Abstract
Leukocyte telomere length (LTL) is ostensibly a biomarker of human aging. Cross-sectional analyses have found that LTL is relatively short in a host of aging-related diseases. These studies have also provided indirect estimates of age-dependent LTL shortening. In this paper, the authors report findings of the first comprehensive longitudinal study of 450 whites and 185 African Americans in Louisiana (aged 31.4 and 37.4 years at baseline (1995–1996) and follow-up (2001–2006) examinations, respectively) participating in the Bogalusa Heart Study. Rate of change in LTL was highly variable among individuals, with some displaying a paradoxical gain in LTL during the follow-up period. The most striking observation was that age-dependent LTL shortening was proportional to LTL at baseline examination. At both baseline and follow-up examinations, African Americans had longer LTLs than whites, and smokers had shorter LTLs than nonsmokers. The longer LTL in African Americans than in whites explained in part the faster rate of LTL shortening observed among African Americans. These findings underscore the complexity of leukocyte telomere dynamics in vivo and suggest that determinants in addition to the “end-replication problem” contribute to telomere shortening in vivo.
Keywords: aging, body mass index, leukocytes, oxidative stress, smoking, telomere
Leukocyte telomere length (LTL) is highly variable at birth (1, 2) and afterward (3–9). In cross-sectional analyses based primarily on comparisons of LTL in individuals of different ages, it seems that LTL progressively shortens linearly with age (3–9). However, cross-sectional analyses hardly capture the rate and trajectory of age-dependent LTL shortening in an individual, as well as variations in these parameters among individuals. Deciphering patterns of LTL shortening and variation in the rate of LTL shortening with age among individuals would be highly relevant because shortened LTL has often been detected in individuals with aging-related disorders, cardiovascular disease in particular (5, 9–12). Factors that predispose to cardiovascular disease, including obesity (4, 13, 14), insulin resistance (8, 13), and cigarette smoking (4, 6, 14), are also associated with shortened LTL. Moreover, a recent cross-sectional analysis observed that LTL was longer, but its age-dependent shortening was faster, in adult African Americans than in whites (15). For these reasons, we examined in a longitudinal study the factors that contribute to variation in LTL shortening among young African-American and white adults in the Bogalusa Heart Study (16). Our findings indicate that age-dependent LTL shortening is proportional to LTL.
MATERIALS AND METHODS
Participants
The Bogalusa Heart Study is a long-term investigation of the natural history of cardiovascular disease beginning in childhood in the biracial community (65% white, 35% African American) of Bogalusa, Louisiana (16). Between 1973 and 2006, 7 cross-sectional surveys of children aged 4–17 years and 9 cross-sectional surveys of adults aged 18–46 years, who had been previously examined as children, were conducted. In the ongoing Bogalusa Heart Study cohort, 1,203 adults aged 24–44 years were examined for cardiovascular risk factors between 2004 and 2006. LTL data were available for 635 participants, who had blood samples collected on 2 occasions—a baseline examination in 1995–1996 and a follow-up examination in 2001–2006. Compared with individuals not included, subjects with longitudinal LTL measurements showed no significant differences in the relevant study variables, except that they were 0.8 years older (P = 0.032). Self-administered questionnaires were used to obtain information on cigarette smoking; those who smoked at least 1 cigarette per week during the past 12 months were defined as current smokers. The study protocol was approved by the institutional review board of Tulane University (New Orleans, Louisiana), and each participant gave written informed consent.
LTL measurements
DNA from frozen blood samples, obtained at baseline and follow-up examinations, was extracted by using the Gentra Puregene Blood Kit (QIAGEN Inc., Valencia, California). DNA integrity was assessed following electrophoresis of 0.02 μg of DNA (200 V for 0.75 hours) on 1% agarose gels and staining with 2X SYBR Green I (Invitrogen Corporation, Carlsbad, California) for 45 minutes.
Telomere-length measurements were performed by obtaining the mean length of the terminal restriction fragments, using the Southern blot method, as previously described (1, 13). In brief, after overnight DNA digestion with 10 U Hinf I and 10 U Rsa I restriction enzymes (Roche Diagnostics Corporation, Indianapolis, Indiana), samples of DNA (3 μg each) were resolved on 0.5% agarose gels at 50 V for 16 hours. DNA was then depurinated in 0.25 N hydrochloric acid, denatured in 0.5 mol/L of sodium hydroxide per 1.5 mol/L of sodium chloride, and neutralized in 0.5 mol/L of tris(hydroxymethyl)aminomethane (pH 8) per 1.5 mol/L of sodium chloride. After transfer to a positively charged nylon membrane, DNA was hybridized to a digoxigenin 3′-end labeled 5′-(CCCTAA)3 telomeric probe (overnight at 65°C), washed 3 times in 2X saline-sodium citrate/0.1% of sodium dodecyl sulfate, and washed once in 2X saline-sodium citrate. Probe was detected by the digoxigenin luminescent detection procedure (Roche Diagnostics) after exposure on Hyperfilm (GE Healthcare, Chalfont St. Giles, United Kingdom). Each sample was resolved in duplicate on different gels, and autoradiograms were digitized for analysis of telomere-length measurement.
Baseline and follow-up terminal restriction fragments from the same individuals were resolved in adjacent lanes of each gel, with samples run in duplicate on different gels. The intraclass correlation coefficient of LTL was 0.972 (P < 0.0001), and the coefficient of variation for the duplicate samples was 1.4%. The laboratory that conducted the LTL measurements was blinded to the identity and characteristics of subjects. The rate of change (loss or gain) in LTL was computed as the difference between LTL value at follow-up minus that at baseline examinations divided by the number of years of follow-up.
Statistical analysis
Mean values and standard deviations were computed for baseline and follow-up LTL and other continuous covariate variables separately for African Americans and whites. LTLs adjusted for age and body mass index (BMI) were calculated as least-squares means for smokers and nonsmokers by holding age and/or BMI to their mean values. Analysis of covariance was performed by using generalized linear models to test differences in baseline and follow-up LTL between African Americans and whites and between smokers and nonsmokers. The relation between LTL attrition and baseline LTL was examined by linear regression models using rate of change in LTL as the dependent variable, adjusting for race, age, sex, BMI, and smoking. Statistical analyses were performed by using SAS version 9.0 software (SAS Institute, Inc., Cary, North Carolina). Three outliers (2 African-American women and 1 white man) whose rate of LTL shortening was more than 250 base pairs (bp)/year or LTL lengthening was more than 250 bp/year were excluded from the analyses.
RESULTS
General characteristics
Major characteristics of the cohort are summarized in Tables 1–3. The age ranges were 20.0–40.0 years at baseline and 25.7–48.2 years at follow-up examinations. Smokers constituted approximately a third of the cohort. African Americans had a higher BMI than whites. Compared with whites, African Americans had longer LTLs at baseline and follow-up examinations and a higher rate of LTL shortening. The follow-up period was slightly shorter for African Americans than for whites (5.7 years vs. 6.0 years), but this difference was not significant (P = 0.1180). Table 2 summarizes LTL parameters for smokers versus nonsmokers. Age-, BMI-, and race-adjusted LTL was significantly shorter in smokers versus nonsmokers by 141 bp (P = 0.0227) at baseline examination and by 164 bp (P = 0.0099) at follow-up examination. However, we found no significant difference in the rate of LTL shortening between smokers and nonsmokers.
Table 1.
Major Characteristics of the Cohort of Young Adults in the Bogalusa Heart Study in Louisiana at Baseline (1995–1996) and Follow-up (2001–2006) Examinationsa
| Parameter | Whites (n = 450) | African Americans (n = 185) | All (n = 635) | P Value (Race)b |
| Baseline examination | ||||
| Age, years | 31.4 (5.0) | 31.4 (5.3) | 31.4 (5.1) | 0.7904 |
| BMI, kg/m2 | 27.1 (6.4) | 30.5 (8.4) | 28.1 (5.1) | <0.0001 |
| Smokers, % | 32.4 | 36.8 | 33.7 | 0.2962 |
| LTLB, bp | 7,288 (735) | 7,847 (734) | 7,451 (777) | <0.0001 |
| Follow-up examination | ||||
| Age, years | 37.4 (4.4) | 37.0 (4.7) | 37.3 (4.5) | 0.5823 |
| BMI, kg/m2 | 28.3 (6.6) | 32.0 (8.9) | 29.4 (7.5) | <0.0001 |
| Smokers, % | 30.7 | 30.8 | 30.7 | 0.9715 |
| LTLFU, bp | 7,076 (721) | 7,603 (767) | 7,230 (772) | <0.0001 |
| Change in LTLc | ||||
| Follow-up, years | 6.0 (2.4) | 5.7 (2.5) | 5.9 (2.4) | 0.1180 |
| LTL, bp/year | −37.8 (41.3) | −47.7 (55.3) | −40.7 (46.0) | 0.0181 |
Abbreviations: BMI, body mass index; bp, base pairs; LTL, leukocyte telomere length (expressed in bp); LTLB, baseline LTL; LTLFU, follow-up LTL.
Unless otherwise specified, data are presented as mean (standard deviation).
P values for race difference in LTL and change in LTL were adjusted for age, sex, and BMI.
Rate of change in LTL (bp/year) = (LTLFU – LTLB)/follow-up years.
Table 2.
Leukocyte Telomere Length Parameters of Interest in Smokers Versus Nonsmokers Participating in the Bogalusa Heart Study in Louisiana at Baseline (1995–1996) and Follow-up (2001–2006) Examinations
| LTL Parameter | Nonsmokers | Smokers | P Value |
| Unadjusted LTL | |||
| LTLB, bp | 7,481 | 7,392 | 0.0660 |
| LTLFU, bp | 7,270 | 7,150 | 0.0219 |
| Age-adjusted LTL | |||
| LTLB, bp | 7,492 | 7,370 | 0.0479 |
| LTLFU, bp | 7,275 | 7,128 | 0.0196 |
| Age- and BMI-adjusted LTL | |||
| LTLB, bp | 7,500 | 7,359 | 0.0227 |
| LTLFU, bp | 7,280 | 7,116 | 0.0099 |
| Change, bp/yeara | −40 | −42 | 0.4630 |
Abbreviations: BMI, body mass index; bp, base pairs; LTL, leukocyte telomere length (expressed in bp); LTLB, baseline LTL; LTLFU, follow-up LTL.
Rate of change in LTL (bp/year) = (LTLFU − LTLB)/follow-up years.
Table 3.
Regression of Baseline (1995–1996) and Follow-up (2001–2006) Leukocyte Telomere Length on Parameters of Interest for Participants in the Bogalusa Heart Study in Louisianaa
| Parameter | Baseline LTL |
Follow-up LTL |
||||||
| β Coefficient | P Value | Partial R2 | Model R2 | β Coefficient | P Value | Partial R2 | Model R2 | |
| Race, African American | 587.8 | <0.0001 | 0.107 | 551.5 | <0.0001 | 0.096 | ||
| Age, years | −13.4 | 0.0220 | 0.009 | −11.7 | 0.0715 | 0.005 | ||
| BMI, kg/m2 | −9.1 | 0.0316 | 0.006 | −8.6 | 0.0312 | 0.007 | ||
| Smoking, yes/no | −141.6 | 0.0227 | 0.006 | −163.5 | 0.0099 | 0.008 | ||
| Sex, female | 56.8 | 0.3456 | 0.002 | 0.130 | 37.0 | 0.5372 | 0.000 | 0.116 |
Abbreviations: BMI, body mass index; LTL, leukocyte telomere length (expressed in base pairs (bp)).
The rate of change in LTL (bp/year) is negative; therefore, negative regression coefficients denote faster LTL attrition.
We next examined the contribution of race, age, smoking, BMI, and sex to variations in LTL at baseline and follow-up examinations. Table 3 shows that race was the main factor explaining variations in LTL at baseline and follow-up examinations and that smoking and BMI also accounted for some of the variations.
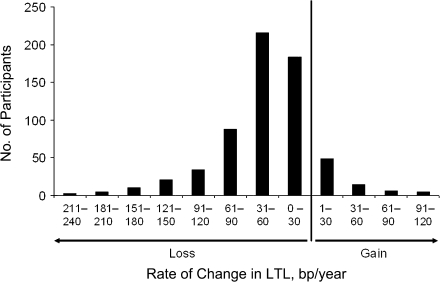
Figure 1 displays the distribution of rate of change in LTL among participants. This parameter varied considerably. The majority of individuals displayed LTL shortening (loss) (85.9% of African Americans and 88.0% of whites). In addition, 1 African American and 5 whites showed no change in LTL between baseline and follow-up examinations. However, a subset of the cohort showed LTL gain. The means for the rates of change for African Americans and whites are displayed in Table 1. For the entire cohort, the mean rate of change was −40.7 bp/year and was higher in African Americans than in whites (−47.7 bp/year vs. −37.8 bp/year, respectively; P = 0.0181).
Figure 1.
Distribution of the rate of change in leukocyte telomere length (LTL) in Louisiana study participants. A total of 561 participants showed LTL shortening, and 74 showed either gain (n = 68) or no change (n = 6) in LTL between baseline (1995–1996) and follow-up (2001–2006) examinations. bp, base pairs.
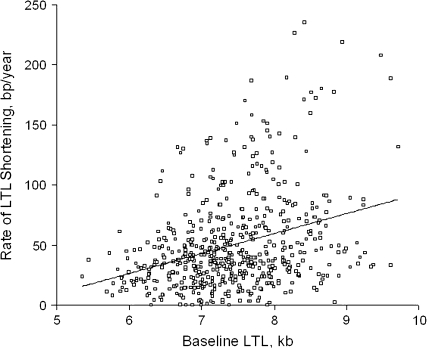
Given that different mechanisms are likely to govern LTL shortening versus gain, we separately examined LTL dynamics in individuals who did not gain LTL during the follow-up period. For these individuals (n = 561), the LTL shortening rate for African Americans was 60.0 (standard deviation, 47.6) bp/year and that for whites was 45.9 (standard deviation, 34.7) bp/year (P = 0.0003). Figure 2 displays the relation between the rate of LTL shortening that took place during the follow-up period and LTL derived from the baseline samples. A strong association was observed between the rate of LTL shortening and baseline LTL (a 17-bp/year increase in the rate of LTL shortening for each kilobase of LTL).
Figure 2.
Relation between rate of leukocyte telomere length (LTL) shortening during follow-up (2001–2006) and LTL derived from the baseline samples (1995–1996) in Louisiana study participants. Δ LTL = 0.017 baseline LTL – 0.073; r = 0.326, P < 0.0001. bp, base pairs.
To evaluate the potential influence of measurement error on the association of LTL and its rate of change, we set a conservative limit of quantification defined as the absolute value of the rate of change in LTL of less than 20 bp/year to denote detectable change. The association between baseline LTL and the rate of change was then examined for the subgroup with smaller (<20 bp/year loss or gain, n = 146) and larger (≥20 bp/year loss or gain, n = 489) changes in LTL. For the smaller-change group, the association was not significant (β = −0.05, P = 0.956), but, in the group with a larger change, the regression coefficient was significant (β = −13.7, P < 0.0001). This subgroup analysis provides confidence that measurement error did not play a significant role in the association noted in this study.
Since African Americans had longer LTL at baseline and follow-up examinations and a higher rate of LTL shortening, we examined the race effect and other pertinent factors on the variations in LTL rate of change for the entire cohort and separately for those individuals who demonstrated no gain in LTL during the follow-up period. The findings are summarized in Table 4. When considered alone, race significantly contributed to the variations in rate of change in LTL. However, when race and LTL at baseline examination were analyzed jointly, only LTL at baseline examination accounted significantly for the rate of change in LTL, meaning that the longer LTL in African Americans was a major factor in the higher rate of LTL shortening in African Americans versus whites. Adding age, BMI, smoking, and sex at baseline examination to the model provided little explanation above that of baseline LTL for interindividual variation in LTL shortening rate. These observations held when the data were analyzed for the entire cohort or after exclusion of individuals who gained LTL between baseline and follow-up examinations.
Table 4.
Regression of the Rate of Change in Leukocyte Telomere Length on Parameters of Interest for Participants in the Bogalusa Heart Study in Louisianaab
| Entire Cohort (n = 635) |
Subjects With no Gain in LTL (n = 561) |
|||||||
| β Coefficient | P Value | Partial R2 | Model R2 | β Coefficient | P Value | Partial R2 | Model R2 | |
| Model I | ||||||||
| Race, African American | −9.9 | 0.0135 | 0.010 | 0.010 | −14.1 | <0.0001 | 0.026 | 0.026 |
| Model II | ||||||||
| Race, African American | −4.6 | 0.2772 | 0.010 | −5.9 | 0.1070 | 0.026 | ||
| LTLB, bp | −9.6 | <0.0001 | 0.023 | 0.033 | −15.6 | <0.0001 | 0.084 | 0.110 |
| Model III | ||||||||
| Race, African American | −3.4 | 0.4309 | 0.010 | −4.4 | 0.2424 | 0.026 | ||
| LTLB, bp | −9.9 | <0.0001 | 0.023 | −16.1 | <0.0001 | 0.084 | ||
| Age, years | −0.6 | 0.1120 | 0.003 | −0.6 | 0.0605 | −0.007 | ||
| BMI, kg/m2 | −0.2 | 0.5464 | 0.000 | −0.3 | 0.1288 | 0.004 | ||
| Smoking, yes/no | −3.6 | 0.3533 | 0.000 | −0.5 | 0.8760 | 0.000 | ||
| Sex, female | −3.6 | 0.3378 | 0.002 | 0.038 | −1.3 | 0.6885 | 0.000 | 0.121 |
Abbreviations: BMI, body mass index; bp, base pairs; LTL, leukocyte telomere length (expressed in bp); LTLB, baseline LTL; LTLFU, follow-up LTL.
The rate of change in LTL (bp/year) = (LTLFU − LTLB)/follow-up years.
The rate of change in LTL is negative; therefore, negative regression coefficients denote faster LTL attrition.
DISCUSSION
Our key finding in this biracial cohort of young adults was that age-dependent LTL shortening rate was proportional to LTL. In addition, we confirmed previous observations in a small sample of the Bogalusa Heart Study with a 10-year follow-up showing that the rate of LTL shortening was highly variable among individuals, with a subset of participants displaying paradoxical lengthening of LTL during the follow-up period (13). A previous cross-sectional analysis of LTL in a combined sample of participants from the Bogalusa Heart Study and the NHLBI Family Heart Study (age range, 19–93 years) found not only that LTL was longer in African Americans than in whites but also that the rate of age-dependent LTL shortening was faster in African Americans (15). The present work indicates that the longer LTL in African Americans partially explained their faster age-dependent LTL shortening compared with that of whites.
Cross-sectional analyses of leukocyte telomere dynamics have suggested that age-dependent LTL shortening might be slower (17, 18) or faster (19) in young than in older adults, but these studies were statistically underpowered to test either of these suppositions (20). Note that, in the present study, cross-sectional analysis revealed only a small effect of age on LTL attrition (Table 3), but this finding was due to the considerable interindividual variation in LTL and the relatively narrow age range of the cohort (20 years for baseline examination and 23 years for follow-up examinations) (20).
What, then, might be the reason for the dependency of leukocyte telomere shortening on LTL itself? In cultured somatic cells, telomeres become shortened with cell division. The same apparently holds for somatic cells in vivo, given that telomere length is substantially longer in skeletal muscle, a postmitotic tissue, than in proliferative tissues (21). The inability of DNA polymerase to replicate nuclear DNA to its terminus—the so-called end-replication problem—would result in telomere shortening with somatic cell division (22, 23), a process that is autonomous of telomere length. However, both theoretical considerations and empirical data suggest that the “end-replication problem” accounts for only a fraction of telomere shortening with replication (24–26).
Telomere length is variable not only among different chromosomes (27) but also between homologous chromosomes (28), and it shortens faster in the homologous chromosome with the longer telomeres. This finding suggests that telomere shortening might depend on telomere length. Telomere shortening that is dependent on telomere length would maintain the length proportionality among different telomeres. In contrast, replication-mediated clipping of a fixed stretch of telomere repeats from telomeres of different lengths would result in loss of proportionality between telomere length in a single chromosome and the mean telomere length of all chromosomes. This possibility does not seem to be the case; proportionality between the length of telomeres in single chromosomes and the mean length of telomeres in all chromosomes is maintained not only in sperm (29) but also in somatic cells such as leukocytes from donors of a wide age range (30). The factor that might cause proportional telomere shortening is oxidative stress (31–34) because longer telomeres are bigger targets for free radicals, which attack the G triplets on the telomeres.
The following 2 suppositions are at the heart of studies exploring the use of LTL as a biomarker of human aging: 1) oxidative stress and inflammation are key elements in the biology of aging and in aging-related disorders (35, 36); and 2) leukocyte telomere dynamics register the accruing burden of oxidative stress and inflammation over the life course of the individual (reviewed by Aviv (37)). Thus, the shortened LTL displayed by individuals who suffer from aging-related diseases presumably results from an accelerated rate of age-dependent LTL shortening. This presumption is based on findings that oxidative stress augments telomere attrition per replication (31–34) and the inflammation heightens the turnover of leukocytes, which would further increase LTL shortening with age. Such a concept supports the tenet that the shortened LTL in persons with atherosclerotic cardiovascular disease (5, 9–12) is due to a protracted increase in the inflammatory and oxidative stress burdens (38, 39).
The findings of the present study support previous observations that LTL was shorter in smokers than in nonsmokers (4, 6, 14) and was inversely correlated with BMI (4, 9, 13–15), presumably because smoking and obesity are associated with accelerated LTL shortening. However, as shown in Table 2, we could not demonstrate a statistically significant difference in the rate of LTL shortening for smokers versus nonsmokers. One potential explanation might be that our method was insufficiently sensitive to detect differences in the rates of LTL shortening between smokers and nonsmokers, although the gap in LTL between the 2 groups did increase with age. Theoretically, LTL might be rapidly shortened after an individual starts smoking. Such rapid shortening would attenuate the rate of shortening thereafter because of the proportionality of LTL loss to LTL itself.
As indicated in our previous work (13), we are uncertain as to the reason for the gain in LTL in a small subset of the cohort. We doubt that it relates to technical matters. Age-dependent LTL shortening would largely mirror telomere attrition in hematopoietic stem cells and progenitor cells, assuming that telomere shortening downstream of hematopoietic stem cells/progenitor cells is relatively constant. However, this constancy might not hold all the time. For instance, acute infection might transiently increase proliferation of peripheral mononuclear cells, resulting in a temporary increase in the difference between LTL and telomere length in hematopoietic stem cells/progenitor cells. This transient effect would not shorten by much telomere length in hematopoietic stem cells/progenitor cells over the life course of the individual, but it might affect LTL results in a longitudinal study of short duration. Another alternative relates to the status of hematopoietic stem cells within bone marrow niches. Evidently, hematopoietic stem cells residing in the osteoblastic niche are largely quiescent; their mobilization into the vascular niche, which might arise from a host of factors, promotes their transformation into proliferative hematopoietic stem cells (40). It might be possible that, in a small subset of participants, a crop of quiescent hematopoietic stem cells were mobilized into the vascular niche between baseline and follow-up examinations, resetting LTL above its length at the baseline examination. Both of these possibilities, if present, would not only account for LTL gain but also contribute to variation in LTL shortening during the follow-up period.
The dependence of LTL shortening on LTL suggests that the rate of LTL shortening declines in older individuals. However, the factors that affect LTL dynamics in the individual are anything but constant throughout adulthood because the precursors of inflammation and oxidative stress progressively increase with age (41–43). Individually or jointly, inflammation and oxidative stress might therefore sustain or even accelerate the rate of LTL shortening in the elderly, even though LTL progressively shortens with age. Accordingly, only longitudinal evaluations of LTL shortening rates in different age groups and diverse sample populations would provide a reliable appraisal of leukocyte telomere dynamics as humans get older.
Regarding potential regression to the mean effect, baseline telomere length was not relevant to the inclusion of subjects in the study. In terms of average change, any masking effect of LTL dynamics was not an issue because strong biologic arguments support LTL shortening with age. The potential latent effect of race may underlie some of our results, but race was independent of inclusionary criteria in the study and was used to further stratify the analysis. In terms of induced correlation between baseline and change from baseline, age adjustment of only those changes from baseline, as well as the existing high level of variation in LTL at baseline and changes from baseline, limited this effect. The use of analysis of covariance also limited unforeseen regression to the mean effects.
In conclusion, this comprehensive longitudinal study of age-dependent LTL shortening underscores the complexity of leukocyte telomere dynamics. Its findings support the role of mechanisms additional to the end-replication problem in telomere dynamics in vivo and explain in part the faster rate of LTL shortening in African Americans than in whites. LTL itself accounts for approximately 10% of the variation in LTL shortening, meaning that a host of other factors influence leukocyte telomere dynamics in both African Americans and whites. However, the effect of LTL on its own shortening rate is sizable and requires careful consideration in longitudinal studies that examine leukocyte telomere dynamics. Such studies might provide valuable information about the biology of human aging.
Acknowledgments
Author affiliations: The Center of Human Development and Aging, University of Medicine and Dentistry of New Jersey, New Jersey Medical School, Newark, New Jersey (Abraham Aviv, Jeffrey P. Gardner, Masayuki Kimura, Michael Brimacombe, Xiaojian Cao); and Tulane Center for Cardiovascular Health, Tulane University Health Sciences Center, New Orleans, Louisiana (The Bogalusa Heart Study) (Wei Chen, Sathanur R. Srinivasan, Gerald S. Berenson).
Supported by R01 grants AG16592 and AG020132 from the National Institute on Aging.
The authors thank Dr. Woodring Wright for his insightful suggestions.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- bp
base pairs
- LTL
leukocyte telomere length
References
- 1.Okuda K, Bardeguez A, Gardner JP, et al. Telomere length in the newborn. Pediatr Res. 2002;52(3):377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Akkad A, Hastings R, Konje JC, et al. Telomere length in small-for-gestational-age babies. BJOG. 2006;113(3):318–323. doi: 10.1111/j.1471-0528.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 3.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55(5):876–882. [PMC free article] [PubMed] [Google Scholar]
- 4.Valdes A, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 5.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 6.Nawrot TS, Staessen JA, Gardner JP, et al. Telomere length and possible link to X chromosome. Lancet. 2004;363(9408):507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 7.Njajou OT, Cawthon RM, Damcott CM, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104(29):12135–12139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 10.Benetos A, Gardner JP, Zureik M, et al. Short telomeres are associated with increased carotid artery atherosclerosis in hypertensive subjects. Hypertension. 2004;43(2):182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 11.Cawthon RM, Smith KR, O'Brien E, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 12.van der Harst P, van der Steege G, de Boer RA, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49(13):1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Gardner JP, Li S, Srinivasan SR, et al. Rise in insulin resistance and gain in adiposity is associated with escalated telomere attrition. Circulation. 2005;111(17):2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 14.Vasan RS, Demissie S, Kimura M, et al. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: the Framingham Heart Study. Circulation. 2008;117(4):1138–1144. doi: 10.1161/CIRCULATIONAHA.107.731794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt SC, Chen W, Gardner JP, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7(4):451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Bogalusa Heart Study 20th Anniversary Symposium. Am J Med Sci. 1995;310:S1–S138. doi: 10.1097/00000441-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95(10):5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordffjäll K, Larefalk A, Limdgern P, et al. Telomere length and heredity: indications of paternal inheritance. Proc Natl Acad Sci U S A. 2005;102(45):16374–16378. doi: 10.1073/pnas.0501724102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unryn BM, Cook LS, Riabowol KT. Paternal age is positively linked to telomere length of children. Aging Cell. 2005;4(2):97–101. doi: 10.1111/j.1474-9728.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 20.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35(6):1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 21.Gardner JP, Kimura M, Chai W, et al. Telomere dynamics in macaques and humans. J Gerontol A Biol Sci Med Sci. 2007;62(4):367–374. doi: 10.1093/gerona/62.4.367. [DOI] [PubMed] [Google Scholar]
- 22.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymatic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41(1):181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 23.Watson ID. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 24.op den Buijs J, van den Bosch PP, Musters MW, et al. Mathematical modeling confirms the length-dependency of telomere shortening. Mech Ageing Dev. 2004;125(6):437–444. doi: 10.1016/j.mad.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 25.op den Buijs J, Musters M, Verrips T, et al. Mathematical modeling of vascular endothelial layer maintenance: the role of endothelial cell division, progenitor cell homing, and telomere shortening. Am J Physiol Heart Circ Physiol. 2007;287(6):H2651–H2658. doi: 10.1152/ajpheart.00332.2004. [DOI] [PubMed] [Google Scholar]
- 26.Proctor CJ, Kirkwood TB. Modeling telomere shortening and the role of oxidative stress. Mech Ageing Dev. 2002;123(4):351–363. doi: 10.1016/s0047-6374(01)00380-3. [DOI] [PubMed] [Google Scholar]
- 27.Martens UM, Zijlmans JM, Poon SS, et al. Short telomeres on human chromosome 17p. Nat Genet. 1998;18(1):76–80. doi: 10.1038/ng0198-018. [DOI] [PubMed] [Google Scholar]
- 28.Goldman F, Bouarich R, Kulkarni S, et al. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc Natl Acad Sci U S A. 2005;102(47):17119–17124. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baird DM, Britt-Compton B, Rowson J, et al. Telomere instability in the male germline. Hum Mol Genet. 2006;15(1):45–51. doi: 10.1093/hmg/ddi424. [DOI] [PubMed] [Google Scholar]
- 30.Kimura M, Barbieri M, Gardner JP, et al. Leukocytes of exceptionally old persons display ultra-short telomeres. Am J Physiol Regul Integr Comp Physiol. 2007;293(6):R2210–R2217. doi: 10.1152/ajpregu.00615.2007. [DOI] [PubMed] [Google Scholar]
- 31.Forsyth NR, Evans AP, Shay JW, et al. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell. 2003;2(5):235–243. doi: 10.1046/j.1474-9728.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 32.Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol. 2007;42(11):1039–1042. doi: 10.1016/j.exger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Tchirkov A, Lansdorp PM. Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Hum Mol Genet. 2003;12(3):227–232. doi: 10.1093/hmg/ddg023. [DOI] [PubMed] [Google Scholar]
- 34.Sitte N, Saretzki G, von Zglinicki T. Accelerated telomere shortening in fibroblasts after extended periods of confluency. Free Radic Biol Med. 1998;24(6):885–893. doi: 10.1016/s0891-5849(97)00363-8. [DOI] [PubMed] [Google Scholar]
- 35.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Finch C, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 37.Aviv A. Telomeres and human somatic fitness. J Gerontol A Biol Sci Med Sci. 2006;61(8):871–873. doi: 10.1093/gerona/61.8.871. [DOI] [PubMed] [Google Scholar]
- 38.Hansson GK, Libby P. The immune response in atherosclerosis; a double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 39.Harrison D, Griendling KK, Landmesser U, et al. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91(3A):7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 40.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116(5):1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5(2):136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Caruso C, Lio D, Cavallone L, et al. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 43.Dröge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6(3):361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]