Abstract
While dying at home may be the choice of many, where people die may be less important than argued. We examined factors associated with parental planning of a child’s location of death (LOD) and its effects on patterns of care and parent’s experience. In a cross-sectional study of 140 parents who lost a child to cancer at one of two tertiary level U.S. pediatric hospitals, 88 (63%) planned the child’s LOD and 97% accomplished their plan. After adjusting for disease and family characteristics, families whose primary oncologist clearly explained treatment options during the child’s end of life (EOL) and who had home care involved were more likely to plan LOD. Planning LOD was associated with more home deaths (72% versus 8% among those who did not plan, P<0.001) and fewer hospital admissions (54% versus 98%, P<0.001). Parents who planned were more likely to feel very prepared for the child’s EOL (33% versus 12%, P=0.007) and very comfortable with LOD (84% versus 40%, P<0.001), and less likely to have preferred a different LOD (2% versus 46%, P<0.001). Among the 73 non-home deaths, planning was associated with more deaths occurring in the ward than in the intensive care unit or other hospital (92% versus 33%, P<0.001), and fewer children being intubated (21% versus 48%, P=0.029). Comprehensive physician communication and home care involvement increase the likelihood of planning a child’s LOD. Opportunity to plan LOD is associated with outcomes consistent with high quality palliative care, even among non-home deaths, and thus may represent a more relevant outcome than actual LOD.
Keywords: Children, cancer, palliative care, terminal care, hospice, home care, location of death, quality of care, attitude to death, death
Introduction
Whereas dying at home may be the choice of many,1–5 the actual place of death may be less important than has been argued. Among nine attributes of a good death examined in our study, patients ranked dying at home as the least important characteristic.6 In another study, patients reported dying at the preferred location as highly important.4 The Institute of Medicine report “When Children Die”7 has recommended advance planning regarding several aspects of end-of-life (EOL) care, including location of care and location of death (LOD). This report suggests that “planning [in advance of an expected death] can… provide parents some comfort as they anticipate and grieve in advance… [and] …help reduce …distressing experiences including unwanted interventions.” However, to date, research in children has focused on predictors,8 9 trends,10 11 and effects12 13 of place of death. Little is known about what factors facilitate the opportunity to plan a child’s LOD and further, what effects, if any, result from planning LOD.
We sought to determine whether modifiable clinical factors, such as communication about EOL care options and use of support services, were associated with parental planning of LOD, after adjusting for child and family characteristics. In addition, we explored whether parental planning of the child’s LOD had any impact on patterns of care and the parent’s experience with the child’s EOL.
Methods
We conducted a retrospective cross-sectional survey of bereaved parents whose children were cared for at the Dana-Farber Cancer Institute/Children’s Hospital Boston (DFCI/CHB), and Children’s Hospitals and Clinics of Minnesota (CHCM). Methods have been described elsewhere.14–16 Parents and physicians of children who died of cancer between 1990 and 1999 were interviewed between 1997 and 2001. Parents were considered eligible if they were English speaking, resided in North America, the child had died of cancer more than one year ahead of data collection, and the child’s physician gave permission to contact the family. Eligible families received a letter of invitation containing a postage-paid “opt-out” (DFCI/CHB) or “opt-in” (CHC) postcard, according to each site’s institutional review board standards. One parent per family was interviewed, chosen by the family. Charts of children of participating parents were also reviewed.
Permission to contact families was denied in 19 cases. Of the 244 eligible parents, we were able to reach 222, and 141 parents completed the surveys, for an overall response rate of 64%. Responders and non-responders did not differ regarding child’s age at death or diagnosis. Most interviews were administered by telephone; 35 were conducted face-to-face based on parent preference. Interviews were conducted a median of 3.3 years after the child’s death (range 1.1–10.8 years). Our results are based on 140 parents who answered the planning LOD question.
Parental Survey
The parental survey is a 390-item semi-structured questionnaire. Whenever possible, previously validated items were chosen, however most items were developed de novo from literature review and using focus groups of parents and medical caregivers.17 All questions reported in this analysis are close-ended questions with yes/no or Likert type response options.
This report focuses on the following items: 1) “Were you able to plan in advance the location of your child’s death?” (yes/no); 2) “Did your child die in the location you had planned?” (yes/no); 3) “How comfortable were you with the location of your child’s death?” (“very comfortable” vs. “somewhat comfortable,” “somewhat” or “very uncomfortable”); 4) “Would you have preferred your child to have died elsewhere?” (yes/no) -- if yes, where?; and 5) “Did you feel well prepared for the circumstances at the time of your child’s death?” (“very prepared” vs. “somewhat,” “a little,” “not at all prepared”). Parents were also asked whether the primary clinician gave a clear explanation about all available treatment options during the EOL period (“agree strongly” vs. “agree somewhat,” “disagree somewhat,” “disagree strongly”), whether they were followed by a psychosocial clinician (yes/no), and whether home care was involved in the child’s care (yes/no). Timing of home care discussion was reported as days before the child’s death (analyzed as < or ≥ 30 days prior to death). Information about LOD (home/treating hospital/other location), sociodemographic, and family characteristics was also collected.
Medical Record Review
Child’s sex, diagnosis (hematological vs. non-hematological cancer), type of death (cancer progression vs. treatment-related complication), patterns of care (admission to hospital during last month of life, visits to emergency department in last week of life, intubation in the last 24 hours, use of cardiopulmonary resuscitation (CPR), withdrawal of supportive measures), and zip code were collected from the medical records. Distance to the hospital was calculated with http://webservices.imacination.com/distance/.
Statistical Methods
All analyses were conducted using the SAS statistical package (SAS Institute, Inc, Cary, NC). Missing data for the outcome and independent variables were handled using the complete case method.18
Determinants of Planning LOD
Determinants of planning LOD were explored using multivariate logistic regression models. The modifiable clinical factors considered as independent variables included (1) strongly agree that oncologist gave clear information about all available treatment options during EOL period, (2) parent was followed by a psychosocial clinician, and (3) home care became involved. All were treated as binary variables. Disease, child, and family characteristics were included as covariates to control for potential confounding. Our model-building strategy began with univariate analyses using Pearson χ2 test for categorical variables and t-test or Wilcoxon rank-sum test for continuous variables. Prior studies indicate that children with hematological cancers are more likely to die in the hospital, have a shorter EOL trajectory, are eligible for intensive treatments such as bone marrow transplant, and are more likely to die from treatment-related complications.9 19–21 Hence, we expected parental planning to be limited in children with hematological cancers and elected to force this variable into the models. Remaining child and family covariates were selected using a stepwise, forward-selection logistic regression procedure setting a P-value <0.15 for variable entry, and a P-value <0.05 for retention in the model. To build our final model, we manually introduced those independent variables with a Wald test P <0.1 in ascending order, adjusting for diagnosis and the child and family covariates previously selected. Potential confounders (change in β estimates >20%) were retained in the model. Given the small sample size, our ability to check for interactions was limited. If significant variables had more than five missing values, we conducted a sensitivity analysis imputing all missing values to each possible response option successively. This conservative approach was based on the risk of instability derived from our small sample size. Finally, we assessed whether physician clustering played a significant role by using generalized estimating equations (PROC GENMOD) to fit the model. Results are reported as odds ratios (OR) with 95% confidence intervals (CI).
Effects of Planning LOD
To explore the effects of planning LOD, univariate analyses were performed with the Pearson χ2 test or Exact Fisher’s test using planning LOD as the independent variable. We examined the following patterns of care: 1) actual LOD, 2) hospital admissions in last month of life (yes/no), and ≥ 3) one emergency department visit in last week of life. In the subgroup of families whose children did not die at home, we also examined 1) being intubated in the last 24 hours of life, 2) CPR attempted, and 3) withdrawal of support measures other than intubation. We also explored impact of planning on the following aspects of the parent’s experience: 1) parents feeling very prepared for medical circumstances around the time of death, 2) parents feeling very comfortable with LOD, and 3) parents reporting they would have preferred another LOD. To explore if the impact of planning extended beyond the actual LOD, we also conducted the same analysis in the subgroup of families whose children did not die at home.
Results
Most parents, 62% (n=88/140), were able to plan their child’s LOD. Table 1 presents child, family, and clinical characteristics of those who planned and those who did not plan LOD.
Table 1.
Characteristics of patients whose parents planned location of death (LOD) and patients whose parents did not plan LOD
| Planned LOD1 | |||
|---|---|---|---|
| YES (N=88) N (%) | NO (N=52) N (%) | p-value2 | |
|
Study Site | |||
| Massachusetts | 62(70) | 40(77) | 0.406 |
|
| |||
| Minnesota | 26(30) | 12(23) | |
|
Disease characteristics | |||
| Type of cancer | |||
| Hematological malignancy | 32/87 (37) | 38 (73) | <0.001 |
| Other | 55/87 (63) | 14 (27) | |
| Type of death | |||
| Disease Progression | 84/87 (97) | 25/51 (49) | <0.0013 |
| Treatment-related | 3/87 (3) | 26/51 (51) | |
|
| |||
|
Child characteristics | |||
| Male Gender | 52(59) | 22 (42) | 0.055 |
| Child’s age at death in years, mean (SD) | 10.6 (6.5) | 9.6 (6.7) | 0.3994 |
|
| |||
|
Parental characteristics | |||
| Parental Age at child’s death in years, mean (SD) | 39.8(8.2) | 37.8(8.2) | 0.1744 |
| Was married before the child died | 77 (88) | 44(85) | 0.630 |
| White race | 82 (93) | 48(92) | 1.0003 |
| Post secondary education | 65(74) | 43(83) | 0.229 |
| Annual Income > 35,000 | 70(80) | 36(69) | 0.169 |
| Private insurance | 82(93) | 43(83) | 0.053 |
| Christian | 67/87(77) | 39(75) | 0.787 |
| Religiousness (very vs. somewhat/not at all religious) | 25(28) | 26/51(51) | 0.008 |
|
| |||
|
Other family characteristics | |||
| Number of other children, median (range) | 2(0–8) | 2(0–5) | 0.9945 |
| Distance from Hospital in miles, median (range) | 20 (2–853) | 24 (2–216) | 0.3825 |
| Time elapsed since child’s death in years, median (range) | 4(1–11) | 3(1–8) | 0.2995 |
| Family had previous loss | 76(86) | 33(64) | 0.002 |
| Age of the deceased in years, mean (SD) | 57.7(21.8) | 67.2(14.4) | 0.0104 |
| Previous loss was a parent, sibling, spouse or child | 46/76(61) | 15/33(46) | 0.145 |
| Previous death due to cancer | 33/76(43) | 14/33(42) | 0.923 |
| Previous death associated with a lot of suffering | 25/70(36) | 16/32(50) | 0.172 |
|
| |||
|
Clinical Factors | |||
| Oncologist gave information about treatments during EOL | 69/83(83) | 29/46(62) | 0.011 |
| Parent was followed by psychosocial clinician | 54/87(62) | 36/50(72) | 0.238 |
| Home care became involved | 71/86(83) | 18/51(36) | <0.001 |
| Home care was discussed ≥ 30 days before death | 48/82 (59) | 14/52 (27) | <0.001 |
Denominator indicated when appropriate
χ2 test
Fisher’s Exact test
Student T test
Wilcoxon Rank Sum test
Determinants of Planning LOD
Disease and Child Characteristics Associated with Planning LOD
Planning was less likely among children with hematological cancers as compared to other diagnoses (P<0.001) (Table 1). Dying from disease progression, as opposed to treatment toxicity, was associated with a higher likelihood of planning LOD (P<0.001). Given that almost all patients with non-hematological malignancies died due to disease progression (69/70) but only 59% (41/69) of those with hematological cancers did so, we considered type of death to be an intermediate variable and excluded it from the multivariate analysis. No other child characteristics were associated with parental planning of LOD.
Parent Characteristics Associated with Planning LOD
Though most sociodemographic characteristics were similar among parents who planned LOD and those who did not, parents who were very religious were less likely to plan (P=0.008) (Table 1). On the other hand, parents who experienced a previous loss were more likely to plan (P=0.002), especially if the deceased was younger (P=0.008). Distance to the hospital was not associated with planning.
In multivariate analysis, adjusted for diagnosis, three family characteristics remained independently associated with planning LOD (Table 2); families who had experienced a previous loss and/or who had private insurance were more likely to have planned their child’s LOD, and those who were very religious were less likely to have planned.
Table 2.
Multivariate analysis of determinants associated with planning a child’s LOD1
| Non-modifiable factors model (disease and family characteristics) |
Adjusted Modifiable factors model2 |
|||
|---|---|---|---|---|
| OR (CI95%)3 | p-value | OR (95%CI)3 | p-value | |
| Disease and family characteristics | ||||
| Child had hematological cancer | 0.20 (0.1–0.5) | <0.001 | 0.34 (0.1–0.9) | 0.036 |
| Family experienced a previous loss | 5.64 (2.1–15.1) | <0.001 | 7.44 (2.4–23.3) | <0.001 |
| Having Private Insurance | 5.60 (1.5–21.5) | 0.012 | 5.33 (1.3–22.9) | 0.024 |
| Being very religious | 0.33 (0.1–0.8) | 0.011 | 0.27 (0.1–0.8) | 0.011 |
|
| ||||
| Clinical factors | ||||
| Oncologist clearly explained treatment options | 3.87 (1.3–11.8) | 0.017 | ||
| Home care was involved | 5.34 (2.0–14.6) | 0.001 | ||
LOD: location of death
14 observations missing. Sensitivity analysis and clustering by physician did not significantly alter results
OR: Odds ratio (95%CI: 95% Confidence intervals)
Modifiable Clinical Factors
Parents who reported that they strongly agreed that physicians communicated about all treatment options at the EOL were more likely to plan LOD as compared to those who did not agree strongly (P=0.011). Home care involvement was also associated with planning LOD (P<0.001) (Table 1). Importantly, parents who planned LOD were more likely to have discussed home care at least 30 days before the child’s death (P<0.001) (Table 1).
After adjusting for diagnosis, previous experience with loss, being very religious, and having private insurance, parents who felt that primary oncologists had clearly explained all treatment options available during the EOL period were 3.87 (95% CI 1.3, 11.8) times more likely to plan LOD as compared to families that felt otherwise; likewise, families that had home care services were 5.34 (95% CI 2.0, 14.6) times more likely to plan LOD compared to families who did not have home care.
In this final multivariate model, there were 14 missing observations, 11 of them related to the variable “oncologist clearly explained treatment options.” Sensitivity analysis did not change results significantly; neither did clustering by physician.
Impact of Planning LOD
Ninety-seven percent (n=85/88) of parents who planned their child’s LOD reported that the child died in the planned location. Table 3 presents patterns of care at the EOL associations with planning LOD. Overall, 67 children died at home and 73 at non-home locations. Children whose parents planned LOD were significantly more likely to die at home (P<0.001), and were less likely to be admitted to the hospital during the last month of life (P<0.001). Among the subgroup of families whose child did not die at home, children whose parents planned LOD were significantly more likely to die in a hospital ward as opposed to the Intensive Care Unit (ICU) or another hospital (P<0.001) and less likely to have been intubated in the last 24 hours of life (P=0.029).
Table 3.
Impact of planning LOD1 on patterns of care at end-of-life
| Planned LOD2 | |||
|---|---|---|---|
| YES N (%) | NO N (%) | P3 | |
| All Deaths (N=140) | N=88 | N=52 | |
|
| |||
| LOD | |||
| Home | 63 (72) | 4 (8) | <0.0014 |
| Hospital | 25 (28) | 39 (75) | |
| Other | 0(0) | 9(17) | |
|
| |||
| Admission to hospital during last month of life | 47/87(54) | 51(98) | <0.001 |
| At least 1 Emergency Room visit in last week | 5/84(6) | 8/50(16) | 0.057 |
|
| |||
| Non-Home deaths (N=73) | N=25 | N=48 | |
|
| |||
| Location of death | |||
| Hospital Ward | 23 (92) | 16 (33) | <0.0014 |
| Intensive Care Unit | 2(8) | 23 (47) | |
| Other Hospital | 0(0) | 9(19) | |
|
| |||
| Number of days admitted during last month of life, median (interquartile range) | 17(4–27) | 21(6–28) | 0.494 |
|
| |||
| Intubated in last 24 hours | 5/24(21) | 21/44(48) | 0.029 |
|
| |||
| CPR5 attempted | 1/24 (4) | 8/43 (19) | 0.1424 |
|
| |||
| Withdrawal of support (other than intubation) | 9/25(36) | 8/42 (19) | 0.123 |
LOD: location of death
Denominator indicated when appropriate
χ2 test
Fisher’s Exact test
CPR: cardiopulmonary resuscitation
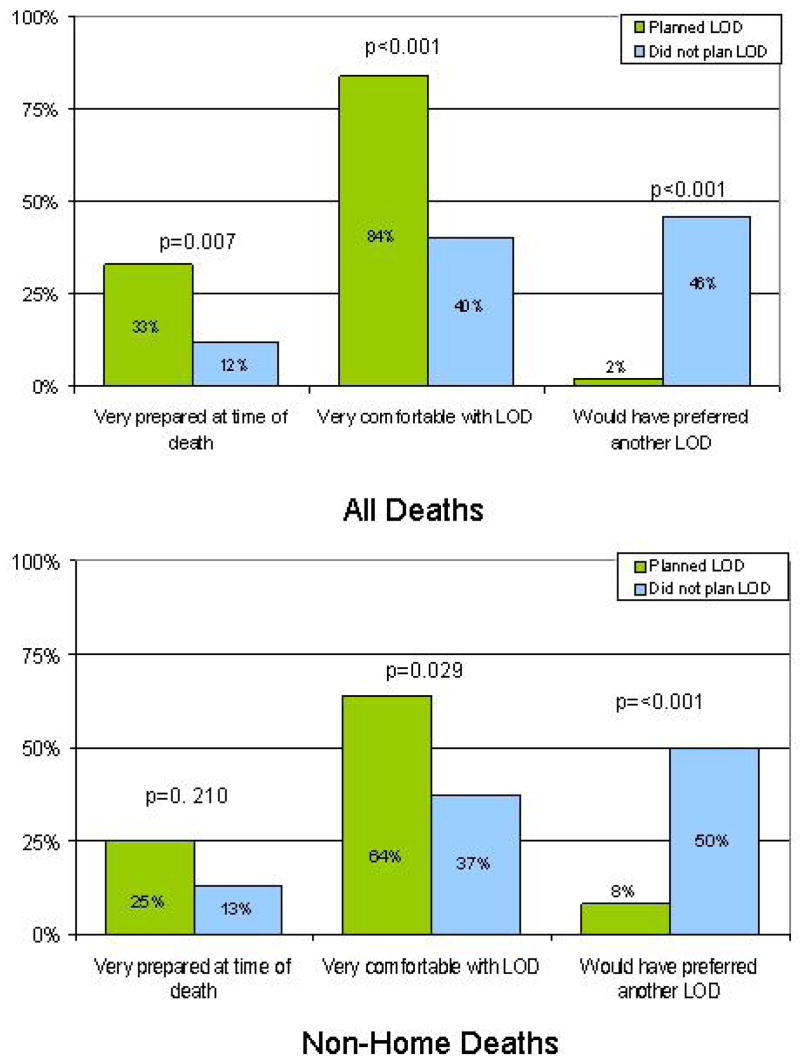
Regarding parent’s experience around the time of the child’s death (Figure 1, upper panel), parents who planned were significantly more likely than their counterparts to feel very prepared for medical circumstances around the time of death (28/85 (33%) versus 6/50 (12%), P=0.007), to report being very comfortable with the LOD (74/88 (84%) versus 20/50 (40%), P<0.001), and less likely to have preferred the death to occur at another location (2/88 (2%) versus 22/48 (46%), P<0.001). Among children who did not die at home, parents who planned were also more likely to report feeling very comfortable with LOD (16/25 (64%) versus 17/46 (37%), P=0.029) and less likely to have preferred another LOD than those who did not plan (2/25 (8%) versus 22/44 (50%), P<0.001). Among parents whose child did not die at home and who would have preferred another LOD, 63% (n=15/24) would have preferred the death to be at home.
Figure. Effects of parental planning of the child’s location of death (LOD).
Upper Panel shows parent reports of their sense of preparedness at the time of death, and level of comfort and regret related to the actual LOD, stratified by whether or not they planned LOD(N=140).
Lower Panel shows the same results for parents of children who did not die at home (N=73).
Discussion
Our study is one of the first to examine determinants and effects of parental planning of their child’s LOD. Dying at home has been signaled as an indicator of good quality EOL care. However, despite numerous efforts and advancement of the palliative care field, pediatric home death rates, although growing, have remained low.22 In this analysis, we were able to take a step back, and look at the opportunity to plan LOD, an outcome that if targeted, may not only help increase home death rates but also bring about high quality EOL care for children who do not die at home. We therefore hope that these results will begin a dialogue about the need for EOL quality indicators that are more aligned with the patient and family perspective.
In comparing our results to those published, we found that studies about effects of planning are scarce. Previously published rates of death at planned or preferred LOD in terminally ill adults range from 33% to 67%,5 23–25 which is much lower than the 97% seen in our study. The higher success rate in children may be due to the unique qualities of the parental role. In fact, terminally ill adults who live with relatives and have support and advocacy from extended family are more likely to die at the preferred LOD25 and to die at home.26 The role of a parent inherently includes advocacy, as well as financial and emotional support, and this may lead to greater opportunities for successful planning.
Our findings indicate that planning is associated with benefits for both children and parents. Most parents who planned chose home for the child’s place of death, which prior research suggests has beneficial effects on family outcomes.13 27 Parental preparedness, which we also found to be associated with planning, has been identified as a key determinant of high quality EOL care.28 In addition, the children of parents who planned were more likely to experience less invasive care around the time of death, and parents reported less decisional regret about the LOD. That the effects of planning remained consistent among children who did not die at home suggests that there is an intrinsic benefit to planning that extends beyond increasing the likelihood of death at home. This becomes particularly relevant when considering that almost 60% of children who die from cancer in the US do not die at home22 and that home death, albeit beneficial for many, imposes extraordinary demands on some families.29 Taken together, our findings suggest that the opportunity to plan LOD may be a better proxy for high quality EOL care than the actual LOD, one that is more inclusive, and better aligned with palliative care principles.
The effects reported in this paper are restricted to the period surrounding death, and as such, extrapolation to complex long-term outcomes, such as grief, should be made with caution. However, there is some evidence suggesting that the experience at the time of death has long-lasting effects. Kreicbergs et al. found that when the child’s actual death was difficult, 80% of parents were still affected by the experience 4–9 years later.30 Another study uncovered that when the death is more expected, parents experience less grief.31 By influencing the experience around the time of death, planning may affect long-term bereavement outcomes. The extent of these potential effects needs to be determined.
By uncovering modifiable factors that facilitate parental planning of the child’s LOD, such as communication and support services, our study identified potential targets for future interventions. Improving physician-patient communication is challenging but can be achieved by providing specific training and modeling.32 33 For example, Alexander et al.34 reported that a short intensive training course focusing on delivering bad news and discussing preferences with patients at the EOL improved residents overall communication skills, though conversations about advance care planning were harder to teach. Additional strategies may be needed to improve physician’s ability to facilitate planning. For example, instruments such as the Seattle Pediatric Palliative Care Project Decision Making Tool,35 may be of help. This tool prompts physicians to document risks and benefits of all treatment options discussed with parents, one of the factors identified by our study to be associated with planning. Preliminary results of the project showed beneficial effects on the child’s quality of life and family satisfaction with EOL care. Further exploration of these types of interventions, as well as a better understanding of the barriers physicians face when talking to parents, may increase the effectiveness of communication at this very challenging time.
Early involvement of home care may also facilitate the opportunity to plan LOD. Unfortunately, home care continues to be integrated late into the care of patients with advanced disease36 and is especially under-utilized in children with life-limiting illnesses.37 38 The probability of physicians referring children to hospice services depends largely on access to qualified programs and a willingness of such programs to continue disease-modifying therapies.38 39 Unfortunately, few home care and hospice services accommodate these needs.37 Thus, to facilitate opportunities for planning LOD, strategies aimed at reducing barriers to home-based pediatric palliative care are needed. In this regard, the state of Massachusetts recently launched a state-wide initiative, the Massachusetts Pediatric Palliative Care Program, which provides coverage of in-home pediatric palliative care consult services to children with life-limiting conditions regardless of their life expectancy, use of disease-directed treatment, or block nursing.40 Broad educational programs, such as the Initiative for Pediatric Palliative Care (IPPC),41 may also serve to broaden access to pediatric palliative care by increasing the number of trained clinicians. Evaluation of these, as well as other initiatives, will inform policy makers and health care payers about ways of improving children’s access to home-based palliative care services.
Our study has a number of limitations primarily related to its cross-sectional design. However, the rigorous approach to analysis lends validity to the results. In assessing determinants of planning, independent variables and covariates were chosen parsimoniously and whenever possible based on prior knowledge.15 26 42 43 This empirically driven model and its stability to sensitivity analysis confer strength to our findings.
One of the limitations in this regard lies in the inability to definitively establish a temporal sequence between home care involvement and planning LOD. In other words, home care involvement may well be a consequence of planning rather than a predictor. However, planning LOD is likely to take place near the time of the child’s death. The fact that parents who planned LOD were more likely to have discussed home care over 30 days before the child’s death increases the likelihood of home care involvement actually preceded planning.
As is the case with observational studies,44 it is possible that important unknown confounders were omitted from our analysis. The SUPPORT Study revealed that decision-making processes are heavily influenced by a myriad of psychological and social forces that are hard to measure and disentangle.45 Hence, although we adjusted for child and family characteristics, there may be other relevant factors that were not taken into account. Moreover, our goal when adjusting by family characteristics was to be able to isolate the effect of variables that clinicians could act upon. In order to understand how family characteristics affect planning in greater depth, a mixed-methods research approach, using both quantitative and qualitative methods, may be more suitable.46
Finally, our parent sample was fairly homogeneous, consisting mainly of white, middle class parents, and therefore the generalizability of our findings may be limited. Feudtner et al.22 have shown racial and ethnic differences regarding LOD in children, with lower home death rates among African Americans and Hispanics. Thus, replication of our findings in a more diverse population and including children with diagnoses other than cancer and whose illness trajectories may differ markedly is warranted. Moreover, there is no reason to believe that our findings only apply to children and families, and thus it would be valuable to similarly evaluate advanced planning of LOD in adult patients.
Conclusions
Planning LOD is facilitated by comprehensive communication and early involvement of homecare, and associated with favorable outcomes such as death in the desired location and minimal parent regret about LOD. Further development and evaluation of palliative care interventions aimed at facilitating advanced care planning, including LOD, may have substantial benefit for dying children and their families.
Acknowledgments
We thank the parents for their willingness to share their stories.
Authors’ work was independent of funders. V. Dussel is the recipient of a fellowship from the Agency for Health Research and Quality (T32HP10018). J. Wolfe was the recipient of Grant No. NCI 5 K07 CA096746 from the National Cancer Institute and a Child Health Research Grant from the Charles H. Hood Foundation. J. Watterson was supported in part by the Pine Tree Apple Tennis Classic Oncology Research Fund. J. Wolfe (guarantor and principal investigator), J.C. Weeks and V. Dussel are responsible for study conception and design; J. Wolfe, J.M. Hilden, J. Watterson, C. Moore, and B.G. Turner conducted data collection; V. Dussel and J. Wolfe were responsible for data analysis and interpretation; V. Dussel, U. Kreicbergs, and J. Wolfe drafted the paper, andall authors revised it.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies B, Deveau E, deVeber B, et al. Experiences of mothers in five countries whose child died of cancer. Cancer Nurs. 1998;21(5):301–311. doi: 10.1097/00002820-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Vickers JL, Carlisle C. Choices and control: parental experiences in pediatric terminal home care. J Pediatr Oncol Nurs. 2000;17(1):12–21. doi: 10.1177/104345420001700103. [DOI] [PubMed] [Google Scholar]
- 3.Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med. 2000;3(3):287–300. doi: 10.1089/jpm.2000.3.287. [DOI] [PubMed] [Google Scholar]
- 4.Tang ST. When death is imminent: where terminally ill patients with cancer prefer to die and why. Cancer Nurs. 2003;26(3):245–251. doi: 10.1097/00002820-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Beccaro M, Costantini M, Rossi PG, et al. Actual and preferred place of death of cancer patients. Results from the Italian survey of the dying of cancer (ISDOC) J Epidemiol Community Health. 2006;60(5):412–416. doi: 10.1136/jech.2005.043646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinhauser KE, Christakis NA, Clipp EC, et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284(19):2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 7.Field MJ, Behrman RE, editors. Institute of Medicine (US), Committee on Palliative and End-of-Life Care for Children and Their Families. Washington, DC: National Academies Press; 2003. When children die: Improving palliative and end-of-life care for children and their families. [PubMed] [Google Scholar]
- 8.Kurashima AY, Latorre Mdo R, Teixeira SA, De Camargo B. Factors associated with location of death of children with cancer in palliative care. Palliat Support Care. 2005;3(2):115–119. doi: 10.1017/s1478951505050194. [DOI] [PubMed] [Google Scholar]
- 9.Surkan PJ, Dickman PW, Steineck G, Onelov E, Kreicbergs U. Home care of a child dying of a malignancy and parental awareness of a child’s impending death. Palliat Med. 2006;20(3):161–169. doi: 10.1191/0269216306pm1139oa. [DOI] [PubMed] [Google Scholar]
- 10.Feudtner C, Silveira MJ, Christakis DA. Where do children with complex chronic conditions die? Patterns in Washington State, 1980–1998. Pediatrics. 2002;109(4):656–660. doi: 10.1542/peds.109.4.656. [DOI] [PubMed] [Google Scholar]
- 11.Ramnarayan P, Craig F, Petros A, Pierce C. Characteristics of deaths occurring in hospitalised children: changing trends. J Med Ethics. 2007;33(5):255–260. doi: 10.1136/jme.2005.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauer ME, Mulhern RK, Wallskog JM, Camitta BM. A comparison study of parental adaptation following a child’s death at home or in the hospital. Pediatrics. 1983;71(1):107–112. [PubMed] [Google Scholar]
- 13.Goodenough B, Drew D, Higgins S, Trethewie S. Bereavement outcomes for parents who lose a child to cancer: are place of death and sex of parent associated with differences in psychological functioning? Psychooncology. 2004;13(11):779–791. doi: 10.1002/pon.795. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342(5):326–333. doi: 10.1056/NEJM200002033420506. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe J, Klar N, Grier HE, et al. Understanding of prognosis among parents of children who died of cancer: impact on treatment goals and integration of palliative care. JAMA. 2000;284(19):2469–2475. doi: 10.1001/jama.284.19.2469. [DOI] [PubMed] [Google Scholar]
- 16.Grunberg SM, editor. Quality of care at the end of life in children with cancer. 40th Annual Meeting of the American Society of Clinical Oncology: New Orleans, Louisiana, 2004; AU: IS THIS AN ABSTRACT? IS THERE A PROCEEDINGS BOOK? [Google Scholar]
- 17.Streiner DL, Norman GR. Health measurement scales: A practical guide to their development and use. 2. New York: Oxford Medical Publications; 1995. [Google Scholar]
- 18.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142(12):1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe J. Suffering in children at the end of life: recognizing an ethical duty to palliate. J Clin Ethics. 2000;11(2):157–163. [PubMed] [Google Scholar]
- 20.Klopfenstein KJ, Hutchison C, Clark C, Young D, Ruymann FB. Variables influencing end-of-life care in children and adolescents with cancer. J Pediatr Hematol Oncol. 2001;23(8):481–486. doi: 10.1097/00043426-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Bradshaw G, Hinds PS, Lensing S, Gattuso JS, Razzouk BI. Cancer-related deaths in children and adolescents. J Palliat Med. 2005;8(1):86–95. doi: 10.1089/jpm.2005.8.86. [DOI] [PubMed] [Google Scholar]
- 22.Feudtner C, Feinstein JA, Satchell M, Zhao H, Kang T. Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. JAMA. 2007;297(24):2725–2732. doi: 10.1001/jama.297.24.2725. [DOI] [PubMed] [Google Scholar]
- 23.Guthrie B, Nelson M, Gazzard B. Are people with HIV in London able to die where they plan? AIDS Care. 1996;8(6):709–713. doi: 10.1080/09540129650125425. [DOI] [PubMed] [Google Scholar]
- 24.Pritchard RS, Fisher ES, Teno JM, et al. Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. J Am Geriatr Soc. 1998;46(10):1242–1250. doi: 10.1111/j.1532-5415.1998.tb04540.x. [DOI] [PubMed] [Google Scholar]
- 25.Tang ST, McCorkle R. Determinants of congruence between the preferred and actual place of death for terminally ill cancer patients. J Palliat Care. 2003;19(4):230–237. [PubMed] [Google Scholar]
- 26.Gomes B, Higginson IJ. Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ. 2006;332(7540):515–521. doi: 10.1136/bmj.38740.614954.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauer ME, Mulhern RK, Schell MJ, Camitta BM. Long-term follow-up of parental adjustment following a child’s death at home or hospital. Cancer. 1989;63(5):988–994. doi: 10.1002/1097-0142(19890301)63:5<988::aid-cncr2820630534>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Mack JW, Hilden JM, Watterson J, et al. Parent and physician perspectives on quality of care at the end of life in children with cancer. J Clin Oncol. 2005;23(36):9155–9161. doi: 10.1200/JCO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Stajduhar KI, Davies B. Death at home: challenges for families and directions for the future. J Palliat Care. 1998;14(3):8–14. [PubMed] [Google Scholar]
- 30.Kreicbergs U, Valdimarsdottir U, Onelov E, et al. Care-related distress: A nationwide study of parents who lost a child to cancer. J Clin Oncol. 2005;23(36):9162–9171. doi: 10.1200/JCO.2005.08.557. [DOI] [PubMed] [Google Scholar]
- 31.Wijngaards-de Meij L, Stroebe M, Schut H, et al. Couples at risk following the death of their child: predictors of grief versus depression. J Consult Clin Psychol. 2005;73(4):617–623. doi: 10.1037/0022-006X.73.4.617. [DOI] [PubMed] [Google Scholar]
- 32.Fallowfield L, Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet. 2004;363(9405):312–319. doi: 10.1016/S0140-6736(03)15392-5. [DOI] [PubMed] [Google Scholar]
- 33.Fellowes D, Wilkinson S, Moore P. Communication skills training for health care professionals working with cancer patients, their families and/or carers. Cochrane Database Syst Rev. 2004;(2):CD003751. doi: 10.1002/14651858.CD003751.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Alexander SC, Keitz SA, Sloane R, Tulsky JA. A controlled trial of a short course to improve residents’ communication with patients at the end of life. Acad Med. 2006;81(11):1008–1012. doi: 10.1097/01.ACM.0000242580.83851.ad. [DOI] [PubMed] [Google Scholar]
- 35.Hays RM, Valentine J, Haynes G, et al. The Seattle pediatric palliative care project: effects on family satisfaction and health-related quality of life. J Palliat Med. 2006;9(3):716–728. doi: 10.1089/jpm.2006.9.716. [DOI] [PubMed] [Google Scholar]
- 36.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 37.Leuthner SR, Boldt AM, Kirby RS. Where infants die: examination of place of death and hospice/home health care options in the state of Wisconsin. J Palliat Med. 2004;7(2):269–277. doi: 10.1089/109662104773709396. [DOI] [PubMed] [Google Scholar]
- 38.Fowler K, Poehling K, Billheimer D, et al. Hospice referral practices for children with cancer: a survey of pediatric oncologists. J Clin Oncol. 2006;24(7):1099–1104. doi: 10.1200/JCO.2005.02.6591. [DOI] [PubMed] [Google Scholar]
- 39.Bluebond-Langner M, Belasco JB, Goldman A, Belasco C. Understanding parents’ approaches to care and treatment of children with cancer when standard therapy has failed. J Clin Oncol. 2007;25(17):2414–2419. doi: 10.1200/JCO.2006.08.7759. [DOI] [PubMed] [Google Scholar]
- 40.Hospice and Palliative Care Federation of Massachusetts. [Accessed June 28, 2007];Pediatric Palliative Care Program. Available at: http://www.hospicefed.org/hospice_pages/pedi_palliative_care.htm.
- 41.Solomon M, Dokken DL, Fleischman AR, et al. The initiative for pediatric palliative care (IPPC): background and goals. Newton, MA: Education Development Center, Inc.; 2002. Available at: www.ippcweb.org. AU: PLS PROVIDE LAST DATE ACCESSED. [Google Scholar]
- 42.Curtis JR, Wenrich MD, Carline JD, et al. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41–49. doi: 10.1111/j.1525-1497.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weitzen S, Teno JM, Fennell M, Mor V. Factors associated with site of death: a national study of where people die. Med Care. 2003;41(2):323–335. doi: 10.1097/01.MLR.0000044913.37084.27. [DOI] [PubMed] [Google Scholar]
- 44.Robins JM. Data, design, and background knowledge in etiologic inference. Epidemiology. 2001;12(3):313–320. doi: 10.1097/00001648-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Lynn J, Arkes HR, Stevens M, et al. Rethinking fundamental assumptions: SUPPORT’s implications for future reform. Study to Understand Prognoses and Preferences and Risks of Treatment. J Am Geriatr Soc. 2000;48(5 Suppl):S214–S221. doi: 10.1111/j.1532-5415.2000.tb03135.x. [DOI] [PubMed] [Google Scholar]
- 46.Dobratz MC. Enriching the portrait: methodological triangulation of life-closing theory. ANS Adv Nurs Sci. 2006;29(3):260–270. doi: 10.1097/00012272-200607000-00008. [DOI] [PubMed] [Google Scholar]