Abstract
Expression of IL-7 receptor α (CD127) is associated with naive and memory (i.e. non-effector) CD8+ T cell phenotypes. Effector CD8+ T cells are predominantly CD127− and most die by apoptosis. Therefore, CD127 appears to be a marker for CD8+ T cell differentiation, yet its role in CD8+ T cell survival and memory development is unclear. To address this, we investigated the cell death and cell division of isolated CD8+CD127+ and CD8+CD127− T cells in response to common IL-2 receptor γ chain (γC) cytokines other than IL-7. We show here that (i) memory cells (CD127+CD45RA−) divide frequently in response to either IL-2, -4 or -15; (ii) IL-2 and -15 enhance cell division in effector–memory-like cells (CD127−CD45RA+) while IL-4 enhances the cell division of effector cells (CD127−CD45RA−); (iii) CD8+CD127+ T cells are more sensitive to the anti-apoptotic effects of IL-2 or IL-15 than CD8+CD127− T cells and (iv) CD8+CD127+ T cell produce more Bcl-2 in response to IL-2 or IL-15 compared with CD8+CD127− T cells. Therefore, CD8+CD127+ and CD8+CD127− T cells differ in their responsiveness to cell division and anti-apoptotic signals from IL-2, -4 and -15. This suggests a role for γC cytokines in the pathogenesis of diseases in which CD127 expression is altered on CD8+ T cells such as in progressive viral infections and cancer.
Keywords: CD8+ T cells, gamma chain cytokines, IL-7 receptor
Introduction
Homeostatic maintenance of CD8+ T cells requires the delivery and balance of cell proliferation, survival and apoptosis-inducing signals. These signals are mainly derived from cytokines whose receptor complexes share a common IL-2 receptor γ chain (γC) (IL-2, 4, 7, 9, 15 and 21) and have similar downstream signaling pathways (1). It is now well established that IL-7 is critical for T cell development and that along with IL-2, -4, -7 and -15 maintains naive and memory T cells (2–6). There is some redundancy among the roles of IL-2 and IL-15 in stimulating T cell proliferation in vitro, yet in vivo, these cytokines are associated with the induction of cell death and survival of T cell clones, respectively (7). Specifically, high concentrations of IL-2 are associated with increased activation-induced cell death of CD8+ T cells, while IL-4 inhibits the latter (8). IL-15 has been well described to have an important role in the survival of naive and memory CD8+ T cells (9–11). It is thought that the extent of cell death incurred following the contraction phase of a CD8+ T cell response is related in part to events determining the extent of cell division and this may influence memory T cell development (12).
A typical CD8+ T cell response includes the activation of naive cells (Tnaive), clonal expansion of effector cells followed by a contraction of effector cells and establishment of memory cells which include central memory (TCM), effector–memory (TEM) and CD45RA+ effector–memory (TEMRA) T cells (13–16). The expression of IL-7 receptor α (CD127) is found primarily on naive and memory T cells (17, 18) while CD127 expression is down-regulated in most effector cells (3). The proportion of CD8+ T cell subsets expressing CD127 in healthy individuals is as follows: naive > TEM and TCM > effector. Functionally, it has been shown that CD8+CD127− T cells produced IFN-γ and tumor necrosis factor-α but not IL-2 and were more spontaneously apoptotic and more prone to activation-induced apoptosis and proliferated somewhat less than CD127+ T cells (19, 20). Therefore, CD127 expression may be a determinant of CD8+ T cell survival (17) and certain cytokines may have a role in determining CD8+ T cell fate and function.
The expression of CD127 on CD8+ T cells is of particular relevance to anti-viral responses as a down-regulation of CD127 expression has been observed in infections with latent viruses such as epstein-barr virus (EBV) and cytomegalovirus (CMV) as well as in chronic viral infections such as HIV and HCV (21–24). In chronic viral infections, this has been correlated with CD8+ T cell exhaustion in the context of persistent exposure to antigen. We and others have demonstrated that significantly fewer CD8+ T cells express CD127 in HIV-infected patients with uncontrolled plasma viremia compared with healthy individuals (22, 23, 25, 26). In a recent report, HIV-infected patients had an increased expansion of effector-like CD8+ T cells lacking CD127 which correlated with markers of disease progression such as plasma viremia and CD4+ T cell depletion, supporting reports in inbred laboratory rodents that CD127 is an important marker of functionally distinct CD8+ T cell subsets (19). The impact of altered CD127 expression and immune system dysfunction in chronic viral infections is not known nor is how this explains the maintenance of CD8+ T cell homeostasis in healthy individuals.
Differences in the cytokine responsiveness of naive, effector and memory CD8+ T cell subsets have been described elsewhere, although not in the context of CD127 expression (3, 10, 13, 27, 28). Evidence suggests that the expression of CD127 is associated with different cell phenotypes and differentiation pathways of CD8+ T cell responses during which significant cell proliferation and cell death occur. Furthermore, the proportion of cells expressing CD127 has been correlated with disease status (22, 23, 25, 26). We hypothesized that the expression of CD127 is associated with CD8+ T cell survival. Therefore, we investigated the survival features of isolated CD8+CD127+ and CD8+CD127− T cells and the influence of γC cytokines.
Materials and methods
Isolation of CD8+CD127+ and CD8+CD127− T cells from human peripheral blood
All research conducted using blood from human subjects were approved by the Ottawa Hospital Research Ethics Board. Blood was collected from healthy volunteers into heparin-containing tubes and PBMC were isolated by Ficoll-Hypaque density gradient centrifugation. Briefly, CD8+ T cells were isolated using the human CD8+ T cell isolation kit II following the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA, USA) along with the autoMACS cell sorter’s ‘deplete’ protocol to remove non-CD8+ T cells (Miltenyi Biotec). Cells were then cultured (1 × 106 cells ml−1) overnight in complete RPMI medium [supplemented with 20% FCS (Cansera, Etobicoke, Ontario, Canada), 100 IU ml−1 each of penicillin and streptomycin (Sigma–Aldrich, Oakville, Ontario, Canada)]. The CD127+ and CD127− cells were separated by labeling CD8+ T cells with PE-conjugated mouse anti-human CD127 mAbs (65 μl per 1 × 107 cells, Beckman Coulter, Mississauga, Ontario, Canada) in PBS + 0.5% bovine serum (Sigma–Aldrich) + 0.05 mM EDTA (binding buffer, 80 μl per 1 × 107 cells) and incubated for 15 min at 4°C. Cells were washed and labeled with mouse anti-PE mAbs conjugated to paramagnetic beads (40 μl per 1 × 107 cells, Miltenyi Biotec) in binding buffer (80 μl per 1 × 107 cells) for 15 min at 4°C. Cells were washed, re-suspended in binding buffer and separated using the autoMACS cell sorter’s ‘possels’ protocol to separate the antibody-labeled CD127+ T cells from the CD127− T cells. The CD8+ T cell isolation typically yielded a >95% pure population (data not shown), with minimal numbers of CD4+ T cells and no contaminating monocytes/macrophages, dendritic cells, B cells or NK cells. The separation of CD127 subsets typically enriched a CD127+ population (>95% CD127+) and a CD127− population (<12% CD127+), comparable to similar methods used to enrich other CD8+ T cell subsets (29). Expression of γC cytokine receptors was assessed using FITC-conjugated anti-IL-2Rα (Beckman Coulter) and anti-IL-2Rβ (R&D Systems), PE-conjugated IL-2Rγ (BD Pharmingen, San Diego, CA, USA), biotinylated IL-4Rα and anti-IL-15Rα (R&D Systems) and streptavidin R-PE conjugate (Sigma-Aldrich).
CFSE labeling and culture conditions for cell cycling experiments
Following isolation, cells were labeled with CFSE (CellTrace™ CFSE Cell Proliferation Kit) using a method modified from the manufacturer’s specifications (Invitrogen Canada Inc., Burlington, Ontario, Canada). A stock solution (5 mM) of CFSE was prepared in dimethyl sulfoxide followed by the preparation of a working solution (8 μM) of Carboxyfluorescein succinimdyl ester (CFSE) in PBS + 0.1% BSA. Cells were re-suspended in CFSE working solution (1 × 107 cells ml−1) and incubated at 37°C, in the dark for 10 min. Cell were incubated with 15 volumes of cold complete RPMI on ice, in the dark for 5 min and then washed and re-suspended in complete RPMI (1 × 106 cells ml−1). Cells were cultured with medium only, PHA (2.5 μg ml−1, Sigma–Aldrich), IL-2 (1, 10, 100 units ml−1), IL-4 or IL-15 (1, 10, 100 ng ml−1) (R&D Biosystems, Minneapolis, MN, USA). The concentration of PHA used was the lowest tested concentration (i.e. submaximal dose) that induced at least two rounds of cell division among activated cells. Cells were cultured for 4 days and then cell division was assessed by flow cytometry.
Quantitative flow cytometry analysis of cell cycling
Following 4 days of cell culture, CFSE-labeled cells were labeled with mouse anti-human CD45RA-ECD and mouse anti-human CD8-PC5 and analyzed by flow cytometry (Beckman Coulter ALTRA flow cytometer and the EXPO version 2.0 software package). Color compensation was conducted in accordance with the fluorescence-minus-one strategy using single- and double-stained samples. A total of 15 000 events were collected for each sample ensuring the inclusion of a sufficient number (1000–3000) of stimulated T cells for analysis. The gating strategy involved the examination of cell division among stimulated T cells identified on the basis of increased forward and side scatter profiles, a known feature of dividing lymphocytes (30). These dividing cells were further subdivided into CD8+CD45RA+ or CD8+CD45RA− T cell subsets to identify the following cell phenotypes: CD8+CD127+CD45RA+ (naive), CD8+CD127+CD45RA− (central memory, TCM), CD8+CD127−CD45RA+ (effector/effector–memory, TE/TEM) and CD8+CD127−CD45RA− (RA+ effector–memory like, TEMRA). Individual cell divisions of the stimulated T cells were noted according to previously described methods involving CFSE analysis of T cell division (31, 32). The proportion of stimulated T cells that have undergone ≥3 divisions as determined by the last detectable division (% CFSElo).
Flow cytometry analysis of surface Fas and intracellular Bcl-2 expression
Isolated CD8+CD127+ and CD8+CD127− T cells were incubated with medium, IL-2 (100 units ml−1, R&D Systems) or IL-15 (100 ng ml−1) for 48 h and then incubated with mouse anti-human CD95 mAb (CH11) (3.3 μg ml−1, Beckman Coulter) to induce Fas-mediated apoptosis or mouse anti-human CD3 mAb (HIT3a) (1 μg ml−1, BD Biosciences, Mississauga, Ontario, Canada) to induce CD3-mediated apoptosis as described previously (33). Intracellular Bcl-2 staining was performed using the BD Biosciences FITC-conjugated Bcl-2 antibody reagent set, following the manufacturer’s protocol. Cells were washed with PBS and re-suspended in fixation buffer (100 μl per 105 cells, eBiosciences, San Diego, CA, USA) and incubated at room temperature for 20 min. Following incubation, cells were washed, re-suspended in permeabilization buffer (100 μl per 105 cells) with FITC-conjugated Bcl-2 antibody (2 μl per 105 cells, BD Biosciences) and incubated at room temperature for 20 min. Samples were washed and then re-suspended in flow staining buffer (0.5 ml per 105 cells, eBiosciences). Analysis was performed by flow cytometry and data are represented as values of mean fluorescence intensity.
The expression of surface Fas on freshly isolated CD8+CD127+ and CD8+CD127− T cells was assessed flow cytometry using mouse-anti-human Fas-FITC mAb (BD Biosciences).
Analysis of apoptosis
Three flow cytometry-based methods were used to assess apoptosis: Annexin V–phosphatidylinositol (PI) staining and analysis of caspase-3 and caspase-8 activity. Isolated CD8+CD127+ and CD8+CD127− T cells were pre-incubated for 48 h with medium, IL-2 (100 units ml−1) or IL-15 (100 ng ml−1) then incubated with anti-human CD95 or anti-human CD3 as described above. Isotype controls were used to confirm Fas- and CD3-specific apoptosis. The Annexin V–PI staining was performed using the Annexin V–FITC Apoptosis Detection Kit I, according to the manufacturer’s instructions (BD Pharmingen). Apoptotic cells (Annexin–V+PI−) were distinguished from dead cells (Annexin–V+PI+) and data are represented as the % of Annexin–V+PI− cells/(% Annexin–V+PI− cells + % Annexin–V−PI− cells), gating on all cells. Caspases-3 and -8 activity was determined using Carboxyfluorescein Caspase Detections Kits following the manufacturer’s instructions (Biocarta, San Diego, CA, USA). These kits detect active caspases in living cells using a carboxyfluorescein (FAM)-labeled peptide fluoromethyl ketone (FMK) caspase inhibitor that irreversibly binds to active caspases (FAM-DEVD-FMK for caspase-3 and FAM-LETD-FMK for caspase-8). Given the observed individual variation in the mean fluorescence intensity of when measuring intracellular caspase staining, data were collected and the relative caspase activity was calculated by dividing % of caspase positive cells in response to apoptotic stimuli with or without cytokine pre-treatment by its appropriate control. In all assays of apoptosis, a sample of cells was incubated with camptothecin (10 μM) (Sigma–Aldrich) as a positive control.
Cytokine signaling
The phosphorylation of STAT5 (pSTAT5) in CD8+ T cells was analyzed by flow cytometry (Alexa Fluor 488 mouse anti-human STAT5 pY694, BD Biosciences, San Jose, CA, USA) following 15 min of culture. Phosphorylated Akt and FOXO3a in cell lysates were assessed by western blot after 2 h of culture. As a loading control, the expression of beta-actin was detected using a mouse anti-human mAb (Cell Signaling Technology, Danvers, MA, USA). Cell pellets were collected by centrifugation and lysed by incubating in lysis buffer (40 μl per 107) for 1 h. Lysates were then centrifuged for 20 min at 14 000 r.p.m., 4°C and supernatants were collected and stored at −20°C. Lysate concentrations were quantified using a BCA Protein Assay Kit (Pierce Biotechnologies, Rockford, IL, USA). A total of 5 μg of protein was resolved by SDS–PAGE and blotted onto polyvinylidene fluoride membrane. Membranes were blocked overnight in 5% skim milk in Tris-buffered saline, probed with a rabbit anti-human pAktser or anti-humanantibody followed by a anti-rabbit-IgG HRP antibody (Cell Signaling Technology, Boston, MA, USA). Proteins were detected by ECL (Pierce Biotechnologies). Densitometry analysis of protein bands was performed using AlphaEase Fc Software 6.0 (Alpha Innotech Corp., San Leandro, CA, USA) and relative changes in protein expression of treated cells were calculated compared with unstimulated controls.
Statistical analysis
Statistical analysis was performed using the Student’s t-test for paired samples (GraphPad Prism 4.0 Software, San Diego, CA, USA). Following flow cytometry analysis, electronic sample files were further analyzed using the FCS Express 2.0 software (De Novo Software, Thornhill, Ontario, Canada). Flow cytometry figures were generated by FCS Express 2.0 software. Data calculations were performed using Microsoft Excel and were subsequently plotted using GraphPad Prism 4.0 software.
Results
Proliferation of CD8+CD127+ and CD8+CD127− T cells differ in response to γC cytokines
It has been well described that IL-2, -4 and -15 promote the survival and proliferation of CD8+ T cells (7, 10, 11, 13, 34). Therefore, we studied the effects of IL-2, IL-4 and IL-15 on cell division of mitogen-stimulated, CFSE-labeled, CD8+CD127+ and CD8+CD127− T cells using a flow cytometry strategy described in Methods and Fig. 1. No appreciable difference in the expression of γC cytokine receptors was observed in either of these populations (Table 1). Incubation of CD8+ T cells with cytokine alone did not result in an appreciable number of cells undergoing multiple cell divisions, i.e. % CFSElo (data not shown). As mentioned in the Methods, a submaximal concentration of PHA was used to stimulate T cells in vitro in order to study cytokine effects.
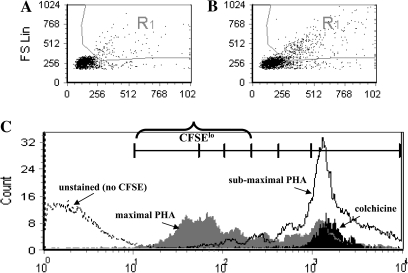
Fig. 1.
Cell gating strategy for the analysis of cell division by flow cytometry. Isolated CD8+CD127+ and CD8+CD127− T cells were stained with CFSE and then cultured for 4 days before analysis. Activated and/or dividing lymphocytes within the isolated subsets were identified on the basis of increased forward and side scatter profiles (R1) relative to the proportionally smaller non-dividing cells. Approximately 10 000 total cells were analyzed for each sample. Representative scatter plots of (A) untreated CD8+ T cells and (B) CD8+ T cells treated with a submaximal concentration of PHA (2.5 μg ml−1). Cells within the R1 gate (1000–5000 cells) were then gated into CD8+CD45RA+ and CD8+CD45RA− subsets for cell division analysis. (C) A representative histogram of gated CD8+ T cells depicts cell frequencies versus CFSE staining. Markers delineating cell divisions are included. A bracket delineates cells that have undergone ≥3 divisions, i.e. ‘CFSElo’ cells. This histrogram displays the CFSE profile of colchicine-treated (mitotically arrested) cells (black), cells stimulated with submaximal (white) and maximal (gray, 10 μg ml−1) concentrations of PHA and non-CFSE-labeled cells (dotted line).
Table 1.
Summary of γC cytokine receptor expression in isolated CD8+CD127+ and CD8+CD127− T cellsa
| CD8+CD127+ |
CD8+CD127− |
|||
| % expressionb | MFIc | % expression | MFI | |
| IL-2Rα | 18.1 ± 1.62 | 15.63 ± 2.05 | 15.16 ± 3.41 | 15.03 ± 4.55 |
| IL-2Rβ | 26.97 ± 3.51 | 17.22 ± 2.72 | 24.76 ± 5.94 | 18.27 ± 3.37 |
| IL-2Rγ | 95.58 ± 6.70 | 27.18 ± 5.90 | 84.74 ± 5.53 | 24.79 ± 2.33 |
| IL-4Rα | 64.07 ± 10.33 | 57.10 ± 9.77 | 58.71 ± 8.17 | 49.00 ± 3.01 |
| IL-15Rα | 32.69 ± 12.28 | 25.00 ± 6.97 | 35.41 ± 13.79 | 26.18 ± 8.27 |
These data summarize the expression of γC cytokine receptor in four individuals as measured by flow cytometry.
Average % cytokine receptor expression ± standard deviations of the mean.
Average MFI: mean fluorescence intensity ± standard deviations of the mean.
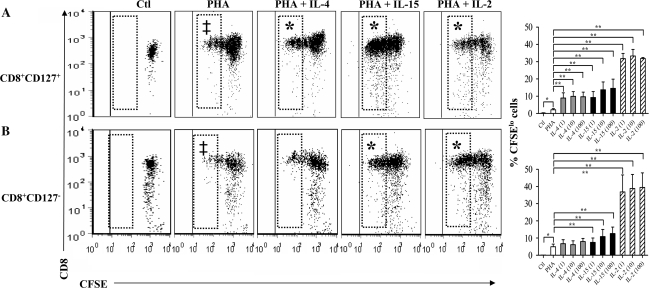
Submaximal concentrations of PHA induced only a modest degree of proliferation in CD8+CD127+ and CD8+CD127− T cells (Fig. 2A and B). The addition of IL-2, -4 or -15 at all concentrations tested significantly increased the proportion of CFSElo CD8+CD127+ T cells compared with cells cultured with PHA alone (Fig. 2A, P ≤ 0.05). Only IL-15 and IL-2 significantly increased the proportion of CFSElo CD8+CD127− T cells compared with PHA alone (Fig. 2B). Therefore, IL-2, -4 and -15 increased the proportion of CFSElo CD8+CD127+ T cells while the CD8+CD127− T cells only responded significantly to IL-2 and IL-15. This suggests that there are differences in the proliferative capacities of these two cell populations, and this may be further pronounced in the study of Tnaive, TCM, TEM and TEMRA subsets.
Fig. 2.
Cell division of CD8+CD127+ and CD8+CD127− T cells in response to γC cytokines. Isolated CD8+CD127+ and CD8+CD127− T cells were cultured with media only (Ctl) and cells incubated with PHA (2.5 μg ml−1), PHA + IL-4 (1, 10 or 100 ng ml−1) or PHA + IL-15 (10 ng ml−1) or PHA + IL-2 (1, 10 or 100 units ml−1). Representative dot plots of cell division profiles of CD8+ T cell subsets are shown: (A) CD8+CD127+ and (B) CD8+CD127−. The CFSElo cells (shown in hatched boxes), have undergone ≥3 divisions. The cytokine concentrations shown here include IL-4 (100 ng ml−1), IL-15 (10 ng ml−1) and IL-2 (100 units ml−1). In addition, the proportion of CFSElo cells from experiments in six individuals is summarized in bar graphs. Statistically significant changes in the proportion of CFSElo cells are indicated with a ‘‡’ compared with unstimulated controls (Ctl) or ‘*’ when comparing PHA + IL-4 (paired Student's t-test, P < 0.05), IL-15 or IL-2 to PHA alone.
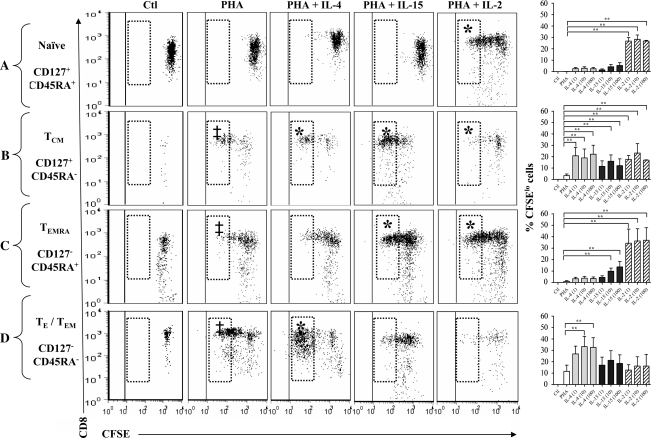
Cell division of CD8+CD127+CD45RA+T cells (naive).
Incubation with a submaximal concentration of PHA increased the proportion of naive (CD45RA+) T cells in the CD127+ T cell cultures that underwent up to two cell divisions compared with unstimulated controls (P = 0.007, Fig. 3A). The addition of IL-4 or IL-15 with PHA did not alter this significantly (Fig. 3A). All tested concentrations of IL-2 significantly increased cell division and the proportion of CFSElo cells (P = 0.001). These results indicate that IL-2, but not IL-4 and IL-15, enhances the proliferation of primed CD8+CD127+CD45RA+ T cells.
Fig. 3.
Cell division of subsets of CD8+CD127+ and CD8+CD127− T cells. Isolated CD8+CD127+ and CD8+CD127− T cells were cultured with media only (Ctl) and cells incubated with PHA (2.5 μg ml−1), PHA + IL-4 (1, 10 or 100 ng ml−1) or PHA + IL-15 (10 ng ml−1) or PHA + IL-2 (1, 10 or 100 units ml−1). Representative dot plots (y-axis, CD8+: x-axis, CFSE) of cell division profiles of CD8+ T cell subsets are shown: (A) CD127+CD45RA+, (B) CD127+CD45RA−, (C) CD127−CD45RA+ and (D) CD127−CD45RA−. The CFSElo cells (shown in hatched boxes) have undergone the maximum number of detectable divisions. The cytokine concentrations shown here include IL-4 (100 ng ml−1), IL-15 (10 ng ml−1) and IL-2 (100 units ml−1). In addition, the proportion of CFSElo cells from experiments in six individuals is summarized in bar graphs. Statistically significant changes in the proportion of CFSElo cells are indicated with a ‘‡’ compared with unstimulated controls (Ctl) or ‘*’ when comparing PHA + IL-4 (paired Student's t-test, P < 0.05), IL-15 or IL-2 to PHA alone.
Cell division of CD8+CD127+CD45RA− T cells (TCM).
The proportion of CFSElo CD8+CD127+CD45RA− T cells significantly increased in response to PHA compared with unstimulated controls (Fig. 3B). The addition of IL-2 (1–100 units ml−1), IL-4 (1–100 ng ml−1) or IL-15 (10–100 ng ml−1) significantly increased the proportion of CFSElo CD127+CD45RA− cells (P ≤ 0.05). Therefore, IL-2, IL-4 and IL-15 increased the proportion of CD8+CD127+CD45RA− T cells undergoing multiple divisions. It is also notable that stimulation with IL-2 or IL-4 tended to result in a greater proportion of CFSElo CD8+CD127+CD45RA− T cells compared with IL-15, although this did not reach statistical significance.
Cell division of CD8+CD127−CD45RA+ T cells (TEMRA).
Culturing CD8+CD127−CD45RA+ T cells with PHA-alone-induced cells to undergo more than one cell division compared with unstimulated controls. In addition, IL-2, IL-4 or IL-15 increased the proportion of dividing CD127−CD45RA+ T cells compared with PHA alone (Fig. 3C). All concentrations of IL-15 (1, 10 and 100 ng ml−1) tested significantly increased the proportion of CFSElo cells (P = 0.031, 0.036 and 0.052, respectively, compared with PHA alone). Similarly, IL-2 (1, 10 and 100 units ml−1) increased the proportion of CFSElo cells (P ≤ 0.001 compared with PHA alone). IL-4 did not increase the proportions of CFSElo cells in this subset. Thus, IL-2 and -15, but not IL-4, significantly increased the proportions of PHA-stimulated CD127−CD45RA+ T cells undergoing multiple cell divisions.
Cell division of CD8+CD127−CD45RA− T cells (TE/TEM).
Incubation of CD8+CD127−CD45RA− T cells with PHA significantly increased the proportion of CFSElo cells (Fig. 3D). Low concentrations of IL-4 (1 and 10 ng ml−1) tended to increase the proportion of CFSElo cells, although this did not reach statistical significance (P = 0.061 and 0.058, respectively); however, 100 ng ml−1 of IL-4 increased the proportion of CFSElo cells (P = 0.035) compared with PHA alone. This appears to be in contrast to the lack of IL-4-induced proliferation in bulk CD8+CD127− T cells (Fig. 2). This is likely due to the fact that CD8+CD127−CD45RA− T cells make up <15% of all CD8+CD127− T cells (data not shown), and hence this proliferative effect may not be detected when analyzing bulk CD127− T cells. In contrast, IL-2 or IL-15 did not significantly increase the proportion of CFSElo cells. Therefore, IL-4, but not IL-2 and IL-15, increased the proportion of CD127−CD45RA− T cells undergoing multiple cell divisions. Analyses of these data for all dividing cells (i.e. not just those that have divided three or more times) yielded similar results (data not shown).
Susceptibility to apoptosis
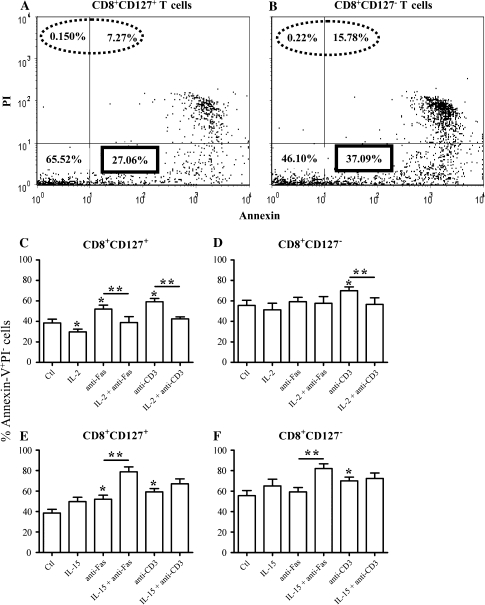
Human CD8+CD127+ T cells are known to undergo less spontaneous apoptosis than CD8+CD127− T cells (19); however, the role of γC cytokines in regulating cell death (an important function of these cytokines) in these subsets has not been described. It should be noted that there were consistently more (>2-fold) Annexin–V+PI+ cells (apoptotic or necrotic cells) in the CD8+CD127− T cells compared with the CD8+CD127+ T cells at the initiation of culture and this was consistent following 4 days of culture (Fig. 4A and B). In addition, a greater proportion of CD8+CD127− T cells were spontaneously apoptotic (Annexin+PI−) compared with CD8+CD127+ T cells (P = 0.010, Fig. 4C and D) as expected. The cytokines tested included IL-2 and IL-15 due to their common and distinct roles in T cell survival (10). The results are described below in detail and are summarized in Figs 4 and 5 and Table 2.
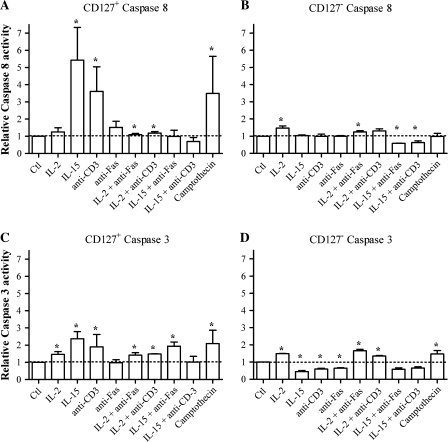
Fig. 4.
Apoptosis in isolated CD8+CD127− and CD8+CD127+ T cells pre-incubated with IL-2 or IL-15. (A and B) Isolated CD8+CD127+ and CD8+CD127− T cells were cultured with media only for 4 days, stained for Annexin–V/PI and analyzed by flow cytometry. The dot plots represent the percentage staining of Annexin–V (x-axis) versus PI (y-axis). The proportion of cells that are either apoptotic or necrotic (Annexin–V+PI+ or Annexin–V−PI+, circled with a dotted line) and are hence indistinguishable from truly apoptotic cells (Annexin–V+PI−). These results were confirmed on a total of six separate experiments. (C–F) Isolated CD8+CD127+ and CD8+CD127− T cells were cultured with medium only (Ctl), IL-2 [100 μ ml−1 (C) and (D)] or IL-15 [100 ng ml−1 (E) and (F)] for 48 h followed by a 48-h incubation with anti-Fas (3.3 μg ml−1) or anti-CD3 (1 μg ml−1) to induce apoptosis. Apoptotic cells (Annexin–V+PI−) were detected by flow cytometry and their proportions were calculated using the following formula: % of Annexin–V+PI− cells/(% Annexin–V+PI− cells + % Annexin–V−PI− cells). Statistically significant effects are indicated with a ‘*’ compared with Ctl or ‘**’ when comparing anti-Fas or anti-CD3 alone compared with anti-Fas or anti-CD3 + IL-2 or IL-15 (Student’s t-test P < 0.05, n = 6).
Fig. 5.
Caspase activity of CD8+CD127+ and CD8+CD127− T cells pre-incubated with IL-2 or IL-15. Cells were treated with medium only (Ctl), IL-2 (100 μ ml−1) or IL-15 (100 ng ml−1) for 48 h followed by a 48-h incubation with anti-Fas (3.3 μg ml−1) or anti-CD3 (1 μg ml−1) to induce apoptosis and caspase-3 and -8 activity were evaluated by flow cytometry. Caspase-8 activity was measured in A) CD8+CD127+ and B) CD8+CD127− T-cells and caspase-3 activity was measured in C) CD8+CD127+ and D) CD8+CD127− T-cells. Data are represented as relative caspase activity. Statistically significant effects are indicated by ‘*’ when compared with Ctl or ‘**’ when comparing anti-Fas or anti-CD3 + IL-2 or IL-15 and anti-Fas or anti-CD3 alone (Student’s t-test P < 0.05, ±SD, n = 4).
Table 2.
Summary of statistically significanta apoptosis-related features of CD8+CD127+ and CD8+CD127− T cellsb
| CD8+CD127+ |
CD8+CD127− |
|||||
| Annexin+ | Caspase-8 | Caspase-3 | Annexin+ | Caspase-8 | Caspase-3 | |
| IL-2 | ↓ | NC | ↑ | NC | ↑ | ↑ |
| IL-15 | NC | ↑ | ↑ | NC | NC | ↓ |
| Anti-CD3 | ↑ | ↑ | ↑ | ↑ | NC | ↓ |
| Anti-Fas | ↑ | NC | NC | NC | NC | ↓ |
| IL-2 + anti-CD3 | ↓ | ↓ | ↓ | ↓ | NC | ↑ |
| IL-2 + anti-Fas | ↓ | ↓ | ↑ | NC | ↑ | ↑ |
| IL-15 + anti-CD3 | NC | ↓ | ↓ | NC | ↓ | NC |
| IL-15 + anti-Fas | ↑ | ↓ | ↑ | ↑ | ↓ | NC |
NC, no change;↑ = significantly increases apoptosis (P < 0.05); ↓ = significantly decreases apoptosis (P < 0.05).
Statistical significance was determined by the Student's t-test (P < 0.05).
Responses to cytokine, anti-CD3 or anti-Fas alone were compared with medium controls while responses involving cytokine pre-treatment followed by anti-Fas or anti-CD3 were compared with anti-Fas or anti-CD3 alone.
Apoptosis of CD8+CD127+ T cells.
When CD8+CD127+ T cells were incubated with anti-CD3, the proportion of Annexin–V+PI− cells and caspase-3 and caspase-8 activity increased significantly compared with unstimulated cells (P = 0.051, Figs 4C, 5A and C). Incubation with anti-Fas increased Annexin–V+PI− staining (P = 0.036) but had no effect on caspase activity. Pre-incubation of CD8+CD127+ T cells with IL-2 significantly reduced the proportion of Annexin–V+PI− cells, caspase-3 and caspase-8 activity induced by anti-CD3 (P = 0.001, 0.006 and 0.050, respectively Figs 4C, 5A and C). Similarly, IL-2 decreased Annexin–V+PI− cells and caspase-8 in anti-Fas-stimulated cells (P = 0.045 and 0.027, respectively, Figs 4C and 5A) but increased caspase-3 activity (Fig. 5C). Pre-treatment with IL-15 did not alter Annexin–V+PI− staining in response to anti-CD3 but it did decrease caspase-3 and caspase-8 activity (P = 0.010 and 0.048, Figs 4E and 5C). The effects of IL-15 on anti-Fas-induced apoptosis were inconsistent: this cytokine increased Annexin–V+PI− staining and caspase-3 activity while decreasing caspase-8 activity. Therefore, in CD8+CD127+ T cells, IL-2 was more protective against anti-Fas- and anti-CD3-induced apoptosis while IL-15 seemed only to have a protective effect by decreasing caspase activity in anti-CD3-stimulated cells.
Apoptosis of CD8+CD127− T cells.
Incubation of CD8+CD127− T cells with anti-CD3 resulted in an increase in Annexin–V+PI− staining (P = 0.021, Fig. 4D and F) but incubation with anti-Fas did not. Similar to CD8+CD127+ T cells, pre-treatment of CD8+CD127− T cells with IL-2 decreased Annexin–V+PI− staining induced by anti-CD3 but increased the effect of anti-CD3 or anti-Fas on caspase-3 activity (P = 0.050, Fig. 5D). Pre-treatment with IL-15 decreased caspase-8 activity of both anti-CD3- and anti-Fas-stimulated CD8+CD127− T cells, but increased anti-Fas-induced Annexin–V+PI− staining and had no effect on caspase-3 activity (Figs 4F and 5D). IL-15 decreased caspase-8 activity in anti-CD3-stimulated cells but had no effect on Annexin–V+/PI− staining or caspase-3 activity. Therefore, pre-treatment of CD8+CD127− T cells with either IL-2 or IL-15 did not consistently decrease anti-Fas- or anti-CD3-induced apoptosis, and in some cases, cytokine pre-treatment resulted in an increase in apoptosis-related signals.
Analysis of the data for ‘dead or dying’ cells (i.e. Annexin–V+ or PI+ cells per all cells) yielded similar results. Therefore, exclusion of dead (PI+) cells from the analysis did not significantly bias the results.
Expression of Bcl-2 and phospo-STAT5
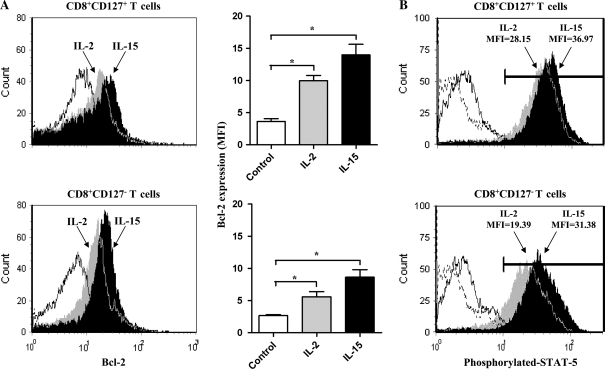
Cell death signals are counterbalanced by survival signals provided to a cell. Since the anti-apoptotic molecule Bcl-2 has an important role in reducing a cell’s susceptibility to apoptosis (35), the expression of Bcl-2 in isolated CD8+CD127+ and CD8+CD127− T cells was investigated. In these experiments, IL-2 was included due to its dual role in cell proliferation and activation-induced cell death and IL-15 was used as it has primarily been associated with cell survival. There was no statistically significant difference in the amount of the anti-apoptotic molecule Bcl-2 expressed in unstimulated CD8+CD127+ T cells compared with CD8+CD127− T cells (Fig. 6A) which concurs with previous findings (36). Disparate production of Bcl-2 between these subsets has been reported in the context of antigen or IL-7 stimulation or specific diseases (21, 36, 37). Incubation of CD8+CD127+ T cells with IL-2 or IL-15 significantly increased Bcl-2 expression by 2.8-fold (P < 0.001) and 3.9-fold (P < 0.001), respectively, compared with medium controls. An increase in Bcl-2 expression in CD8+CD127− T cells incubated with IL-2 or IL-15 was also observed though was somewhat lower (increases of 2.2- and 3.2-fold, respectively; P < 0.0001). The amount of Bcl-2 produced in CD8+CD127+ T cells in response to IL-2 or IL-15 was significantly greater than in CD8+CD127− T cells (P = 0.002 and 0.012, respectively). Thus, although these two subsets do not significantly differ in their basal level of Bcl-2 expression, CD8+CD127+ T cells are more responsive to IL-2 or IL-15 than CD8+CD127− T cells in this regard.
Fig. 6.
The expression of Bcl-2 in CD8+CD127+ T cells and CD8+CD127− T cells in response to IL-2 or IL-15. (A) Representative flow cytometry histograms depict the expression of Bcl-2 by isolated CD8+CD127+ and CD8+CD127− T cells cultured for 48 h with medium only (Ctl, white fill), IL-2 (100 units ml−1, gray fill) or IL-15 (100 ng ml−1, black fill). Unstained cells (similar to isotype control) are shown with a dotted line, proximal to the y-axis. The expression of Bcl-2 in these two subsets in six individuals is expressed as mean fluorescence intensities (MFI). Statistically significant differences compared with Ctl are indicated with ‘*’ (Student’s t-test P < 0.05, ±SD). (B) The expression of phosphorylated STAT5 in CD8+CD127+ and CD8+CD127− T cells cultured for 15 min with IL-2 (100 units ml−1) or IL-15 (100 ng ml−1) is shown in flow cytometry histograms. Mean fluorescence intensity values are indicated. Data represent the results from one of four individuals tested.
The expression of Bcl-2 by several γC cytokines is increased in part by signaling pathways that phosphorylate STAT proteins (38). The intracellular expression of phosphorylated-STAT5, a signaling molecule induced by IL-2 and IL-15, was assessed by flow cytometry. Culturing CD8+CD127+ T cells with IL-2 (100 units ml−1) or IL-15 (100 ng ml−1) induced more phospho-STAT5 than CD8+CD127− T cells (Fig. 6B). This may explain in part the discrepancies in the magnitude of Bcl-2 expression by the two cell subsets in response to IL-2 or IL-15.
Expression of Fas, phospho-FOXO3a and phospho-Akt
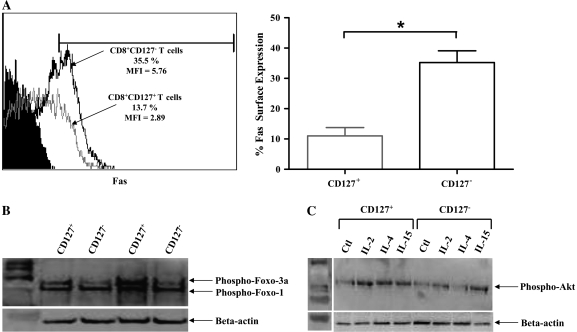
One of the most common pathways used by cytotoxic effector T cells is initiated by Fas signaling (39, 40). To determine if CD8+CD127+ and CD8+CD127− T cell subsets differ in their potential susceptibility to receptor-mediated apoptosis, the expression of the Fas receptor was analyzed. Isolated CD8+CD127− T cells expressed significantly more Fas than CD8+CD127+ T cells (P = 0.0003, Fig. 7) and concurs with recent findings that suggest a similar dichotomy of Fas expression among T cell subset in the context of CD127 expression (41). These results suggest that cytotoxic effector CD8+ T cells, which are typically CD127−, express more Fas than non-cytotoxic CD8+ T cells. However, in these experiments, subset differences in susceptibility to anti-Fas-induced apoptosis were inconsistent as the observed anti-Fas effects were relatively weak and variable in isolated CD8+CD127+ and CD8+CD127− T cells (Figs 4 and 5; Table 2).
Fig. 7.
Expression of Fas on CD8+CD127− T cells and CD8+CD127+ T cells. (A) A representative figure of Fas expression on freshly isolated CD8+CD127+ (gray line) and CD8+CD127− T cells (black line) as evaluated by flow cytometry. Unstained cells are shown in black fill. The expression of Fas is graphed as % Fas-positive cells within isolated CD8+CD127+ and CD8+CD127− T cells. Statistical significance is denoted by ‘*’ as calculated by analysis of variance analysis (P < 0.05, n = 7). (B) The baseline expression of phosphorylated FOXO3a by CD8+CD127+ and CD8+CD127− T cells is shown by western blot analysis, depicting the results of two individuals as representatives of six individuals tested. The expression of FOXO1 was also detected by the FOXO-specific antibody. The expression of beta-actin was used as a loading control. (C) The expression of phosphorylated-Akt CD8+CD127+ and CD8+CD127− T cells cultured for 2 h with medium, IL-2 (100 units ml−1), IL-4 (100 ng ml−1) or IL-15 (100 ng ml−1) is shown by western blot. The data represent the results of one individual as a representative of four individuals tested.
Baseline anti-apoptotic phospho-FOXO3a expression was assessed by western blot. Isolated CD8+CD127+ T cells expressed more phospho-FOXO3a compared with CD8+CD127− T cells (Fig. 7B). This compliments the observed increase in spontaneous apoptosis in CD8+CD127− T cells (Fig. 4B). Differences in cytokine-induced proliferation may be due to altered activation of phospho-Akt, a mitogen-activated protein kinase pathway molecule common to γC cytokines and important for T cell proliferation (42). The IL-2, IL-4 and IL-15 cytokines induced a higher level of phosphorylated Akt in CD8+CD127+ T cells than in CD8+CD127− T cells (Fig. 7C). This may explain observed proliferation patterns to cytokines such as the increase in IL-4- and IL-15-induced proliferation of CD8+CD127+ T cells compared with CD8+CD127− T cells (Fig. 2).
Discussion
The present study is the first to directly compare the cell division and susceptibility to apoptosis of human CD8+CD127+ and CD8+CD127− T cells in response to γC cytokines. It has been hypothesized that regaining or maintaining the expression of CD127 and increased Bcl-2 expression through the effector stage may result in the generation of long-lived memory cells while lack of CD127 predicts cell death (43, 44). This has also been associated with IL-7- and IL-15-dependent production of long-term memory CD8+ T cells (45). A recent report has shown that when cultured with IL-7, naive CD8+ T cells enter the cell cycle with delayed kinetics compared with memory cells (46). Despite this delay, continuous IL-7 stimulation caused a significantly greater number of naive cells to divide for a longer period of time than memory CD8+ T cells that tended to exit the cell cycle earlier (46). We and others have shown that IL-7 down-regulates CD127 expression on CD8+ T cells in both mice (47) and humans (25, 46, 48–50), and this may impede the delivery of signals for cell survival and memory cell development. However, a recent report has shown that CD127 expression is insufficient to reduce cell death in the contraction phase, and that CD127 expression does not necessarily preclude susceptibility to apoptosis (51). This supports the notion that γC cytokines other than IL-7 may determine the fate of effector cells.
Isolated CD8+CD127− T cells from healthy individuals were recently shown to be more susceptible to apoptosis than CD8+CD127+ T cells, as reported herein (Figs 4 and 5; Table 2), and this difference was enhanced in HIV-infected individuals (19). The mechanisms responsible for these observed differences in apoptosis in CD8+ T cells are unclear. We have reported for the first time that CD8+CD127− T cells express significantly more Fas than CD8+CD127+ T cells (Fig. 7A) which may explain this finding. However, in the present experiments, there was no difference between the susceptibility of CD8+CD127+ T cells versus CD8+CD127− T cells to Fas-induced apoptosis, questioning the significance of differential Fas expression in these subsets. We demonstrated that CD8+CD127− T cells are more prone to spontaneous apoptosis than CD8+CD127+ T cells (Fig. 4). This may be explained by CD8+CD127− T cells’ lower expression of anti-apoptotic molecule FOXO3a (Fig. 7B), a molecule associated with promoting memory T cell longevity (52) and resistance to apoptosis in HIV+ elite controllers (53). The greater amount of Bcl-2 in CD8+CD127+ T cells and the incremental cytokine-increased Bcl-2 expression suggests that this subset would be less prone to apoptosis than CD8+CD127− T cells (Fig. 6A). Increased cytokine-induced phosphorylation of STAT5 in CD8+CD127+ T cells may be a mechanism underlying the subset differences in Bcl-2 expression (Fig. 6B) as STAT5 phosphorylation has been shown to increase Bcl-2 transcription (38). However, not all forms of apoptotic stimuli are inhibited by Bcl-2, which acts primarily on mitochondrial-associated apoptosis and does not inhibit Fas-mediated apoptosis (54).
In the present report, IL-2 and IL-15 signaling seemed to decrease the effects of anti-Fas/anti-CD3-induced apoptosis in CD8+CD127+ T cells while CD8+CD127− T cells were less responsive to the anti-apoptotic effects of these cytokines (Table 2). Although IL-2 and IL-15 employ the same β and γ receptors (55), they have distinct roles in inhibiting and promoting CD8+ T cell survival, respectively (13, 56). IL-15 is largely known for its proliferative and anti-apoptotic effects. We observed that pre-incubation with IL-15 decreased caspase-8 activity in CD8+CD127+ and CD8+CD127− T cells in response to anti-Fas but increased Annexin–V+PI− staining and caspase-3 activity (Table 2). A previous report showed that IL-15-stimulated lymphocytes are rendered more susceptible to apoptosis compared with lymphocytes stimulated with IL-2 (57) and this is confirmed here in CD127+ T cells treated with IL-15 only (Fig. 5A). In addition, IL-15-induced proliferation did not result in a proportional decrease in the total number of apoptotic cells, particularly with high concentrations of cytokine (57) as used here. Similarly, the induction of caspase activity by IL-2 in CD8+CD127− T cells may be in part due to IL-2’s reportedly potent ability to both induce proliferation while increasing susceptibility to apoptosis and suggests that this cell subset is more susceptible to apoptosis than CD8+CD127+ T cells (11).
To facilitate the evaluation of cytokine effects, isolated CD8+CD127+ and CD8+CD127− T cells were treated with submaximal concentrations of PHA (2.5 μg ml−1). There was no quantitative difference in the proportion of cells undergoing multiple divisions (% CFSElo cells) between these two populations when incubated with this concentration of PHA. This would appear to be in contrast to a previous report in which a high concentration of PHA (10 μg ml−1) significantly increased CD8+CD127+ T cell division compared with CD8+CD127− T cells (19). We report that IL-2, IL-4 and IL-15 enhance the proliferation of CD8+CD127+ T cells while only IL-2 and IL-15 enhance CD8+CD127− T cell division (Fig. 5). We further subdivided these two populations into specific subsets. Phenotypic and functional characteristics of CD8+CD127+ and CD8+CD127− T cells suggest that Tnaive and TCM cells are CD8+CD127+ while effector cells (including TEM and TEMRA) are mostly CD8+CD127− (19). Specifically, the pattern of CD127 and CD45RA expression has been associated with distinct CD8+ T cell subsets (58). Therefore, for the evaluation of γC cytokine-induced cell division, these markers were used here to distinguish between Tnaive, TE/EM, TEMRA and TCM CD8+ T cells, all of which are known to have both redundant and unique cytokine requirements for proliferation (13). For the purpose of discussion, cell subsets are referred to according to cell surface markers based on T cell phenotypes published previously (14–16): naive cells (CD8+CD127+CD45RA+), effector cells (CD8+CD127−CD45RA−), memory cells (CD8+CD127+CD45RA−) and RA+ effector–memory-like cells (CD8+CD127−CD45RA+). Although there may be limitations to this phenotyping of T cells, there is sufficient rationale to suggest that the expression of CD127 identifies phenotypically and functionally distinct cell subsets (19). We have previously shown that the majority of CD8+CD127+CD45RA+ T cells derived from PBMC is naive, i.e. CD62L+ (48). We observed that when stimulated PHA in the presence or absence of cytokines, both CD8+CD127+ and CD8+CD127− T cells decreased the intensity of CD45RA expression while still remaining CD45RA+ and this was associated with increased cell division (data not shown). This would not appreciably affect the analysis of cell subsets using the above strategy. It is also acknowledged that the CD8+CD127+ T cell subset is a heterogenous population of naive and TCM cells, the latter of which may respond more rapidly to proliferative signals and subsequently produce cytokines that could affect the naive T cells.
Interestingly, cell division responses to IL-2, IL-4 or IL-15 preferentially enhance the cell division of the two effector subsets. Incubation of CD8+CD127− T cells with IL-4 enhanced the proportion of CFSElo effector CD8+ T cells (CD127−CD45RA−) while neither IL-2 nor IL-15 had any significant effects (Fig. 3). In contrast, both IL-2 and IL-15 increased the proportion of CFSElo TEMRA cells while IL-4 had no significant effect. These results cannot be explained by cytokine receptor expression since the expression of IL-2Rβ, IL-2Rγ, IL-4Rα and IL-15Rα is similar in CD8+CD127+ and CD8+CD127− T cells (Table 1). It is possible that proliferation differences are due in part to CD8+CD127− T cells’ increased magnitude of cytokine-induced phosphorylation of Akt (Fig. 7C), suggesting inherent subsets differences in signaling pathways associated with proliferation (38, 42). The present data indicate some significant dose responses to cytokine treatment; however, it appears that despite the titrations used here, there was not always a consistent dose-dependent proliferation response. This is not the first occasion in which a cytokine dose response could not be titrated. For example, STAT5 signaling in CD8+ T cells, common to many γC cytokines, appears to require a threshold of cytokine to induce detectable signal and increasing signaling strength is indistinguishable when higher concentrations of cytokines are used (A. M. Crawley, S. Faucher, J. B. Angel, unpublished results).
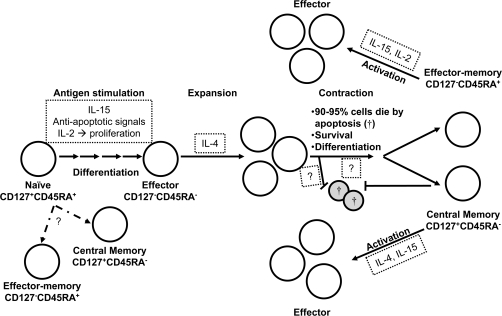
Based on currently accepted models of CD8+ T cell activation and differentiation and the present findings, we propose a revised model regarding the survival and susceptibility to apoptosis of CD8+CD127+ and CD8+CD127− T cell subsets (Fig. 8). Activated CD8+ Tnaive cells respond to IL-2 signaling and will differentiate into effector cells which then clonally expand in response to IL-2 or IL-4. The factors regulating the survival of developing memory cells from effector cells following the contraction phase are not known. Lastly, IL-2, IL-4 or IL-15 can enhance the expansion of activated CD8+ TCM cells and resistance of these cells to apoptosis is augmented by IL-2 or IL-15.
Fig. 8.
A model for the cell division and susceptibility to apoptosis of CD8+CD127+ and CD8+CD127− T cell subsets. The present findings, identified by text boxes with small dotted lines, have been inserted into a version of the currently accepted model for three main elements of a CD8+ T cell response (1). T cell activation results in the down-regulation of CD127 on naive CD8+CD127+ T cells and their susceptibility to apoptosis is inhibited by IL-2 and IL-15, resulting in the expansion of CD8+CD127−CD45RA− effector T cells which can be enhanced by IL-4 (2). The signals, that either inhibit the apoptosis of the majority of the effector cells or ensure the survival of remaining cells (≈5%) into memory cells following the contraction phase, are unclear (3). The expansion of activated central memory cells (CD8+CD127+CD45RA−) is enhanced by IL-4 or IL-15 while activated effector–memory cells (CD8+CD127−CD45RA+) divide in response to IL-15. This model does not describe CD8+ T cell development as a linear event as suggested by the potential for the differentiation of naive CD8+ T cells into either central or RA+ effector–memory cells shown with dashed arrows. Cells undergoing apoptosis are identified by a cross symbol.
Collectively, these data indicate that CD8+CD127+ T cells are more consistently responsive to the anti-apoptotic effects of IL-2 and IL-15 than CD8+CD127− T cells. In addition, the cell division kinetics of cells expressing or lacking CD127 differ in response to IL-2, IL-4 or IL-15. The frequency of cell division may relate to different functional characteristics of CD8+ T cells, although this has not yet been described. These results are of particular relevance for diseases in which the expression of CD127 is altered. The expression of CD127 is down-regulated in several viral infections, and in HIV infection this parallels disease progression (22–24, 26). In breast cancer patients, down-regulation of CD127 expression on CD8+ T cells is associated with decreased expression of STAT5, a signaling molecule shared by other γC cytokines (59). Mechanisms responsible for the homeostatic maintenance and proliferation of T cells within a full T cell compartment are now known to differ from T cell restoration in response to acute T-lymphopenia as seen in progressive HIV infection where responsiveness to survival cytokines such as IL-7 is compromised (60, 61). This may be explained in part by different T cell responses to cytokine survival and proliferation signals (27) and as described here, with the differential effects of IL-2, -4 and -15 on CD8+ T cell subset survival features. Understanding cell survival and differentiation signals is of the utmost importance for the design of therapies meant to improve CD8+ T cell responses, including therapeutics that consist of exogenous cytokines.
Funding
Ontario HIV Treatment Network (ROGB131); Canadian Institutes of Health Research (HOP84649).
Acknowledgments
The authors thank Drs Paul MacPherson and Ashok Kumar for helpful discussion and critical review of the manuscript. A.M.C. is a recipient of an Ontario HIV Treatment Network (OHTN) fellowship and J.B.A. is an OHTN Career Scientist.
Contribution: A.M.C. designed and performed most of the research, analyzed data and wrote the paper; T.K. performed a number of the experiments; K.P. assisted with the flow cytometry analysis and J.B.A. assisted in the design of the research, data analysis and aided in the writing of the paper. The authors also gratefully acknowledge James MacLeod for his assistance with some of these experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Glossary
Abbreviations
- CFSE
carboxyfluorescein succinimdyl ester
- FAM
carboxyfluorescein
- FMK
fluoromethyl ketone
- PI
phosphatidylinositol
- γC
γ chain
References
- 1.Ziegler SE, Morella KK, Anderson D, et al. Reconstitution of a functional interleukin (IL)-7 receptor demonstrates that the IL-2 receptor gamma chain is required for IL-7 signal transduction. Eur. J. Immunol. 1995;25:399. doi: 10.1002/eji.1830250214. [DOI] [PubMed] [Google Scholar]
- 2.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 2005;174:6571. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 3.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 4.Benczik M, Gaffen SL. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol. Invest. 2004;33:109. doi: 10.1081/imm-120030732. [DOI] [PubMed] [Google Scholar]
- 5.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002;168:4827. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 7.Li XC, Demirci G, Ferrari-Lacraz S, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat. Med. 2001;7:114. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 8.Riou C, Dumont AR, Yassine-Diab B, Haddad EK, Sekaly RP. IL-4 influences the differentiation and the susceptibility to activation-induced cell death of human naive CD8+ T cells. Int. Immunol. 2006;18:827. doi: 10.1093/intimm/dxl019. [DOI] [PubMed] [Google Scholar]
- 9.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J. Immunol. 2003;170:5018. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 10.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 2006;24:657. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 11.Lenardo M, Chan KM, Hornung F, et al. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 1999;17:221. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 12.Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells but does not enhance memory. J. Immunol. 2006;177:4458. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 2003;3:269. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 14.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 15.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 16.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 17.Huster KM, Busch V, Schiemann M, et al. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl Acad. Sci. USA. 2004;101:5610. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 2005;175:4686. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 19.Paiardini M, Cervasi B, Albrecht H, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 2005;174:2900. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- 20.Zaunders JJ, Moutouh-de Parseval L, Kitada S, et al. Polyclonal proliferation and apoptosis of CCR5+ T lymphocytes during primary human immunodeficiency virus type 1 infection: regulation by interleukin (IL)-2, IL-15, and Bcl-2. J. Infect. Dis. 2003;187:1735. doi: 10.1086/375030. [DOI] [PubMed] [Google Scholar]
- 21.Lang KS, Recher M, Navarini AA, et al. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 2005;35:738. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- 22.Koesters SA, Alimonti JB, Wachihi C, et al. IL-7Ralpha expression on CD4(+) T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur. J. Immunol. 2006;36:336. doi: 10.1002/eji.200535111. [DOI] [PubMed] [Google Scholar]
- 23.Boutboul F, Puthier D, Appay V, et al. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005;19:1981. doi: 10.1097/01.aids.0000191919.24185.46. [DOI] [PubMed] [Google Scholar]
- 24.Golden-Mason L, Burton JR, Jr, Castelblanco N, et al. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44:1098. doi: 10.1002/hep.21365. [DOI] [PubMed] [Google Scholar]
- 25.Sasson SC, Zaunders JJ, Zanetti G, et al. Increased plasma interleukin-7 level correlates with decreased CD127 and increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J. Infect. Dis. 2006;193:505. doi: 10.1086/499309. [DOI] [PubMed] [Google Scholar]
- 26.MacPherson PA, Fex C, Sanchez-Dardon J, Hawley-Foss N, Angel JB. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2001;28:454. doi: 10.1097/00042560-200112150-00008. [DOI] [PubMed] [Google Scholar]
- 27.Goldrath AW, Sivakumar PV, Glaccum M, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 2002;195:1515. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodolce J, Burkett P, Koka R, et al. Interleukin-15 and the regulation of lymphoid homeostasis. Mol. Immunol. 2002;39:537. doi: 10.1016/s0161-5890(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 29.Chiu WK, Fann M, Weng NP. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J. Immunol. 2006;177:7802. doi: 10.4049/jimmunol.177.11.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coico R. Current Protocols in Immunology. John Wiley & Sons, Inc, Hoboken, NJ, USA.; 1998. [Google Scholar]
- 31.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J. Immunol. Methods. 2000;243:147. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 32.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J. Clin. Invest. 1997;100:3173. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badley AD, Parato K, Cameron DW, et al. Dynamic correlation of apoptosis and immune activation during treatment of HIV infection. Cell Death Differ. 1999;6:420. doi: 10.1038/sj.cdd.4400509. [DOI] [PubMed] [Google Scholar]
- 34.Morrot A, Hafalla JC, Cockburn IA, Carvalho LH, Zavala F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J. Exp. Med. 2005;202:551. doi: 10.1084/jem.20042463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokoyama T, Tanahashi M, Kobayashi Y, et al. The expression of Bcl-2 family proteins (Bcl-2, Bcl-x, Bax, Bak and Bim) in human lymphocytes. Immunol. Lett. 2002;81:107. doi: 10.1016/s0165-2478(02)00003-2. [DOI] [PubMed] [Google Scholar]
- 36.Sasson SC, Zaunders JJ, Kelleher AD. The IL-7/IL-7 receptor axis: understanding its central role in T-cell homeostasis and the challenges facing its utilization as a novel therapy. Curr. Drug Targets. 2006;7:1571. doi: 10.2174/138945006779025365. [DOI] [PubMed] [Google Scholar]
- 37.Colle JH, Moreau JL, Fontanet A, et al. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients—effects of antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2006;42:277. doi: 10.1097/01.qai.0000214823.11034.4e. [DOI] [PubMed] [Google Scholar]
- 38.Kittipatarin C, Khaled AR. Interlinking interleukin-7. Cytokine. 2007;39:75. doi: 10.1016/j.cyto.2007.07.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrack P, Kappler J. Control of T cell viability. Annu. Rev. Immunol. 2004;22:765. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 40.Teh SJ, Dutz JP, Motyka B, Teh HS. Fas (CD95)-independent regulation of immune responses by antigen-specific CD4-CD8+ T cells. Int. Immunol. 1996;8:675. doi: 10.1093/intimm/8.5.675. [DOI] [PubMed] [Google Scholar]
- 41.Fluur C, De Milito A, Fry TJ, et al. Potential role for IL-7 in Fas-mediated T cell apoptosis during HIV infection. J. Immunol. 2007;178:5340. doi: 10.4049/jimmunol.178.8.5340. [DOI] [PubMed] [Google Scholar]
- 42.Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood. 2007;109:1034. doi: 10.1182/blood-2006-06-027912. [DOI] [PubMed] [Google Scholar]
- 43.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 44.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 2004;5:809. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 45.Buentke E, Mathiot A, Tolaini M, Di Santo J, Zamoyska R, Seddon B. Do CD8 effector cells need IL-7R expression to become resting memory cells? Blood. 2006;108:1949. doi: 10.1182/blood-2006-04-016857. [DOI] [PubMed] [Google Scholar]
- 46.Swainson L, Verhoeyen E, Cosset FL, Taylor N. IL-7R alpha gene expression is inversely correlated with cell cycle progression in IL-7-stimulated T lymphocytes. J. Immunol. 2006;176:6702. doi: 10.4049/jimmunol.176.11.6702. [DOI] [PubMed] [Google Scholar]
- 47.Park JH, Yu Q, Erman B, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Vranjkovic A, Crawley AM, Gee K, Kumar A, Angel JB. IL-7 decreases IL-7 receptor alpha (CD127) expression and induces the shedding of CD127 by human CD8+ T cells. Int. Immunol. 2007;19:1329. doi: 10.1093/intimm/dxm102. [DOI] [PubMed] [Google Scholar]
- 49.Colle JH, Moreau JL, Fontanet A, et al. CD127 expression and regulation are altered in the memory CD8 T cells of HIV-infected patients—reversal by highly active anti-retroviral therapy (HAART) Clin. Exp. Immunol. 2006;143:398. doi: 10.1111/j.1365-2249.2006.03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young CD, Angel JB. Optimization of culture and storage conditions for an in vitro system to evaluate thymocyte phenotype and function. J. Immunol. Methods. 2006;312:157. doi: 10.1016/j.jim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J. Immunol. 2005;175:4400. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- 52.Riou C, Yassine-Diab B, Van grevenynghe J, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J. Exp. Med. 2007;204:79. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Grevenynghe J, Procopio FA, He Z, et al. Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat. Med. 2008;14:266. doi: 10.1038/nm1728. [DOI] [PubMed] [Google Scholar]
- 54.Strasser A, Harris AW, Huang DC, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 57.Naora H, Gougeon ML. Interleukin-15 is a potent survival factor in the prevention of spontaneous but not CD95-induced apoptosis in CD4 and CD8 T lymphocytes of HIV-infected individuals. Correlation with its ability to increase BCL-2 expression. Cell Death Differ. 1999;6:1002. doi: 10.1038/sj.cdd.4400575. [DOI] [PubMed] [Google Scholar]
- 58.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 2006;211:236. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 59.Vudattu NK, Magalhaes I, Schmidt M, Seyfert-Margolis V, Maeurer MJ. Reduced numbers of IL-7 receptor (CD127) expressing immune cells and IL-7-signaling defects in peripheral blood from patients with breast cancer. Int. J. Cancer. 2007;121:1512. doi: 10.1002/ijc.22854. [DOI] [PubMed] [Google Scholar]
- 60.Rethi B, Fluur C, Atlas A, et al. Loss of IL-7Ralpha is associated with CD4 T-cell depletion, high interleukin-7 levels and CD28 down-regulation in HIV infected patients. AIDS. 2005;19:2077. doi: 10.1097/01.aids.0000189848.75699.0f. [DOI] [PubMed] [Google Scholar]
- 61.Oehen S, Brduscha-Riem K. Naive cytotoxic T lymphocytes spontaneously acquire effector function in lymphocytopenic recipients: a pitfall for T cell memory studies? Eur. J. Immunol. 1999;29:608. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]