Abstract
Alterations in gamma-frequency oscillations are implicated in psychiatric disorders, and polymorphisms in NRG-1 and ERBB4, genes encoding Neuregulin-1 (NRG-1) and one of its receptors, designated ErbB4, are associated with schizophrenia. Here we show that NRG-1 selectively increases the power of kainate-induced, but not carbachol-induced, gamma oscillations in acute hippocampal slices. NRG-1β is more effective than NRG-1α, a splice variant with lower affinity for ErbB receptors, and neither isoform affects the network activity without prior induction of gamma oscillations. NRG-1β dramatically increases gamma oscillation power in hippocampal slices from both rats (2062 ± 496%) and mice (710 ± 299%). These effects of NRG-1β are blocked by PD158780, a pan-specific antagonist of ErbB receptors, and are mediated specifically via ErbB4 receptors, because mice harboring a targeted mutation of ErbB4 do not respond to NRG-1. Moreover, we demonstrate that 50% of gamma-amino butyric acidergic parvalbumin (PV)–positive interneurons, which heavily contribute to the generation of gamma oscillations, express ErbB4 receptors. Importantly, both the number of PV-immunoreactive interneurons (−31%) and the power of kainate-induced gamma oscillations (−60%) are reduced in ErbB4 knockout mice. This study provides the first plausible link between NRG-1/ErbB4 signaling and rhythmic network activity that may be altered in persons with schizophrenia.
Keywords: ErbB4, hippocampus, interneuron, mouse, parvalbumin, rat
Introduction
The synchronization of neuronal network activity in the human cortex and hippocampus at gamma frequencies (30–80 Hz) is important for cognition, learning and memory (Engel and Singer 2001). Spontaneous long-lasting gamma oscillations have been recorded in vivo in the hippocampus and their frequency is modulated by gamma-aminobutyric acidergic (GABAergic) basket cells (Bragin et al. 1995; Fisahn et al. 1998; Csicsvari et al. 2003; Mann et al. 2005). Similar gamma rhythms can be evoked in rodent acute hippocampal slices by bath application of carbachol (Fisahn et al. 1998) or kainic acid (Hajos et al. 2000; Fisahn et al. 2004) resulting in the activation of muscarinic or kainate receptors, respectively. Both carbachol-induced and kainate-induced gamma oscillations are driven by the complex network of the CA3 area and rely on interplay of excitatory and inhibitory synaptic transmission (Fisahn et al. 1998). Whereas fast inhibitory and fast excitatory neurotransmission is necessary for both types of gamma oscillations, differences are emerging. For example, carbachol-induced activity depends on the recruitment of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors whereas kainate-induced activity does not. Furthermore, it has been suggested that the dominance of inhibitory versus excitatory neurotransmission may be different in the various induction paradigms for gamma oscillation and that different paradigms recruit distinct subgroups of GABAergic interneurons (Palhalmi et al. 2004).
Recent studies indicate that the power of gamma oscillations in subjects diagnosed with schizophrenia is reduced (Kwon et al. 1999; Wilson et al. 2007), and that regional reaction time phase-lock of oscillations is correlated with either the positive or negative symptoms of the disorder (Spencer et al. 2004). In addition, the amounts of RNA and immunoreactivity for parvalbumin (PV) are reduced in post-mortem tissue from the frontal cortex and the hippocampus of schizophrenic patients (Reynolds et al. 2002; Zhang and Reynolds 2002; Hashimoto et al. 2003; Torrey et al. 2005), and a direct relation to the changes in gamma oscillations has been suggested (Lewis et al. 2005).
Neuregulin-1 (NRG-1) is a trophic and differentiation factor that signals via ErbB receptor tyrosine kinases (ErbB2–ErbB4) and that harbors an epidermal growth factor (EGF)--like domain mediating its biological effects (Buonanno and Fischbach 2001). The isoforms NRG-1α and NRG-1β are generated by alternate splicing of the EGF-like domain; NRG-1β is more highly expressed in brain and has the highest affinity for ErbB receptors. In the hippocampus pro-NRG-1β is presynaptically expressed in CA3 pyramidal neurons, and it is cleaved and released in an activity-dependent manner (Eilam et al. 1998; Loeb et al. 2002; Ozaki et al. 2004). NRG-1 binds to either ErbB3 or ErbB4, causing a conformational change that promotes receptor dimerization and autophosphorylation, and subsequent activation of downstream signaling pathways. Although ErbB2 does not bind NRG-1, it functions as coreceptor after heterodimerization with ErbB3 or ErbB4; ErbB4 can also function as a homodimer (Buonanno and Fischbach 2001). ErbB receptors are widely and differentially expressed in the adult brain. In general, ErbB2 is expressed in most cells, ErbB3 is enriched in glial populations, and ErbB4 is mainly found in neurons. ErbB4 transcripts and protein are most highly expressed in interneurons (Lai and Lemke 1991; Gerecke et al. 2001; Yau et al. 2003; Longart et al. 2007), where it localizes at glutamatergic postsynaptic densities and interacts with PSD-95 (Garcia et al. 2000; Huang et al. 2000).
NRG-1 and ERBB4 were identified as schizophrenia susceptibility genes (Stefansson et al. 2002; Norton et al. 2006; Silberberg et al. 2006), and these finding have been supported by numerous subsequent genetic-association studies worldwide (Corfas et al. 2004; Harrison and Weinberger 2005; Li et al. 2006). Biochemical studies using post-mortem brain samples, recently revealed that NRG-1 signaling and ErbB4-PSD95 interactions are altered in dorsal prefrontal cortices of schizophrenia patients (Hahn et al. 2006). Moreover, subjects harboring an “at-risk” NRG-1 polymorphism manifest decreased frontal and temporal lobe activation, decreased IQ and augmented psychotic symptoms (Hall et al. 2006). Animal studies have also shown that NRG-1 and ErbB4 hypomorphic mice, but not ErbB2 or ErbB3 mutant mice (Gerlai et al. 2000), exhibit deficits in sensory gating that are ameliorated by treatment with the antipsychotic clozapine (Stefansson et al. 2002).
Given the associations of gamma oscillations, basket cell function and the NRG/ErbB signaling pathway with schizophrenia, we were interested in investigating a potential role of NRG-1 in regulating gamma oscillatory activity.
Material and Methods
Animals
Electrophysiological experiments were performed with male Wistar rats (4–5 weeks), and with ErbB4MHC-ErbB4−/− (Tidcombe et al. 2003) and C57BL/6 wild-type (WT) mice (5–8 weeks); immunohistological experiments used adult mice from both groups (9–12 weeks). ErbB4MHC-ErbB4−/− mice were backcrossed for 15 generations into C57BL/6. Animals were raised under a 12-h light/12-h dark cycle with food and water provided ad libitum. Procedures were approved and followed the NIH guidelines for the care and use of laboratory animals.
Electrophysiology
Horizontal 300-μm-thick hippocampal slices were maintained at room temperature at the interface between humidified carbogen gas (95% O2/5% CO2) and artificial cerebrospinal fluid (ACSF), pH 7.3, containing (in mM): NaCl 124, KCl 3.5, NaH2PO4 1.25, MgCl2 1.5, CaCl2 1.5, NaHCO3 30, glucose 10, for at least 1 h prior to recording. Extracellular field recordings were made in stratum pyramidale (sp) of CA3 in an interface recording chamber (36 °C; rat slices) or a submerged recording chamber (32 °C; mouse slices) using glass microelectrodes containing ACSF (resistance 3–5 MΩ). Drugs: kainic acid (Sigma-Aldrich, St. Louis, MO), NRG-1α and NRG-1β EGF-like domain, PD158780 (R&D Systems, Minneapolis, MN). Different batches of NRG-1 peptides were tested for their capacity to elicit equal ErbB receptor phosphorylation. Data were recorded with a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and stored using pClamp 9.2 software (Molecular Devices). Fast Fourier Transformations for power spectra were computed from 60-s traces using Axograph software (Molecular Devices). Power values are derived from integrating power spectra between 20 and 80 Hz.
Cell Quantification
Paraformaldehyde-fixed 50-μm horizontal sections were processed for either PV/ErbB4 double-immunofluorescence (6 sections per animal; WT: n = 2; ErbB4MHC-ErbB4−/−: n = 2) or PV immunohistochemistry (16–20 sections per animal; WT: n = 4; ErbB4MHC-ErbB4−/−: n = 4) using standard procedures. Primary antibodies: rabbit polyclonal anti-PV (Swant, Bellinzona, Switzerland), mouse monoclonal anti-ErbB4 (AB-1, Lab Vision, Fremont, CA). Numerical cell density, location in layers, and degree of coexpression were determined for PV and ErbB4 immunoreactive cell somata in hippocampal CA3 on either confocal or brightfield images. The specificity of the staining was tested by standard procedures, including application of the ErbB4 antibody on ErbB4MHC-ErbB4−/− sections.
Data Analysis and Statistics
Analysis of electrophysiological data (paired and unpaired Student t-test) was carried out in KaleidaGraph software (Synergy Software, Reading, PA). Significance of the cell density of PV neurons in WT vs. ErbB4MHC-ErbB4−/− mice (n = 4 each) was evaluated with a 2-tailed Student's t-test (Fig. 3D). The distribution of PV cells across layers in CA3 was analyzed with 2-way ANOVA (WT: n = 2, ErbB4MHC-ErbB4−/−: n = 2; Fig. 3E). Significance level for all tests was set to P = 0.05. All data are means ± SEM.
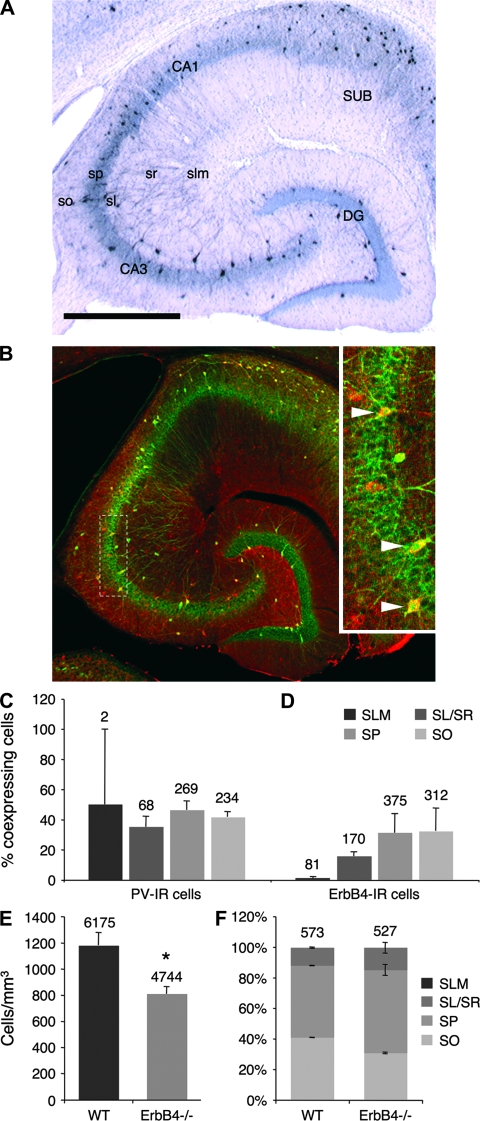
Figure 3.
PV-expressing neurons coexpress ErbB4 in WT hippocampus and are reduced in ErbB4MHC-ErbB4−/− mice. (A) PV immunohistochemistry, and (B) mounted image of ErbB4 (red) and PV (green) double-immunofluorescence on sections from WT mice. ErbB4-positive somata are distributed throughout all layers, whereas PV-expressing somata are mostly near to pyramidal cells. Scale bar = 600 μm. Insert: Pyramidal cell layer of CA3, showing coexpression of ErbB4 and PV (arrowheads). (C) Almost 50% of PV-immunoreactive (IR) cells coexpress ErbB4 throughout all layers; (D) however, less ErbB4-IR cells coexpress PV, notably in slm. (E) PV-expressing cells are reduced by 31% in ErbB4MHC-ErbB4−/− mice (WT vs. ErbB4MHC-ErbB4−/− mice, n = 4 each, 16–20 sections per animal, P = 0.017). (F) Quantitative analysis confirms that most PV-IR somata are near pyramidal cells, either in the so, sp, or lower stratum radiatum (sr), whereas slm is almost devoid of PV-IR cells. WT and ErbB4MHC-ErbB4−/− mice do not differ in across-layer distribution of PV-positive cells (n = 2 each). (C–F) Total numbers of counted cells are presented on top of the columns.
Results
Neuregulin-1 Increases the Power of Gamma-Frequency Oscillations
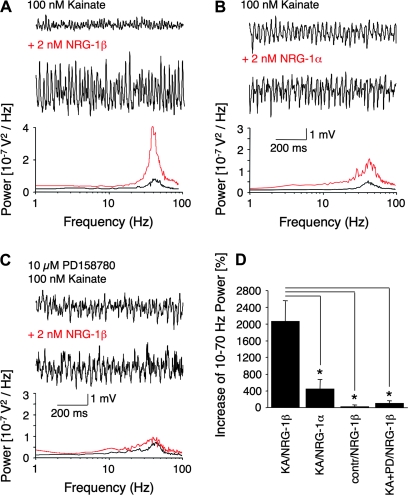
Given the associations of gamma oscillations and NRG-ErbB signaling with schizophrenia, we investigated whether NRG-1 modulates hippocampal rhythmic activity. We first tested the effects of NRG-1β, the splice variant predominantly expressed in brain with highest affinity for ErbB receptors (Buonanno and Fischbach 2001), on kainate-induced (100 nM) gamma oscillations in rat hippocampal slices. Perfusion of slices with 2 nM NRG-1β dramatically increased the power of gamma oscillations (Fig. 1A; P = 0.015), whereas application of the less active NRG-1α isoform resulted in a smaller, but still significant increase (Fig. 1B; P = 0.031). NRG-1β increased oscillation power by 2062 ± 496% (n = 6), which was significantly higher than the 442 ± 220% (n = 4) increase elicited by NRG-1α (Fig. 1D, P = 0.027). Measurements taken at 5, 10, and 15 min after NRG-1β application indicate its effects on kainate-induced gamma oscillation power do not change significantly by prolonging perfusion beyond 10 min (5 min: 1.02 × 10−03 ± 0.36 × 10−03 V2; 10 min: 1.30 × 10−03 ± 0.45 × 10−03 V2; 15 min: 1.16 × 10−03 ± 0.27 × 10−03 V2; n = 8; 5 min/10 min P = 0.016; 10 min/15 min P = 0.531). Consequently, all subsequent measurements were taken after 10-min perfusion. Interestingly, application of 2 nM NRG-1β without prior induction of gamma oscillations by kainate neither generated rhythmic network activity nor significantly increased unspecific network activity (Fig. 1D; 23 ± 46%, n = 4, P = 0.73). A lack of NRG-1β effect at basal activity levels is consistent with our prior work showing that basal glutamatergic synaptic transmission is not modified by NRG-1β and requires Schaffer collateral stimulation (Kwon et al. 2005).
Figure 1.
Modulation of kainate-induced gamma oscillations by NRG-1 in rat hippocampal slices. (A–C) Representative sample traces (top) and power spectra (bottom) of kainate-induced gamma oscillations in rat slices (black: control; red: NRG-1 treated); note difference in scales. (A) NRG-1β (n = 6; P = 0.015) and (B) NRG-1α (n = 4; P = 0.031) significantly increase the power of kainate-induced gamma oscillations, whereas (C) preincubation with the ErbB inhibitor PD158780 (PD) prior to NRG-1β treatment prevents an increase (n = 6; P = 0.13). (D) Quantification of relative gamma power in response to different NRG-1 and ErbB inhibitor treatments. NRG-1β increases the power of kainate-induced (KA) gamma oscillations more strongly than NRG-1α (β: n = 6, α: n = 4, P = 0.027) or NRG-1β after ErbB inhibitor treatment (KA + PD/NRG-1β, n = 6, P = 0.013). NRG-1β has no effect in control slices without prior application of kainate (contr/NRG-1β, n = 4, P = 0.73).
An ErbB Receptor Antagonist Blocks the Neuregulin-1 Effect
Next, we tested for the specificity of the NRG-1β effect on gamma oscillations by treating slices with PD158780, a pan-ErbB specific antagonist that targets the intracellular tyrosine phosphorylation site of these receptors (Fig. 1C). When slices were pretreated with 10 μM PD158780 for 30 min, to allow penetration of the inhibitor into the cell, subsequent application of 2 nM NRG-1β did not alter significantly the power of kainate-induced gamma oscillations (Fig. 1D; 105 ± 58%; n = 6; P = 0.13). Taken together, our results indicate that the effects of NRG-1β are mediated specifically by ErbB receptors because the efficacy of NRG-1 isoforms to increase gamma oscillation power correlates with their binding affinities for the receptors (β isoforms being more active than α isoforms), and because NRG-1 effects are significantly blocked by PD158780. However, pharmacological approaches do not resolve which ErbB receptor subtype mediates the NRG-1 effect because, to our knowledge, selective antagonists for the distinct receptor proteins do not exist. Although the widely used antagonists AG1478 and PD158780 specifically target ErbB receptors, they are not selective for a receptor subtype. ErbB4 kinase activity, as well as those of ErbB1 (EGFR) and ErbB2, are all blocked by both antagonists (Egeblad et al. 2001; Stoll et al. 2001; Brignola et al. 2002). Because the NRG-1 ligand binds ErbB3 and ErbB4, and ErbB3 is restricted to glia in the hippocampus, we hypothesized that ErbB4 is the necessary receptor for NRG-1-mediation of hippocampal gamma oscillations. To address this issue, we had to resort to using genetically targeted mutant mice.
Neuregulin-1 Modulates Kainate-Induced, but not Carbachol-Induced, Gamma Oscillations
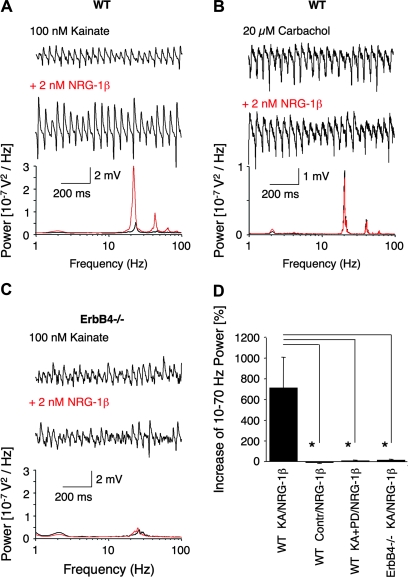
With the goal of investigating NRG-1 effects in genetically altered mice, we began by characterizing how NRG-1β modulates gamma oscillations in mouse hippocampal slices. As shown in Figure 2A,D, 2 nM NRG-1β also dramatically increased the power of kainate-induced gamma oscillations in WT C57BL/6 mouse hippocampal slices (710 ± 299%; n = 12; P = 0.004). The differences between rat and mouse experiments in NRG-1β-induced gamma oscillation frequency and relative power increase are due to differing recording conditions (see Methods and Supplementary Fig. 1). Consistent with previous work showing that NRG-1β action on synaptic function endures after washout (Kwon et al. 2005), we observed that its effects on gamma oscillations could not be reversed by 30-min washout (−16 ± 9%; n = 6, P = 0.1; data not shown). NRG-1β failed to generate any rhythmic network activity when applied without prior induction of gamma oscillations (−8 ± 7%; n = 10; P = 0.18; Fig. 2D), and its effect was blocked by 30-min preincubation of slices with 10μM PD158780 (5 ± 4%; n = 8; P = 0.47; compare NRG-1β: 710 ± 299%). However, PD158780 had no significant effect on kainate-induced gamma oscillations when it was added after NRG-1β treatment (data not shown).
Figure 2.
NRG-1β increases kainate-induced, but not carbachol-induced, gamma oscillations and its effect is absent in slices from ErbB4MHC-ErbB4−/− mice. (A–C) Representative sample traces (top) and power spectra (bottom) of gamma oscillations; note difference in scales. (A) Kainate-induced (n = 12; P = 0.004) or (B) carbachol-induced (n = 8; P = 0.2) gamma oscillations in slices from WT mice (black: control; red: NRG-1β treated). (C) Kainate-induced gamma oscillations in slices from ErbB4MHC-ErbB4−/− mice (black: control; red: NRG-1β treated). (D) Quantification of the effects of NRG-1β on kainate-induced gamma oscillations in hippocampal slices from WT or ErbB4MHC-ErbB4−/− mice. NRG-1β increases significantly the power of kainate-induced oscillations (n = 12; P = 0.004), whereas NRG-1β (2 nM) has no effect in WT slices without prior application of kainate (n = 10; P = 0.18). Coapplication of 10 μM PD158780 prevents NRG-1β modulation of kainate-induced gamma oscillations (n = 8, P = 0.47), which differs from NRG-1β + kainate-induced oscillations (P = 0.038). The NRG-1β modulation of kainate-induced gamma oscillations in ErbB4MHC-ErbB4−/− slices is absent (n = 14, P = 0.26) and therefore significantly different from WT littermates (P = 0.018), demonstrating that the effects of NRG-1β require signaling via ErbB4 receptors.
To determine whether the effects of NRG-1β on network activity are selective to kainate-induced gamma oscillations, we investigated its effects on mouse slices treated with carbachol (Fig. 2B), another induction paradigm for gamma oscillations (Fisahn et al. 1998). The NRG-1β-induced increase in the power of kainate-induced gamma oscillations is totally absent in carbachol-induced gamma oscillations (−10 ± 7%; n = 8; P = 0.2; Fig. 2B). The differential modulation by NRG-1β is most likely due to differing neuronal mechanisms involved in the kainate and carbachol induction paradigms (A. Fisahn, unpublished data), for example, recruitment of AMPA receptors in carbachol- but not kainate-induced gamma oscillations (Bartos et al. 2007), and indicates a site-specific action of NRG-1/ErbB signaling on the kainate-related pathway.
Neuregulin-1 Modulation of Gamma Oscillations is ErbB4 Receptor Dependent
Based on the cellular and subcellular distribution of ErbB4 receptors (Gerecke et al. 2001), and the behavioral similarities between NRG-1 and ErbB4 hypomorphic mice (Stefansson et al. 2002) that is not shared by ErbB2 and ErbB3 mutant mice (Gerlai et al. 2000), we hypothesized that the ErbB4 receptor mediates the NRG-1 effects on gamma oscillations. We tested this hypothesis using adult ErbB4 knockout mice rescued from embryonic lethality by transgenic expression of ErbB4 specifically in heart (ErbB4MHC-ErbB4−/−; Tidcombe et al. 2003). As shown in Figure 2C,D, the NRG-1β-induced increase in gamma oscillation power is entirely absent in hippocampal slices from ErbB4MHC-ErbB4−/− mice (10 ± 10%; n = 14; P = 0.26; compared with WT littermate controls: 710 ± 299%; P = 0.018). This result, together with the block of NRG-1β-induced increase in gamma oscillation power in WT slices by PD158780, demonstrates that the modulatory role of NRG-1β on kainate-induced gamma oscillations specifically requires signaling via ErbB4 receptors.
We went on to investigate possible differences in kainate-induced oscillations between WT and ErbB4MHC-ErbB4−/− mutant mice. We found that in ErbB4MHC-ErbB4 null mice gamma oscillation power was reduced by 60% (WT: 2.78 × 10−04 ± 8.20 × 10−05 V2, n = 20; ErbB4MHC-ErbB4−/−: 1.09 × 10−04 ± 2.40 × 10−05 V2, n = 14; P = 0.0386), whereas the peak frequency was not altered (WT: 28.97 ± 1.12 Hz, n = 20; ErbB4MHC-ErbB4−/−: 27.43 ± 1.38 Hz, n = 14; P = 0.331, unpaired t-test). We also observed that hippocampal slices from ErbB4MHC-ErbB4−/− mice were more prone to generate epileptiform activity in response to 100 nM kainate (A. Fisahn, unpublished observation). Taken together, these changes indicate a reduction or impairment of network inhibition in ErbB4MHC-ErbB4−/− mice.
Coexpression of ErbB4 and PV in Interneurons
Because the importance of PV-immunoreactive interneurons for gamma oscillations is well established (Bartos et al. 2007), we went on to investigate the hippocampal interneuron populations that express PV and ErbB4. As shown in Figure 3A,B, most PV-positive cells are located close to pyramidal cell somata in strata oriens (so), sp, and lucidum (sl), whereas ErbB4 cells also reside in strata radiatum (sr) and lacunosum moleculare (slm). We found that in WT mice almost half of PV cells coexpress ErbB4 (44%, 250 of 573 cells) throughout all layers in cornu ammonis (Fig. 3C: slm: 50 ± 50%, sl/sr: 35.2 ± 7%, sp: 46.5 ± 5.8%, so: 41.7 ± 3.6%), whereas only 27% (250 of 938) of ErbB4 cells coexpress PV, with a strong gradient across layers (Fig. 3D: slm: 1.1 ± 1.1%, sl/sr: 15.8 ± 2.8%, sp: 31.3 ± 12.6%, so: 32.5 ± 15.3%). We would like to emphasize that coexpression was determined by counting only neurons that were clearly, and independently, identifiable in both the red and green color channels, rather than solely on appearance of yellow pixels in the overlay picture; therefore, it is unlikely that we overestimated the number of neurons that coexpress ErbB4 and PV.
Reduced Number of PV-Immunoreactive Neurons in Mutant Mice
Next, we compared the number of PV-positive neurons in hippocampal sections from WT and ErbB4MHC-ErbB4−/− mice. This analysis revealed that the numerical density (cells/mm3) of PV-positive cell bodies is reduced by 31% in cornu ammonis of ErbB4MHC-ErbB4−/− compared with WT mice (Fig. 3E; WT: 1183 ± 98, n = 4; ErbB4MHC-ErbB4−/−: 812 ± 58, n = 4; P = 0.017). Our results on the numerical density of PV cells in the hippocampus of WT animals are in line with previously published data of both mice (Jinno and Kosaka 2002) and rats (Keilhoff et al. 2004). The 31% loss in ErbB4MHC-ErbB4−/−, however, does not change the distribution of PV cells across layers (P = 0.787; Fig. 3F).
Discussion
We show pharmacologically and with ErbB4MHC-ErbB4−/− mice that NRG-1 dramatically, and in an activity-dependent fashion, increases the power of hippocampal gamma oscillations via ErbB4 receptors, which are coexpressed by half of PV-containing interneurons. As a possible mechanism, we suggest that the NRG-1–induced increase in gamma oscillation power results from an activation of ErbB4 receptors on PV-containing interneurons, because perisomatic feedback inhibition crucially contributes to extracellular gamma oscillations (Mann et al. 2005). An increase of precisely timed GABA release via ErbB4 receptor activation (Woo et al. 2007; A. Buonanno, unpublished data) from perisomatic-targeting interneurons would lead to increased synchronization of pyramidal cell activity and, via recurrent collaterals and additional mechanisms, to recruitment of more interneurons (Mann and Paulsen 2007). Consequently, inhibition of ErbB4 receptors or deleting them outright would abolish the NRG-1–induced increase of both GABA release and gamma oscillations. However, although the activation of ErbB4-expressing PV neurons provides a plausible mechanism for the NRG-1 effect, we presently cannot exclude the possibility that other interneurons also contribute to the increase in oscillation power. Given the high density of ErbB4-expressing interneurons in the hippocampus it is likely that additional populations of interneurons coexpress the receptor. Further studies are needed to identify these neurons and investigate whether they contribute to the NRG-1 effects on gamma oscillations. Our results in ErbB4MHC-ErbB4−/− mice clearly demonstrate that ErbB4 is necessary for the NRG-1 effect; however, we currently cannot rule out if ErbB2 might contribute to the response through heterodimerization with ErbB4.
ErbB4 is crucially involved in the proliferation, differentiation and migration of interneuronal precursor cells (Yau et al. 2003; Anton et al. 2004). It was recently reported that GABA-immunoreactivity is reduced by 40% in the P20 hippocampus of ErbB4MHC-ErbB4−/− mice (Flames et al. 2004), which is close to the 31% reduction of PV-immunoreactivity that we observe in adult mutant mice. It is therefore tempting to speculate that the interneurons that coexpress PV and ErbB4 are most susceptible to disruption of the NRG-1-ErbB4 signaling pathway. This is interesting given the selective loss of PV-positive interneurons reported in post-mortem hippocampus of schizophrenia patients (Zhang and Reynolds 2002; Lewis et al. 2005). The reduction in PV-immunoreactivity in cornu ammonis of ErbB4MHC-ErbB4−/− mice correlates with a reduction in power of gamma oscillations by 60% in vitro, whereas the peak frequency is not significantly changed. A clear relation of a loss or impairment of PV-immunoreactivity to changes in cortical gamma oscillations has yet to be established (Vreugdenhil et al. 2003; Fuchs et al. 2007). However, ongoing experiments indicate that hippocampal slices of ErbB4MHC-ErbB4−/− mice are also more susceptible to epileptiform activity than WT slices (A. Fisahn, unpublished observation), which supports the idea of dysfunctional changes in hippocampal circuitry of ErbB4MHC-ErbB4−/− mice. Generally, the lack of a clear correlation between reduced PV-immunoreactivity and changes in gamma oscillation suggests that PV-expressing interneurons might be differently affected in different brain areas, different experimental paradigms, and schizophrenia.
There is increasing evidence for a role of acute NRG-1/ErbB signaling in the regulation of neural plasticity at glutamatergic synapses. At hippocampal CA3-to-CA1 synapses NRG-1 induces changes in AMPA receptor, but not NMDA receptor, surface expression and excitatory postsynaptic potentials (EPSPs; Kwon et al. 2005; Li et al. 2007). In prefrontal cortical pyramidal cells, however, NRG-1 was reported to reduce NMDA, but not AMPA, receptor currents (Gu et al. 2005). Acute reductions in neocortical glutamatergic transmission by application of NMDA antagonists can induce aberrant gamma oscillations in vivo with both simultaneously increased and decreased frequencies in different cortical areas (Pinault 2008). These regional differences suggest that NRG-1 effects on glutamatergic transmission, and on gamma oscillations, could also be region-specific, probably due to different cellular and subcellular localization of ErbB receptors in neocortical versus hippocampal areas.
Numerous studies suggest that alterations in neuronal connectivity, in particular a regional reduction of specific interneurons, may underlie the pathophysiology in schizophrenia and other psychoses (Green and Nuechterlein 1999; Ford et al. 2007) that are associated with impaired sensory information processing and reductions in the power of gamma oscillations (Kwon et al. 1999; Wynn et al. 2005; Wilson et al. 2007). This study is the first to directly link NRG-1/ErbB4 signaling and gamma band activity involved in higher brain processes. However, the single nucleotide polymorphisms (SNPs) in the NRG-1 and ERBB4 genes that are associated with schizophrenia will likely cause only subtle changes in the expression and the biological activity of these proteins, and it is yet unknown whether a reduction of PV neurons and gamma oscillations is a primary effect, or rather compensatory for other genetic and nongenetic risk-factors of schizophrenia.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Research Council of Sweden to A.F.; National Institute of Child Health and Human Development, IRP, National Institutes of Health to A.B., J.N., L.Y.
Supplementary Material
Acknowledgments
Confocal microscopy imaging was performed at the Microscopy & Imaging Core (National Institute of Child Health and Human Development, NIH) with the assistance of Dr Vincent Schram and Chip Dye. We are grateful to Daniel Abebe and Irina Karavanova for their assistance with breeding of mutant mice. Conflict of Interest: None declared.
References
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, et al. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignola PS, Lackey K, Kadwell SH, Hoffman C, Horne E, Carter HL, Stuart JD, Blackburn K, Moyer MB, Alligood KJ, et al. Comparison of the biochemical and kinetic properties of the type 1 receptor tyrosine kinase intracellular domains. Demonstration of differential sensitivity to kinase inhibitors. J Biol Chem. 2002;277:1576–1585. doi: 10.1074/jbc.M105907200. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Mortensen OH, van Kempen LC, Jaattela M. BIBX1382BS, but not AG1478 or PD153035, inhibits the ErbB kinases at different concentrations in intact cells. Biochem Biophys Res Commun. 2001;281:25–31. doi: 10.1006/bbrc.2001.4302. [DOI] [PubMed] [Google Scholar]
- Eilam R, Pinkas-Kramarski R, Ratzkin BJ, Segal M, Yarden Y. Activity-dependent regulation of Neu differentiation factor/neuregulin expression in rat brain. Proc Natl Acad Sci USA. 1998;95:1888–1893. doi: 10.1073/pnas.95.4.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ. Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci. 2004;24:9658–9668. doi: 10.1523/JNEUROSCI.2973-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci USA. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Cortical oscillations and schizophrenia: timing is of the essence. Arch Gen Psychiatry. 1999;56:1007–1008. doi: 10.1001/archpsyc.56.11.1007. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, Thomson PA, Porteous DJ, Cunningham-Owens DG, Johnstone EC, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Patterns of expression of calcium binding proteins and neuronal nitric oxide synthase in different populations of hippocampal GABAergic neurons in mice. J Comp Neurol. 2002;449:1–25. doi: 10.1002/cne.10251. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor ErbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- Loeb JA, Hmadcha A, Fischbach GD, Land SJ, Zakarian VL. Neuregulin expression at neuromuscular synapses is modulated by synaptic activity and neurotrophic factors. J Neurosci. 2002;22:2206–2214. doi: 10.1523/JNEUROSCI.22-06-02206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longart M, Chatani-Hinze M, Gonzalez CM, Vullhorst D, Buonanno A. Regulation of ErbB-4 endocytosis by neuregulin in GABAergic hippocampal interneurons. Brain Res Bull. 2007;73:210–219. doi: 10.1016/j.brainresbull.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mann EO, Radcliffe CA, Paulsen O. Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back. J Physiol. 2005;562:55–63. doi: 10.1113/jphysiol.2004.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T. Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J Neurochem. 2004;91:176–188. doi: 10.1111/j.1471-4159.2004.02719.x. [DOI] [PubMed] [Google Scholar]
- Palhalmi J, Paulsen O, Freund TF, Hajos N. Distinct properties of carbachol- and DHPG-induced network oscillations in hippocampal slices. Neuropharmacology. 2004;47:381–389. doi: 10.1016/j.neuropharm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Beasley CL, Zhang ZJ. Understanding the neurotransmitter pathology of schizophrenia: selective deficits of subtypes of cortical GABAergic neurons. J Neural Transm. 2002;109:881–889. doi: 10.1007/s007020200072. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll SW, Kansra S, Peshick S, Fry DW, Leopold WR, Wiesen JF, Sibilia M, Zhang T, Werb Z, Derynck R, et al. Differential utilization and localization of ErbB receptor tyrosine kinases in skin compared to normal and malignant keratinocytes. Neoplasia. 2001;3:339–350. doi: 10.1038/sj.neo.7900170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2008;18(2):371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.