Abstract
Inflammatory activation of monocytes is an essential part of both innate immune responses and the pathogenesis of conditions such as atherosclerosis. However, the mechanisms which modulate the response of monocytes to inflammatory stimuli are still poorly understood. Here, we report that tribbles-2 (trb-2) is a novel regulator of inflammatory activation of monocytes. Down-regulation of trb-2 levels potentiates LPS-induced IL-8 production via enhanced activation of the extracellular signal-regulated kinase and jun kinase mitogen-activated protein kinase (MAPK) pathways. In keeping with this, the endogenous level of trb-2 expression in human primary monocytes is inversely correlated to the cell’s ability to produce IL-8. We show that trb-2 is a binding partner and a negative regulator of selected MAPKs. The potential in vivo relevance of these findings is highlighted by the observation that modified low-density lipoprotein profoundly down-regulates trb-2 expression, which may, in turn, significantly contribute to the inflammatory processes in the development of vascular disease. Taken together, our results define trb-2 as a potent novel regulator of monocyte biology, controlling the activation of these cells.
Keywords: inflammation, IL-8, MAP kinases, tribbles
Introduction
The family of tribbles proteins has recently been identified as potent regulators of signal processing in a number of physiological and pathological processes. Of the three vertebrate tribbles, tribbles-2 (trb-2) was first described as a gene up-regulated by mitogens in dog thyroid cells (1, 2). Most of the recent data on this gene and its protein product are observational, describing differential expression in prostate cancer (3), autoimmune uveitis (4) and inflammatory conditions (5). Functional studies on trb-2 have demonstrated its involvement in the progression of mitosis in Xenopus embryos and that it is necessary for the normal development of the eye and the neuronal system (6). A role for trb-2 in cell division is suggested as gene expression is up-regulated in a subset of acute myeloid leukaemias (AML), and retroviral over-expression of trb-2 induces AML in mice possibly via enhancing degradation of certain C/EBP protein forms (7, 8). These results are in line with previous work in Drosophila, which demonstrated that levels of the fly homologue of C/EBP (slbo) are critical for programmed mitosis and that slbo turnover is regulated by tribbles (9).
While the potential importance of tribbles is implicated by these reports and other literature, less is known about the molecular mechanism of their action. Members of tribbles family have been reported to interact and modulate the activity of signal transduction pathways, including the phosphoinositide 3-kinase (PI3K)/Akt (10, 11) and the mitogen-activated protein kinase (MAPK) (12, 13) systems. The functional relevance of tribbles-mediated regulation of these pathways has been highlighted by a recent report suggesting that trb-2 controls adipocyte differentiation (14). We have shown recently that trb-1 regulates the proliferative capacity of vascular smooth muscle cells via action on MAPK pathways and found this gene to be up-regulated in the wall of atherosclerotic arteries (15). These observations prompted us to further investigate the potential involvement of tribbles proteins in cell types, which play a central role in innate immune responses as well as in the chronic inflammatory disease of the vessel wall.
Monocytes play a central role in the initiation of innate inflammatory responses. Once activated by pathogenic stimuli or cytokines, they normally reside at the sites of inflammation, differentiate to macrophages and coordinate local responses via the expression of a range of cytokines and chemokines, which attract and activate other cell types. It is therefore of crucial importance to the physiological resolution of inflammation to have effective control mechanisms in place which control monocyte/macrophage responses. Activation and recruitment of monocytes to the developing lesion of the arterial wall are essential in the progression of atherosclerosis. Once in the lesion, monocytes produce chemokines, such as IL-8 and cytokines, including IL-1, that further perpetuate the local inflammation. Monocytes differentiate into macrophages that can internalize oxidized low-density lipoprotein (oxLDL) particles, leading to their differentiation into foam cells. In addition, binding of modified LDL will itself regulate monocyte function.
Among others, activation of MAPK has been widely reported as a key intracellular signalling network, contributing to inflammatory activation of monocytes, as measured by the production of IL-8 chemokine, for instance (16–18). However, mechanisms for the down-regulation of these pathways are still ill defined. With the recent identification of tribbles proteins as regulators of MAPK activation (12, 13), we set out to investigate the role of tribbles in the inflammatory activation of monocytes. We (5) and others (1, 2, 19–21) have reported that tribbles expression is regulated by a range of stimuli, including stress, mitogens and cytokines, suggesting that control of signalling systems may be achieved through the modulation of expression of tribbles proteins.
We report here that expression of trb-2 is selectively down-regulated by acetylated low-density lipoprotein (AcLDL). The consequence of reduced trb-2 expression is the potentiation of IL-8 production both in THP-1 cells and in human primary monocytes. Our results demonstrate that trb-2 acts via inhibition of extracellular signal-regulated kinase (ERK) and jun kinase (JNK) activation. The molecular action of trb-2 is at the level of trb-2–mitogen activated protein kinase (MAPKK) complexes, MKK7 and MEK1, that are binding partners of this protein in monocyte/macrophages. Further, we show that the kinase-like domain of trb-2 is sufficient for binding to MAPKKs, as well as to exert its bioactivity. Co-stimulation of THP-1 cells as well as human primary monocytes with LPS and modified low-density lipoprotein (LDL) leads to the enhancement of IL-8 production, probably via down-regulation of trb-2. This raises the possibility that regulation of trb-2 levels could be a novel control mechanism of both physiological and pathological inflammatory activation of monocytes. Putting these observations together, we suggest that in vivo modulation of trb-2 expression may be an important regulatory mechanism in monocyte biology.
Methods
All the experiments described in this study were performed multiple times (N > 2) and representative datasets are shown.
Ethics
The human samples were obtained under the ethical approval granted by the North Sheffield Research Ethics Committee. This study conforms to the principles outlined in the Declaration of Helsinki.
Cells
THP-1 and Raw 264.7 cells were purchased from American Type Culture Collection and maintained in RPMI (Invitrogen, CA, USA) supplemented with 10% FCS (L-glutamine) and penicillin–streptomycin.
Plasmid constructs
Trb-1 and trb-2 expression plasmids were generated by using pCDNA3.1(+) (Invitrogen, Paisley, UK) as a backbone. Protein fragment complementation assay (PCA) fusion proteins were constructed as described before (15). To generate trb-2DN and -DC-truncated protein expression constructs, amino acid residues 1–60 and 309–343 were deleted by PCR-aided mutagenesis. All constructs have been fully sequence verified.
THP-1 transfection with siRNA
Transfections were performed using Nucleofector (Amaxa, Cologne, Germany) using program U-001 and Cell line Nucleofector Kit V solution (Amaxa). For most experiments, 1.0 × 106 cells were used per nucleofection.
siRNA SmartPool against human trb-2, MKK4, MKK7 and MEK1 were purchased from Dharmacon (Chicago, IL, USA) and used according to the manufacturer’s recommendation.
MAPK inhibitor treatment
MEK1 inhibitor (PD98059), p38 MAPK inhibitor (SB203580) and JNK MAPK inhibitor (SP600125) were purchased from Calbiochem (Gibbstown, NJ, USA) and used 20 μM for MEK1 and JNK MAPK inhibitors and 0.2 μM for p38 MAPK inhibitor. The cells were treated for 1 h with inhibitors before the LPS treatment.
Immunoblot
Anti-MKK7, Anti-MKK4 and Anti-MEK1 antibodies were purchased from Cell Signalling Technology (Danvers, MA, USA) and used according to the manufacturer’s recommendation. Polyclonal antibody against trb-2 was raised against the N-terminal region of trb-2 by standard techniques.
Protein fragment complementation assay
To confirm the physical interaction between trb-2 and MEK1, MKK4 and MKK7 in live monocytes, we used the yellow fluorescent protein (YFP)-based PCA (22, 23). This approach is based on the observation that the interaction of two fusion proteins, expressing the proteins of interest, coupled to the N- or C-terminal portion of EYFP will lead to re-folding of the YFP fragments and will generate a functional fluorophore. Therefore, by using this approach, the interaction of proteins can be visualized in live cells. The PCA plasmids trb-2-V2 and MEK1, MKK4/7 -V1 were generated by tagging trb-2 and MKKs with half of Venus mutant YFP (V1 and V2) as described before (24). The Venus variant of YFP was used in this assay since it provides a higher signal than EYFP. MKKs and Trb-2 were fused to the N-terminal (V1) or to the C-terminal portions of Venus YFP (V2), respectively. The two expression constructs (trb-2 and one of the MKKs) were co-transfected and the YFP signal was visualized by FACS (Figs 4C and 5A–D) and fluorescent microscopy (Fig. 4E). As controls, proteins expressing a leucine–zipper in fusion with the N- or C-terminal region of Venus protein (zip-V1 and Zip-V2, respectively) were used. When these two proteins are co-expressed, a strong fluorophore is formed. Therefore, we used this construct pair as a positive control. To detect the level of ‘background’ in this assay arising from unspecific interactions, the combination of zip-V1 and trb2-V2 constructs (which are not expected to interact) was co-expressed.
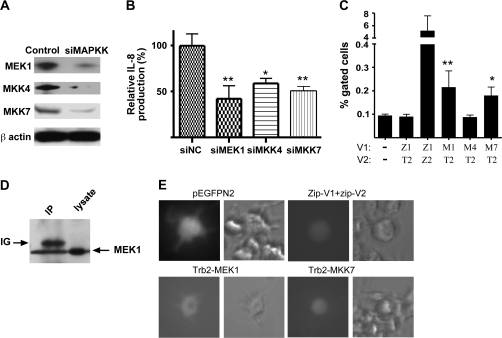
Fig. 4.
Trb-2 interacts with MEK1 and MKK7 but not with MKK4. (A) MEK1, MKK4 and MKK7 are endogenously expressed in THP-1 cells and their protein expression levels can be down-regulated by specific siRNAs (24 h post-transfection). (B) THP-1 cells were transfected with siMAPKK or control siRNA, as indicated, and stimulated by LPS. Production of IL-8 was detected by ELISA. One-way analysis of variance (ANOVA) with Dunnett’s Multiple Comparison Test was performed to analyse the results. *P < 0.05, **P < 0.01 (C) Interaction between trb-2 and the above MAPKKs was also investigated in THP-1 cells, using the MAPKK-V1 and trb2-V2 fusion protein expression constructs and analysed by FACS. Abbreviations: Z, zip; T, trb; M, MAPKK. Co-transfection of zip-v1/trb2-V2 pair was used as a negative control and the zip-v1/zip-v2 pair as a positive control. One-way ANOVA with Dunnett’s Multiple Comparison Test (with mock) was performed to analyse the results. *P < 0.05, **P < 0.01 (D) Interaction between MEK1 and trb-2 was detected in THP-1 cells by co-immunoprecipitation, which were transfected by trb2-V2 and MEK1 expression constructs. Trb-2 protein was precipitated by anti-GFP antibody and the binding partner was detected by an anti-MEK1 antibody (left lane). As control, total cell lysate was loaded and probed for MEK1 (right lane). Contamination of Ig heavy chain is indicated on the figure (E) Trb–MKK complexes were visualized in Raw264.7 cells. These cells were transfected with the indicated pair of expression vectors, using electroporation, as for the THP-1 cells. pEGFPN2 was used as a control of transfection efficiency and the zip-V1 and zip-2 constructs were co-transfected to as positive controls for PCA in these cells.
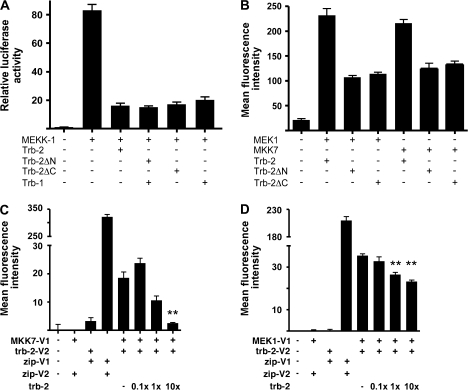
Fig. 5.
Trb-2 kinase-like domain is required for inhibiting AP-1 activation and binding to MKKs. (A) The ability of wild-type trb-2 and truncated protein forms, lacking either the N- or the C-terminal protein domain, to inhibit MEKK1-induced AP-1 activation was measured in a luciferase assay system, using HeLa cells as a test system, as before (12, 13). As a control for inhibition of AP-1 activation, wild-type trb-1 protein expression construct was used. (B) The capacity of trb-2 proteins, lacking the N- or C-terminal protein domains, was assessed by PCA, as above. MEK1 and MKK7 were expressed in fusion with the N-terminal V1 fragment of YFP; full-length and mutant trb-2 was fused to the C-terminal V2 fragment, as previously. (C) (D) FACS was used to show the specificity of interaction between trb-2 and MEK1 and MKK7. Three doses of untagged trb-2 expression plasmid (relative to the amount of trb2-V2) were co-transfected (1/10, 1/1 and 10/1) with the above PCA constructs and the mean fluorescence intensity was calculated in the varying samples. One-way analysis of variance with Dunnett’s Multiple Comparison Test (with no untagged trb-2 transfected) was performed to analyse the results. **P < 0.01.
Results
AcLDL reduces trb-2 expression in monocytes
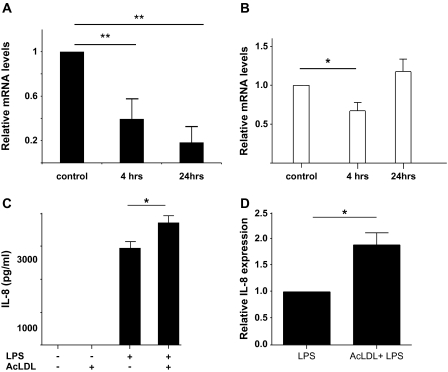
In order to identify molecular regulators of monocyte responses by modified LDL in the context of inflammation, we first characterized the regulation of trb-2 expression by AcLDL and LPS in a monocytic cell line, THP-1 cells. Given the recent reports highlighting an important regulatory function for trb-2 in myeloid cells (7, 8, 25), we focussed our studies on this member of the tribbles family. oxLDL and AcLDL share many basic mechanisms of their action, including receptors (although oxLDL may sometimes use additional receptors as well) and signalling pathways (26–28). THP-1 cells were incubated with AcLDL or LPS and expression of trb-2 was investigated by quantitative real-time PCR (Fig. 1A and B). While AcLDL incubation resulted in a profound down-regulation of trb-2 expression, LPS-induced activation resulted in a significant but transient down-regulation of trb-2 expression. Next, we tested whether AcLDL stimulation of THP-1 cells has an impact on inflammatory activation of monocytes, specifically on LPS-induced IL-8 production. Our results show that AcLDL treatment significantly potentiates maximally induced IL-8 production by LPS in these cells (Fig. 1C). AcLDL treatment alone did not induce IL-8 production, ruling out LPS contamination as a source of potentiation in the combined treatment. To validate our findings, the ability of human primary monocytes to produce IL-8 in response to LPS stimulation was similarly assessed. As shown in Fig. 1(D), co-incubation with AcLDL increased the amount of IL-8 produced in response to LPS, similar to that seen in the monocytic cell line, THP-1.
Fig. 1.
AcLDL and LPS modulates trb-2 expression and inflammatory activation of monocytes. (A–B) trb-2 messenger RNA expression was measured in response to AcLDL (A) and LPS (100 ng μl−1) (B) treatment (5 μg ml−1). In order to assess statistical significance, one-way analysis of variance (ANOVA) test with Dunnett’s Multiple Comparison Test was performed. *P < 0.05, **P < 0.01 (C) The impact of 24 h AcLDL and LPS treatment alone and in combination was studied on IL-8 protein production in THP-1 cells. Cells were lysed and IL-8 levels were quantified by ELISA (R&D Systems). One-way ANOVA with Dunnett’s Multiple Comparison Test was performed to analyse the results. *P < 0.05 (D) Responsiveness of primary monocytes from four healthy volunteers was assessed by stimulating cells with LPS or with co-stimulation by LPS and AcLDL. IL-8 levels, in response to LPS alone, were used as a unit for normalization for each donor and values measured in samples with LPS-AcLDL co-stimulation were expressed relative to these. AcLDL treatment alone did not induce the production of IL-8 (data not shown).
trb-2 expression controls IL-8 production of monocytes
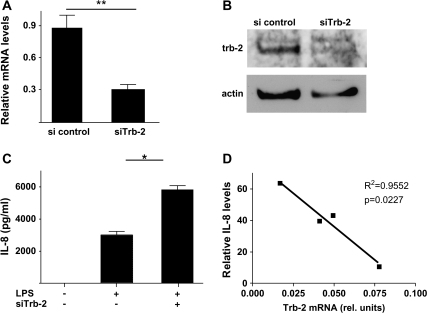
To examine whether the observed down-regulation of trb-2 is mechanistically involved in LPS-induced monocyte activation, we reduced trb-2 levels by transfecting siRNA against trb-2 and confirmed suppression as measured by trb-2 RNA (Fig. 2A) and protein expression (Fig. 2B). As a biologically relevant marker of monocyte activation, IL-8 production was induced in siRNA-transfected cells by LPS treatment and measured by ELISA. The results in Fig. 2(C) show that sitrb-2-treated THP-1 cells produced significantly higher levels of IL-8, compared with cells transfected with control siRNA. To further characterize the importance of trb-2 expression in monocytes, human primary monocytic cells were isolated from blood, stimulated with LPS and IL-8 production was assessed by ELISA. We found that the level of IL-8 inversely correlated to the expression of endogenous trb-2 in primary monocytes (Fig. 2D).
Fig. 2.
Down-regulation of trb-2 expression leads to increased IL-8 production. (A) The efficiency of trb-2 knockdown by siRNA was assessed using qRT–PCR [Student’s t-test was performed to analyse the results. **P < 0.01 (A)] or western blot (B). In both cases, β-actin was used as a housekeeping control. (C) The impact of reduced trb-2 levels on LPS-induced IL-8 production was measured by ELISA. Student’s t-test was performed to analyse the results. P = 0.041 (D) Responsiveness of primary monocytes from four healthy volunteers were assessed by stimulating cells with stimulation by LPS. The relative level of IL-8 produced is expressed as fold induction of unstimulated cells. The relationship between the amount of IL-8 produced and trb-2 expression levels was measured by linear regression. trb-2 levels were normalized to the β-actin qRT–PCR signal in the same sample.
Activation of MAPK pathways controls IL-8 production and is modulated by trb-2
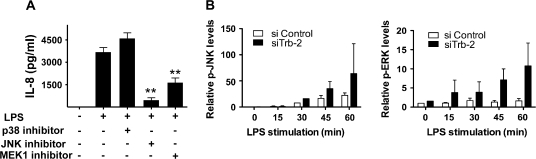
Previous reports have demonstrated that the expression of IL-8 is regulated by MAPK pathways (29–33). In agreement with this, blocking of JNK or MEK1, but not of p38 activity by pharmacological kinase inhibitors, resulted in the inhibition of LPS-induced IL-8 production (Fig. 3A). As the above data indicated a negative role for trb-2 in IL-8 production, we hypothesized that trb-2 may exert its effect through reducing MAPK activation. This was tested by comparing JNK and ERK activation in LPS-stimulated THP-1 cells at normal or reduced trb-2 levels (Fig. 3B). Western blotting analysis of MAPK phosphorylation levels showed that sitrb-2 treatment potentiated LPS-dependent MAPK activation. These observations are compatible with a negative regulatory role for trb-2 in control of inflammatory activation of specific MAPK pathways.
Fig. 3.
Trb-2 is an inhibitor of the activation of MAPK pathways in THP-1 cells. (A) The involvement of MAPK pathways in LPS-induced IL-8 production was measured by the use of inhibitors of specific MAPK pathways. (B) THP-1 cells were transfected with control- or trb-2-specific siRNA and the level of activation for MAPKs (ERK and JNK), contributing to IL-8 production, was assayed by western blotting. The images were digitized and the intensity of the signal quantified from at least three independent experiments. pMAPK levels were normalized to β-actin. Two-way analysis of variance was performed to assess statistical significance of the results. phospho-JNK: P = 0.0027; phospho-ERK: P = 0.0268.
Trb-2 modulates IL-8 production via interaction with MAPKKs
Previously, we demonstrated that tribbles-1 and -3 proteins interact with MAPKKs and regulate their activity (12, 13). The experiments shown above have demonstrated that reduced Trb-2 levels lead to elevated IL-8 production through enhanced activation of the JNK and ERK pathways. This suggests a similar mechanism of action to trb-1 and -3, for trb-2. Therefore, we investigated the interaction of trb-2 with MKK4/SEK-1, MKK7 and MEK1, known activators of JNK or ERK, respectively. Our results show that all three MAPKKs are endogenously expressed in THP-1 cells and that their expression can be inhibited by specific siRNA treatment (Fig. 4A). As expected, down-regulation of levels of these MAPKKs by siRNA led to impaired IL-8 production, in response to LPS (Fig. 4B), indicating that all three proteins contribute to the activation of IL-8 expression in these settings.
Next, we investigated whether trb-2 physically interacts with the above MAPKKs in monocytic cells by using protein fragment complementation assay (PCA) as described previously (24). Trb-2 binding to MKK7 and MEK1 but not to MKK4 was detected by FACS analysis (Fig. 4C). As an additional control, the MEK1–trb-2 complex was also detected in a co-immunoprecipitation system, as shown in Fig. 4(D). Further to the above, trb-2/MEK1 and trb-2–MKK7 complexes were visualized by fluorescent microscopy, in the macrophage cell line Raw264.7 cells, by PCA (Fig. 4E). In agreement with the FACS results, no interaction between trb-2 and MKK4 was detected in this assay (data not shown). Thus, we conclude that trb-2 specifically interacts with MEK1 and MKK7 but not with MKK4 in monocyte/macrophage cells.
The kinase-like domain of trb-2 is necessary for MAPKK binding and for the inhibition of activating protein-1 activation
Tribbles proteins are comprised of an N-terminal, proline-rich N-terminal domain, a central domain, which is similar to serine–threonine kinases but thought to be catalytically inactive, and a short C-terminal domain of unknown function (34). We have demonstrated previously that the central kinase-like domain of tribbles-1 is required for its ability to inhibit AP-1 activation, which is a well-characterized target of stress kinases (13). In addition, we showed recently that this domain is sufficient for the formation of trb-1–MKK4 complexes (15). However, there are no data available to date to evaluate whether the kinase-like domain is a generic requirement for tribbles action within the protein family. Here, we performed a similar analysis of trb-2–MAPKK complexes, using HeLa cells, where we have characterized and validated the biological mechanism of action of tribbles proteins previously (12). First, we expressed full-length or truncated trb-2 proteins, the latter lacking either the N- or the C-terminal tribbles domains. The ability of these proteins to inhibit activating protein-1 (AP-1) activation was assessed in a luciferase reporter system, where stress kinase pathways, leading to the phosphorylation and thus the activation of AP-1 transcription factor was induced by over-expressed MEKK1 (Stratagene, Pathdetect system). We have also shown previously that AP-1 activation by a range of cytokines is blocked by tribbles-3 in this system, thus validating the biological importance of tribbles activity in this assay. As shown in Fig. 5(A), both truncated trb-2 versions as well as the full-length protein inhibited AP-1 activation. In addition, PCA analysis demonstrated that both MEK1 and MKK7 are able to form complexes with the truncated trb-2 proteins (Fig. 5B). However, we note that the level of PCA signal was reduced when truncated trb-2 were expressed, suggesting that the terminal regions may contribute to the stability of tribbles–MKK complexes. Experiments testing this hypothesis are currently underway. The specificity of trb-2/MAPKK interactions, as detected by PCA, was assessed by FACS (Fig. 5C and D). Co-expression of an increasing amount of ‘unlabelled’ trb-2 led to a dose-dependent elimination of the YFP signal both in the trb-2/MKK7 (Fig. 5C) and trb-2–MEK1 (Fig. 5D) complexes, as detected by FACS. In addition, neither MKK-Venus nor trb-2-Venus fusion proteins interacted with their zip-Venus counterparts (these were used as positive control constructs in the system), which further supports the specific nature of this interaction.
Discussion
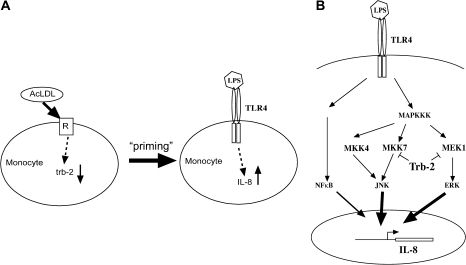
Appreciation of the molecular events occurring in response to signalling through pattern recognition receptors within monocytes is essential to understanding innate immunity, as well as conditions when these pathways and processes are subverted in disease pathogenesis. The data presented here illuminate the role of trb-2, a novel, negative regulator of MAPK signalling in the context of LPS stimulation of monocytes. AcLDL, which signals in a manner similar to oxLDL, down-regulates the expression of trb-2. This, in turn, leads to enhanced IL-8 production in LPS-stimulated monocyte cells. Data in other systems have revealed the capacity for tribbles proteins to interact with a range of cell signalling pathways, including members of the MAPK and PI3K network and a number of transcription factors [reviewed in (34, 35)]. We show here that IL-8 expression is controlled by specific MAPK pathways, as demonstrated by pharmacological inhibition of the ERK and JNK pathways and by siRNA-mediated suppression of MEK1 and MKK4, MKK7 expression, respectively. In line with the proposed inhibitory action of trb-2, siRNA-mediated down-regulation of trb-2 expression leads to a significant increase in JNK and ERK activation. Our experiments investigating physical interaction between proteins have shown that trb-2 can interact with MKK7 or MEK1, activators of ERK and JNK. These data indicate that trb-2 is an important negative regulator of monocyte IL-8 production in response to LPS and controls the augmentation of this response by AcLDL (Fig. 6A and B).
Fig. 6.
A model for the role of trb-2 in monocyte biology in inflammatory settings. (A) AcLDL uptake by monocytes triggers reduction of trb-2 expression, resulting in a hypersensitive state towards inflammatory stimuli, as exemplified by LPS-induced IL-8 production. (B) The molecular basis of trb-2 regulatory function of MAPKK pathways in the expression of IL-8.
We have shown that trb-2 comes into close physical proximity with MEK1 and MKK7 but not MKK4. This is in keeping with the findings of others which support the mechanism of action of tribbles through physical interaction with other signal transduction proteins. This has led to their description as scaffold/regulatory proteins. Our current observations are in strong support of this model. However, molecular details of tribbles/MAPKK interactions and the detailed mode of tribbles action on activation of kinase cascades remain to be determined. It is also noteworthy that trb-2 has been reported to be expressed primarily in the cytoplasm, while trb-1 and -3 are believed to be nuclear proteins (13, 34). Currently, there are no published data on the nature and relevance of interaction between MAPK pathways and trb-2. We suggest that the basic molecular mechanisms responsible for tribbles action are similar for trb-2 to those we have reported to trb-1 previously (12, 15). We have carried out preliminary analysis to characterize the domains of trb-2 protein necessary for the observed MAPK inhibitory activity and investigated, whether the domain minimally required for the inhibition of MAPK activation is also sufficient for the formation of MAPKK–trb-2 complexes. Our data demonstrate that the trb-2 kinase-like domain is sufficient for both activities. These results are in line with our previous reports on trb-1.
The experiments in this study provide evidence that tribbles, a novel group of proteins, can act as regulators of innate immune responses in monocytes. Modulation of such key components may be particularly attractive to pharmacotherapy as the predicted outcome of trb-2 up-regulation (or prevention of down-regulation) would be to reduce innate immune responsiveness without its total abolition. Based on this, we believe that further studies exploring the regulation of tribbles expression in inflammatory disease are warranted to determine whether inhibition of tribbles expression occurs in these conditions.
Funding
British Heart Foundation project grants (PG/02/122, PG/05/100); Short Term Visiting Fellowship of European Federation of Immunological Societies to K.E.
Acknowledgments
The authors thank Prof. Stephen Michnick for the Venus PCA constructs.
Glossary
Abbreviations
- AcLDL
acetylated low-density lipoprotein
- AML
acute myeloid leukaemias
- AP-1
activating protein-1
- ERK
extracellular signal-regulated kinase
- JNK
jun kinase
- LDL
low-density lipoprotein
- MAPK
mitogen-activated protein kinase
- oxLDL
oxidized low-density lipoprotein
- PCA
protein fragment complementation assay
- PI3K
phosphoinositide 3-kinase
- trb-1(2)
tribbles-1(2)
- YFP
yellow fluorescent protein
References
- 1.Wilkin F, Suarez-Huerta N, Robaye B, et al. Characterization of a phosphoprotein whose mRNA is regulated by the mitogenic pathways in dog thyroid cells. Eur. J. Biochem. 1997;248:660. doi: 10.1111/j.1432-1033.1997.t01-1-00660.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilkin F, Savonet V, Radulescu A, Petermans J, Dumont JE, Maenhaut C. Identification and characterization of novel genes modulated in the thyroid of dogs treated with methimazole and propylthiouracil. J. Biol. Chem. 1996;271:28451. doi: 10.1074/jbc.271.45.28451. [DOI] [PubMed] [Google Scholar]
- 3.Bisoffi M, Klima I, Gresko E, et al. Expression profiles of androgen independent bone metastatic prostate cancer cells indicate up-regulation of the putative serine-threonine kinase GS3955. J. Urol. 2004;172:1145. doi: 10.1097/01.ju.0000135117.40086.fa. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Davis JL, Li W. Identification of tribbles homolog 2 as an autoantigen in autoimmune uveitis by phage display. Mol. Immunol. 2005;42:1275. doi: 10.1016/j.molimm.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Sung HY, Francis SE, Crossman DC, Kiss-Toth E. Regulation of expression and signalling modulator function of mammalian tribbles is cell-type specific. Immunol. Lett. 2006;104:171. doi: 10.1016/j.imlet.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Saka Y, Smith JC. A Xenopus tribbles orthologue is required for the progression of mitosis and for development of the nervous system. Dev. Biol. 2004;273:210. doi: 10.1016/j.ydbio.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Keeshan K, He Y, Wouters BJ, et al. Tribbles homolog 2 inactivates C/EBPalpha and causes acute myelogenous leukemia. Cancer Cell. 2006;10:401. doi: 10.1016/j.ccr.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keeshan K, Shestova O, Ussin L, Pear WS. Tribbles homolog 2 (Trib2) and HoxA9 cooperate to accelerate acute myelogenous leukemia. Blood Cells Mol. Dis. 2008;40:119. doi: 10.1016/j.bcmd.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Rorth P, Szabo K, Texido G. The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol. Cell. 2000;6:23. doi: 10.1016/s1097-2765(05)00008-0. [DOI] [PubMed] [Google Scholar]
- 10.Iynedjian PB. Lack of evidence for a role of TRB3/NIPK as inhibitor of PKB-mediated insulin signaling in primary hepatocytes. Biochem. J. 2004;386:113. doi: 10.1042/BJ20041425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 12.Kiss-Toth E, Bagstaff SM, Sung HY, et al. Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J. Biol. Chem. 2004;279:42703. doi: 10.1074/jbc.M407732200. [DOI] [PubMed] [Google Scholar]
- 13.Kiss-Toth E, Wyllie DH, Holland K, et al. Functional mapping and identification of novel regulators for the Toll/interleukin-1 signalling network by transcription expression cloning. Cell. Signal. 2006;18:202. doi: 10.1016/j.cellsig.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Naiki T, Saijou E, Miyaoka Y, Sekine K, Miyajima A. TRB2, a mouse tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPbeta. J. Biol. Chem. 2007;282:24075. doi: 10.1074/jbc.M701409200. [DOI] [PubMed] [Google Scholar]
- 15.Sung HY, Guan H, Czibula A, et al. Human tribbles-1 controls proliferation and chemotaxis of smooth muscle cells via MAPK signaling pathways. J. Biol. Chem. 2007;282:18379. doi: 10.1074/jbc.M610792200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giri RK, Selvaraj SK, Kalra VK. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J. Immunol. 2003;170:5281. doi: 10.4049/jimmunol.170.10.5281. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72:847. [PubMed] [Google Scholar]
- 18.Marie C, Roman-Roman S, Rawadi G. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or Mycoplasma fermentans membrane lipoproteins. Infect. Immun. 1999;67:688. doi: 10.1128/iai.67.2.688-693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corcoran CA, Luo X, He Q, Jiang C, Huang Y, Sheikh MS. Genotoxic and endoplasmic reticulum stresses differentially regulate TRB3 expression. Cancer Biol. Ther. 2005;4:1063. doi: 10.4161/cbt.4.10.2205. [DOI] [PubMed] [Google Scholar]
- 21.Schwarzer R, Dames S, Tondera D, Klippel A, Kaufmann J. TRB3 is a PI 3-kinase dependent indicator for nutrient starvation. Cell. Signal. 2005;18:899. doi: 10.1016/j.cellsig.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Nyfeler B, Michnick SW, Hauri HP. Capturing protein interactions in the secretory pathway of living cells. Proc. Natl Acad. Sci. USA. 2005;102:6350. doi: 10.1073/pnas.0501976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remy I, Michnick SW. Mapping biochemical networks with protein-fragment complementation assays. Methods Mol. Biol. 2004;261:411. doi: 10.1385/1-59259-762-9:411. [DOI] [PubMed] [Google Scholar]
- 24.Remy I, Michnick SW. A cDNA library functional screening strategy based on fluorescent protein complementation assays to identify novel components of signaling pathways. Methods. 2004;32:381. doi: 10.1016/j.ymeth.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Argiropoulos B, Palmqvist L, Yung E, et al. Linkage of Meis1 leukemogenic activity to multiple downstream effectors including Trib2 and Ccl3. Exp. Hematol. 2008;36:845. doi: 10.1016/j.exphem.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J. Biol. Chem. 1991;266:2282. [PubMed] [Google Scholar]
- 27.Kamps JA, Kruijt JK, Kuiper J, van Berkel TJ. Characterization of the interaction of acetylated LDL and oxidatively modified LDL with human liver parenchymal and Kupffer cells in culture. Arterioscler. Thromb. 1992;12:1079. doi: 10.1161/01.atv.12.9.1079. [DOI] [PubMed] [Google Scholar]
- 28.Yancey PG, Miles S, Schwegel J, Jerome WG. Uptake and trafficking of mildly oxidized LDL and acetylated LDL in THP-1 cells does not explain the differences in lysosomal metabolism of these two lipoproteins. Microsc. Microanal. 2002;8:81. doi: 10.1017/s1431927601020013. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharyya A, Pathak S, Datta S, Chattopadhyay S, Basu J, Kundu M. Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem. J. 2002;368:121. doi: 10.1042/BJ20020555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobreva I, Waeber G, James RW, Widmann C. Interleukin-8 secretion by fibroblasts induced by low density lipoproteins is p38 MAPK-dependent and leads to cell spreading and wound closure. J. Biol. Chem. 2006;281:199. doi: 10.1074/jbc.M508857200. [DOI] [PubMed] [Google Scholar]
- 31.Fu Y, Luo N, Lopes-Virella MF. Upregulation of interleukin-8 expression by prostaglandin D2 metabolite 15-deoxy-delta12, 14 prostaglandin J2 (15d-PGJ2) in human THP-1 macrophages. Atherosclerosis. 2002;160:11. doi: 10.1016/s0021-9150(01)00541-x. [DOI] [PubMed] [Google Scholar]
- 32.Kibayashi E, Urakaze M, Kobashi C, et al. Inhibitory effect of pitavastatin (NK-104) on the C-reactive-protein-induced interleukin-8 production in human aortic endothelial cells. Clin. Sci. (Lond). 2005;108:515. doi: 10.1042/CS20040315. [DOI] [PubMed] [Google Scholar]
- 33.Scholz H, Yndestad A, Damas JK, et al. 8-Isoprostane increases expression of interleukin-8 in human macrophages through activation of mitogen-activated protein kinases. Cardiovasc. Res. 2003;59:945. doi: 10.1016/s0008-6363(03)00538-8. [DOI] [PubMed] [Google Scholar]
- 34.Hegedus Z, Czibula A, Kiss-Toth E. Tribbles: a family of kinase-like proteins with potent signalling regulatory function. Cell. Signal. 2007;19:238. doi: 10.1016/j.cellsig.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Hegedus Z, Czibula A, Kiss-Toth E. Tribbles: novel regulators of cell function; evolutionary aspects. Cell. Mol. Life Sci. 2006;63:1632. doi: 10.1007/s00018-006-6007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]