Abstract
It has been argued (e.g., the Wilcox-Russell hypothesis) that (low) birth weight is a correlate of adverse birth outcomes but is not on the “causal” pathway to infant mortality. However, the US national policy for reducing infant mortality is to reduce low birth weight. If these theoretical views are correct, lowering the rate of low birth weight may have little effect on infant mortality. In this paper, the authors use the “covariate density defined mixture of logistic regressions” method to formally test the Wilcox-Russell hypothesis that a covariate which influences birth weight, in this case maternal age, can influence infant mortality directly but not indirectly through birth weight. The authors analyze data from 8 populations in New York State (1985–1988). The results indicate that among the populations examined, 1) maternal age significantly influences the birth weight distribution and 2) maternal age also affects infant mortality directly, but 3) the influence of maternal age on the birth weight distribution has little or no effect on infant mortality, because the birth-weight-specific mortality curve shifts accordingly to compensate for changes in the birth weight distribution. These results tend to support the Wilcox-Russell hypothesis for maternal age.
Keywords: birth weight, infant mortality, latent variable, logistic regression, mixture of normal distributions
Current national health policy is to lower US infant mortality by reducing the rate of low birth weight, as stated in Healthy People 2010 (1). This is supported by a large body of literature demonstrating that low birth weight is a risk factor for infant mortality (2–7). Nevertheless, theoreticians often question whether birth weight lies within the “causal” pathway to infant mortality or is simply an indicator of adverse conditions (7–14). If these theoretical views are correct, prevention strategies that target birth weight might not have the intended effect of lowering infant mortality.
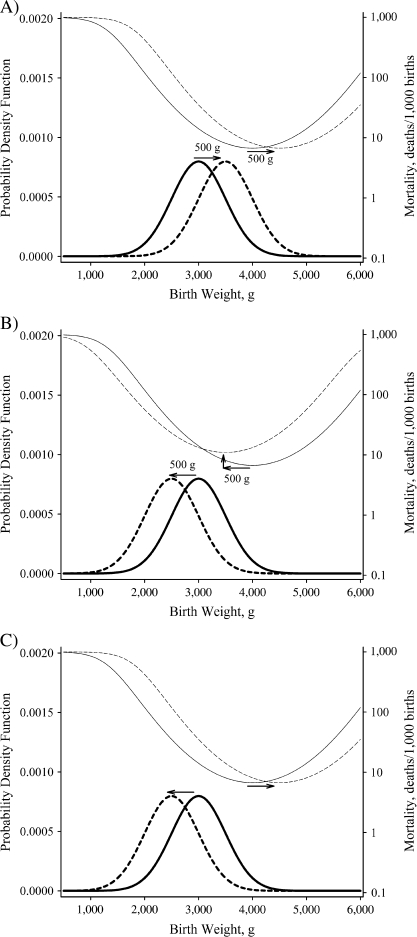
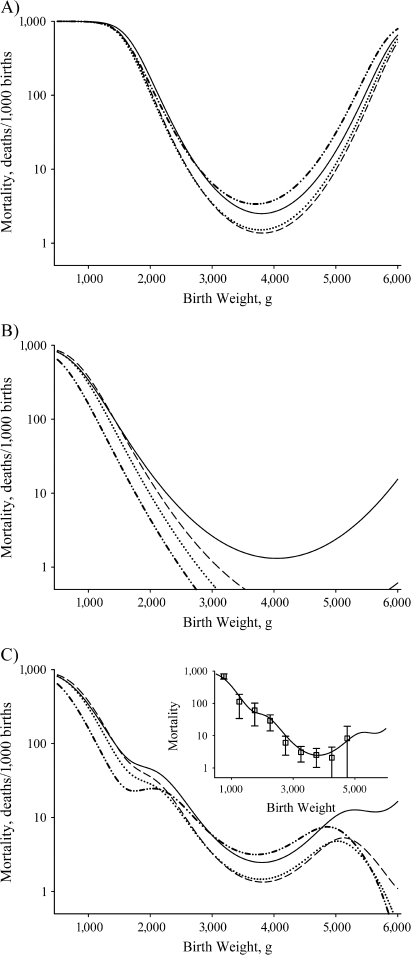
One of these theoretical views, the Wilcox-Russell hypothesis (7, 13–16), is sufficiently detailed to be explicitly testable. In this theory, the relation between birth weight and infant mortality among “normal” births—that is, those from the dominant Gaussian portion of the birth weight distribution—is influenced by 2 phenomena. First, the birth weight distribution may shift in response to an exogenous covariate (e.g., altitude), but the reverse-J-shaped birth-weight-specific mortality curve also shifts horizontally by a similar amount in the same direction (Figure 1, part A), so that there is no change in infant mortality; that is, no indirect effects of the factor operate through birth weight. Second, a covariate (e.g., maternal smoking) may have direct effects on infant mortality by increasing or decreasing the birth-weight-specific mortality curve vertically at all birth weights after the horizontal shifts in the birth weight distribution and the birth-weight-specific mortality curve have been accounted for (Figure 1, part B). This paradigm does not account for all of the potential “causal” pathways by which birth weight might influence infant mortality; for example, it only refers to “normal” births and not the remaining “residual” births, and it does not account for the reverse-J shape of the infant mortality curve (12). However, if the Wilcox-Russell hypothesis is correct for a particular variable under study, it would eliminate a potentially important pathway by which the variable could influence infant mortality—that is, the pathway through birth weight. In any event, all that is required to falsify the Wilcox-Russell hypothesis in relation to a particular variable is to show that the birth weight distribution and the birth-weight-specific mortality curve do not shift horizontally together to the same extent (Figure 1, part C)—that is, that there are significant indirect effects operating through birth weight.
Figure 1.
Characteristic changes in mortality with respect to changes in birth weight based on the Wilcox-Russell “causality” theory (7, 13–16), New York State, 1985–1988. A) No indirect effect, shift in birth weight (bold lines, solid to dashed) and corresponding shift in birth-weight-specific mortality curve (thin lines, solid to dashed), no overall change in infant mortality. B) No indirect effect plus a direct effect, shift in birth weight (bold lines, solid to dashed) and corresponding shift in birth-weight-specific mortality curve (thin lines, solid to dashed), no overall change in infant mortality due to shift in birth weight but direct effect increases mortality at all birth weights and overall. C) Indirect effect but no direct effect, shift in birth weight (bold lines, solid to dashed) not identical to the shift in birth-weight-specific mortality curve (thin lines, solid to dashed), infant mortally changes due to shift in birth weight. Only the graph in part C suggests that birth weight lies within the “causal” pathway. See Wilcox (14) for details.
The Wilcox-Russell hypothesis has never been statistically tested for any particular variable because of the lack of proper statistical techniques. Our primary aim in this paper was to apply the “covariate density defined mixture of logistic regressions” (CDDmlr) method to test the Wilcox-Russell hypothesis with respect to a continuous covariate, in this case maternal age. In particular, the CDDmlr model can statistically distinguish horizontal and vertical shifts in the birth-weight-specific infant mortality curve separately for both “normal” and “residual” (referred to here as “compromised”) births, and thus it can test the hypothesis that the horizontal shifts in the birth weight distribution and the birth-weight-specific mortality curve are identical. In this paper, we apply the model to 1985–1988 New York State birth cohorts to estimate the role that birth weight plays with regard to the impact of maternal age on infant mortality. The results are stratified by sex, parity, and African-American versus European-American race/ethnicity. Other racial/ethnic groups were omitted because of small samples.
MATERIALS AND METHODS
Mathematical model
CDDmlr in its application to birth outcomes (17, 18) is defined as the product of the conditional mortality submodel and the birth weight density submodel :
| (1) |
where x, y, θ, and β represent birth weight, the occurrence of death, the parameters modeling the birth weight distribution, and the parameters modeling the birth-weight-specific mortality, respectively.
In the case of 2 Gaussian subpopulations (corresponding to Wilcox and Russell's “normal” and “residual” births (7, 14, 15, 19)),
| (2) |
, the mixing proportion, is defined as the proportion of births belonging to the less numerous of the 2 subpopulations—that is, the secondary subpopulation (s) as opposed to the primary subpopulation (p). For i = p and s, represents the Gaussian density, truncated at 0, with mean and variance .
The probability of death conditioned on x is given by
| (3) |
where is the conditional probability of an infant with birth weight x belonging to subpopulation s. The birth weight density submodel (equation 2) determines
| (4) |
and, for i = p and s, is the corresponding probability of death for subpopulation i (standard logistic regression). Birth-weight- and subpopulation-specific mortality is generally assumed to be reverse-J-shaped in this model, following the Wilcox-Russell theory (7, 13–16); hence the quadratic parameterization:
| (5) |
Here the original model is extended in 2 ways. First, a continuous exogenous covariate, t, is incorporated into the birth weight density submodel (equation 2) by defining the respective parameters as functions of t—that is, by assuming nonlinear (second-degree polynomial) effects:
| (6) |
| (7) |
| (8) |
Second, the standardized birth weight (; i.e., the birth weight (x) standardized on the basis of the mean () and the standard deviation () of the respective subpopulation) and the covariate t are incorporated into the mortality submodel (equation 5); that is,
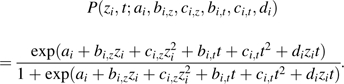 |
(9) |
Overall, there are 27 parameters (Table 1): 15 for the birth weight density submodel and 12 for the mortality submodel.
Table 1.
Definitions of the Model Parameters Used in an Application of the CDDmlr Method to Test the Wilcox-Russell Hypothesis With Respect to Maternal Age, New York State, 1985–1988
| Symbol | Definition |
| Birth weight density submodel parameters for the i subpopulation ( and p)a | |
| Mixing proportion (% secondary subpopulation) | |
| Constant in | |
| Linear term in | |
| Square term in | |
| Mean birth weight | |
| Constant in | |
| Linear term in | |
| Square term in | |
| Standard deviation of birth weight | |
| Constant in | |
| Linear term in | |
| Square term in | |
| Conditional mortality submodel parameters for the i subpopulation ( and p)b | |
| Constant | |
| Linear term for standardized birth weight (z) | |
| Square term for z | |
| Linear term for maternal age (t) | |
| Square term for t | |
| Interaction term for z and t |
Abbreviation: CDDmlr, covariate density defined mixture of logistic regressions.
Coefficients of a nonlinear function of a continuous exogenous covariate t.
Coefficients of a second-degree bivariate polynomial function.
Adding covariate t to the logistic regression (equation 9) while defining the birth weight density submodel parameters as a function of t (equations 6–8) represents the covariate's direct effect on infant mortality (i.e., the vertical shift, ) and its indirect effect through birth weight on infant mortality (i.e., any difference between the horizontal shifts of birth weight and the mortality curve, ). Thus, a significant interaction term () indicates a rejection of the Wilcox-Russell hypothesis for that covariate.
Data and methods
The data for this analysis consisted of all non-Hispanic African-American and European-American singleton livebirths occurring in New York State during the period 1985–1988. We used these data instead of data on more recent birth cohorts because the higher death rates in these data increased the power of the analysis (20) but the data were nevertheless accurately and consistently collected. Births with missing information on sex, parity, race/ethnicity, maternal age, or birth weight were omitted. We also omitted births to mothers with third- and higher-order parity to reduce heterogeneity in the multiparous strata. Analyses were carried out with stratification by race/ethnicity, sex, and parity (primiparous (parity = 0) vs. multiparous (parity = 1 or 2)).
The CDDmlr model is fitted to individual-level data by using the maximization function ms() in the S-PLUS library (21) to maximize the joint likelihood—that is, both submodels are fitted under 1 likelihood (17, 18). Details on the fitting procedures used and the statistical properties of CDDmlr are presented elsewhere (20). The CDDmlr model is identified when the birth weight density submodel is identified and when the individual logistic regressions are identified (e.g., covariate matrix full rank) (20). The birth weight density submodel is identified by specifying that the majority subpopulation is the primary subpopulation (20, 22). It is also identified when continuous exogenous covariates (e.g., maternal age) are introduced. However, some care must be taken when examining dichotomous exogenous covariates (23). Statistical significance is examined by using bias-adjusted bootstrap percentile confidence intervals at the 95% level. The bootstraps consist of 200 replicates, the first 100 of which are used to estimate the bias of the fitting procedure and the second 100 of which are used to estimate the width of the confidence interval. This is a relatively small sample of bootstraps. Larger samples require excessive computing resources. To determine whether this results in stable confidence intervals, 800 additional bootstrap iterations were carried out for 4 European-American cohorts. Comparison of the results obtained using the total sample of 1,000 bootstraps and those based on 200 bootstraps showed that they were similar. Thus, the smaller samples appeared to provide reasonable results.
RESULTS
The demographic and birth weight characteristics of the 8 birth cohorts are presented in Table 2. The parameter estimates and the bias-adjusted 95% confidence intervals for the birth weight distribution and the conditional mortality submodels are presented in Table 3 and Table 4, respectively. With the exception of the interaction term specifying the Wilcox-Russell hypothesis, the use of second-degree polynomials limits the biologic interpretability of the individual parameters. Therefore, the results are presented graphically.
Table 2.
Characteristics of the Sample Populations Used in an Application of the CDDmlr Method to Test the Wilcox-Russell Hypothesis With Respect to Maternal Age, New York State, 1985–1988
| Birth Cohort | African Americans |
European Americans |
||||||
| Females |
Males |
Females |
Males |
|||||
| Parity 0 | Parity 1 or 2 | Parity 0 | Parity 1 or 2 | Parity 0 | Parity 1 or 2 | Parity 0 | Parity 1 or 2 | |
| No. of births | 22,981 | 24,801 | 24,028 | 25,931 | 111,203 | 124,120 | 117,657 | 130,811 |
| No. of deaths | 294 | 263 | 366 | 336 | 547 | 594 | 730 | 776 |
| Crude death ratea | 12.79 | 10.60 | 15.23 | 12.96 | 4.92 | 4.79 | 6.20 | 5.93 |
| Birth weight, g | ||||||||
| Minimum | 195 | 140 | 120 | 116 | 78 | 100 | 170 | 113 |
| 5th percentile | 2,041 | 2,155 | 2,146 | 2,250 | 2,500 | 2,608 | 2,552 | 2,693 |
| 25th percentile | 2,807 | 2,835 | 2,920 | 2,977 | 3,033 | 3,118 | 3,147 | 3,232 |
| 50th percentile | 3,120 | 3,175 | 3,260 | 3,317 | 3,345 | 3,402 | 3,459 | 3,550 |
| 75th percentile | 3,450 | 3,515 | 3,572 | 3,640 | 3,657 | 3,720 | 3,799 | 3,884 |
| 95th percentile | 3,941 | 4,026 | 4,082 | 4,167 | 4,139 | 4,206 | 4,309 | 4,394 |
| Maximum | 6,522 | 6,120 | 6,350 | 6,719 | 7,999 | 7,919 | 7,709 | 7,940 |
| Maternal age, years | ||||||||
| Minimum | 12 | 14 | 12 | 14 | 11 | 15 | 10 | 15 |
| 5th percentile | 17 | 19 | 17 | 19 | 19 | 21 | 19 | 21 |
| 25th percentile | 20 | 24 | 20 | 23 | 23 | 25 | 23 | 26 |
| 50th percentile | 24 | 27 | 24 | 27 | 26 | 29 | 26 | 29 |
| 75th percentile | 28 | 31 | 28 | 31 | 30 | 32 | 30 | 32 |
| 95th percentile | 34 | 37 | 34 | 37 | 35 | 37 | 35 | 36 |
| Maximum | 45 | 47 | 48 | 48 | 47 | 55 | 55 | 58 |
Abbreviation: CDDmlr, covariate density defined mixture of logistic regressions.
No. of deaths per 1,000 births.
Table 3.
Parameter Estimates and Significance for the Birth Weight Density Submodel in an Application of the CDDmlr Method to Test the Wilcox-Russell Hypothesis With Respect to Maternal Age, New York State, 1985–1988
| Birth Cohort | African Americans |
European Americans |
||||||
| Females |
Males |
Females |
Males |
|||||
| Parity 0 | Parity 1 or 2 | Parity 0 | Parity 1 or 2 | Parity 0 | Parity 1 or 2 | Parity 0 | Parity 1 or 2 | |
| α0 | −1.62a | −2.95a | −0.80a | −2.21a | −1.18a | 0.25a | 0.49a | 1.17a |
| α1 | −2.72 | 7.45 | −12.24a | −1.86 | −12.35a | −21.60a | −23.06a | −28.01a |
| α2 (×10−2) | 0.06 | −0.15 | 0.28a | 0.08 | 0.25a | 0.36a | 0.43a | 0.47a |
| γs,0 | 1,645.56a | 321.96a | 2,369.52a | 1,559.23a | 1,844.67a | 1,670.83a | 1,677.73a | 1,969.81a |
| γs,1 | 84.61 | 164.85a | 3.47 | 52.54 | 81.92 | 103.52 | 92.43 | 77.21 |
| γs,2 | −2.04 | −2.96a | −0.05 | −0.52 | −1.63 | −1.97 | −1.63 | −1.38 |
| λs,0 | 896.83a | 921.35a | 841.51a | 997.82a | 936.87a | 766.81a | 880.49a | 1,064.26a |
| λs,1 | 8.07a | 7.20a | 12.12a | 4.93a | 5.62a | 13.08a | 7.33a | 3.59a |
| λs,2 (×10−3) | 0.04a | −0.12a | 0.06 | −0.23a | 2.15a | 0.09a | 0.29a | 0.09a |
| γp,0 | 2,909.58a | 2,703.25a | 2,721.13a | 2,665.97a | 3,100.22a | 2,758.24a | 3,246.73a | 2,784.72a |
| γp,1 | 17.45a | 29.91a | 42.46a | 42.46a | 20.32a | 43.38a | 19.51a | 51.81a |
| γp,2 | −0.25a | −0.40a | −0.73a | −0.62a | −0.38a | −0.68a | −0.37a | −0.82a |
| λp,0 | 453.29a | 490.84a | 327.96a | 333.39a | 514.28a | 558.66a | 507.36a | 574.49a |
| λp,1 | −4.57 | −5.19 | 9.22 | 9.63 | −5.92a | −8.06a | −2.99 | −8.05a |
| λp,2 | 0.15 | 0.14 | −0.16 | −0.17 | 0.12a | 0.14a | 0.05 | 0.16a |
Abbreviation: CDDmlr, covariate density defined mixture of logistic regressions.
Significant on the basis of 95% bias-corrected confidence intervals.
Table 4.
Parameter Estimates and Significance for the Conditional Mortality Submodel in an Application of the CDDmlr Method to Test the Wilcox-Russell Hypothesis With Respect to Maternal Age, New York State, 1985–1988
| Birth Cohort | African Americans |
European Americans |
||||||
| Females |
Males |
Females |
Males |
|||||
| Parity 0 | Parity 1 or 2 | Parity 0 | Parity 1 or 2 | Parity 0 | Parity 1 or 2 | Parity 0 | Parity 1 or 2 | |
| as | −10.36a | 0.46a | −6.83a | 4.20a | 2.68a | 1.55a | −1.82a | 0.87a |
| bs,z | −3.16a | −1.77 | −1.89 | −4.21 | 0.76a | −0.16a | −0.44a | 0.50a |
| cs,z | 1.07a | 1.21 | 0.94 | −0.63 | 0.65a | 0.67a | 0.66a | 0.72a |
| bs,t | 0.13 | −0.65 | 0.04 | −0.79 | −0.63a | −0.58 | −0.27 | −0.36a |
| cs,t (×10−2) | 0.13 | 1.27 | −0.01 | 0.85 | 0.88 | 1.02 | 0.33 | 0.44a |
| ds | 0.03 | −0.02 | −0.04 | −0.13 | −0.13 | −0.06 | −0.06 | −0.07a |
| ap | −4.53a | −0.64a | −4.71a | −2.06a | −4.72a | −3.64a | −1.87a | −0.91a |
| bp,z | −0.86a | −0.48a | −0.36a | −0.66a | −0.69a | 0.06a | −0.45a | −0.35a |
| cp,z | 0.21a | 0.33a | 0.25a | 0.23a | 0.28a | 0.32a | 0.30a | 0.23a |
| bp,t | −0.08 | −0.36a | −0.10 | −0.23a | −0.13 | −0.14 | −0.32a | −0.32a |
| cp,t (×10−1) | 0.01 | 0.06a | 0.03 | 0.04a | 0.02 | 0.01 | 0.06a | 0.05a |
| dp (×10−2) | 1.30 | 0.31 | −0.04 | 1.05 | 1.02 | −1.58 | 0.18 | −0.21 |
Abbreviation: CDDmlr, covariate density defined mixture of logistic regressions.
Significant on the basis of 95% bias-corrected confidence intervals.
Relation of maternal age and the birth weight distribution
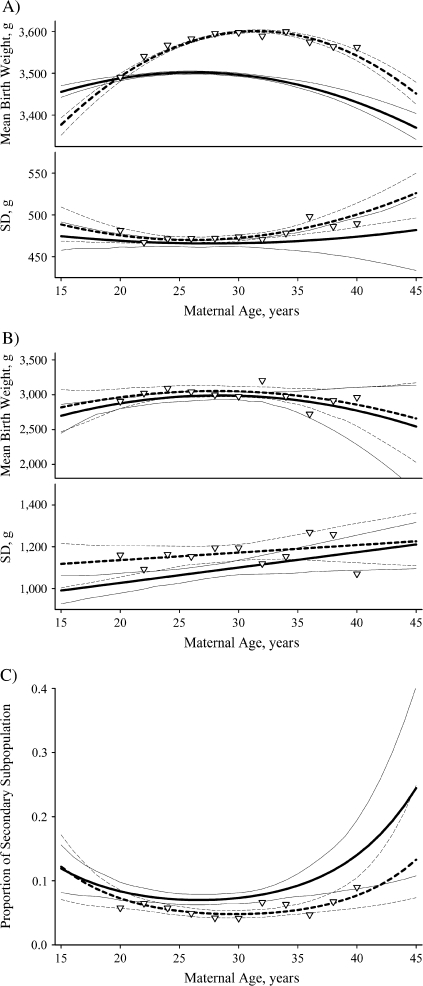
Maternal age had a strong effect on the mean birth weight of the primary subpopulation and the variance of the secondary subpopulation (Figure 2, Table 3). In particular, the effects of both the linear and the quadratic coefficients for maternal age on the primary subpopulation mean birth weight were significant at all parities in all populations examined. Primary subpopulation infants born to multiparous mothers were significantly larger than their peers born to primiparous mothers, particularly at maternal ages greater than 20 years (Figure 2, part A). On the other hand, maternal-age effects on the mean birth weight of the secondary subpopulation were significant in only 1 of 8 populations tested (African-American multiparous females) (Table 3). There were no significant differences in the mean birth weight between primiparous and multiparous secondary subpopulation births across the range of maternal age (Figure 2, part B). Maternal age influenced the standard deviation in birth weight of the secondary subpopulation in all birth cohorts examined, but it only affected the standard deviation in birth weight of the primary subpopulation in 2 of the 8 birth cohorts examined (Table 3). Thus, the most consistent effects concern the primary subpopulation mean and the secondary subpopulation standard deviation of birth weight. In general, maternal-age-specific primary subpopulation mean birth weight had an inverted-U shape, with maximum values between ages 25 years and 35 years (Figure 2, part A). The standard deviation of the secondary subpopulation generally increased monotonically with maternal age (Figure 2, part B), except for European-American multiparous female births, where it declined again at older maternal ages (not shown).
Figure 2.
Characteristic changes in the birth weight distribution by maternal age and parity, New York State, 1985–1988. A) primary subpopulation; B) secondary subpopulation; C) mixing proportion. The solid lines represent primiparous births (parity = 0), and the dashed lines represent multiparous births (parity = 1 or 2). The thin lines represent the respective bias-adjusted 95% confidence intervals. The inverted triangles (∇) represent the estimated values for multiparous births obtained by applying the “covariate density defined mixture of logistic regressions” method stratified by maternal age (bin size = 2 year) rather than using maternal age as a covariate. The results are for European-American males and are similar to the results for all populations examined, except as noted in the text. SD, standard deviation.
Maternal age had significant effects on the mixing proportion of all 4 European-American cohorts but only 1 of the African-American cohorts (Table 3). Among European-American births, was significantly U-shaped with maternal age for all parities (Figure 2, part C). However, European-American multiparous births had a significantly lower proportion of secondary subpopulation births compared with primiparous births, particularly among mothers aged 25–35 years (Figure 2, part C). These parity-specific differences with maternal age were not significant for 3 of the 4 African-American cohorts, although the trends were similar.
Relation of maternal age to infant mortality
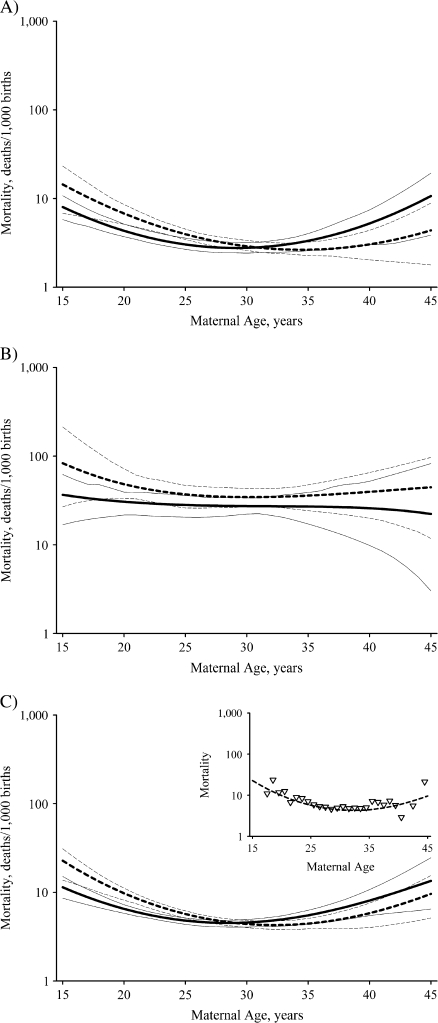
In general, infant mortality tended to decline with maternal age to a minimum and then increase again at older ages, particularly among primary subpopulation births (Figure 3). However, among African Americans, secondary subpopulation infant mortality among primiparous births increased monotonically with maternal age (not shown), while for multiparous European-American female births, secondary subpopulation infant mortality increased to a maximum and then declined at older ages (not shown).
Figure 3.
Characteristic changes in infant mortality by maternal age, New York State, 1985–1988. A) primary subpopulation; B) secondary subpopulation; C) total infant mortality. The solid lines represent primiparous births (parity = 0), and the dashed lines represent multiparous births (parity = 1 or 2). The thin lines represent the respective bias-adjusted 95% confidence intervals. The results are for European-American males and are similar to the results for all populations examined, except as noted in the text. The insert in part C shows a comparison of the observed mortality rates (∇) with the model-estimated mortality rates for multiparous births.
Significant direct effects of maternal age on infant mortality occurred in 4 of the 8 primary subpopulations and 3 of the 8 secondary subpopulations (Table 4). On the other hand, there was little if any statistically detectable indirect effect of maternal age on infant mortality (Table 4). The birth weight × maternal age interaction coefficients (i.e., ds and dp) were all insignificant, with the exception of secondary subpopulation multiparous European-American males. Thus, there were significant shifts in birth weight distributions, principally in the primary subpopulation mean; but in all primary subpopulations and most of the secondary subpopulations, birth-weight-specific infant mortality shifted along with the shifts in the birth weight distribution.
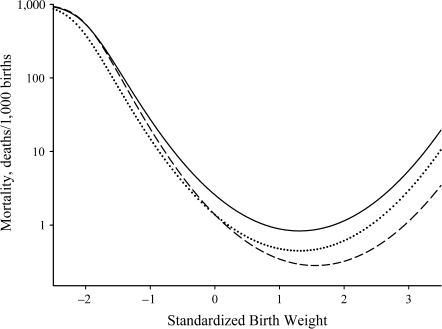
The value of ds was −0.07 (95% confidence interval: −0.02, −0.13) among secondary subpopulation multiparous European-American males (Table 4). The result was a significant horizontal shift in the birth-weight-specific mortality curve to the right relative to mean birth weight and an accompanying vertical shift in the birth-weight-specific mortality curve towards a lower optimal mortality. Interestingly, mean birth weight did not change in this case (Table 3). Figure 4 presents the results for maternal age at 20 and 25 years, but the shifts were similar at all ages.
Figure 4.
Model-estimated birth-weight-specific infant mortality curves for secondary subpopulation European-American males born to multiparous mothers aged 20 years (solid line) and 25 years (dashed line), New York State, 1985–1988. The dotted line shows the mortality curve for mothers aged 25 years assuming “no indirect effect.”
A maternal age pediatric paradox
Figure 5 presents characteristic model-estimated birth-weight-specific infant mortalities at several maternal ages. Of particular interest is the “pediatric paradox” with respect to maternal age. For example, in Figure 5, part C, infants born to women aged 26 and 34 years have higher estimated mortality at birth weights below 2,200 g but lower estimated mortality at birth weights above 2,200 g in comparison with infants born to older women. Whether estimates for older and/or young women display this effect varies by parity and race/ethnicity, with the effect being stronger for female births to African-American teenagers and primiparous births to older European-American mothers. In addition, primiparous male births to African-American teenagers have lower estimated mortality up to 3,500 g. However, repeated childbearing for adolescent mothers results in significantly higher estimated mortality (not shown), as is commonly observed (24).
Figure 5.
Characteristic model-estimated birth-weight-specific infant mortality curves for European-American males born to primiparous mothers, by maternal age, New York State, 1985–1988. A) primary subpopulation; B) secondary subpopulation; C) total cohort. The solid, dashed, dotted, and dashed-dotted-dotted lines represent mothers aged 18, 26, 34, and 42 years, respectively. The insert in part C shows a comparison of the observed mortality rates based on binned data (bin size = 500 g) (with corresponding 95% confidence intervals (T-shaped bars)) with the model-estimated mortality rates. Note that the decline in infant mortality which occurs at high birth weights for most maternal ages is due to the quadratic specification of subpopulation-specific infant mortality and the paucity of data at these birth weights (i.e., the large observed standard errors). The results are similar for the other ages.
DISCUSSION
These results suggest that in the populations studied, the effects of maternal age on infant mortality among “normal” (primary subpopulation) births were direct and not indirect (Table 4), which supports the Wilcox-Russell hypothesis (7, 13–16) with regard to maternal age. In particular, maternal age did significantly influence the birth weight distribution among “normal” births, but these changes were compensated for by shifts in birth-weight-specific infant mortality, so that “normal” infant mortality was unaffected.
The Wilcox-Russell hypothesis does not address “residual/compromised” (secondary subpopulation) births. Nevertheless, the results presented above suggest few, if any, indirect effects of maternal age on infant mortality among “compromised” births (Table 3). In the 1 significant case, males born to European-American multiparous mothers, the secondary subpopulation birth weight distribution appeared to remain fixed, while, in addition to a direct effect (i.e., the vertical shift), the secondary subpopulation birth-weight-specific mortality curve shifted to the right with increasing maternal age. This was the only significant indirect effect observed in the 16 tests conducted. It is possible that this is simply Type I error. Additional data will be necessary to confirm these results.
Maternal age also influenced birth weight and infant mortality through the proportion of “normal” births versus “compromised” births (Figure 2, part C), since these subpopulations differed significantly with respect to their birth weight distributions (Figure 2, parts A and B) and birth-weight-specific infant mortality (Figure 5, parts A and B) (17, 25). In our analysis, the effects of maternal age on the mixing proportion were all significant among European Americans but not among most African-American birth cohorts (Table 3). The lack of significant results could be due to the smaller African-American samples. Analyses with larger samples will be necessary to determine whether this racial/ethnic difference is correct.
The Wilcox-Russell hypothesis (7, 13–16) and the analysis presented above have several limitations. First, a limitation of our implementation for maternal age is the specification of indirect effects as a linear interaction (logit) with maternal age (i.e., ). The finding that indirect effects are not significant could be due to nonlinearity in this response. For example, the birth-weight-specific mortality curve might shift relative to birth weight in 1 direction during the early childbearing years and then back again in the later childbearing years. This is most likely to be a problem among African Americans, given the substantial proportion of births to women under the age of 20 years (Table 2). However, additional analyses by 5-year maternal-age segment (not shown) suggest that the linear interaction assumption is a reasonable approximation in all populations examined. Analyses with larger samples, particularly at the youngest and oldest maternal ages, will be necessary to determine whether this trend is in fact linear on a logarithmic scale over the entire range of childbearing years.
A second limitation of our model is that the birth weight density submodel employed here is not identical to Wilcox's semiparametric birth weight model (7, 14, 15, 19), upon which the Wilcox-Russell hypothesis is conceptually based. Wilcox and Russell's model assumes an uncontaminated Gaussian distribution in the middle of the birth weight range. This requires constraints on the fitting procedure that degrade the goodness of fit (19). Experimentation with 2- and 3-subpopulation Gaussian density submodels (26) and biologically reasonable alternative parametric specifications (27) indicates that for our data, the central part of the birth weight density is not a pure Gaussian distribution. In other respects, however, these 2 mixture models are very similar. In particular, they are both interpreted in the same way—that is, the primary subpopulation is one undergoing “normal” fetal development, while the secondary/“residual” subpopulation represents births that were “compromised” during fetal development (7, 14, 15, 17–19, 25, 28)—for example, by preterm delivery (we did not exclude preterm births). Additional applications will be necessary to confirm this view. Nevertheless, we believe that the 2-subpopulation Gaussian mixture submodel provides a reasonable separation between “normal” and “compromised/residual” births.
A final limitation is that the Wilcox-Russell hypothesis assumes that the primary birth-weight-specific mortality curve is reverse-J-shaped. Birth weight could be the “cause” of the reverse-J shape of the mortality curve. Basso et al. (12) theorize that the reverse-J shape is a result of confounding among 3 Gaussian subpopulations, each with constant birth-weight-specific mortality. The Basso et al. paradigm (12) can also be examined statistically using CDDmlr, but this was beyond the scope of the present analysis.
Nevertheless, at least in the case of maternal age, the Wilcox-Russell hypothesis appears to be correct. Horizontal shifts in the birth weight density appear to be compensated for by shifts in the birth-weight-specific infant mortality curve, so that overall mortality is not affected by the shift in birth weight. The major effects on infant mortality identified are consistent with direct effects of the covariate. The implication is that part of the observed association between birth weight and mortality is due to the direct influences of maternal age on infant mortality and on birth weight. Analyses of additional covariates are needed to confirm or qualify these findings.
Policies aimed at reducing infant mortality by influencing birth weight make the most sense if a shift in birth weight necessarily results in a change in infant mortality. The findings presented above, however, suggest that shifts in the birth weight density do not necessarily produce changes in overall infant mortality. Thus, an intervention that improves birth weight may not necessarily improve overall mortality. On the other hand, the results suggest that intervening on the basis of the proportion of “normal” births versus “compromised” births will influence both birth weight and infant mortality. A policy, such as the US policy (1), which focuses on reducing low birth weight in an effort to reduce infant mortality might be effective if it lowers the proportion of “compromised” births. However, more efficient interventions might be developed by targeting “compromised” births directly.
In conclusion, CDDmlr is a new method for examining the relation of birth weight to infant mortality. Here it was used to test the Wilcox-Russell hypothesis (7, 13–16). Examination of the effects of maternal age on birth weight and infant mortality tended to support the Wilcox-Russell hypothesis. In particular, we found statistically significant direct effects of maternal age on infant mortality but few if any indirect effects of maternal age on infant mortality operating through birth weight, despite significant effects of maternal age on the distribution of birth weight itself. We also found that maternal age affected the overall birth weight distribution and total infant mortality through its effects on the proportion of “normal” births versus “compromised” births. These results suggest that interventions targeting birth weight could have little effect on infant mortality. More effective interventions might be designed by targeting the direct effects and/or the proportion of “compromised” births.
Acknowledgments
Author affiliations: Department of Anthropology, College of Arts and Sciences, University at Albany–State University of New York, Albany, New York (Timothy B. Gage, Fu Fang, Erin O’Neill) and Department of Epidemiology and Biostatistics, School of Public Health, University at Albany–State University of New York, Albany, New York (Timothy B. Gage, Howard Stratton).
This work was supported by National Institute of Child Health and Human Development grant RO1-HD37405.
The authors thank Dr. Louise-Anne McNutt for comments on the manuscript.
Conflict of interest: none declared.
Glossary
Abbreviation
- CDDmlr
covariate density defined mixture of logistic regressions
References
- 1.Healthy People 2010. Washington, DC: US Department of Health and Human Services; 2000. Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. [Google Scholar]
- 2.Karn MN, Penrose LS. Birth weight and gestation time in relation to maternal age, parity and infant survival. Ann Eugen. 1952;16(4):147–164. [PubMed] [Google Scholar]
- 3.Guyer B, Martin JA, MacDorman MF, et al. Annual summary of vital statistics—1996. Pediatrics. 1997;100(6):905–918. doi: 10.1542/peds.100.6.905. [DOI] [PubMed] [Google Scholar]
- 4.Buehler JW, Kleinman JC, Hogue CJ, et al. Birth-weight-specific infant mortality, United States, 1960 and 1980. Public Health Rep. 1987;102(2):151–161. [PMC free article] [PubMed] [Google Scholar]
- 5.Singh GK. Infant mortality in the United States: trends, differentials, and projections, 1950 through 2010. Am J Public Health. 1995;85(7):957–964. doi: 10.2105/ajph.85.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312(2):82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox AJ. On the importance—and the unimportance—of birthweight. Int J Epidemiol. 2001;30(6):1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 8.Wise PH. The anatomy of a disparity in infant mortality. Annu Rev Public Health. 2003;24(1):341–362. doi: 10.1146/annurev.publhealth.24.100901.140816. [DOI] [PubMed] [Google Scholar]
- 9.Haig D. Meditations on birth weight: is it better to reduce the variance or increase the mean? Epidemiology. 2003;14(4):490–492. doi: 10.1097/01.EDE.0000070402.42917.f4. [DOI] [PubMed] [Google Scholar]
- 10.Savitz DA, Hertz-Picciotto I, Poole C, et al. Epidemiologic measures of the course and outcome of pregnancy. Epidemiol Rev. 2002;24(2):91–101. doi: 10.1093/epirev/mxf006. [DOI] [PubMed] [Google Scholar]
- 11.Savitz DA, Dole N, Herring AH. Methodologic issues in the design and analysis of epidemiologic studies of pregnancy outcome. Stat Method Med Res. 2006;15(2):93–102. doi: 10.1191/0962280206sm433oa. [DOI] [PubMed] [Google Scholar]
- 12.Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? Am J Epidemiol. 2006;164(4):303–311. doi: 10.1093/aje/kwj237. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox AJ, Russell IT. Why small black infants have a lower mortality rate than small white infants: the case for population-specific standards for birth weight. J Pediatr. 1990;116(1):7–10. doi: 10.1016/s0022-3476(05)81638-5. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox AJ. The Analysis of Birth Weight and Infant Mortality: An Alternative Hypothesis. Durham, NC: Epidemiology Branch, National Institute of Environmental Health Sciences; 2002. [Google Scholar]
- 15.Wilcox AJ, Russell IT. Birthweight and perinatal mortality: I. On the frequency distribution of birthweight. Int J Epidemiol. 1983;12(3):314–318. doi: 10.1093/ije/12.3.314. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox AJ, Russell IT. Perinatal mortality: standardizing for birthweight is biased. Am J Epidemiol. 1983;118(6):857–864. doi: 10.1093/oxfordjournals.aje.a113704. [DOI] [PubMed] [Google Scholar]
- 17.Gage TB, Bauer MJ, Heffner N, et al. Pediatric paradox: heterogeneity in the birth cohort. Hum Biol. 2004;76(3):327–342. doi: 10.1353/hub.2004.0045. [DOI] [PubMed] [Google Scholar]
- 18.Fang F, Stratton H, Gage TB. Multiple mortality optima due to heterogeneity in the birth cohort: a continuous model of birth weight by gestational age-specific infant mortality. Am J Hum Biol. 2007;19(4):475–486. doi: 10.1002/ajhb.20607. [DOI] [PubMed] [Google Scholar]
- 19.Umbach DM, Wilcox AJ. A technique for measuring epidemiologically useful features of birthweight distributions. Stat Med. 1996;15(13):1333–1348. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1333::AID-SIM271>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Gage TB, Bauer MJ, Fang F, et al. Mixed Logistic Regressions With Covariate Density Defined Components: Applications to Birth Outcomes. (CSDA Working Paper Series 2004-1) Albany, NY: Center for Social and Demographic Analysis, University at Albany–State University of New York; 2004. [Google Scholar]
- 21.Bates DM, Chambers JM. Nonlinear models. In: Chambers JM, Hastie TJ, editors. Statistical Models in S. Pacific Grove, CA: Wadsworth and Brooks; 1992. pp. 421–454. [Google Scholar]
- 22.McLachlan GJ, Peel D. Finite Mixture Models. New York, NY: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 23.Hennig C. Identifiability of models for clusterwise linear regression. J Classif. 2000;17(2):273–296. [Google Scholar]
- 24.Nelson PB. Repeat pregnancy among adolescent mothers: a review of the literature. J Natl Black Nurses Assoc. 1990;4(4):38–34. [PubMed] [Google Scholar]
- 25.Gage TB. Birth-weight-specific infant and neonatal mortality: effects of heterogeneity in the birth cohort. Hum Biol. 2002;74(2):165–184. doi: 10.1353/hub.2002.0020. [DOI] [PubMed] [Google Scholar]
- 26.Gage TB. Classification of births by birth weight and gestational age: an application of multivariate mixture models. Ann Hum Biol. 2003;30(5):589–604. doi: 10.1080/03014460310001592678. [DOI] [PubMed] [Google Scholar]
- 27.Gage TB. Modeling birthweight and gestational age distributions: additive vs. multiplicative processes. Am J Hum Biol. 2002;14(6):728–734. doi: 10.1002/ajhb.10089. [DOI] [PubMed] [Google Scholar]
- 28.Gage TB, Therriault G. Variability of birth-weight distributions by sex and ethnicity: analysis using mixture models. Hum Biol. 1998;70(3):517–534. [PubMed] [Google Scholar]