Abstract
The Lewis (LEW) strain of rat appears more sensitive to nicotine than other strains in self administration, conditioned place preference, and drug discrimination behavioral studies. The present study sought to further evaluate the behavioral effects of chronic nicotine treatment in the LEW strain by assessing spontaneous activity, which has consistently revealed sensitization to chronic nicotine administration in Sprague Dawley (SD) rats. High active and low active male and female LEW rats (N = 8 per group) were treated twice daily with either nicotine (0.4 mg/kg, sc) or vehicle for 14 consecutive days. Regardless of baseline activity level or sex, spontaneous activity was significantly decreased, compared to saline-treated rats, after a single nicotine injection. However, spontaneous activity increased in both low and high activity rats (both sexes) over the two-weeks of nicotine administration to levels that were significantly higher than saline-treated rats. Based on these findings, acute and chronic nicotine administration had greater suppressive and enhancing effects on spontaneous activity in LEW rats compared to other strains of rats previously studied. These results further clarify the behavioral sensitivity of the LEW strain of rat to nicotine exposure and lend credence to the role of genetics in the individual susceptibility to nicotine dependence.
Keywords: nicotine, Lewis, activity, sensitization, rat, chronic
1. Introduction
A major question in drug dependence research concerns the susceptibility of individual humans to develop dependency on tobacco (Rosecrans and Karan, 1993). Nicotine, the principal active component in tobacco products, has been studied extensively in rats, and different responses to nicotine have been found across strains. In particular, two inbred strains of rats, Lewis (LEW) and Fischer 344 (F-344), exhibit differences in their responses to nicotine, as well as to other drugs of abuse (for review, see Kosten and Ambrosio, 2002). Nicotine self-administration is more readily established in LEW rats than in F-344 rats (Shoaib et al., 1997; Brower et al., 2002). Moreover, LEW rats have been found to more rapidly learn to self-administer cocaine (Kosten et al., 1997) and other drugs of abuse (Suzuki et al., 1988; Suzuki et al., 1992) than F-344 rats. LEW rats exhibit a greater sensitivity to nicotine compared to F-344 rats in conditioned place preference (Horan et al., 1997; Philibin et al., 2005), and the nAChR antagonist mecamylamine elicits conditioned place aversion in LEW, but not F-344, rats (Suzuki et al., 1999). LEW rats also exhibit a greater conditioned taste aversion to nicotine (Pescatore et al., 2005) than F-344 rats. LEW rats have been found to be more sensitive to nicotine than F-344 rats in drug discrimination, as evidenced by the fact that LEW rats can be trained to discriminate 0.4 mg/kg nicotine from saline whereas F-344 rats require a higher nicotine dose, 0.9 mg/kg, to acquire this discrimination (Philibin et al., 2005). Furthermore, nicotine-induced increases in dopamine release in the nucleus accumbens have been found to be significantly greater in LEW compared to F-344 rats (Sziraki et al., 2001).
There is also extensive evidence that nicotine’s effects vary depending on the individual’s baseline level of physiological arousal. For example, in male and female SD and F-344 rats ranked according to high and low baseline activity, repeated administration of nicotine (0.4 mg/kg) has been shown to decrease activity in high-activity rats, while increasing activity in low-activity rats (Rosecrans, 1971b; Rosecrans, 1971a; Rosecrans and Schechter, 1972; Rosecrans, 1995). These “behavioral normalizing” effects of nicotine have also been observed in both male and female Sprague Dawley (SD) rats using several models, including habituation and startle (Rosecrans and Chance, 1978).
The present investigation was designed to further illuminate nicotine’s subtle effects on behavior by studying male and female LEW rats selected for low and high levels of baseline spontaneous activity. The LEW rat was chosen for study because, as reviewed above, it is especially sensitive to nicotine relative to other strains of rats (Philibin et al., 2005). The present studies compared the acute and chronic effects of nicotine on locomotor activity, using methods established in this laboratory to study male and female SD rats (e.g., Rosecrans, 1971a; Rosecrans, 1972).
2. Methods
2.1 Subjects
This study was conducted in accordance with the National Institute of Health Guidelines for the Care and Use of Animals in Research and the protocols were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. Sixty-four (64) male and female LEW rats (Harlan Laboratories, Indianapolis, IN) were purchased and housed in individual plastic cages in an animal facility maintained at constant temperature and humidity. Animal rooms were set on a 0600–1800 hr light-dark cycle. Rats were 60 days old when received and were acclimated for 7 days after arrival, during which time they were handled daily. Rats had ad lib access to food and water. Male and female LEW rats were studied in separate groups within 60 days of each other, using identical methods.
2.2 Drugs
Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) was dissolved in a 0.01 M phosphate buffer (pH=7.4). Nicotine and vehicle were administered subcutaneously at a volume of 1 ml/kg. All nicotine concentrations are expressed as free-base.
2.3 Initial activity assessment
One week after arrival to the vivarium, activity levels were assessed by photobeam crosses using open field test chambers (ENV 515, Med-Associates, St. Albans, VT) for 3-min daily sessions over 5 consecutive days. Using the average activity number of photobeam crosses from days 4 and 5 for each subject, subjects of each sex (N=32) were ranked and separated into High (H) and Low (L) Activity (A) groups, with 16 subjects per group. Subjects in the both the HA and LA groups were further subdivided into two groups of equal size (nicotine and vehicle groups; N=8) and matched for average activity using activity counts from days 4 and 5.
2.4 Behavioral testing regimen
All subjects received 0.4 mg/kg (sc) nicotine or vehicle (0.01M phosphate buffer, pH=7.4; sc), depending on group assignment, twice per day for 14 consecutive days, which has been shown to be more effective than once-a-day treatments for eliciting upregulation of nAChRs (Rowell and Li, 1997). All injections were performed at approximately 0900 and 1700 hours daily. Nicotine or vehicle was administered to each rat in the behavioral laboratory 5 min prior to each test session following doses 1, 13 or 27 (i.e., after a single injection, 1 week and 2 weeks of injections, respectively). During test sessions, each subject’s cumulative activity level was recorded for a period of 20 min.
2.5 Data Analysis
The number of photobeam crosses for the 20 min locomotor activity test sessions were recorded and the mean (± the standard error of the mean [SEM]) number of crosses for each group was calculated. Two-factor repeated measures ANOVAs were used to analyze the effects of the following factors on the number of photobeam crosses within each activity group for male and female rats: 1) treatment (nicotine vs. vehicle) and 2) number of sessions (day 1 vs. week 1 vs. week 2). Statistical comparisons between male and female rats were not conducted because males and female rats were studied at separate times.
3. Results
3.1 Initial locomotor activity
Male rats selected for HA (N=16) exhibited a mean baseline rate over days 4 and 5 of 308 ± 13 counts/3 min (range = 242 to 423 mean counts/3 min), while male rats selected for LA (N=16) exhibited a baseline rate over days 4 and 5 of 185 ± 10 counts/3min (range = 74 to 241 mean counts/3 min). Female rats selected for HA (N=16) averaged 448 ± 11 counts/3 min over days 4 and 5 (range = 372 to 509 mean counts/3 min) and those selected for LA (N=16) exhibited a rate of 287 ± 15 counts/3 min (range = 183 to 362 mean counts/3 min). Females appeared more active than male subjects, which has been a consistent finding in this laboratory using the same behavioral protocol. HA and LA rats were equally divided into groups that received either nicotine (N=8) or vehicle (N=8). However, one subject was later removed from the male LA vehicle group and one subject from the male LA nicotine group due to poor health.
3.2 Effects of nicotine on spontaneous activity in male rats
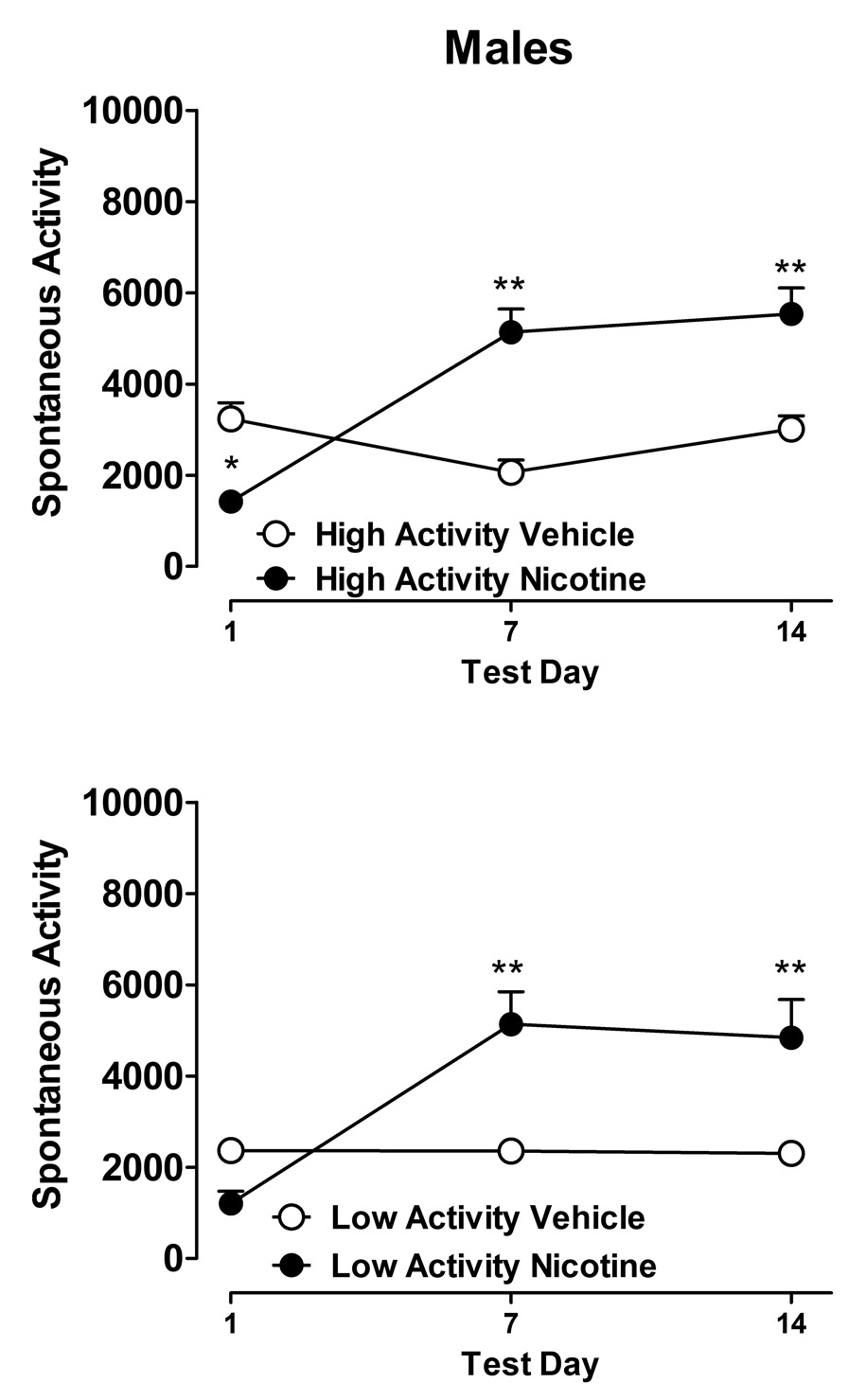
The effects of nicotine or vehicle on spontaneous activity in the male rats are shown in figure 1. In the HA activity rats, statistically significant effects on activity were found for treatment (nicotine vs. saline, F1,15=10.27, p<0.01), test sessions (day 1, day7 and day 14, F2,30=15.70, p < 0.01) and for the interaction between treatment and test sessions (F2,30=28.71, p<0.01). Newman-Keuls post hoc analyses revealed that in HA rats, the first injection of nicotine (test session 1) produced a statistically significant reduction in activity compared to vehicle-treated rats (Figure 1, top panel). However, there was a significant increase in spontaneous activity after 7 and 14 days administration of nicotine (i.e. after 13 and 27 injections of nicotine or vehicle, respectively) compared to vehicle-treated rats.
Figure 1.
Locomotor activity per 20 min test session during two weeks of nicotine (0.4 mg/kg twice daily, sc; filled symbols) or vehicle (saline, open symbols) administration in male LEW rats. Rats were further divided into high activity (HA) and low activity (LA) groups based upon their baseline level of activity prior to drug or vehicle administration. Symbols represent mean cumulative number of photobeam crosses (+/−SEM) for each test session for n=7–8 per group. *P < 0.05 and ** P < 0.01 for comparisons between nicotine- and vehicle-treated groups at each time point by Newman-Keuls test.
In the LA rats, there was also a statistically significant effect of treatment (F1,12=6.94, p < 0.05), test sessions (F2,24=16.79, p < 0.01), and for the interaction between treatment and test sessions (F2,24=17.39, p < 0.01). Newman-Keuls post hoc analyses revealed that the first injection of nicotine failed to produce a significant difference in activity compared to vehicle-treated rats, whereas both 7 and 14 days of nicotine administration produced a significant increase in activity compared to vehicle treated rats.
For test day three (i.e., after 14 days of nicotine or saline administration), a two-factor ANOVA was conducted to compare activity in nicotine- and saline-treated rats in both the HA and LA groups. There were no statistically significant differences between the HA and LA activity groups, nor was there a significant interaction between activity group and treatment condition. However, nicotine was found to significantly increase activity (F1,27=22.75, p < 0.01), which is consistent with the effects of nicotine observed within both the HA and LA groups after 14 days of nicotine administration.
3.3 Effects of nicotine on spontaneous activity in female rats
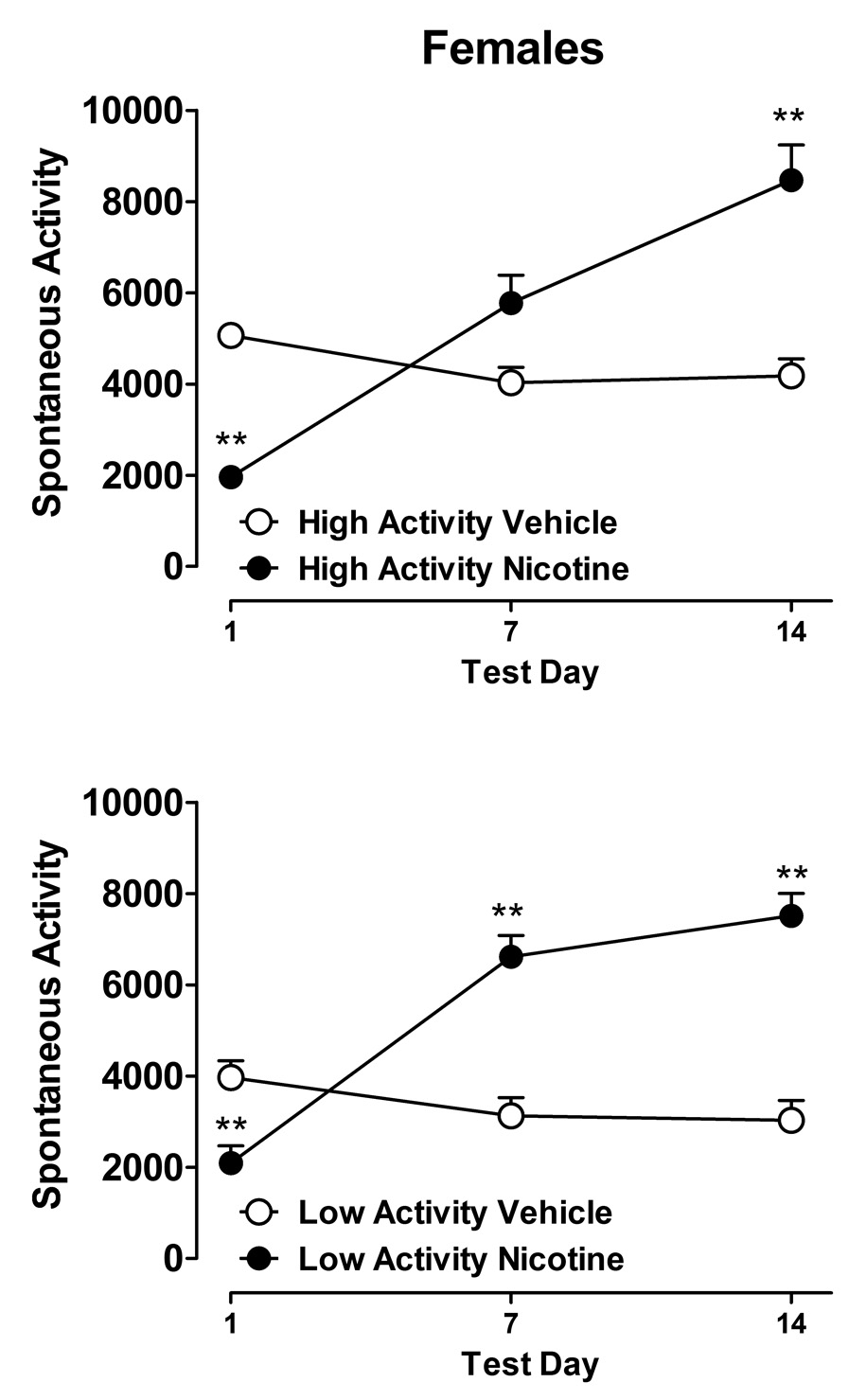
The effects of nicotine or vehicle on spontaneous activity in the female rats are shown in figure 2. In the HA rats, statistically significant effects on activity were found for treatment (F1,14=5.10, p < 0.05), test sessions (F2,28=20.29, p < 0.01), and for the interaction between treatment and test sessions (F2,28=36.20, p < 0.01). Subsequent Newman-Keuls analyses revealed that nicotine significantly decreased activity after the first administration, and after 7 days of nicotine treatment, spontaneous activity increased to levels that were not significantly different from vehicle-treated rats. After 14 days of nicotine administration, spontaneous activity was significantly increased compared to vehicle control rats (figure 2, top panel).
Figure 2.
Locomotor activity during two weeks of nicotine or vehicle administration in female Lewis rats. *P < 0.05 and ** P < 0.01 for comparisons between nicotine- and vehicle-treated groups at each time point. See figure 1 for further details.
In the LA rats, statistically significant effects on activity were also found for treatment (F1,14=17.85, p < 0.01), test sessions (F2,28=29.53, p < 0.01), and for the interaction between treatment and test sessions (F2,28=60.28, p < 0.01). Newman-Keuls analyses revealed that nicotine significantly reduced activity after the first administration, but significantly increased activity after 7 and 14 days administration compared to saline controls (figure 2, bottom panel).
For test day three, a two-factor ANOVA was conducted to compare activity in nicotine- and vehicle-treated rats in both the HA and LA groups. There were no statistically significant differences between the HA and LA activity groups, nor was there a significant interaction between activity group and treatment condition. However, nicotine was found to significantly increase activity (F1,28=65.92, p < 0.01), which is consistent with the effects of nicotine observed within both the HA and LA groups after 14 days of nicotine administration.
4. Discussion
This is the first investigation comparing the chronic effects of nicotine on spontaneous activity in LEW rats, a strain that is thought to be more sensitive to the effects of nicotine than SD and F-344 rats (Horan et al., 1997; Suzuki et al., 1999; Philibin et al., 2005). In the present study, repeated administration of nicotine (0.4 mg/kg) initially suppressed spontaneous activity by nicotine on the first day of treatment and then enhanced activity after the first and second weeks of nicotine treatment. This sensitization effect occurred in rats identified prior to drug administration as low activity (LA) and high activity (HA) rats.
The LEW rats responded to nicotine differently than SD rats did in previous studies conducted in this laboratory using virtually identical designs (Rosecrans, 1995; Pehrson et al., 2008). A preliminary study found that the LEW rat exhibited symptoms of neurotoxicity, including seizures, following 0.8 mg/kg of nicotine, and therefore, 0.4 mg/kg of nicotine was used in the present study instead of the 0.8 mg/kg dose used in the SD studies. A single nicotine injection did not affect activity in the LA male SD rats, whereas, similar to the HA LEW rats tested in the present study, it suppressed locomotor activity in the HA male SD rats. After one week of nicotine administration, nicotine increased locomotor activity in LA male SD rats, while locomotor activity remained suppressed in HA male SD rats. However, after two weeks of nicotine administration, both LA and HA male SD rats exhibited greater levels of locomotor activity compared to vehicle-treated rats (Rosecrans, 1995; Pehrson et al., 2008). The same experimental design used in the male SD rats was also used in female SD rats, with different results. Female SD control rats (i.e., vehicle-treated rats) exhibited significantly greater activity levels, often three-fold higher, at baseline and throughout the study, compared to male SD control rats. In the female SD rats, a single nicotine injection suppressed activity in both LA and HA groups, whereas no consistent differences in activity level were observed between nicotine- and vehicle-treated rats after one and two weeks of treatment (Rosecrans, 1995; Pehrson et al., 2008). Thus, the effects of repeated nicotine administration in male and female SD rats are quite different from the effects of repeated nicotine administration in male and female LEW rats. In the present study, both male and female LEW rats exhibited a reduction in locomotor activity upon the first injection of nicotine and an increase in locomotor activity after 1 and 2 weeks of nicotine treatment.
Increases in response to nicotine after repeated administration may be a consequence of upregulation of nAChRs (Marks et al., 1983; Marks et al., 1985; Wonnacott, 1990; Mochizuki et al., 1998; Zhang et al., 2000). nAChRs have been found to rapidly desensitize when bound by an agonist in vitro (Bertrand et al., 1990; Marks et al., 1994; Corringer et al., 2000) and can remain desensitized after prolonged repeated exposure to nicotine (Ogden and Colquhoun, 1985; Bertrand et al., 1990). Tolerance to acute administration of nicotine is observed for several hours (James et al., 1994; Rosecrans et al., 1995). The repeated administration of nicotine results in up-regulation of nAChRs in vivo in animals (Marks et al., 1983; Marks et al., 1985; Wonnacott, 1990; Mochizuki et al., 1998; Zhang et al., 2000, Vann et al., 2006) and man (Benwell et al., 1988; Breese et al., 1997) and has been shown to produce behavioral sensitization to nicotine in rats (Clarke and Kumar, 1983; Clarke et al., 1988; Rosecrans, 1995; Miller et al., 2001). Repeated administration of nicotine, would therefore, be expected to have compensatory effects on receptor availability through up-regulation, due to the repeated and prolonged deactivation of these receptors.
Behavioral sensitization to nicotine after chronic nicotine administration (Clarke and Kumar, 1983; Clarke et al., 1988; Rosecrans, 1995; Miller et al., 2001) as well as acute behavioral tolerance to nicotine, when nicotine is administered while nAChRs are in a desensitized state (James et al., 1994; Rosecrans et al., 1995; Zhang et al., 2000; Robinson et al., 2006; Prus et al. 2007), are well-established effects. Based on spontaneous activity data from male SD rats (described above), Rosecrans and others hypothesized that the relatively slower onset of behavioral sensitization to nicotine in the HA rats is due to less desensitization of nAChRs than occurs in the LA rats. This initial hypothesis led to the present study in the LEW rat, a strain of rat found to be more sensitive to nicotine in many behavioral models, and may therefore exhibit longer periods of receptor desensitization compared to the SD and F-344 rats. In all of the LEW rats, regardless of sex or baseline level of activity, behavioral sensitization to repeated nicotine exposure was observed.
Although the effects that nicotine has on spontaneous activity are presumed to be mediated by nAChRs, it should be noted that repeated administration of nicotine also produces an accumulation of the active nicotine metabolite nornicotine in the brain (Papke et al., 2007). Although nornicotine metabolite levels after acute nicotine administration have not been found to be sufficient to alter neurotransmission, nornicotine concentrations after repeated nicotine administration have been found to reach levels that are known to elicit DA release in the striatum and nucleus accumbens (Dwoskin et al., 1993; Green et al., 2001). Nornicotine, like nicotine, has been shown to inhibit locomotor activity after a single dose, but has been shown to increase activity in rats chronically treated with nicotine (Stolerman and Jarvis, 1995). Furthermore, nornicotine produces nicotine-like discriminative stimulus effects in rats (Rosecrans and Meltzer, 1981; Goldberg et al., 1989; Bardo et al., 1997; Desai et al., 1999; Desai et al., 2003). Thus, nornicotine may contribute to the actions of nicotine, and there may be individual and strain differences in the production of this metabolite. The differential metabolism of nicotine between strains and species is an area that needs further investigation.
The present study was conducted in order to assess the behavioral characteristics of nicotine after acute and repeated nicotine in the LEW rat, a strain of rat thought to have a greater sensitivity to nicotine, compared to other strains of rats. Although the sex and baseline level of physiological arousal was found to be important for the development of nicotine sensitization in SD rats (Rosecrans, 1995; Pehrson et al. 2008), LEW rats were found to exhibit behavioral sensitization to nicotine regardless of sex or baseline physiological arousal, suggesting that genetic differences between these two strains were the most important determinants of differences in behavioral sensitization to nicotine. Further elucidating the behavioral effects of nicotine sensitization and investigating the neurobiological mechanisms mediating this sensitization in LEW and other strains of rats may contribute greatly to our understanding of individual susceptibility to nicotine dependence.
Acknowledgments
Research described in this article was supported by Philip Morris USA Inc. and Philip Morris International and by National Institute of Health grant # DA-0052
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardo MT, Bevins RA, Klebaur JE, Crooks PA, Dwoskin LP. (−)-Nornicotine partially substitutes for (+)-amphetamine in a drug discrimination paradigm in rats. Pharmacol Biochem Behav. 1997;58:1083–1087. doi: 10.1016/s0091-3057(97)00303-1. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1998;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Ballivet M, Rungger D. Activation and blocking of neuronal nicotinic acetylcholine receptor reconstituted in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990;87:1993–1997. doi: 10.1073/pnas.87.5.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930:12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmacol. 1983;80:587–594. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Fu DS, Jakubovic A, Fibiger HC. Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther. 1988;246:701–708. [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Commons KG. Alpha 4 nicotinic acetylcholine receptor subunit links cholinergic to brainstem monoaminergic neurotransmission. Synapse. 2003;49:195–205. doi: 10.1002/syn.10218. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Asymmetric generalization between the discriminative stimulus effects of nicotine and cocaine. Behav Pharmacol. 1999;10:647–656. doi: 10.1097/00008877-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl) 2003;167:335–343. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Buxton ST, Jewell AL, Crooks PA. S(−)-nornicotine increases dopamine release in a calcium-dependent manner from superfused rat striatal slices. J Neurochem. 1993;60:2167–2174. doi: 10.1111/j.1471-4159.1993.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Engberg G, Erhardt S, Sharp T, Hajos M. Nicotine inhibits firing activity of dorsal raphe 5-HT neurones in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:41–45. doi: 10.1007/s002100000252. [DOI] [PubMed] [Google Scholar]
- Foynes MM, Riley AL. Lithium-chloride-induced conditioned taste aversions in the Lewis and Fischer 344 rat strains. Pharmacol Biochem Behav. 2004;79:303–308. doi: 10.1016/j.pbb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Shaw AE, Riley AL. Cocaine-induced conditioned taste aversions: comparisons between effects in LEW/N and F344/N rat strains. Psychopharmacology (Berl) 1994;114:229–232. doi: 10.1007/BF02244841. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behavior and discriminative properties in rats. Psychopharmacology (Berl) 1989;97:295–302. doi: 10.1007/BF00439441. [DOI] [PubMed] [Google Scholar]
- Green TA, Crooks PA, Bardo MT, Dwoskin LP. Contributory role for nornicotine in nicotine neuropharmacology: nornicotine-evoked [3H]dopamine overflow from rat nucleus accumbens slices. Biochem Pharmacol. 2001;62:1597–1603. doi: 10.1016/s0006-2952(01)00838-3. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Freet CS. The suppressive effects of sucrose and cocaine, but not lithium chloride, are greater in Lewis than in Fischer rats: evidence for the reward comparison hypothesis. Behav Neurosci. 2000;114:353–363. doi: 10.1037//0735-7044.114.2.353. [DOI] [PubMed] [Google Scholar]
- Grinevich VP, Letchworth SR, Lindenberger KA, Menager J, Mary V, Sadieva KA, Buhlman LM, Bohme GA, Pradier L, Benavides J, Lukas RJ, Bencherif M. Heterologous expression of human {alpha}6{beta}4{beta}3{alpha}5 nicotinic acetylcholine receptors: binding properties consistent with their natural expression require quaternary subunit assembly including the {alpha}5 subunit. J Pharmacol Exp Ther. 2005;312:619–626. doi: 10.1124/jpet.104.075069. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–1119. [PubMed] [Google Scholar]
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR., Jr (−)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26:93–94. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- James JR, Villanueva HF, Johnson JH, Arezo S, Rosecrans JA. Evidence that nicotine can acutely desensitize central nicotinic acetylcholinergic receptors. Psychopharmacology (Berl) 1994;114:456–462. doi: 10.1007/BF02249336. [DOI] [PubMed] [Google Scholar]
- Johnson JH, Zhao C, James JR, Rosecrans JA. Individual variability of dopamine release from nucleus accumbens induced by nicotine. Brain Res Bull. 2000;51:249–253. doi: 10.1016/s0361-9230(99)00226-9. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Lancellotti D, Bayer BM, Glowa JR, Houghtling RA, Riley AL. Morphine-induced conditioned taste aversions in the LEW/N and F344/N rat strains. Pharmacol Biochem Behav. 2001;68:603–610. doi: 10.1016/s0091-3057(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther. 1983;226:291–302. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–628. [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Yang JM, Lippiello PM, Collins AC. Desensitization of nicotine-stimulated 86Rb+ efflux from mouse brain synaptosomes. J Neurochem. 1994;63:2125–2135. doi: 10.1046/j.1471-4159.1994.63062125.x. [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Guzman-Marin R, Drucker-Colin R. Nicotine stimulation of dorsal raphe neurons: effects on laterodorsal and pedunculopontine neurons. Eur Neuropsychopharmacol. 2001;11:359–366. doi: 10.1016/s0924-977x(01)00104-3. [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Guzman-Marin R, Dominguez Mdel C, Drucker-Colin R. Mechanisms of nicotine actions on dorsal raphe serotoninergic neurons. Eur J Pharmacol. 2002;452:77–82. doi: 10.1016/s0014-2999(02)02244-6. [DOI] [PubMed] [Google Scholar]
- Miller DK, Wilkins LH, Bardo MT, Crooks PA, Dwoskin LP. Once weekly administration of nicotine produces long-lasting locomotor sensitization in rats via a nicotinic receptor-mediated mechanism. Psychopharmacology (Berl) 2001;156:469–476. doi: 10.1007/s002130100747. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Villemagne VL, Scheffel U, Dannals RF, Finley P, Zhan Y, Wagner HN, Jr, Musachio JL. Nicotine induced up-regulation of nicotinic receptors in CD-1 mice demonstrated with an in vivo radiotracer: gender differences. Synapse. 1998;30:116–118. doi: 10.1002/(SICI)1098-2396(199809)30:1<116::AID-SYN15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J Neurochem. 2004;90:40–49. doi: 10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- Ogden DC, Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985;225:329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem. 2007;101:160–167. doi: 10.1111/j.1471-4159.2006.04355.x. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Philibin SD, Gross D, Robinson SE, Vann RE, Rosecrans JA, James JR. The effects of acute and repeated nicotine doses on spontaneous activity in male and female Sprague Dawley rats: Analysis of brain area epibatidine binding and cotinine levels. Pharmacol Biochem Behav. 2008;89:424–431. doi: 10.1016/j.pbb.2008.01.018. Epub 2008 Feb 2. [DOI] [PubMed] [Google Scholar]
- Pescatore KA, Glowa JR, Riley AL. Strain differences in the acquisition of nicotine-induced conditioned taste aversion. Pharmacol Biochem Behav. 2005;82:751–757. doi: 10.1016/j.pbb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Philibin SD, Vann RE, Varvel SA, Covington HE, 3rd, Rosecrans JA, James JR, Robinson SE. Differential behavioral responses to nicotine in Lewis and Fischer-344 rats. Pharmacol Biochem Behav. 2005;80:87–92. doi: 10.1016/j.pbb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Maxwell AT, Baker KM, Rosecrans JA, James JR. Acute behavioral tolerance to nicotine in the conditioned taste aversion paradigm. Drug Dev Res. 2007;68:522–528. [Google Scholar]
- Robinson SE, James JR, Lapp LN, Vann RE, Gross DF, Philibin SD, Rosecrans JA. Evidence of cellular nicotinic receptor desensitization in rats exhibiting nicotine-induced acute tolerance. Psychopharmacology. 2006;184:306–313. doi: 10.1007/s00213-005-0049-9. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Effects of nicotine on behavioral arousal and brain 5-hydroxytryptamine function in female rats selected for differences in activity. Eur J Pharmacol. 1971a;14:29–37. doi: 10.1016/0014-2999(71)90119-1. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Effects of nicotine on brain area 5-hydroxytryptamine function in male and female rats separated for differences of activity. Eur J Pharmacol. 1971b;16:123–127. doi: 10.1016/0014-2999(71)90067-7. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology. 1972;11:863–870. doi: 10.1016/0028-3908(72)90045-7. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Schechter MD. Brain 5-hydroxytryptamine correlates of behavior in rats: strain and sex variability. Physiol Behav. 1972;8:503–510. doi: 10.1016/0031-9384(72)90336-8. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Meltzer LT. Central sites and mechanisms of action of nicotine. Neurosci Biobehav Rev. 1981;5:497–501. doi: 10.1016/0149-7634(81)90020-8. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Karan LD. Neurobehavioral mechanisms of nicotine action: role in the initiation and maintenance of tobacco maintenance. J Subst Abuse Treat. 1993;10:161–170. doi: 10.1016/0740-5472(93)90041-y. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. The psychopharmacological basis of nicotine's differential effects on behavior: individual subject variability in the rat. Behav Genet. 1995;25:187–196. doi: 10.1007/BF02196927. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Wiley JL, Bass CE, Karan LD. Nicotine-induced acute tolerance: studies involving schedule-controlled behavior. Brain Res Bull. 1995;37:359–362. doi: 10.1016/0361-9230(95)00007-0. [DOI] [PubMed] [Google Scholar]
- Rowell PP, Li M. Dose-response relationship for nicotine-induced up-regulation of rat brain nicotinic receptors. J Neurochem. 1997;68:1982–1989. doi: 10.1046/j.1471-4159.1997.68051982.x. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Thorndike E, Schindler CW, Goldberg SR. Discriminative stimulus effects of nicotine and chronic tolerance. Pharmacol Biochem Behav. 1997;56:167–173. doi: 10.1016/s0091-3057(96)00174-8. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Pharmacol Exp Ther. 1988;245:164–170. [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Etonitazene delivered orally serves as a reinforcer for Lewis but not Fischer 344 rats. Pharmacol Biochem Behav. 1992;42:579–586. doi: 10.1016/0091-3057(92)90002-w. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in Lewis and Fischer 344 inbred rat strains. Eur J Pharmacol. 1999;369:159–162. doi: 10.1016/s0014-2999(99)00086-2. [DOI] [PubMed] [Google Scholar]
- Sziraki , Lipovac MN, Hashim A, Sershen H, Allen D, Cooper T, Czobor P, Lajtha A. Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochem Res. 2001;26:609–617. doi: 10.1023/a:1010979018217. [DOI] [PubMed] [Google Scholar]
- Vann RE, James JR, Rosecrans JA, Robinson SE. Nicotinic receptor inactivation after acute and repeated in vivo nicotine exposures in rats. Brain Res. 2006;1086:98–103. doi: 10.1016/j.brainres.2006.02.075. [DOI] [PubMed] [Google Scholar]
- Wei X, Sumithran SP, Deaciuc AG, Burton HR, Bush LP, Dwoskin LP, Crooks PA. Identification and synthesis of novel alkaloids from the root system of Nicotiana tabacum: affinity for neuronal nicotinic acetylcholine receptors. Life Sci. 2005;78:495–505. doi: 10.1016/j.lfs.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]
- Zhang X, Paterson D, James R, Gong ZH, Liu C, Rosecrans J, Nordberg A. Rats exhibiting acute behavioural tolerance to nicotine have more [125I]alpha-bungarotoxin binding sites in brain than rats not exhibiting tolerance. Behav Brain Res. 2000;113:105–115. doi: 10.1016/s0166-4328(00)00205-9. [DOI] [PubMed] [Google Scholar]