127195. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Large brains in autism: the challenge of pervasive abnormality. Neuroscientist. 2005;11:417–440. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput Biol. 2006;2:e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hofman MA. Size and shape of the cerebral cortex in mammals 1: The cortical surface. Brain Behav Evol. 1985;27:28–40. doi: 10.1159/000118718. [DOI] [PubMed] [Google Scholar]
- Hofman MA. Size and shape of the cerebral cortex in mammals 2: The cortical volume. Brain Behav Evol. 1988;32:17–26. doi: 10.1159/000116529. [DOI] [PubMed] [Google Scholar]
- Hofman MA. On the evolution and geometry of the brain in mammals. Prog Neurobiol. 1989;32:137–158. doi: 10.1016/0301-0082(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008 doi: 10.1093/cercor/bhm244. doi:10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relation between corpus callosum size and forebrain volume. Cereb Cortex. 1997;7:48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Steinmetz H. Brain size: a possible source of interindividual variability in corpus callosum morphology. In: Zaidel E, Iacoboni M, Pascual-Leone AP, editors. The role of the corpus callosum in sensory motor integration: anatomy, physiology, and behavior; individual differences and clinical applications. New York: Plenum Press; 1998. [Google Scholar]
- Jerison HJ. Brain size and the evolution of mind. New York: American Museum of Natural History; 1991. [Google Scholar]
- Jungers WL, Falsetti AB, Wall CE. Shape, relative size, and size-adjustments in morphometrics. Am J Phys Anthropol. 1995;38:137–161. [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–996. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Why is brain size so important: design problems and solutions as neocortex gets bigger or smaller. Brain and Mind. 2000;1:7–23. [Google Scholar]
- Kaiser M, Hilgetag CC. Modelling the development of cortical systems networks. Neurocomputing. 2004;58:297–302. [Google Scholar]
- Karbowski J. Optimal wiring principle and plateaus in the degree of separation for cortical neurons. Phys Rev Lett. 2001;86:3674–3677. doi: 10.1103/PhysRevLett.86.3674. [DOI] [PubMed] [Google Scholar]
- Karbowski J. How does connectivity between cortical areas depend on brain size? Implications for effficient computation. J Comput Neurosci. 2003;15:347–356. doi: 10.1023/a:1027467911225. [DOI] [PubMed] [Google Scholar]
- Karbowski J. Global and regional brain metabolic scaling and its functional consequences. BMC Biol. 2007;5:18. doi: 10.1186/1741-7007-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, Folstein SE. Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry. 1997;36:282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Chen Y, Schlaug G. Corpus callosum: musician and gender effects. NeuroReport. 2003;14:205–209. doi: 10.1097/00001756-200302100-00009. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Courchesne E. 2004. Pathological brain overgrowth leads to reduced interhemispheric connectivity. [Google Scholar]
- Lewis JD, Courchesne E, Elman JL. Rate of growth and hemispheric specialization. The 23rd International Summer School of Brain Research, August 25-29, 2003, Amsterdam, the Netherlands. 2003 [Google Scholar]
- Lewis JD, Courchesne E, Elman JL. Growth trajectories and cortico-cortical connections. The 37th Annual Gatlinburg Conference: On Research & Theory in Intellectual & Developmental Disabilities, March 10-14, 2004, San Diego, CA. 2004 [Google Scholar]
- Lewis JD, Elman JL. Growth-related neural reorganization and the autism phenotype: a test of the hypothesis that altered brain growth leads to altered connectivity. Dev Sci. 2008;11:135–155. doi: 10.1111/j.1467-7687.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Elman JL, Courchesne E. Brain overgrowth and reduced long-distance connectivity in autism. The 11th Annual Meeting of the Organization for Human Brain Mapping, June 12-16, 2005, Toronto, ON, Canada. 2005 [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006;17:1103–1106. doi: 10.1097/01.wnr.0000227987.77304.cc. [DOI] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62:270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares R, Montiel J, Aboitiz F. Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain Behav Evol. 2001;57:98–105. doi: 10.1159/000047229. [DOI] [PubMed] [Google Scholar]
- Öztürk AH, Tasçioglu B, Aktekin M, Kurtoglu Z, Erdin I. Morphometric comparison of the human corpus callosum in professional musicians and non-musicians by using in vivo magnetic resonance imaging. J Neuroradiol. 2002;29:29–34. [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Park H-J, Kubicki M, Shenton ME, Guimond A, McCarley RW, Maier SE, Kikinis R, Jolesz FA, Westin C-F. Spatial normalization of diffusion tensor MRI using multiple channels. Neuroimage. 2003;20:1995–2009. doi: 10.1016/j.neuroimage.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Swan GE, Carmelli D. Brain structure in men remains highly heritable in the seventh and eighth decades of life. Neurobiol Aging. 2000;21:63–74. doi: 10.1016/s0197-4580(00)00086-5. [DOI] [PubMed] [Google Scholar]
- Prothero J. Scaling of cortical neuron density and white matter volume in mammals. Brain Res. 1997;38:513–524. [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junqué C, Martí-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Rauch RA, Jinkins JR. Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behav Brain Res. 1994;64:65–78. doi: 10.1016/0166-4328(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. Differential expansion of neural projection systems in primate brain evolution. Neuroreport. 1999a;10:1453–1459. doi: 10.1097/00001756-199905140-00012. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J Hum Evol. 1999b;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Ringo JL. Neuronal interconnection as a function of brain size. Brain Behav Evol. 1991;38:1–6. doi: 10.1159/000114375. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Hiorns RW, Powell TP. The basic uniformity in structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Scamvougeras A, Kigar DL, Jones D, Weinberger DR, Witelson SF. Size of the human corpus callosum is genetically determined: an MRI study in mono and dizygotic twins. Neurosci Lett. 2003;338:91–94. doi: 10.1016/s0304-3940(02)01333-2. [DOI] [PubMed] [Google Scholar]
- Schlenska G. Volumen und Oberflächenmessungen an Gehirnen verschiedener Säugetiere im Vergleich zu einem errechneten Modell. J Hirnforsch. 1974;15:401–408. [Google Scholar]
- Schüz A, Miller R. Cortical areas unity and diversity Conceptual advances in brain research. London: Taylor & Francis; 2002. [Google Scholar]
- Schüz A, Preissl H. Basic connectivity of the cerebral cortex and some considerations on the corpus callosum. Neurosci Biobehav Rev. 1996;20:567–570. doi: 10.1016/0149-7634(95)00069-0. [DOI] [PubMed] [Google Scholar]
- Smith R. Relative Size versus Controlling for Size: Interpretation of Ratios in Research on Sexual Dimorphism in the Human Corpus Callosum. Current Anthropology. 2005;46:249–273. [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ. Small worlds inside big brains. Proc Natl Acad Sci USA. 2006;103:19219. doi: 10.1073/pnas.0609523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Zwi JD. The small world of the cerebral cortex. NeuroInformatics. 2004;2:145–162. doi: 10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- Tomasch J. Size, distribution, and number of fibres in the human corpus callosum. Anat Rec. 1954;119:119–135. doi: 10.1002/ar.1091190109. [DOI] [PubMed] [Google Scholar]
- Tower DB. Structural and functional organization of mammalian cerebral cortex: the correlation of neuron density with brain size. J Comp Neurol. 1954;101:9–52. doi: 10.1002/cne.901010103. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of'small-world'networks. Nature. 1998;393:409–410. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Conduction in myelinated, unmyelinated, and demyelinated fibers. Arch Neurol. 1977;34:585–589. doi: 10.1001/archneur.1977.00500220019003. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. PNAS. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]