Abstract
The involvement of the mannose-induced Acanthamoeba cytopathic protein (MIP-133) in tissue injury and activation of metalloproteinase of corneal and stromal cells was examined in vitro.
Activation of MMP-1, MMP-2, MMP-3, and MMP-9 induced by MIP-133 on human corneal epithelial and stromal cell cultures was examined by reverse transcriptase polymerase chain reaction (RT-PCR), and ELISA.
MMP-1, MMP-2, MMP-3, and MMP-9 mRNA were expressed in both cultured human corneal epithelial and stromal cells. When the epithelial cells were exposed to MIP-133 protein, the mRNA expression for MMP-1 and MMP-9 was unchanged. However, the transcript for MMP-2 and MMP-3 was decreased by two fold. By contrast, the expression of MMP-2 and MMP-3 was significantly up-regulated (2–4 fold) in the corneal stromal cells 1, 4, and 8 hours after MIP-133 stimulation. At the protein level, there was no significant difference in the level of MMPs between the corneal epithelial cells before and after stimulation with MIP-133. By contrast, the levels of MMP-2 and MMP-3 were significantly higher in the corneal stromal cells stimulated with MIP-133. The supernatants from corneal stromal cells stimulated with MIP-133 were incubated with PMSF and MIP-133 antibody and the level of MMP-2 was measured by ELISA. Activation of MMP-2 by MIP-133 was inhibited in the supernatants pretreated with the serine protease inhibitor, PMSF, and anti-MIP-133. Supernatants pretreated with the cysteine protease inhibitor E6 or control antibody produced the same amount of MMP-2 as the untreated supernatants. To verify the possible of homology between MMPs and A. castellanii proteases, the mRNA from A. castellanii was prepared and analyzed for the expression of MMP genes by PT-PCR. The results showed that A. castellanii did not express mRNA for MMP-1, MMP-2, MMP-3, or MMP-9. Thus, A. castellanii mRNA does not cross react with human MMPs. Furthermore, ELISA was used to determine the cross reactivity of MMP antibodies with the MIP-133 protein. Monoclonal antibodies against MMPs did not cross react with either the MIP-133 protein or BSA (negative control antigen).
The results indicate that the MIP-133 protein modulates MMP-2 and-3 expression differently in human corneal epithelial and stromal cells.
Keywords: Acanthamoeba, Keratitis, cornea, Mannose Induced Protein, Metalloproteinases
1. Introduction
Acanthamoeba keratitis is a sight-threatening corneal disease caused by pathogenic free-living amoebae (McCulley et al., 2000). Acanthamoeba spp. are ubiquitous organisms that can be isolated from a wide variety of environments, including public water supplies, swimming pools, fresh water reservoirs, salt water, hot tubs, ventilation ducts, soil, bottled water, eyewash stations, and even contact lenses and lens cases (Auran et al., 1987; Brown et al., 1982; Nerad et al., 1995; Rivera et al., 1981). The disease is often associated with contact lens wear, which appears to be an important risk factor in infection.
The pathogenic cascade of Acanthamoeba keratitis involves a series of processes that include: (1) binding of the trophozoites to the corneal epithelial cells via lectin-glycoprotein interactions (Leher et al., 1999; Morton et al., 1991; Panjwani et al., 1990; Panjwani et al., 1992), (2) generation of cytopathic factors that destroy the corneal epithelium and stromal cells (Cao et al., 1998; Leher et al., 1998), (3) production of proteolytic enzymes that facilitate the invasion and penetration of trophozoites through the basement membrane and stroma (Cao et al., 1998; Hadas & Mazur, 1993; Mitra et al., 1995; Mitro et al., 1994), (4) elaboration of collagenolytic enzymes that degrade types I and IV collagens, which constitute the corneal matrix (Badenoch et al., 1990; Mitro et al., 1994)
We have shown that trophozoites exposed to free mannose for 48 hr or longer are induced to release a soluble 133-kDa cytolytic factor (MIP-133) that mediates contact-independent cytolysis of corneal epithelial cells in vitro (Leher et al., 1998). Therefore, the parasite’s binding to the mannose receptors induces the generation of cytopathic factors that destroy the corneal epithelial and stromal cells and is an important step in the pathogenicity of Acanthamoeba keratitis.
Stromal disease occurs late and is characterized by a ring infiltrate or abscesses containing single, multiple, or overlapping rings (McCulley et al., 2000). Since Acanthamoeba trophozoites produce a variety of extracellular proteases that may be important pathogenic factors in destruction of corneal tissues (Hadas & Mazur, 1993; Mitro et al., 1994), it is possible that trophozoites directly contribute to stromal degradation and the pathogenic cascade through the elaboration of collagenolytic enzymes. We and others have reported that Acanthamoeba spp. secrete collagenolytic enzymes, which digest collagen shields and purified type I collagen in vitro (Badenoch et al., 1990; He et al.,, 1990; Kong et al., 2000, Mitro et al., 1994). Moreover, ocular isolates of A. castellanii constitutively secrete a collagenolytic enzyme that produces severe stromal necrosis, edema, inflammation, and a ring–like infiltrate following intracorneal injection in rats (Badenoch et al., 1990). We have found that the mannose-induced protein (MIP-133), which is secreted by trophozoites, can degrade human collagen types I and IV (Hurt et al., 2003).
Collagenases are a small group of the matrix metalloproteinase (MMP) family, and are highly specific proteases capable of causing hydrolytic cleavage in the triple-helical region of collagen molecules (Mallya et al., 1992). In contrast to mammalian collagenase, which cleaves the collagen helix at a single site, bacterial collagenases attack multiple sites along the helix (Mallya et al., 1992).
In addition to pathogenic microorganisms, corneal cells have been shown to produce matrix metalloproteinases (Fini et al., 1992). These MMPs are usually secreted as inactive precursors and are activated extracellularly via pathway dependent proteases.
MMPs are a family of zinc dependent neutral endopeptidases and participate in tissue degradation and remodeling under physiological and pathological conditions. The proteolysis of the extracellular matrix seems to be a key initiating event in the progression of the inflammatory process. MMPs are synthesized and secreted by multiple cell types, including corneal epithelial and stromal cells. They are secreted as inactive enzymes (pro-MMPs) and their activation is a crucial step in the destruction and remodeling of the extracellular matrix and is specifically regulated by specific tissue inhibitors of MMPs (TIMPs). Activation of pro MMPs can be achieved in vitro by proteinases such as trypsin, elastase, cathepsin G, and plasmin (Lijnen et al., 1998; Tyagi et al., 1993).
Corneal injury and infection activate keratocytes to synthesize both extracelluar matrices and MMPs (Dong et al., 2001; Fini et al., 1998). It has been shown that the culture supernatant of Pseudomonas. aeruginosa degrades collagen directly, and stimulates in vitro collagen degradation by rabbit keratocytes (Haoet al., 1999). Moreover, it has been demonstrated that factors derived from P. aeruginosa activate the latent pro-matrix metalloproteinases produced by keratocytes (Hao et al., 1999; Matsumoto et al., 1993; Nagano et al., 2001). Kernacki et al.,(1997) demonstrated that increased proteolytic activity induced by MMP-2 and MMP-9 in ocular tissues during P. aeruginosa infection may contribute to the corneal damage observed during the infection.
We hypothesized that the MMP-133 protein can activate the corneal MMPs, which in turn contribute to the degradation of extracellular matrix. We used human cultured epithelial and stromal cells to examine the effect of MIP-133 protein on the activation of the three main groups of MMPs, gelatinases (MMP-2 and -9), collagenases (MMP-1) and stromelysins (MMP-3) in cultured human corneal epithelial and stromal cells. Moreover, several studies have shown that these MMPs are the major MMPs that are expressed in the human corneal epithelium and stroma during wound healing, bacterial infections, or other disorders of the cornea (Fini et al., 1992; Saghizadeh et al., 2001).
2. Materials and methods
2.1 Cell lines
Human telomerase-immortalized corneal epithelial cells (HCE) and normal human keratocytes (NHKs) were a generous gift from Dr. James Jester (The University of California at Irvine). HCE cells were cultured in KGM with Bullet Kit (CC-3111, Clonetics, Walkersville, MD). NHK cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; BioWhittaker) containing 10 mM HEPES buffer solution; 1% nonessential amino acid solution; 1% L-glutamine; 1% penicillin, streptomycin, and amphotericin B; 1% sodium pyruvate.
2.2. MIP-133 isolation
The MIP-133 protein was purified as stated previously (Hurt et al., 2003). Briefly, 10-fold-concentrated supernatants from A. castellanii cultures grown with 100 mM methyl-α-D-mannopyranoside, were analyzed by SDS-4 to 15% PAGE Ready Gels (Bio-Rad) under both reducing and nonreducing conditions. Supernatants were taken from trophozoites at mid-log phase. For fast protein liquid chromatography, culture supernatants of mannose-stimulated A. castellanii trophozoites were concentrated 10-fold with Ultrafree-15 centrifuge concentrators with a molecular cutoff of 5 kDa (Millipore, Bedford, Mass.). Samples were centrifuged at 3,000 × g for 20 min and passed in 0.5-ml volumes over a Superdex 200 (Amersham Pharmacia Biotech, Piscataway, N.J.) column with phosphate-buffered saline (PBS) (pH. 7.2). Fractions were collected every 0.5 ml and examined by SDS-4 to 15% PAGE, fractions containing the mannose-induced cytolytic protein were pooled and concentrated 10-fold, and the buffer was exchanged three times with 10 mM Tris buffer (pH 8.0) with Ultrafree concentrators. Two-hundred-microliter samples containing 1 mg of protein were applied to a DEAE ionic-exchange column by using 10 mM Tris buffer, pH 8.0 (buffer A). Adsorbed protein was eluted by using a gradient of 10 mM Tris buffer, pH 8.0, with 1 M NaCl (buffer B). Fractions were examined for the ~133-kDa protein by SDS-4 to 15% PAGE. An initial 15% buffer B step removed contaminating proteins, and the ~133-kDa protein was eluted between 15 to 30% buffer B run at 0.1 ml/min. Fractions containing the 133-kDa protein were pooled and concentrated 10-fold and washed three times with PBS (pH 7.2) to exchange the buffer. The protein concentration of the purified MIP133 was determined by bicinchoninic acid protein assay (BCA) with bovine serum albumin used as a standard (Smith et al., 1985).
2.3. mRNA isolation and RT-PCR
For MMP gene expression analysis, the corneal epithelial and stromal cells were treated with 7.5µg of MIP-133 protein /ml. Untreated cells served as a control. mRNA from HCE and NHK cells was isolated using Oligotex Direct mRNA Mini Kit (QIAGEN Inc, CA) as described previously (Alizadeh et al.,, 2005). The samples were treated with DNase I (0.2 U/µl; Ambion, Austin, TX) to remove possible DNA contamination. The RT-PCR was carried out by using SuperScript One –Step RT-PCR with Platium Tag (Invitrogen, CA) following the manufacturer’s instruction. Relatively equal amounts of mRNA (50ng) from each sample were used for this reaction. Cycle parameters were generally a 1 cycle of 45–55° C for 15–30 min and 94° C for 2 min for cDNA synthesis and pre-denaturation, 35 cycles of: 94° C for 30 s, 60–64° C for 30 s, 68–72° C for 1 min for PCR amplification, and 1 cycle of 72° C for 7 min for final extension. The PCR products were visualized in 2.0% agarose gels. β-actin mRNA levels were used as an internal control. The primer pairs for MMP1, 2, 3, and 9 were obtained from R&D systems (R&D Systems, Inc, Minneapolis, MN). The gene expression was quantified by Image Quant software analysis and the results are expressed as fold increase over β-actin gene expression.
2.4. Supernatant preparation
The MIP-133 protein was added at 7.5µg of protein /ml to flasks of confluent monolayers of HCE and NHK cells in serum-free medium and incubated for 24 hours at 37 ° C in 5 ml. Supernatants from HCE and NHK were collected and concentrated with Ultrafree-15 centrifuge concentrators with a molecular cutoff of 5 kDa (Millipore, Bedford, Mass.). Unstimulated supernatants from HCE and NHK cells were processed similarly. Supernatants were stored at −20° C for ELISA analysis.
2.5. ELISA
96-well assay plates were coated with 50 ul of supernatants (50 ug protein) overnight in carbonate buffer. Plates were washed four times with PBS containing 0.05% Tween-20 (wash buffer; Sigma), then blocked with 0.5% BSA in PBS for 1 hour at room temperature. All MMPs antibodies were diluted in blocking buffer and different dilutions of rabbit anti human MMP-1, MMP-2, MMP-3, and MMP-9 monoclonal antibodies (Chemicon Temecula, CA) were added and incubated at room temperature for 2 hrs. The plates were then washed in PBS and incubated in a 1:2,000 dilution of peroxidase conjugated goat anti-mouse IgG (Vector Laboratories, Inc. Burlingame, CA) in blocking buffer. After 1 h, the membrane was washed and developed using ABC detection kit (Vector Laboratories) with DAB as chromogen as recommended by the manufacturer. After development, 100 ul of 10% SDS (Sigma) was added per well prior to reading on a microplate reader at 405 nm.
Inhibition assays involved incubating MIP-133 stimulated stromal cell supernatants with serine protease inhibitors, 1 mM phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich), or MIP-133 antibody. Supernatants incubated with a cysteine protease inhibitor (E6) or a control antibody (Chicken IgY) served as controls. All experiments were performed in triplicate.
2.12. Statistical analysis
All statistical analyses were performed using the unpaired Student's t-test. A value of P < 0.05 was considered statistically significant.
3. Results
3.1 Regulation of MMPs gene expression in cultured human epithelial and stromal cells and activation by MIP-133 protein
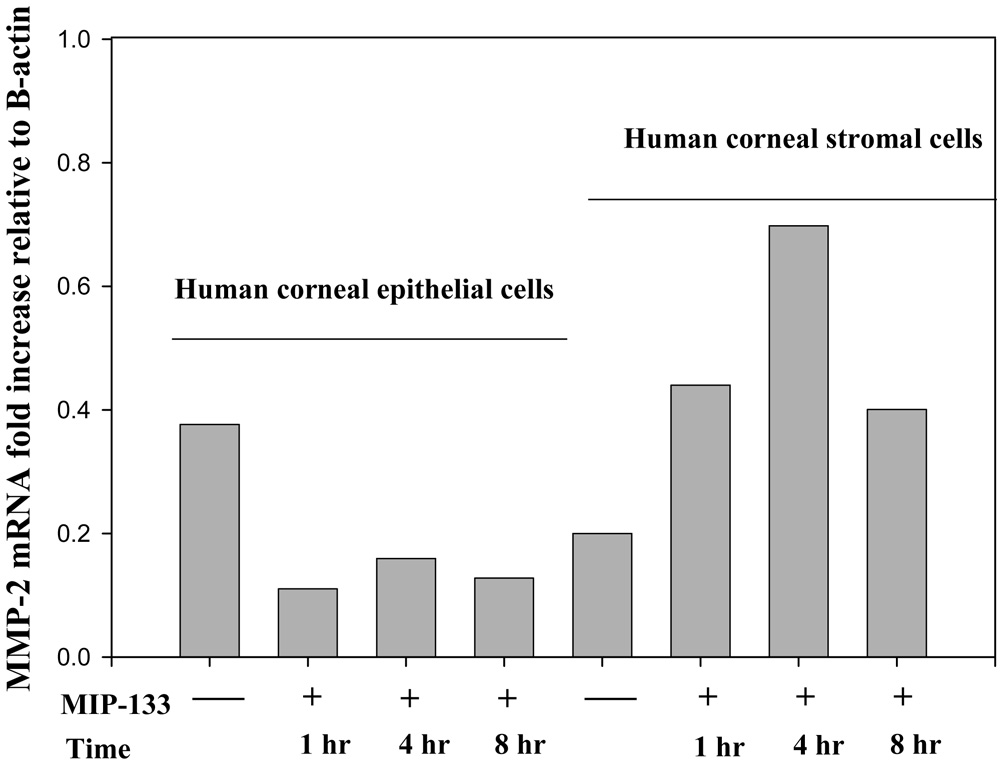
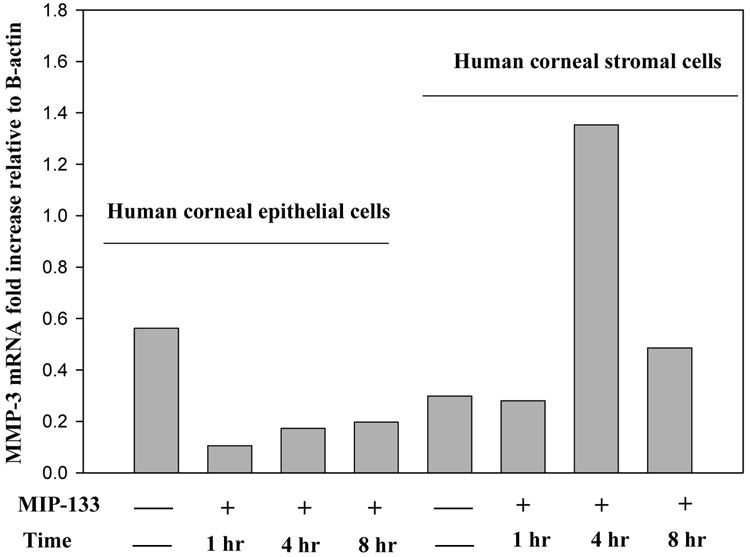
We determined whether normal human corneal epithelial and stromal cells express mRNA for MMP-1, MMP-2, MMP-3, and MMP-9, and whether MMP-133 protein modulates MMPs expression in vitro. Accordingly, the expression of MMP-1 (interstitial collagenase) MMP-2 and MMP-9 (representative of MMP’s responsible for degradation of collagens type I and IV), and MMP-3 (stromelysin) was determined in human corneal epithelial and stromal cells. The 133-kDa protein (7.5 µg/well) was added to 96 well plates containing human corneal epithelial or stromal cells and incubated for 2–8 hr. Cells cultured without the 133-kDa protein served as a control. Corneal epithelial and stromal cells were collected from each well and analyzed for the expression of MMP genes by RT-PCR. mRNA from A. castellanii without corneal cells served as a control. β-actin was used as an internal control. The results showed that MMP-1, MMP-2, MMP-3, and, MMP-9 was expressed in both cultured corneal epithelial and stromal cells. When the epithelial cells were exposed to MIP-133 protein, the mRNA expression for MMP-1 and MMP-9 was unchanged (data not shown). However, the transcripts for MMP-2 and MMP-3 were decreased by two fold (Figure 1 and Figure 2). By contrast, the expression of MMP-2 and MMP-3 was significantly upregulated (2–4 fold) in the stromal cells 1, 4, and 8 hours after MIP-133 stimulation (Figure 1 and Figure 2). The expression of MMP-1 and MMP-9 mRNA expression in stroma cells was not appreciably affected by MIP-133 stimulation. The results indicate that the MIP-133 protein modulates MMP-2 and MMP-3 gene expression differently in human corneal epithelial and stromal cells.
Fig. 1.
Semi-quantitative RT-PCR analysis of MMP-2 gene expression in the human corneal epithelial (HCE) and stromal (NHK) cells before and 1, 4, and 8 hours after stimulation with the mannose induced protein (MIP-133). β-actin was used as an internal control.
Fig. 2.
Semi-quantitative RT-PCR analysis of MMP-3 gene expression in the human corneal epithelial (HCE) and stromal (NHK) cells before and 1 , 4, and 8 hours after stimulation with the mannose induced protein (MIP-133). β-actin was used as an internal control.
To verify the possibility of homology between MMPs and A. castellanii proteases, the mRNA from A. castellanii was prepared and analyzed for the expression of MMP genes by PT-PCR. The results showed that A. castellanii did not express mRNA for MMP-1, MMP-2, MMP-3, or MMP-9 (data not shown).
3.2 Regulation of MMPs protein expression in cultured human epithelial and stromal cells and activation by MIP-133 protein
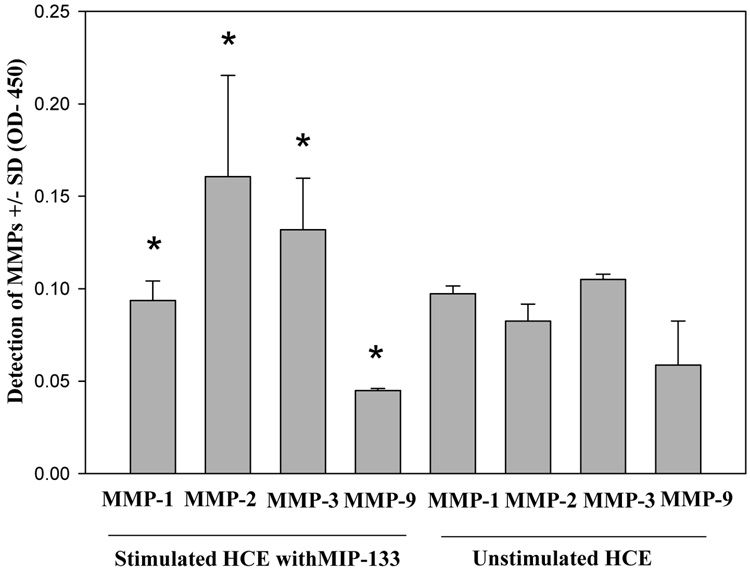
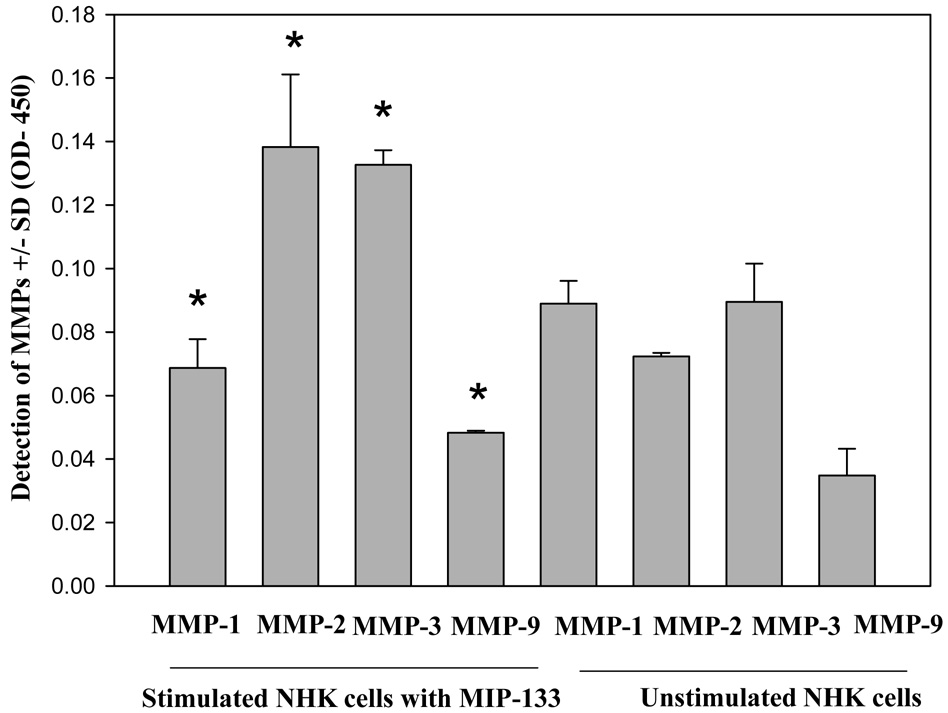
Corneal epithelial and stromal cells were stimulated with MIP-133 and the supernatants were collected after 24 hrs and the levels of MMP-1, MMP-2, MMP-3, and MMP-9 proteins were measured by ELISA. There was no significant difference in the level of MMPs between the corneal epithelial cells before or after stimulation with MIP-133 (Fig. 3). By contrast, the levels of MMP-2 and MMP-3 were significantly higher in the corneal stromal cells stimulated with MIP-133 (Fig. 4).
Fig. 3.
Effect of MIP-133 on MMPs released by human corneal epithelial cells. Cells were incubated with 7.5ug/ml MIP-133 for 24 hours and the amount of MMPs in the supernatants was determined by ELISA. *, Not significantly different from corresponding controls (P > 0.05).
Fig. 4.
Effect of MIP-133 on MMPs released by human stromal cells. Cells were incubated with 7.5ug/ml MIP-133 for 24 hours and the amount of MMPs in the supernatants was determined by ELISA. *, significantly different from corresponding controls (P < 0.05).
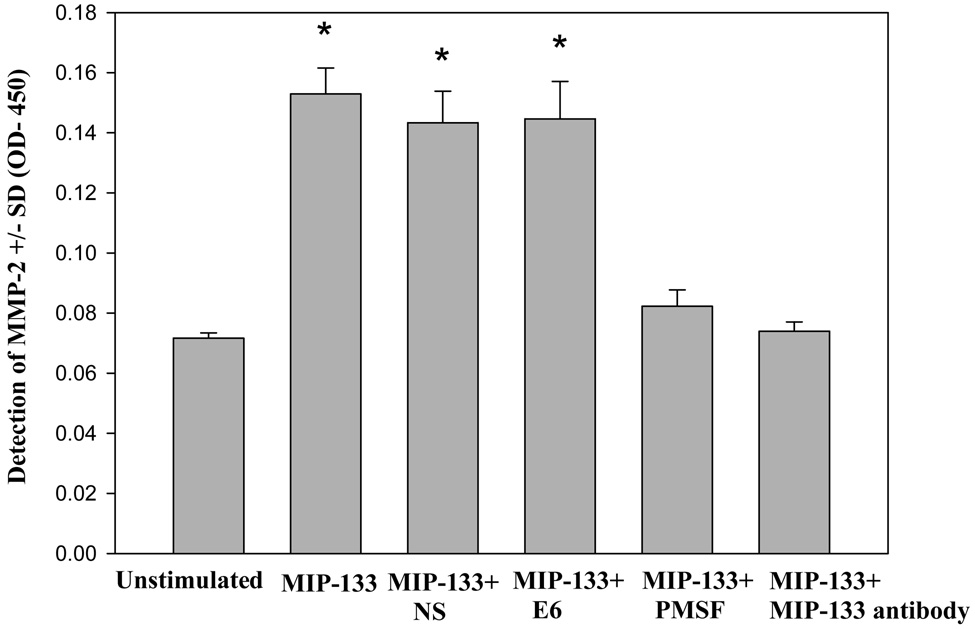
3.3 Inhibition of MIP-133 induced the release of MMP-2 in the human stromal cells by anti-MIP-133 antibody and protease inhibitor
Previous studies in our lab have shown that the MIP-133 function could be inhibited by the serine protease inhibitor PMSF and MIP-133 antibody (Hurt et al., 2003). Therefore, it was important to determine if inhibition of MIP-133 can block activation of MMP-2 and MMP-3 by the corneal stromal cells.
The supernatants from corneal stromal cells stimulated with MIP-133 were incubated with PMSF and MIP-133 antibody and the level of MMP-2 was measured by ELISA as described above. As a control, supernatants were incubated with a cysteine protease inhibitor (E6) or control antibody (Chicken IgY). MIP-133 induced significant increase on the level of MMP-2 in the corneal stromal cells (Fig. 5). Activation of MMP-2 by MIP-133 was inhibited in the supernatants pretreated with PMSF and anti-MIP-133 (Fig. 5). Supernatants pretreated with the cysteine protease inhibitor E6 or control antibody produced the same amount of MMP-2 as the untreated supernatants (Fig. 5). Production of MMP-3 was also inhibited in the supernatants pretreated with PMSF and MIP-133 antibody (data not shown). Likewise, pretreatment of unstimulated supernatants from corneal stromal cells with PMSF and MIP-133 had no significant effect on MMPs activity (data not shown).
Fig. 5.
Inhibition of MIP-133 prevents MMP-2 released by human corneal stromal cells. Human corneal stromal cells were incubated with 7.5ug/ml MIP-133 in the presence of 1:100 dilution of the chicken anti-MIP-133 antiserum (IMS). Normal chicken serum (NS) was used as a control. Additional controls included 1.0 mM of phenylmethylsulfonyl fluoride (PMSF, serine protease inhibitor) and 10 µM of E6 (cytosine protease inhibitor). After 24 hours, the supernatants were collected and the level of MMP-2 was determined by ELISA. Plates were then read at OD 405 nm. *, significantly different from MIP-133, MIP-33+NS, and MIP-133 +E6 (P < 0.05).
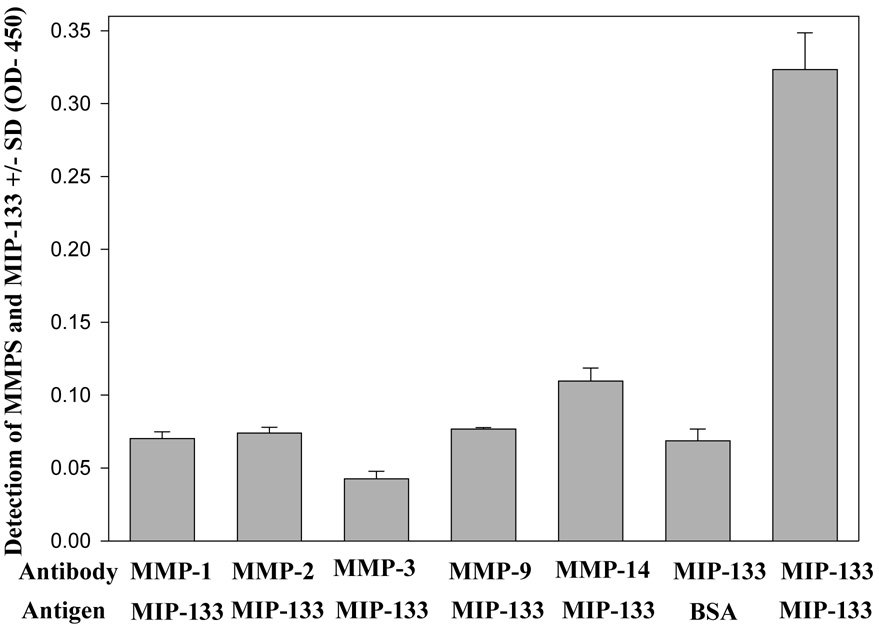
3.4 Cross reactivity of MMPs with MIP-133 protein
ELISA was used to determine the cross reactivity of MMP antibodies with the MIP-133 protein. Monoclonal antibodies against the various MMPs did not cross react with either the 133-kDa protein or BSA (Fig. 6). The results indicate that MIP-133 protein do not cross react with human MMPs antibodies.
Fig. 6.
Cross reactivity of MMPs anybodies with MIP-133 protein. 96-well plates were coated with 50ug of the MIP-133 protein and allowed to dry in carbonate buffer. Sample wells were then washed 3 times, incubated with mouse anti-MMPs antibodies as the primary, followed by goat anti-mouse IgG-HRP as described in the Materials and Methods section. Plates were then read at OD 405. As a control, the MIP-133 protein or BSA was co-incubated with 1:100 chicken anti-MIP-133 antiserum or pre-immune chicken serum. Wells were then washed and incubated with Goat anti-chicken -horse radish peroxidase. ELISA plates were developed and read at 405nm.
DISUSSION
Acanthamoeba mannose induced protein (MIP-133) may contribute to the pathogenesis of Acanthamoeba keratitis in several ways. For example, as shown in earlier studies, this proteolytic enzyme was efficient at killing corneal epithelial cells, the first mechanistic barrier of the ocular surface, by inducing apoptosis via a caspase-3-dependent pathway. Subsequent steps in pathogenesis require the amoebae to penetrate and degrade both human types I and IV collagen (Hurt et al., 2003). The purpose of this study was to determine the effect of MIP-133 on the modulation of matrix metalloproteinases of cultured human epithelial and stromal cells in vitro. We hypothesized that MIP-133 can activate the corneal pro MMPs, which in turn contribute to the degradation of extracellular matrix.
The results showed that MMP1, MMP-2, MMP-3, and MMP-9 mRNA was expressed in both cultured corneal epithelial and stromal cells. These results indicate that MMPs are constitutively produced, at least under our experimental conditions. The mRNA expression for these MMPs did not change 1 hr after exposure to MIP-133. However, these matrix MMPs are no longer detectable when corneal epithelial cells are treated with MIP-133 for 4 and 8 hours. Disappearance of activated MMPs in corneal epithelial cells 4 and 8 hours after exposure to MIP-133 might be due to degradation of MMPs RNA by MIP-133 or the result of injury to the epithelial cells. However, we have shown that 7.5 ug/ ml MIP-133 is not toxic to epithelial cells during these incubation times. Previous studies have shown that MMP-9 is down regulated in the rabbit cornea by Pseudomonas elastase (Miyajima et al., , 2001). The mRNA for all these MMPs was expressed in normal stromal cells. However, the expression of MMP-2, and MMP-3 mRNA was upregulated after treatment with MIP-133. The expression of MMP-1 and 9 was no longer detectable in stromal cells treated with MIP-133 for 8 hours. At the protein level, treatment of the corneal stromal cells with MIP-133 induced an increase in the expression of MMP-2 and MMP-3, while the levels of these MMPs were not altered in the corneal epithelial cells after MIP-133 stimulation. Thus, the accumulation of matrix components seems to be an important effect of MIP-133 in fibroblastic cells as compared with epithelial cells. Our study suggests that one possible consequence of increased MIP-133 proteolytic activity on the ocular surface would be stimulated production of MMPs by the corneal stromal cells. Theses results also suggest that increased MMP-2 and MMP-3 secretion by stromal cells after MIP-133 stimulation may be associated with pathologic processes such as corneal melting in Acanthamoeba keratitis. We have shown that MIP-133 has proteolytic activity which can degrade both human types I and IV collagen (Hurt et al., 2003). The present results indicate that inhibition of MIP-133 activity by PMSF and MIP-133 antibody inhibits MMP-2 and MMP-3 activity by the corneal stromal cells. These results suggest that MIP-133 is a potent activator of MMPs with the effect being produced by functionally active MIP-133. Our results also indicate that MIP-133 does not cross react with human MMP since anti-MMPs antibodies did not react with MIP-133 in the ELISA’s.
It is not known how proteolytic molecules induce upregulation or activation of MMPs in corneal tissues. We have shown that MIP-133 induces apoptosis of corneal epithelial and stromal cells through a caspase-dependent pathway. Thus, it is possible that apoptosis of corneal stromal cells induces upregultaion of proapoptotic molecules such a TNF-α and FasL that may be involved in activation and upregulation of MMPs in the corneal tissues. It has been shown that Pseudomonas aeruginosa induces apoptosis of host cells through the upregulation of Fas /Fas ligand on the cell surface. Upregulation of MMP-3 expression by IL-1β or TNF-α in human ocular stromal fibroblast and other tissues has been reported previously (Girard et al., 1991; Li et al., 2000). More recently it has been shown that apoptosis of human lung fibroblasts by P. aeruginosa was due to activation of phosophlipase A2 on the lung fibroblasts and arachidonic acid release (Kirschnek & Gulbins, 2006). We have shown that MIP-133 induced significant arachidonic acid release from the cells that are susceptible to apoptosis (unpublished findings). Therefore, it is possible that inflammatory mediators that are induced by MIP-133 modulate MMP production in corneal tissues. It has been shown that cyclooxygenase-2-derived prostaglandins downregulate MMP-1 expression in fibroblast –like synoviocytes (Pillinger et al., 2003). It is known that the regulation of MMP activation and activity is strongly dependent on the levels of tissue inhibitors of metalloproteinases (TIMPs) (Brew et al., 2000). Our results indicate that the133-kDa protein modulates MMP-2 and-3 expression differently in human corneal epithelial and stromal cells. Therefore, the difference in levels of TIMPs and their regulation by certain inflammatory factors might explain the different patterns of MMPs in the corneal epithelium and stromal cells. It has been shown that TIMP-1 and TIMP-2 were expressed strongly in the corneal epithelium of mice infected with herpes simplex virus as compared to keratocytes (Yang et al., 2003). Thus, a balance between MMPs and their inhibitors may determine the net enzymatic activity present in the corneal cells.
In conclusion, our results suggest a unique pathological function of MIP-133 related to the production and activation of MMPs in corneal epithelial and stromal cells, which may facilitate the invasion of Acanthamoeba trophozoites and intensify the severity of Acanthamoeba keratitis. Thus, MMPs as well as MIP-133 may become targets for therapeutic agents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alizadeh H, Neelam S, Hurt M, Niederkorn JY. Role of contact lens wear, bacterial flora, and mannose-induced pathogenic protease in the pathogenesis of amoebic keratitis. Infect Immun. 2005;73:1061–1068. doi: 10.1128/IAI.73.2.1061-1068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auran JD, Starr MB, Jakobiec FA. Acanthamoeba keratitis. A review of the literature. Cornea. 1987;6:2–26. [PubMed] [Google Scholar]
- Badenoch PR, Johnson AM, Christy PE, Coster DJ. Pathogenicity of Acanthamoeba and a Corynebacterium in the rat cornea. Arch Ophthalmol. 1990;108:107–112. doi: 10.1001/archopht.1990.01070030113040. [DOI] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Cursons RT, Keys EA. Amoebae from antarctic soil and water. Appl Environ Microbiol. 1982;44:491–493. doi: 10.1128/aem.44.2.491-493.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Jefferson DM, Panjwani N. Role of carbohydrate-mediated adherence in cytopathogenic mechanisms of Acanthamoeba. J Biol Chem. 1998;273:15838–15845. doi: 10.1074/jbc.273.25.15838. [DOI] [PubMed] [Google Scholar]
- Dong Z, Katar M, Alousi S, Berk RS. Expression of membrane-type matrix metalloproteinases 4, 5, and 6 in mouse corneas infected with P. aeruginosa. Invest Ophthalmol Vis Sci. 2001;42:3223–3227. [PubMed] [Google Scholar]
- Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998;290 Suppl:12–23. doi: 10.1007/pl00007449. [DOI] [PubMed] [Google Scholar]
- Fini ME, Yue BY, Sugar J. Collagenolytic/gelatinolytic metalloproteinases in normal and keratoconus corneas. Curr Eye Res. 1992;11:849–862. doi: 10.3109/02713689209033483. [DOI] [PubMed] [Google Scholar]
- Girard MT, Matsubara M, Fini ME. Transforming growth factor-beta and interleukin-1 modulate metalloproteinase expression by corneal stromal cells. Invest Ophthalmol Vis Sci. 1991;32:2441–2454. [PubMed] [Google Scholar]
- Hadas E, Mazur T. Biochemical markers of pathogenicity and virulence of Acanthamoeba sp. strains. Parasitol Res. 1993;79:696–698. doi: 10.1007/BF00932513. [DOI] [PubMed] [Google Scholar]
- Hao JL, Nagano T, Nakamura M, Kumagai N, Mishima H, Nishida T. Effect of galardin on collagen degradation by Pseudomonas aeruginosa. Exp Eye Res. 1999;69:595–601. doi: 10.1006/exer.1999.0755. [DOI] [PubMed] [Google Scholar]
- He YG, Niederkorn JY, McCulley JP, Stewart GL, Meyer DR, Silvany R, Dougherty J. In vivo and in vitro collagenolytic activity of Acanthamoeba castellanii. Invest Ophthalmol Vis Sc. 31;1990:2235–2240. [PubMed] [Google Scholar]
- Hurt M, Neelam S, Niederkorn J, Alizadeh H. Pathogenic Acanthamoeba spp secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease. Infect Immun. 2003;71:6243–6255. doi: 10.1128/IAI.71.11.6243-6255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernacki KA, Fridman R, Hazlett LD, Lande MA, Berk RS. In vivo characterization of host and bacterial protease expression during Pseudomonas aeruginosa corneal infections in naive and immunized mice. Curr Eye Res. 1997;16:289–297. doi: 10.1076/ceyr.16.4.289.10686. [DOI] [PubMed] [Google Scholar]
- Kirschnek S, Gulbins E. Phospholipase A2 functions in Pseudomonas aeruginosa-induced apoptosis. Infect Immun. 2006;74:850–860. doi: 10.1128/IAI.74.2.850-860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Kim TH, Chung DI. Purification and characterization of a secretory serine proteinase of Acanthamoeba healyi isolated from GAE. J Parasitol. 2000;86:12–17. doi: 10.1645/0022-3395(2000)086[0012:PACOAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Leher H, Kinoshita K, Alizadeh H, Zaragoza FL, He Y, Niederkorn J. Impact of oral immunization with Acanthamoeba antigens on parasite adhesion and corneal infection. Invest Ophthalmol Vis Sci. 1998;39:2337–2343. [PubMed] [Google Scholar]
- Leher H, Zaragoza F, Taherzadeh S, Alizadeh H, Niederkorn JY. Monoclonal IgA antibodies protect against Acanthamoeba keratitis. Exp Eye Res. 1999;69:75–84. doi: 10.1006/exer.1999.0678. [DOI] [PubMed] [Google Scholar]
- Li DQ, Shang TY, Kim HS, Solomon A, Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, -10, and -11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:2928–2936. doi: 10.1167/iovs.02-0874. [DOI] [PubMed] [Google Scholar]
- Lijnen HR, Silence J, Van Hoef B, Collen D. Stromelysin-1 (MMP-3)-independent gelatinase expression and activation in mice. Blood. 1998;91:2045–2053. [PubMed] [Google Scholar]
- Mallya SK, Partin JS, Valdizan MC, Lennarz WJ. Proteolysis of the major yolk glycoproteins is regulated by acidification of the yolk platelets in sea urchin embryos. J Cell Biol. 1992;117:1211–1221. doi: 10.1083/jcb.117.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Shams NB, Hanninen LA, Kenyon KR. Cleavage and activation of corneal matrix metalloproteases by Pseudomonas aeruginosa proteases. Invest Ophthalmol Vis Sci. 1993;34:1945–1953. [PubMed] [Google Scholar]
- McCulley JP, Alizadeh H, Niederkorn JY. The diagnosis and management of Acanthamoeba keratitis. CLAO J. 2000;26:47–51. [PubMed] [Google Scholar]
- Mitra MM, Alizadeh H, Gerard RD, Niederkorn JY. Characterization of a plasminogen activator produced by Acanthamoeba castellanii. Mol Biochem Parasitol. 1995;73:157–164. doi: 10.1016/0166-6851(94)00109-z. [DOI] [PubMed] [Google Scholar]
- Mitro K, Bhagavathiammai A, Zhou OM, Bobbett G, McKerrow JH, Chokshi R, Chokshi B, James ER. Partial characterization of the proteolytic secretions of Acanthamoeba polyphaga. Exp Parasitol. 1994;78:377–385. doi: 10.1006/expr.1994.1041. [DOI] [PubMed] [Google Scholar]
- Miyajima S, Akaike T, Matsumoto K, Okamoto T, Yoshitake J, Hayashida K, Negi A, Maeda H. Matrix metalloproteinases induction by pseudomonal virulence factors and inflammatory cytokines in vitro. Microb Pathog. 2001;31:271–281. doi: 10.1006/mpat.2001.0470. [DOI] [PubMed] [Google Scholar]
- Morton LD, McLaughlin GL, Whiteley HE. Adherence characteristics of three strains of Acanthamoeba. Rev Infect Dis. 1991;13:S424. doi: 10.1093/clind/13.supplement_5.s424. [DOI] [PubMed] [Google Scholar]
- Nagano T, Hao JL, Nakamura M, Kumagai N, Abe M, Nakazawa T, Nishida T. Stimulatory effect of pseudomonal elastase on collagen degradation by cultured keratocytes. Invest Ophthalmol Vis Sci. 2001;42:1247–1253. [PubMed] [Google Scholar]
- Nerad TA, Sawyer TK, Lewis EJ, McLaughlin SM. Acanthamoeba pearcei n. sp. (Protozoa: Amoebida) from sewage contaminated sediments. J Eukaryot Microbiol. 1995;42:702–705. doi: 10.1111/j.1550-7408.1995.tb01619.x. [DOI] [PubMed] [Google Scholar]
- Panjwani N, Zaidi TS, Gigstad JE, Jungalwala FB, Barza M, Baum J. Binding of Pseudomonas aeruginosa to neutral glycosphingolipids of rabbit corneal epithelium. Infect Immun. 1990;58:114–118. doi: 10.1128/iai.58.1.114-118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani N, Zhao Z, Baum J, Pereira M, Zaidi T. Acanthamoebae bind to glycolipids of rabbit corneal epithelium. Infect Immun. 2002;60:3460–3463. doi: 10.1128/iai.60.8.3460-3463.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger MH, Rosenthal PB, Tolani SN, Apsel B, Dinsell V, Greenberg J, Chan ES, Gomez PF, Abramson SB. Cyclooxygenase-2-derived E prostaglandins down-regulate matrix metalloproteinase-1 expression in fibroblast-like synoviocytes via inhibition of extracellular signal-regulated kinase activation. J Immunol. 2003;171:6080–6089. doi: 10.4049/jimmunol.171.11.6080. [DOI] [PubMed] [Google Scholar]
- Rivera F, Galvan M, Robles E, Leal P, Gonzalez L, Lacy AM. Bottled mineral waters polluted by protozoa in Mexico. J Protozool. 1981;28:54–56. doi: 10.1111/j.1550-7408.1981.tb02803.x. [DOI] [PubMed] [Google Scholar]
- Saghizadeh M, Brown DJ, Castellon R, Chwa M, Huang GH, Ljubimova JY, Rosenberg S, Spirin KS, Stolitenko RB, Adachi W, Kinoshita S, Murphy G, Windsor LJ, Kenney MC, Ljubimov AV. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: a possible mechanism of basement membrane and integrin alterations. Am J Pathol. 2001;158:723–734. doi: 10.1016/S0002-9440(10)64015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Solomon A, Li DQ, Lee SB, Tseng SC. Regulation of collagenase, stromelysin, and urokinase-type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2000;41:2154–2163. [PubMed] [Google Scholar]
- Tyagi SC, Ratajska A, Weber KT. Myocardial matrix metalloproteinase(s): localization and activation. Mol Cell Biochem. 1993;126:49–59. doi: 10.1007/BF01772207. [DOI] [PubMed] [Google Scholar]
- Yang YN, Bauer D, Wasmuth S, Steuhl KP, Heiligenhaus A. Matrix metalloproteinases (MMP-2 and 9) and tissue inhibitors of matrix metalloproteinases (TIMP-1 and 2) during the course of experimental necrotizing herpetic keratitis. Exp Eye Res. 2003;77:227–237. doi: 10.1016/s0014-4835(03)00112-x. [DOI] [PubMed] [Google Scholar]