Abstract
Background
Entropy™ is an anaesthetic EEG monitoring method, calculating two numerical parameters: State Entropy (SE, range 0–91) and Response Entropy (RE, range 0–100). Low Entropy numbers indicate unconsciousness. SE uses the frequency range 0.8–32 Hz, representing predominantly the EEG activity. RE is calculated at 0.8–47 Hz, consisting of both EEG and facial EMG. RE–SE difference (RE−SE) can indicate EMG, reflecting nociception. We studied RE−SE and EMG in patients anaesthetized without neuromuscular blockers.
Methods
Thirty-one women were studied in propofol–nitrous oxide (P) or propofol–nitrous oxide–remifentanil (PR) anaesthesia. Target SE value was 40–60. RE−SE was measured before and after endotracheal intubation, and before and after the commencement of surgery. The spectral content of the signal was analysed off-line. Appearance of EMG on EEG was verified visually.
Results
RE, SE, and RE−SE increased during intubation in both groups. Elevated RE was followed by increased SE values in most cases. In these patients, spectral analysis of the signal revealed increased activity starting from low (<20 Hz) frequency area up to the highest measured frequencies. This was associated with appearance of EMG in raw signal. No spectral alterations or EMG were seen in patients with stable Entropy values.
Conclusions
Increased RE is followed by increased SE at nociceptive stimuli in patients not receiving neuromuscular blockers. Owing to their overlapping power spectra, the contribution of EMG and EEG cannot be accurately separated with frequency analysis in the range of 10–40 Hz.
Keywords: anaesthetics i.v., propofol; analgesics opioid, remifentanil; measurement techniques, electromyography; monitoring, electroencephalography
EEG is the method of choice in measuring the hypnotic component of anaesthesia. During the last decade, several measures have been introduced for this purpose. The most widely adopted EEG measure of anaesthetic drug effect is Bispectral Index (BIS™ monitor, Aspect Medical Systems, Newton, MA, USA).1 BIS utilizes a sensor placed in the forehead and temple of the patient to collect EEG. Although the approach is easy to use for anaesthetists, the placement is prone to facial EMG contamination,2 which is shown to represent impending arousal or nociception,3 even during deep hypnosis with propofol.4 Therefore, a single-number index like BIS can never tell with certainty the impact of EMG on the information received from the analysed signal.
To at least partly overcome the problem described above, another EEG monitoring device, the M-ENTROPY™ module (GE Healthcare, Helsinki, Finland), was introduced.5 The M-ENTROPY™ module calculates the characteristics of the upper facial biosignal with an analysis of time–frequency balanced spectral entropy, taking into account the special characteristics of suppressed EEG signal. The resulting index of EEG and EMG activity is called Entropy, which has been shown to be a valid indicator of the hypnotic effect of propofol, thiopental, isoflurane, sevoflurane, and desflurane.6
Entropy consists of two parameters: State Entropy (SE) and Response Entropy (RE). SE is computed over the frequency range from 0.8 to 32 Hz, which represents the EEG-dominant part of the frequency spectrum. Therefore, SE reflects the cortical state of the patient. RE is computed over the frequency range from 0.8 to 47 Hz, covering both the EEG-dominant and the EMG-dominant areas of the spectrum. Consequently, RE−SE difference serves at least partly as an indicator of upper facial EMG activation.5
The RE and SE are both calculated with 5 s intervals from the preceding 1.92 and 15 s segments of EEG, respectively, and the new values are shown on the screen and output from the monitor.
The appearance of upper facial EMG indicates that the patient is responding to an external stimulus,7 which is usually nociceptive. Therefore, RE−SE difference may indicate nociception or inadequate anaesthesia, an assumption supported by one recently published article,8 but disputed by another.9
The present study was designed to prospectively investigate RE, SE, RE−SE difference, and upper facial EMG during propofol–nitrous oxide or propofol–nitrous oxide–remifentanil anaesthesia in patients without neuromuscular blocking agent (NMBA) medication (i) before and after intubation and (ii) before and after beginning of laparoscopic surgery. The primary aim of the study was to evaluate the behaviour of RE, SE, and RE−SE difference between the groups. Secondary aims were to compare the Entropy indices between intubation and surgery, to study the impact of visually verified EMG on the indices, and to make comparisons between heart rate (HR) and RE−SE difference. As remifentanil is a very potent opioid, we hypothesized that smaller increase in RE, and therefore, smaller RE−SE difference would be seen in patients receiving remifentanil as an adjuvant of their propofol−nitrous oxide anaesthesia. We also hypothesized that EMG would react more precisely than EEG to nociceptive stimuli, and that changes of RE−SE difference would associate with alterations in HR. To our knowledge, this is the first clinical study where the appearance of EMG is verified visually both from the original biosignal and from its spectral presentation, and compared with the behaviour of the Entropy parameters.
Methods
Patients
This study followed the design of a prospective clinical study. The local Ethics Committee and Finnish National Agency for Medicines approved the study protocol. All patients gave their written informed consent. Inclusion criteria were the following: patients undergoing elective laparoscopic gynaecological surgery (expected duration >30 min) between ages 18 and 60 yr, and ASA physical status I or II. Patients were excluded if they had a disease or injury affecting the central nervous system, alcohol or drug abuse, or BMI >28. All patients fasted overnight before surgery. A total of 33 patients were enrolled.
Electroencephalogram acquisition
The forehead biosignal was collected with a disposable electrode strip (Entropy Sensor, GE Healthcare) for Entropy measurement. After degreasing of the forehead skin using isopropanol 70%, the strip was positioned as recommended by the manufacturer. The signal was acquired from two electrodes of the strip: one frontally in the midline, 2 cm above the eyebrows, and the other 2 cm laterally from the outer canthus of the left eye. The first electrode is on the frontal muscle, the second on the orbicularis oculi and temporal muscles. Entropy was collected with an M-ENTROPY™ module of the S/5™ Anesthesia Monitor (GE Healthcare). The sampling rate was 400 Hz. High- and low-pass filters of 0.5 and 118 Hz (−3 dB; 10 dB per decade), respectively, were applied. Power line artifact was not filtered. All the monitored values were collected on a laptop computer.
Anaesthesia and study protocol
RE, SE, and vital signs were collected under two anaesthetic regimens, both of which included premedication with diazepam 10 mg p.o. An i.v. route was established for all patients and an infusion of isotonic saline was started. Intermittent non-invasive arterial pressure was recorded every 5 min. Electrocardiogram, inspired fractions and end-tidal concentrations of anaesthetic gases and CO2, and peripheral oxygen saturation were continuously monitored with the Datex-Ohmeda S/5™ Anesthesia Monitor.
Entropy monitoring started before induction of anaesthesia and continued uninterrupted until the end of the study. Seventeen patients were anaesthetized with propofol 3 mg kg−1 i.v., followed by manually controlled ventilation of nitrous oxide 67% in oxygen via face mask. Endotracheal intubation was performed, as gently as possible, 150 s later. If intubation difficulties were met, mask ventilation was re-started and another intubation attempt was done 1–3 min later. If the second attempt was unsuccessful, rocuronium was given and the patient was discarded from the study. After securing the airway, controlled mechanical ventilation was started with a fresh flow of 6 litre min−1 (67% nitrous oxide in oxygen), and propofol infusion was started. The infusion rate of propofol was adjusted to keep the Entropy parameters between 40 and 60, the target being 50.
Sixteen patients were anaesthetized the same way as described above, but target-controlled infusion (TCI, Asena™ PK, Alaris Medical Systems, Basingstoke, UK) of remifentanil was added to the anaesthetic regimen. Remifentanil was infused with the estimated effect-site concentration (Ce) of 4.0 ng ml−1 from the beginning of anaesthetic induction (the pharmacokinetic model of Minto and colleagues)10 until the end of the study. Laryngoscopy for endotracheal intubation was initiated after reaching the remifentanil Ce of 4.0 ng ml−1.
After endotracheal intubation, patients were not touched or otherwise disturbed for 5 min to discover the magnitude of RE−SE difference, and the presence or absence of EMG, during anaesthesia without surgery. After 5 min, the patient was prepared for operation and permission to start laparoscopy was granted. A propofol bolus of 50 mg was given as a rescue medication if the Entropy values exceeded 60, and repeated 90 s later, if needed. The study was completed 1 min after setting the first laparoscopy trochar. Thereafter, the administration of nitrous oxide was discontinued and patient's lungs were ventilated with air/oxygen. Fentanyl (the propofol group) and rocuronium were given according to clinical needs.
Analyses of the biosignal
RE and SE values were analysed off-line as a mean of 15 s (three consecutive readings) at the following time points: awake, 90 s after anaesthetic induction, 30 s before intubation, 30 s after intubation, after a 5 min equilibrium period, 60 s before skin incision, 30 s after skin incision, 30 s after setting of the needle of Veress, 30 s after beginning of gas insufflation, at the end of gas insufflation, and 30 s after setting of the first laparoscopy trochar. RE−SE was calculated by subtracting the SE value from the corresponding RE value.
Spectrogram, that is, a presentation of the spectral content of a biosignal, was produced with Somnologica™ sleep analysis program (Medcare Flaga, Reykjavik, Iceland). Power spectra of consecutive 1.0 s samples of the signal are calculated and presented vertically along y-axis, plotted against time in x-axis. The density (i.e. ‘darkness’) of spectrogram reveals the amount of activity at respective frequency. Thus, spectrogram presents the same information as successive power spectra, but in a compressed form. Power line artifact is seen in spectrogram as a sharp activity band at 50 or 60 Hz, and ECG or EOG artifacts are typically located at rather low frequency range. Therefore, EMG is virtually the only artifact that is displayed over a wide frequency range.
Both the raw biosignal and a spectrogram at the time points of interest were inspected visually off-line, without knowledge of the behaviour of the Entropy indices, by an experienced clinical neurophysiologist (V.J.), to judge the presence or absence of EMG. Later on, the traces and classifications were analysed jointly by all authors, to ensure the agreement of the detections. To demonstrate the effect of EMG on the EEG spectrum, spectral analyses before and after intubation or commencement of surgery were drawn in four patients. In analyses, a window of 15 s was used.
All patients were interviewed during the first postoperative day, regarding their possible anaesthesia and surgery-related memories and intubation-associated sequelae.
Statistical methods
The study was designed to have a power of 80% to detect statistical significance, assuming two-sided α-level of 0.05, with surgery-associated mean RE−SE differences of 7.0 and 2.0 units (sd 4.5 units) between the propofol and the propofol−remifentanil groups, respectively. The power calculation was based on the previous unpublished Entropy data of our own. To meet the criteria of power calculation, 14 patients per group were needed. All statistical analyses were performed using SPSS for Windows software (version 16.0, SPSS, Chicago, IL, USA). One-way analysis of variance followed by t-tests was used for parametric data, and Mann–Whitney and χ2 tests were used for non-parametric comparisons, where appropriate. Pearson's correlation analysis was used to compare the change of HR with that of RE−SE difference at endotracheal intubation. P<0.05 was considered statistically significant.
Results
Two enrolled patients, both receiving propofol−nitrous oxide without remifentanil, were excluded because of problems in laryngoscopy and intubation. All others were successfully intubated with one or two attempts. Therefore, 15 patients in the propofol−nitrous oxide group and 16 patients in the propofol−nitrous oxide−remifentanil group were studied. Patient characteristics did not differ between the groups. The mean ages of patients not receiving remifentanil and those with remifentanil were 39 vs 32 yr, mean weight was 62 vs 61 kg, and mean height was 166 vs 165 cm, respectively.
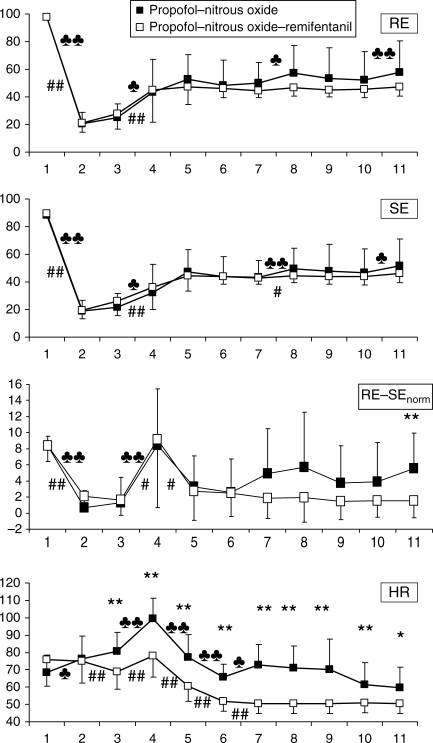
The RE or SE values did not differ between the groups along the study. The RE−SE difference showed significant between-group difference awake and from skin incision to the end of the study. Awake, the mean of propofol−nitrous oxide group was 1.22 units higher (P<0.042). Therefore, the groups were baseline-corrected by subtracting 1.22 units from all means in the propofol−nitrous oxide group. After such correction, the only detected between-group difference was after setting the first laparoscopy trochar. The behaviour of RE, SE, baseline-corrected RE−SE difference, and HR in both groups is presented in Figure 1.
Fig 1.
The behaviour of RE, SE, baseline-corrected RE−SE, and HR [mean (sd)] during the study in patients receiving propofol–nitrous oxide or propofol−nitrous oxide−remifentanil anaesthesia. 1, awake; 2, 90 s after anaesthetic induction; 3, 30 s before intubation; 4, 30 s after intubation; 5, after a 5 min equilibrium period; 6, 60 s before skin incision; 7, 30 s after skin incision; 8, 30 s after setting of the needle of Veress; 9, 30 s after beginning of gas insufflation; 10, at the end of gas insufflation; 11, 30 s after setting of the first laparoscopy trochar. ♣P<0.05, ♣♣P<0.01 within the propofol–nitrous oxide group, respectively. #P<0.05, ##P<0.01, and *P<0.05, **P<0.01 within the propofol–nitrous oxide–remifentanil group, and between the groups, respectively.
The SE and RE values increased more commonly after intubation than after skin incision (Table 1). RE increased ≥10 units in 27 events. This increase in RE values was rapidly followed by ≥10 units increase of SE in 21/27 cases, thereby decreasing the calculated RE−SE difference.
Table 1.
Number of patients with small (<10), moderate (10 to ≤30) or large (>30 units) change in SE or RE values at intubation or skin incision. The mean of three consecutive index values before intubation or skin incision is subtracted from that of three consecutive values 1 min after the event. *P=0.006, **P=0.001 for SE and RE change values, respectively, between intubation and skin incision (two-tailed Mann–Whitney test)
| Magnitude of change in index values | Intubation*, ** |
Incision |
||||||
|---|---|---|---|---|---|---|---|---|
| Propofol |
Propofol–remifentanil |
Propofol |
Propofol–remifentanil |
|||||
| SE | RE | SE | RE | SE | RE | SE | RE | |
| <10 units | 7 | 4 | 8 | 7 | 9 | 9 | 16 | 15 |
| 10 to ≤30 units | 4 | 7 | 6 | 5 | 5 | 5 | 0 | 1 |
| >30 units | 4 | 4 | 2 | 4 | 1 | 1 | 0 | 0 |
| Σ | 15 | 15 | 16 | 16 | 15 | 15 | 16 | 16 |
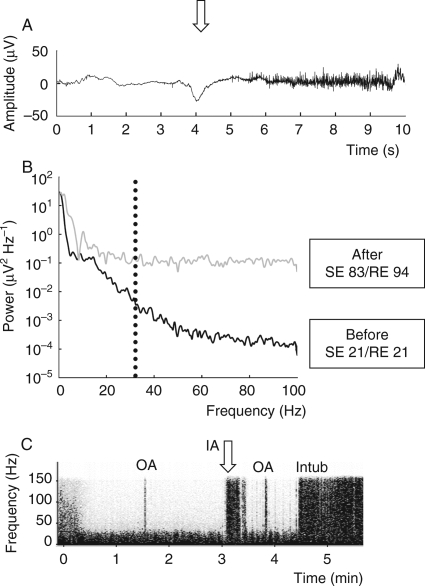
When all blindly analysed episodes of interventions were pooled together, the EMG was present more frequently in recordings of patients in the propofol group (79 EMG findings in 165 episodes) than in the propofol–remifentanil group (33 findings in 176 episodes). Incidence of EMG in both groups and the association between EMG and Entropy RE−SE difference are given in Table 2. In some patients, EMG changed the power spectrum of the biosignal from 15 Hz up to ∼150 Hz, that is, to the highest detectable frequencies. The presence of EMG in the raw biosignal of an example patient, and its effect on power spectrum, Entropy values, and spectrogram, is depicted in Figure 2.
Table 2.
The occurrence of visible EMG in EEG signal along the course of study in patients receiving propofol–nitrous oxide or propofol–remifentanil–nitrous oxide anaesthesia, and the association of EMG with RE–SE difference
| Time point | EMG present/absent (n) |
RE–SE [mean (sd)] |
||||
|---|---|---|---|---|---|---|
| Propofol | Propofol–remifentanil | P-value (Fisher's exact test) | EMG present | EMG absent | P-value (t-test) | |
| Awake | 15/0 | 16/0 | 1.0 | 8.96 (1.70) | ||
| Induction+90 s | 5/10 | 4/12 | 0.704 | 2.44 (2.35) | 1.72 (1.83) | 0.43 |
| Before intubation | 1/14 | 0/16 | 0.484 | 12 (0) | 1.73 (1.86) | <0.001 |
| After intubation | 15/0 | 13/3 | 0.226 | 9.96 (7.6) | 0.67 (0.33) | <0.001 |
| Steady state | 4/11 | 0/16 | 0.043 | 8 (1.63) | 2.93 (3.6) | 0.001 |
| Before skin incision | 2/13 | 0/16 | 0.226 | 12 (1.41) | 2.52 (2.73) | 0.027 |
| After skin incision | 6/9 | 0/16 | 0.007 | 12.17 (3.49) | 1.96 (2.13) | <0.001 |
| Needle of Veress | 6/9 | 0/16 | 0.007 | 13 (6.48) | 2.28 (3.01) | 0.009 |
| Start of gas insufflation | 7/8 | 0/16 | 0.002 | 7.57 (5.16) | 1.83 (2.44) | 0.025 |
| End of gas insufflation | 8/7 | 0/16 | 0.001 | 8.38 (4.5) | 1.52 (1.81) | 0.003 |
| Laparoscopy trochar | 10/5 | 0/16 | <0.001 | 9.1 (2.92) | 1.62 (1.96) | <0.001 |
Fig 2.
(a) The appearance of EMG activity in EEG signal after laryngoscopy (arrow downward) and attempted intubation in a patient not receiving remifentanil. EEG signal remains suppressed, despite strong EMG contamination. (b) Power spectrum of the EEG signal before (black line) and after (grey line) laryngoscopy in the same situation as in (a). EMG activity changes the spectrum after laryngoscopy. The spectrum with EMG impact starts to change already below 20 Hz. Dotted vertical line represents the 32 Hz frequency, which is used in Entropy calculation to differentiate EEG activity (<32 Hz) from EMG activity (>32 Hz). In Entropy calculation, low SE value increases rapidly after appearance of EMG, because the power at <32 Hz area increases. (c) EEG spectrogram (frequency vs time) of the same patient as in (a) and (b). Fast activity disappears in the beginning of anaesthesia (0–30 s). Continuous activity below 30 Hz is merely EEG. Placement of oropharyngeal airway (OA) at 1 min 40 s elicits short EMG response, shown as a vertical bar up to 150 Hz. Laryngoscopy and attempted intubation (IA) at arrow is associated with longer EMG response. Replacement of oropharyngeal airway at 3 min 50 s, and successful intubation (Intub) at 4 min 30 s. Long-lasting EMG activity ensues.
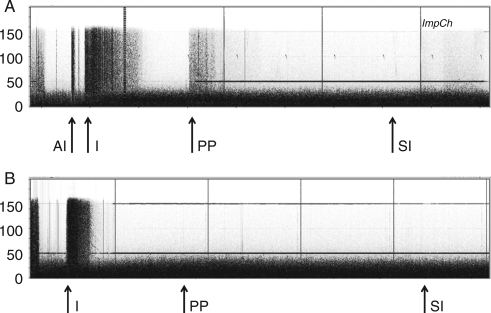
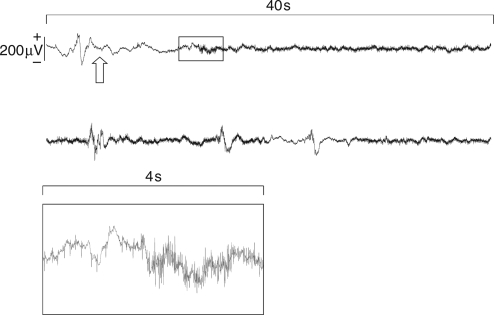
Figure 3 presents the spectrograms from the whole study period in two patients, one in the propofol group and another in the propofol−remifentanil group. In some patients, the EMG was seen on the top of suppressed EEG signal in association with noxious stimulus (Fig. 4).
Fig 3.
EEG spectrograms presenting the whole study periods in two patients: (a) without and (b) with remifentanil infusion. Fast activity disappears in the beginning of anaesthesia. EEG activity is seen in both recordings as a continuous activity below 30 Hz. EMG contamination is seen as vertical bars up to 150 Hz. AI, attempted intubation; I, intubation; PP, patient positioning; SI, skin incision. Laryngoscopy and intubation are associated with strong EMG activity in both recordings. Thereafter, no EMG is seen in (b), that is, the patient receiving remifentanil. In (a), both noxious and non-noxious stimuli (like positioning the patient) elicit EMG activity. Horizontal bars at 50 and 150 Hz are power line (50 Hz) artifacts and its harmonic (150 Hz). Vertical bars (ImpCh) indicate automatic Entropy sensor impedance checks every 10 min.
Fig 4.
Two successive 40 s samples of EEG which show development of EMG after intubation (arrow upward) during EEG burst suppression. The box below is an enlarged 4 s piece of the signal, showing the beginning of EMG activity.
The mean time intervals from induction of anaesthesia to intubation were 161 and 168 s for groups without and with remifentanil, respectively (NS). The same figures for intervals from anaesthetic induction to skin incision were 29 and 32 min, respectively (NS).
HR was similar in both groups, awake and 90 s after induction of anaesthesia. From intubation to the end of the study period, HR was higher in patients without remifentanil (Fig. 1). The correlation between the change of HR and that of RE−SE difference at endotracheal intubation was poor: r=0.179 (P=0.336).
Rescue medication due to elevated SE values was needed 18 times in 12 patients (nine in the propofol group and three in the propofol–remifentanil group). None of the patients, however, had recall, sore throat, or other complaints in the postoperative interview.
Discussion
The first finding in our study was that RE, SE, and RE−SE difference increased in both groups during laryngoscopy and endotracheal intubation, along with continuous EMG activity in the biosignal, in spite of remifentanil (4.0 ng ml−1) in one group. The second finding was that the intubation-associated increase in RE−SE difference was only transient, because the increase in RE was often rapidly followed by an increase in SE. These findings suggest that RE−SE difference cannot be used as a long-lasting, reliable indicator of nociception. Although regularly detected in the initial phase of stimulation, RE−SE difference soon disappears due to increasing SE.
The small, though statistically significant, difference in RE−SE awake was most likely due to random factors,11 because no abnormal EEG was seen in visual evaluation. However, this difference in RE−SE awake was taken into account by normalizing the groups in the way described in Results. After baseline correction according to the awake values, the RE−SE difference was higher in patients not receiving remifentanil only at setting of the first trochar.
The third finding was that intubation was associated with greater increases in RE, SE, and RE−SE difference than commencement of surgery. None of the indices showed increased values at skin incision. This is in line with earlier literature, ranking endotracheal intubation as a stronger nociceptive stimulus than skin incision.12
The fourth and perhaps the most significant finding was that increased index values during nociceptive stimuli were strongly associated with the presence of EMG, confirmed by visual analysis of the raw biosignal and by the analysis of the spectrogram; both performed without knowledge of anaesthesia regimen. Spectral analysis of the biosignal demonstrated an EMG-associated change in the spectrum at <20–100 Hz.
After endotracheal intubation, no continuous EMG activity in the raw signal, or significant RE−SE difference in Entropy values, was noted in patients receiving remifentanil. EMG was more frequently present during surgical manipulation in patients without remifentanil. The reactivity of upper facial biosignal was seen in nearly all patients during intubation, in spite of lower RE and SE values than was the case during commencement of surgery. This could be explained by the high stimulus intensity of laryngoscopy and intubation13,14 that elicits EMG activity also in patients receiving remifentanil. Another postulation might be the modelling of remifentanil pharmacokinetics: as the estimated effect-site remifentanil concentration is based on the cortical electrical activity,10 it is theoretically possible that the drug concentration was lower than assumed in the areas controlling EMG. The EMG recorded is likely to be both from facial- and trigeminal-innervated muscles as the electrodes are on frontal and temporal muscles. The pathways to frontal and temporal muscles are the facial and trigeminal nerves, respectively, both originating from the brain stem.15 The Ke0 for remifentanil effect at the brain stem might differ from that measured as a change in EEG. The mean duration of 30 min from anaesthetic induction to skin incision, on the other hand, was sufficient to complete the saturation of remifentanil, abolishing the reactivity of EMG. Finally, as one of the electrodes in the Entropy strip is placed on the patient's temple, it is possible that the EMG activity during laryngoscopy and intubation comes partly from the temporal muscle, instead of the frontal muscle. Further research is needed to elucidate the behaviour of EMG during intubation.
In analysis of Entropy and raw EEG signal, the presence of EMG dictated Entropy values during nociceptive events. Typically, RE values increased with short delay, as described earlier by Vakkuri and colleagues.6 In the majority of cases, the increase of RE was soon followed by an increase in SE values, decreasing the RE−SE difference. The increase in SE values was most probably due to the increase in biosignal activity below 32 Hz, as depicted in Figure 2. While all activity below 32 Hz is regarded as EEG in the analysis of Entropy,5 strong EMG impact starts to change the spectrum already at 20 Hz.16 This may explain the unexpected behaviour of SE during nociceptive stimuli, when anaesthesia regimen does not include adequate anti-nociception or effective neuromuscular block. Nociception-associated EMG activity may increase SE values, because the content of the biosignal starts to change already below 20 Hz (Fig. 2).
HR is a standard indicator of nociception. In this study, HR differed between the groups from intubation to the end of the study. Higher values were seen in patients without remifentanil. This may indicate stronger nociceptive input in these patients, although the direct effect of remifentanil on HR should also be considered.17 The poor correlation between HR and RE−SE difference was most probably due to the widespread nociception-elicited alterations in EEG, reducing the RE−SE difference, as described above.
On the basis of our results, it is appropriate to conclude that Entropy RE−SE difference may increase during strong nociceptive stimuli, but this increase is often transient, due to increasing SE values. Therefore, although high SE values serve as an indicator of impending awareness, EMG contamination can associate with high SE values during otherwise sufficient hypnotic state of anaesthesia. Adequate anti-nociceptive medication, however, decreases the risk of EMG contamination, as seen in our patients receiving remifentanil.
One might argue that increasing SE levels in our study indicate less adequate anaesthetic depth and impending arousal. From a theoretical point of view, that is well possible. However, the connection between EMG, spectral characteristics of EEG, and SE was well demonstrated in the present study. EMG is generated at brain stem level; therefore, the presence of EMG is not a direct indicator of awareness. Moreover, appearance of EMG does not necessarily alter the underlying EEG signal during anaesthesia. As an example, in Figure 4, the burst suppression EEG signal remained unaltered, although strong EMG activity contaminated the signal. Finally, none of our patients had recall despite high SE values, although the study remains underpowered to examine awareness.
The biosignal was visually analysed by a single neurophysiologist with considerable experience on anaesthesia-related EEG. No artifacts that could contribute significantly to the frequency bands above 32 Hz were detected in this analysis. As shown in Figure 3, electrical noise appears as a narrow band at 50 Hz, whereas electro-oculogram and ECG artifacts are limited at low frequency areas, instead of widespread EMG activity. With the aid of spectral presentation, EMG is reliably detected in EEG.
The intraoperative EMG contamination of EEG is challenging for an anaesthetist, because such low-amplitude activity is not always readily seen on monitor, even though the displaying of ‘raw’ EEG signal is highly recommended. The EMG-associated increase in SE value does not directly possess the risk of unintentional awareness during anaesthesia, because high SE value usually leads to re-adjustment of hypnotics. When remaining undetected, the situation could, however, cause inappropriate and potentially harmful use of anaesthetics.
The anaesthetic regimen of this study does not reflect our routine clinical practice and necessitates a comment. Our aim was to study the magnitude of RE−SE difference with or without strong anti-nociceptive medication, and without confounding impact of NMBAs. Nitrous oxide, however, was used as a baseline anti-nociceptive agent in our intubated patients under controlled ventilation. In most cases, endotracheal intubation and commencement of surgery went smoothly, whereas difficulties in intubation were faced in two patients, who were discarded from the study. Owing to the gentle manipulating techniques and used rescue medication, none of the patients reported any anaesthesia-related sequelae. With this technique, we were able to demonstrate the effects of EMG on the Entropy indices RE and, especially, SE. As neuromuscular blockers were not used, the impact of them remains to be studied later on.
In conclusion, we showed that Entropy RE−SE difference cannot reliably be used as an indicator of nociception in patients anaesthetized with propofol, nitrous oxide, and remifentanil without NMBAs. EMG activity can contaminate the interpretation, especially by increasing SE values. Further research is needed to elucidate the effects of EMG in detail, and to study the effect of neuromuscular blocking agents on EMG signal.
References
- 1.Johansen JW, Sebel PS. Development and clinical application of electroencephalographic bispectrum monitoring. Anesthesiology. 2000;93:1336–44. doi: 10.1097/00000542-200011000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Vivien B, Di Maria S, Ouattara A, Langeron O, Coriat P, Riou B. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99:9–17. doi: 10.1097/00000542-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Paloheimo M, Edmonds HL, Jr, Wirtavuori K, Tammisto T. Assessment of anaesthetic adequacy with upper facial and abdominal wall EMG. Eur J Anaesthesiol. 1989;6:111–9. [PubMed] [Google Scholar]
- 4.Jäntti V, Sloan T. EEG and anesthetic effects. Intraoperative monitoring of neural function. In: Nuwer MR, editor. Handbook of Clinical Neurophysiology. Amsterdam: Elsevier BV; 2008. pp. 77–93. [Google Scholar]
- 5.Viertiö-Oja H, Maja V, Särkelä M, et al. Description of the Entropy™ algorithm as applied in the Datex-Ohmeda S/5™ Entropy module. Acta Anaesthesiol Scand. 2004;48:154–61. doi: 10.1111/j.0001-5172.2004.00322.x. [DOI] [PubMed] [Google Scholar]
- 6.Vakkuri A, Yli-Hankala A, Talja P, et al. Time–frequency balanced spectral entropy as a measure of anesthetic drug effect in central nervous system during sevoflurane, propofol, and thiopental anesthesia. Acta Anaesthesiol Scand. 2004;48:145–53. doi: 10.1111/j.0001-5172.2004.00323.x. [DOI] [PubMed] [Google Scholar]
- 7.Paloheimo MPJ, Wilson RC, Edmonds HL, Jr, Lucas LF, Triantafillou AN. Comparison of neuromuscular blockade in upper facial and hypothenar muscles. J Clin Monit. 1988;4:256–60. doi: 10.1007/BF01617322. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler P, Hoffman WE, Baughman VL, Koenig H. Response entropy increases during painful stimulation. J Neurosurg Anesthesiol. 2005;17:86–90. doi: 10.1097/01.ana.0000151408.62650.b5. [DOI] [PubMed] [Google Scholar]
- 9.Valjus M, Ahonen J, Jokela R, Korttila K. Response Entropy™ is not more sensitive than State Entropy™ in distinguishing the use of esmolol instead of remifentanil in patients undergoing gynaecological laparoscopy. Acta Anaesthesiol Scand. 2006;50:32–9. doi: 10.1111/j.1399-6576.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 10.Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanil. II. Model application. Anesthesiology. 1997;86:24–33. doi: 10.1097/00000542-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Brown SL, Schwartz GE. Relationships between facial electromyography and subjective experience during affective imaginery. Biol Psychol. 1980;11:49–62. doi: 10.1016/0301-0511(80)90026-5. [DOI] [PubMed] [Google Scholar]
- 12.Kazama T, Ikeda K, Morita K. Reduction by fentanyl of the Cp sub 50 values of propofol and hemodynamic responses to various noxious stimuli. Anesthesiology. 1997;87:213–27. doi: 10.1097/00000542-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Katoh T, Ikeda K. The minimum alveolar concentration of sevoflurane in humans. Anesthesiology. 1987;66:301–3. doi: 10.1097/00000542-198703000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kimura T, Watanebe S, Asakura N, Inomata S, Okada M, Taguchi M. Determination of end-tidal sevoflurane concentration for tracheal intubation and minimum alveolar anesthetic concentration in adults. Anesth Analg. 1994;79:378–81. doi: 10.1213/00000539-199408000-00032. [DOI] [PubMed] [Google Scholar]
- 15.Wilson-Pauwels L, Akesson EJ, Stewart PA. Cranial Nerves, Anatomy and Clinical Comments. Toronto: B.C. Decker Inc.; 1988. [Google Scholar]
- 16.van Boxtel A. Optimal signal bandwidth for the recording of surface EMG activity on facial, jaw, oral and neck muscles. Psychophysiology. 2001;38:22–34. [PubMed] [Google Scholar]
- 17.Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007;62:1266–80. doi: 10.1111/j.1365-2044.2007.05221.x. [DOI] [PubMed] [Google Scholar]