Abstract
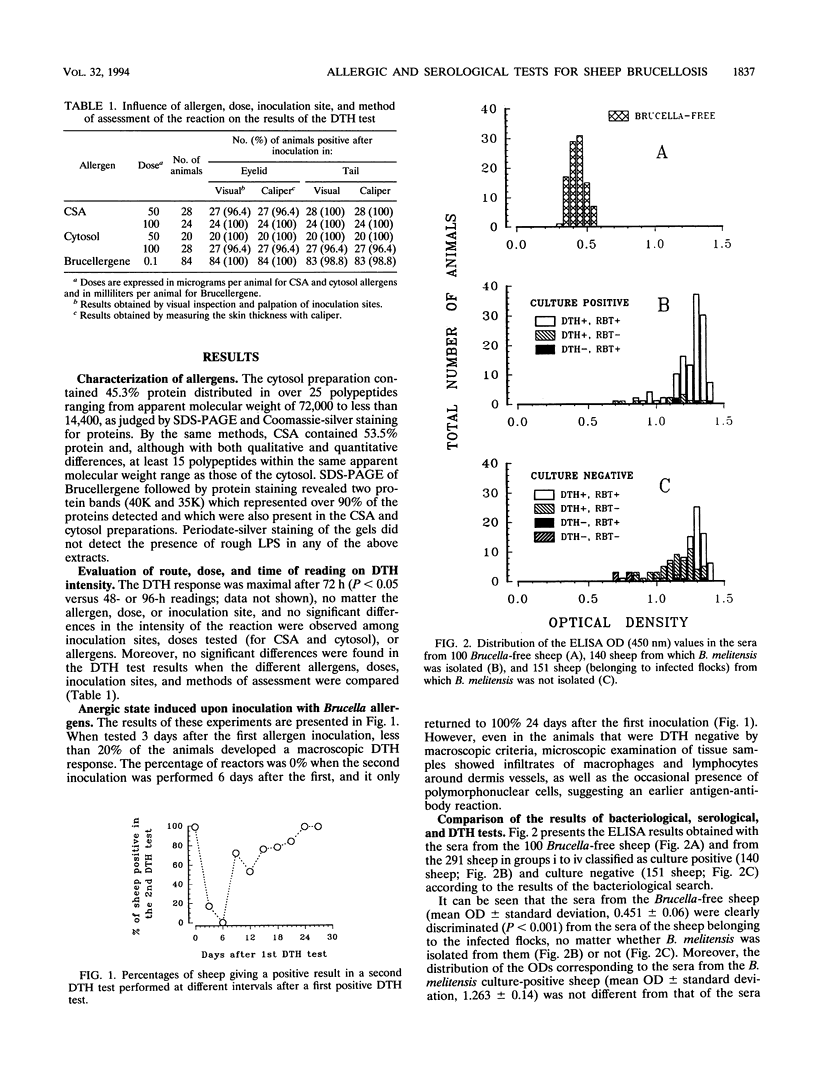
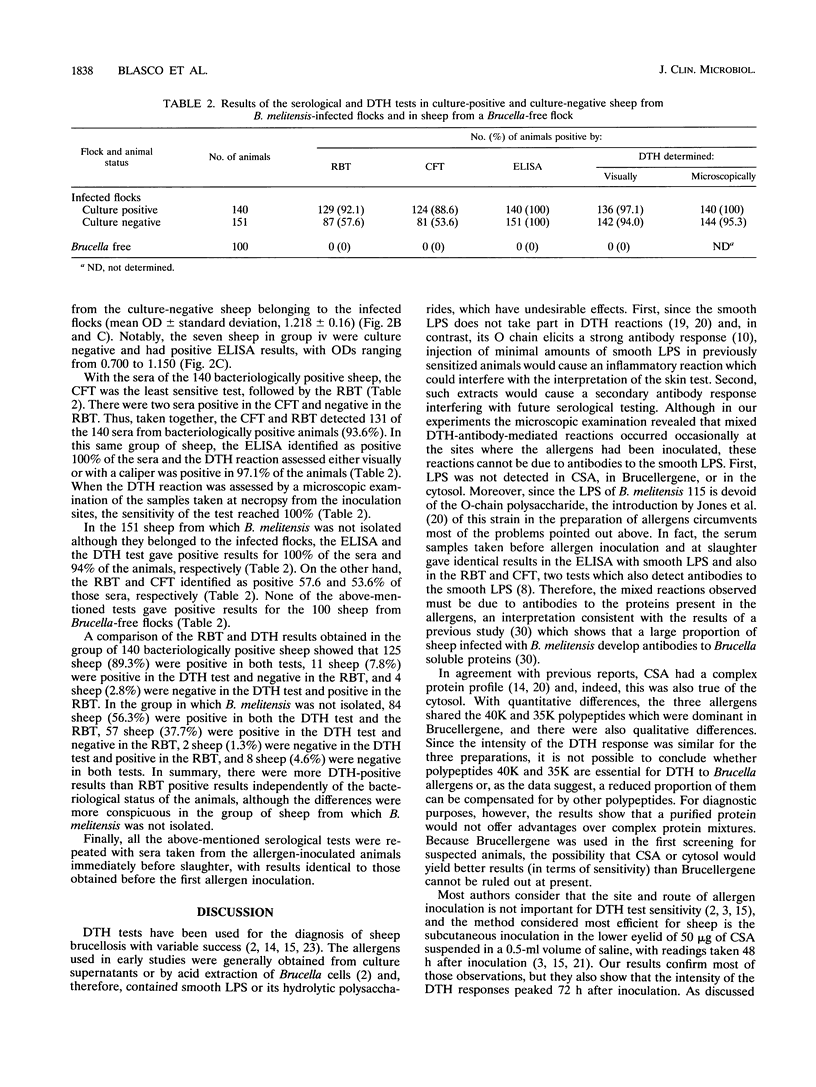
A total of 291 unvaccinated sheep from Brucella melitenesis-infected flocks were examined for delayed-type hypersensitivity (DTH) responses with Brucellergene commercial allergen and with cold saline extract and cytosol from rough B. melitensis 115, and their sera were tested in the rose bengal test (RBT), complement fixation test (CFT), and enzyme-linked immunosorbent assay (ELISA) with lipopolysaccharide. DTH reactions were maximal after 72 h, with no intensity differences among allergens, inoculation sites (eyelid and tail), and doses tested. There were no differences in the results recorded by visual inspection and palpation of inoculation sites, by measuring skin thickness with a caliper, or by microscopic examination of samples taken at necropsy. Six days after DTH testing, energy was observed in 100% of the animals, and 100% reactivity was recovered only after 24 days. All animals were necropsied, and thorough bacteriological searches were performed. The sensitivities found with the 140 animals from which B. melitensis was isolated were ELISA, 100%; DTH, 97.1%; RBT, 92.1%; and CFT, 88.6%. Those results put into question the value of RBT and CFT as screening and confirmatory tests for sheep brucellosis and at least indicate that their standardization should be modified. For 151 tested sheep from which B. melitensis was not isolated, the percentages of positive animals were ELISA, 100%; DTH, 94.0%; RBT, 57.6%; and CFT, 53.6%. All tests were negative for 100 tested sheep from Brucella-free flocks. The different results of bacteriological and immunological tests suggest the usefulness of developing indirect tests able to distinguish truly infected animals from those that have developed an immunological response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhongbhibhat N., Elberg S., Chen T. H. Characterization of brucella skin-test antigens. J Infect Dis. 1970 Jul-Aug;122(1):70–82. doi: 10.1093/infdis/122.1-2.70. [DOI] [PubMed] [Google Scholar]
- Blasco J. M., Garin-Bastuji B., Marin C. M., Gerbier G., Fanlo J., Jiménez de Bagués M. P., Cau C. Efficacy of different Rose Bengal and complement fixation antigens for the diagnosis of Brucella melitensis infection in sheep and goats. Vet Rec. 1994 Apr 16;134(16):415–420. doi: 10.1136/vr.134.16.415. [DOI] [PubMed] [Google Scholar]
- Brown G. M., Ranger C. R., Kelley D. J. Selective media for the isolation of Brucella ovis. Cornell Vet. 1971 Apr;61(2):265–280. [PubMed] [Google Scholar]
- De Moreno M. R., Smith J. F., Smith R. V. Silver staining of proteins in polyacrylamide gels: increased sensitivity through a combined Coomassie blue-silver stain procedure. Anal Biochem. 1985 Dec;151(2):466–470. doi: 10.1016/0003-2697(85)90206-4. [DOI] [PubMed] [Google Scholar]
- Diaz R., Levieux D. Rôle respiectif en sérologie de la brucellose bovine des antigènes et des immunoglobulines G 1 et G 2 dans les tests d'agglutination, de Coombs et au Rose Bengale ainsi que dans le phénomène de zone. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 6;274(10):1593–1596. [PubMed] [Google Scholar]
- Díaz-Aparicio E., Aragón V., Marín C., Alonso B., Font M., Moreno E., Pérez-Ortiz S., Blasco J. M., Díaz R., Moriyón I. Comparative analysis of Brucella serotype A and M and Yersinia enterocolitica O:9 polysaccharides for serological diagnosis of brucellosis in cattle, sheep, and goats. J Clin Microbiol. 1993 Dec;31(12):3136–3141. doi: 10.1128/jcm.31.12.3136-3141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadi A., Zowghi E. The use of allergic test in the diagnosis of Brucella melitensis infection in sheep. Br Vet J. 1983 Sep-Oct;139(5):456–461. doi: 10.1016/s0007-1935(17)30392-5. [DOI] [PubMed] [Google Scholar]
- Farrell I. D. The development of a new selective medium for the isolation of Brucella abortus from contaminated sources. Res Vet Sci. 1974 May;16(3):280–286. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Jiménez de Bagüs M. P., Marín C. M., Blasco J. M., Moriyón I., Gamazo C. An ELISA with Brucella lipopolysaccharide antigen for the diagnosis of B. melitensis infection in sheep and for the evaluation of serological responses following subcutaneous or conjunctival B. melitensis strain Rev 1 vaccination. Vet Microbiol. 1992 Feb;30(2-3):233–241. doi: 10.1016/0378-1135(92)90117-c. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Taylor A. G. Characterization of allergens prepared from smooth and rough strains of Brucella melitensis. Br J Exp Pathol. 1973 Oct;54(5):492–508. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loquerie R., Durand M. P. Applications cliniques d'un test cutané d'hypersensibilité retardée pour dépistage de la brucellose ovine et caprine au moyen d'un allergène commercial. Dev Biol Stand. 1984;56:407–410. [PubMed] [Google Scholar]
- Radunz B. L., Lepper A. W. Suppression of skin reactivity to bovine tuberculin in repeat tests. Aust Vet J. 1985 Jun;62(6):191–194. doi: 10.1111/j.1751-0813.1985.tb07294.x. [DOI] [PubMed] [Google Scholar]
- Trap D., Gaumont A. J. Le diagnostic sérologique de la brucellose bovine et ovine par l'épreuve à l'antigène tamponné. Dev Biol Stand. 1976;31:136–140. [PubMed] [Google Scholar]
- Trap D., Gaumont R. Comparaison entre électrosynérèse et épreuves sérologiques classiques dans le diagnostic de la brucellose ovine. Ann Rech Vet. 1982;13(1):33–39. [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]


