Abstract
A colonial protochordate, Botryllus schlosseri, undergoes a natural transplantation reaction in the wild that results alternatively in colony fusion (chimera formation) or inflammatory rejection. A single, highly polymorphic histocompatibility locus (called Fu/HC) is responsible for rejection versus fusion. Gonads are seeded and gametogenesis can occur in colonies well after fusion, and involves circulating germ-line progenitors. Buss proposed that colonial organisms might develop self/non-self histocompatibility systems to limit the possibility of interindividual germ cell “parasitism” (GCP) to histocompatible kin [Buss, L. W. (1982) Proc. Natl. Acad. Sci. USA 79, 5337–5341 and Buss, L. W. (1987) The Evolution of Individuality (Princeton Univ. Press, Princeton]. Here we demonstrate in laboratory and field experiments that both somatic cell and (more importantly) germ-line parasitism are a common occurrence in fused chimeras. These experiments support the tenet in Buss’s hypothesis that germ cell and somatic cell parasitism can occur in fused chimeras and that a somatic appearance may mask the winner of a gametic war. They also provide an interesting challenge to develop formulas that describe the inheritance of competing germ lines rather than competing individuals. The fact that fused B. schlosseri have higher rates of GCP than unfused colonies additionally provides a rational explanation for the generation and maintenance of a high degree of Fu/HC polymorphism, largely limiting GCP to sibling offspring.
With the exception of pregnancy, vertebrates do not undergo natural transplantation, and therefore do not become chimeras. However, many marine invertebrates naturally undergo transplantation interactions, which can result in chimeras with fused vascular systems (1, 2). One such invertebrate, the colonial tunicate Botryllus schlosseri, is a protochordate that has a dispersal stage known as a tadpole larvae, which, like vertebrates, possess a notochord, neural tube, and segmented musculature (3). When its tadpole larvae disperse from a mother colony, they rapidly settle and metamorphose into a founder individual (oozooid) that loses its chordate characteristics and, like other clonal invertebrates, through asexual reproduction grow into a multi-individual colony composed of genetically identical clonemates that are interconnected by extracorporeal blood vessels (4). If two oozooids settle next to each other or two adult colonies meet as they spread over a marine surface, one of two things occurs: vascular fusion leading to blood chimerism (Fig. 1), or an inflammatory rejection reaction, which maintains the physical boundaries of the contacting individuals (5–7). As demonstrated by Oka and Watanabe (6) and confirmed by Scofield et al. (8), whether fusion or rejection occurs in this species is determined by the genotypes of the contacting individuals at a single allorecognition locus, called Fu/HC (fusibility/histocompatibility). This locus is highly polymorphic within natural populations (9, 10) and, thus, the principal locus for natural allorecognition in these colonial tunicates, like the major allorecognition locus in vertebrates (the major histocompatibility complex or MHC), essentially allows allele matching only with close relatives (8). While MHC polymorphism in vertebrates is believed to be maintained by the role of MHC products in presenting foreign (largely infectious agent) peptides, the nature of the genes for Fu/HC allorecognition, and the selective forces for their allelic polymorphism are undetermined. Here we provide evidence supporting the hypothesis that allorecognition in colonial tunicates serves to prevent parasitism of their gonads by circulating germ-line stem cells of Fu/HC disparate individuals (1).
Figure 1.

A ventral view of a newly fused chimera of B. schlosseri showing the fusion of the common tunic and blood vascular system as well as what tissues were sampled in the laboratory fusion study. Note the color difference among the component colonies. These colonies originate from a single founder individual and grow through asexual multiplication (budding) of the original founder. As they enlarge, the genetically identical individuals or zooids become arranged in star-shaped systems. Within and between systems the zooids are connected to each other by a blood vascular system and a common overlying tunic. Reproductive colonies are hermaphroditic with each zooid containing both testes and ovaries (4). Located around the periphery of each colony are the ampullae, which are bulb-like end points of the blood vascular system. Fusion or rejection occurs when the ampullae of two adjacent colonies come into contact.
During embryonic vertebrate development germ-line stem cells pass from the inner cell mass to the extraembryonic mesoderm, and from there back to the body proper (upon vascular connection between yolk sac and embryo), where they colonize the gametogenic microenvironments provided by the genital ridges (11). In colonial tunicates gonadal development occurs in the asexual phase of their life history and, like vertebrates, their gametogenic gonadal microenvironments receive blood-borne germ-line stem cell progenitors (12). In a remarkable set of experiments Sabbadin and Zaniolo (13) demonstrated that within chimeras of the colonial tunicate B. schlosseri, where the blood supplies of all the component individuals are interconnected, it is possible for the germ-line cells of one member of a chimera to migrate and establish themselves within the gonads of its chimeric partners. Intriguingly, they also found that the germ-line cells of one individual were often more effective than the germ-line cells of its partner in gaining access to germ-line positions, suggesting that chimerism can often result in either germ cell or somatic cell parasitism (abbreviated hereafter as G/SCP) depending on what cell lines are involved. In their experiments, genetically defined pigment markers were used to type the progeny of crosses involving laboratory colonies that were experimentally fused and then later separated. Recently, Pancer et al. (14) and independently ourselves (see below) confirmed these results for laboratory B. schlosseri in experiments that used microsatellites as genetic markers. Whether G/SCP also occurs naturally in the field was not addressed by these experiments.
Buss (1) and others (15, 16) proposed that chimerism may result in demographic benefits such as increased size, growth rates, reproduction, and survivorship. However, Chadwick-Furman and Weissman (17) have shown both in the laboratory and the field that chimerism actually reduces fitness by causing a decrease in growth, reproduction, and survivorship. Buss (1) and others (15, 16) alternatively suggested that fusion could be beneficial if it were limited to close relatives because fitness losses due to G/SCP might be compensated by an increase in inclusive fitness (that is, a heritable advantage to the germ lines of relatives). They further proposed that because the allorecognition systems of clonal invertebrates limits fusion to close relatives, they may have evolved as a mechanism to minimize the fitness costs of G/SCP. We show here that G/SCP occurs extensively within the laboratory, and more importantly, field chimeras of B. schlosseri and as Buss (1) hypothesized unfused adjacent colonies largely lack G/SCP. Our finding that fused colonies will often contain germ-line contributions from more than one genotype within a chimera, suggests that not only is the individual no longer the unit of inheritance [as is assumed in most evolutionary theory (18)], but germ cell lineage competition may be the principal force for selection.
METHODS
Fused chimeras of B. schlosseri were produced by identifying fusible pairs of colonies (see ref. 19 for methods), cutting these colonies into four equal-sized subclones, and placing one subclone from each compatible colony adjacent to each other on a glass slide. Colonies generally fused with each other within 2–3 days after being placed on the slide. The four pairs of colonies were randomly assigned to one of four treatments. They were either allowed to fuse and harvested after (i) 1 week, (ii) 4 weeks, (iii) 8 weeks, or (iv) the pairs were separated 1 week after fusion and harvested 7 weeks later (Fig. 2). Harvesting involved collecting tissue samples at several sites along a linear transect that bisected the plane of fusion. At each collection site, samples were taken of a single bud (to test somatic chimerism) and sperm liberated from the testes of an adjacent adult zooid (to test gametic chimerism). Harvesting also involved taking blood samples with a glass capillary tube from either the entire chimera or from both separated colonies in the case of treatment 4. Although care was taken to exclude blood cells from bud collections, contamination at low levels was possible. Genetic typing of the tissues from all colonies before and after fusion was performed by extracting the DNA from the tissues and using PCR to amplify several microsatellite loci [D.S.S., J. M. Quattro, and I.L.W., unpublished work) and Pancer et al. (20)].
Figure 2.
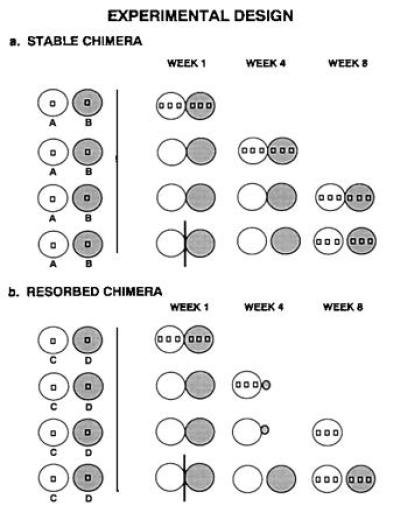
(a) Experimental protocol used in the 8-week laboratory experiment to sample stable chimeras. Circles represent subclones and squares within circles represent sampling sites. (b) Experimental protocol used in the 8-week laboratory experiment when one of the chimeric partners was resorbed. The experimental protocol was identical except that after resorption the sampling of tissues was restricted to just the remaining chimeric partner.
RESULTS
Laboratory Colony Fusions: Somatic Chimerism.
Nine fused chimeras of B. schlosseri were experimentally produced in the laboratory and the temporal and spatial patterns of genetic admixture within these chimeras were followed using microsatellite loci. Upon fusion a second, heritable allorecognition event known as resorption can occur, which results in the death of all zooids within one colony and their resorption by the viable partner (21). Because patterns of genetic admixture varied according to whether resorption occurred (4 of 9 chimera), in Table 1 and in our discussion of results we distinguish chimeras that underwent resorption from those that remained stable. Within 8 weeks sampled blood and/or tissues showed the genotypes of both partners in all nine chimeric pairs, but there was considerable variation in both the rate and spatial pattern of admixture (Table 1, Fig. 3a). At week 1, three of four chimeras that later showed resorption were chimeric in one partner’s buds, whereas none of five partners that had remained fused (in this observation period) had a detectable amount of partner DNA in buds, although in all distinguishable pairs the blood was chimeric (Fig. 3a). By 4 weeks, and sustained through week 8, the still-fused partners contained buds that were mixes of both genotypes. By week 8 the genetic material of one of the four “losers” in the resorbed chimera could no longer be detected. Spatially, chimeras exhibited either a sectorial pattern in which both genotypes were detected within some systems but not in others (Table 1, lines 10, 11, 22, and 23), or a uniform pattern in which tissues throughout the entire chimera exhibited both genotypes (Table 1, lines 13, 14, 16, 17, 19, 20, 25, 26, 28, and 29).
Table 1.
Spatial and temporal pattern of genetic admixture within nine laboratory produced chimeras
| Line no. | Chimera (genotypes) | Locus | Tissue type | Week 1 | Week 4 | Week 8 | Separated |
|---|---|---|---|---|---|---|---|
| Resorbed partners | |||||||
| 1 | 10.63 v 38 | PBC1 | Bud | AB,AB | B,B | R | B,AB,B | R | B,B,B,B,B | AB,– | B,B |
| 2 | (AA | BB) | Sperm | A,– | B,B | R | B,B,B | R | B,B,B,B,B | AB,AB | B,B | |
| 3 | Blood | AB | B | B | AB | B | ||
| 4 | 745un v 10.63 | PB41 | Bud | AB,AB | AB,AB | AB,AB,AB,AB,AB | R | AB,AB,AB | R | ND |
| 5 | (AB | AA) | Sperm | –,– | –,– | AB,–,–,AB,– | R | AB,AB,AB | R | ND | |
| 6 | Blood | AB | AB | AB | ND | ||
| 7 | 745un v 10.63 | PB49 | Bud | A,A | AB,AB | A,A,A,A,A | R | A,A,A | R | ND |
| 8 | (AA | AB) | Sperm | –,– | –,– | AB,–,–,A,– | R | A,AB,AB | R | ND | |
| 9 | Blood | AB | A | A | ND | ||
| 10 | 151 v 3.30 | PBC1 | Bud | A,A | B,B | AB,AB,AB | R | AB,AB,AB | R | AB,AB,AB | B,B |
| 11 | (AA | BB) | Sperm | A,A | B,B | BAB,AB | R | BABB | R | AB,AB,AB | B,B | |
| 12 | Blood | AB | AB | AB | AB | B | ||
| 13 | 45.2 v 158 | PB49 | Bud | AB | AB,AB | R | AB,AB | R | AB,AB | A,A | AB,AB |
| 14 | (AA | AB) | Sperm | AB | AB,AB | R | AB,– | R | –,– | A,A | A,A | |
| 15 | Blood | AB | AB | AB | A | AB | ||
| Chimeric partners | |||||||
| 16 | 6 v 152 | PB41 | Bud | BC,BC | A,A | ABC | ABC,ABC | ABC,ABC | ABC,ABC | ND |
| 17 | (BC | AA) | Sperm | BC,BC | A,A | ABC | ABC,ABC | ABC,ABC | ABC,ABC | ND | |
| 18 | Blood | ABC | ABC | ABC | ND | ||
| 19 | 240 v 165b | PB41 | Bud | AB,AB | AC,AC | ABC,ABC | ABC,ABC | ABC,ABC | ABC,ABC | ABC,ABC,ABC | ABC,ABC |
| 20 | (AB | AC) | Sperm | AB,AB | AC,AC | ABC,ABC | ABC,ABC | ABC,ABC | ABC,ABC | ABC,ABC,ABC | ABC,ABC | |
| 21 | Blood | ABC | ABC | ABC | ABC | ABC | ||
| 22 | 15 v 154 | PB49 | Bud | B,B | AB,AB | B,AB,B | AB,AB | AB,B | AB,AB | ND |
| 23 | (BB | AB) | Sperm | B,B | AB,AB | B,AB,B | B,B | AB,B | ABB | ND | |
| 24 | Blood | AB | AB | AB | ND | ||
| 25 | d25.1 v 88 | PB41 | Bud | AB,AB | AC,AC | ABC,ABC,ABC | ABC,ABC | ABC,ABC | ABC,ABC,ABC | ABC,ABC | ABC,ABC |
| 26 | (AB | AC) | Sperm | AB,AB | AC,AC | ABC,ABC,ABC | ABABC | ABC,ABC | –,–,– | ABC,– | ABC,ABC | |
| 27 | Blood | ABC | ABC | ABC | ABC | ABC | ||
| 28 | 41.5 v 220.53 | PB41 | Bud | AB,AB | AC,AC | ABC,ABC | AC,AC | ND | AB,AB | ABC,ABC |
| 29 | (AB | AC) | Sperm | –,– | –,– | ABC,ABC | ABC,ABC | ND | –,– | –,– | |
| 30 | Blood | ABC | ABC | ND | ABC,ABC | ABC,ABC | ||
Listed are the microsatellite bands observed for a particular tissue within a particular system. The site of fusion is indicated by a |. The systems closest to the site of fusion are listed next to the |, and the systems farther away are listed either to the left or right of the closest systems. –, no sample was taken for a particular tissue; ND, no data were collected because a particular chimera died before the appropriate sampling time; □, replacement of endogenous tissue with fused partner’s tissue; R, resorption. Note that lines 4–6 and 7–9 are for the same chimera analyzed with two different microsatellite loci.
Figure 3.
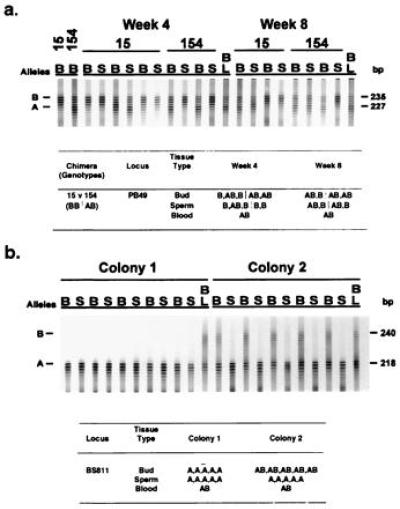
(a) An autoradiograph and interpretative table (data organized as in Table 1) that show the results of genotypic sampling of buds (B), sperm (S), and blood (BL) from a stable laboratory chimeric pair in which G/SCP was observed. To produce this chimeric pair, colony 15 genotyped with the microsatellite locus PB49 as having allele B was fused with colony 154, which was genotyped as sharing allele B and having a unique allele A. One week after fusion, the blood showed the hybrid genotype AB, whereas the other tissues showed their original genotype (Table 1, lines 22 and 23). By week 4, the blood still had genotype AB, one of the systems in colony 15 showed genetic admixture in both the somatic and gametic tissues, and in colony 154 there was complete (at least 96%) replacement of the host gametic tissue by a cell line having genotype B of colony 15. Similar results were obtained for the paired subclone sampled 8 weeks after fusion. (b) An autoradiograph and interpretative table (data organized as in Table 1) that show the results of genotypic sampling of buds (B), sperm (S), and blood (BL) from a field chimera in which G/SCP was observed. The tissues were typed using microsatellite locus BS811 (14). Based on the spatial pattern of genotypes within the somatic tissues of the chimera at the time of collection it is presumed that the initial genotypes for the two colonies were AA (Left) and AB (Right). After fusion, both the somatic and gametic tissues of colony AA appear to remain unchanged. In contrast, while the somatic tissues of colony AB retained their original genotype its gametic tissues were almost completely replaced by tissues having genotype AA. This replacement is interpreted as an example of G/SCP.
In the laboratory colonies, replicate pairs that fused at time 0 were surgically separated at 1 week, then analyzed at 8 weeks. In the resorbed chimeras set zero of four tested had detectable bud chimerism. In the set whose replicates remained fused at 8 weeks, five of five tested were chimeric in their buds although none of the five were detectable bud chimeras at week 1.
None of the bud chimeras were entirely derived from the fused partner’s cells. This indicates not only that asexually derived buds are not clonal (consistent with histological evidence that bud development involves several distinct cell types), but also that somatic parasitism is not complete in the cases studied.
Laboratory Colony Fusions: Germ Cell Parasitism (GCP).
Individual testes were isolated from fused colonies and tested for genotypes of each partner. In the four chimeras of the resorbed set, two had partner’s genotype (Table 1, lines 8 and 11). In the still-fused set, all five had testes that contained partner’s genotype (Table 1, lines 17, 20, 23, 26, and 29). Separated colonies in both sets also showed GCP. In a few chimeric pairs (e.g., line 14), genetic admixture eventually led to the detection of partner but not host elements in the testes. This apparent replacement of gametic tissues was completed in as little as 4 weeks and persisted for at least 8 weeks after fusion (Table 1, lines 11, 14, 23, and 26). Remarkably, it was observed in both chimeras that remained fused, as well as in resorbed chimeras (Table 1, lines 11, 14, 23, and 26; Fig. 3a). The phenomenon of complete replacement could result from clonogenic seeding of a testis or from cell lineage competition.
It could be claimed that in several instances the chimerism and/or replacement of somatic or testicular cells is an artifact of the assay method, that is, that absence of detection of a particular microsatellite marker may not result from absence of cells carrying that marker. However, in all of these experiments concurrent titration controls were carried out, and for the probes used, the limits of sensitivity were about 2–4% of the opposite partner’s type (the range of DNA mixtures tested was from 1:50 to 50:1 at the same final DNA concentration (1 ng/μl). Thus, undetectable levels of a particular microsatellite band would mean that at least 96–98% of the DNA tested came from the positive partner’s genome.
Two lines of evidence from the laboratory experiment suggest that the phenomenon of G/SCP results from the differentiation of pluripotent cells that migrate within the common circulatory system of the chimeras. First, the partner’s genotype was often found mixed with the host genotype in the chimeras that had been separated for 1 month (Table 1, lines 1–3, 10–12, 19–21, 25–27, and 28–30). Second, within chimeras that remained fused, the competing genotype was found not only in tissues adjacent to the site of fusion but also at sites that were far removed (Table 1, lines 16, 17, 19, 20, 22, 23, 25, 26, 28, and 29). Tissue replacement could be occurring either as a result of chance or as a result of direct cell lineage competition. Chance would be most likely if tissues such as the testis are initially formed by a single cell and its descendants (thus clonal), whereas cell lineage competition would be more likely if tissues are formed through the coalescence of several cell lines. Clonal markers will be required to resolve this point.
Field Chimeras: Somatic Chimerism.
G/SCP is also an important phenomenon in nature as we show by genetic examination of the tissues of 10 chimeras [initially identified by a color polymorphism (22)], collected from a marina in Monterey, California. The number of genetically distinct colonies forming these chimeras was either two or three. Using five microsatellite loci to type tissues (D.S.S., J. M. Quattro, and I.L.W., unpublished work), we detected the genotypes of two or more partners in the somatic tissues of 8 of 10 chimeras (Table 2, lines 1, 4, 7, 10, 13, 16, 22, and 25) and in the blood of all 10 chimeras (Table 2, lines 3, 6, 9, 12, 15, 18, 21, 24, 27, and 30). When there were three colonies forming a chimera, the chimeric buds would sometimes be a genetic mixture of just two neighboring colonies (Table 2, line 1) and other times would be a mixture of all three colonies (Table 2, lines 1 and 10). Unlike the laboratory examples, in 2 of 10 field chimeras there was also complete replacement of some bud tissues by a partner (Table 2, lines 13 and 22). When this replacement occurred, there was no corresponding blood cell color variation among zooids, presumably as a result of the long-term mixture of circulating pigment cells within the chimera prior to collection.
Table 2.
Microsatellite banding patterns of 10 field chimeras
| Line no. | Chimera | Locus (genotype) | Tissue | Colony 1 | Colony 2 | Colony 3 |
|---|---|---|---|---|---|---|
| 1 | 1 | PB41 | Bud | BC,BC,BC,BC,BC | AC,AC,AC,AC,AC | ABC,ABC,ABC,ABC,ABC |
| 2 | (B | C | A) | Sperm | BC,B,BC,BC,B | C,C,C,C,C | A,A,A,ABC,ABC | |
| 3 | Blood | ABC | ABC | ABC | ||
| 4 | 2 | PB41 | Bud | ABC,ABC,ABC,ABC,ABC | C,C,C,C,C | |
| 5 | (AB | C) | Sperm | AB,AB,AB,ABC,ABC | C,C,C,C,C | ||
| 6 | Blood | ABC | ABC | |||
| 7 | 3 | PB41 | Bud | ABC,BC,BC,ABC,BC | AC,ABC,AC,AC,AC | |
| 8 | (BC | AC) | Sperm | BC,BC,BC,ABC,BC | AC,AC,AC,AC,AC | ||
| 9 | Blood | ABC | ABC | |||
| 10 | 4 | PB41 | Bud | ABCD,ABCD,ABCD,ABCD,ABCD | ABCD,ABCD,ABCD,ABCD,ABCD | CD,CD,CD,ABCD,CD |
| 11 | (BC | AD | CD) | Sperm | BC,BC,BC,ABCD,ABC | AD,AD,ABCD,ABCD | CD,CD,CD,ABCD,CD | |
| 12 | Blood | ABCD | ABCD | ABCD | ||
| 13 | 5 | PB41 | Bud | C,C,C,CB | B,BC,BC,BC,B | |
| 14 | (C | B) | Sperm | BCB,B,BBC | BCC,CBC,BC | ||
| 15 | Blood | BC | BC | |||
| 16 | 6 | PB41 | Bud | AB,AB,AB,AB,AB | A,AB | |
| 17 | (AB | A) | Sperm | AB,AB,AB,AB,AB | –,– | ||
| 18 | Blood | AB | AB | |||
| 19 | 7 | BS811 | Bud | A,A,A,A,A | AB,AB,AB,AB,AB | |
| 20 | (A | AB) | Sperm | A,A,A,A,A | A,A,A,A,A | ||
| 21 | Blood | AB | AB | |||
| 22 | 8 | PB41 | Bud | AC,AC,AC,AC,AC | ACB,B,B,B | |
| 23 | (AC | B) | Sperm | AC,AC,AC,AC,AC | ACB,B,B,B | ||
| 24 | Blood | ABC | ABC | |||
| 25 | 9 | PB41 | Bud | BC,BC,BC,BC,BC | ABC,ABC,ABC,ABC,ABC | |
| 26 | (BC | A) | Sperm | BC,BC,BC,BC,BC | A,A,A,A,A | ||
| 27 | Blood | ABC | ABC | |||
| 28 | 10 | PB41 | Bud | AB,AB,AB,AB,AB | BC,BC,BC,BC,BC | |
| 29 | (AB | BC) | Sperm | AB,AB,AB,AB,AB | AB,AB,AB,AB,AB | ||
| 30 | (AC | BC) | Blood | ABC | ABC |
Columns 5–7 represent the banding patterns for the 2–3 individuals that formed each of the 10 field chimeras. The data are presented as in Table 1 with the exception that data for the chimeric partners are presented in separate columns. When there are more than two colonies per chimera the data are presented so that the systems farthest (closest) away from any plane of fusion are listed to the left (right).
Field Chimeras: GCP.
Evidence of germ cell transfer and establishment into partner’s testes could be detected in 8 of 10 chimeras. Two or more genotypes were detected in the testes of at least one partner in 5 of 10 field chimeras (Table 2, lines 2, 5, 8, 11, and 14) and GCP was detected in 4 of 10 field chimeras (Table 2, lines 14, 20, 23, and 29). In two of four of the chimeras showing GCP the degree of reproductive parasitism was much greater than ever observed in the laboratory; almost the entire spermatic output was usurped by just one of the chimeric partners (Table 2, lines 20 and 29; Fig. 3b). Differences between laboratory and field chimeras in the frequency and extent of GCP might be related to the length of time since fusion (Table 1), but confirmation of this hypothesis awaits long-term field studies in which chimeras are continuously monitored after fusion.
Field Chimeras: Analysis of GCP by Progeny Testing.
It was important to test whether GCP resulted in allogeneic reproductive success (as measured by oozooid production), as well as spermatic success. The test involved setting up crosses (as described in ref. 23) initiated by separating field colonies in half, mating them to a third colony from the laboratory, and scoring the microsatellite genotypes of the progeny. Within a cross the chimeric halves served as either father or mother. The results, shown in Table 3, demonstrate that in crosses involving the halves, where testicular GCP was not detected by PCR analysis (8.1 and 10.1), no offspring were produced that were sired by the other partner. In contrast, when the parasitized partner (8.2 and 10.2) served as the sperm donor, crosses produced offspring sired by the parasitizing partner in numbers roughly equivalent to the proportion of testes bearing the partner’s microsatellite genotype (Table 3). Note particularly that in the cross involving colony 10.2, the chimeric half, which appeared from microsatellite analysis of testes to be completely parasitized, all offspring had the partner’s genotype. Similar results were observed in crosses where the “parasitized” half served as the mother. In crosses involving 10.2, all offspring were derived from 10.1 oocytes, and in crosses involving 8.2, 12 of 27 oocytes were derived from 8.1 (Table 3). Thus, GCP involves eggs as well as sperm. The sperm output of each genotype appears to predict both male and female reproductive output as assayed by the progeny. Results from crosses further suggest that genotypes determined through the use of microsatellites are not affected by contamination from surrounding tissues because otherwise the results from typing sperm would not have matched the results of typing offspring from the crosses. They also confirm that when a band(s) representing a particular genotype is absent in a particular assay, it indicates that either no cells or very few cells having this genotype remain in the tissue.
Table 3.
Number of progeny sired by the chimeric partner of the colony that contributed sperm or eggs to a defined cross
| Defined cross: paternal × maternal colony | Total no. of progeny | No. of progeny sired by chimeric partner |
|---|---|---|
| 8.1 × 745un | 32 | 0 |
| 8.2 × BBYD73 | 32 | 4 |
| 745un × 8.2 | 27 | 12 |
| 10.1 × 670.1 | 24 | 0 |
| 10.2 × 670.2 | 24 | 24 |
| 670.2 × 10.2 | 24 | 24 |
The distribution of genotypes of progeny sired by the chimeric partner (8.1 or 10.1) were as expected under the assumption that they were the product of just two parental colonies.
Analysis of Adjacent Unfused Field Colonies.
If allorecognition evolved and/or is maintained to reduce the costs of G/SCP, there should be little or no evidence of G/SCP within rejecting colonies. To test this idea we collected 15 pairs of colonies from the Monterey marina that were in physical contact along a broad border but were not at the time fused. We harvested and genotyped the tissues from these colonies as described above for the chimeric field colonies. Genetic analysis of these tissues revealed no evidence of either genetic admixture or G/SCP in 13 of the 15 colony pairs. Surprisingly, one partner in a pair showed evidence of both GCP and SCP, and in another pair a low level of SCP was found in one partner. A G test found that the estimated rate of G/SCP in putatively rejecting colonies (6.7%) was significantly lower than the estimated rate for fused chimeras (40%) (G = 4.1, df = 1, P < 0.05) as would be expected if allorecognition evolved to reduce the costs of G/SCP. The unfused pairs are categorized as putatively rejecting because we were unable to determine whether these paired colonies had in the past undergone either rejection or fusion. (A prospective study of rejecting field colonies would require up to 1 year to complete.) Rejecting colonies usually exhibit brown points of rejection near their periphery. Unfortunately, none of the 15 unfused paired colonies showed these signs of rejection. The lack of any physical evidence of rejection does not mean that rejection never occurred. The physical manifestations of rejection often disappear within 1–3 weeks after they first form. However, it is also possible that the paired colonies had previously fused and then separated as they sometimes do in the laboratory (24). Still another possibility is that for various reasons the unfused pairs never even reacted to each other (25).
CONCLUSIONS
In this paper we provide evidence supporting Buss’s hypothesis (1) that invertebrate allorecognition systems may have evolved to prevent cell lineage competition resulting from the introduction of somatic and gametic progenitors that can accompany colony fusion. First we confirmed the results of Sabbadin and Zaniolo (13) and Pancer et al. (14) that G/SCP can occur within laboratory produced chimeras of B. schlosseri. We then extended these results to the field by demonstrating that G/SCP also occurs within chimeras of B. schlosseri, which have been growing naturally in the wild. The laboratory experiments also suggest that (i) the temporal and spatial pattern of genetic admixture covaries with whether colony resorption takes place and (ii) GCP and SCP occur as a result of the migration and subsequent establishment of foreign circulating germ-line cells and totipotent somatic stem cells. The importance of G/SCP as a selective factor may be quite dramatic in some populations. The rate of chimerism within natural populations of B. schlosseri can reach levels as high as 20% (9, 26) and our data suggest a 40% incidence of G/SCP within those chimeras. Finally, as predicted by Buss’s hypothesis (1), we showed that the rate of G/SCP drops considerably when colony rejection occurs instead of fusion. However, a more critical test than performed here involving prospective rather than retrospective analyses of rejecting field colonies needs to be undertaken to demonstrate more conclusively such a reduction in G/SCP.
The replacement of one cell line by another within the gametic tissues of B. schlosseri chimeras was characterized here as a form of parasitism because the “winner’s” excess reproduction is obtained through the usurpation of the somatic resources of the “loser.” Indeed, in some cases the somatic tissues of the winner are completely resorbed by the losing colony and thus the winner can only exist through the usurpation of the loser’s somatic resources. Still to be determined though is whether certain genotypes exist that are parasitic specialists, and like good parasites are capable of spreading infectiously throughout a population. Answering this question will entail breeding studies to determine whether the ability to “win” in cell line competition is a heritable trait subject to selection and long-term field studies to determine whether over time the winners of G/SCP tend to spread locally in a population. In addition to B. schlosseri, G/SCP has been reported to occur in a cellular slime mold, a colonial hydroid and Neurospora (1, 27, 28). In the case of the cellular slime mold, specialized parasitic genotypes that must usurp the loser’s somatic resources to survive have been identified (1). It has been proposed that these winners are successful because instead of using some of their resources to construct a soma, they invest all of their energy into reproduction.
Due to the ancestral position of ascidians in the phylum chordata, there is the possiblity that the Fu/HC locus is an ancestral form of the vertebrate MHC or may have shared a common ancestor with the MHC (discussed in refs. 29 and 30). Indeed, the mechanism of action in Fu/HC is reminiscent of native allorecognition displayed by natural killer cells. Natural killer recognition and killing is dependent on the presence or absence of self-MHC class I alleles. Until the Fu/HC gene is cloned and sequenced it cannot be determined if the biological similarity is structural or merely semantic (31). If the two genes turn out to be structurally homologous, studies of the Fu/HC locus might reveal much about the origins and diversity of the MHC. For instance, the results presented here would suggest that if homologous to Fu/HC the MHC may not have initially evolved as a defense mechanism against infectious microorganisms and that the well-developed polymorphism in MHC molecules was present prior to the development of the vertebrate T-cell receptor recognition system. Even if the similarity between vertebrate MHC allorecognition and protochordate Fu/HC allorecognition is semantic rather than structural, the recognition events found in B. schlosseri may also turn up in vertebrates. For example, although natural transplantation in vertebrates is rare, pregnancies with multiple, genetically distinct individuals are common in most vertebrates. In cattle (and probably many other vertebrate species), wombmates can share a common fetal circulation; they are born as immunologically tolerant hematopoietic chimeras (32), but appear to retain their germ-line integrity (33), despite the likely passage of germ-line precursors from extra-embryonic mesoderm to genital ridges via the blood (34). Perhaps Fu/HC (or MHC)-like allorecognition is responsible. It is also interesting that the descendants of the “link” between vertebrate and invertebrates, like vertebrates, sequester germ-line progenitors that later seed the incipient gonads (13, 35). It is conceivable that the analysis of these phenomenon can proceed best with this model organism.
The finding that G/SCP occurs within a natural population of a clonal invertebrate has a number of implications for future studies on the population and conservation biology of these organisms. First, G/SCP could result in mis-estimation of genetic diversity within a population when such estimates are based on counts of the number of phenotypically distinct somatic individuals. Second, G/SCP can lead to a misclassification of breeding systems, because when there is a difference between the number of genetic and somatic individuals within a population, a population can be more or less outbred than a count of somatic individuals would predict. Third, G/SCP opens up the possibility that genetic structure within a population can develop through the spread of an infectious winner over a limited area. The effect of this phenomenon would be a genetic homogenization that mistakenly could be attributed to the actions of inbreeding and in the long term could lead to increased inbreeding and enhanced kin associations. Finally, the existence of a form of inheritance in colonial species that results from multiple origins of germ-line cells disturbs any formal genetics that assumes that the individual is the unit of inheritance, and that the collective complexity of the genome of that individual is represented in its germ-line output. In B. schlosseri, colonies are the unit of selection somatically, but their germ line varies according to the input from fused partners. We are presently at a loss to formulate mathematically the likely outcomes of such an inheritance mode.
Acknowledgments
We thank K. Ishizuka and K. Palmeri for extensive technical help, J. Quattro and J. Smith for help in the development of microsatellite loci, and N. Chadwick-Furman, A. DeTomaso, B. Magor, B. Rinkevich, J. Smith, J. Stimson, and P. Yund for comments on previous drafts of the manuscript. This research was supported by a National Science Foundation Research Fellowship in Ocean Science and Biotechnology to D.S.S. and by Systemix/Sandoz grants to I.L.W.
Footnotes
Abbreviations: MHC, major histocompatibility complex; G/SCP, germ cell or somatic cell parasitism; GCP, germ cell parasitism.
References
- 1.Buss L W. Proc Natl Acad Sci USA. 1982;79:5337–5341. doi: 10.1073/pnas.79.17.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buss L W. The Evolution of Individuality. Princeton: Princeton Univ. Press; 1987. [Google Scholar]
- 3.Millar R H. In: Advances in Marine Biology. Russell R H, Yonge M, editors. London: Allen & Unwin; 1971. pp. 1–100. [Google Scholar]
- 4.Milkman R. Biol Bull. 1967;132:229–243. doi: 10.2307/1539891. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft F W. Proc Calif Acad Sci. 1903;3:138–186. [Google Scholar]
- 6.Oka H, Watanabe H. Bull Mar Biol Stn Asamushi. 1960;10:153–155. [Google Scholar]
- 7.Scofield V L, Nagashima L S. Biol Bull. 1983;165:733–744. doi: 10.2307/1541475. [DOI] [PubMed] [Google Scholar]
- 8.Scofield V L, Schlumpburger J M, West L A, Weissman I L. Nature (London) 1982;295:499–502. doi: 10.1038/295499a0. [DOI] [PubMed] [Google Scholar]
- 9.Rinkevich B, Porat R, Goren M. Proc R Soc London B. 1995;259:319–324. [Google Scholar]
- 10.Grosberg R K, Quinn J F. Nature (London) 1986;322:456–459. [Google Scholar]
- 11.Gilbert S F. Developmental Biology. Sunderland, MA: Sinauer; 1994. [Google Scholar]
- 12.Berrill N J, Liu C K. Q Rev Biol. 1948;23:124–132. doi: 10.1086/396241. [DOI] [PubMed] [Google Scholar]
- 13.Sabbadin A, Zaniolo G J. J Exp Zool. 1979;207:289–304. [Google Scholar]
- 14.Pancer Z, Gershon H, Rinkevich B. Biol Bull. 1995;189:106–112. doi: 10.2307/1542460. [DOI] [PubMed] [Google Scholar]
- 15.Grosberg R K. Q Rev Biol. 1988;63:377–412. [Google Scholar]
- 16.Rinkevich R, Weissman I L. Symbiosis. 1987;4:117–134. [Google Scholar]
- 17.Chadwick-Furman N E, Weissman I L. Proc R Soc London B. 1995;262:157–162. doi: 10.1098/rspb.1995.0190. [DOI] [PubMed] [Google Scholar]
- 18.Fisher R A. The Genetical Theory of Natural Selection. Oxford: Clarendon; 1930. [Google Scholar]
- 19.Rinkevich R, Weissman I L. J Zool. 1987;213:717–733. [Google Scholar]
- 20.Pancer Z, Gershon H, Rinkevich B. Biochim Biophys Acta. 1994;203:646–651. doi: 10.1006/bbrc.1994.2231. [DOI] [PubMed] [Google Scholar]
- 21.Rinkevich R, Weissman I L. Oikos. 1992;63:119–124. [Google Scholar]
- 22.Sabbadin A, Graziani G. Nature (London) 1967;213:815–816. [Google Scholar]
- 23.Boyd H C, Weissman I L, Saito Y. Biol Bull. 1990;178:239–250. doi: 10.2307/1541825. [DOI] [PubMed] [Google Scholar]
- 24.Rinkevich B, Weissman I L. Bull Mar Sci. 1989;45:213–227. [Google Scholar]
- 25.Rinkevich B, Weissman I L. J Exp Zool. 1992;263:105–111. doi: 10.1002/jez.1402630111. [DOI] [PubMed] [Google Scholar]
- 26.Grosberg R K. Evolution. 1987;41:372–384. doi: 10.1111/j.1558-5646.1987.tb05804.x. [DOI] [PubMed] [Google Scholar]
- 27.Hauenschild V C. Wilhelm Roux’s Arch Entwicklungsmach Org. 1954;147:1–41. doi: 10.1007/BF00576821. [DOI] [PubMed] [Google Scholar]
- 28.Pittenger T H, Brawner T B. Genetics. 1961;46:1645–1663. doi: 10.1093/genetics/46.12.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berrill N J. The Origins of Vertebrates. London: Oxford Univ. Press; 1955. [Google Scholar]
- 30.Weissman I L, Scofield V L, Saito Y, Boyd H, Rinkevich B. In: Invertebrate Historecognition. Grosberg R K, Hedgecock D, Nelson K, editors. New York: Plenum; 1987. pp. 67–78. [Google Scholar]
- 31.Hall B K. Homology: The Heirarchical Basis of Comparative Biology. San Diego: Academic; 1994. [Google Scholar]
- 32.Owen R D. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 33.Ohno S, Trujillo J M, Stenius C, Christian L C, Teplitz R L. Cytogenetics. 1962;1:258–265. doi: 10.1159/000129735. [DOI] [PubMed] [Google Scholar]
- 34.Mintz, B. (1968) J. Anim. Sci. 27, Suppl. 1, 51–60. [PubMed]
- 35.Mukai H, Watanabe H. J Morphol. 1976;148:337–362. doi: 10.1002/jmor.1051480306. [DOI] [PubMed] [Google Scholar]


