1 Introduction
Understanding environmental variability and the ways in which organisms respond to such variability over short- and long timescales is of considerable importance to the field of evolutionary physiology, and more generally to ecology and to conservation biology. This is as true for insects as it is for other organisms (Prosser, 1986; Spicer and Gaston, 1999; Chown and Nicolson, 2004), and these topics form the substance of this review. After sketching the modern ecological and evolutionary contexts within which evolutionary physiology must now be done, and providing a survey of sources of environmental variability and their effects on insect populations, we move on to explore environmental variation and the various ways in which it may be quantified. Some environmental variables are relatively simple and straightforward, both to measure and to control, whereas others pose substantially greater problems from both perspectives. Even variables that are seemingly easy to measure might act in ways that are difficult to identify (Stenseth and Mysterud, 2005).
Next we briefly revisit definitions of phenotypic plasticity and acclimation. Given their significance it is not surprising that these issues have enjoyed considerable attention over the last decade (e.g. Huey and Berrigan, 1996; Huey et al., 1999; Relyea, 2002; Wilson and Franklin, 2002; Piersma and Drent, 2003; West-Eberhard, 2003; DeWitt and Scheiner, 2004; Pigliucci, 2005; Angilletta et al., 2006), and in many cases remain the subject of controversy.
Then we examine insect responses to the thermal environment over a variety of spatial and temporal scales, focussing on recent developments in the field. In doing so, we do not suggest that other abiotic or biotic features of the environment (such as water loss, solar radiation, wind, landscape structure, and species interactions) are insignificant. Indeed, the importance of water availability for insect survival and the determination of distribution and abundance patterns has been widely demonstrated (see Hadley, 1994; Tauber et al., 1998; Addo-Bediako et al., 2001; Hawkins et al., 2003; Chown and Nicolson, 2004). Rather, we examine thermal aspects of the environment because they are of considerable significance in determining large- and small-scale patterns of diversity at several scales (Andrewartha and Birch, 1954; Chown and Gaston, 1999; Allen et al., 2002; Hawkins et al., 2003; Willig et al., 2003; Chown et al., 2004a; Evans et al., 2005).
Finally, we return to the question of what lessons insect evolutionary physiologists might have to offer ecology and conservation biology. In particular, we consider how evolutionary physiology can offer ecologists a set of useful general rules in some cases and can unveil the nature of local contingency in others (see Lawton, 1992, 1999; Chown and Nicolson, 2004; Simberloff, 2004). Although migration ability has a significant influence on the evolution of environmental responses, we do not discuss the costs of flight and the physiology of wing polymorphism and its environmental determinants here (see Zera and Denno, 1997; Shiga et al., 2002; Zhao and Zera, 2002, 2004a,b; Cadet et al., 2003; Zera and Zhao, 2003 for access to this literature).
2 Evolutionary physiology in a changing world
2.1 Humans and Ecological Change
Humans are altering the modern environment in several ways that affect biodiversity. Most noteworthy among these are habitat destruction and alteration, changes to global, and consequently local climates, pollution (including nutrient enrichment), and the introduction of species to areas from which they were previously absent and in which they subsequently become invasive (Mack et al., 2000; Sala et al., 2000; Tilman et al., 2001; Gaston et al., 2003; Palmer et al., 2004; Thomas et al., 2004; Millenium Ecosystem Assessment, 2005). All of these processes have brought about substantive changes to populations, either by causing local increases or declines in abundance, by promoting changes to life history characteristics so affecting birth and/or death rates, or by affecting rapid local extirpations or introductions. In some cases, these have led to extinction of all populations of some species.
Climate change has resulted in the colonization of higher latitude areas and the establishment of new populations in several northern-hemisphere insect species. This has resulted in substantial range shifts (Parmesan et al., 1999), although changes in both range size and position have depended on interactions between the life-history characteristics and habitat requirements of the species concerned, and landscape structure (Hill et al., 1999; Thomas et al., 2001; Parmesan and Yohe, 2003; Root et al., 2003; Simmons and Thomas, 2004; Hill et al., 2006). In many cases, climate change effects are negative and have either resulted in or are predicted to give rise to species extinctions (Thomas et al., 2004; Pounds et al., 2006). Habitat destruction and alteration have likewise substantially affected populations, sometimes changing the entire structure of local assemblages, with subsequent downstream effects on ecosystem functioning (e.g. Steenkamp and Chown, 1996; Cunningham, 2000; Donaldson et al., 2002; Rickman and Connor, 2003; Stefanescu et al., 2004; Samways et al., 2005). The intentional (e.g. for biological control) or accidental introduction of individuals to an area from which they were previously absent has also led to substantial population changes. In the case of the introduced species, new populations are typically established and subsequently increased in abundance (e.g. Dennill et al., 1993; Ernsting, 1993; Moller, 1996; McGeoch and Wossler, 2000; Tsutsui et al., 2000), while resident, often indigenous, populations are negatively affected (Chown and Smith, 1993; O’Dowd et al., 2003; Sanders et al., 2003; Holway and Suarez, 2006). The effects of introductions can often be subtle initially, with more pronounced impacts accumulating slowly through time (Chown and Block, 1997; Ernsting et al., 1999; Goulson, 2003; Ness, 2004). Nonetheless, in many systems, introductions have resulted in species extinctions (Blackburn et al., 2004), and substantial changes to system functioning (Mooney and Hobbs, 2000; Hansen et al., 2002; Goulson, 2003; O’Dowd et al., 2003; Blancafort and Gómez, 2005). Finally, the effects of pollution on populations have long been appreciated by freshwater ecologists (see reviews in McGeoch, 1998, in press). However, the sheer pervasiveness and substantial effects of pollution, and especially those of nutrient enrichment, are only now beginning to be appreciated (Millennium Ecosystem Assessment, 2005).
In some instances, the impacts of these processes are likely to be mediated directly by biotic interactions, with only a minimal role played by the abiotic environment. Habitat destruction can lead directly to the loss of populations and species owing to absence of appropriate resources (Brooks et al., 1999, 2002; Beier et al., 2002; Dunn, 2005), and co-extinctions can exacerbate these impacts (Koh et al., 2004). Habitat alteration can cause mesopredator release, thus having knock-on effects on other trophic levels (Crooks and Soulé, 1999), and similar outcomes for particular populations have been documented following invasive species eradication or control efforts (Zavaleta et al., 2001). Following climate change or habitat destruction, the incidence of disease can increase, benefiting the disease and, where it is vector-borne, disease vectors, but typically not the host(s) (Patz et al., 2000; Harvell et al., 2002; Kutz et al., 2005; Vittor et al., 2006). The opposite situation has also been demonstrated (e.g. Randolph and Rogers, 2000), and is thought to be one of the major ways in which autonomous control of tsetse and trypanosomiasis will be affected (Rogers and Randolph, 2002). Similarly, the effects of invasive alien species on indigenous populations is often direct, either by way of herbivory, predation, or parasitism (Chapuis et al., 1994; Mack et al., 2000; Blackburn et al., 2004), or as a consequence of competition, although the role of invasive species as ‘drivers’ or ‘passengers’ in the latter case has yet to be fully resolved (Didham et al., 2005).
However, in many situations, impacts on populations of the above mentioned processes have been or will be a direct consequence of changes in the abiotic environment, or have taken place via indirect effects of abiotic factors on other species. This is certainly true of climate change (Bale et al., 2002; Walther et al., 2002; Root et al., 2003). It is well established that the thermal and hygric environments encountered by animals have direct effects on survival, growth, and reproduction (Tauber et al., 1998; Denlinger et al., 2001; Hochachka and Somero, 2002; Chown and Nicolson, 2004; Koz³owski et al., 2004). Nutrient availability, which is being altered by global changes in CO2 and tropospheric ozone levels, also plays a significant role in influencing insect life histories and population dynamics (Slanksy and Rodriguez, 1987; Fagan et al., 2002; Woods et al., 2003). Likewise, predator–prey and plant–insect interactions can be influenced substantially by the conditions of the abiotic environment (Park, 1962; Chase, 1996; Davis et al., 1998; Coviella and Trumble, 1999; Karnosky et al., 2003).
Many of the effects of habitat alteration and pollution, and of species introductions are either being realized in similar ways to those described or are substantially influenced by the conditions of the abiotic environment. Habitat destruction has considerable effects on the abiotic environment, which in turn affects population dynamics. Indeed, the coupling between climate and vegetation is well established (Bonan, 2002). For example, in the Atlantic forest region of south-eastern Brazil, a strong positive relationship exists between tree cover and rainfall, indicating that anthropogenic deforestation has resulted in reductions in rainfall (Webb et al., 2006). Small forest patches are likely to suffer further degradation owing to local climate responses to landscape alteration. Large-scale, historical deforestation for agriculture in the United States cooled the climate and led to an increase in the incidence of frost (Bonan, 1999, 2002). These abiotic changes have had large effects on species resident in the landscape.
Perhap, one of the most striking examples of the effects of land use change on insect mortality, via changes in abiotic conditions, is the case of Monarch butterflies overwintering in oyamel fir forests in Mexico. The adult butterflies are susceptible to freezing (freezing and dying at c. −8.7 °C), especially by inoculation if they become wet (freezing at c. −3.7–−4.5 °C) (Alonso-Mejía et al., 1992; Larsen and Lee, 1994). Forest cover not only forms an umbrella offering protection from direct rainfall, but it also prevents wind-blown spray from reaching the butterflies (Anderson and Brower, 1996). Clustering by butterflies in the forest promotes retention of high lipid reserves (Alonso-Mejía et al., 1997), and a well-developed understory enables adults that have been knocked from clusters to regain height, so avoiding dew and benefiting from the aggregations (Alonso-Mejía et al., 1992, 1997). Forest thinning and understory removal, as a consequence of human activities, therefore poses substantial threats to these butterflies by increasing overwintering mortality. Global climate change forecasts suggest that cool-weather precipitation is likely to increase in the overwintering sites, thus bringing additional risk, especially if forest cover is thinned. These changes will render many present sites wholly unsuitable within 50 years (Oberhauser and Peterson, 2003). Other studies have demonstrated effects of microclimate changes on insect assemblages (e.g. Perfecto and Vandermeer, 1996).
The likelihood of establishment and subsequent spread of a species alien to a given area is, at least to some extent, a function of the interaction between individuals of that species and the abiotic environment. It is widely appreciated that a match in climate between native and introduced ranges is a reasonable, though not the only or a foolproof (see Samways et al., 1999), predictor of success of an alien species in its introduced range, whether the species is an unintentional introduction, or a biological control agent (e.g. Dennill and Gordon, 1990; Duncan et al., 2003; Robertson et al., 2004). Similarly, both productive and ambient energy are strong correlates of broad-scale variation in alien species richness (Chown et al., 2005; Richardson et al., 2005).
These examples clearly illustrate that comprehension of human impacts on modern diversity requires an understanding of the effects of the abiotic environment on individuals and populations (of different species), and the ways in which individuals and populations respond to the environment and its spatial and temporal variation. Such knowledge is also necessary for predicting what interventions might be required given a future of ongoing change (Hannah et al., 2002; Walther et al., 2002; Williams et al., 2005; Xenopoulos et al., 2005). While several bioclimatic modelling approaches (see Pearson and Dawson, 2003; Huntley et al., 2004; Segurado and Araújo, 2004) are available that provide a first, and much-needed, estimate of likely species abundances and occurrences (Rogers and Randolph, 1991; Jeffree and Jeffree, 1996; Robinson et al., 1997a,b; Randolph and Rogers, 2000; Rogers, 2000; Erasmus et al., 2002; Pearson and Dawson, 2003; Tatem et al., 2003; Huntley et al., 2004; Thomas et al., 2004), they are based almost solely on climatic correlates of abundance and distribution, and have, in consequence, been criticized (e.g. Davis et al., 1998; Samways et al., 1999). From a physiological perspective, concerns have come from three principal perspectives. Spatial variation in population responses to the environment is often not considered (Davis and Shaw, 2001); the rapid alterations to phenotypes that might take place via phenotypic plasticity in the form of developmental plasticity, acclimation, and hardening are typically ignored (Helmuth et al., 2005); and the likely outcome of covariation among abiotic variables, and their interaction with other components of the environment, such as risk of predation and intensity of competition, are often not adequately assessed (Rogers and Randolph, 2000; Angilletta et al., 2006). Spatial and temporal variability in phenotypes might substantially alter predicted responses to change (Stillman, 2003), especially if this variability varies among traits (Chown, 2001; Hoffmann et al., 2003a). Consequently, it has been proposed that physiological investigations and biophysical modelling should be used in concert with large-scale bioclimatic investigations of species responses to understand what the future might hold for various taxa in a climate of change (Helmuth et al., 2005). Thus, it is clear that evolutionary physiologists face substantial challenges, not only in deepening understanding of how organisms respond to their changing environments, but also in addressing the demands being made of them by ecologists and conservation biologists concerned about the appropriate actions to take in the face of rapid, global environmental change (Angilletta et al., 2006; Wikelski and Cooke, 2006).
2.2 Variability and Change in Populations
Physiological responses to changes in the environment take place over a range of time scales, from rapid, phenotypic adjustments to longer-term, evolutionary changes that might also alter the phenotypic response to the environment (Hochachka and Somero, 2002; West-Eberhard, 2003; Chown and Nicolson, 2004). The likelihood that one or more of these responses will be realized depends on the nature of the environment in which the population finds itself, and the extent to which the population is connected to others by dispersal, whether or not this dispersal takes place in a metapopulation landscape.
Physiologists have long appreciated that environmental conditions and their variability have an influence on phenotypic plasticity (see Section 4). It is widely thought that acclimatization is more likely in species from temperate than those from less variable tropical and polar environments (Spicer and Gaston, 1999; Ghalambor et al., 2006), and less likely in stenothermal (narrow temperature tolerance) species (Somero et al., 1996; Pörtner et al., 2000), although tropical species might be more eurythermal (wide temperature tolerance) than their polar counterparts (Somero, 2005). More generally, the environmental circumstances under which adaptive population differentiation, phenotypic plasticity, or some combination thereof arise form the subject of a large and growing theoretical field (e.g. West-Eberhard, 2003; Berrigan and Scheiner, 2004; Pigliucci, 2005). Somewhat surprisingly, this field and work examining the evolution of thermal physiology remain reasonably distinct (though see Lynch and Gabriel, 1987; Gilchrist, 1995), even though the physiological models often struggle to explain the high frequency of eurythermic strategies (see reviews in Angilletta et al., 2002, 2003, 2006). Hence, we focus on the former plasticity models, noting parallels with the thermal physiology models where appropriate.
Many investigations have shown that greater environmental variability tends to favour phenotypic plasticity within populations, as long as cue reliability and accuracy of the response (which is a function of environmental lability and unpredictability, and of the extent to which the response lags behind the environmental change) is high, and the cost of plasticity is low (Lively, 1986; Moran, 1992; Scheiner, 1993; Tufto, 2000). This conclusion holds for both optimality and quantitative genetic (environmental threshold) models (Hazel et al., 2004). Recent modelling work has also shown that the likelihood of this outcome is affected strongly by migration between different populations (Tufto, 2000; Sultan and Spencer, 2002). With little or no migration, and different environments, adaptive differentiation between populations in each of these environments readily evolves. Increases in migration rate, by contrast, lead to fixation of the plastic phenotype even though it might not be the best type anywhere (i.e. relative to adaptively differentiated habitat specialists) (Tufto, 2000; Sultan and Spencer, 2002). Nonetheless, if response accuracy is low (i.e. no better than random for at least one environmental state), the specialist phenotype is favoured, and the same is likely to be true if the global cost of plasticity is high (though evidence for the latter is scarce) (Van Tienderen 1991, 1997; Moran 1992; Sultan and Spencer, 2002, but see also Relyea 2002; van Kleunen and Fischer, 2005). In addition, environmental-threshold models show that with low cue reliability and low frequency of benign patches, a reversed (counter–intuitive) conditional, but unstable, strategy is favoured (Hazel et al. 2004).
In the context of insect physiological responses, four outcomes of these models are most notable. First, plastic responses are likely to be common across a broad range of conditions in the presence of even relatively low levels of migration. Second, plastic phenotypes might be favoured globally even when in any given environment they have a lower fitness than a habitat specialist (Sultan and Spencer, 2002). The plastic responses might also be the reverse of what is expected under a given set of circumstances (Hazel et al., 2004). Third, variation in trait response lag times, such as between developmental change and acclimation (see Section 4), might account for differences in plasticity among traits. Finally, these outcomes will be affected by the level of within-site homogeneity, the number of sites in any given broader environment, and the frequency of different kinds of patches. In insects, populations connected by migration (whether or not a true metapopulation system is demonstrated – e.g. Harding and McNamara, 2002) are relatively common (see Schneider, 2003; Roslin and Kotze, 2005 for discussion), suggesting that plasticity will be regularly found in many traits and might account for a substantial proportion of the ‘population differentiation’ found between them. Evidence is accumulating that this is indeed the case, as reflected in recent assessments of the contribution of plasticity to population variation in thermal tolerance traits of several taxa, including Drosophila (Ayrinhac et al., 2004; Hoffmann et al., 2005a), weevils (Klok and Chown, 2003), and tsetse (Terblanche et al., 2006). Significantly, in the case of the weevils, the single widespread species investigated, which is present on two islands separated by thousands of kilometres, thus precluding dispersal, showed substantial inter-island population differentiation in lower thermal limits that could not be accounted for by phenotypic plasticity, in keeping with theoretical predictions. These findings also suggest that genetic accommodation (also more narrowly thought of as epigenetic assimilation) has been significant in the evolution of thermal tolerances in insects (see Pigliucci and Murren, 2003; West-Eberhard, 2003).
If plastic phenotypes are favoured globally, even if their fitness is not highest at any particular site, then negative tests of the beneficial acclimation hypothesis under a particular set of conditions might not be unexpected. Together with the tendency for many tests of beneficial acclimation to focus on developmental changes (which might be less likely to be demonstrably beneficial because of response lag times) (Wilson and Franklin, 2002), this might account for recent conclusions that acclimation (a form of plasticity) is typically not beneficial (Huey et al., 1999; Wilson and Franklin, 2002), despite the fact that theoretical models demonstrate a wide range of scenarios under which adaptive phenotypic plasticity might evolve.
2.3 Dispersal, Plasticity, and Range Edges
That population connectivity has a strong influence on the likelihood of local adaptation has also been recognized in the context of the mechanisms determining species range margins (Hoffmann and Blows, 1994; Lenormand, 2002). While the proximate determinants of range margins might appear to be the inability of a population to cope with a given set of circumstances that lie just beyond its range, the ultimate determinants of range margins have to do with the inability of a population to respond to these circumstances, which it must do to achieve either colonization, population growth, or stasis (Carter and Prince, 1981; Gaston, 2003). Populations might be unable to persist in a given area as a consequence of an absence of suitable habitat patches, an increase in extinction rate such that population persistence is impossible (i.e. reflecting lack of adaptation in the broadest sense), or a decline in dispersal or colonization rate (Holt and Keitt, 2000). In other words, gradients in any of these factors might result in range margins. Why a population should be unable to adapt to local circumstances beyond its range, thus reducing extinction probability, increasing colonization success, or enabling a change in habitat use, is thought to be a result of low genetic variation, low heritability, genetic trade-offs, mutation accumulation, and the need for changes in several components of the phenotype simultaneously (Hoffmann and Blows, 1994; Gaston, 2003; Blows and Hoffmann, 2005). It is also thought to be a result of swamping of genotypes in marginal populations by those from central populations via immigration (Case and Taper, 2000; Gaston, 2003; Alleaume-Benharira et al., 2006). In other words, gene flow inhibits local adaptation. Kirkpatrick and Barton (1997) showed that random dispersal results in a flow of genes to the periphery of the species’ range so turning peripheral populations into ‘sinks’ where death rates are higher than birth rates. Paradoxically, while gene flow maintains the number of individuals, it also has the effect of ensuring that the peripheral population remains a sink. Nonetheless, in several situations a balance is struck between local adaptation and gene flow, which then sets a species’ range limits. This balance can be altered by relatively small changes in the parameters in Kirkpatrick and Barton’s (1997) model, thus explaining the rapid expansions of populations that are sometimes seen. Subsequent modelling work has shown that if sink populations are variable and this variation is temporally autocorrelated (as is the case of almost all abiotic variation), then adaptation in peripheral populations can take place even in the face of gene flow (Holt et al., 2004a). In essence, a favourable period may lower the extent of maladaptation in sink environments for long enough to allow population growth, which in turn would reduce the effects of gene flow from immigrants. Low levels of migration also mitigate the negative effects of genetic drift and may reduce stochastic variation around the mean phenotype that is the consequence of drift (Alleaume-Benharira et al., 2006). In consequence, depending on population size and the strength of the environmental gradient, the optimal migration rate (see Alleaume-Benharira et al., 2006) is an intermediate one (see also Forde et al., 2004; Holt et al., 2004b).
Typically, models of the influence of gene flow on range limits have not considered the simultaneous influence of migration on the evolution of phenotypic plasticity. Kirkpatrick and Barton (1997) acknowledged that the tendency for gene flow to swamp local adaptation might be ameliorated by phenotypic plasticity, but did not take the matter further. Likewise, Holt et al. (2004a) gave no attention to the likelihood that instead of promoting local adaptation, autocorrelated environmental variability (which would improve response accuracy) is likely to promote the evolution of phenotypic plasticity. In consequence, it is difficult to ascertain what the influence of phenotypic plasticity on the evolution of range edges might be (see also Sultan, 2004). On the one hand, it might promote local adaptation of a kind by allowing populations to persist (Kirkpatrick and Barton, 1997), and perhaps to grow out of the substantial effects of gene flow on local adaptation (Holt et al., 2004a,b; see also West-Eberhard, 2005). On the other hand, it seems equally likely that phenotypic plasticity might prevent local adaptation because it is the favoured strategy everywhere, despite lower fitness in some locations (Sultan and Spencer, 2002). This would frustrate local adaptation and prevent range expansion. Clearly, there is a need to link models investigating the effects of migration on local adaptation (Kirkpatrick and Barton, 1997; Holt et al., 2004a), and those investigating the conditions that promote phenotypic plasticity (Tufto, 2000; Sultan and Spencer, 2002). This amounts to an understanding of the role of genetic accommodation in setting and/or altering range limits (see Pigliucci and Murren, 2003; West-Eberhard, 2003; Pigliucci et al., 2006).
2.4 Implications for Insect Physiology
Models of the kinds described provide considerable insight into the significance of phenotypic plasticity for mediating species responses to environmental change. Thus, not only is it important to understand the extent to which various traits show phenotypic plasticity, but it is also important to comprehend the conditions that promote such variability relative to changes in basal responses. It is not just understanding of the ways in which populations might avoid extinction that can be informed by such investigations. Recently, Wiens (2004) has argued that comprehension of the reasons why populations are unable to expand their ranges is likely to provide considerable insight into what causes new lineages to arise – i.e. what is the cause of speciation in allopatry. Understanding what traits determine the inability of species to occupy certain habitats (and these are often likely to be physiological (Gaston, 2003; Wiens, 2004)), and why these show little capacity for change in some instances and considerable capacity for change in others (West-Eberhard, 2003) is therefore significant in the context of both extinction and speciation, the ultimate determinants of species richness variation on the planet (West-Eberhard, 1989; Gaston and Chown, 1999; Chown and Gaston, 2000).
3 Abiotic environmental variation and its measurement
That weather and climate have significant effects on insect populations has long been appreciated by ecologists (Shelford, 1911; Andrewartha and Birch, 1954; Messenger, 1959; Kingsolver, 1989; Roy et al., 2001). The coincidence of species range edges with particular climatic features (Chown and Gaston, 1999), robust relationships between climatic variables and insect abundances and distributions (Jeffree and Jeffree, 1996; Robinson et al. 1997a,b), and the recent response of species range edges to global climate change (Parmesan et al., 1999), all serve to emphasize that climate exerts a significant effect on insect populations. Fluctuations in abundance through time, as a consequence of changes in birth rates, death rates or both, in synchrony with changes in weather, similarly highlight the significance of weather for the population dynamics of many insect species (Andrewartha and Birch, 1954; Kingsolver, 1989; Roy et al., 2001; Hargrove, 2004). Appreciation for the fact that microclimatic measurements are of considerable importance for understanding insect responses to the environment is also well developed (Willmer, 1982; Leather et al., 1993; Danks, 1996, 1999; Hodkinson, 2003). More recently, the emphasis of investigations has shifted to variability and unpredictability (Kingsolver and Huey, 1998; Angilletta et al., 2006; but see also Levins, 1968), the intensity of extreme conditions (e.g. Gaines and Denny, 1993; Parmesan et al., 2000), and the frequency, rate of approach to, and duration of particular conditions (Sømme, 1996; Kelty and Lee, 1999; Sinclair, 2001a; Robertson, 2004a; Rako and Hoffmann, 2006).
3.1 Means and Extremes
Owing to their availability, even before the advent of widely available remotely sensed information and geographic information systems, data on the annual means (e.g. of temperature) or totals (e.g. precipitation) of Stevenson Screen data across broad geographic scales were regularly used as independent variables for examination of large-scale variation in physiological traits. Recent studies have adopted similar approaches, documenting significant and sometimes strong relationships between the variables of interest and the climatic parameter used (see e.g. Addo-Bediako et al., 2001, 2002; Hoffmann et al., 2003b; Parkash et al., 2005).
The use of mean annual climatic data has proven controversial, however. It has been argued that insects are unlikely to experience these mean temperatures because of microhabitat selection and inactivity of certain stages at particular times of the year (see e.g. Hodkinson, 2003). Undoubtedly this is true, as many studies have demonstrated (see Kevan, 1975; Bale, 1987; Leather et al., 1993; Sinclair and Chown, 2005a, for examples). However, the crux of the matter lies in the question being posed and the scale at which it is investigated. For large-scale, comparative studies it is unlikely that microclimate data will be available for every site from which the study organisms have been collected. Many individual studies simply provide a locality name and a broad description of prevailing local climates, and if climatic data are provided they are often supplied from the nearest meteorological station (i.e. Stevenson Screen values). Anyone interested in large-scale patterns in variation must then come to a decision about what parameters to use, and ‘macroclimatological’ variables are certainly more informative than none at all (see Chown et al., 2003). In addition, these can be useful in revealing the likely cause of variation in a given biological variable. For example, along the east coast of Australia, highest daily maximum temperature in the hottest month does not vary with latitude, but mean daily maximum temperature declines with latitude. Thus, the number of warm days declines as latitude increases and this variation is probably the cause of clinal variation of the 56H8 heat-shock protein (hsp70) allele in Drosophila melanogaster (Bettencourt et al., 2002).
It has been argued that large-scale studies are perhaps of little value because of uncertainties associated both with the microhabitats occupied by the species (or populations) and the biology of the species concerned (Hodkinson, 2003). Such tension between broader-scale and finer-scale approaches is not new (e.g. Feder, 1987), and has been discussed in detail recently in the context of population dynamics and community ecology (Lawton, 1992, 1999; Simberloff, 2004). In our view, both approaches have their merits and drawbacks, and each reveals patterns and mechanisms that would simply have remained undetected had the approach not been followed (see also Chown et al., 2004b). For example, it is only through local-scale work, with fine temporal resolution, that the responses of the goldenrod gall fly, Eurosta solidaginis, to its thermal and hygric environments have come to be comprehended (Storey et al., 1981; Storey and Storey, 1986; Storey, 1990; Joanisse and Storey, 1994a; Lee et al., 1995; Irwin and Lee, 2000; Williams et al., 2004). And it is this work that is enabling novel insights to be gained into the role of hypoxia-inducing-factor-1α in mediating resistance to cold, freezing, and anoxia (Morin et al., 2005). By contrast, in the absence of large-scale work, it would not have become clear that over broad spatial scales, upper lethal limits are much less variable than lower lethal limits (Addo-Bediako et al., 2000; Gibert and Huey, 2001; Kimura, 2004). Nor would it be apparent that substantial differences exist between the high latitude northern and southern hemispheres in the cold hardiness strategies adopted by insects, and that these differences may be driven in part by differences in unpredictability of freezing events owing to the ‘mean’ climates of the two hemispheres (Sinclair et al., 2003a; Sinclair and Chown, 2005a).
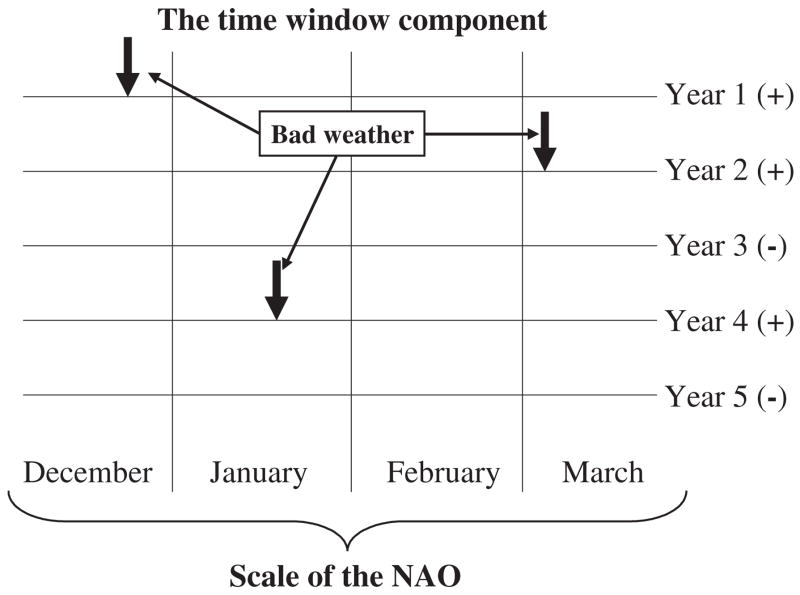
Several recent studies, especially of mammalian and avian population dynamics and life histories, have shown why measured variables such as temperature and precipitation, might be much less adequate at explaining population responses than broader climate indices such as the North Atlantic Oscillation (NAO) or El Niño Southern Oscillation (ENSO) (Hallett et al., 2004; Stenseth and Mysterud, 2005). In essence, by integrating a variety of weather variables across spatial and temporal scales that are of significance for the animals, these indices often provide a much better estimate of the overall quality of a season than do short-term measurements such as temperature or snowfall at a given site for specific months (Fig. 1). In consequence, where specific climatic variables differ from year to year in their relationship with aspects of population dynamics, and fail to capture the complexities of environmental effects on animals (such as the importance of combinations of variables such as low temperature and high precipitation – see the discussion of monarch butterfly mortality in Alonso-Mejía et al., 1992), the climate indices are effective in representing the overall quality of a season and so can explain much of the variation in population responses. Therefore, these indices can also provide substantial predictive capability and an indication of longer-term change associated with changing broad-scale climatic patterns (Stenseth and Mysterud, 2005). While the relationships between climatic indices (sometimes called teleconnection patterns) and insect responses have not been fully explored, a growing number of studies indicate that these relationships bear closer scrutiny (e.g. Holmgren et al., 2001; Ottersen et al., 2001; Sinclair, 2001a; Gagnon et al., 2002; Conrad et al., 2003; Briers et al., 2004). ‘Biologically relevant’ guides to these indices and discussion of their relationships with local weather variables are becoming more common, making their use accessible to a wide range of disciplines (Stenseth et al., 2003).
FIG. 1.
Climate indices such as the North Atlantic Oscillation (NAO) integrate a variety of weather variables across spatial and temporal scales. Here, poor weather in years two, three, and four takes place in different months. However, the sign of the climate index (in this case NAO) indicates that these years have been poor irrespective of when the worst conditions have been experienced. Redrawn from Stenseth and Mysterud (2005, p. 1196) with permission from the British Ecological Society.
To understand the effects of extreme weather on insects and their likely physiological responses, often in anticipation of these extremes, local scale, temporally explicit studies are nonetheless necessary. That extreme abiotic conditions have significant effects on population dynamics, and even population persistence has been demonstrated on several occasions (Leather et al., 1993; Roy et al., 2001). For example, populations of Euphydryas editha (Lepidoptera, Nymphalidae) were driven to extinction as a consequence of three extreme weather events, and human landscape alteration. In one year, minimal snow led to early April (rather than June) emergence of adults and their subsequent starvation owing to an absence of nectar. A year later emergence was once again early for the same reason, and a “normal” snowstorm in May resulted in high mortalities. In 1992, unusually low temperatures killed most of the host plants, leaving caterpillars with no source of food (Thomas et al., 1996; Parmesan et al., 2000). Winter mortality has also been shown to be the source of population (larval) mortality in the butterfly Atalopedes campestris, and warming climates have meant enhanced survival of this species and population persistence in some previously uninhabitable areas (Crozier, 2003, 2004).
It is not only the intensity and occurrence of extreme events that are important, but also the duration of the events, the rates at which they are approached, and the likelihood of their occurrence within a given time frame (i.e. their frequency or return time) (Gaines and Denny, 1993; Gutschick and BassiriRad, 2003). The significance of the intensity and duration of stressful conditions, and their interactions, has long been appreciated by physiologists (for discussion see Cossins and Bowler, 1987; Hochachka and Somero, 2002), and continues to attract the attention of insect physiologists (e.g. Sømme, 1996; Nedved, 1998; Shintani and Ishikawa, 1999; Irwin and Lee 2000, 2002; Jing and Kang, 2003; Neargarder et al., 2003; Renault et al., 2004; Rako and Hoffmann, 2006). Recent work has shown that sublethal exposures may also have substantial impacts. For example, repeated sublethal exposures to high temperature induce substantial mortality in the flesh fly, Sarcophaga crassipalpis, although this thermosensitivity can be overcome by a hardening treatment (Denlinger and Yocum, 1998). In the caterpillars of the tineid moth, Pringleophaga marioni, repeated sublethal low-temperature exposures affect gut functioning, thus depressing growth rates relative to control larvae, and in consequence sublethal events have a negative effect on fitness (Sinclair and Chown, 2005b). In the fly, Syrphus ribesii, repeated stressful exposures result in substantial mortality and an altered cold hardiness strategy (Brown et al., 2004). Such a change in strategy and effect of repeated stressful, typically sublethal mortality events has also been documented in the beetle, Hydromedion sparsutum (Bale et al., 2001).
The rate at which a particular stressful event is approached is important. While early work demonstrated that some variables, such as crystallization temperature (or supercooling point, SCP) are little influenced by changes in rate (Salt, 1966, see also Sinclair et al., 2006), other variables can be profoundly affected. For example, cooling rate may be significant in determining survival of freezing-tolerant insects (Miller, 1978; Shimada and Riihimaa, 1990). In other freezing-tolerant insects, the rate of freezing is important because it affects the likelihood of intra-cellular ice formation (Worland et al., 2004). Cooling rate has a substantial effect on mortality caused by low temperatures and on critical thermal minima because low rates of cooling can provide opportunities for a rapid cold hardening response (Kelty and Lee, 1999, 2001). Likewise, the rate at which conditions return to more benign values is significant, especially following exposure to cold and desiccation, because a return to more normal conditions has profound physiological effects, and might cause stress responses (e.g. Yocum et al., 1991; Joanisse and Storey, 1998; Hayward et al., 2004a; Nielsen et al., 2005).
Recognition of the fact that intensity, duration, and frequency of, rate of approach to, and rate of departure from extreme events all have significant effects on physiological responses and the fitness of insect populations has re-invigorated interest in documenting climate variability in the field. The wide availability of appropriate sensors and datalogging equipment has made such documentation more tractable. Fortunately, a variety of techniques is available for analyzing both more conventional microclimatic data and those relevant for the assessment of extreme values (e.g. Gaines and Denny, 1993; Ferguson and Messier, 1996; Sinclair, 2001b; Vasseur and Yodzis, 2004).
3.2 Variability and Unpredictability
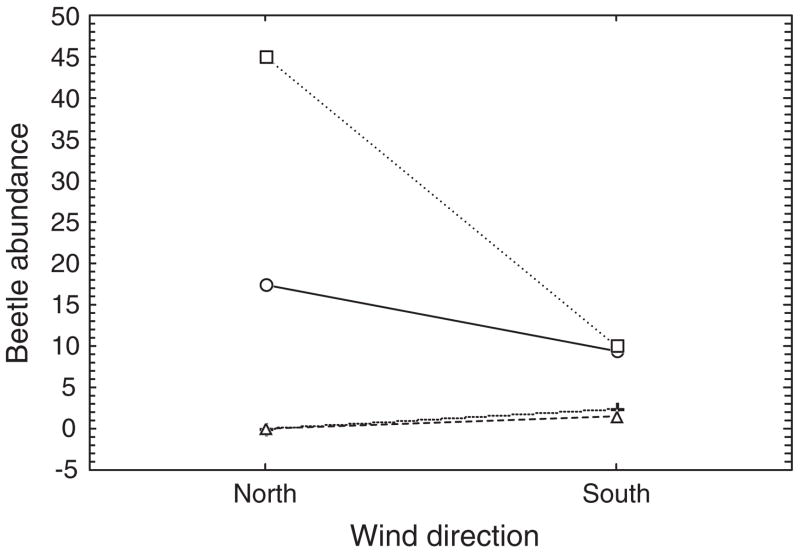
Insects show a variety of behavioural responses to small-scale temporal and spatial variation in abiotic conditions. For example, short-term selection of sunlit patches is one of the most common mechanisms for regulating body temperature (May, 1979; Dreisig, 1980), which at least in some cases results in a close match between preferred body temperature and body temperatures realized in the field (Ward and Seely, 1996). These responses depend on the mechanisms of physiological regulation open to the species (see also Angilletta et al., 2006 for a vertebrate perspective). In the case of thermoregulation, they differ substantially between species of different size, those capable of basking and those able to generate endogenous heat, and between these species and those that employ neither mechanism (see e.g. Herrera, 1997; Sformo and Doak, 2006; and discussion in Chown and Nicolson, 2004). Evolution of tolerance of extreme conditions may also enable species to make use of resources that are typically unavailable under more benign conditions owing to inter-specific interactions. Such a daily partitioning of the thermal environment is thought to represent one of the mechanisms enabling coexistence of competing ant species (Cerdá et al., 1998; Bestelmeyer, 2000; Parr et al., 2005). The varying regulatory abilities (physiological and/or behavioural) of species also contribute to their apparent daily and seasonal abundances, or variation in activity and phenology (Hodkinson et al., 1996; Danks, 1999; Gordon et al., 2001). For example, during their summer-activity peak, adults of Bothrometopus brevis on Heard Island are most active during comparatively warm, north-wind and light-rain conditions (Fig. 2). During heavy rain, activity is low, and it is negligible when low temperatures, associated with south winds, prevail, especially if these are accompanied by snow and sleet (Chown et al., 2004c). Relatively poor resistance to desiccation in the group of weevils to which the species belongs is likely responsible for this behaviour and for their tendency to shelter in rock crevices during dry conditions (Chown, 1993; Chown and Klok, 2003).
FIG. 2.
Interaction plot of mean numbers of adult Bothrometopus brevis weevils active at a site on sub-Antarctic Heard Island for each combination of weather conditions prevailing at the site over the course of a summer, including the two major wind directions (north or south) and either no precipitation (○), light rain (□), snow (+), or heavy precipitation of either kind (△). Redrawn from Chown et al. (2004c).
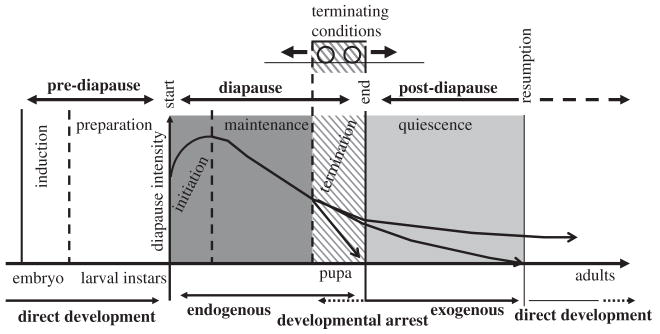
Perhaps, the most well-investigated responses in insects to varying conditions are those associated with seasonal changes in the environment. Dormancy, and especially the endogenous, centrally mediated change that controls diapause has received the most attention. It is the subject of a large literature that has recently been reviewed from several perspectives. Notable amongst these is the clarification of the ecophysiological phases of insect diapause, and their associated terminology (Koštál, 2006), and an overview of the molecular regulation of diapause (Denlinger, 2002). These reviews also refer to further syntheses of work describing the hormonal and physiological changes that are associated with the major phases of diapause: induction of, preparation for, and the initiation, maintenance, and termination of diapause (Fig. 3). One of the most thoroughly explored of these changes is the development of cold hardiness, or a programmed response to cold (Chown and Nicolson, 2004). Although the development of cold hardiness is not always associated with diapause, the two ‘programmes’ are often intimately related (Denlinger, 1991, 2002). Insect responses to changing seasonal conditions have been reviewed from a wide variety of perspectives with much emphasis being placed on the seasonal progression of physiological states and their underlying molecular mechanisms (e.g. Leather et al., 1993; Sømme, 1995; Hallman and Denlinger, 1998; Storey, 2002; Storey and Storey, 2004).
FIG. 3.
Schematic depiction of the major phases of diapause, viz. pre-diapause, diapause, and post-diapause, as defined by Koštál (2006). Further division into subphases, viz. induction, preparation, initiation, maintenance, termination, and quiescence is indicated by vertical lines. Redrawn with permission from Elsevier.
Several recent studies have also indicated that ‘unpredictable’ or unusual events, associated with inter-annual climatic variability, can have a substantial effect on survival of overwintering insects. For example, unseasonably warm Arctic weather can lead to surface ice formation (following rain rather than the usual snow), resulting in substantial mortality of soil-dwelling species (Coulson et al., 2000). Similarly, in Eurosta solidaginis, overwintering at mild temperatures results in a substantial decline in survival as well as in mass of the larvae at the end of the overwintering period (Irwin and Lee, 2000). Mass loss is significant because it translates to a decline in fitness owing to lower fecundity in lighter adult females. The effect of milder temperatures is also reflected in a latitudinal cline in overwinter mass loss, from c. 7.5% in the colder northern parts of the species’ range to c. 20% in milder southern areas. At finer spatial scales, galls that remain below the snow experience milder conditions, as a consequence of insulation by the snow and greater warming during spring days, than exposed galls. Larvae in the former consume much of their stored energy, resulting in lower fecundity, and it seems likely that this has resulted in strong selection for overwintering above the snow and substantial freeze tolerance (Irwin and Lee, 2003).
Inter-annual variability in precipitation can have substantial effects on insect-development rates, leading to considerable population variance. In herbivorous caterpillars, this unpredictable variation in growth rates, coupled with the unpredictable variation in climate has substantial effects on parasitoid dynamics. In areas with a high-precipitation coefficient of variation (c.v.), parasitism frequency is low, whereas it is much higher in areas of low precipitation c.v. (Stireman et al., 2005). These effects are much more pronounced in host-specific parasitoids, which have little opportunity for exploiting alternative resources. If this spatial relationship applies through time, then increases in the variability of the abiotic environment, as are predicted to occur in many areas as a component of global environmental change (Watson, 2002), will mean increased caterpillar outbreaks. Such outbreaks have substantial ecosystem effects, cascading through multiple trophic levels, with both economic and conservation implications (e.g. Myers, 1988; Hódar et al., 2003).
Given that unseasonable weather events can have substantial influences on survival, that climatic variation can influence host–parasitoid interactions through differential responses of these groups to abiotic factors, and that both variability and unpredictability are key factors influencing the likely evolution of phenotypic plasticity, documenting variability and unpredictability is of considerable importance. Such documentation must recognize that both of these environmental characteristics vary at a variety of scales (Kingsolver and Huey, 1998), and that the significance of the scale of variation will depend on both the size and longevity of the insect stage that is being investigated. For example, investigation of inter-annual variation and predictability of winter minima is unlikely to be directly significant for the short-lived adults of an insect species such as a goldenrod gall fly, but might be of considerable indirect significance because such temperatures will determine adult fecundity via energetic effects on the larval stage (see also Angilletta et al., 2006).
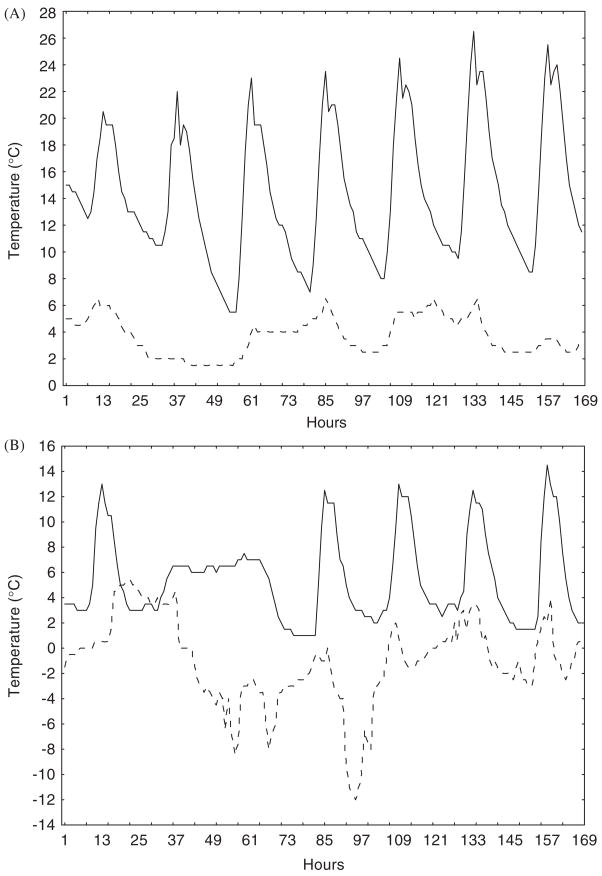
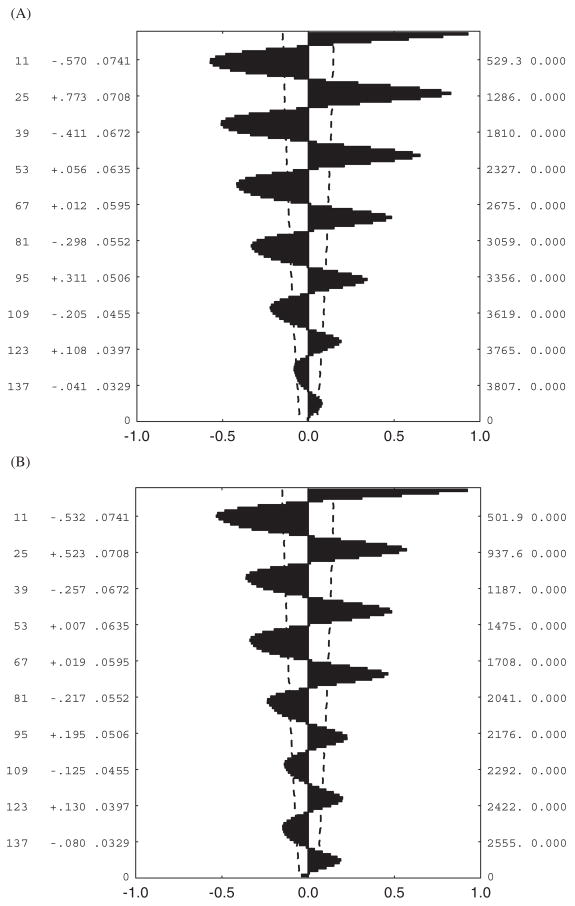
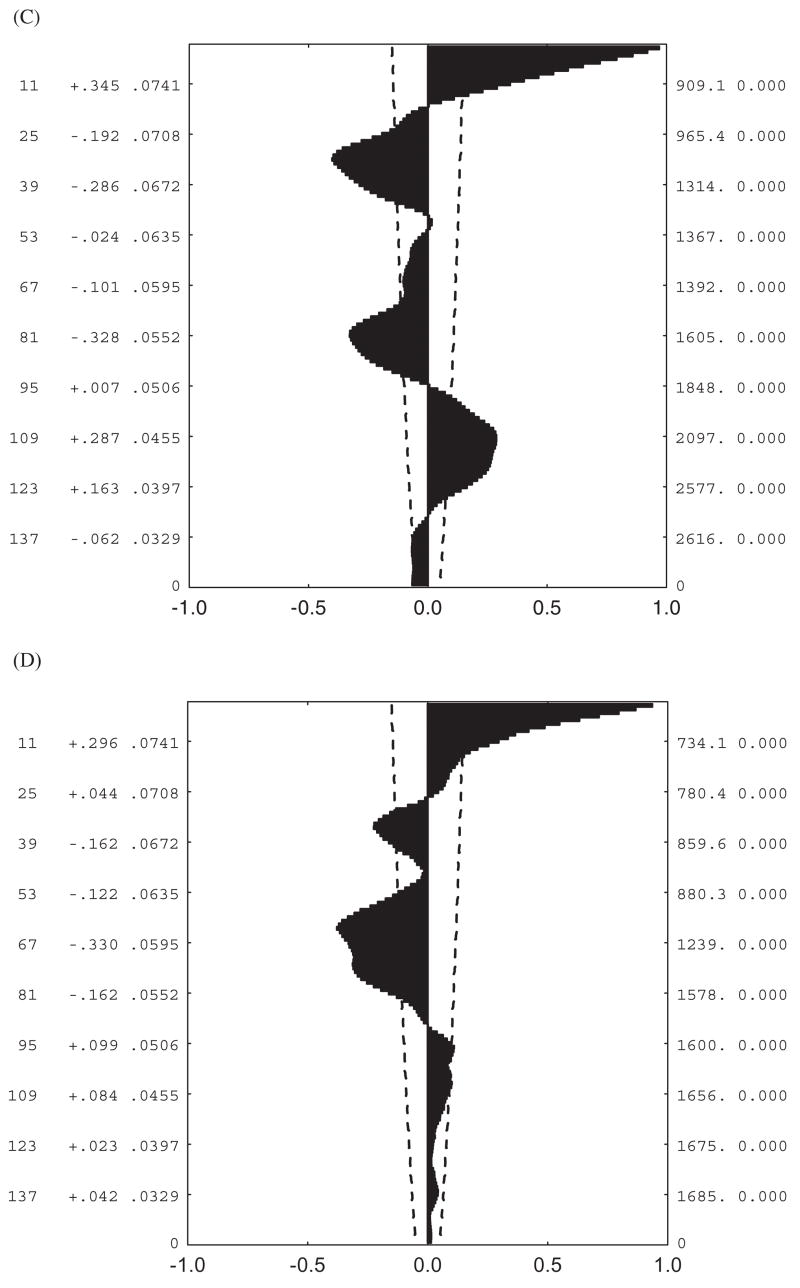
Although standard measures of variation, such as differences in seasonal means, minima, and temperature ranges, and coefficients of variation, provide considerable insight into variability in conditions, they are less appropriate for understanding predictability of these conditions. Several approaches allow the latter to be done. One of the most straightforward approaches is to examine correlations of conditions over a variety of temporal scales, which can reveal differences in predictability at the scale of the individual’s lifetime, between seasons, and between years (Kingsolver and Huey, 1998). In addition, examination of the autocorrelation plots of the time series in question provide a rapid way of assessing predictability of a particular environmental variable. For example, hourly soil temperatures over a weeklong period at a site on the west coast of South Africa (Lambert’s Bay) are perfectly predictable from day to day (Fig. 4a), and this is reflected in significantly positive autocorrelations at lags of 24 h and multiples thereof (Fig. 5a). Although conditions at higher altitudes are a little less predictable (Fig. 4b), a similar autocorrelation pattern can be seen (Fig. 5b). By contrast, sea level and high altitude soil temperatures at sub-Antarctic Marion Island are far less predictable (Fig. 4a, b), as is immediately obvious from the autocorrelation plots. In the sea-level example (Fig. 5c), temperatures are significantly dissimilar to those experienced 24 h previously than would be expected by chance, and at the higher elevation the signal rapidly becomes indistinguishable from white noise (Fig. 5d). Fourier analyses provide similar conclusions, with the South-African site data showing greatest spectral density at 24 h, and the Marion Island site data showing rather weaker signals at 55 h at sea level, and no significant signal for the higher elevation site.
FIG. 4.
Hourly temperatures at the soil surface over a week long period in August 2002 for (A) a sea-level site at Lambert’s Bay on the west coast of South Africa (solid line) and a sea-level site at sub-Antarctic Marion Island (dashed line), and (B) a site (Sneeukop) at 1960 m above sea level 50 km distant from the Lambert’s Bay site (solid line) and at 800 m on Marion Island (dashed line). Note the difference in predictability of temperatures for the Lambert’s Bay and Marion Island sites.
FIG. 5.
Autocorrelation plots for hourly temperatures shown in Fig. 4. (A) Lambert’s Bay sea-level data, (B) Sneeukop close to Lambert’s Bay, (C) Marion Island sea level, (D) Marion Island 800- m site. The dashed lines on each figure represent the 95% confidence intervals, while the values reported to the right of the lags on the y-axis are the autocorrelation coefficients and their standard errors.
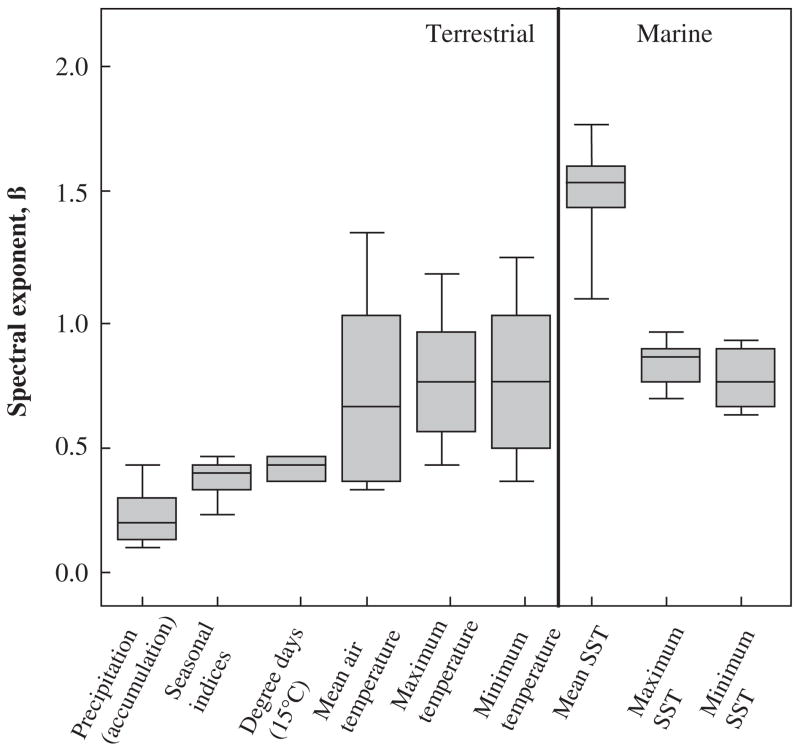
The calculation of spectral densities is being widely applied in ecology as a means of investigating the form and significance of environmental noise for populations and other levels in the ecological hierarchy. The importance of the colour of noise was first raised in an ecological context by Steele (1985) and has since been the subject of much attention (e.g. Lawton, 1988; Halley, 1996; Cohen et al., 1998; Storch et al., 2002). White noise contains an equal mix of all frequencies, with a flat spectral density. It is a special case of a family of noise forms in which variance scales with frequency according to an inverse power law, 1/fβ (Halley, 1996; Vasseur and Yodzis, 2004). In the case of white noise, β = 0. If the spectral density is greater at low than at high frequencies then the spectrum is said to be reddened: low frequency cycles dominate. Brown noise refers to a signal generated by Brownian process or a random walk. By contrast, pink noise lies midway between brown and white noise. In a comprehensive assessment of long-term variability (with the seasonal component removed), Vasseur and Yodzis (2004) showed that in the case of mean environmental temperature, noise colour varies from mostly white (0 ≤ β ≤ 0.5) in terrestrial locations to red–brown (red: 0.5 ≤ β ≤ 1.5; brown: 1.5 ≤ β ≤ 2) at coastal locations, to brown for sea-surface temperature data. By contrast, monthly minima and maxima have reddened spectra, whilst precipitation and seasonal indices are characterized by pink noise (Fig. 6). The difference between mostly white spectra at terrestrial locations and reddened noise in marine systems is probably the consequence of the substantial buffering capacity of the sea (Vasseur and Yodzis, 2004). This buffering capacity can probably also explain the mostly white spectra of minimum temperature between 30 and 60° of latitude in the northern hemisphere, and the reddened spectrum in the same areas in the southern hemisphere. Between 30 and 60° N, the land:water proportion is approximately 1:1, whereas between 30 and 60° S, it is 1:15 (Chown et al., 2004a). The absence of a difference in the spectral exponent for maximum air temperature among the hemispheres is readily explained by the fact that absolute maxima differ little among them, whereas variation in absolute minima is much more pronounced (Addo-Bediako et al., 2000; Chown et al., 2004a).
FIG. 6.
Box plots of the spectral components for several environmental variables, including sea surface temperature (SST) for terrestrial and marine systems. Lines indicate the median, 75th and 90th percentiles. Redrawn from Vasseur and Yodzis (2004, p. 1149) with permission from the Ecological Society of America.
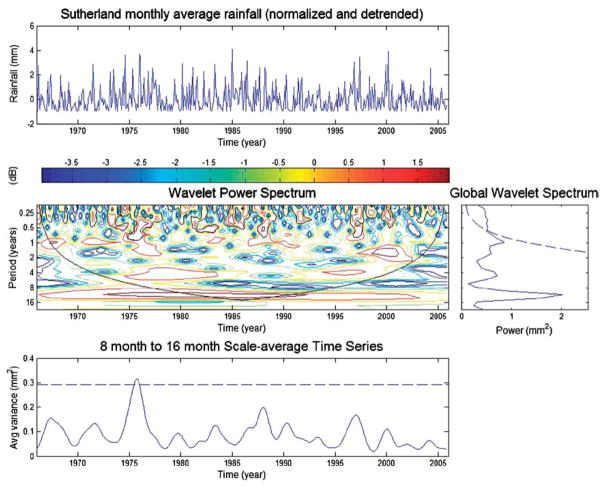
If changes in periodic behaviour over a long period take place (i.e. the data lack stationarity), Fourier analyses and assessments of the relationship between spectral density and frequency will not reveal them. Although the scales of variation of the entire series will be apparent, any sequence in these data will remain hidden (Grenfell et al., 2001). Wavelet analysis is a powerful technique that can be used to explore variation in frequency as time progresses by time–frequency analysis of the signal (Fig. 7). Although it is a relatively complex analytical approach, several clear guides to its use are available (e.g. Torrence and Compo, 1998), and it is no longer confined to the geophysical applications in which it has been most popular (e.g. Mélice et al., 2003). Rather, it is being applied to a wide variety of population-level data. For example, wavelet analyses unveiled a substantial change in inter-annual variability of the populations and breeding success of three Antarctic seabird species associated with a shift in environmental conditions (Jenouvrier et al., 2005). Klvana et al. (2004) used wavelet analysis to demonstrate a strong coherence between porcupine feeding scar data and the solar cycle: the first demonstration of a population cycle in mammals that is related to both local climatic fluctuations and the solar cycle. In insect herbivores, a similar relationship was demonstrated at virtually the same time, though using a less complex analytical approach (Selås et al., 2004). The populations of several moth species in Norway are inversely correlated with sunspot activity. This seems to be a consequence of enhanced UV-B radiation during low sunspot activity, which requires pigment production by the host plant. Caterpillars prefer leaves exposed to elevated UV-B because the leaves incur metabolic costs producing pigments, so reducing resistance to the herbivores.
FIG. 7.
Wavelet analysis (see Torrence and Compo, 1998) of monthly rainfall data from Sutherland, a high altitude, semi-arid area in the Karoo of South Africa. The upper panel shows the detrended normalized data. The central panel, the wavelet power spectrum with period on the y-axis and years on the x-axis, and the dark line the cone of influence (with no zero padding), and the global power wavelet spectrum shown to the right thereof. The averaged time series is shown in the lower panel.
Investigations of changes in long-term periodic behaviour are not common in the insect physiological literature. However, it seems likely that they will prove to be useful, especially in understanding long-term changes in insect responses to the environment. These kinds of changes are not unknown in the physiological literature. For example, in the overwintering larvae of Dendroides canadensis and Cucujus clavipes, initial studies indicated that individuals are freezing tolerant, while investigations in the following years revealed a switch to freeze intolerance (Kukal and Duman, 1989). This shift in cold hardiness strategy is thought to have been a consequence of changes in the thermal environment of the species, though no detailed time-series analyses were undertaken. In the cricket, Conocephalus discolor, long-term changes in the environment have resulted in high frequency of an extra-long-winged form in newly established populations of the species, which must have been affected through changes in hormonal regulation of wing production in the species (Thomas et al., 2001). The influence of variability and predictability on the evolution of plasticity also means that long-term assessments of the likely stationarity of the environment may provide considerable insight into species responses that might be mediated by plasticity (Stillman, 2003; Helmuth et al., 2005).
4 Phenotypic plasticity
Although circumstances exist where a specialist will be favoured over a conditional strategist (Berrigan and Scheiner, 2004; van Kleunen and Fischer, 2005), plasticity is optimal under a wide range of conditions (Section 2.2). Appreciation for the commonness of phenotypic plasticity has long existed in the literature on physiological and morphological traits (review in DeWitt and Scheiner, 2004), but it is only relatively recently that its importance in evolution has been realized (West-Eberhard, 1989, 2005). The literature in the field is now substantial, and the idea here is not to review the field, nor to dwell on debates, such as the merits of the character state and polynomial approaches to investigating plasticity, that have long characterized the field. Recent comprehensive reviews and perspectives provide ready access to this literature, including resolution of several of the debates (e.g. Nylin and Gotthard, 1998; Schlichting and Pigliucci, 1998; Schlichting, 2002; Pigliucci and Murren, 2003; West-Eberhard, 2003; De-Witt and Scheiner, 2004; Pigliucci, 2005). Rather, we focus on several issues that are significant for physiologists concerned with phenotypic plasticity, especially in its more common guises of acclimation or acclimatization. Initially, we dwell briefly on semantic issues, not because we think that creating specific terminology for different forms of plasticity is especially helpful (see West-Eberhard, 2003 for this view and Piersma and Drent, 2003, for a contrary opinion), but because in some cases it is not yet entirely clear how similar or different are the mechanisms underlying responses at different time scales (e.g. Bowler, 2005; Loeschcke and Sørensen, 2005; Sinclair and Roberts, 2005, but see also Chown and Nicolson, 2004, Ch. 5).
4.1 Terminology
Phenotypic plasticity can be defined as ‘the ability of an organism to react to an environmental input with a change in form, state, movement, or rate of activity’ (West-Eberhard, 2003). It is often also defined as ‘the environmentally sensitive production of alternative phenotypes by given genotypes’ (DeWitt and Scheiner, 2004), although in the singular, such a definition could result in neglect of the fact that the initial phenotype of an individual is typically a structure provided by the parent, and therefore is not the product of one genotype (West-Eberhard, 2003; Huestis and Marshall, 2006). The former definition includes all forms of plasticity, and indeed, can be simplified to ‘intra-individual variability’. Further, formal, qualification of the term plasticity, and hence a restriction of its definition, has long been used to distinguish between non-adaptive and adaptive responses, active and passive responses, reversible, irreversible and cyclic responses, continuous and discontinuous responses, and those which take place following development, or shorter-term exposures to different environments (see Piersma and Drent, 2003; Bowler, 2005; Seebacher, 2005 for recent examples, and West-Eberhard, 2003, for review of the older literature). By contrast, West-Eberhard (2003) suggested that special terms for these kinds of plasticity are not necessary, but rather that descriptive adjectives should be used to make appropriate distinctions where these are necessary. In a similar vein, DeWitt and Scheiner (2004) argued for broad applicability of the term plasticity, pointing out that the significant issue is the focus on genotype-environment interactions.
These more ‘liberal’ approaches are well suited to investigations of plasticity in insects. For example, the definitions provided by Piersma and Drent (2003), initially seem appropriate for studies of intra-individual environmental responses in this group. However, on further consideration it is clear that they are problematic. Thus, ‘developmental plasticity’ in Piersma and Drent’s (2003) sense is not thought to take place within a single individual, whereas this contradicts widely accepted views on plasticity, probably as a consequence of the fact that the distinction between population and individual levels was not explicitly made (see Pigliucci, 2005, Box 1). Likewise, Piersma and Drent (2003) argue that ‘developmental plasticity’ precludes reversible phenotypic change. However, several recent studies have shown that developmental plasticity in a variety of traits may be either reversible or irreversible. In Bicyclus anynana, rearing temperature has a substantial effect on egg size, which is largely reversible by holding adults at different temperatures (Fischer et al., 2003, 2006), and in Lycaena tityrus, the effects of developmental plasticity on cold shock are similarly reversible in the adult stage (Zeilstra and Fischer, 2005). In the tsetse, Glossina pallidipes, developmental plasticity (pupal exposures only) of critical thermal minima and desiccation rate are irreversible following treatments at 29 °C relative to 25 °C, but the pupal treatment was either reversed or had little effect following a 21 °C treatment for these traits, and following both treatments in the case of metabolic rate and critical thermal maximum (Terblanche and Chown, 2006). Similarly, in D. melanogaster, mortality induced by cold shock following rearing at a high developmental temperature is little affected by adult acclimation, whereas chill coma recovery time is strongly affected by adult acclimation (Rako and Hoffmann, 2006).
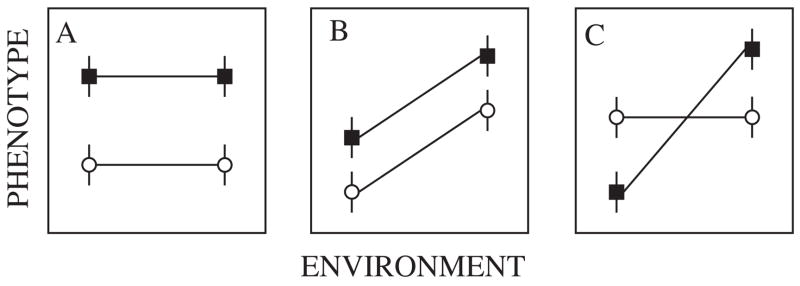
Some confusion has also arisen in the literature as a consequence of distinctions made between responses considered to be genetic (≈ adaptive) and those thought to be non-genetic (≈ plastic). Although widespread, such a distinction is, in DeWitt and Scheiner’s (2004) words ‘enduring and perennially misleading’. As they point out, plastic responses have a genetic basis, and may be active or passive (see also West-Eberhard, 2003; Fischer et al., 2006). Moreover, as has long been clear, genotypes and the environment interact (often simply stated as a G × E interaction). Unfortunately, the terms plasticity and G × E interactions are also sometimes confused because of usage of the terms at both the level of individual genotypes and populations of genotypes (Pigliucci, 2005). Because plasticity is defined as the ability of an organism to react to an environmental input, a slope (positive or negative) in the environment–phenotype space indicates plasticity at the individual level, and plasticity at the population level if the average difference among environments across genotypes is considered (Fig. 8). At the population level, statistically significant G × E interactions refer to the differences in slope of the reaction norms (DeWitt and Scheiner, 2004), whereas at the individual level genotype by environment interactions represent the idea that the genotype and environment interact continuously during an individual’s development (Pigliucci, 2005). Much of the insect physiological literature on thermal tolerances and water balance is concerned with population-level responses, and discussion of these responses should bear in mind the distinctions between plasticity and G × E interactions made so clearly by DeWitt and Scheiner (2004).
FIG. 8.
Reaction norms for two families (circles and squares, mean and standard errors for the phenotypes are shown) demonstrating (A) significant genetic variance, (B) significant genetic and environmental variance, (C) significant genetic, environmental, and genetic by environmental interaction variance. Based on De-Witt and Scheiner (2004, p. 4).
A final potential complication arises when performance curves (see Huey and Kingsolver, 1993; Huey and Berrigan, 1996) or performance functions (Angilletta et al., 2002) and reaction norms (the form a phenotypic response to the environment takes, see Huey and Berrigan, 1996) are equated. There is no reason why a performance curve should not be considered a reaction norm (see Angilletta et al., 2003), and the statistics for analysing aspects of the two are similar in many respects (compare Gilchrist, 1996 and David et al., 1997, see also Kingsolver et al., 2001). However, the complication arises when the response of the performance curves themselves, or components thereof, to various environmental conditions are assessed. Thus, the shape of the performance curve as well as its position, breadth, height, and other components (see Huey and Kingsolver, 1993; Angilletta et al., 2002) might all respond in different ways to environmental conditions imposed during any part of an individual’s life (Fig. 9). The form of these responses also constitutes a reaction norm. Arguably, the most appropriate way to deal with such potential complications is to be explicit about what the subject is of the work. Where variation in performance curves is being assessed, use of the term ‘reaction norm’ should be restricted to the response of the curves, rather than being meant to imply the curves too.
FIG. 9.
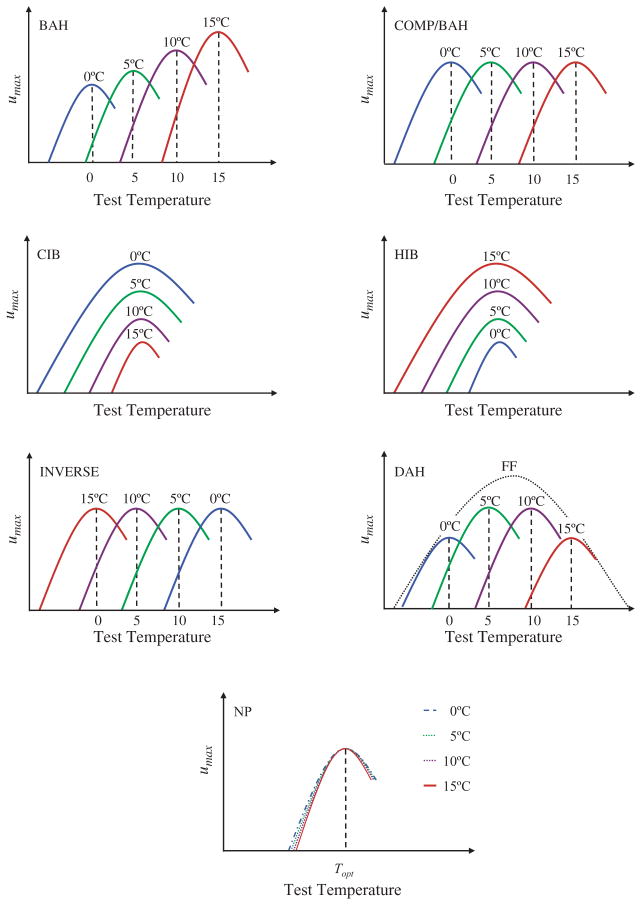
Predictions from each of the major hypotheses for the response of individual performance curves to acclimation. In each case four acclimation temperatures from low to high are indicated (blue (0 °C), green (5 °C), purple (10 °C), red (15 °C)), and in one case the expectation for field fresh (FF) individuals is also shown. BAH = beneficial acclimation hypothesis, COMP/BAH = complete temperature compensation (an instance of BAH), CIB = colder is better, HIB = hotter is better, IAH = inverse acclimation hypothesis, DAH = deleterious acclimation hypothesis, NP = no plasticity. Redrawn from Deere and Chown (2006).
4.2 Acclimation as a Form of Plasticity
With the exception of metabolic scaling, few topics in evolutionary physiology have generated as much recent, vigorous discussion as has acclimation and whether or not it is beneficial (reviews in Huey et al., 1999; Angilletta et al., 2006). Acclimation (in the laboratory) and acclimatization (in the field) are both terms coined to describe intra-individual variability. Therefore, they describe forms of phenotypic plasticity (see Huey and Berrigan, 1996; Huey et al., 1999). Physiologists have long held the view that phenotypic change by an individual in advance of, or in response to, a changing environment is beneficial (see Prosser, 1986; Cossins and Bowler, 1987; Hochachka and Somero, 2002 for access to the literature, and Shreve et al., 2004, for a recent example). This view has been recast as the beneficial acclimation hypothesis (BAH), defined by Leroi et al. (1994) as ‘acclimation to a particular environment gives an organism a performance advantage in that environment over another organism that has not had the opportunity to acclimate to that particular environment’. While it does not explicitly cover responses in anticipation of an environmental change, this definition has been used by many recent investigations as a departure point for examining the extent to which acclimation can be considered beneficial (see Huey et al., 1999 for review). In addition, the majority of these studies have included explicit a priori alternatives, a strong inference approach that was typically lacking from the previous physiological literature (Huey and Berrigan, 1996). Most of these more recent studies have found little support for the beneficial acclimation hypothesis (Leroi et al., 1994; Zamudio et al., 1995; Bennet and Lenski, 1997; Sibly et al., 1997; Woods, 1999; Gibert et al., 2001; Gilchrist and Huey, 2001; Woods and Harrison, 2001; Stillwell and Fox, 2005). Rather, in each case one or more of the alternative hypotheses (Fig. 9) could not be rejected.
Considering the wide range of scenarios under which plasticity is likely to be favoured (Section 2.2; also Scheiner, 1993; Agrawal, 2001), this lack of support for the BAH is counter intuitive. However, as is clear from Section 2.2, several circumstances exist in which acclimation is unlikely to be beneficial. Moreover, under some conditions, such as if lag times are substantial, plasticity might not readily evolve. Wilson and Franklin (2002) argued that the majority of thermal acclimation tests of the BAH are neither direct nor complete because they assess the adaptive significance of ‘developmental plasticity’, rather than investigating what comparative physiologists regard as acclimation (or acclimatization) (see Spicer and Gaston, 1999, pp. 32–38; Willmer et al., 1999, pp. 9–12). That is, many past assessments of phenotypic plasticity have involved alteration of rearing regimes and subsequent assessment of adults (which implies substantial lag times), rather than assessment of phenotypic alterations within a given life stage. Thus, in Wilson and Franklin’s (2002) view, these tests are confounded by the fact that several different kinds of plasticity are being assessed simultaneously. It has also been suggested that some of the alternative hypotheses are not mutually exclusive and that it is, in consequence, difficult to design experiments to distinguish between them (Angilletta et al., 2006). Finally, stressful environmental treatments might have compromised tests of the BAH by impairing organismal performance (Wilson and Franklin, 2002; Woods and Harrison, 2002), and a focus on the entire suite of characters that constitute fitness is likewise problematic (Woods and Harrison, 2002).
Several proposals have been made to resolve what appears to be a hung jury on the question of beneficial acclimation. These include adopting a strong inference approach and selecting environmental conditions with care to ensure that the effects of stressful conditions are fully assessed (and perhaps using independent measures of stress such as the presence of heat shock proteins) (see discussion in Hoffmann, 1995; Hoffmann and Hewa-Kapuge, 2000; Loeschcke and Hoffmann, 2002; Wilson and Franklin, 2002; Woods and Harrison, 2002). Careful consideration of the alternative hypotheses in the context of appropriate statistical methods (e.g. orthogonal polynomial contrasts in ANOVA – Huey et al., 1999) should also alleviate problems associated with hypothesis testing. For example, Angilletta et al. (2006) suggested that the ‘colder is better’ and ‘developmental buffering’ hypotheses are not mutually exclusive because the former posits increased body size at low temperatures whereas the latter is based on a size-independent mechanism. However, as Huey et al. (1999) made clear, ‘colder is better’ also suggests that performance could be enhanced following low-temperature treatments by mechanisms not associated with size. Therefore, the two hypotheses could be mutually exclusive (see Fig. 9). Explicitly assessing different forms of plasticity (e.g. hardening, acclimation within a life stage, and developmental plasticity) can also provide a fresh perspective on the question. For example, exposure of Drosophila melanogaster to low-temperature treatments for brief periods of a few hours (hardening), two days (acclimation), and for two generations (developmental plasticity) revealed substantial complexity in fly responses, some of which could be considered beneficial (Rako and Hoffmann, 2006; see also Nielsen et al., 2005). Broader application of these approaches is essential if the significance of phenotypic plasticity for the evolution of physiological traits, and for changes in the distribution and abundance of organisms are to be more fully comprehended (Section 2.4; Sultan, 2004; Dybdahl and Kane, 2005).
4.3 ‘Unintentional’ Acclimation
Any population exposed to a novel environment is expected, at least in the longer term, to adapt to that environment, or at the very least respond to selection imposed by that environment. Reponses to selection are indeed common both in the laboratory and in the field (e.g. Huey et al., 1991; Gibbs, 1999; Hoekstra et al., 2001; Kingsolver et al., 2001). One unintended consequence of this response is that organisms held in the laboratory for several generations adapt to the laboratory conditions (Harshman and Hoffmann, 2000; Matos et al., 2000; Sgròand Partridge, 2000). Differences between laboratory colonies and field populations have been documented for many traits and species, including cold and heat tolerance in flies (Zatsepina et al., 2001), antennal sensilla chemo- and mechanoreceptors in Hemiptera (Catala et al., 2004), pheromone communication between sexes in the screwworm Cochliomyia hominivorax (Hammack, 1991), and CO2 anaesthesia effects on knockdown and recovery times in cockroaches (Branscome et al., 2005). Such laboratory adaptation can also take the form of a relatively rapid decline in stress resistance. For example, in Drosophila melanogaster, starvation and desiccation resistance declined from LT50 values of 50.1–35.9 h, and 14.3–8.9 h, respectively over a period of four years (Hoffmann et al., 2001). However, not all traits respond so strongly to long-term laboratory culture (Krebs et al., 2001).
Therefore, rapid responses to selection often seen in the laboratory might represent the reacquisition of responses to more stressful conditions experienced by the population before it was taken into culture. The accumulation of mutations in culture, which can have significant effects on responses to laboratory selection, also appears to be pervasive (Harshman and Hoffmann, 2000). In consequence, investigations using laboratory selection, which provides a useful and essential complement to comparative studies (Kingsolver and Huey, 1998; Gibbs, 1999; Feder and Mitchell-Olds, 2003), must take due cognisance of laboratory adaptation.
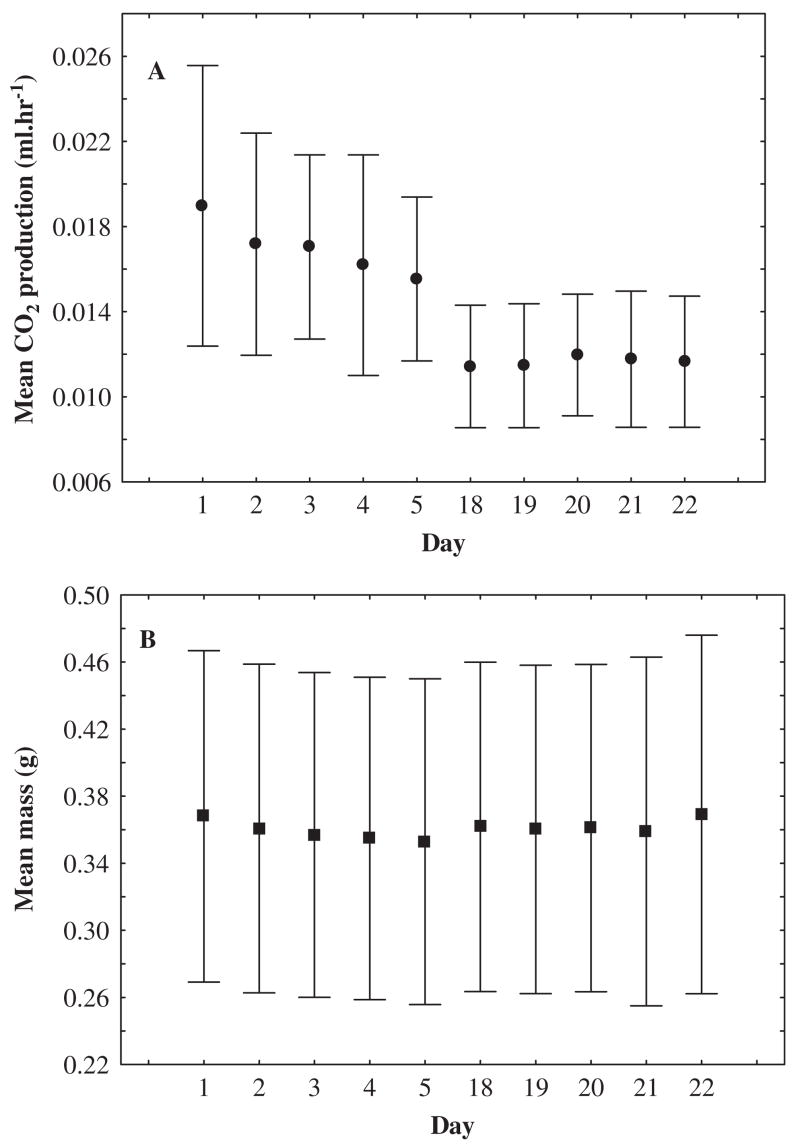
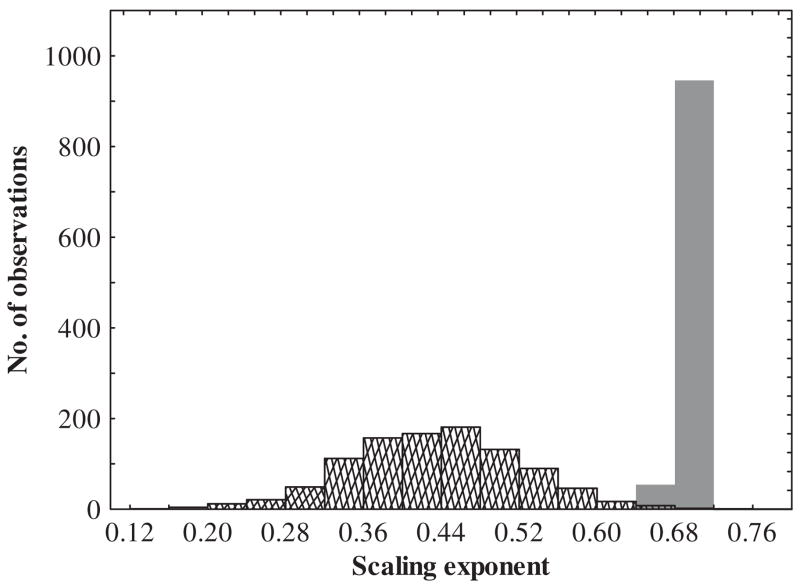
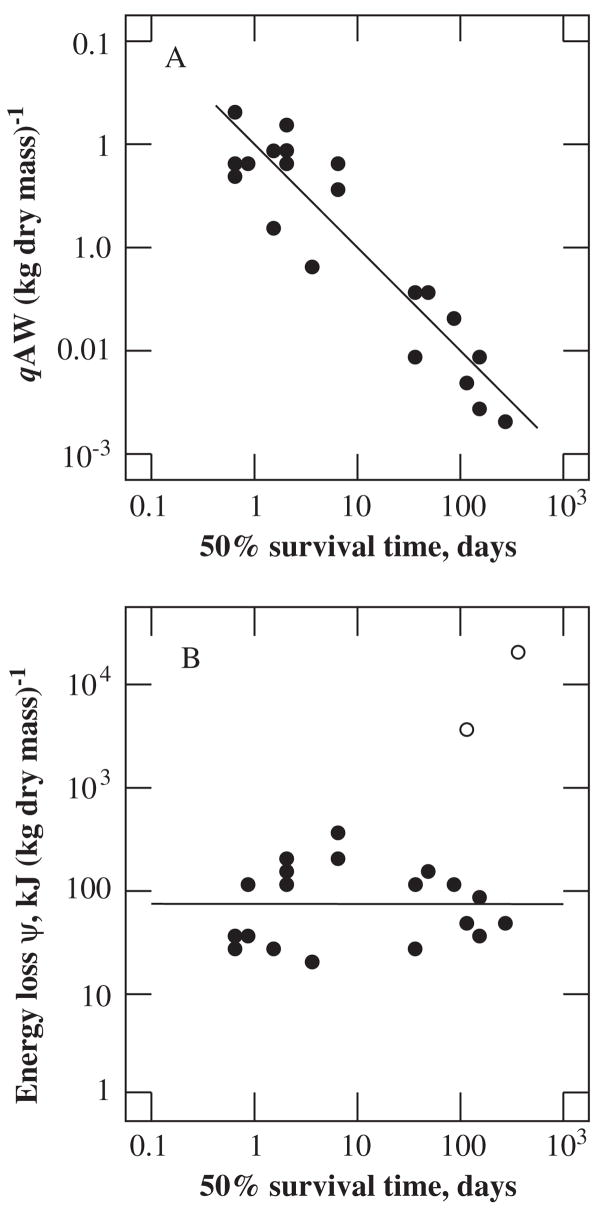
In a similar fashion, holding organisms for substantial periods in the laboratory could give rise to substantial, unintended, acclimation effects. It is widely appreciated that insects can respond rapidly to a given environmental treatment and to its relaxation (e.g. Lee et al., 1987a; Hoffmann et al., 2003b; Chown and Nicolson, 2004; Rako and Hoffmann, 2006; Terblanche et al., 2006). Such laboratory responses form the basis of a large and proliferating physiological field aimed at investigating the nature, time course, and mechanistic underpinnings of phenotypic plasticity. What is perhaps less widely acknowledged is that unintended acclimation can confound investigations (though see Spicer and Gaston, 1999). One recent demonstration of the significance of this problem is provided by an investigation of the scaling of avian metabolic rate (McKechnie et al., 2006). Captive birds have a shallower metabolic rate–body mass relationship than wild birds because small birds tend to upregulate basal metabolic rate in captivity, while the converse is true in large birds. The same kinds of responses could confound physiological investigations in arthropods. In the whip-spider, Damon annulatipes, mean metabolic rate declined substantially, from 30.2 to 21.8 μl CO2 h−1, despite no change in mean body mass, following two weeks in the laboratory (Terblanche et al., 2004). The same trend has been found in the scorpion Uroplectes carinatus (Fig. 10). These declines in metabolic rate are likely a consequence of reduced temperature variation, less demanding foraging requirements, and absence of the need to avoid predators (Hoffmann et al., 2001; Terblanche et al., 2004). Simple simulations illustrate that, if these kinds of effects are more common than has been assumed, they will have to be taken into consideration in future, especially, comparative studies. Assume that metabolic rate scales as mass0.70, with little variation as a consequence of different life histories – a simplistic assumption (Koz³owski et al., 2003; Brown et al., 2004; Clarke, 2004), but one useful for present purposes. If metabolic rate declines with an exponential decay function (y = MR e−0.15t, where t = hypothetical time in the laboratory), and the amount of time spent in the laboratory varies at random among the species (or individuals/populations) of interest, then the form of the relationship can change substantially (Fig. 11). Most notably, there is considerable increase in the variation of slope coefficients.
FIG. 10.
A rapid decline is found in whole-animal metabolic rate (A) but not in body mass (B) with introduction to stable laboratory conditions in the scorpion Uroplectes carinatus. Mean standard metabolic rate (CO2 ml h−1±95% confidence intervals) recorded using flow-through respirometry at 25 °C and body mass (in g) from each trial day during acclimation to constant conditions (25 °C) in the laboratory.
FIG. 11.
Variation induced by laboratory acclimation around a hypothetical metabolic rate-body mass scaling relationship. The solid bars represent a hypothetical metabolic rate-body mass scaling relationship for animals (n = 50 individuals, using 1000 random numbers re-sampled with replacement using Microsoft Excel) that are all in the same acclimation state (i.e. only field collected). An exponential decay function (y = MR e −0.15t,where t = hypothetical time in the laboratory) was applied to these data to simulate a possible acclimation-induced decline in metabolic rate and how this may affect the scaling exponents (hatched bars). A random series of time intervals were generated (ranging from 0 to 5) and applied to metabolic rate data using the exponential decay function.
What unintended laboratory acclimation means for past comparative investigations is not obvious, although the nature of the question and the likely signal-to-noise ratio of the study will determine the importance of the unintended effects (see discussion in Chown et al., 2003; Hodkinson, 2003). Nonetheless, it is clear that comparison of individuals freshly retrieved from the field with those held in the laboratory, bearing in mind that seasonal acclimatization is also common (Chown et al., 2003; Chown and Nicolson, 2004), could go some way to resolving these issues. Similarly, field-cage experiments (such as reciprocal transplants) (e.g. Jenkins and Hoffmann, 1999; Hoffmann et al., 2003c) may be revealing. However, it should not be forgotten that in many instances the very subject of investigation is phenotypic change in response to manipulation of one or more environmental variables while all others are held constant. In this case, laboratory treatments and investigations of individual responses are the only way to proceed, but their unintentional consequences should not be neglected.
5 Sensing
Any response to the environment, whether it is a conditional response, or one that eventually becomes fixed, requires a sensing mechanism or receptor (Denlinger et al., 2001; Danks, 2003). Lag times, unpredictability, and inscrutability of the environment are widely discussed in the literature on the evolution of phenotypic plasticity, as are the nature and time course of, and mechanisms underlying organismal responses. The perception of the environment dictates the speed of response to change (see also Robertson, 2004a). Therefore, knowledge of the mechanisms that underlie perception is important for determining the way in which the animal is likely to perceive and respond to a changing environment. Accurate environmental perception enables insects to take advantage of optimal conditions, ultimately contributing to the animal’s success in a particular environment. The relative timing and reliability of cues not only has behavioural implications, but also has both physiological and ecological consequences, ranging from the preparation for and response to diurnal and seasonal physiological changes, to physiological reorganization during dormancy, and the likelihood that phenotypic plasticity will evolve. Nonetheless, in the context of plasticity, sensing mechanisms (or receptors) have typically received much less attention than other physiological traits. Consequently, this is a fertile field for investigation, though it presupposes that much of the basic information on sensing is available (see Chown and Storey, 2006 for an analogous discussion). As we show in this section, progress in modern understanding of temperature and moisture (hygro-) sensing differs appreciably, and has some way to go before it can be readily integrated into investigations of whole-animal physiological responses to the environment.
5.1 Detecting Changes in External Environmental Temperature
For at least the past century, it has been clear that insects are capable of sensing and responding to temperature variation (reviewed in Blum, 1985; Chown and Nicolson, 2004), as is attested by studies of body temperature regulation. For example, behavioural thermoregulation in a temperature gradient has been shown in a wide variety of species, including cockroaches (Murphy and Heath, 1983), grasshoppers (Lactin and Johnson, 1996; Forsman et al., 2002), bugs (Lazzari, 1991; Guarneri et al., 2003; Minoli and Lazari, 2003; Schilman and Lazzari, 2004), moths (Kuhrt et al., 2006), beetles (Roberts et al., 1991; Ybarrondo, 1995; Jian et al., 2002), flies (Huyton and Brady, 1975; Yamamoto, 1994), and ants (Roces and Nunez, 1995). It is largely assumed that body temperature preferences evolve (see Garland et al., 1991; Angilletta et al., 2006), although only a few demonstrations exist. Thus, it is not clear to what degree natural selection has been responsible for the origin and maintenance of preferred body temperature variation among insect species. However, a positive response to laboratory selection has been observed in Drosophila melanogaster (Good, 1993). After 10 generations, flies reared at warmer temperatures showed an increase in preferred body temperature (Fig. 12). Partial reversal of the shift in preferred body temperature provided evidence for an environmental component to the change (Good, 1993; see also Murphy, 1986; Forsman et al., 2002). These findings also suggest that geographic clines in preferred body temperature should not be uncommon, and this seems to be the case in some species (e.g. the grasshoppers Melanoplus sanguinipes and Xanthippus corallipes) (Ashby, 1997; Rourke, 2000; Samietz et al., 2005), but not others (e.g. Drosophila immigrans and D. virilis, Yamamoto, 1994).
FIG. 12.
Mean preferred temperatures (± SE) in Drosophila melanogaster. Values represent tenth generation 25, 27, and 30°C-reared populations and reverse temperature treatment populations (i.e. reversible plastic component), 25/30 °C and 30/25 °C (females only). The 25, 27, and 30 °C groups are significantly different, while the 25/30 °C and 30/25 °C do not differ (although both of the latter reverse treatments differ from the 30 °C group). Figure redrawn from Good (1993) with permission from Elsevier.
Other factors may also play a crucial role in determining temperature preference. Remarkably, individuals of the nematode Caenorhabditis elegans select temperatures at which they were reared, while specifically avoiding temperatures at which they were starved (Mori, 1999). Thus, while temperature optimization may be critical in determining organism survival, more immediate fitness consequences may force animals to use behavioural means to override physiological function under certain circumstances (Huey et al., 2003). However, such a mechanism has not yet been demonstrated in insects (Forsman et al., 2002).
The proximate mechanisms for sensing environmental temperature are specialized sensillae, which may be found on various body parts in terrestrial arthropods (see Must et al., 2006), and in particular on the antennae in insects. Central temperature receptors, which measure body temperature, for example, in the prothoracic ganglion of the cockroach, also exist (Murphy and Heath, 1983; Janiszewski, 1986).
Much evidence for temperature sensing has been provided by electrophysiological studies, used effectively to show that temperature causes depolarization of specific antennal cells (reviewed in Altner and Loftus, 1985; Merivee et al., 2003; Must et al., 2006), which, in the case of Drosophila, are usually located in the third antennal segment (e.g. Shanbhag et al., 1995; Sayeed and Benzer, 1996). These peripheral temperature receptors increase their firing rate as the temperature is lowered (in the case of cold receptors; e.g. Loftus, 1968; Nishikawa et al., 1992; Merivee et al., 2003) or raised (in heat receptors in vertebrates, see Patapoutian et al., 2003), but display little electrophysiological activity in response to baseline temperature within the normal range (Nishikawa et al., 1992). Consequently, thermosensory neurons respond in a phasic-tonic manner to rapid temperature changes, and several cold cell responses can occur (including changes in peak frequency and action potential (firing) rate). However, the specific characteristics of both the phasic and tonic changes can vary substantially among cells within sensilla, individuals, and species (Merivee et al., 2003; Must et al., 2006).
In temperature sensitive neurons, the receptor cells probably do not function as a simple thermometer. Rather, neuronal firing rates are also influenced by the direction and rate of temperature change (e.g. Nishikawa et al., 1992; and reviewed in Patapoutian et al., 2003; Must et al., 2006). Thus, the relationship between temperature sensing at the neuron and temperature perception in the central nervous system (CNS) is complex because different cells respond in different ways to variation in temperature (Nishikawa et al., 1992; and see Merivee et al., 2003). Additional complexity arises because different cooling rates elicit different neuronal firing rates among the various receptor types (Nishikawa et al., 1992). The magnitude of temperature change in, for example, step-wise changes can also influence the steady-state firing rates (Nishikawa et al., 1992; Ehn and Tichy, 1994), and considerable temporal changes in firing rates, often with rapid phasic (i.e. transient) changes occurring during the first few seconds of a response, have also been found (e.g. Ehn and Tichy, 1994; Must et al., 2006). A variety of temperature response types for neuronal firing rates can be distinguished in terrestrial arthropods, and are discussed in detail by Must et al. (2006).
In the case of steady-state changes in temperature, for most insects investigated to date, cold cells provide information on warmer temperatures via a reduction in neuronal firing rates (Must et al., 2006; see also Nishikawa et al., 1992; Merivee et al., 2003). During rapid temperature changes, warming results in a long inter-spike period followed by a similar reduction in nerve impulse activity (Merivee et al., 2003). Currently, however, there is relatively little evidence in insects for heat receptors which respond with an increase in firing rates to warmer, stable temperatures (but see Must et al., 2006). During cooling to a new cold temperature, the phasic component indicates temperature decrease at the start of a temperature change, while the tonic component decreases more slowly (depending on the magnitude of the temperature change) and stabilizes at the new level of constant temperature. Thus, a considerable amount of information is transmitted to the CNS regarding the insect’s immediate thermal environment, although how this information is processed and integrated remains unclear at present (Merivee et al., 2003).
Body temperature preference typically represents a value well within the range of temperatures experienced in an organism’s natural environment, and variation in precision, and thus possibly sensitivity, of temperature regulation on a thermal gradient is not uncommon (Yamamoto, 1994; Sayeed and Benzer, 1996; see also Murphy, 1986). Consequently, different levels of temperature sensitivity (i.e. variation in neuronal responses to temperature stimuli) among insect species are unsurprising (discussed in Nishikawa et al., 1992), and some evidence exists for inter-specific variation in the ability to discriminate temperature changes (i.e. resolution). For example, sensory neurons in Drosophila larvae can detect temperature variation at a resolution of <1 °C (Nishikawa et al., 1992; Liu et al., 2003), while Speophyes lucidulus is conservatively estimated to be capable of resolving temperature changes of 0.7 °C (Altner and Loftus, 1985; see also Hess and Loftus, 1984). The ground beetle, Pterostichus aethiops, shows changes in firing rates of campaniform sensilla induced by temperature changes of as little as 0.1 °C (Merivee et al., 2003). By contrast, a single warm cell from the spider Cupiennius salei can resolve differences in warming to 0.4 °C (Ehn and Tichy, 1994), while a tropical tick’s warm cell can resolve temperature to 0.6 °C (Hess and Loftus, 1984).
In insects, the cellular mechanisms of temperature sensation have received less attention than, for example, in vertebrates (Clapham, 2003; Liu et al., 2003; Patapouitian et al., 2003). Nonetheless, despite the underlying differences in thermal biology of ectothermic invertebrates and endothermic vertebrates, it appears that their thermal sensory mechanisms may be conserved at the molecular level (Liu et al., 2003; Patapouitian et al., 2003). Accumulated evidence suggests that the primary temperature sensors in the sensory nerve endings of mammals belong to the temperature receptor protein superfamily of cation channels and that these proteins underlie the cellular processes that result in nerve depolarization (Voets et al., 2004). At the cellular level, only recently has it been shown that the invertebrate temperature-activated transient receptor potential ion channel (thermoTRP) families found in Drosophila and the nematode worm, Caenorhabditis elegans, can be directly activated by temperature (Viswanath et al., 2003) although there is some variation among the vertebrate and invertebrate TRP systems. Specifically, Viswanath et al. (2003) showed that the Drosophila orthologue of the mammalian cold-activated ion channel ANKTM1 responds to warming rather than cooling. Therefore, while the thermosensing function may be well conserved from an evolutionary perspective (i.e. the proteins themselves are present in both vertebrate and invertebrate organisms; see also Rosenzweig et al., 2005) a large degree of flexibility in the TRP responses to temperature can be found (Viswanath et al., 2003).
Typically, TRPs are identified by their homology rather than by ligand function, and can serve multiple purposes, many of which are not necessarily related to temperature sensation (Clapham, 2003). Several different mechanisms have been proposed for how the TRPs act as ion gates (reviewed in Clapham, 2003 and Voets et al., 2004 for thermoTRPs). Temperature variation could result in production and binding of ligands that activate channels. By contrast, the channel proteins could undergo some form of temperature-dependent structural changes, thereby resulting in channel opening. Finally, changes in membrane tension, facilitated by lipid bilayer re-arrangements, may cause temperature-dependent activation of thermoTRPs (Clapham, 2003). In mammalian cells, Voets et al. (2004) found that temperature sensitivity is regulated by the trans-membrane voltage and ambient temperature variation results in graded shifts in the voltage dependence of channel activation.
Marked variation in the expression and temperature sensitivity of thermoTRPs exists, hence they are grouped into distinct types of sensory neurons according to function. For insects, two key families, with several forms of thermoTRPs in each family, are described: the melastatin family (containing e.g. TRPM8 and ANKTM1), and the vanilloid family (containing TRPV1-4 and TRPA1). In mammals, at least six families are recognized (Clapham, 2003). It is not clear from the available literature if these other thermoTRP families are important, or whether they are present at all, in insects. It has been suggested that TRPA1 may play an important role in thermotaxis in Drosophila (Rosenzweig et al., 2005). TRPA1 knockout (using RNA interference) eliminates the avoidance of high temperatures in a thermal gradient, and the expression of this family of ion channel protein occurs in cells not previously thought to have a function in thermosensation (Rosenzweig et al., 2005). (It is worth noting that similar results occur in peripheral temperature receptor ablation experiments in cockroaches, i.e. high temperatures are no longer avoided; Murphy (1986)). For example, Rosenzweig et al., (2005) found some evidence for TRP1A expression in two pairs of cells adjacent to the mouthhooks and in the developing gut. Generally, however, receptors in the melastatin family respond to temperatures in the 17–25 °C range, while the vanilloid receptors are sensitive across the 33–52 °C range. Currently, there is little information available documenting how, if at all, thermoTRP family composition in sensilla may vary among insect taxa or within species (e.g. along geographic clines).
Five recent findings in temperature sensing strike us as being important from an evolutionary and ecological physiology perspective. First, the perception of temperature can interact with mechanical, electrical (Godde and Haug, 1990), and hygric stimuli (Nishikawa et al., 1992; Inoshita and Tanimura, 2006) to alter the neuronal signal (firing rate) (and see Voets et al., 2004). Second, temperature (both heat and cold) activation of thermoTRPs can occur in cell-free areas, thereby suggesting that temperature-dependent binding of second messengers is not an important process in the activation of TRPs (Voets et al., 2004). This is important because it markedly distinguishes thermoTRPs from classical ion-channels. Third, temperature sensitivity is at least partially dependent on the trans-membrane voltage and not solely on temperature, and therefore this voltage can contribute to the fine-tuning of cold and heat sensitivity in sensory cells (Voets et al., 2004). Fourth, in insects, temperature sensation can occur in cells located outside of the antennae (see e.g. Sayeed and Benzer, 1996; Liu et al., 2003), and more specifically, in cells not previously thought to have thermosensory functions (e.g. Rosenzweig et al., 2005). Furthermore, it has also been suggested that thermoTRPs can sense intra- and extracellular temperature variation (Clapham, 2003). Finally, it has been demonstrated that circadian clock proteins (FRQ) in the yeast, Neurospora crassa, could be regulated by thermosensitive gene splicing (at the gene-translation level) and may play a crucial role in temperature sensing (Diernfellner et al., 2005), although the importance of such a mechanism requires confirmation in insects.
5.2 Detecting Changes in Water Availability
For reasons similar to those outlined in the previous section, it is apparent that insects can detect changes in external moisture conditions. These include the presence of hygrosensors located on the antennal arista in flies (e.g. Rees, 1970; Sayeed and Benzer, 1996) and antennae of cockroaches (Yokohari, 1978; Tichy, 2003), the demonstration of hygropreference in a humidity gradient (e.g. Hayward et al., 2000, 2001; Steidle and Reinhard, 2003; Walters and Mackay, 2003), and electrophysiological studies showing changes in nerve impulse frequency with altered ambient humidity (e.g. Yokohari, 1978; Tichy, 2003). However, the mechanisms of hygrosensing in insects are less clearly elucidated than those of thermosensing, probably because of the perceived intractability of the approaches required for its investigation (though see Tichy, 2003), despite its importance (Edney, 1977; Hadley, 1994; Tauber et al., 1998; Chown and Nicolson, 2004). Regardless of the reasons, it is clear that knowledge of thermosensory mechanisms in insects, especially at the cellular level, is more advanced than that of hygrosensory mechanisms. Consequently, in this section we also draw on information from non-insect arthropod taxa (e.g. Collembola).
That terrestrial arthropods should have particular hygropreferences seems a reasonable proposition, although this has not been demonstrated frequently in insects. Where hygropreference is demonstrated, this is typically done for pests of stored products (e.g. Jian et al., 2005) and their potential control agents (e.g. Steidle and Reinhard, 2003), or for vectors of diseases (e.g. Lorenzo and Lazzari, 1999). Non-random hygropreferences have often been demonstrated by means of only two humidity options (either high or low) (e.g. Prange and Hamilton, 1992; Jian et al., 2005) and more seldom using a range of humidities, as is usually the case in temperature gradient experiments (but see e.g. Roces and Kleineidam, 2000; Hayward et al., 2001; Walters and Mackay, 2003). Regardless, ecological evidence suggesting variation in species’ ambient moisture preferences is seldom linked with hygropreference in a gradient (but see Hayward et al., 2004b). Specifically for insects, clear humidity preferences have been demonstrated in ants (North, 1991; Walters and Mackay, 2003; but see Roces and Kleineidam, 2000), bugs (Roca and Lazzari, 1994; Lorenzo and Lazzari, 1999; Guarneri et al., 2003), beetles (Weston and Hoffman, 1991; Weissling and Giblindav, 1993), and wasps (Steidle and Reinhard, 2003).
In some terrestrial arthropods, no clear hygropreference has been found. For example, the ant, Atta sexdens rubropilosa (Roces and Kleineidam, 2000), and the mite, Lauroppia translamellata (Hayward et al., 2000), do not show distinct humidity preferences. However, hygropreference may be influenced by the physiological state (e.g. desiccation) and ambient temperature (see e.g. Jones, 1950; Hayward et al., 2001), or even photoperiod (e.g. North, 1991) experienced by individuals. Therefore, demonstrations of a lack of hygropreference need to consider hydration state before concluding a lack of behavioural hygroregulation exists in a species. For example, in mites (Jones, 1950) and ticks (Lees, 1948), prior desiccation resulted in higher preferred humidity levels. Evidence also exists for a preference in Cryptopygus antarcticus for higher humidity levels at elevated temperatures (Hayward et al., 2001) (Fig. 13), possibly reflecting a similar physiological process to the former example. An alternative argument, however, may be that species with higher desiccation resistance do not require careful hygro-preference (discussed in Hayward et al., 2000, 2004b). When faced with a high and low humidity option, at higher temperatures (>40 °C) grasshoppers prefer low humidity, possibly to facilitate evaporative cooling (Prange and Hamilton, 1992). In these examples, understanding the cellular-level mechanism of hygrosensing and comparing these responses with desiccation, resistance/tolerance could shed light on the underlying mechanisms.
FIG. 13.
Mean frequencies (± SE) indicating the distribution of Cryptopygus antarcticus within a linear humidity gradient at 5, 10, and 20 °C. These data show that at higher temperatures C. antarcticus prefers higher relative humidity. Figure redrawn from Hayward et al. (2001) with permission from Elsevier.
Electrophysiological studies have confirmed the presence of hygroreceptors on, among others, the antennae of caterpillars, bees, mosquitoes, locusts, bugs, flies, stick insects, and cockroaches (see Altner and Prillinger, 1980; Tichy and Loftus, 1996; Tichy, 2003). For example, Periplaneta americana has hygroreceptors that increase neuron impulse frequency in response to higher humidity (moist receptors) and dry receptors that increase impulse frequency in response to lowered humidity (see e.g. Waldow, 1970; Yokahari, 1978; Tichy, 2003). Both the moist and dry receptors can be present in the same sensillum, and it has been suggested that the integration of their signals in the CNS may be important for functional responses to altered ambient moisture (reviewed in Altner and Prillinger, 1980). Only recently, however, has it been possible to demonstrate that impulse frequencies of moist and dry receptors are also sensitive to the rate of change in relative humidity (Tichy, 2003). Typically, previous electrophysiological experiments focused on step-wise changes rather than graded responses. High neuron impulse frequencies of the moist cells signal high humidity and vice versa. However, at a given humidity level, the response frequency is even higher when the humidity continues rising. The hygroreceptors are most sensitive to low rather than high rates of humidity change. These results therefore suggest continuous input about the state of the moisture in the ambient air, and may provide an early warning of changing humidity conditions (Tichy, 2003). Some evidence also exists for inter-specific variation in hygrosensing abilities. The spider, Cupiennius salei, is capable of discriminating between relative humidities differing by 10% (Ehn and Tichy, 1994), while perception of humidity change in Periplaneta is approximately double the resolution, with responses to humidity in the order of 5% at the CNS (Nishino et al., 2003).
Within the context of humidity transduction, the structure and function of insect hygroreceptors have been thoroughly reviewed by Tichy and Loftus (1996). Several models have been proposed to explain the humidity transduction process, but three of these seem to be the most likely (Tichy and Loftus, 1996). First, evaporation rate results in changes of chemical concentration, osmotic pressure, or mechanical stress in the receptor cells (the so-called electrochemical hygrometer model). Second, evaporation causes a temperature differential detected by heat cells and thus the system functions like a psychrometer. Third, changes in cell volume as a result of water uptake or loss are detected, and this constitutes a mechanical model. The latter mechanical hygrometer theory is perhaps the most favoured model, although several aspects of this model remain poorly elucidated (Yokahari, 1978; Tichy and Loftus, 1996), and much of the work is based on only a handful of model organisms (e.g. Periplaneta).
Considerable structural variation has been found between insect hygroreceptors in the species that have been investigated (see e.g. Shields, 1994; Tichy and Loftus, 1996; Bland et al., 1998; Hunger and Steinbrecht, 1998), and some evidence for functional variation also exists (Tichy and Loftus, 1996; Wolfrum, 1997). Consequently, it has been suggested that several possible models may apply to terrestrial arthropod moisture transduction rather than one ubiquitous system (Tichy and Loftus, 1996; see also Ziegler and Altner, 1995). As Tichy and Loftus (1996) have noted, ‘there is still much to be learned’ regarding mechanisms of insect hygroreceptor transduction, particularly within an evolutionary and ecological framework.
6 Responses to the thermal environment
The thermal environment holds considerable significance for most, if not all levels, of the biological and genealogical hierarchies (Cossins and Bowler, 1987; Gillooly et al., 2001, 2005; Allen et al., 2002; Hochachka and Somero, 2002; Clarke, 2003, 2006; Evans et al., 2005). The form of temperature’s effect at various organizational levels, and the behavioural, physiological, and morphological ways in which organisms modify the potential effects of temperature are therefore central to much of physiology and ecology, and continue to engender debate (see e.g. Gillooly et al., 2001; Clarke, 2004; Clarke and Fraser, 2004; Gillooly et al., 2006; Clarke, 2006). The effects of temperature on an individual insect can be represented in two ways: if resistance responses are under consideration then a thermobiological scale (e.g. Vannier, 1994) is convenient (Fig. 14), while if capacity responses are being considered then a performance curve (Fig. 15) might be more useful, although the distinction between capacity and resistance responses is artificial (Angilletta et al., 2002; Chown and Nicolson, 2004). That a mismatch between oxygen supply and demand might be responsible for setting thermal limits in many non-insect species (Pörtner, 2001) nicely makes this point.
FIG. 14.
The thermobiological scale proposed by Vannier (1994). Redrawn with permission from Elsevier.
FIG. 15.
An idealized thermal performance curve showing the optimum(To), performance breadth(B80), and critical limits. Redrawn from Angilletta et al. (2002, p. 250) with permission from Elsevier.
The literature on the effects of temperature on insects and the responses they mount to counter these effects, or to modify the relationship between the temperatures they experience and their survival probability, is substantial, and has been reviewed recently in several guises (Bale, 2002; Vernon and Vannier, 2002; Sinclair et al., 2003b; Hoffmann et al., 2003b; Chown and Nicolson, 2004; Korsloot et al., 2004). Nonetheless, owing both to the rapid development of molecular tools, and the pressing need to comprehend the likely biological impacts of global change, advances in the field are rapid. Hence, in this section we will provide an appropriate, though not comprehensive, background to the injurious effects of high and low temperatures and individual responses to them at a variety of scales, but will focus more on recent advances in the field.
6.1 Low-Temperature Injury
6.1.1 Freezing injury
One of the most significant physical thresholds for organisms is the transition of water between the liquid and solid phases. Most insects cannot survive freezing, although they typically freeze a few degrees below 0 °C owing to the colligative effects of their body fluids (Zachariassen, 1985). When the temperature of an organism declines below the melting point of its bodily fluids there is a risk of ice formation. Crystallization may take place either by aggregation of water molecules into an ice nucleus (homogeneous nucleation) or via their aggregation around some substance or irregularity (heterogeneous nucleation). When freezing takes place, additional water is added to the nucleus or nuclei, and effectively the animal begins to desiccate. The removal of water from the solution causes an increase in solute concentration. Progressive concentration of the body fluids may lead to changes in pH, protein denaturation, and alterations of membrane properties, thus affecting electrochemical gradients and transport properties. In addition, cellular shrinkage may occur owing to removal of water from the cells and this may damage the cell membrane to such an extent that it cannot recover following thawing (the critical minimum cell volume hypothesis) (Zachariassen, 1985; Denlinger and Lee, 1998; Ramløv, 2000; Kristiansen and Zachariassen, 2001). The critical minimum cell volume that can be endured likely also sets the lower lethal temperature in insects that are tolerant of freezing (Storey and Storey, 1996). Insects that are able to limit or avoid these injuries, and therefore survive freezing, are also challenged by anoxia because diffusion through ice takes place slowly. Typically, tracheoles are fluid filled when oxygen demand is low, as it is at low temperatures preceding freezing (Irwin and Lee, 2002; Sinclair et al., 2004), and the movements that are typical of air sacs and tracheae (Herford, 1938; Westneat et al., 2003; Chown and Nicolson, 2004) will also be limited. In consequence, the frozen state is an ischemic one (Morin et al., 2005).
Recent work has taken further early investigations of those tissues most sensitive to freezing injury (e.g. Lee et al., 1993). Differential sensitivity of tissues to freezing appears to be species specific, although the numbers of species investigated is low. In the Antarctic midge, Belgica antarctica, the fat body has the lowest cell viability following freezing, followed by the gut, Malpighian tubules, and salivary glands (Lee et al., 2006). Similarly, in Eurosta solidaginis, the Malpighian tubules and fat body are more sensitive to freezing than the gut, although in this case the integumentary muscles, haemocytes, and tracheae are most sensitive to low temperatures (Yi and Lee, 2003). Earlier ultrastructural work suggested that the nervous system is especially sensitive to freezing (Collins et al., 1997). In the alpine cockroach, Celattoblatta quinquemaculata, the gut is most sensitive to freezing, while the Malpighian tubules and fat body are most susceptible to temperatures below the freezing point (Worland et al., 2004). In Chilo suppressalis, the gut is most sensitive in overwintering larvae, whereas in non-diapausing individuals it is the fat body (Izumi et al., 2005).
6.1.2 Chilling injury
Cold shock, or direct chilling injury is a form of injury that results from rapid cooling in the absence of extracellular ice formation. Direct chilling injury is usually distinguished from the consequences of a long-term exposure to low temperatures, which is known as indirect chilling injury. In both cases, the absence of ice formation distinguishes these kinds of injury from those associated with freezing of an insect’s body fluids. Several investigations of insect responses to short- and long-term low-temperature exposure suggest that these two forms of injury might be different (Chen and Walker, 1994; McDonald et al., 1997, 2000). However, comparative assessments of the injuries induced by direct and indirect chilling injury are rare, and much speculation surrounds their relationship and the likelihood that responses to one may alleviate the effects of the other (Sinclair and Roberts, 2005).
Chill coma coincides with the temperature at which the excitability of nerves and muscles is lost (Goller and Esch, 1990; Xu and Robertson, 1994), associated with declining resting potentials. The inability of Na+/K+-ATPases to function at low temperatures is thought to be a major cause of chill coma (Hosler et al., 2000). Although chill coma is reversible, it appears that chilling injury represents ongoing damage to membranes and a marked impact on neuromuscular transmission (Yocum et al., 1994; Kelty et al., 1996), with downstream effects on reproduction (Denlinger and Lee, 1998; Rinehart et al., 2000a; Shreve et al., 2004). Direct chilling injury induces fluid-to-gel phase transitions in membranes, which result in separation of membrane proteins and lipids, change membrane permeability, and cause a decline in the activity of membrane-bound enzymes. Direct chilling injury is also a consequence of protein structural changes and denaturation, a decrease in enzyme activity, (Ramløv, 2000; Yocum, 2001), and a possible increase in oxidative stress (Rojas and Leopold, 1996). Fat body and Malpighian tubule cells are particularly prone to direct chilling injury (Worland et al., 2004).
Although the mechanisms underlying indirect chilling injury are less well understood than those associated with direct chilling, one recent study has suggested that equilibration of transmembrane ion gradients are important (Koštál et al., 2004). In the species investigated, the bug Pyrrochoris apterus, absence of energy is not the cause of Na+/K+-ATPase failure. Rather, the ability of the enzyme to exploit available energy is impaired, suggesting that damage to membrane function at least partially explains indirect chilling injury. Clearly, additional work is required to unveil the mechanisms of indirect chilling injury and their relationship to those responsible for direct injury. However, impacts on membrane pumps, especially the Na+/K+-ATPase, appear to be common to both. Both direct and indirect chilling injury are likely also a consequence of depletion of substrates (Hoffmann et al., 2003b; Renault et al., 2004), which may be one reason why a biphasic response to low temperatures is found (Karan and David, 2000). Whatever the cause of the injury, it translates to downstream effects on fitness (e.g. Chakir et al., 2005).
Repeated, short-term sublethal low-temperature exposures are a substantial source of injury. In plant-feeding caterpillars of Pringleophaga marioni, repeated cooling results in a substantial decline in growth rate, which is a consequence of damage to the gut (Sinclair and Chown, 2005b). How this damage is incurred is not clear, but changes to metabolic rates during cooling and longer-term alterations in lipid content suggest one way in which this could happen. At the critical thermal minimum, or onset of chill coma (−2 °C), metabolic rate plummets (Q10 of 2 × 103) (Sinclair et al., 2004), which Makarieva et al. (2006) interpret as the point at which metabolic control is abandoned (see Section 6.2.2). In caterpillars consuming plant material, oxidation of phenolics can generate reactive oxygen species, which in turn cause membrane lipid peroxidation (Krishnan and Kodrík, 2006). Polyunsaturated fatty acids are especially susceptible to free radical damage (Storey, 1996), and are known to increase in abundance in membranes in response to low temperature (Logue et al., 2000; Hulbert, 2003; Overgaard et al., 2005). If abandoned metabolic control results in cessation of production of anti-oxidants (see Krishnan and Kodrík, 2006), then gut damage may be a consequence of oxidative damage. If this is the likely route of damage to the gut, then it might be expected that in plant chewing, but perhaps not other species, the gut would be most sensitive to low temperature. Currently, insufficient information exists to test this idea.
Failure in organismal performance at low temperatures may also arise at higher levels of organization. The most comprehensive formulation of this idea is the oxygen limitation hypothesis (Pörtner et al., 1998, 2000; Pörtner, 2001, 2002a). In essence, it is thought that in complex organisms (with distinct oxygen acquisition and circulation systems), critical temperatures that affect fitness are not set by cellular level damage, but rather by a transition to anaerobic metabolism. At low temperature these deleterious temperatures (called pejus by Pörtner, 2001) result from insufficient aerobic capacity of mitochondria, and a concomitant decline in ventilation and circulation, which leads to a mismatch between oxygen supply and demand, a drop in aerobic scope, transition to anaerobiosis, and cessation of higher physiological function. At high temperature, insufficient oxygen uptake and distribution by ventilation and circulation to meet mitochondrial demands results in a similar mismatch between supply and demand, and eventual physiological collapse. Pörtner (2001, 2002a) argued that thermal limits in the majority of animals are set by oxygen limitation.
Whether thermal tolerances are set by oxygen limitation has not been widely explored in insects, although several recent studies suggest that cellular level processes are more important. Although temperature–PO2 interactions are found in the eggs of Manduca sexta, diffusive supply of O2, rather than ventilation and circulation, is limiting at high temperatures (Woods and Hill, 2004). Declining egg metabolic rates at high temperature are not set by low or falling O2, but either by direct effects of temperature on protein stability or some other, unknown factor. Because the eggs of M. sexta are representative of those of many insect species (Woods and Hill, 2004), these findings suggest that oxygen limitation of thermal tolerance is unimportant for insect eggs. In adults of the tenebrionid beetle, Gonocephalum simplex, oxygen limitation of upper thermal tolerance does not appear to be significant either (Klok et al., 2004), because changes in ambient PO2 have no effect on thermal tolerance. If oxygen limitation of thermal tolerance is important, thermal tolerance limits should increase at high PO2 and decline at low PO2 (Pörtner, 2001, 2002b).
At low temperatures, it appears that dysfunctional ion pumps are not a consequence of unavailability of ATP, or ventilatory/circulatory problems, but rather as a consequence of the inability of the pumps to utilize ATP (Koštál et al., 2004). However, the available data are somewhat equivocal because in the freezing tolerant caterpillars of Pringleophaga marioni, individuals that are exposed to a temperature lower than their lethal limit and then thawed have metabolic rates identical to those that have not been killed by freezing, but higher water-loss rates, suggesting that what is lost is central control of processes, rather than cellular-level capabilities (Sinclair et al., 2004). In the context of the oxygen limitation of thermal tolerance hypothesis, the data suggest that upper limits are probably set by cellular level damage and insect responses to this damage, while lower limits are set by some combination of cellular and whole-organismal level responses. Decoupling of upper and lower lethal temperatures at a wide variety of levels (Chown, 2001; Chown and Nicolson, 2004) support this conclusion. Nonetheless, these data mostly come from insects at rest. What the situation is in active insects is far from clear, although pronounced effects of hypoxia and hyperoxia on functioning and growth are known from a variety of species (Joos et al., 1997; Harrison and Lighton, 1998; Frazier et al., 2001).
6.2 Responses to Low Temperature
6.2.1 Responses to short-term chilling
Debate has recently arisen concerning the terminology for the length of exposure of animals to cold (and heat), and the responses they show in consequence (Bowler, 2005; Loeschcke and Sørenson, 2005; Sinclair and Roberts, 2005, see also Spicer and Gaston, 1999). In part, this debate has arisen because both treatments and responses tend to be labelled in the same fashion. Short-term exposures (of minutes to hours) to sublethal conditions are typically termed hardening (Hoffmann et al., 2003b) as are the responses shown to these conditions (Bowler, 2005), while cold shock is the stress imposed by these conditions (Denlinger et al., 1991). Long-term exposures (days to weeks) to temperatures within the normal viable range of the organism, and the responses shown by the animals, are normally termed acclimation. In the field, animals also respond to low temperatures with substantial long-term alterations to their physiology (Zachariassen, 1985; Storey, 1990; Bale, 2002), which has been termed a ‘programmed response to cold’ (Chown and Nicolson, 2004) to distinguish it from shorter-term laboratory treatments. Exposure of immature stages to a given temperature regime may also alter the physiology of later stages, which has been termed developmental plasticity (Piersma and Drent, 2003). All of these changes constitute phenotypic plasticity (Section 4), and the debate has centred largely on whether mechanistic responses (and presumably sources of injury) are similar across the range of responses. The essence of the question is the nature of the time by intensity effect of low-temperature stress. That is, whether the injuries caused by the stress, and the subsequent responses, can be readily divided because of the existence of threshold effects, or whether the time by intensity response space is continuous. Present data do not allow this question to be fully addressed, especially because the full suite of responses has rarely been examined for a given population. However, progress is being made in this area.
In Drosophila melanogaster, a comparison has been made of the effects of rearing temperature (developmental effects), a two-day exposure (acclimation), and hardening (a few hours) on fly mortality, chill coma recovery, and recovery during exposure to stress (Rako and Hoffmann, 2006). The responses shown by the flies are complex. Hardening improves survival following a cold shock, but has no effect on chill coma recovery times. Flies reared at 19 °C have lower mortality levels than those reared at 25 °C, and acclimation at 12 °C further reduces mortality in the 19 °C group, but has little effect on the 25 °C group. Flies reared at 19 °C also have longer chill coma recovery times than those reared at 25 °C, and acclimation has a larger effect on the latter than on the former group of flies. When subject to 30 generations of selection, every alternative generation, for decreased chill coma recovery time, this measure of resistance declines, as does mortality following cold shock. However, hardening capability is little effected (though not in males) (Anderson et al., 2005). These studies suggest that the mechanisms underlying longer-term responses of chill coma recovery and survival of low temperature are similar, in keeping with conclusions of earlier work (review in Chown and Nicolson, 2004). However, the mechanisms underlying shorter-term responses probably differ, given that hardening affects mortality but not chill coma recovery, and that the protein synthesis inhibitor, cycloheximide, affects cold shock tolerance, but not tolerance if it is preceded by hardening (Misener et al., 2001, see also Hoffmann et al., 2003b).
Differences in the intensity of stress also affect chill coma recovery. In Drosophila subobscura, chill coma recovery time increases with declining temperature in a non-linear fashion. Initially it increases with declining temperature, then remains unchanged, and subsequently increases again (David et al., 2003). This pattern is also evident in D. melanogaster (Macdonald et al., 2004; Rako and Hoffmann, 2006). Such a biphasic response suggests that two different mechanisms are responsible for responses to low temperature stress. These effects could be realized in different ways to result in the response plateau. Two exponential processes could be involved, with a relatively rapid transition from one to the other, or alternatively, one of the processes could be exponential and the other could show a declining sigmoid shape (David et al., 2003). The latter is possible only if some process is increasingly damaged at lower temperature up to some maximum level.
What mechanisms underlie acclimation responses to low temperature and the rapid cold hardening response have yet to be fully resolved. Those underlying the longer-term seasonal responses associated with cold hardiness are well understood and the time course and biochemistry of these have been reviewed many times, providing a convenient entry to this large literature (Zachariassen, 1985; Block, 1990; Storey, 1990, 1997; Storey and Storey, 1996, 2004; Denlinger and Lee, 1998; Sømme, 1999; Duman, 2001; Bale, 2002; Chown and Nicolson, 2004). The molecular underpinnings of such mechanisms are now being explored more fully (Morin et al., 2005), and the subtleties of responses, including interactions with diapause and their hormonal regulation are being uncovered (Chen et al., 2005a; Hayward et al., 2005; Tachibana et al., 2005). At a biochemical level, mechanisms include the production of low molecular weight cryoprotectants such as polyhydric alcohols (e.g. glycerol, sorbitol), sugars (trehalose), and amino acids such as proline, the production of antifreeze proteins, and either the removal and masking of ice nucelators (in freeze-intolerant species) or the production of protein or lipoprotein ice nucleators in freezing-tolerant species (Chown and Nicolson, 2004).
The mechanisms underlying rapid cold hardening are now beginning to be explored. Initially, it was thought that glycerol plays some role in the response. At least in pharate adults of S. crassipalpis, rapid cold hardening is associated with a threefold increase in glycerol levels to 81.4 mM. Although this change is insufficient to have a colligative effect on cold hardiness, glycerol is thought to play a role in protecting membranes against low-temperature damage associated with phase transitions, and in stabilizing proteins (Lee et al., 1987a; Kostal et al., 2001), although this role has yet to be confirmed. In S. bullata, glycerol is produced in response to cold shock and to short-term desiccation and anoxia, but only following a return to higher temperature. In all cases, the glycerol production is not as extensive as in seasonal responses, but it does improve survival (Yoder et al., 2006). Moreover, exogenous treatment with glycerol also confers cold hardiness, and ligation of larvae indicates that glycerol production is under central control. These results, and those of Yi and Lee (2004), support the idea that the initial response to cold shock is under local cellular control, and is subsequently complemented by input from the CNS (Yoder et al., 2006). They also provide further evidence that short-term responses to low temperature are biphasic. Thus, one, possibly local cellular response generates almost immediate protection, while the second remains active for a longer period.
In other species, such as D. melanogaster and the moth, Lymantria dispar, glycerol is not produced in response to cold shock (Yocum et al., 1991; Kelty and Lee, 1999). In L. dispar and in Sarcophaga crassipalpis, and the beetle Leptinotarsa decemlineata, cold shock results in upregulation of heat-shock protein synthesis (Denlinger and Lee, 1998; Yocum et al., 1998; Yocum, 2001). Nonetheless, this typically only takes place once the animals have been returned to a higher temperature (Joplin et al., 1990; Rinehart et al., 2000b; Yocum, 2001). Moreover, in D. melanogaster, Hsps are not synthesized in response to brief low-temperature treatments, but rather only following more extended exposures (Kelty and Lee, 2001; Sejerkilde et al., 2003; Overgaard et al., 2005; Nielsen et al., 2005). In this species, rapid cold hardening is accompanied by changes in the composition of membrane phospholipids fatty acids and an increase in the extent of membrane unsaturation (Overgaard et al., 2005). Taken together, these results point to the fact that prevention of damage to membranes, and possibly proteins and the cytoskeleton (see Michaud and Denlinger, 2005) is likely a major component of the rapid cold hardening response, though different routes to such protection are likely, and the intricacies of such mechanisms are far from resolved.
Unsaturation of membranes is a well-documented response to low temperature. It prevents membrane fluid-to-gel transitions (Logue et al., 2000; Hochachka and Somero, 2002, Hulbert, 2003), and the role of polyols in stabilizing membranes and proteins is also well established. Recent work has demonstrated that small heat shock proteins protect membranes by improving fluidity of high-temperature melting lipids (Tsvetkova et al., 2002), and they may have this role in several species following rapid cold hardening. For example, in Sarcophaga crassipalpis, RNA interference of hsp23 causes a significant and substantial reduction of survival of cold shock following hardening. By contrast RNAi of hsp70 has little effect on survival following hardening, leaving the upregulation of Hsp70 during rapid cold hardening in the species unexplained (Michaud and Denlinger, 2005; Chown and Storey, 2006). Whatever the mechanisms underlying rapid cold hardening finally turn out to be, it is clear that the protective effects of the response to rapid cold hardening extend not only to survival, but also to several other components of fitness (Rinehart et al., 2000a).
The mechanisms that underlie improvement of survival following longer-term exposures to low temperature, such as those used during investigations of acclimation (several days) have not been investigated to the same extent. Past overviews have tended not to draw a distinction between investigations of the rapid hardening and acclimation responses (e.g. Chown and Nicolson, 2004). Low-temperature treatments of several days result in expression of Hsps in Drosophila melanogaster, Lymantria dispar, and in several species of Drosophila (Burton et al., 1988; Denlinger et al., 1992; Goto and Kimura, 1998; Goto et al., 1998). This expression typically takes place only following a return to high temperatures. Recent work on Drosophila has suggested that increases in Hsp70 following long-term cold acclimation may have to do with repair of damage induced both by low temperature and by re-heating (Goto and Kimura, 1998; Sejerkilde et al., 2003; Nielsen et al., 2005; Overgaard et al., 2005). Several studies have demonstrated that alternating temperatures (i.e. cessation of chilling and return to higher temperatures) improve the survival of chilling (Chen and Denlinger, 1992; Coulson and Bale, 1996; Hanc and Nedved, 1999; Renault et al., 2004), and this may be a consequence of the synthesis of Hsps and possibly also polyhydric alcohols at the higher temperatures. Additional responses to longer-term cold exposure include elevation of energy reserves (Chen and Walker, 1994; Misener et al., 2001). However, the role of polyhydric alcohols has not been well explored.
6.2.2 Programmed responses to cold
Seasonal changes in physiology in anticipation of declining environmental temperatures, and the variety of strategies which insects employ to overcome low temperatures in temperate and polar regions, have received much attention (Bale, 1987, 1993, 2002; Sinclair, 1999; Vernon and Vannier, 2002; Sinclair et al., 2003b; review in Chown and Nicolson, 2004). What is much less clear is what circumstances might promote each of the various strategies, or what their fitness costs and benefits are (Block, 1991; Voituron et al., 2002). It is widely accepted that the basal response shown by insects to freezing is freeze intolerance (Vernon and Vannier, 2002). Therefore, freeze tolerance is a derived strategy, though it probably originated several times (Sinclair et al., 2003a). Early work (see Zachariassen, 1985) pointed to the importance of freezing tolerance in areas with extremely low temperatures, especially given that supercooling in freeze-intolerant species is a metastable state. This work also suggested that freezing tolerance promotes cold hardiness in insects that retain ice-nucleating agents in their haemo-lymph and gut, such as those exposed to regular freezing events. These early ideas were further developed to show that regular freeze-thaw events associated with environmental unpredictability are likely the major environmental factor selecting for moderate freeze tolerance (Sinclair et al., 2003a; Sinclair and Chown, 2005a). Thus, the proposed advantages to freezing tolerance over freeze intolerance can be summarized as follows:
Nucleation Hypothesis I: Non-zero risk of freezing during long-term exposure in freeze-intolerant individuals.
Nucleation Hypothesis II: Short-term risk of inoculative freezing in freeze-intolerant individuals, especially in moist environments.
Desiccation avoidance hypothesis: Supercooled insects are in vapour pressure deficit if surrounded by ice.
Extreme survival hypothesis: At very low temperatures the supercooled state may be stable for short periods only.
Energy conservation hypothesis: Freezing reduces metabolic rate and the latter is apparently insensitive to changes in temperature in frozen animals.
Environmental variability hypothesis: Freezing tolerance enables animals to survive cold snaps at any time without metabolically costly synthesis of additional cryoprotectants, and enables them to take advantage of warm spells to continue with growth and development.
To some extent these hypotheses do not recognize the complexity of responses, which may include mixed strategies and changes in strategies following exposures to low temperature (Kukal and Duman, 1989; Bale et al., 2001; Brown et al., 2004). Moreover, the remaining qualitative hypotheses which, while useful, lack the rigour of the models applied to many other problems in insect life-history theory (see Roff, 2002).
One attempt to place the costs and consequences of cold hardiness strategies on a more quantitative footing is the energetic model of cold hardiness developed by Voituron et al. (2002). They assumed that the strategy adopted is the one that maximizes fitness as measured by available energy at the end of winter, which can be represented by the fitness differences of the two strategies, Ψ, from the equation:
| (1) |
where W0 is the metabolizable energy reserve at the start of winter, ST the energetic cost of a freezing tolerance strategy, N the number of freezing days, Nmax the maximum number of freezing days before death, SA the energetic cost of freeze intolerance, a the sensitivity of freeze intolerance to climate (energy required to produce reliable cyroprotection for NT), T the cold intensity, and θ the shape of the change in fitness (or WT – energy available at end of winter) of a freezing tolerant individual as Nmax is approached.
When Ψ>0, then freezing tolerance will be favoured and when Ψ < 0, freeze intolerance is favoured. Analytical and simulation work by Voituron et al. (2002) has demonstrated that freezing tolerance is favoured by low stress associated with freezing, low initial energy content, high number of freezing days, and a high value of θ. By contrast, freeze intolerance is favoured by a low number of freezing days, low stress associated with supercooling, low sensitivity to climate, and high initial energy content. The model also indicates that harsher conditions should favour a mixed strategy.
The majority of these outcomes appear to be in keeping with empirical findings, especially for extreme strategies such as strong freezing tolerance and freeze avoidance (Chown and Nicolson, 2004). As a result, it is a much needed and useful first step towards a quantitative understanding of the costs and benefits of each of the strategies. Nonetheless, as recognized by Voituron et al. (2002), the model is less able to deal with other strategies and requires further development to do so (see also Sinclair et al., 2003a). Additionally, several of the basic assumptions made by the model either have been poorly explored, or remain theoretical constructs only, in need of empirical evaluation.
The value of θ, or the form of the relationship between number of days remaining until Nmax and WT, has a significant influence on the likelihood that freezing tolerance will be favoured, but has not been explored at all. If frozen individuals are largely anoxic (Storey and Storey, 1996; Morin et al., 2005), then duration of survival is likely to be a function not of energy stores, but rather of the extent of damage owing to chaotic biochemical reactions (Knickerbocker and Lutz, 2001; Milton et al., 2003a; Makarieva et al., 2006). Anoxic organisms die after the cumulative energetic yield of chaotic anoxic biochemical processes has passed c. 70–100 kJ (kg dry mass)−1, which means that the more effectively an organism can suppress the accumulation of disorder, the longer it will survive (Fig. 16). Consequently, it might be argued that θ should typically have a value substantially less than 1, which is a situation unlikely to promote freezing tolerance (Voituron et al., 2002, p. 262). The energy available to an organism in advance of freezing has little influence on whether or not a freezing tolerant strategy will be followed, by contrast with the assumptions of the model. Rather, it is the extent of biochemical conservation of tissues in advance of the anoxic condition, or limitation of damage by chaotic reactions, that is of most significance (Makarieva et al., 2006).
FIG. 16.
Metabolic rate, energy loss, and survival time under anoxic conditions. (A) Mass-specific rates, qA, of energy dissipation by organisms capable of surviving more than half a day of anoxia. (B) Energy loss ψ during anoxia is independent of survival time (filled circles). The open circles indicate normoxic energy losses of bears during hibernation and ticks during prolonged starvation. Redrawn from Makarieva et al. (2006, p. 90).
Another assumption of the modelling approach is temperature independence of metabolic costs in frozen animals (Voituron et al., 2002, p. 257). The logic used is that anoxic individuals meet their energetic demands through anaerobic pathways, and the energy cost of frozen animals is therefore temperature independent. Unfortunately, empirical evidence for this idea is at best weak. Although several studies have measured metabolic rates of frozen and supercooled insects, they are confounded by the use of closed system respirometry, which makes detection of activity difficult (reviewed in Sinclair et al., 2004). In addition, estimates of energy expenditure at very low temperatures in frozen insects can probably only be made reliably using calorimetric (see Hansen et al., 2004, 2006) or biochemical methods, owing to the fact that metabolism is either anaerobic and even if it is not, diffusion through ice is extremely low. Nonetheless, evidence gained from other studies of species experiencing anoxia suggests that the relationship between metabolic rate (which in this instance is like a measure of chaotic biochemical reactions) and temperature is positive, with a Q10 of approximately 2.8 (Makarieva et al., 2006). Therefore, it appears that several assumptions made in the model developed by Voituron et al. (2002) require further exploration, as does consideration of the reasons for the development of the two major cold hardiness strategies.
One possibility is that the freezing-tolerance and freeze-intolerance responses represent the two major alternatives for surviving stress, i.e. abandoned metabolic control and minimum metabolic control, respectively (Makarieva et al., 2006). In the case of abandoned metabolic control, the organism maximally protects its cellular structures against degradation and then switches off most other metabolic processes. The disadvantage of this strategy is that no matter how low, the rate of spontaneous degradation of structures is never zero. However, in this case, repair is not possible until favourable conditions return. Therefore, once a critical threshold has passed (which appears to be c. 100 kJ (kg dry mass)−1), the animal dies. Survival time is dependent on the rate of spontaneous degradation, and decreases with increasing temperature because degradation has a Q10 of approximately 2.8. In addition, the energy losses tolerated in the regime of abandoned metabolic control are much lower than those tolerated under minimum metabolic control. In the case of minimum metabolic control, the organism survives the stress by continually sustaining order at a minimum metabolic level, which is independent of both body mass and temperature. The period of survival depends on energy stored by the organism. Hence, much greater rates of energy loss per unit body mass can be tolerated because the loss is non-random and occurs in storage tissues without threatening organismal integrity. For example, hibernating bears can tolerate losses in the region of 16 000 – 40 000 kJ (kg dry mass)−1, and starved ticks can tolerate energy losses of 4300–20 000 kJ (kg dry mass)−1. Nonetheless, some damage may accumulate, which might also account for the periodic arousal that is typical of many hibernating organisms (Makarieva et al., 2006, see also McNab, 2002).
At least some evidence suggests that this categorization of cold hardiness strategies is appropriate. In freeze-tolerant insect species, metabolic rate drops rapidly just in advance of freezing or as it occurs (Lundheim and Zachariassen, 1993; Irwin and Lee, 2002; Sinclair et al., 2004), which Makarieva et al. (2006) have interpreted as the point at which metabolic control is abandoned. Furthermore, in the freeze-tolerant Eurosta solidaginis, metabolic rate (which is largely anoxic in the frozen state, Joanisse and Storey, 1994a, 1996) is strongly related to temperature in the frozen state, and increases in temperature reduce survival, in keeping with the expectations of abandoned metabolic control (Irwin and Lee, 2000, 2002, 2003; Irwin et al., 2001). Moreover, antioxidant enzymes show a decline in winter (Joanisse and Storey, 1996), and this species shows no overwinter heat-shock protein response to cold stress (Lee et al., 1995), indicating that most metabolic processes are shut down (although accumulation of some polyols may continue and anaerobic metabolism certainly proceeds – see Joanisse and Storey 1994a, b). Degradation of mitchondria over winter in the strongly freezing-tolerant caterpillars of Gynaephora groenlandica (Kukal et al., 1989) is also indicative of abandoned metabolic control, especially since these structures might be most susceptible to chaotic metabolic reactions (Makarieva et al., 2006).
Whether freeze intolerance represents minimum metabolic control is more difficult to ascertain. Previous studies have claimed a strong relationship between temperature and metabolic rate in the supercooled state, although the technical approach used precludes assessment of the influence of insect activity (see Sinclair et al., 2004). However, some findings support the idea that aerobic metabolism with ongoing damage repair characterizes freeze intolerance. Thus, in the goldenrod gall moth, Epiblemma scudderiana, metabolism is clearly aerobic (Joanisse and Storey, 1994b, 1996), and in several other freeze-intolerant species, a heat-shock protein response is shown (Denlinger, 2002; Chen et al., 2005a), suggesting ongoing damage control and repair. In diapausing, freeze-intolerant pupae of flesh flies, periodic increases in metabolic rate (infradian cycles) are apparent (Denlinger et al., 1972), suggesting that minimum metabolic rates are unable to sustain all damage repair and that periodic increase in metabolism are required to do so. Similar oscillating patterns have been found in overwintering individuals of Megachile rotundata (Yocum et al., 2005).
Thus, despite the fact that a large literature exists on insect responses to winter cold, it is clear that much remains to be done to understand the environmental and life-history contexts of these responses. For example, the effects of moulting on SCPs is now only beginning to be understood in some species, and it appears that much of the variation in the frequency distributions of SCPs, which has long occupied physiologists, is likely nonadaptive (Fig. 17) (Worland, 2005; Worland et al., 2006). Nonetheless, an excellent start has been made at understanding the life-history contexts of cold hardiness responses especially in the context of the metabolic costs of the strategies. Indeed, interactions between metabolism and cold hardiness have not been explored to any large extent, despite the fact that they are likely to prove significant for understanding the evolution of low-temperature tolerance (Voituron et al., 2002; see also Hoffmann et al., 2005b).
FIG. 17.
Supercooling point (SCP) distributions from the springtail Ceratophysella denticulata on sub-Antarctic Marion Island from (A) an ‘arbitrary’ field sample, (B) pre-moulting animals from the same main sample, (C) recently moulted animals, and (D) recently moulted animals that had been fed for one day (10 °C). Note the substantial decline in supercooling point associated with the moulting process and the increase thereof following feeding. Redrawn from Worland et al., 2006. result in unfolding. In this unfolded state, exposed amino acid side groups, especially hydrophobic residues, can lead to interactions between these ‘non-native’ proteins and folded proteins, inducing the latter to unfold. The result is irreversible aggregations of unfolded proteins. These unfolded proteins reduce the cellular pool of functional proteins and may also be cytotoxic (Feder, 1996, 1999; Feder and Hofmann, 1999; Kregel, 2002; Korsloot et al., 2004).
6.3 Responses to High Temperature
6.3.1 High-temperature injury
High-temperature injury results from disruption of the structure of membranes (Hochachka and Somero, 2002) and in consequence their function, especially those of neurons (Robertson, 2004a; Klose and Robertson, 2004). The ways in which the structure of membranes are disrupted by high temperatures have been reviewed in considerable detail (Hochachka and Somero, 2002), and will not be considered here. High temperature also results in alterations in the cell microenvironment, and especially affects the cytoskeleton (Klose and Robertson, 2004) and pH (Denlinger and Yocum, 1998), perturbation of protein structure, and DNA lesions (Somero, 1995; Feder, 1999). Intense thermal stress can perturb the structure of an organism’s proteins. During normal cellular functioning, proteins are generally folded, but may be unfolded during transport, synthesis of polypeptides, and assembly of multimeric proteins. Stress may also
Neuronal phenomena are characterized by two main types of thermal sensitivity. The first is a consequence of temperature-dependence of conduction, action potential duration, and synapse functioning. In turn these reflect temperature dependence of activation and inactivation of ion channels, which are the result of conformational changes in protein structure. When a neuronal parameter is made ineffective by high or low temperature then limits are set (Robertson, 2004a). The second is a consequence of a high thermal dose (duration and intensity of stress), which causes disturbance or damage eventually leading to failure of neuronal function. A primary site of thermal damage is the cytoskeleton, which contributes to processes underlying synaptic plasticity (Klose et al., 2004). Stress causes disruption of actin microfilament integrity, resulting in dissociation from membranes, disassembly of microtubules, and collapse of intermediate filaments towards the nucleus (Klose and Robertson, 2004). Synaptic damage is also a major consequence of thermal stress (Karunanithi et al., 2002; Robertson, 2004a), and damage to neuronal functioning takes place as a consequence of a decrease in amplitude and duration of the action potential. The latter is a consequence of rapid activation of K+ currents, which overwhelm the Na+ current before the latter can develop fully (Robertson, 2004a), as well as an extracellular accumulation of K+ (Robertson, 2004b). These effects are of considerable significance given the importance of the nervous system in enabling an organism to sense and respond to its environment (Klose and Robertson, 2004).
The damage wrought by sublethal thermal stress affects development, muscular contraction, flight ability, fertility, and several other processes at higher organizational levels (Denlinger and Yocum, 1998; Rohmer et al., 2004; Chakir et al., 2005; Krebs and Thompson, 2005; Jørgensen et al., 2006). The organizational level at which thermal stress has most effect has not been fully resolved. However, it seems likely that it is not at the level of acquisition and transport of oxygen (i.e. oxygen limitation of thermal tolerance) as has been suggested by Pörtner (2001) (see Section 6.1.2). That insect upper thermal tolerances show relatively little geographic variation, at least by comparison with lower lethal limits, and that thermal limits are invariant with changing oxygen concentrations provide support for this idea (Chown, 2001; Klok et al., 2004). However, the number of species investigated in the latter case remains small, and the response to a prolonged stress has not been assessed.
6.3.2 Basal responses
The temperature at which heat induces injury and/or death varies both through space and in time. Because differences in methods of measurement usually assess different traits (e.g. knockdown resistance vs. survival), resulting in dissimilar outcomes, it is difficult to reach general conclusions regarding upper thermotolerance limits (see Chown and Nicolson, 2004). However, in insects they generally do not exceed about 53 °C, and are usually not much lower than 30 °C, although these values depend on the trait being measured. There are examples of very low tolerance levels in some species such as alpine grylloblattids, and tolerance may increase dramatically in dormant, virtually anhydrobiotic stages such as eggs. Ignoring these extreme values, substantial variation in tolerances remains, although it is typically less than that found for lower lethal limits (Addo-Bediako et al., 2000; Chown, 2001; Hoffmann et al., 2005a).
The physiological basis of this variation in thermotolerance is much less clear. It has been suggested that constitutively expressed heat shock proteins (Hsps, see later) might be responsible for both survival of potentially lethal temperatures and for improved knockdown resistance (McColl et al., 1996; Gilchrist et al., 1997; but see also Nielsen et al., 2005). Alternatively, alterations in cell membrane composition or changes in allozymes or their concentrations might also be involved (Hochachka and Somero, 2002). One explanation for increased basal thermotolerance is the cost of a low-level, induced-stress response. Continuous expression of heat shock proteins reduces survival and fecundity, inhibits growth and thus affects development time (Krebs and Loeschcke, 1994; Feder and Krebs, 1998; Feder, 1999), and impairs locomotion (Robertson, 2004b). It also acts as a substrate sink, and interferes with cellular functioning (Zatsepina et al., 2001). Consequently, at high temperatures there might be a considerable premium for reduction of this response, and probably an increase in basal thermotolerance allowing the organisms to cope with what are otherwise potentially injurious temperatures. This basal thermotolerance may be a consequence of constitutively expressed hsps (Lansing et al., 2000), the presence of osmolytes (Wolfe et al., 1998), or alterations in membranes and allozymes (Zatsepina et al., 2001).
6.3.3 Induced tolerance and its underlying mechanisms
It has long been appreciated that injury caused by high temperature can be ameliorated by prior exposure to a sublethal, or moderately high temperature (Denlinger et al., 1991; Hoffmann and Watson, 1993; Robertson et al., 1996). This acclimation response lasts for several hours, but is nonetheless transient (Krebs and Loeschcke, 1995). Induced thermotolerance responds strongly both to artificial selection (Krebs and Loeschcke, 1996), and to laboratory natural selection (Cavicchi et al., 1995), and it is clear that this trait shows considerable genetic variation (Krebs and Loeschcke, 1997; Loeschcke et al., 1997).
The expression of heat shock proteins, which act as molecular chaperones to proteins, is now recognized as one of the most widespread and conserved responses to thermal and other stresses (Feder and Hofmann, 1999; Kregel, 2002). Molecular chaperones interact with the unfolded proteins to minimize their harmful effects by binding to the exposed side groups, preventing unfolded proteins from interacting. In an ATP-dependent manner, they also release the proteins so that they can fold properly, and target proteins for degradation or removal from the cell (Parsell and Lindquist, 1993). Hsps have a significant role in protecting cytoskeletal integrity during thermal stress, and are also of considerable significance for retention of neuronal functioning (Karunanithi et al., 1999; Klose et al., 2004). These heat shock proteins comprise several families that are recognized by their molecular weight, and include Hsp100, Hsp90, Hsp70, Hsp60, and a family of smaller proteins (Denlinger et al., 2001). The roles of these families differ, and the smaller Hsps also have a function in membrane stabilization (Tsvetkova et al., 2002).
The best known of these families in insects is Hsp70, especially because of its dramatic increase in Drosophila in response to high-temperature stress. Conclusive demonstrations of the association between Hsp70 expression and thermotolerance have come from investigations of isofemale lines and genetically engineered strains of D. melanogaster (Krebs and Feder, 1997; Feder and Krebs, 1998; Feder and Hofmann, 1999), as well as investigations of Sarcophaga crassipalpis (Denlinger and Yocum, 1998), and the locust nervous system (Robertson, 2004a, b). Heat shock is also known to induce expression of Hsp70 and to confer thermotolerance and undertake chaperoning roles in several other insect species such as locusts, whiteflies, beetles, moths, ants, fruit flies, and parasitic wasps (Denlinger et al., 1991, 1992; Gehring and Wehner, 1995; Thanaphum and Haymer, 1998; Maisonhaute et al., 1999; Salvucci et al., 2000; Landais et al., 2001; Qin et al., 2003; Neargarder et al., 2003; Mahroof et al., 2005). Therefore, it seems likely that Hsp70 will be identified as a common component of the heat shock response in most taxa, although the nature and complexity of the response is likely to vary. Nonetheless, ongoing expression of Hsps can have significant deleterious effects (see above). This may explain the decline in the expression of Hsp70 and inducible thermotolerance when either laboratory strains (Sørenson et al., 1999; Lerman and Feder, 2001) or wild populations (Sørenson et al., 2001; Zatsepina et al., 2001; Qin et al., 2003) evolve at high temperatures.
In aphids and whiteflies, the synthesis of protective osmolytes, specifically polyhydric alcohols, also confers thermotolerance (Wolfe et al., 1998; Salvucci, 2000; Salvucci et al., 2000). Sorbitol accumulates to levels as high as 0.44 M within 3 h of exposure to high temperatures in the whitefly, Bemisia argentifolii, and appears to serve the same protective role as heat shock proteins. At physiological concentrations, sorbitol increases the thermal stability of proteins by stabilizing their structure and preventing heat-induced aggregation, thus maintaining catalytic activity at high temperatures (Salvucci, 2000). If the insects are deprived of nutrients, sorbitol production declines and heat shock proteins assume greater importance in protecting proteins against thermal stress. Nonetheless, it appears that sorbitol is routinely produced as a rapid response to high temperature, via an unusual synthetic pathway involving fructose and an NADPH-dependent ketose reductase.
Further responses to high temperature stress involve modulation of potassium conductance in the neuronal system. Prior heat shock improves tolerance of stress in a variety of nervous tissue preparations (see Klose and Robertson, 2004; Robertson, 2004a, b for review). At least one consequence of this heat shock is a reduction in whole cell K+ conductance, which is mimicked by the application of serotonin (5-hydroxytryptamine) (Ramirez et al., 1999; Wu et al., 2002). This results in an increase in action potential duration and a reduction in the extracelleular accumulation of potassium ions. Both have substantial effects on neuronal functioning (Robertson, 2004b). Nonetheless, the response is complex and differs both between tissues and between species (Robertson, 2004a). Prior heat shock reduces recovery time in the ventilatory central pattern generator following thermal stress, and it seems likely that heat shock somehow activates the Na+/K+-ATPase, although how this happens has not been investigated. Nonetheless, it is clear that prior heat shock confers substantial thermal tolerance across the nervous system, at the presynaptic, synaptic and axonic levels.
6.4 Relationships Between High- and Low-Temperature Tolerance
6.4.1 Organismal level responses
Given that both polyols and heat shock proteins are expressed in response to cold and heat shock, and that pretreatment at a high temperature increases tolerance of cold shock (Chen et al., 1991; Sinclair and Chown, 2003), and vice versa (Sejerkilde et al., 2003), depending on the species, it might seem that the responses to heat and cold are similar. However, although heat shock proteins are synthesized rapidly in response to both cold and heat treatments, considerable differences exist between these two responses. First, the time course of the response differs. Hsps are often not produced in response to rapid cold hardening, but are expressed following cold acclimation, and usually they are produced only once individuals return to higher temperatures. By contrast, during heat shock, Hsps are synthesized during the stress. Second, the duration of the response differs dramatically between the two forms of shock. Usually, synthesis of Hsps in response to high temperature is brief and ceases almost immediately on cessation of the stress (Yocum and Denlinger, 1992), while in response to low temperature, Hsp synthesis may continue for days (Yocum et al., 1991). Third, during heat shock, normal protein synthesis is almost entirely replaced by stress protein synthesis, whereas following a cold shock normal protein synthesis and the production of stress proteins occur concurrently. Finally, upregulation of serine proteinase genes associated with immune function also differs between cold and heat shock (Chen et al., 2005b).
Nonetheless, other responses, such as stabilization of membranes by polyols and modulation of membrane pumps may be common to both high- and low-temperature responses, as is the improvement to stress resistance under alternating temperature regimes (Pétavy et al., 2001). In addition, central regulation of responses involves similar mechanisms. Thus, while the initial response to cold and heat shock takes place at the cellular level (Hochachka and Somero, 2002; Yi and Lee, 2004), subsequent responses are centrally regulated (Denlinger and Yocum, 1998; Yoder et al., 2006). Hormonal responses may also turn off downstream functions, such as reproduction, that would otherwise be negatively affected by thermal stress (Pszczolkowski and Chiang, 2000; Gruntenko et al., 2000, 2003a, b; Irwin et al., 2001; Pszczolkowski and Gelman, 2004).
Interactions between hormonal regulation of insect development and stress resistance have been especially well explored for cold hardiness and diapause. In the flesh fly, Sarcophaga crassipalpis, non-diapausing pupae are much more sensitive to low temperature than pupae in diapause, which can survive prolonged exposure to temperatures approaching their SCP (c. −23 °C) (Lee and Denlinger, 1985; Chen et al., 1987; Lee et al., 1987b). Ecdysteroid titre drops rapidly at the onset of diapause and it seems likely that genes associated with the action of these hormones are essential for regulating diapause (Denlinger, 2002; Hayward et al., 2005). Transcripts for heat shock protein 90 (hsp90) are downregulated during diapause, and their expression is likely controlled by 20-hydroxyecdysone (Rinehart and Denlinger, 2000). Exposure to both cold and heat shock results in upregulation of hsp90, and exposure to cold, but not heat results in upregulation of heat shock cognate 70 (hsc70) (Rinehart et al., 2000b). By contrast, hsp23 and hsp70 are upregulated at the start of diapause and downregulated rapidly when diapause is terminated (Yocum et al., 1998; Denlinger et al., 2001). During diapause neither heat shock nor cold shock result in further upregulation of these heat shock proteins, possibly as a consequence of upregulation of hsp90.
Given that the continued expression of heat shock proteins is known to be deleterious, their continued upregulation during diapause in S. crassipalpis initially appears remarkable. However, cell cycle arrest plays an important role in diapause in S. crassipalpis. Therefore, if the majority of negative effects of Hsp expression have to do with reduced cellular growth and differentiation, Hsps may have little adverse effect during diapause and may even assist in the maintenance of diapause (Hayward et al., 2005), as well as serving to protect diapausing individuals from thermal and other stresses. The downregulation of hsp90 at the onset of diapause, and its upregulation following diapause termination, or in response to heat or cold shock, is also readily comprehensible within this framework. Hsp90 keeps unstable proteins ready for activation until they are stabilized during signal transduction. Thus, given relative cell inactivity during diapause, Hsp90 is unlikely to be required, but because of its ability to stabilize proteins, it remains responsive to thermal stress. However, this pattern of expression is not common to all insect species (e.g. Goto et al., 1998; Goto and Kimura, 2004; Chen et al., 2005a; Tachibana et al., 2005). Therefore, the role of Hsps during diapause, and their hormonal regulation deserve further exploration.
6.4.2 Geographic variation
At higher levels of organization, substantial differences between tolerance to high and low temperatures are particularly evident. Geographic variation in response to cold and heat shock have been investigated in several species (e.g. Goto and Kimura, 1998), but the comparison of flesh flies from tropical and temperate areas made by Chen et al. (1990) is one of the most comprehensive. While all the species show an inducible tolerance to heat shock, only the species from temperate and alpine areas show rapid cold hardening. As might be expected, basal tolerance of cold is greater in the temperate and alpine species than in the tropical ones, but this is true also of basal heat tolerance. Although this appears somewhat unusual, it should be kept in mind that mid-latitude areas are often characterized by very high temperatures (Sømme, 1995), and that global variation of absolute maximum temperatures is much less than that of absolute minima.
This difference in global temperature variation lies at the heart of similar large-scale patterns in insect thermal tolerances. Latitudinal variation in upper lethal limits, though significant (a range of about 30 °C), is much less pronounced than spatial variation in lower lethal temperatures (a range of about 60 °C) (Addo-Bediako et al., 2000). Similar patterns are found across smaller geographic ranges both within and between species (e.g. Chown, 2001; Ayrinhac et al., 2004; Hoffmann et al., 2005a; Terblanche et al., 2006), and in a variety of stages (e.g. Shintani and Ishikawa, 1999; Jing and Kang, 2003; Wang and Kang, 2005). Similar clines in genes associated with the response to thermal stress are now also being demonstrated (e.g. Bettencourt et al., 2002; Frydenberg et al., 2003). Much of the variation within species is environmentally induced. In other words, common garden experiments reveal that differences among populations can largely be accounted for by phenotypic plasticity. The significance of phenotypic plasticity in shaping responses to the environment has also been demonstrated for altitudinal clines (e.g. Klok and Chown, 2003), where many of the intraspecific thermal tolerance patterns, and indeed interspecific patterns, are similar to those found across latitude (e.g. Collinge et al., 2006), although exceptions can be found (e.g. Sørensen et al., 2005). Laboratory selection experiments have revealed similar, differential responses to heat and cold (reviews in Chown, 2001; Chown and Storey, 2006).
6.5 Low Temperature, Dehydration, and Starvation
It has been widely accepted for at least the past decade that a physiological link exists between an insect’s ability to withstand cold and its ability to survive dehydration (reviewed in Ring and Danks, 1994; Block, 1996; Denlinger and Lee, 1998; Danks, 2000; Chown and Nicolson, 2004). This is at least partly a consequence of the recognition that the damage caused by desiccation and by freezing is similar (for review see Storey and Storey, 1996). Indeed, cellular hydration state probably acts as a trigger for many of the mechanisms that enable survival of subzero temperatures and dry conditions (reviewed in Schliess and Haüssinger, 2002). It is widely accepted that cell and whole-animal regulatory processes that are affected by hydration state, including processes directly resulting in cell death, are directly influenced by cell volume changes (Chamberlin and Strange, 1989; Parker, 1993; Schliess and Haüssinger, 2002), although this evidence has come primarily from mammalian tissues (though see Chamberlin and Strange, 1989). Changes in cell hydration state may be sensed by a variety of mechanisms including stretch-activated ion channels, cytoskeletal elements, and changes in membrane structure (reviewed in Chamberlin and Strange, 1989; Parker, 1993). The interactions among cell volume, osmotic status and stress responses are complex. Changes in cell volume associated with dehydration and rehydration, and which are mediated to some degree by the osmotic state of the cell (Parker, 1993; Lang et al., 1998; Schliess and Haüssinger, 2002), can also induce other stress responses (Schliess et al., 1999). Cell hydration state is also closely coupled with oxidative stress responses. In the case of the latter, there is some evidence to suggest that oxidative stress can be converted into osmotic stress and that the converse may also be true (Qin et al., 1997; Schliess and Haüssinger, 2002), although this type of response may be restricted to specific mammalian tissues or selected cell types. The critical minimum cell volume is widely acknowledged to be a threshold for cellular functioning (Storey and Storey, 1996), and iso-osmotic declines in cell volume can directly result in apoptotic cell death (Schliess and Haüssinger, 2002). Typically, the association of these stress responses with cellular hydration status, either by volume or cell concentration changes, have not been well elucidated for even the most common model organisms (Parker, 1993; Schliess and Haüssinger, 2002).
Given the similarities in the likely damage caused by low temperature and dehydration, it has been suggested by several authors that the biochemical mechanisms enabling survival of low temperatures may simply be shared stress pathways which are also utilized under desiccation stress (Pullin, 1996; Worland and Block, 2003). There are several reasons why these views have arisen, although the degree to which these traits have co-evolved, or if the co-related responses may be considered adaptive, is not yet clear (Sinclair et al., 2003b). The principal mechanism linking cold tolerance and desiccation resistance is at least partly one of physical chemistry, such that a smaller volume of fluid will freeze at lower temperatures (Salt, 1956; Worland, 1996; Denlinger and Lee, 1998; Worland, 2005). It is, therefore, no coincidence that the most desiccation- and cold-tolerant ectotherm species on the planet are also the smallest (Watanabe et al., 2002; Alpert, 2006). This loss of body water also results in the concentration of molecules in solution, and can be another advantage of dehydration during cold exposure (Salt, 1956; Worland, 1996; Chown and Nicolson, 2004). As a result, cryoprotective dehydration is now recognized as an important strategy which some arthropods and several other invertebrates use to survive overwintering (Holmstrup et al., 2002a; Chown and Nicolson, 2004; Bennett et al., 2005). For example, in the collembolan, Onychuirus arcticus, loss of water to surrounding ice enables the SCP to drop from −6.5 to c. −17 °C (Worland et al., 1998; Holmstrup et al., 2002a).
However, simple changes in body water alone do not necessarily explain why some terrestrial arthropods show increased survival at temperatures well above their freezing point, as in freeze-intolerant species, nor does it explain enhanced survival of cold stress at temperatures above 0 °C. These can be explained by the wide array of intracellular sugar and polyhydric alcohol cryoprotectants, thermal hysteresis proteins, heat shock proteins, and membrane-bound proteins that are synthesized in response to either cold or dehydration and that, in many instances, underlie cross tolerance (see above and Koštál and Šimek, 1996; Storey and Storey, 1996; Chown and Nicolson, 2004; Bennett et al., 2005). For example, in the collembolan, Folsomia candida, changes in total membrane phospholipid fatty acid composition during humidity acclimation is similar to those observed during cold exposure, in conjunction with the accumulation of intracellular cryoprotectants (Holmstrup et al., 2002b). Moreover, an enhanced cold tolerance follows the humidity treatment (Bayley et al., 2001; Holmstrup et al., 2002b). In larvae of the freeze-tolerant Pringleophaga marioni increased tolerance of low temperature follows a desiccation pre-treatment (Sinclair and Chown, 2003), and those individuals of Anthonomus pomorum which survive desiccation best are those that have high trehalose contents, a pattern similar to individuals that are most tolerant of low temperature (Koštál and Šimek, 1996).
In many terrestrial arthropods, both desiccation and low temperature stimulate the production of glycerol (e.g. reviewed in Chown and Nicolson, 2004, see also Yoder et al., 2006). The accumulation of low molecular weight organic molecules has also been implicated in the absorption of atmospheric water for springtails (Bayley and Holmstrup, 1999) and for protection of cells against osmotic damage during extreme dehydration (Danks, 1999, 2000). In Eurosta solidaginis larvae during the early onset of winter, reductions in water loss rates are not correlated with changes in cold tolerance, nor are they associated with changes in haemolymph osmolality or body water content (Williams et al., 2004). However, a second phase of increased desiccation resistance was associated with an increase in haemolymph osmolality. Williams et al. (2004) speculated that interactions between cryoprotectants such as glycerol, which bind water, making it resistant to freezing and removal by dehydration, and anti-freeze proteins (or in the specific case of E. solidaginis, a dehydrin-like protein) act to lower the permeability of the cuticular barrier. In the flesh fly, Sarcophaga bullata, treatment with an exogenous glycerol dose increases both low-temperature tolerance and dehydration resistance (Yoder et al., 2006).
The expression of heat shock proteins in response to desiccation has also been investigated in several species (Tammariello et al., 1999; Bayley et al., 2001; Hayward et al., 2004a), although a relationship between cold shock and desiccation has not always been found (Goto et al., 1998). In Sarcophaga crassipalpis, which shows a complex pattern of up- and down-regulation of different heat shock proteins over the course of diapause (Hayward et al., 2005), heat shock protein expression in response to dehydration and rehydration has recently been carefully explored (Hayward et al., 2004a). In non-diapausing pupae, Hsp23 and Hsp70 are upregulated by desiccation, although the threshold for expression depends on dehydration rate. The upregulation results in a delay in eclosion, which is in keeping with previous findings that certain Hsps may interfere with the cell cycle (see Denlinger, 2002). By contrast, in diapausing pupae, which up-regulate Hsp23 and Hsp70 on entry into diapause (Hayward et al., 2005), no Hsps were upregulated. During rehydration, both Hsp90 and Hsc70 (constitutive heat shock protein or the heat shock cognate) are upregulated in diapausing and non-diapausing pupae, and it appears that this response is very similar to the one shown following exposure to low temperatures. The role of Hsps in stabilizing both proteins and membranes clearly accounts for their significant roles during dehydration and rehydration, and it seems unlikely that they act in isolation from other cellular processes (Arispe et al., 2002; Tsvetkova et al., 2002; Hayward et al., 2004a).
Although much of the literature has been concerned with cross tolerance, and the identification of the similarities between responses to low temperature and dehydration, trade-offs in responses might also occur especially given modifications in lipid content and type. In D. melanogaster, it is well known that in response to starvation substantial changes in lipid content take place (by contrast with response to desiccation which more typically involve changes in glycogen stores – see Gibbs et al., 2003). Hence, a tradeoff between starvation resistance and low-temperature tolerance was predicted by Hoffmann et al. (2005b). This is indeed what they found in D. melanogaster. Following selection for starvation resistance, low-temperature tolerance declined, although the response was sex specific. The biochemistry underlying the response remains poorly investigated, and it is not yet clear how widespread such trade-offs might be in other species. This response is also different to the one more typically investigated in the context of cold tolerance: the decline in SCPs with gut clearance or starvation (see Klok and Chown, 1998; Salin et al., 2000; Chown and Nicolson, 2004).
Thus, for many terrestrial arthropod species, survival of low temperatures is based on mechanisms that are similar to those required for surviving dehydration, and in many instances, the stresses are simultaneous. Moreover, the nature of the low-temperature response might affect the extent of dehydration, which may feed back to alter the former. For example, a supercooled insect is much more likely to experience dehydration in the presence of ice than is a frozen insect (Lundheim and Zachariassen, 1993). The likelihood of dehydration might substantially influence the cold tolerance strategy that is adopted (see above and Section 6.2.2). Although little evidence exists for a direction of evolution, given the presumed date of origin of many insect taxa (see Shear and Kukalová-Peck, 1990; Labandeira and Sepkoski, 1993), and the likely conditions of the planet at the time (Stanley, 1989; Behrensmeyer et al., 1992), it seems most plausible to presume that the first responses were to desiccation, and that they formed a suite of mechanisms which were subsequently honed and modified to accommodate tolerance of low temperatures.
7 Conclusions
We commenced this review by pointing out that humans are affecting fundamental changes to the landscape and climate of the planet, and suggesting that understanding and prediction of the consequences of these changes will require comprehension of the physiological responses of insects to their environments. This view is shared by many evolutionary physiologists (e.g. Hoffmann and Parsons, 1997; Helmuth et al., 2005; Parsons, 2005), and by an increasingly wide variety of ecologists (e.g. Brown et al., 2004; Owen-Smith, 2005; Wiens and Graham, 2005). In several ways, these fields, which separated in the middle of the last century, are once again beginning to be integrated (see discussions in Spicer and Gaston, 1999; Chown and Storey, 2006).
Here, we have sought to demonstrate that such integration of a variety of approaches, including models of range limits and the development of plasticity, the assessment of environmental variability, and the exploration of responses at a wide variety of spatial and temporal scales, is of considerable value. The likely role of interactions between plasticity and stress in affecting responses to rapidly changing environments is especially significant, and has been identified as a key component missing from many assessments of the responses of organisms to environmental change (Helmuth et al., 2005). Indeed, recent work has suggested that stress responses might act as a capacitor for evolution (Garland and Kelly, 2006), which might substantially alter predictions of change.
For example, the responsiveness of Hsp90 to proteins denatured by heat stress may also be the cause of the expression of phenocopies, or developmental abnormalities that resemble specific mutations and by genetic accommodation these might later be permanently expressed (Denlinger and Yocum, 1998). Hsp90 has been identified as a capacitor of morphological evolution in several species (Rutherford and Lindquist, 1998; Queitsch et al., 2002), although the process is complex and may be trait specific (Milton et al., 2003b). Evolution of phenotypes by genetic accommodation might also take place via changes in hormonal titres, as has been demonstrated for a colour polyphenism in Manduca sexta caterpillars (Suzuki and Nijhout, 2006). Such environmental perturbations might extend beyond traits that are immediately affected, and could result in changes to others such as the extent of wing venation and development, especially if alterations in hormonal titres and heat shock protein responses are involved (Roff, 1986; Marcus, 2001). Consequently, changes in mobility as well as physiological traits might evolve in response to stress, in ways that are not intuitively obvious. Certainly, in the context of rapidly changing dispersal capabilities in insects that are experiencing substantial landscape and climate change these interactions deserve closer attention (see e.g. Thomas et al., 2001, but also Simmons and Thomas, 2004).
Whether the kinds of integration we have sought to promote will reveal fundamental biological laws or remain a documentation of individual responses to different conditions is a significant question. A similar question has recently occupied ecologists (see Lawton, 1999; Simberloff, 2004), and has emerged in discussions of the value of macrophysiology (Chown et al., 2003, 2004b; Hodkinson, 2003). Clearly, several broad generalizations are emerging from investigations of individual and population responses to the thermal environment. For example, virtually all populations that have been examined to date show plastic responses to low-temperature treatments (see also Rako and Hoffmann, 2006). Likewise, irrespective of the level of analysis, it appears that upper lethal limits and responses to high temperature show a much narrower range of variation than do lower lethal limits and responses to low temperatures (Chown, 2001; Chown and Nicolson, 2004). However, exceptions do exist (e.g. Sinclair and Chown, 2003 for lack of responsiveness to low-temperature acclimation in P. marioni). Like Simberloff (2004), we do not consider this a problem for the field, given that both understanding and prediction are essential components of the scientific endeavour, and that the two may not be related in any way (Casti, 1991). In other words, understanding of the responses of a given population or species might not result in subsequent predictive capacity, nor might prediction of the effect of a given environmental or other manipulation necessarily presuppose complete understanding of the underlying mechanisms. However, what is critical, especially in the context of the demands being placed on evolutionary physiologists by conservation biologists and ecologists, is the identification of those cases where understanding is required for prediction. It is here that insect evolutionary physiology faces its greatest challenges.
Acknowledgments
We thank Stephen Simpson for inviting us to write this review, Jacques Deere for providing us with the BAH figures, Elrike Marais, Ken Storey, Anastassia Makarieva, Brent Sinclair, Elliot Krafsur, and Emilie Gray for discussion of several of the issues raised here, Anel Garthwaite for assistance, and Elrike Marais and Stephen Simpson for commenting on a previous draft of the manuscript. This work was supported by the DST-NRF Centre of Excellence for Invasion Biology and by National Institutes of Health Grant AI-52456 to E.S. Krafsur.
References
- Addo-Bediako A, Chown SL, Gaston KJ. Thermal tolerance, climatic variability and latitude. Proc R Soc Lond B. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addo-Bediako A, Chown SL, Gaston KJ. Revisiting water loss in insects: a large scale view. J Insect Physiol. 2001;47:1377–1388. doi: 10.1016/s0022-1910(01)00128-7. [DOI] [PubMed] [Google Scholar]
- Addo-Bediako A, Chown SL, Gaston KJ. Metabolic cold adaptation in insects: a large-scale perspective. Funct Ecol. 2002;16:332–338. [Google Scholar]
- Agrawal AA. Phenotypic plasticity in the interaction and evolution of species. Science. 2001;294:321–326. doi: 10.1126/science.1060701. [DOI] [PubMed] [Google Scholar]
- Alleaume-Benharira M, Pen IR, Ronce O. Geographical patterns of adaptation within a species’ range: interactions between drift and gene flow. J Evol Biol. 2006;19:203–215. doi: 10.1111/j.1420-9101.2005.00976.x. [DOI] [PubMed] [Google Scholar]
- Allen AP, Brown JH, Gillooly JF. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science. 2002;297:1545–1548. doi: 10.1126/science.1072380. [DOI] [PubMed] [Google Scholar]
- Alonso-Mejía A, Arellano-Guillermo A, Brower LP. Influence of temperature, surface body moisture and height aboveground on survival of monarch butterflies overwintering in Mexico. Biotropica. 1992;24:415–419. [Google Scholar]
- Alonso-Mejía A, Rendon-Salinas E, Montesinos-Patiño E, Brower LP. Use of lipid reserves by monarch butterflies overwintering in Mexico: implications for conservation. Ecol Appl. 1997;7:934–947. [Google Scholar]
- Alpert P. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? J Exp Biol. 2006;209:1575–1584. doi: 10.1242/jeb.02179. [DOI] [PubMed] [Google Scholar]
- Altner H, Loftus R. Ultrastructure and function of insect thermo- and hygro-receptors. Annu Rev Entomol. 1985;30:273–295. [Google Scholar]
- Altner H, Prillinger L. Ultrastructure of invertebrate chemo-, thermo- and hygro-receptors and its functional significance. Int Rev Cytol. 1980;67:69–139. [Google Scholar]
- Anderson AR, Hoffmann AA, McKechnie SW. Response to selection for rapid chill-coma recovery in Drosophila melanogaster: physiology and life history traits. Gen Res. 2005;85:15–22. doi: 10.1017/s0016672304007281. [DOI] [PubMed] [Google Scholar]
- Anderson JB, Brower LP. Freeze-protection of overwintering monarch butterflies in Mexico: critical role of the forest as a blanket and an umbrella. Ecol Entomol. 1996;21:107–116. [Google Scholar]
- Andrewartha HG, Birch LC. The Distribution and Abundance of Animals. Chicago: University of Chicago Press; 1954. [Google Scholar]
- Angilletta MJ, Bennett AF, Guderley H, Navas CA, Seebacher F, Wilson RS. Coadaptation: a unifying principle in evolutionary thermal biology. Physiol Biochem Zool. 2006;79:282–294. doi: 10.1086/499990. [DOI] [PubMed] [Google Scholar]
- Angilletta MJ, Niewiarowski PH, Navas CA. The evolution of thermal physiology in ectotherms. J Thermal Biol. 2002;27:249–268. [Google Scholar]
- Angilletta MJ, Wilson RS, Navas CA, James RS. Tradeoffs and the evolution of thermal reaction norms. Trends Ecol Evol. 2003;18:234–240. [Google Scholar]
- Arispe N, Doh M, De Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp90. Cell Stress Chaperon. 2002;7:330–338. doi: 10.1379/1466-1268(2002)007<0330:lidtca>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby PD. Conservation of mass-specific metabolic rate among high- and low-elevation populations of the acridid grasshopper Xanthippus corallipes. Physiol Zool. 1997;70:701–711. doi: 10.1086/515877. [DOI] [PubMed] [Google Scholar]
- Ayrinhac A, Debat V, Gibert P, Kister AG, Legout H, Moreteau B, Vergilino R, David JR. Cold adaptation in geographical populations of Drosophila melanogaster: phenotypic plasticity is more important than genetic variability. Funct Ecol. 2004;18:700–706. [Google Scholar]
- Bale JS. Insect cold hardiness: freezing and supercooling – an ecophysiological perspective. J Insect Physiol. 1987;33:899–908. [Google Scholar]
- Bale JS. Classes of insect cold hardiness. Funct Ecol. 1993;7:751–753. [Google Scholar]
- Bale JS. Insects and low temperatures: from molecular biology to distributions and abundance. Philos Trans R Soc Lond B. 2002;357:849–861. doi: 10.1098/rstb.2002.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol. 2002;8:1–16. [Google Scholar]
- Bale JS, Worland MR, Block W. Effects of summer frost exposures on the cold tolerance strategy of a sub-Antarctic beetle. J Insect Physiol. 2001;47:1161–1167. doi: 10.1016/s0022-1910(01)00097-x. [DOI] [PubMed] [Google Scholar]
- Bayley M, Holmstrup M. Water vapour absorption in arthropods by accumulation of myoinositol and glucose. Science. 1999;285:1909–1911. doi: 10.1126/science.285.5435.1909. [DOI] [PubMed] [Google Scholar]
- Bayley M, Petersen SO, Knigge T, Köhler HR, Holmstrup M. Drought acclimation confers cold tolerance in the soil collembolan Folsomia candida. J Insect Physiol. 2001;47:1197–1204. doi: 10.1016/s0022-1910(01)00104-4. [DOI] [PubMed] [Google Scholar]
- Behrensmeyer AK, Damuth JD, DiMichele WA, Potts R, Sues H-D, Wing SL. Evolutionary Paleo-ecology of Terrestrial Plants and Animals. Chicago: University of Chicago Press; 1992. Terrestrial Ecosystems through Time. [Google Scholar]
- Beier P, Van Drielen M, Kankam BO. Avifaunal collapse in West African forest fragments. Conserv Biol. 2002;16:1097–1111. [Google Scholar]
- Bennett AF, Lenski RE. Evolutionary adaptation to temperature. VI Phenotypic acclimation and its evolution in Escherichia coli. Evolution. 1997;51:36–44. doi: 10.1111/j.1558-5646.1997.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Bennett VA, Sformo T, Walters K, Toien O, Jeannet K, Hochstrasser R, Pan Q, Serianni AS, Bernes BM, Duman JG. Comparative overwintering physiology of Alaska and Indiana populations of the beetle Cucujus clavipes (Fabricius): roles of antifreeze proteins, polyols, dehydration and diapause. J Exp Biol. 2005;208:4467–4477. doi: 10.1242/jeb.01892. [DOI] [PubMed] [Google Scholar]
- Berrigan DM, Scheiner SM. Phenotypic plasticity. Functional and conceptual approaches. In: DeWitt TJ, Scheiner SM, editors. Modelling the Evolution of Phenotypic Plasticity. Oxford: Oxford University Press; 2004. pp. 82–97. [Google Scholar]
- Bestelmeyer BT. The trade-off between thermal tolerance and behavioural dominance in a subtropical South American ant community. J Anim Ecol. 2000;69:998–1009. [Google Scholar]
- Bettencourt BR, Kim I, Hoffmann AA, Feder ME. Response to natural and laboratory selection at the Drosophila HSP70 genes. Evolution. 2002;56:1796–1801. doi: 10.1111/j.0014-3820.2002.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ. Avian extinction and mammalian introductions on oceanic islands. Science. 2004;305:1955–1958. doi: 10.1126/science.1101617. [DOI] [PubMed] [Google Scholar]
- Blancafort X, Gómez C. Consequences of the Argentine ant, Line-pithema humile (Mayr), invasion on pollination of Euphorbia characias (L.) (Euphorbiaceae) Acta Oecol. 2005;28:49–55. [Google Scholar]
- Bland RG, Slaney DP, Weinstein P. Antennal sensilla on cave species of Australian Paratemnopteryx cockroaches (Blattaria: Blattellidae) Int J Ins Morph Embryol. 1998;27:83–93. [Google Scholar]
- Block W. Cold tolerance of insects and other arthropods. Philos Trans R Soc Lond B. 1990;326:613–633. [Google Scholar]
- Block W. To freeze or not to freeze? Invertebrate survival of sub-zero temperatures. Funct Ecol. 1991;5:284–290. [Google Scholar]
- Block W. Cold or drought – the lesser of two evils for terrestrial arthropods? Eur J Entomol. 1996;93:325–339. [Google Scholar]
- Blows MW, Hoffmann AA. A reassessment of genetic limits to evolutionary change. Ecology. 2005;86:1371–1384. [Google Scholar]
- Blum MS. Fundamentals of Insect Physiology. New York: Wiley; 1985. [Google Scholar]
- Bonan GB. Frost followed the plow: impacts of deforestation on the climate of the United States. Ecol Appl. 1999;9:1305–1315. [Google Scholar]
- Bonan GB. Concepts and Applications. Cambridge: Cambridge University Press; 2002. Ecological Climatology. [Google Scholar]
- Bowler K. Acclimation, heat shock and hardening. J Thermal Biol. 2005;30:125–130. [Google Scholar]
- Branscome DD, Koehler PG, Oi FM. Influence of carbon dioxide gas on German cockroach (Dictyoptera: Blattellidae) knockdown, recovery, movement and feeding. Physiol Entomol. 2005;30:144–150. [Google Scholar]
- Briers RA, Gee JHR, Geoghegan R. Effects of the North Atlantic oscillation on growth and phenology of stream insects. Ecography. 2004;27:811–817. [Google Scholar]
- Brooks TM, Pimm SL, Kapos V, Ravilious C. Threat from deforestation to montane and lowland birds and mammals in insular South-east Asia. J Anim Ecol. 1999;68:1061–1078. [Google Scholar]
- Brooks TM, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Rylands AB, Konstant WR, Flick P, Pilgrim J, Oldfield S, Magin G, Hilton-Taylor C. Habitat loss and extinction in the hotspots of biodiversity. Conserv Biol. 2002;16:909–923. [Google Scholar]
- Brown CL, Bale JS, Walters KFA. Freezing induces a loss of freeze tolerance in an overwintering insect. Proc R Soc Lond B. 2004;271:1507–1511. doi: 10.1098/rspb.2004.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Gillooly JF, Allen AP, Savage V, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- Burton V, Mitchell HK, Young P, Petersen NS. Heat shock protection against cold stress of Drosophila melanogaster. Mol Cell Biol. 1988;8:3550–3552. doi: 10.1128/mcb.8.8.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet C, Ferriere R, Metz JAJ, van Baalen M. The evolution of dispersal under demographic stochasticity. Am Nat. 2003;162:427–441. doi: 10.1086/378213. [DOI] [PubMed] [Google Scholar]
- Carter RN, Prince SD. Epidemic models used to explain biogeographical distribution limits. Nature. 1981;293:644–645. [Google Scholar]
- Case TJ, Taper ML. Interspecific competition, environmental gradients, gene flow, and the coevolution of species’ borders. Am Nat. 2000;155:583–605. doi: 10.1086/303351. [DOI] [PubMed] [Google Scholar]
- Casti JL. What Science can Know about the Future. London: Abacus; 1991. Searching for Certainty. [Google Scholar]
- Catala SS, Maida DM, Caro-Riaño H, Jaramillo N, Moreno J. Changes associated with laboratory rearing in antennal sensilla patterns of Triatoma infestans, Rhodnius prolixus, and Rhodnius pallescens (Hemiptera, Reduviidae, Triatominae) Mem Inst Oswaldo Cruz. 2004;99:25–30. doi: 10.1590/s0074-02762004000100005. [DOI] [PubMed] [Google Scholar]
- Cavicchi S, Guerra D, La Torre V, Huey RB. Chromosonal analysis of heat-shock tolerance in Drosophila melanogaster evolving at different temperatures in the laboratory. Evolution. 1995;49:676–684. doi: 10.1111/j.1558-5646.1995.tb02304.x. [DOI] [PubMed] [Google Scholar]
- Cerdá X, Retana J, Manzaneda A. The role of competition by dominants and temperature in the foraging of subordinate species in Mediterranean ant communities. Oecologia. 1998;117:404–412. doi: 10.1007/s004420050674. [DOI] [PubMed] [Google Scholar]
- Chakir M, Chafik A, Moreteau B, Gibert P, David JR. Male sterility thresholds in Drosophila: D. simulans appears more cold-adapted than its sibling D. melanogaster. Genetica. 2005;114:195–205. doi: 10.1023/a:1015154329762. [DOI] [PubMed] [Google Scholar]
- Chamberlin ME, Strange K. Anisoosmotic cell volume regulation: a comparative view. Am J Physiol. 1989;257:C159–C173. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- Chapuis JL, Boussès P, Barnaud G. Alien mammals, impact and management in the French subantarctic islands. Biol Conserv. 1994;67:97–104. [Google Scholar]
- Chase JM. Abiotic controls of trophic cascades in a simple grassland food chain. Oikos. 1996;77:495–506. [Google Scholar]
- Chen B, Kayukawa T, Jiang H, Monteiro A, Hoshizaki S, Ishikawa Y. DaTrypsin, a novel clip-domain serine protease gene up-regulated during winter and summer diapauses of the onion maggot, Delia antiqua. Gene. 2005b;347:115–123. doi: 10.1016/j.gene.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Chen B, Kayukawa T, Monteiro A, Ishikawa Y. The expression of the HSP90 gene in response to winter and summer diapauses and thermal-stress in the onion maggot, Delia antiqua. Ins Mol Biol. 2005a;14:697–702. doi: 10.1111/j.1365-2583.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- Chen CP, Denlinger DL. Reduction of cold injury in flies using an intermittent pulse of high temperature. Cryobiology. 1992;29:138–143. [Google Scholar]
- Chen CP, Denlinger DL, Lee RE. Cold-shock injury and rapid cold hardening in the flesh fly Sarcophaga crassipalpis. Physiol Zool. 1987;60:297–304. [Google Scholar]
- Chen CP, Lee RE, Denlinger DL. A comparison of the responses of tropical and temperate flies (Diptera: Sarcophagidae) to cold and heat stress. J Comp Physiol B. 1990;160:543–547. [Google Scholar]
- Chen CP, Lee RE, Denlinger DL. Cold shock and heat shock: a comparison of the protection generated by brief pretreatment at less severe temperatures. Physiol Entomol. 1991;16:19–26. [Google Scholar]
- Chen CP, Walker VK. Cold-shock and chilling tolerance in Drosophila. J Insect Physiol. 1994;40:661–669. [Google Scholar]
- Chown SL. Desiccation resistance in six sub-Antarctic weevils (Coleoptera: Curculionidae): humidity as an abiotic factor influencing assemblage structure. Funct Ecol. 1993;7:318–325. [Google Scholar]
- Chown SL. Physiological variation in insects: hierarchical levels and implications. J Insect Physiol. 2001;47:649–660. doi: 10.1016/s0022-1910(00)00163-3. [DOI] [PubMed] [Google Scholar]
- Chown SL, Addo-Bediako A, Gaston KJ. Physiological diversity: listening to the large-scale signal. Funct Ecol. 2003;17:562–572. [Google Scholar]
- Chown SL, Block W. Comparative nutritional ecology of grass-feeding in a sub-Antarctic beetle: the impact of introduced species on Hydromedion sparsutum from South Georgia. Oecologia. 1997;111:216–224. doi: 10.1007/s004420050228. [DOI] [PubMed] [Google Scholar]
- Chown SL, Gaston KJ. Exploring links between physiology and ecology at macro-scales: the role of respiratory metabolism in insects. Biol Rev. 1999;74:87–120. [Google Scholar]
- Chown SL, Gaston KJ. Areas, cradles and museums: the latitudinal gradient in species richness. Trends Ecol Evol. 2000;15:311–315. doi: 10.1016/s0169-5347(00)01910-8. [DOI] [PubMed] [Google Scholar]
- Chown SL, Gaston KJ, Robinson D. Macrophysiology: large-scale patterns in physiological traits and their ecological implications. Funct Ecol. 2004b;18:159–167. [Google Scholar]
- Chown SL, Hull B, Gaston KJ. Human impacts, energy availability, and invasion across Southern Ocean Islands. Global Ecol Biogeogr. 2005;14:521–528. [Google Scholar]
- Chown SL, Klok CJ. Water balance characteristics respond to changes in body size in sub-Antarctic weevils. Physiol Biochem Zool. 2003;76:634–643. doi: 10.1086/376919. [DOI] [PubMed] [Google Scholar]
- Chown SL, Klok CJ, McGeoch MA. Weather to go out: activity of Bothrometopus brevis (Curculionidae) at Heard Island. Polar Biol. 2004c;27:217–221. [Google Scholar]
- Chown SL, Nicolson SW. Mechanisms and Patterns. Oxford: Oxford University Press; 2004. Insect Physiological Ecology. [Google Scholar]
- Chown SL, Sinclair BJ, Leinaas HP, Gaston KJ. Hemispheric asymmetries in biodiversity – a serious matter for ecology. PLoS Biol. 2004a;2(e406):1701–1707. doi: 10.1371/journal.pbio.0020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown SL, Smith VR. Climate change and the short-term impact of feral house mice at the sub-Antarctic Prince Edward Islands. Oecologia. 1993;96:508–516. doi: 10.1007/BF00320508. [DOI] [PubMed] [Google Scholar]
- Chown SL, Storey KB. Linking molecular physiology to ecological realities. Physiol Biochem Zool. 2006;79:314–323. doi: 10.1086/499989. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clarke A. Costs and consequences of evolutionary temperature adaptation. Trends Ecol Evol. 2003;18:573–581. [Google Scholar]
- Clarke A. Is there a universal temperature dependence of metabolism? Funct Ecol. 2004;18:252–256. [Google Scholar]
- Clarke A. Temperature and the metabolic theory of ecology. Funct Ecol. 2006;20:405–412. [Google Scholar]
- Clarke A, Fraser KPP. Why does metabolism scale with temperature? Funct Ecol. 2004;18:243–251. [Google Scholar]
- Cohen AE, Gonzalez A, Lawton JH, Petchey OL, Wildman D, Cohen JE. A novel experimental apparatus to study the impact of white noise and 1/f noise on animal populations. Proc R Soc Lond B. 1998;265:11–15. doi: 10.1098/rspb.1998.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge JE, Hoffmann AA, Mckechnie SW. Altitudinal patterns for latitudinally varying traits and polymorphic markers in Drosophila melanogaster from eastern Australia. J Evol Biol. 2006;19:473–482. doi: 10.1111/j.1420-9101.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- Collins SD, Allenspach AL, Lee RE. Ultrastructural effects of lethal freezing on brain, muscle, and Malpighian tubule from freeze-tolerant larvae of the gall fly, Eurosta solidaginis. J Insect Physiol. 1997;43:39–45. [PubMed] [Google Scholar]
- Conrad KF, Woiwood IP, Perry JN. East Atlantic teleconnection pattern and the decline of a common arctiid moth. Global Change Biol. 2003;9:125–130. [Google Scholar]
- Cossins AR, Bowler K. Temperature Biology of Animals. London: Chapman & Hall; 1987. [Google Scholar]
- Coulson SJ, Bale JS. Supercooling and survival of the beech leaf mining weevil Rhynchaenus fagi L. (Coleoptera: Curculionidae) J Insect Physiol. 1996;42:617–623. [Google Scholar]
- Coulson SJ, Leinaas HP, Ims RA, Søvik G. Experimental manipulation of the winter surface ice layer: the effects on a high Arctic soil microarthropod community. Ecography. 2000;23:299–306. [Google Scholar]
- Coviella CE, Trumble JT. Effects of elevated atmospheric carbon dioxide on insect-plant interactions. Conserv Biol. 1999;13:700–712. [Google Scholar]
- Crooks KR, Soulé ME. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999;400:563–565. [Google Scholar]
- Crozier L. Winter warming facilitates range expansion: cold tolerance of the butterfly Atalopedes campestris. Oecologia. 2003;135:648–656. doi: 10.1007/s00442-003-1219-2. [DOI] [PubMed] [Google Scholar]
- Crozier L. Warmer winters drive butterfly range expansion by increasing survivorship. Ecology. 2004;85:231–241. [Google Scholar]
- Cunningham SA. Depressed pollination in habitat fragments causes low fruit set. Proc R Soc Lond B. 2000;267:1149–1152. doi: 10.1098/rspb.2000.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks HV. The wider integration of studies on insect cold hardiness. Eur J Entomol. 1996;93:383–403. [Google Scholar]
- Danks HV. Life cycles in polar arthropods–flexible or programmed? Eur J Entomol. 1999;96:83–102. [Google Scholar]
- Danks HV. Dehydration in dormant insects. J Insect Physiol. 2000;46:837–852. doi: 10.1016/s0022-1910(99)00204-8. [DOI] [PubMed] [Google Scholar]
- Danks HV. Studying insect photoperiodism and rhythmicity: components, approaches and lessons. Eur J Entomol. 2003;100:209–221. [Google Scholar]
- David JR, Gibert P, Gravot E, Pétavy G, Morin JP, Karan D, Moreteau B. Phenotypic plasticity and developmental temperature in Drosophila: analysis and significance of reaction norms of morphometrical traits. J Thermal Biol. 1997;22:441–451. [Google Scholar]
- David JR, Gibert P, Moreteau B, Gilchrist GW, Huey RB. The fly that came in from the cold: geographic variation of recovery time from low-temperature exposure in Drosophila subobscura. Funct Ecol. 2003;17:425–430. [Google Scholar]
- Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. [DOI] [PubMed] [Google Scholar]
- Davis MB, Shaw RG. Range shifts and adaptive responses to quaternary climate change. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. [DOI] [PubMed] [Google Scholar]
- Deere JA, Chown SL. Testing the beneficial acclimation hypothesis and its alternatives for locomotor performance. American Naturalist. 2006;168:630–644. doi: 10.1086/508026. [DOI] [PubMed] [Google Scholar]
- Denlinger DL. Relationship between cold hardiness and diapause. In: Lee RE, Denlinger DL, editors. Insects at Low Temperature. New York: Chapman & Hall; 1991. pp. 174–198. [Google Scholar]
- Denlinger DL. Regulation of diapause. Annu Rev Entomol. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Giebultowicz JM, Saunders DS, editors. Insect Timing: Circadian Rhythmicity to Seasonality. Amsterdam: Elsevier; 2001. [Google Scholar]
- Denlinger DL, Joplin KH, Chen C-P, Lee RE. Cold shock and heat shock. In: Lee RE, Denlinger DL, editors. Insects at Low Temperature. New York: Chapman & Hall; 1991. pp. 131–148. [Google Scholar]
- Denlinger DL, Lee RE. Physiology of cold sensitivity. In: Hallman GJ, Denlinger DL, editors. Temperature Sensitivity in Insects and Application in Integrated Pest Management. Boulder, CO: Westview Press; 1998. pp. 55–95. [Google Scholar]
- Denlinger DL, Lee RE, Yocum GD, Kukal O. Role of chilling in the acquisition of cold tolerance and the capacitation to express stress proteins in diapausing pharate larvae of the gypsy moth, Lymantria dispar. Arch Ins Biochem Physiol. 1992;21:271–280. [Google Scholar]
- Denlinger DL, Willis JH, Fraenkel G. Rates and cycles of oxygen consumption during pupal diapause in Sarcophaga flesh flies. J Insect Physiol. 1972;18:871–882. doi: 10.1016/0022-1910(72)90026-1. [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Yocum GD. Physiology of heat sensitivity. In: Hallman GJ, Denlinger DL, editors. Temperature Sensitivity in Insects and Application in Integrated Pest Management. Boulder, CO: Westview Press; 1998. pp. 7–53. [Google Scholar]
- Dennill GB, Donnelly D, Chown SL. Expansion of host plant range of a biocontrol agent Trichilogaster acaciaelongifoliae released against the weed Acacia longifolia. Agric Ecosyst Environ. 1993;43:1–10. [Google Scholar]
- Dennill GB, Gordon AJ. Climate-related differences in the efficacy of the Australian gall wasp (Hymenoptera: Pteromalidae) released for the control of Acacia longifolia in South Africa. Environ Entomol. 1990;19:130–136. [Google Scholar]
- DeWitt TJ, Scheiner SM, editors. Functional and Conceptual Approaches. Oxford: Oxford University Press; 2004. Phenotypic Plasticity. [Google Scholar]
- Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ. Are invasive species the drivers of ecological change? Trends Ecol Evol. 2005;20:470–474. doi: 10.1016/j.tree.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Diernfellner ACR, Schafmeier T, Merrow MW, Brunner M. Molecular mechanisms of temperature sensing by the circadian clock of Neurospora crassa. Genes Dev. 2005;19:1968–1973. doi: 10.1101/gad.345905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J, Nänni I, Zachariades C, Kemper J. Effects of habitat fragmentation on pollinator diversity and plant reproductive success in renos-terveld shrublands of South Africa. Conserv Biol. 2002;16:1267–1276. [Google Scholar]
- Dreisig H. Daily activity, thermoregulation and water loss in the tiger beetle Cicindela hybrida. Oecologia. 1980;44:376–389. doi: 10.1007/BF00545242. [DOI] [PubMed] [Google Scholar]
- Duman JG. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu Rev Physiol. 2001;63:327–357. doi: 10.1146/annurev.physiol.63.1.327. [DOI] [PubMed] [Google Scholar]
- Duncan RP, Blackburn TM, Sol D. The ecology of bird introductions. Annu Rev Ecol Evol Syst. 2003;34:71–98. [Google Scholar]
- Dunn RR. Modern insect extinctions, the neglected majority. Conserv Biol. 2005;19:1030–1036. [Google Scholar]
- Dybdahl MF, Kane SL. Adaptation vs. phenotypic plasticity in the success of a clonal invader. Ecology. 2005;86:1592–1601. [Google Scholar]
- Edney EB. Water Balance in Land Arthropods. Berlin: Springer; 1977. [Google Scholar]
- Ehn R, Tichy H. Hygro- and thermoreceptive tarsal organ in the spider Cupiennius salei. J Comp Physiol A. 1994;174:345–350. [Google Scholar]
- Erasmus BFN, van Jaarsveld AS, Chown SL, Kshatriya M, Wessels KJ. Vulnerability of South African animal taxa to climate change. Global Change Biol. 2002;8:679–693. [Google Scholar]
- Ernsting G. Observations on life cycle and feeding ecology of two recently introduced predatory beetle species at South Georgia, sub-Antarctic. Polar Biol. 1993;13:423–428. [Google Scholar]
- Ernsting G, Brandjes GJ, Block W, Isaaks JA. Life-history consequences of predation for a subantarctic beetle: evaluating the contribution of direct and indirect effects. J Anim Ecol. 1999;68:741–752. [Google Scholar]
- Evans KL, Warren PH, Gaston KJ. Species–energy relationships at the macroecological scale: a review of the mechanisms. Biol Rev. 2005;79:1–25. doi: 10.1017/s1464793104006517. [DOI] [PubMed] [Google Scholar]
- Fagan WF, Siemann E, Mitter C, Denno RF, Huberty AF, Woods HA, Elser JJ. Nitrogen in insects: implications for trophic complexity and species diversification. Am Nat. 2002;160:784–802. doi: 10.1086/343879. [DOI] [PubMed] [Google Scholar]
- Feder ME. The analysis of physiological diversity: the prospects for pattern documentation and general questions in ecological physiology. In: Feder ME, Bennett AF, Burggren WW, Huey RB, editors. New Directions in Ecological Physiology. Cambridge: Cambridge University Press; 1987. pp. 38–75. [Google Scholar]
- Feder ME. Ecological and evolutionary physiology of stress proteins and the stress response: the Drosophila melanogaster model. Animals and Temperature. In: Johnston IA, Bennett AF, editors. Phenotypic and Evolutionary Adaptation. Cambridge: Cambridge University Press; 1996. pp. 79–102. [Google Scholar]
- Feder ME. Engineering candidate genes in studies of adaptation: the heat-shock protein Hsp70 in Drosophila melanogaster. Am Nat Suppl. 1999;154:s55–s66. doi: 10.1086/303283. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Feder ME, Krebs RA. Natural and genetic engineering of the heat-shock protein Hsp70 in Drosophila melanogaster: consequences for thermotolerance. Am Zool. 1998;38:503–517. [Google Scholar]
- Feder ME, Mitchell-Olds T. Evolutionary and ecological functional genomics. Nat Rev Genet. 2003;4:649–655. doi: 10.1038/nrg1128. [DOI] [PubMed] [Google Scholar]
- Ferguson SH, Messier F. Ecological implications of a latitudinal gradient in inter-annual climatic varibility: a test using fractal and chaos theories. Ecography. 1996;19:382–392. [Google Scholar]
- Fischer K, Bauerfiend SS, Fielder K. Temperature-mediated plasticity in egg and body size in egg-size selected lines of a butterfly. J Thermal Biol. 2006;31:347–354. [Google Scholar]
- Fischer K, Eenhorn E, Bot ANM, Brakefield PM, Zwaan BJ. Cooler butterflies lay larger eggs: developmental plasticity versus acclimation. Proc R Soc Lond B. 2003;270:2051–2056. doi: 10.1098/rspb.2003.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde SE, Thompson JN, Bohannan BJM. Adaptation varies through space and time in a coevolving host–parasitoid interaction. Nature. 2004;431:841–844. doi: 10.1038/nature02906. [DOI] [PubMed] [Google Scholar]
- Forsman A, Ringblom K, Civantos E, Ahnesjo J. Coevolution of color pattern and thermoregulatory behaviour in polymorphic pygmy grasshoppers Tetrix undulata. Evolution. 2002;56:349–360. doi: 10.1111/j.0014-3820.2002.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Frazier MR, Woods HA, Harrison JF. Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol Biochem Zool. 2001;74:641–650. doi: 10.1086/322172. [DOI] [PubMed] [Google Scholar]
- Frydenberg J, Hoffmann AA, Loeschcke V. DNA sequence variation and latitudinal associations in hsp23, hsp26 and hsp27 from natural populations of Drosophila melanogaster. Mol Ecol. 2003;12:2025–2032. doi: 10.1046/j.1365-294x.2002.01882.x. [DOI] [PubMed] [Google Scholar]
- Gagnon AS, Smoter-Tomic KE, Bush ABG. The El Niño southern oscillation and malaria epidemics in South America. Intern J Biometeorol. 2002;46:81–99. doi: 10.1007/s00484-001-0119-6. [DOI] [PubMed] [Google Scholar]
- Gaines SD, Denny MW. The largest, smallest, highest, lowest, longest, and shortest: extremes in ecology. Ecology. 1993;74:1677–1692. [Google Scholar]
- Garland T, Huey RB, Bennett AF. Phylogeny and coadaptation of thermal physiology in lizards – a reanalysis. Evolution. 1991;45:1969–1975. doi: 10.1111/j.1558-5646.1991.tb02703.x. [DOI] [PubMed] [Google Scholar]
- Garland T, Kelly SA. Phenotypic plasticity and experimental evolution. J Exp Biol. 2006;209:2344–2361. doi: 10.1242/jeb.02244. [DOI] [PubMed] [Google Scholar]
- Gaston KJ. The Structure and Dynamics of Geographic Ranges. Oxford: Oxford University Press; 2003. [Google Scholar]
- Gaston KJ, Chown SL. Geographic range size and speciation. In: Magurran AE, May RM, editors. Evolution of Biological Diversity. Oxford: Oxford University Press; 1999. pp. 236–259. [Google Scholar]
- Gaston KJ, Jones AG, Hänel C, Chown SL. Rates of species introduction to a remote oceanic island. Proc R Soc Lond B. 2003;270:1091–1098. doi: 10.1098/rspb.2003.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Wehner R. Heat shock protein synthesis and thermo-tolerance in Cataglyphis, an ant from the Sahara desert. Proc Natl Acad Sci USA. 1995;92:2994–2998. doi: 10.1073/pnas.92.7.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. Are mountain passes higher in the tropics? Janzen’s hypothesis revisited. Integr Comp Biol. 2006;46:5–17. doi: 10.1093/icb/icj003. [DOI] [PubMed] [Google Scholar]
- Gibert P, Huey RB. Chill-coma temperature in Drosophila: effects of developmental temperature, latitude, and phylogeny. Physiol Biochem Zool. 2001;74:429–434. doi: 10.1086/320429. [DOI] [PubMed] [Google Scholar]
- Gibert P, Huey RB, Gilchrist GW. Locomotor performance of Drosophila melanogaster: interactions among developmental and adult temperatures, age, and geography. Evolution. 2001;55:205–209. doi: 10.1111/j.0014-3820.2001.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Gibbs AG. Laboratory selection for the comparative physiologist. J Exp Biol. 1999;202:2709–2718. doi: 10.1242/jeb.202.20.2709. [DOI] [PubMed] [Google Scholar]
- Gibbs AG, Fukuzato F, Matzkin LM. Evolution of water conservation mechanisms in Drosophila. J Exp Biol. 2003;206:1183–1192. doi: 10.1242/jeb.00233. [DOI] [PubMed] [Google Scholar]
- Gilchrist GW. Specialists and generalists in changing environments. I Fitness landscapes of thermal sensitivity. Am Nat. 1995;146:252–270. [Google Scholar]
- Gilchrist GW. A quantitative genetic analysis of thermal sensitivity in the locomotor performance curve of Aphidius ervi. Evolution. 1996;50:1560–1572. doi: 10.1111/j.1558-5646.1996.tb03928.x. [DOI] [PubMed] [Google Scholar]
- Gilchrist GW, Huey RB. Parental and developmental temperature effects on the thermal dependence of fitness in Drosophila melanogaster. Evolution. 2001;55:209–214. doi: 10.1111/j.0014-3820.2001.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Gilchrist GW, Huey RB, Partridge L. Thermal sensitivity of Drosophila melanogaster: evolutionary responses of adults and eggs to laboratory natural selection at different temperatures. Physiol Zool. 1997;70:403–414. doi: 10.1086/515853. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Allen AP, Savage VM, Charnov EL, West GB, Brown JH. Response to Clarke and Fraser: effects of temperature on metabolic rate. Funct Ecol. 2006;20:400–404. [Google Scholar]
- Gillooly JF, Allen AP, West GB, Brown JH. The rate of DNA evolution: effects of body size and temperature on the molecular clock. Proc Natl Acad Sci USA. 2005;102:140–145. doi: 10.1073/pnas.0407735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- Godde J, Haug T. Analysis of the electrical responses of antennal thermo- and hygroreceptors of Antherea (Saturniidae, Lepidoptera) to thermal, mechanical, and electrical stimuli. J Comp Physiol A. 1990;167:391–401. [Google Scholar]
- Goller F, Esch H. Comparative study of chill-coma temperatures and muscle potentials in insect flight muscles. J Exp Biol. 1990;150:221–231. [Google Scholar]
- Good DS. Evolution of behaviours in Drosophila melanogaster in high temperatures: genetic and environmental effects. J Insect Physiol. 1993;39:537–544. [Google Scholar]
- Gordon DM, Moses L, Falkovitz-Halpern M, Wong EH. Effect of weather on infestation of buildings by the invasive Argentine ant, Linepithema humile (Hymenoptera: Formicidae) Am Midl Nat. 2001;146:321–328. [Google Scholar]
- Goto SG, Kimura MT. Heat- and cold-shock responses and temperature adaptations in subtropical and temperate species of Drosophila. J Insect Physiol. 1998;44:1233–1239. doi: 10.1016/s0022-1910(98)00101-2. [DOI] [PubMed] [Google Scholar]
- Goto SG, Kimura MT. Heat-shock-responsive genes are not involved in the adult diapause of Drosophila triauraria. Gene. 2004;326:117–122. doi: 10.1016/j.gene.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Goto SG, Yoshida KM, Kimura MT. Accumulation of Hsp70 mRNA under environmental stresses in diapausing and non-diapausing adults of Drosophila triauraria. J Insect Physiol. 1998;44:1009–1015. doi: 10.1016/s0022-1910(97)00143-1. [DOI] [PubMed] [Google Scholar]
- Goulson D. Effects of introduced bees on native ecosystems. Annu Rev Ecol Evol Syst. 2003;34:1–26. [Google Scholar]
- Grenfell BT, Bjørnstad ON, Keppey J. Travelling waves and spatial hierarchies in measles epidemics. Nature. 2001;414:716–723. doi: 10.1038/414716a. [DOI] [PubMed] [Google Scholar]
- Gruntenko NE, Bownes M, Terashima J, Sukhanova MZ, Raushenbach IY. Heat stress affects oogenesis differently in wild-type Drosophila virilis and a mutant with altered juvenile hormone and 20-hydroxyecdysone levels. Ins Mol Biol. 2003a;12:393–404. doi: 10.1046/j.1365-2583.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Gruntenko NE, Chentsova NA, Andreenkova EV, Bownes M, Segal D, Adonyeva NV, Rauschenbach IY. Stress response in a juvenile hormone-deficient Drosophila melanogaster mutant apterous (56f) Ins Mol Biol. 2003b;12:353–363. doi: 10.1046/j.1365-2583.2003.00419.x. [DOI] [PubMed] [Google Scholar]
- Gruntenko NE, Wilson TG, Monastirioti M, Rauschenbach IY. Stress-reactivity and juvenile hormone degradation in Drosophila melanogaster strains having stress-related mutations. Ins Bioch Mol Biol. 2000;30:775–783. doi: 10.1016/s0965-1748(00)00049-7. [DOI] [PubMed] [Google Scholar]
- Guarneri AA, Lazzari C, Xavier AAP, Diotaiuti L, Lorenzo MG. The effect of temperature on the behaviour and development of Triatoma brasiliensis. Physiol Entomol. 2003;28:185–191. [Google Scholar]
- Gutschick VP, BassiriRad H. Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytol. 2003;160:21–42. doi: 10.1046/j.1469-8137.2003.00866.x. [DOI] [PubMed] [Google Scholar]
- Hadley NF. Water Relations of Terrestrial Arthropods. San Diego, CA: Academic Press; 1994. [Google Scholar]
- Hallett TB, Coulson T, Pilkington JG, Clutton-Brock TH, Pemberton JM, Grenfell BT. Why large-scale climate indices seem to predict ecological processes better than local weather. Nature. 2004;430:71–75. doi: 10.1038/nature02708. [DOI] [PubMed] [Google Scholar]
- Halley JM. Ecology, evolution and 1/f-noise. Trends Ecol Evol. 1996;11:33–37. doi: 10.1016/0169-5347(96)81067-6. [DOI] [PubMed] [Google Scholar]
- Hallman GJ, Denlinger DL. Temperature Sensitivity in Insects and Application in Itegrated Pest Management. Boulder, CO: Westview Press; 1998. [Google Scholar]
- Hammack L. Sex-pheromone communication in the screwworm, Cochliomyia hominivorax – ontogenetic and strain effects. J Chem Ecol. 1991;17:2143–2154. doi: 10.1007/BF00987997. [DOI] [PubMed] [Google Scholar]
- Hanc Z, Nedved O. Chill injury at alternating temperatures in Orchesella cincta (Collembola: Entomobryidae) and Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae) Eur J Entomol. 1999;96:165–168. [Google Scholar]
- Hannah L, Midgley GF, Lovejoy T, Bond WJ, Bush M, Lovett JC, Scott D, Woodward FI. Conservation of biodiversity in a changing climate. Conserv Biol. 2002;16:264–268. doi: 10.1046/j.1523-1739.2002.00465.x. [DOI] [PubMed] [Google Scholar]
- Hansen DM, Olesen JM, Jones CG. Trees, birds and bees in Mauritius: exploitative competition between introduced honeybees and endemic nectarivorous birds? J Biogeogr. 2002;29:721–734. [Google Scholar]
- Hansen LL, Ramløv H, Westh P. Metabolic activity and water vapour absorption in the mealworm Tenebrio molitor L. (Coleoptera, Ten-ebrionidae): real-time measurements by two-channel microcalorimetry. J Exp Biol. 2004;207:545–552. doi: 10.1242/jeb.00762. [DOI] [PubMed] [Google Scholar]
- Hansen LL, Westh P, Wright JC, Ramløv H. Metabolic changes associated with active water vapour absorption in the mealworm Tenebrio molitor L. (Coleoptera, Tenebrionidae): a microcalorimetric study. J Insect Physiol. 2006;52:291. doi: 10.1016/j.jinsphys.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Harding KC, McNamara JM. A unifying framework for meta-population dynamics. Am Nat. 2002;160:173–185. doi: 10.1086/341014. [DOI] [PubMed] [Google Scholar]
- Hargrove JW. Tsetse population dynamics. In: Maudlin I, Holmes PH, Miles MA, editors. The Trypanosomiases. Wallingford, CT: CABI Publishing; 2004. pp. 113–138. [Google Scholar]
- Harrison JF, Lighton JRB. Oxygen-sensitive flight metabolism in the dragonfly Erythemis simplicicollis. J Exp Biol. 1998;201:1739–1744. doi: 10.1242/jeb.201.11.1739. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Hoffmann AA. Laboratory selection experiments using Drosophila: what do they really tell us? Trends Ecol Evol. 2000;15:32–36. doi: 10.1016/s0169-5347(99)01756-5. [DOI] [PubMed] [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Hawkins BA, Field R, Cornell HV, Currie DJ, Guegan JF, Kaufman DM, Kerr JT, Mittelbach GG, Oberdorf T, O’Brien EM, Porter EE, Turner JRG. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- Hayward SAL, Bale JS, Worland MR, Convey P. Influence of temperature on the hygropreference of the Collembolan, Cryptopygus antarcticus, and the mite, Alaskozetes antarcticus from the maritime Antarctic. J Insect Physiol. 2001;47:11–18. doi: 10.1016/s0022-1910(00)00088-3. [DOI] [PubMed] [Google Scholar]
- Hayward SAL, Pavlides SC, Tammariello SP, Rinehart JP, Denlinger DL. Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J Insect Physiol. 2005;51:631–640. doi: 10.1016/j.jinsphys.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Hayward SAL, Rinehart JP, Denlinger DL. Desiccation and rehydration elicit distinct heat shock protein transcript responses in flesh fly pupae. J Exp Biol. 2004a;207:963–971. doi: 10.1242/jeb.00842. [DOI] [PubMed] [Google Scholar]
- Hayward SAL, Worland MR, Bale JS, Convey P. Temperature and the hygropreference of the Arctic Collembolan Onychiurus arcticus and the mite Lauroppia translamellata. Physiol Entomol. 2000;25:266–272. [Google Scholar]
- Hayward SAL, Worland MR, Convey P, Bale JS. Habitat moisture availability and the local distribution of the Antarctic Collembola Cryptopygus antarcticus and Frisea grisea. Soil Biol Biochem. 2004b;36:927–934. [Google Scholar]
- Hazel W, Smock R, Lively CM. The ecological genetics of conditional strategists. Am Nat. 2004;163:888–900. doi: 10.1086/386313. [DOI] [PubMed] [Google Scholar]
- Helmuth B, Kingsolver JG, Carrington E. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu Rev Physiol. 2005;67:177–201. doi: 10.1146/annurev.physiol.67.040403.105027. [DOI] [PubMed] [Google Scholar]
- Herford GM. Tracheal pulsation in the flea. J Exp Biol. 1938;15:327–338. [Google Scholar]
- Herrera CM. Thermal biology and foraging responses of insect pollinators to the forest floor irradiance mosaic. Oikos. 1997;78:601–611. [Google Scholar]
- Hess E, Loftus R. Warm and cold receptors of two sensilla on the foreleg tarsi of the tropical bont tick Amblyomma variegatum. J Comp Physiol A. 1984;155:187–195. [Google Scholar]
- Hill JK, Hughes CL, Dytham C, Searle JB. Genetic diversity in butterflies: interactive effects of habitat fragmentation and climate-driven range expansion. Biol Lett. 2006;2:152–154. doi: 10.1098/rsbl.2005.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JK, Thomas CD, Huntley B. Climate and habitat availability determine 20th century changes in a butterfly’s range margin. Proc R Soc Lond B. 1999;266:1197–1206. [Google Scholar]
- Hochachka PW, Somero GN. Mechanisms and Processes in Physiological Evolution. New York: Oxford University Press; 2002. Biochemical Adaptation. [Google Scholar]
- Hódar JA, Castro J, Zamora R. Pine processionary caterpillar Thaumetopoea pityocampa as a new threat for relict Mediterranean Scots pine forests under climatic warming. Biol Conserv. 2003;110:123–129. [Google Scholar]
- Hodkinson ID. Metabolic cold adaptation in arthropods: a smaller-scale perspective. Funct Ecol. 2003;17:562–567. [Google Scholar]
- Hodkinson ID, Coulson SJ, Webb NR, Block W, Strathdee AT, Bale JS, Worland MR. Temperature and the biomass of flying midges (Diptera: Chironomidae) in the high Arctic. Oikos. 1996;75:241–248. [Google Scholar]
- Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hoang A, Hill CE, Beerli P. Strength and tempo of directional selection in the wild. Proc Natl Acad Sci USA. 2001;98:9157–9160. doi: 10.1073/pnas.161281098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA. Acclimation: increasing survival at a cost. Trends Ecol Evol. 1995;10:1–2. [Google Scholar]
- Hoffmann AA, Blows MW. Species borders: ecological and evolutionary perspectives. Trends Ecol Evol. 1994;9:223–227. doi: 10.1016/0169-5347(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Hallas RJ, Anderson AR, Telonis-Scott M. Evidence for a robust sex-specific trade-off between cold resistance and starvation resistance in Drosophila melanogaster. J Evol Biol. 2005b;18:804–810. doi: 10.1111/j.1420-9101.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Hallas RJ, Dean JA, Schiffer M. Low potential for climatic stress adaptation in a rainforest Drosophila species. Science. 2003a;301:100–102. doi: 10.1126/science.1084296. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Hallas R, Sinclair C, Partridge L. Rapid loss of stress resistance in Drosophila melanogaster under adaptation to laboratory culture. Evolution. 2001;55:436–438. doi: 10.1111/j.0014-3820.2001.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Hewa-Kapuge S. Acclimation for heat resistance in Trichogramma nr. brassicae: can it occur without costs? Funct Ecol. 2000;14:55–60. [Google Scholar]
- Hoffmann AA, Parsons PA. Extreme Environmental Change and Evolution. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Hoffmann AA, Scott M, Partridge L, Hallas R. Overwintering in Drosophila melanogaster: outdoor field cage experiments on clinal and laboratory selected populations help to elucidate traits under selection. J Evol Biol. 2003c;16:614–623. doi: 10.1046/j.1420-9101.2003.00561.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Shirriffs J, Scott M. Relative importance of plastic versus genetic factors in adaptive differentiation: geographical variation for stress resistance in Drosophila melanogaster from eastern Australia. Funct Ecol. 2005a;19:222–227. [Google Scholar]
- Hoffmann AA, Sørensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Thermal Biol. 2003b;28:175–216. [Google Scholar]
- Hoffmann AA, Watson M. Geographical variation in the acclimation responses of Drosophila to temperature extremes. Am Nat. 1993;142:S93–S113. doi: 10.1086/285525. [DOI] [PubMed] [Google Scholar]
- Holmgren M, Scheffer M, Ezcurra E, Gutiérrez JR, Mohren GMJ. El Niño effects on the dynamics of terrestrial ecosystems. Trends Ecol Evol. 2001;16:89–94. doi: 10.1016/s0169-5347(00)02052-8. [DOI] [PubMed] [Google Scholar]
- Holmstrup M, Bayley M, Ramløv H. Supercool or dehydrate? An experimental analysis of overwintering strategies in small permeable artic invertebrates. Proc Natl Acad Sci USA. 2002a;99:5716–5720. doi: 10.1073/pnas.082580699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrup M, Hedlund K, Boriss H. Drought acclimation and lipid composition in Folsomia candida: implications for cold shock, heat shock and acute desiccation stress. J Insect Physiol. 2002b;48:961–970. doi: 10.1016/s0022-1910(02)00175-0. [DOI] [PubMed] [Google Scholar]
- Holt RD, Barfield M, Gomulkiewicz R. Temporal variation can facilitate niche evolution in harsh sink environments. Am Nat. 2004a;164:187–200. doi: 10.1086/422343. [DOI] [PubMed] [Google Scholar]
- Holt RD, Keitt TH. Alternative causes for range limits: a meta-population perspective. Ecol Lett. 2000;3:41–47. [Google Scholar]
- Holt RD, Knight TM, Barfield M. Allee effects, immigration, and the evolution of species’ niches. Am Nat. 2004b;163:253–262. doi: 10.1086/381408. [DOI] [PubMed] [Google Scholar]
- Holway DA, Suarez AV. Homogenization of ant communities in mediterranean California: the effects of urbanization and invasion. Biol Conserv. 2006;127:319–326. [Google Scholar]
- Hosler JS, Burns JE, Esch HE. Flight muscle resting potential and species-specific differences in chill-coma. J Insect Physiol. 2000;46:621–627. doi: 10.1016/s0022-1910(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Huestis DL, Marshall JL. Interaction between maternal effects and temperature affects diapause occurrence in the cricket Allonemobius socialis. Oecologia. 2006;146:513–520. doi: 10.1007/s00442-005-0232-z. [DOI] [PubMed] [Google Scholar]
- Huey RB, Berrigan D. Testing evolutionary hypotheses of acclimation. Animals and Temperature. In: Johnston IA, Bennett AF, editors. Phenotypic and Evolutionary Adaptation. Cambridge: Cambridge University Press; 1996. pp. 205–237. [Google Scholar]
- Huey RB, Berrigan D, Gilchrist GW, Herron JC. Testing the adaptive significance of acclimation: a strong inference approach. Am Zool. 1999;39:323–336. [Google Scholar]
- Huey RB, Hertz PE, Sinervo B. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am Nat. 2003;161:357–366. doi: 10.1086/346135. [DOI] [PubMed] [Google Scholar]
- Huey RB, Kingsolver JG. Evolution of resistance to high temperature in ectotherms. Am Nat. 1993;142:S21–S46. [Google Scholar]
- Huey RB, Partridge L, Fowler K. Thermal sensitivity of Drosophila melanogaster responds rapidly to laboratory natural selection. Evolution. 1991;45:751–756. doi: 10.1111/j.1558-5646.1991.tb04343.x. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. Life, death and membrane bilayers. J Exp Biol. 2003;206:2303–2311. doi: 10.1242/jeb.00399. [DOI] [PubMed] [Google Scholar]
- Hunger T, Steinbrecht RA. Functional morphology of a double-walled multiporous olfactory sensillum: the sensillum coeloconicum of Bombyx mori (Insecta, Lepidoptera) Tissue and Cell. 1998;30:14–29. doi: 10.1016/s0040-8166(98)80003-7. [DOI] [PubMed] [Google Scholar]
- Huntley B, Green RE, Collingham YC, Hill JK, Willis SG, Bartlein PJ, Cramer W, Hagemeijer WJM, Thomas CJ. The performance of models relating species geographical distributions to climate is independent of trophic level. Ecol Lett. 2004;7:417–426. [Google Scholar]
- Huyton PM, Brady J. Some effects of light and heat on feeding and resting behavior of tsetse flies, Glossina morsitans Westwood. J Entomol A. 1975;50:23–30. [Google Scholar]
- Inoshita T, Tanimura T. Cellular identification of water gustatory receptor neurons and their central projection pattern in Drosophila. Proc Natl Acad Sci USA. 2006;103:1094–1099. doi: 10.1073/pnas.0502376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin JT, Bennet VA, Lee RE. Diapause development in frozen larvae of the goldenrod gall fly, Eurosta solidaginis Fitch (Diptera: Tephritidae) J Comp Physiol B. 2001;171:181–188. doi: 10.1007/s003600000154. [DOI] [PubMed] [Google Scholar]
- Irwin JT, Lee RE. Mild winter temperatures reduce survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis (Diptera: Tephritidae) J Insect Physiol. 2000;46:655–661. doi: 10.1016/s0022-1910(99)00153-5. [DOI] [PubMed] [Google Scholar]
- Irwin JT, Lee RE. Energy and water conservation in frozen vs. supercooled larvae of the goldenrod gall fly, Eurosta solidaginis (Fitch) (Diptera: Tephritidae) J Exp Zool. 2002;292:345–350. doi: 10.1002/jez.10082. [DOI] [PubMed] [Google Scholar]
- Irwin JT, Lee RE. Cold winter microenvironments conserve energy and improve overwintering survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis. Oikos. 2003;100:71–78. [Google Scholar]
- Izumi Y, Sonoda S, Yoshida H, Tsumuki H. Identification of tissues showing the lowest tolerance to freezing in larvae of the rice stem borer, Chilo suppressalis. Physiol Entomol. 2005;30:324–331. [Google Scholar]
- Janiszewski J. The effect of temperature changes on the spontaneous activity in the neural ganglia of the cockroach, Periplaneta americana. J Therm Biol. 1986;11:191–197. [Google Scholar]
- Jeffree CE, Jeffree EP. Redistribution of the potential geographical ranges of Mistletoe and Colorado Beetle in Europe in response to the temperature component of climate change. Funct Ecol. 1996;10:562–577. [Google Scholar]
- Jenkins NL, Hoffmann AA. Limits to the southern border of Drosophila serrata: cold resistance, heritable variation, and trade-offs. Evolution. 1999;53:1823–1834. doi: 10.1111/j.1558-5646.1999.tb04565.x. [DOI] [PubMed] [Google Scholar]
- Jenouvrier S, Weimerskirch H, Barbraud C, Park YH, Cazelles B. Evidence of a shift in the cyclicity of Antarctic seabird dynamics linked to climate. Proc R Soc Lond B. 2005;272:887–895. doi: 10.1098/rspb.2004.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian F, Jayas DS, White NDG. Movement and distribution of adult Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) in stored wheat in response to temperature gradients, dockage, and moisture differences. J Stored Prod Res. 2005;41:401–422. [Google Scholar]
- Jian FJ, Jayas DS, White NDG, Muir WE. Temperature and geotaxis preference by Cryptolestes ferrugineus (Coleoptera : Laemophloeidae) adults in response to 5 degrees C/m temperature gradients at optimum and hot temperatures in stored wheat and their mortality at high temperature. Environ Entomol. 2002;31:816–826. [Google Scholar]
- Jing XH, Kang L. Geographical variation in egg cold hardiness: a study on the adaptation strategies of the migratory locust Locusta migratoria L. Ecol. Entomol. 2003;28:151–158. [Google Scholar]
- Joanisse DR, Storey KB. Enzyme activity profiles in an overwintering population of freeze-tolerant larvae of the gall fly, Eurosta solidaginis. J Comp Physiol B. 1994a;164:247–255. [Google Scholar]
- Joanisse DR, Storey KB. Mitochondrial enzymes during overwintering in two species of cold-hardy gall insects. Insect Biochem Mol Biol. 1994b;24:145–150. [Google Scholar]
- Joanisse DR, Storey KB. Oxidative stress and antioxidants in overwintering larvae of cold-hardy goldenrod gall insects. J Exp Biol. 1996;199:1483–1491. doi: 10.1242/jeb.199.7.1483. [DOI] [PubMed] [Google Scholar]
- Joanisse DR, Storey KB. Oxidative stress and antioxidants in stress and recovery of cold-hardy insects. Ins Biochem Mol Biol. 1998;28:23–30. [Google Scholar]
- Jones BM. The sensory physiology of the harvest mite Trombicula autumnalis Shaw. J Exp Biol. 1950;27:461–494. doi: 10.1242/jeb.27.3.461. [DOI] [PubMed] [Google Scholar]
- Joos B, Lighton JRB, Harrison JF, Suarez RK, Roberts SP. Effects of ambient oxygen tension on flight performance, metabolism, and water loss of the honeybee. Physiol Zool. 1997;70:167–174. doi: 10.1086/639570. [DOI] [PubMed] [Google Scholar]
- Joplin KH, Yocum GD, Denlinger DL. Cold shock elicits expression of heat shock proteins in the flesh fly, Sarcophaga crassipalpis. J Insect Physiol. 1990;36:825–834. [Google Scholar]
- Jørgensen KT, Sørensen JG, Bundgaard J. Heat tolerance and the effect of mild heat stress on reproductive characters in Drosophila buzzatii males. J Thermal Biol. 2006;31:280–286. [Google Scholar]
- Karan D, David JR. Cold tolerance in Drosophila: adaptive variations revealed by the analysis of starvation survival reaction norms. J Thermal Biol. 2000;25:345–351. doi: 10.1016/s0306-4565(99)00106-0. [DOI] [PubMed] [Google Scholar]
- Karnosky DF, Zak DR, Pregitzer KS, Awmack CS, Bockheim JG, Dickson RE, Hendrey GR, Host GE, King JS, Kopper BJ, Kruger EL, Kubiske ME, Lindroth RL, Mattson WJ, McDonald EP, Noormets A, Oksanen E, Parsons WFJ, Percy KE, Podila GK, Riemenschneider DE, Sharma P, Thakur R, Sôber A, Sôber J, Jones WS, Anttonen S, Vapaavuori E, Mankovska B, Heilman W, Isebrands JG. Tropospheric O3 moderates responses of temperate hardwood forests to elevated CO2: a synthesis of molecular to ecosystem results from the Aspen FACE project. Funct Ecol. 2003;17:289–304. [Google Scholar]
- Karunanithi S, Barclay JW, Brown IR, Robertson RM, Atwood HL. Neuroprotection at Drosophila synapses conferred by prior heat shock. J Neurosci. 1999;19:4360–4369. doi: 10.1523/JNEUROSCI.19-11-04360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithi S, Barclay JW, Brown IR, Robertson RM, Atwood HL. Enhancement of presynaptic performance in transgenic Drosophila over-expressing heat shock protein HSP70. Synapse. 2002;44:8–14. doi: 10.1002/syn.10048. [DOI] [PubMed] [Google Scholar]
- Kelty JD, Killian KA, Lee RE. Cold shock and rapid cold-hardening of pharate adult flesh flies (Sarcophaga crassipalpis): effects on behaviour and neuromuscular function following eclosion. Physiol Entomol. 1996;21:283–288. [Google Scholar]
- Kelty JD, Lee RE. Induction of rapid cold hardening by cooling at ecologically relevant rates in Drosophila melanogaster. J Insect Physiol. 1999;45:719–726. doi: 10.1016/s0022-1910(99)00040-2. [DOI] [PubMed] [Google Scholar]
- Kelty JD, Lee RE. Rapid cold-hardening of Drosophila melanogaster (Diptera: Drosophilidae) during ecologically based thermoperiodic cycles. J Exp Biol. 2001;204:1659–1666. doi: 10.1242/jeb.204.9.1659. [DOI] [PubMed] [Google Scholar]
- Kevan PG. Sun-tracking solar furnaces in High Arctic flowers: significance for pollination and insects. Science. 1975;189:723–726. doi: 10.1126/science.189.4204.723. [DOI] [PubMed] [Google Scholar]
- Kimura MT. Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia. 2004;140:442–449. doi: 10.1007/s00442-004-1605-4. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG. Weather and the population dynamics of insects: integrating physiological and population ecology. Physiol Zool. 1989;62:314–334. [Google Scholar]
- Kingsolver JG, Gomulkiewicz R, Carter PA. Variation, selection and evolution of function-valued traits. Genetica. 2001;112:87–104. [PubMed] [Google Scholar]
- Kingsolver JG, Huey RB. Evolutionary analyses of morphological and physiological plasticity in thermally variable environments. Am Zool. 1998;38:545–560. [Google Scholar]
- Kirkpatrick M, Barton NH. Evolution of a species’ range. Am Nat. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- Klok CJ, Chown SL. Interactions between desiccation resistance, host–plant contact and the thermal biology of a leaf-dwelling sub-antarctic caterpillar, Embryonopsis halticella (Lepidoptera: Yponomeutidae) J Insect Physiol. 1998;44:615–628. doi: 10.1016/s0022-1910(98)00052-3. [DOI] [PubMed] [Google Scholar]
- Klok CJ, Chown SL. Resistance to temperature extremes in sub-Antarctic weevils: interspecific variation, population differentiation and acclimation. Biol J Linn Soc. 2003;78:401–414. [Google Scholar]
- Klok CJ, Sinclair BJ, Chown SL. Upper thermal tolerance and oxygen limitation in terrestrial arthropods. J Exp Biol. 2004;207:2361–2370. doi: 10.1242/jeb.01023. [DOI] [PubMed] [Google Scholar]
- Klose MK, Armstrong G, Robertson RM. A role for the cyto-skeleton in heat-shock-mediated thermoprotection of locust neuromuscular junctions. Neurobiology. 2004;60:453–462. doi: 10.1002/neu.20058. [DOI] [PubMed] [Google Scholar]
- Klose MK, Robertson RM. Stress-induced thermoprotection of neuromuscular transmission. Integr Comp Biol. 2004;44:14–20. doi: 10.1093/icb/44.1.14. [DOI] [PubMed] [Google Scholar]
- Klvana I, Berteaux D, Cazelles B. Porcupine feeding scars and climatic data show ecosystem effects of the solar cycle. Am Nat. 2004;164:283–297. doi: 10.1086/423431. [DOI] [PubMed] [Google Scholar]
- Knickerbocker DL, Lutz PL. Slow ATP loss and the defense of ion homeostasis in the anoxic frog brain. J Exp Biol. 2001;204:3547–3551. doi: 10.1242/jeb.204.20.3547. [DOI] [PubMed] [Google Scholar]
- Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, Smith VS. Species coextinctions and the biodiversity crisis. Science. 2004;305:1632–1634. doi: 10.1126/science.1101101. [DOI] [PubMed] [Google Scholar]
- Korsloot A, van Gestel C, van Straalen N. Environmental Stress and Cellular Response in Arthropods. Boca Raton, FL: CRC Press; 2004. [Google Scholar]
- Koštál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52:113. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Koštál V, Simek P. Biochemistry and physiology of aestivo-hibernation in the adult apple blossom weevil, Anthonomus pomorum (Coleoptera: Curculionidae) J Insect Physiol. 1996;42:727–733. [Google Scholar]
- Koštál V, Slachta M, Šimek P. Cryoprotective role of polyols independent of the increase in supercooling capacity in diapausing adults of Pyrrhocoris apterus (Heteroptera: Insecta) Comp Biochem Physiol B. 2001;130:365–374. doi: 10.1016/s1096-4959(01)00441-9. [DOI] [PubMed] [Google Scholar]
- Koštál V, Vambera J, Bastl J. On the nature of pre-freeze mortality in insects: water balance, ion homeostasis and energy charge in the adults of Pyrrhocoris apterus. J Exp Biol. 2004;207:1509–1521. doi: 10.1242/jeb.00923. [DOI] [PubMed] [Google Scholar]
- Koz³owski J, Czarnoleski M, Dañko M. Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr Comp Biol. 2004;44:480–493. doi: 10.1093/icb/44.6.480. [DOI] [PubMed] [Google Scholar]
- Koz³owski J, Konarzewski M, Gawelczyk AT. Cell size as a link between noncoding DNA and metabolic rate scaling. Proc Natl Acad Sci USA. 2003;100:14080–14085. doi: 10.1073/pnas.2334605100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Natural variation in the expression of the heat-shock protein Hsp70 in a population of Drosophila melanogaster and its correlation with tolerance of ecologically relevant thermal stress. Evolution. 1997;51:173–179. doi: 10.1111/j.1558-5646.1997.tb02398.x. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Loeschcke V. Costs and benefits of activation of the heat-shock response in Drosophila melanogaster. Funct Ecol. 1994;8:730–737. [Google Scholar]
- Krebs RA, Loeschcke V. Resistance to thermal stress in adult Drosophila buzzatii: acclimation and variation among populations. Biol J Linn Soc. 1995;56:505–515. [Google Scholar]
- Krebs RA, Loeschcke V. Acclimation and selection for increased resistance to thermal stress in Drosophila buzzatii. Genetics. 1996;142:471–479. doi: 10.1093/genetics/142.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RA, Loeschcke V. Estimating heritability in a threshold trait: heat-shock tolerance in Drosophila buzzatii. Heredity. 1997;79:252–259. doi: 10.1038/hdy.1997.152. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Roberts SP, Bettencourt BR, Feder ME. Changes in thermotolerance and Hsp70 expression with domestication in Drosophila melanogaster. J Evol Biol. 2001;14:75–82. doi: 10.1046/j.1420-9101.2001.00256.x. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Thompson KA. A genetic analysis of variation for the ability to fly after exposure to thermal stress in Drosphila mojavensis. J Thermal Biol. 2005;30:335–342. [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Krishnan N, Kodrik D. Antioxidant enzymes in Spodoptera littoralis (Boisduval): are they enhanced to protect gut tissues during oxidative stress? J Insect Physiol. 2006;52:11–20. doi: 10.1016/j.jinsphys.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Kristiansen E, Zachariassen KE. Effect of freezing on the trans-membrane distribution of ions in freeze-tolerant larvae of the wood fly Xylophagus cinctus (Diptera, Xylophagidae) J Insect Physiol. 2001;47:585–592. doi: 10.1016/s0022-1910(00)00157-8. [DOI] [PubMed] [Google Scholar]
- Kuhrt U, Samietz J, Dorn S. Thermal response in adult codling moth. Physiol Entomol. 2006;31:80–88. [Google Scholar]
- Kukal O, Duman JG. Switch in the overwintering strategy of two insect species and latitudinal differences in cold hardiness. Can J Zool. 1989;67:825–827. [Google Scholar]
- Kukal O, Duman JG, Serianni AS. Cold-induced mitochondrial degradation and cryoprotectant synthesis in freeze-tolerant arctic caterpillars. J Comp Physiol. 1989;158:661–671. doi: 10.1007/BF00693004. [DOI] [PubMed] [Google Scholar]
- Kutz SJ, Hoberg EP, Polley L, Jenkins EJ. Global warming is changing the dynamics of Arctic host–parasite systems. Proc R Soc Lond B. 2005;272:2571–2576. doi: 10.1098/rspb.2005.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira CC, Sepkoski JJ. Insect diversity in the fossil record. Science. 1993;261:310–315. doi: 10.1126/science.11536548. [DOI] [PubMed] [Google Scholar]
- Lactin DJ, Johnson DL. Behavioural optimization of body temperature by nymphal grasshoppers (Melanoplus sanguinipes, Orthoptera: Acrididae) in temperature gradients established using incandescent bulbs. J Therm Biol. 1996;21:231–238. [Google Scholar]
- Landais I, Pommet JM, Mita K, Nohata J, Gimenez S, Fournier P, Devauchelle G, Duonor-Cerutti M, Ogliastro M. Characterization of the cDNA encoding the 90 kDA heat-shock protein in the Lepidoptera Bombyx mori and Spodoptera frugiperda. Gene. 2001;271:223–231. doi: 10.1016/s0378-1119(01)00523-6. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Haüssinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lansing E, Justesen J, Loeschcke V. Variation in the expression of Hsp70, the major heat-shock protein, and thermotolerance in larval and adult selection lines in Drosophila melanogaster. J Thermal Biol. 2000;25:443–450. doi: 10.1016/s0306-4565(00)00008-5. [DOI] [PubMed] [Google Scholar]
- Larsen KJ, Lee RE. Cold tolerance including rapid cold-hardening and inoculative freezing of fall migrant monarch butterflies in Ohio. J Insect Physiol. 1994;40:859–864. [Google Scholar]
- Lawton JH. More time means more variation. Nature. 1988;344:563. [Google Scholar]
- Lawton JH. There are not 10 million kinds of population dynamics. Oikos. 1992;63:337–338. [Google Scholar]
- Lawton JH. Are there general laws in ecology? Oikos. 1999;84:177–192. [Google Scholar]
- Lazzari CR. Temperature preference in Triatoma infestans (Hemiptera: Reduviidae) Bull Entonol Res. 1991;81:273–276. [Google Scholar]
- Leather SR, Walters KFA, Bale JS. The Ecology of Insect Overwintering. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Lee RE, Chen CP, Denlinger DL. A rapid cold-hardening process in insects. Science. 1987a;238:1415–1417. doi: 10.1126/science.238.4832.1415. [DOI] [PubMed] [Google Scholar]
- Lee RE, Chen C, Meacham MH, Denlinger DL. Ontogenetic patterns of cold-hardiness and glycerol production in Sarcophaga crassipalpis. J Insect Physiol. 1987b;33:587–592. [Google Scholar]
- Lee RE, Denlinger DL. Cold tolerance in diapausing and non-diapausing stages of the flesh fly, Sarcophaga crassipalpis. Physiol Entomol. 1985;10:309–315. [Google Scholar]
- Lee RE, Dommel RA, Joplin KH, Denlinger DL. Cryobiology of the freeze-tolerant gall fly Eurosta solidaginis: overwintering energetics and heat shock proteins. Climate Res. 1995;5:61–67. [Google Scholar]
- Lee RE, Elnitsky MA, Rinehart JP, Hayward SAL, Sandro LH, Denlinger DL. Rapid cold-hardening increases the freezing tolerance of the Antarctic midge Belgica antarctica. J Exp Biol. 2006;209:399–406. doi: 10.1242/jeb.02001. [DOI] [PubMed] [Google Scholar]
- Lee RE, McGrath JJ, Morason RT, Taddeo RM. Survival of intracellular freezing, lipid coalescence and osmotic fragility in fat body cells of the freeze-tolerant gall fly Eurosta solidaginis. J Insect Physiol. 1993;39:445–450. [Google Scholar]
- Lees AD. The sensory physiology of the sheep tick Ixodes ricinus L. J Exp Biol. 1948;25:145–207. [Google Scholar]
- Lenormand T. Gene flow and the limits to natural selection. Trends Ecol Evol. 2002;17:183–189. [Google Scholar]
- Lerman DN, Feder ME. Laboratory selection at different temperatures modifies heat-shock transcription factor (HSF) activation in Drosophila melanogaster. J Exp Biol. 2001;204:315–323. doi: 10.1242/jeb.204.2.315. [DOI] [PubMed] [Google Scholar]
- Leroi AM, Bennett AF, Lenski RE. Temperature acclimation and competitive fitness: an experimental test of the beneficial acclimation assumption. Proc Natl Acad Sci USA. 1994;91:1917–1921. doi: 10.1073/pnas.91.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins R. Evolution in Changing Environments. Princeton: Princeton University Press; 1968. [Google Scholar]
- Liu L, Yermolaieva O, Johnson WA, Abboud FM, Welsh MJ. Identification and function of thermosensory neurons in Drosophila larvae. Nat Neurosci. 2003;6:267–273. doi: 10.1038/nn1009. [DOI] [PubMed] [Google Scholar]
- Lively CM. Canalization versus developmental conversion in a spatially variable environment. Am Nat. 1986;128:561–572. [Google Scholar]
- Loeschke V, Hoffmann AA. The detrimental acclimation hypothesis. Trends Ecol Evol. 2002;17:407–408. [Google Scholar]
- Loeschcke V, Krebs RA, Dahlgaard J, Michalak P. High-temperature stress and the evolution of thermal resistance in Drosophila. In: Bijlsma R, Loeschcke V, editors. Environmental Stress, Adaptation and Evolution. Basel: Birkhäuser; 1997. pp. 175–190. [DOI] [PubMed] [Google Scholar]
- Loeschcke V, Sørensen JG. Acclimation, heat shock and hardening –a response from evolutionary biology. J Thermal Biol. 2005;30:255–257. [Google Scholar]
- Loftus R. The response of the antennal cold receptor of Periplaneta americana to rapid temperature changes and to steady temperature. Z Vergl Physiol. 1968;52:413–455. [Google Scholar]
- Logue JA, de Vries AL, Fodor E, Cossins AR. Lipid compositional correlates of temperature-adaptive interspesific differences in membrane physical structure. J Exp Biol. 2000;203:2105–2115. doi: 10.1242/jeb.203.14.2105. [DOI] [PubMed] [Google Scholar]
- Lorenzo MG, Lazzari CR. Temperature and relative humidity affect the selection of shelters by Triatoma infestans, vector of chagas disease. Acta Trop. 1999;72:241–249. doi: 10.1016/s0001-706x(98)00094-1. [DOI] [PubMed] [Google Scholar]
- Lundheim R, Zachariassen KE. Water balance of over-wintering beetles in relation to strategies for cold tolerance. J Comp Physiol B. 1993;163:1–4. [Google Scholar]
- Lynch M, Gabriel W. Environmental tolerance. Am Nat. 1987;129:283–303. [Google Scholar]
- Macdonald SS, Rako L, Batterham P, Hoffmann AA. Dissecting chill coma recovery as a measure of cold resistansb: evidence for a biphasic response in Drosophila melanogaster. J Insect Physiol. 2004;50:695–700. doi: 10.1016/j.jinsphys.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl. 2000;10:689–710. [Google Scholar]
- Mahroof R, Zhu KY, Neven L, Subramanyamm B, Bai J. Expression patterns of three heat shock protein 70 genes among developmental stages of the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae) Comp Biochem Physiol A. 2005;141:247–256. doi: 10.1016/j.cbpb.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Maisonhaute C, Chihrane J, Laugé G. Induction of thermotolerance in Trichogramma brassicae (Hymenoptera: Trichogrammatidae) Environ Entomol. 1999;28:116–122. [Google Scholar]
- Makarieva AM, Gorshkov VG, Li BL, Chown SL. Size- and temperature-independence of minimum life-supporting metabolic rates. Funct Ecol. 2006;20:83–96. [Google Scholar]
- Marcus JM. The development and evolution of crossveins in insect wings. J Anat. 2001;199:211–216. doi: 10.1046/j.1469-7580.2001.19910211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos M, Rose MR, Pité MTR, Rego C, Avelar T. Adaptation to the laboratory environment in Drosophila subobscura. J Evol Biol. 2000;13:9–19. [Google Scholar]
- May ML. Insect thermoregulation. Annu Rev Entomol. 1979;24:313–349. [Google Scholar]
- McColl G, Hoffmann AA, McKechnie SW. Response of two heat shock genes to selection for knockdown heat resistance in Drosophila melanogaster. Genetics. 1996;143:1615–1627. doi: 10.1093/genetics/143.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JR, Bale JS, Walters KFA. Rapid cold hardening in the western flower thrips Frankliniella occidentalis. J Insect Physiol. 1997;43:759–766. doi: 10.1016/s0022-1910(97)00033-4. [DOI] [PubMed] [Google Scholar]
- McDonald JR, Head J, Bale JS, Walters KFA. Cold tolerance, overwintering and establishment potential of Thrips palmi. Physiol Entomol. 2000;25:159–166. [Google Scholar]
- McGeoch MA. The selection, testing and application of terrestrial insects as bioindicators. Biol Rev. 1998;73:181–201. [Google Scholar]
- McGeoch MA. Insects and bioindication: theory and progress. In: Stewart AJA, Lewis OT, New TR, editors. Insect Conservation Biology. London: CABI Publishing; in press. [Google Scholar]
- McGeoch MA, Wossler TC. Range expansion and success of the weed biocontrol agent Trichilogaster acaciaelongifoliae (Froggatt) (Hymenoptera: Pteromalidae) in South Africa. Afr Entomol. 2000;8:273–280. [Google Scholar]
- McKechnie AE, Freckleton RP, Jetz W. Phenotypic plasticity in the scaling of avian basal metabolic rate. Proc R Soc Lond B. 2006;273:931–937. doi: 10.1098/rspb.2005.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab BK. The Physiological Ecology of Vertebrates: A View from Energetics. Ithaca, NY: Cornell University Press; 2002. [Google Scholar]
- Mélice JL, Lutjeharms JRE, Rouault M, Ansorge IJ. Sea-surface temperatures at the sub-Antarctic islands Marion and Gough during the past 50 years. S Afr J Sci. 2003;99:363–366. [Google Scholar]
- Merivee E, Vanatoa A, Luik A, Rahi M, Sammelselg V, Ploomi A. Electrophysiological identification of cold receptors on the antennae of the ground beetle Pterostichus aethiops. Physiol Entomol. 2003;28:88–96. [Google Scholar]
- Messenger PS. Bioclimatic studies with insects. Annu Rev Entomol. 1959;4:183–206. [Google Scholar]
- Michaud MR, Denlinger DL. Molecular modalities of insect cold survival: current understanding and future trends. In: Morris S, Vosloo A, editors. Animals and Environments. Amsterdam: Elsevier International Congress Series; 2005. pp. 32–46. 1275. [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiversity Synthesis. Washington, DC: World Resources Institute; 2005. [Google Scholar]
- Miller LK. Freezing tolerance in relation to cooling rate in an adult insect. Cryobiology. 1978;15:345–349. doi: 10.1016/0011-2240(78)90046-9. [DOI] [PubMed] [Google Scholar]
- Milton CC, Huynh B, Batterham P, Rutherford SL, Hoffmann AA. Quantitative trait symmetry independent of Hsp90 buffering: distinct modes of genetic canalization and developmental stability. Proc Natl Acad Sci USA. 2003b;100:13396–13401. doi: 10.1073/pnas.1835613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton SL, Manuel L, Lutz PL. Slow death in the leopard frog Rana pipiens: neurotransmitters and anoxia tolerance. J Exp Biol. 2003a;206:4021–4028. doi: 10.1242/jeb.00647. [DOI] [PubMed] [Google Scholar]
- Minoli SA, Lazari CR. Chronobiological basis of thermo-preference in the haematophagous bug Triatoma infestans. J Insect Physiol. 2003;49:927–932. doi: 10.1016/s0022-1910(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Misener SR, Chen CP, Walker VK. Cold tolerance and proline metabolic gene expression in Drosophila melanogaster. J Insect Physiol. 2001;47:393–400. doi: 10.1016/s0022-1910(00)00141-4. [DOI] [PubMed] [Google Scholar]
- Moller H. Lessons for invasion theory from social insects. Biol Conserv. 1996;78:125–142. [Google Scholar]
- Mooney HA, Hobbs RJ. Invasive Species in a Changing World. Washington, DC: Island Press; 2000. [Google Scholar]
- Moran NA. The evolutionary maintenance of alternative phenotypes. Am Nat. 1992;139:971–989. [Google Scholar]
- Mori I. Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu Rev Genet. 1999;33:399–422. doi: 10.1146/annurev.genet.33.1.399. [DOI] [PubMed] [Google Scholar]
- Morin P, McMullen DC, Storey KB. HIF-1α involvement in low temperature and anoxia survival by a freeze tolerant insect. Mol Cell Biochem. 2005;280:99–106. doi: 10.1007/s11010-005-8236-x. [DOI] [PubMed] [Google Scholar]
- Murphy BF. The effect of acclimation temperature and the removal of peripheral temperature receptors on body temperature preference in the cockroach, Periplaneta americana. J Thermal Biol. 1986;11:209–212. [Google Scholar]
- Murphy BF, Heath JE. Temperature sensitivity in the prothoracic ganglion of the cockroach Periplaneta americana and its relationship to thermo-regulation. J Exp Biol. 1983;105:305–315. [Google Scholar]
- Must A, Merivee E, Luik A, Mand M, Heidemaa M. Responses of antennal campaniform sensilla to rapid temperature changes in ground beetles of the tribe platynini with different habitat preferences and daily activity rhythms. J Insect Physiol. 2006;52:506–513. doi: 10.1016/j.jinsphys.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Myers JH. Can a general hypothesis explain population cycles of forest Lepidoptera. Adv Ecol Res. 1988;18:179–242. [Google Scholar]
- Neargarder G, Dahlhoff EP, Rank NE. Variation in thermal tolerance is linked to phosphoglucose isomerase genotype in a montane leaf beetle. Funct Ecol. 2003;17:213–221. [Google Scholar]
- Nedved O. Modelling the relationship between cold injury and accumulated degree days in terrestrial arthropods. Cryo Lett. 1998;19:267–274. [Google Scholar]
- Ness JH. Forest edges and fire ants alter the seed shadow of an ant-dispersed plant. Oecologia. 2004;138:448–454. doi: 10.1007/s00442-003-1440-z. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Overgaard J, Sorensen JG, Holmstrup M, Justesen J, Loeschcke V. Role of HSF activation for resistance to heat, cold and high-temperature knock-down. J Insect Physiol. 2005;51:1320–1329. doi: 10.1016/j.jinsphys.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Yokohari F, Ishibashi T. Response characteristics of two types of cold receptors on the antennae of the cockroach, Periplaneta americana. J Comp Physiol A. 1992;171:299–307. [Google Scholar]
- Nishino H, Yamashita S, Yamazaki Y, Nishikawa M, Yokohari F, Mizunami M. Projection neurons originating from thermo- and hygrosensory glomeruli in the antennal lobe of the cockroach. J Comp Neurol. 2003;455:40–55. doi: 10.1002/cne.10450. [DOI] [PubMed] [Google Scholar]
- North RD. Transpiration and humidity preference in a temperate wood ant Formica rufa L. J Insect Physiol. 1991;37:279–286. [Google Scholar]
- Nylin S, Gotthard K. Plasticity in life-history traits. Annu Rev Entomol. 1998;43:63–83. doi: 10.1146/annurev.ento.43.1.63. [DOI] [PubMed] [Google Scholar]
- Oberhauser K, Peterson AT. Modeling current and future potential wintering distributions of eastern North American monarch butterflies. Proc Natl Acad Sci USA. 2003;100:14063–14068. doi: 10.1073/pnas.2331584100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd DJ, Green PT, Lake PS. Invasional ‘meltdown’ on an oceanic island. Ecol Lett. 2003;6:812–817. [Google Scholar]
- Ottersen G, Planque B, Belgrano A, Post E, Reid PC, Stenseth NC. Ecological effects of the North Atlantic oscillation. Oecologia. 2001;128:1–14. doi: 10.1007/s004420100655. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Sørensen JG, Petersen SO, Loeschcke V, Holmstrup M. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J Insect Physiol. 2005;51:1173–1182. doi: 10.1016/j.jinsphys.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Owen-Smith N. Incorporating fundamental laws of biology and physics into population ecology: the metaphysiological approach. Oikos. 2005;111:611–615. [Google Scholar]
- Palmer M, Bernhardt E, Chornesky E, Collins S, Dobson A, Duke C, Gold B, Jacobson R, Kingsland S, Kranz R, Mappin M, Martinez ML, Micheli F, Morse J, Pace M, Pascual M, Palumbi S, Reichman OJ, Simons A, Townsend A, Turner M. Ecology for a crowded planet. Science. 2004;304:1251–1252. doi: 10.1126/science.1095780. [DOI] [PubMed] [Google Scholar]
- Park T. Beetles, competition, and populations. Science. 1962;138:1369–1375. doi: 10.1126/science.138.3548.1369. [DOI] [PubMed] [Google Scholar]
- Parkash R, Tyagi PK, Sharma I, Rajpurohit S. Adaptations to environmental stress in altitudinal populations of two Drosophila species. Phys Entomol. 2005;30:353–361. [Google Scholar]
- Parker JC. In defense of cell volume? Am J Physiol. 1993;265:C1191–C1200. doi: 10.1152/ajpcell.1993.265.5.C1191. [DOI] [PubMed] [Google Scholar]
- Parmesan C, Root TL, Willig MR. Impacts of extreme weather and climate on terrestrial biota. Bull Am Meteorol Soc. 2000;81:443–450. [Google Scholar]
- Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, Huntley B, Kaila L, Kullberg J, Tammaru T, Tennent WJ, Thomas JA, Warren M. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Parr CL, Sinclair BJ, Andersen AN, Gaston KJ, Chown SL. Constraint and competition in assemblages: a cross continental and modeling approach for ants. Am Nat. 2005;165:481–493. doi: 10.1086/428292. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress toleransb: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Parsons PA. Environments and evolution: interactions between stress, resource inadequacy and energetic efficiency. Biol Rev. 2005;80:589–610. doi: 10.1017/S1464793105006822. [DOI] [PubMed] [Google Scholar]
- Patapouitian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol Biogeogr. 2003;12:361–371. [Google Scholar]
- Perfecto I, Vandermeer J. Microclimate changes and the indirect loss of ant diversity in a tropical agroecosystem. Oecologia. 1996;108:577–582. doi: 10.1007/BF00333736. [DOI] [PubMed] [Google Scholar]
- Pétavy G, David JR, Gibert P, Moreteau B. Viability and rate of development at different temperatures in Drosophila: a comparison of constant and alternating thermal regimes. J Thermal Biol. 2001;26:29–39. doi: 10.1016/s0306-4565(00)00022-x. [DOI] [PubMed] [Google Scholar]
- Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol. 2003;18:228–233. [Google Scholar]
- Pigliucci M. Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol. 2005;20:481–486. doi: 10.1016/j.tree.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Murren CJ. Perspective: genetic assimilation and a possible evolutionary paradox: can macroevolution sometimes be so fast as to pass us by? Evolution. 2003;57:1455–1464. doi: 10.1111/j.0014-3820.2003.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Murren CJ, Schlichting CD. Phenotypic plasticity and evolution by genetic assimilation. J Exp Biol. 2006;209:2362–2367. doi: 10.1242/jeb.02070. [DOI] [PubMed] [Google Scholar]
- Pörtner HO. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- Pörtner HO. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A. 2002a;132:739–761. doi: 10.1016/s1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Pörtner HO. Physiological basis of temperature-dependent biogeography: trade-offs in muscle design and performance in polar ectotherms. J Exp Biol. 2002b;205:2217–2230. doi: 10.1242/jeb.205.15.2217. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Hardewig I, Sartoris FJ, van Dijk PLM. Energetic aspects of cold adaptation: critical temperatures in metabolic, ionic and acid-base regulation? In: Pörtner HO, Playle R, editors. Cold Ocean Physiology. Cambridge: Cambridge University Press; 1998. pp. 88–120. [Google Scholar]
- Pörtner HO, van Dijk PLM, Hardewig I, Sommer A. Levels of metabolic cold adaptation: tradeoffs in eurythermal and stenothermal ecto-therms. In: Davison W, Howard-Williams C, Broady PA, editors. Antarctic Ecosystems: Models for Wider Ecological Understanding. Christchurch, New Zealand: New Zealand Natural Sciences; 2000. pp. 109–122. [Google Scholar]
- Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR, Sánchez-Azofeifa GA, Still CJ, Young BE. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- Prange HD, Hamilton GB. Humidity selection by thermoregulating grasshoppers. J Therm Biol. 1992;17:353–355. [Google Scholar]
- Prosser CL. Adaptational Biology: Molecules to Organisms. New York: Wiley; 1986. [Google Scholar]
- Pszczolkowski MA, Chiang AS. Effects of chilling stress on allatal growth and juvenile hormone synthesis in the cockroach, Diploptera punctata. J Insect Physiol. 2000;46:923–931. doi: 10.1016/s0022-1910(99)00199-7. [DOI] [PubMed] [Google Scholar]
- Pszczolkowski MA, Gelman DB. Chilling stress effects on corpus allatum proliferation in the Hawaiian cockroach, Diploptera punctata: a role for ecdysteroids. J Insect Physiol. 2004;50:203–208. doi: 10.1016/j.jinsphys.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Pullin AS. Physiological relationships between insect diapause and cold toleransb: coevolution or coincidence? Eur J Entomol. 1996;93:121–129. [Google Scholar]
- Qin SF, Minami Y, Hibi M, Kurosaki T, Yamamura H. Syk-dependent and – independent signaling cascades in B cells elicited by osmotic and oxidative stress. J Biol Chem. 1997;272:2098–2103. doi: 10.1074/jbc.272.4.2098. [DOI] [PubMed] [Google Scholar]
- Qin W, Tyshenko MG, Wu BS, Walker VS, Robertson RM. Cloning and characterization of a member of the hsp70 gene family from Locusta migratoria a highly thermotolerant insect. Cell Stress Chaperon. 2003;8:144–152. doi: 10.1379/1466-1268(2003)008<0144:cacoam>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Rako L, Hoffmann AA. Complexity of the cold acclimation response in Drosophila melanogaster. J Insect Physiol. 2006;52:94–104. doi: 10.1016/j.jinsphys.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Elsen FP, Robertson RM. Long-term effects of prior heat shock on neuronal potassium currents recorded in a novel insect ganglion slice preparation. J Neurophysiol. 1999;81:795–802. doi: 10.1152/jn.1999.81.2.795. [DOI] [PubMed] [Google Scholar]
- Ramløv H. Aspects of natural cold tolerance in ectothermic animals. Hum Reprod. 2000;15:26–46. doi: 10.1093/humrep/15.suppl_5.26. [DOI] [PubMed] [Google Scholar]
- Randolph SE, Rogers DJ. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proc R Soc Lond B. 2000;267:1741–1744. doi: 10.1098/rspb.2000.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees CJC. The primary process of reception on the type 3 (‘water’) receptor cell of the fly, Phormia terranovae. Proc Roy Soc Lond B. 1970;174:469–490. [Google Scholar]
- Relyea RA. Costs of phenotypic plasticity. Am Nat. 2002;159:272–282. doi: 10.1086/338540. [DOI] [PubMed] [Google Scholar]
- Renault D, Nedved O, Hervant F, Vernon P. The importance of fluctuating thermal regimes for repairing chill injuries in the tropical beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae) during exposure to low temperature. Physiol Entomol. 2004;29:139–145. [Google Scholar]
- Richardson DM, Rouget M, Ralston SJ, Cowling RM, van rensburg BJ, Thuiller W. Species richness of alien plants in South Africa: environmental correlates and the relationship with indigenous plant species richness. Ecoscience. 2005;12:391–402. [Google Scholar]
- Rickman JK, Connor EF. The effect of urbanization on the quality of remnant habitats for leaf-mining lepidoptera on Quercus agrifolia. Ecography. 2003;26:777–787. [Google Scholar]
- Rinehart JP, Denlinger DL. Heat-shock protein 90 is down-regulated during pupal diapause in the flesh fly, Sarcophaga crassipalpis, but remains responsive to thermal stress. Ins Mol Biol. 2000;9:641–645. doi: 10.1046/j.1365-2583.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- Rinehart JP, Yocum GD, Denlinger DL. Thermotolerance and rapid cold hardening ameliorate the negative effects of brief exposures to high or low temperatures on fecundity in the flesh fly, Sarcophaga crassipalpis. Physiol Entomol. 2000a;25:330–336. [Google Scholar]
- Rinehart JP, Yocum GD, Denlinger DL. Developmental up-regulation of inducible hsp70 transcripts, but not the cognate form, during pupal diapause in the flesh fly, Sarcophaga crassipalpis. Ins Biochem Mol Biol. 2000b;30:515–521. doi: 10.1016/s0965-1748(00)00021-7. [DOI] [PubMed] [Google Scholar]
- Ring RA, Danks HV. Desiccation and cryoprotection: overlapping adaptations. Cryo Lett. 1994;15:181–190. [Google Scholar]
- Roberts CS, Seely MK, Ward D, Mitchell D, Campbell JD. Body temperatures of Namib Desert tenebrionid beetles: their relationship in laboratory and field. Physiol Entomol. 1991;16:463–475. [Google Scholar]
- Robertson MP, Villet MH, Palmer AR. A fuzzy classification technique for predicting species’ distributions: applications using invasive alien plants and indigenous insects. Divers Distrib. 2004;10:461–474. [Google Scholar]
- Robertson RM. Modulation of neural circuit operation by prior environmental stress. Integr Comp Biol. 2004a;44:21–27. doi: 10.1093/icb/44.1.21. [DOI] [PubMed] [Google Scholar]
- Robertson RM. Thermal stress and neural function: adaptive mechanisms in insect model systems. J Thermal Biol. 2004b;29:351–358. [Google Scholar]
- Robertson RM, Xu H, Shoemaker KL, Dawson-Scully K. Exposure to heat shock affects thermosensitivity of the locust flight system. J Neurobiol. 1996;29:367–383. doi: 10.1002/(SICI)1097-4695(199603)29:3<367::AID-NEU8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Robinson T, Rogers D, Williams B. Univariate analysis of tsetse habitat in the common fly belt of Southern Africa using climate and remotely sensed vegetation data. Med Vet Entomol. 1997a;11:223–234. doi: 10.1111/j.1365-2915.1997.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Robinson T, Rogers D, Williams B. Mapping tsetse habitat suitability in the common fly belt of Southern Africa using multivariate analysis of climate and remotely sensed vegetation data. Med Vet Entomol. 1997b;11:235–245. doi: 10.1111/j.1365-2915.1997.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Roca MJ, Lazzari CR. Effects of relative humidity on the haematophagous bug Triatoma infestans: hygropreference and eclosion success. J Insect Physiol. 1994;40:901–907. [Google Scholar]
- Roces F, Kleineidam C. Humidity preference for fungus culturing by workers of the leaf-cutting ant Atta sexdens rubropilosa. Insectes Soc. 2000;47:348–350. [Google Scholar]
- Roces F, Nunez JA. Thermal sensitivity during brood care in workers of two Camponotus ant species: circadian variation and its ecological correlates. J Insect Physiol. 1995;41:659–669. [Google Scholar]
- Roff DA. The evolution of wing dimorphism in insects. Evolution. 1986;40:1009–1020. doi: 10.1111/j.1558-5646.1986.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Roff DA. Life History Evolution. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Rogers DJ. Satellites, space, time and the African trypanosomisases. Adv Parasitol. 2000;47:129–171. doi: 10.1016/s0065-308x(00)47008-9. [DOI] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. Mortality rates and population density of tsetse flies correlated with satellite imagery. Nature. 1991;351:739–741. doi: 10.1038/351739a0. [DOI] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289:1763–1766. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. A response to the aim of eradicating tsetse from Africa. Trends Parasitol. 2002;18:534–536. doi: 10.1016/s1471-4922(02)02422-4. [DOI] [PubMed] [Google Scholar]
- Rohmer C, David JR, Moreteau B, Joly D. Heat induced male sterility in Drosophila melanogaster: adaptive genetic variations among geographic populations and role of the Y chromosome. J Exp Biol. 2004;207:2735–2743. doi: 10.1242/jeb.01087. [DOI] [PubMed] [Google Scholar]
- Rojas RR, Leopold RA. Chilling injury in the house fly: evidence for the role of oxidative stress between pupariation and emergence. Cryobiology. 1996;33:447–458. [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–425. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roslin T, Kotze DJ. Preface: insects and plants in space. Ann Zool Fenn. 2005;42:291–294. [Google Scholar]
- Rourke BC. Geographic and altitudinal variation in water balance and metabolic rate in a California grasshopper, Melanoplus sanguinipes. J Exp Biol. 2000;203:2699–2712. doi: 10.1242/jeb.203.17.2699. [DOI] [PubMed] [Google Scholar]
- Roy DB, Rothery P, Moss D, Pollard E, Thomas JA. Butterfly numbers and weather: predicting historical trends in abundance and the future effects of climate change. J Anim Ecol. 2001;70:201–217. [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Salin C, Renault D, Vannier G, Vernon P. A sexually dimorphic response in supercooling temperature, enhanced by starvation, in the lesser mealworm Alphitobius diaperinus (Coleoptera: Tenebrionidae) J Thermal Biol. 2000;25:411–418. doi: 10.1016/s0306-4565(00)00003-6. [DOI] [PubMed] [Google Scholar]
- Salt RW. Influence of moisture content and temperature on cold-hardiness of hibernating insects. Can J Zool. 1956;34:283–294. [Google Scholar]
- Salt RW. Effect of cooling rate on the freezing temperatures of supercooled insects. Can J Zool. 1966;44:655–659. [Google Scholar]
- Salvucci ME. Sorbitol accumulation in whiteflies: evidence for a role in protecting proteins during heat stress. J Thermal Biol. 2000;25:353–361. doi: 10.1016/s0306-4565(99)00107-2. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Stecher DS, Henneberry TJ. Heat shock proteins in whiteflies, an insect that accumulates sorbitol in response to heat stress. J Thermal Biol. 2000;25:363–371. doi: 10.1016/s0306-4565(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Samietz J, Salser MA, Dingle H. Altitudinal variation in behavioural thermoregulation: local adaptation vs plasticity in California grasshoppers. J Evol Biol. 2005;18:1087–1096. doi: 10.1111/j.1420-9101.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- Samways MJ, Osborne R, Hastings H, Hattingh V. Global climate change and accuracy of prediction of species geographical ranges: establishment success of introduced ladybirds (Coccinellidae, Chilocorus spp) worldwide. J Biogeogr. 1999;26:795–812. [Google Scholar]
- Samways MJ, Taylor S, Tarboton W. Extinction reprieve following alien removal. Conserv Biol. 2005;19:1329–1330. [Google Scholar]
- Sanders NJ, Gotelli NJ, Heller NE, Gordon DM. Community disassembly by an invasive species. Proc Natl Acad Sci USA. 2003;100:2474–2477. doi: 10.1073/pnas.0437913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci USA. 1996;93:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner SM. Genetics and evolution of phenotypic plasticity. Annu Rev Ecol Syst. 1993;24:35–68. [Google Scholar]
- Schilman PE, Lazzari CR. Temperature preference in Rhodnius prolixus, effects and possible consequences. Acta Trop. 2004;90:115–122. doi: 10.1016/j.actatropica.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Schlichting CD. Phenotypic plasticity in plants. Plant Species Biol. 2002;17:85–88. [Google Scholar]
- Schlichting CD, Pigliucci M. Phenotypic Evolution: A Reaction Norm Perspective. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- Schliess F, Haüssinger D. The cellular hydration state: a critical determinant for cell death and survival. Biol Chem. 2002;383:577–583. doi: 10.1515/BC.2002.059. [DOI] [PubMed] [Google Scholar]
- Schliess F, Wiese S, Haüssinger D. Osmotic regulation of the heat shock response in H4IIE rat hepatoma cells. FASEB J. 1999;13:1557–1564. doi: 10.1096/fasebj.13.12.1557. [DOI] [PubMed] [Google Scholar]
- Schneider C. The influence of spatial scale on quantifying insect dispersal: an analysis of butterfly data. Ecol Entomol. 2003;28:252–256. [Google Scholar]
- Seebacher F. A review of thermoregulation and physiological performance in reptiles: what is the role of phenotypic flexibility? J Comp Physiol B. 2005;175:453–461. doi: 10.1007/s00360-005-0010-6. [DOI] [PubMed] [Google Scholar]
- Segurado P, Araújo MB. An evaluation of methods for modelling species distributions. J Biogeogr. 2004;31:1555–1568. [Google Scholar]
- Sejerkilde M, Sørensen JG, Loeschcke V. Effects of cold- and heat hardening on thermal resistance in Drosophila melanogaster. J Insect Physiol. 2003;49:719–726. doi: 10.1016/s0022-1910(03)00095-7. [DOI] [PubMed] [Google Scholar]
- Selås V, Hogstad O, Kobro S, Rafoss T. Can sunspot activity and ultraviolet-B radiation explain cyclic outbreaks of forest moth pest species? Proc R Soc Lond B. 2004;271:1897–1901. doi: 10.1098/rspb.2004.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sformo T, Doak P. Thermal ecology of interior Alaska dragonflies (Odonata: Anisoptera) Funct Ecol. 2006;20:114–123. [Google Scholar]
- Sgrò CM, Partridge L. Evolutionary responses of the life history of wild-caught Drosophila melanogaster to two standard methods of laboratory culture. Am Nat. 2000;156:341–353. [Google Scholar]
- Shanbhag SR, Singh K, Singh RN. Fine structure and primary sensory projections of sensilla located in the sacculus of the antenna of Drosophila melanogaster. Cell Tiss Res. 1995;282:237–249. doi: 10.1007/BF00319115. [DOI] [PubMed] [Google Scholar]
- Shear WA, Kukalová-Peck J. The ecology of Paleozoic terrestrial arthropods: the fossil evidence. Can J Zool. 1990;68:1807–1834. [Google Scholar]
- Shelford VE. Physiological animal geography. J Morphol. 1911;22:551–618. [Google Scholar]
- Shields VDC. Ultrastructure of the aporous sensilla on the galea of larval Mamestra configurata (Walker) (Lepidoptera: Noctuidae) Can J Zool. 1994;72:2032–2054. doi: 10.1002/(SICI)1097-4687(199604)228:1<89::AID-JMOR7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Shiga S, Yasuyama K, Okamura N, Yamaguchi T. Neural- and endocrine control of flight muscle degeneration in the adult cricket, Gryllus bimaculatus. J Insect Physiol. 2002;48:15–24. doi: 10.1016/s0022-1910(01)00137-8. [DOI] [PubMed] [Google Scholar]
- Shimada K, Riihimaa A. Cold-induced freezing tolerance in diapausing and non-diapausing larvae of Chymomyza costata (Diptera: Drosophilidae) with accumulation of trehalose and proline. Cryo Lett. 1990;11:243–250. [Google Scholar]
- Shintani Y, Ishikawa Y. Geographic variation in cold hardiness of eggs and neonate larvae of the yellow-spotted longicorn beetle Psacothea hilaris. Physiol Entomol. 1999;24:158–164. [Google Scholar]
- Shreve SM, Kelty JD, Lee RE. Preservation of reproductive behaviors during modest cooling: rapid cold-hardening fine-tunes organismal response. J Exp Biol. 2004;207:1797–1802. doi: 10.1242/jeb.00951. [DOI] [PubMed] [Google Scholar]
- Sibly RM, Winokur L, Smith RH. Interpopulation variation in phenotypic plasticity in the speckled wood butterfly, Pararge aegeria. Oikos. 1997;78:323–330. [Google Scholar]
- Simberloff D. Community ecology: is it time to move on? Am Nat. 2004;163:787–799. doi: 10.1086/420777. [DOI] [PubMed] [Google Scholar]
- Simmons AD, Thomas CD. Changes in dispersal during species’ range expansions. Am Nat. 2004;164:378–395. doi: 10.1086/423430. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ. Insect cold tolerance: how many kinds of frozen? Eur J Entomol. 1999;96:157–164. [Google Scholar]
- Sinclair BJ. Field ecology of freeze tolerance: interannual variation in cooling rates, freeze–thaw and thermal stress in the microhabitat of the alpine cockroach Celatoblatta quinquemaculata. Oikos. 2001a;93:286–293. [Google Scholar]
- Sinclair BJ. Biologically relevant environmental data: macros to make the most of microclimate recordings. Cryo Lett. 2001b;22:125–134. [PubMed] [Google Scholar]
- Sinclair BJ, Addo-Bediako A, Chown SL. Climatic variability and the evolution of insect freeze tolerance. Biol Rev. 2003a;78:181–195. doi: 10.1017/s1464793102006024. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, Chown SL. Rapid responses to high temperature and desiccation but not to low temperature in the freeze tolerant sub-Antarctic caterpillar Pringleophaga marioni (Lepidoptera, Tineidae) J Insect Physiol. 2003;49:45–52. doi: 10.1016/s0022-1910(02)00225-1. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, Chown SL. Climatic variability and hemispheric differences in insect cold tolerance: support from southern Africa. Funct Ecol. 2005a;19:214–221. [Google Scholar]
- Sinclair BJ, Chown SL. Deleterious effects of repeated cold exposure in a freeze-tolerant sub-Antarctic caterpillar. J Exp Biol. 2005b;208:879–969. doi: 10.1242/jeb.01455. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, Klok CJ, Chown SL. Metabolism of the sub-Antarctic caterpillar Pringleophaga marioni during cooling, freezing and thawing. J Exp Biol. 2004;207:1287–1294. doi: 10.1242/jeb.00880. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, Roberts SP. Acclimation, shock and hardening in the cold. J Thermal Biol. 2005;30:557–562. [Google Scholar]
- Sinclair BJ, Terblanche JS, Scott MB, Blatch GL, Klok CJ, Chown SL. Environmental physiology of three species of Collembola at Cape Hallett, North Victoria Land, Antarctica. J Insect Physiol. 2006;52:29–50. doi: 10.1016/j.jinsphys.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, Vernon P, Klok CJ, Chown SL. Insects at low temperatures: an ecological perspective. Trends Ecol Evol. 2003b;18:257–262. [Google Scholar]
- Slansky F, Rodriguez JG. Nutritional Ecology of Insects, Mites, Spiders and Related Invertebrates. New York: Wiley; 1987. [Google Scholar]
- Somero GN. Proteins and temperature. Annu Rev Physiol. 1995;57:43–68. doi: 10.1146/annurev.ph.57.030195.000355. [DOI] [PubMed] [Google Scholar]
- Somero GN. Linking biogeography to physiology: evolutionary and acclimatory adjustments of thermal limits. Front Zool. 2005;2:1–9. doi: 10.1186/1742-9994-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero GN, Dahlhoff E, Lin JJ. Stenotherms and eurytherms: mechanisms establishing thermal optima and tolerance ranges. Animals and Temperature. In: Johnston IA, Bennett AF, editors. Phenotypic and Evolutionary Adaptation. Cambridge: Cambridge University Press; 1996. pp. 53–78. [Google Scholar]
- Sømme L. Invertebrates in Hot and Cold Arid Environments. Berlin: Springer; 1995. [Google Scholar]
- Sømme L. The effect of prolonged exposures at low temperatures in insects. Cryoletters. 1996;17:341–346. [Google Scholar]
- Sømme L. The physiology of cold hardiness in terrestrial arthropods. Eur J Entomol. 1999;96:1–10. [Google Scholar]
- Sørensen JG, Dahlgaard J, Loeschcke V. Genetic variation in thermal tolerance among natural populations of Drosophila buzzatii: down regulation of Hsp70 expression and variation in heat stress resistance traits. Funct Ecol. 2001;15:289–296. [Google Scholar]
- Sørensen JG, Michalak P, Justesen J, Loeschcke V. Expression of the heat-shock protein Hsp70 in Drosophila buzzatii lines selected for thermal resistance. Hereditas. 1999;131:155–164. doi: 10.1111/j.1601-5223.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- Sørensen JG, Norry FM, Scannapieco AC, Loeschcke V. Altitudinal variation for stress resistance traits and thermal adaptation in adult Drosophila buzzatii from the New World. J Evol Biol. 2005;18:829–837. doi: 10.1111/j.1420-9101.2004.00876.x. [DOI] [PubMed] [Google Scholar]
- Spicer JI, Gaston KJ. Physiological Diversity and its Ecological Implications. Oxford: Blackwell Science; 1999. [Google Scholar]
- Stanley SM. Earth and Life through Time. 2. New York: W.H. Freeman; 1989. [Google Scholar]
- Steele JH. A comparison of terrestrial and marine ecological systems. Nature. 1985;313:355–358. [Google Scholar]
- Steenkamp HE, Chown SL. Influence of dense stands of an exotic tree, Prosopis glandulosa Benson, on a savanna dung beetle (Coleoptera: Scarabaeinae) assemblage in southern Africa. Biol Conserv. 1996;78:305–311. [Google Scholar]
- Stefanescu C, Herrando S, Paramo F. Butterfly species richness in the north-west Mediterranean Basin: the role of natural and human-induced factors. J Biogeogr. 2004;31:905–915. [Google Scholar]
- Steidle JLM, Reinhard J. Low humidity as a cue for habitat preference in the parasitoid Lariophagus distinguendus. BioControl. 2003;48:169–175. [Google Scholar]
- Stenseth NC, Mysterud A. Weather packages: finding the right scale and composition of climate in ecology. J Anim Ecol. 2005;74:1195–1198. [Google Scholar]
- Stenseth NC, Ottersen G, Hurrell JW, Mysterud A, Lima M, Chan KS, Yoccoz NG, Adlandsvik B. Studying climate effects on ecology through the use of climate indices: the North Atlantic oscillation, El Niño southern oscillation and beyond. Proc R Soc Lond B. 2003;270:2087–2096. doi: 10.1098/rspb.2003.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman JH. Acclimation capacity underlies susceptibility to climate change. Science. 2003;301:65. doi: 10.1126/science.1083073. [DOI] [PubMed] [Google Scholar]
- Stillwell RC, Fox CW. Complex patterns of phenotypic plasticity: interactive effects of temperature during rearing and oviposition. Ecology. 2005;86:924–934. [Google Scholar]
- Stireman JO, III, Dyer LA, Janzen DH, Singer MS, Lill JT, Marquis RJ, Ricklefs RE, Gentry GL, Hallwachs W, Coley PD, Barone JA, Greeney HF, Connahs H, Barbosa P, Morais HC, Diniz IR. Climatic unpredictability and parasitism of caterpillars: implications of global warming. Proc Natl Acad Sci USA. 2005;102:17384–17387. doi: 10.1073/pnas.0508839102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch D, Gaston KJ, Cepák J. Pink landscapes: 1/f spectra of spatial environmental variability and bird community composition. Proc R Soc Lond B. 2002;269:1791–1796. doi: 10.1098/rspb.2002.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB. Biochemical adaptation for cold hardiness in insects. Philos Trans R Soc Lond B. 1990;326:635–654. [Google Scholar]
- Storey KB. Oxidative stress: animal adaptations in nature. Brazil J Med Biol Res. 1996;29:1715–1733. [PubMed] [Google Scholar]
- Storey KB. Organic solutes in freezing tolerance. Comp Biochem Physiol A. 1997;117:319–326. doi: 10.1016/s0300-9629(96)00270-8. [DOI] [PubMed] [Google Scholar]
- Storey KB. Life in the slow lane: molecular mechanisms of estivation. Comp Biochem Physiol A. 2002;133:733–754. doi: 10.1016/s1095-6433(02)00206-4. [DOI] [PubMed] [Google Scholar]
- Storey KB, Baust JG, Storey JM. Intermediary metabolism during low temperature acclimation in the overwintering gall fly larva, Eurosta solidaginis. J Comp Physiol. 1981;144:183–190. [Google Scholar]
- Storey JM, Storey KB. Winter survival of the gall fly larva, Eurosta solidaginis: profiles of fuel reserves and cryoportectants in a natural population. J Insect Physiol. 1986;32:549–556. [Google Scholar]
- Storey KB, Storey JM. Natural freezing survival in animals. Annu Rev Ecol Syst. 1996;27:365–386. [Google Scholar]
- Storey KB, Storey JM. Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev. 2004;79:207–233. doi: 10.1017/s1464793103006195. [DOI] [PubMed] [Google Scholar]
- Sultan SE. Promising directions in plant phenotypic plasticity. Perspect Plant Ecol Evol Syst. 2004;6:227–233. [Google Scholar]
- Sultan SE, Spencer HG. Metapopulation structure favors plasticity over local adaptation. Am Nat. 2002;160:271–283. doi: 10.1086/341015. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nijhout HF. Evolution of a polyphenism by genetic accommodation. Science. 2006;311:650–652. doi: 10.1126/science.1118888. [DOI] [PubMed] [Google Scholar]
- Tachibana SI, Numata H, Goto SG. Gene expression of heat-shock proteins (Hsp23, Hsp70 and Hsp90) during and after larval diapause in the blow fly Lucilia sericata. J Insect Physiol. 2005;51:641–647. doi: 10.1016/j.jinsphys.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Tammariello SP, Rinehart JP, Denlinger DL. Desiccation elicits heat shock protein transcription in the flesh fly, Sarcophaga crassipalpis, but does not enhance tolerance to high or low temperatures. J Insect Physiol. 1999;45:933–938. doi: 10.1016/s0022-1910(99)00073-6. [DOI] [PubMed] [Google Scholar]
- Tatem AJ, Bayliss M, Mellor PS, Purse BV, Capela R, Pena I, Rogers DJ. Prediction of bluetongue vector distribution in Europe and North Africa using satellite imagery. Vet Microbiol. 2003;97:13–29. doi: 10.1016/j.vetmic.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Tauber MJ, Tauber CA, Nyrop JP, Villani MG. Moisture, a vital but neglected factor in the seasonal ecology of insects: hypotheses and tests of mechanisms. Environ Entomol. 1998;27:523–530. [Google Scholar]
- Terblanche JS, Chown SL. The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae) J Exp Biol. 2006;209:1064–1073. doi: 10.1242/jeb.02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche JS, Klok CJ, Krafsur ES, Chown SL. Phenotypic plasticity and geographic variation in thermal tolerance and water loss of the tsetse Glossina pallidipes (Diptera: Glossinidae): implications for distribution modeling. Am J Trop Med Hyg. 2006;74:786–794. [PMC free article] [PubMed] [Google Scholar]
- Terblanche JS, Klok CJ, Marais E, Chown SL. Metabolic rate in the whip-spider, Damon annulatipes (Arachnida: Amblypygi) J Insect Physiol. 2004;50:637–645. doi: 10.1016/j.jinsphys.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Thanaphum S, Haymer DS. A member of the hsp70 gene family from the Mediterranean fruit fly, Ceratitis capitata. Ins Mol Biol. 1998;7:63–72. doi: 10.1046/j.1365-2583.1998.71051.x. [DOI] [PubMed] [Google Scholar]
- Thomas CD, Bodsworth EJ, Wilson RJ, Simmons AD, Davies ZG, Musche M, Conradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. [DOI] [PubMed] [Google Scholar]
- Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, de Siqueira MF, Grainger A, Hannah L, Hughes L, Huntley B, van Jaarsveld AS, Midgley GF, Miles L, Ortega-Huerta MA, Peterson AT, Phillips OL, Williams SE. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Thomas CD, Singer MC, Boughton DA. Catastrophic extinction of population sources in a butterfly metapopulation. Am Nat. 1996;148:957–975. [Google Scholar]
- Tichy H. Low rates of change enhance effect of humidity on the activity of insect hygroreceptors. J Comp Physiol A. 2003;189:175–179. doi: 10.1007/s00359-003-0397-z. [DOI] [PubMed] [Google Scholar]
- Tichy H, Loftus R. Hygroreceptors in insects and a spider: humidity transduction models. Naturwissenschaften. 1996;83:255–263. [Google Scholar]
- Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D. Forecasting agriculturally driven global environmental change. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo GP. A practical guide to wavelet analysis. Bull Am Meteorol Soc. 1998;79:61–78. [Google Scholar]
- Tsutsui ND, Suarez AV, Holway DA, Case TJ. Reduced genetic variation and the success of an invasive species. Proc Natl Acad Sci USA. 2000;97:5948–5953. doi: 10.1073/pnas.100110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova NM, Horváth I, Török Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vigh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci USA. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufto J. The evolution of plasticity and nonplastic spatial and temporal adaptations in the presence of imperfect environmental cues. Am Nat. 2000;156:121–130. doi: 10.1086/303381. [DOI] [PubMed] [Google Scholar]
- Van Kleunen M, Fischer M. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 2005;166:49–60. doi: 10.1111/j.1469-8137.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- Vannier G. The thermobiological limits of some freezing tolerant insects: the supercooling and thermostupor points. Acta Oecol. 1994;15:31–42. [Google Scholar]
- Van Tienderen PH. Evolution of generalists and specialists in spatially heterogeneous environments. Evolution. 1991;45:1317–1331. doi: 10.1111/j.1558-5646.1991.tb02638.x. [DOI] [PubMed] [Google Scholar]
- Van Tienderen PH. Generalists, specialists, and the evolution of phenotypic plasticity in sympatric populations of distinct species. Evolution. 1997;51:1372–1380. doi: 10.1111/j.1558-5646.1997.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Vasseur DA, Yodzis P. The color of environmental noise. Ecology. 2004;85:1146–1152. [Google Scholar]
- Vernon P, Vannier G. Evolution of freezing susceptibility and freezing tolerance in terrestrial arthropods. Comptes Rendus Biol. 2002;325:1185–1190. doi: 10.1016/s1631-0691(02)01536-6. [DOI] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Voituron Y, Mouquet N, de Mazancourt C, Clobert J. To freeze or not to freeze? An evolutionary perspective on the cold-hardiness strategies of overwintering ectotherms. Am Nat. 2002;160:255–270. doi: 10.1086/341021. [DOI] [PubMed] [Google Scholar]
- Waldow U. Electrophysiological investigations of moist, dry and cold receptors on antenna of migratory locust. Z Vergl Physiol. 1970;69:249–283. [Google Scholar]
- Walters AC, Mackay DA. An experimental study of the relative humidity preference and survival of the Argentine ant, Linepithema humile (Hymenoptera, Formicidae): comparisons with a native Iridomyrmex species in South Australia. Insectes Soc. 2003;50:355–360. [Google Scholar]
- Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Wang XH, Kang L. Differences in egg thermotolerance between tropical and temperate populations of the migratory locust Locusta migratoria (Orthoptera: Acridiidae) J Insect Physiol. 2005;51:1277. doi: 10.1016/j.jinsphys.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Ward D, Seely MK. Behavioral thermoregulation of six Namib Desert tenebrionid beetle species (Coleoptera) Ann Entomol Soc Am. 1996;89:442–451. [Google Scholar]
- Watanabe M, Kikawada T, Minagawa N, Yukuhiro F, Okuda T. Mechanism allowing an insect to survive complete dehydration and extreme temperatures. J Exp Biol. 2002;205:2799–2802. doi: 10.1242/jeb.205.18.2799. [DOI] [PubMed] [Google Scholar]
- Watson RT, editor. Climate Change 2001: Synthesis Report. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Webb TJ, Gaston KJ, Hannah L, Woodward FI. Coincident scales of forest feedback on climate and conservation in a diversity hot spot. Proc R Soc Lond B. 2006;273:757–765. doi: 10.1098/rspb.2005.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissling TJ, Giblindav RM. Water-loss dynamics and humidity preference of Rhynchophorus cruentatus (Coleoptera, Curculionidae) adults. Environ Entomol. 1993;22:93–98. [Google Scholar]
- West-Eberhard MJ. Phenotypic plasticity and the origins of diversity. Annu Rev Ecol Syst. 1989;20:249–278. [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford University Press; 2003. [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci USA. 2005;102:6543–6549. doi: 10.1073/pnas.0501844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westneat MW, Betz O, Blob RW, Fezzaa K, Cooper WJ, Lee WK. Tracheal respiration in insects visualized with synchrotron X-ray imaging. Science. 2003;299:558–560. doi: 10.1126/science.1078008. [DOI] [PubMed] [Google Scholar]
- Weston PA, Hoffman SA. Humidity and tactile responses of Sitophilus zeamais (Coleoptera, Curculionidae) Environ Entomol. 1991;20:1433–1437. [Google Scholar]
- Wiens JJ. What is speciation and how should we study it? Am Nat. 2004;163:914–923. doi: 10.1086/386552. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Graham CH. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst. 2005;36:519–539. [Google Scholar]
- Wikelski M, Cooke SJ. Conservation physiology. Trends Ecol Evol. 2006;21:38–46. doi: 10.1016/j.tree.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Williams JB, Ruehl NC, Lee RE. Partial link between the seasonal acquisition of cold-tolerance and desiccation resistance in the goldenrod gall fly Eurosta solidaginis (Diptera: Tephritidae) J Exp Biol. 2004;207:4407–4414. doi: 10.1242/jeb.01320. [DOI] [PubMed] [Google Scholar]
- Williams P, Hannah L, Andelman S, Midgley G, Araújo M, Hughes G, Manne L, Martinez-Meyer E, Pearson R. Planning for climate change: identifying minimum-dispersal corridors for the Cape Proteaceae. Conserv Biol. 2005;19:1063–1074. [Google Scholar]
- Willig MR, Kaufman DM, Stevens RD. Latitudinal gradients of biodiversity: pattern, process, scale and synthesis. Annu Rev Ecol Syst. 2003;34:273–309. [Google Scholar]
- Willmer PG. Microclimate and the environmental physiology of insects. Adv Ins Physiol. 1982;16:1–57. [Google Scholar]
- Willmer P, Stone G, Johnston IA. Environmental Physiology of Animals. Oxford: Blackwell Science; 1999. [Google Scholar]
- Wilson RS, Franklin CE. Testing the beneficial acclimation hypothesis. Trends Ecol Evol. 2002;17:66–70. [Google Scholar]
- Wolfe GR, Hendrix DL, Salvucci ME. A thermoprotective role for sorbitol in the silverleaf whitefly, Bemisia argentifolii. J Insect Physiol. 1998;44:597–603. doi: 10.1016/s0022-1910(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Wolfrum U. Cytoskeletal elements in insect sensilla. Int J Ins Morphol Embryol. 1997;26:191–203. [Google Scholar]
- Woods HA. Patterns and mechanisms of growth of fifth-instar Manduca sexta caterpillars following exposure to low- or high-protein food during early instars. Physiol Biochem Zool. 1999;72:445–454. doi: 10.1086/316678. [DOI] [PubMed] [Google Scholar]
- Woods HA, Harrison JF. The beneficial acclimation hypothesis versus acclimation of specific traits: physiological change in water stressed Manduca sexta. Physiol Biochem Zool. 2001;74:32–44. doi: 10.1086/319302. [DOI] [PubMed] [Google Scholar]
- Woods HA, Harrison JF. Interpreting rejections of the beneficial acclimation hypothesis: when is physiological plasticity adaptive? Evolution. 2002;56:1863–1866. doi: 10.1111/j.0014-3820.2002.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Woods HA, Hill RI. Temperature-dependent oxygen limitation in insect’s eggs. J Exp Biol. 2004;207:2267–2276. doi: 10.1242/jeb.00991. [DOI] [PubMed] [Google Scholar]
- Woods HA, Makino W, Cotner JB, Hobbie SE, Harrison JF, Acharya K, Elser JJ. Temperature and the chemical composition of poikilothermic organisms. Funct Ecol. 2003;17:237–245. [Google Scholar]
- Worland MR. The relationship between water content and cold tolerance in the arctic collembolan Onychiurus arcticus (Collembola: Onychiuridae) Eur J Entomol. 1996;93:341–348. [Google Scholar]
- Worland MR. Factors that influence the freezing in the sub-Antarctic springtail Tullbergia antarctica. J Insect Physiol. 2005;51:881–894. doi: 10.1016/j.jinsphys.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Worland MR, Block W. Desiccation stress at sub-zero temperatures in polar terrestrial arthropods. J Insect Physiol. 2003;49:193–203. doi: 10.1016/s0022-1910(02)00264-0. [DOI] [PubMed] [Google Scholar]
- Worland MR, Grubor-Lajsic G, Montiel P. Partial desiccation induced by sub-zero temperatures as a component of the survival strategy of the Arctic collembolan Onychiurus arcticus (Tullberg) J Insect Physiol. 1998;44:211–219. doi: 10.1016/s0022-1910(97)00166-2. [DOI] [PubMed] [Google Scholar]
- Worland MR, Leinaas HP, Chown SL. Supercooling point frequency distributions in Collembola are affected by moulting. Funct Ecol. 2006;20:323–329. [Google Scholar]
- Worland MR, Wharton DA, Byars SG. Intracellular freezing and survival in the freeze tolerant alpine cockroach Celatoblatta quinquemaculata. J Insect Physiol. 2004;50:225–232. doi: 10.1016/j.jinsphys.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Wu BS, Lee JK, Thompson KM, Walker VK, Moyes CD, Robertson RM. Anoxia induces thermotolerance in the locust flight system. J Exp Biol. 2002;205:815–827. doi: 10.1242/jeb.205.6.815. [DOI] [PubMed] [Google Scholar]
- Xenopoulos MA, Lodge DM, Alcamo J, Märker ML, Schulze K, van Vuuren D. Scenarios of freshwater fish extinctions from climate change and water withdrawal. Global Change Biol. 2005;11:1557–1564. [Google Scholar]
- Xu H, Robertson RM. Effects of temperature on properties of flight neurons in locust. J Comp Physiol A. 1994;175:193–202. [Google Scholar]
- Yamamoto AH. Temperature preference of Drosophila immigrans and D. virilis: intra- and inter-population genetic variation. Jpn J Gen. 1994;69:67–76. doi: 10.1266/jjg.69.67. [DOI] [PubMed] [Google Scholar]
- Ybarrondo BA. Habitat selection and thermal preference in two species of water scavenger beetles (Coleoptera: Hydrophilidae) Physiol Zool. 1995;68:749–771. [Google Scholar]
- Yi SX, Lee RE. Detecting freeze injury and seasonal cold-hardening of cells and tissues in gall fly larvae, Eurosta solidaginis (Diptera, Tephritidae) using fluorescent vital dyes. J Insect Physiol. 2003;49:999–1004. doi: 10.1016/s0022-1910(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Yi SX, Lee RE. In vivo and in vitro rapid cold-hardening protects cells from cold-shock injury in the flesh fly. J Comp Physiol B. 2004;174:611–615. doi: 10.1007/s00360-004-0450-4. [DOI] [PubMed] [Google Scholar]
- Yocum GD. Differential expression of two HSP70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. J Insect Physiol. 2001;47:1139–1145. doi: 10.1016/s0022-1910(01)00095-6. [DOI] [PubMed] [Google Scholar]
- Yocum GD, Denlinger DL. Prolonged thermotolerance in the flesh fly, Sarcophaga crassipalpis, does not require continuous expression or persistence of the 72 kDa heat-shock protein. J Insect Physiol. 1992;38:603–609. [Google Scholar]
- Yocum GD, Joplin KH, Denlinger DL. Expression of heat shock proteins in response to high and low temperatures in diapausing pharate larvae of the gypsy moth, Lymantria dispar. Archiv Insect Biochem Physiol. 1991;18:239–249. [Google Scholar]
- Yocum GD, Joplin KH, Denlinger DL. Upregulation of a 23 kDa small heat shock protein transcript during pupal diapause in the flesh fly Sarcophaga crassipalpis. Ins Biochem Mol Biol. 1998;28:677–682. doi: 10.1016/s0965-1748(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Yocum GD, Kemp WP, Bosch J, Knoblett JN. Temporal variation in overwintering gene expression and respiration in the solitary bee Megachile rotundata. J Insect Physiol. 2005;51:621–629. doi: 10.1016/j.jinsphys.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Yocum GD, Z̆dárek J, Joplin KH, Lee RE, Smith DC, Manter KD, Denlinger DL. Alteration of the eclosion rhythm and eclosion behavior in the flesh fly, Sarcophaga crassipalpis, by low and high temperature stress. J Insect Physiol. 1994;40:13–21. [Google Scholar]
- Yoder JA, Benoit JB, Denlinger DL, Rivers DB. Stress-induced accumulation of glycerol in the flesh fly, Sarcophaga bullata: evidence indicating anti-desiccant and cryoprotectant functions of this polyol and a role for the brain in coordinating the response. J Insect Physiol. 2006;52:202. doi: 10.1016/j.jinsphys.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Yokahari F. Hygroreceptor mechanism in the antenna of the cockroach Periplaneta. J Comp Physiol. 1978;124:53–60. [Google Scholar]
- Zachariassen KE. Physiology of cold tolerance in insects. Physiol Rev. 1985;65:799–832. doi: 10.1152/physrev.1985.65.4.799. [DOI] [PubMed] [Google Scholar]
- Zamudio KR, Huey RB, Crill WD. Bigger isn’t always better: body size, developmental and parental temperature and male territorial success in Drosophila melanogaster. Anim Behav. 1995;49:671–677. [Google Scholar]
- Zatsepina OG, Velikodvorskaia VV, Molodtsov VB, Garbuz D, Lerman DN, Bettencourt BR, Feder ME, Evgenev MB. A Drosophila melanogaster strain from sub-equatorial Africa has exceptional thermotolerance but decreased Hsp70 expression. J Exp Biol. 2001;204:1869–1881. doi: 10.1242/jeb.204.11.1869. [DOI] [PubMed] [Google Scholar]
- Zavaleta ES, Hobbs RJ, Mooney HA. Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol. 2001;16:454–459. [Google Scholar]
- Zeilstra I, Fischer K. Cold tolerance in relation to developmental and adult temperature in a butterfly. Physiol Entomol. 2005;30:92–95. [Google Scholar]
- Zera AJ, Denno RF. Physiology and ecology of dispersal polymorphism in insects. Annu Rev Entomol. 1997;42:207–231. doi: 10.1146/annurev.ento.42.1.207. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Zhao ZW. Morph-dependent fatty acid oxidation in a wing-polymorphic cricket: implications for the trade-off between dispersal and reproduction. J Insect Physiol. 2003;49:933–943. doi: 10.1016/s0022-1910(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zera AJ. Differential lipid biosynthesis underlies a tradeoff between reproduction and flight capability in a wing-polymorphic cricket. Proc Natl Acad Sci USA. 2002;99:16829–16834. doi: 10.1073/pnas.262533999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zera AJ. The hemolymph JH titer exhibits a large-amplitude, morph-dependent, diurnal cycle in the wing-polymorphic cricket, Gryllus firmus. J Insect Physiol. 2004a;50:93–102. doi: 10.1016/j.jinsphys.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zera AJ. A morph-specific daily cycle in the rate of JH biosynthesis underlies a morph-specific daily cycle in the hemolymph JH titer in a wing-polymorphic cricket. J Insect Physiol. 2004b;50:965–973. doi: 10.1016/j.jinsphys.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Altner H. Are the most numerous sensilla of terrestrial isopods hygroreceptors? Ultrastructure of the dorsal tricorn sensilla of Porcellio scaber. Cell Tiss Res. 1995;282:135–145. doi: 10.1007/BF00319140. [DOI] [PubMed] [Google Scholar]