Abstract
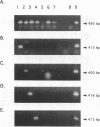
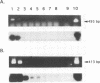
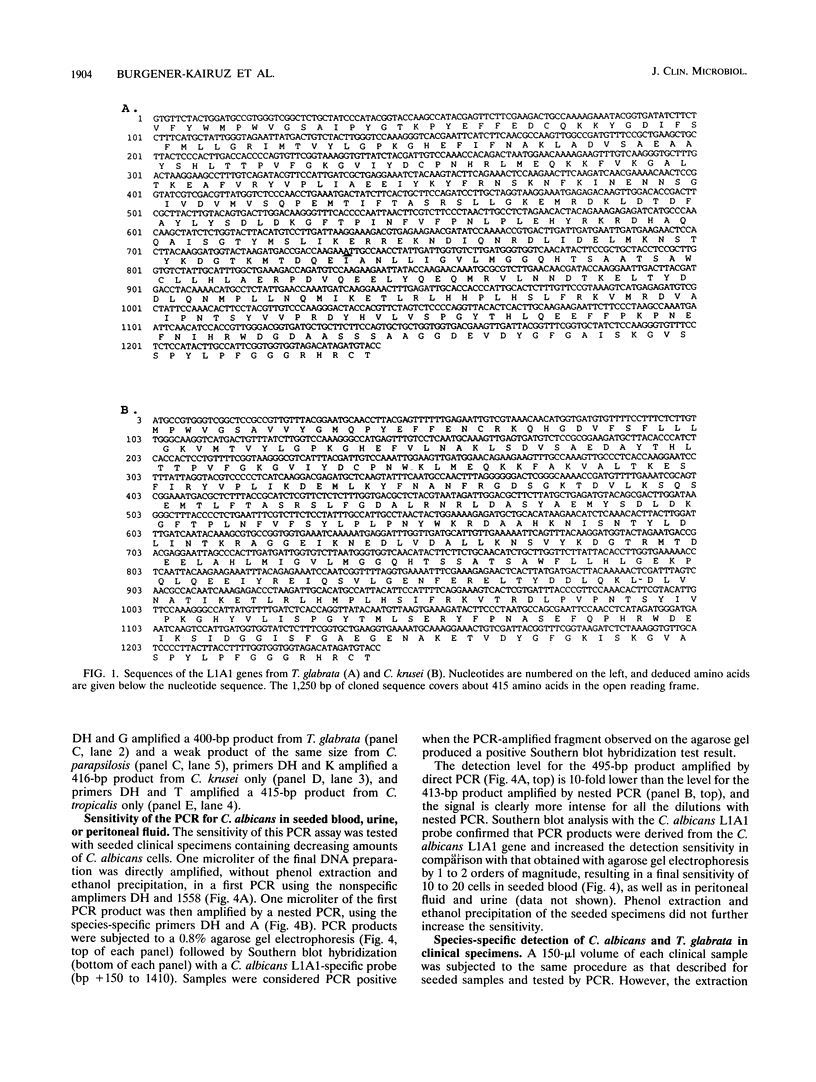
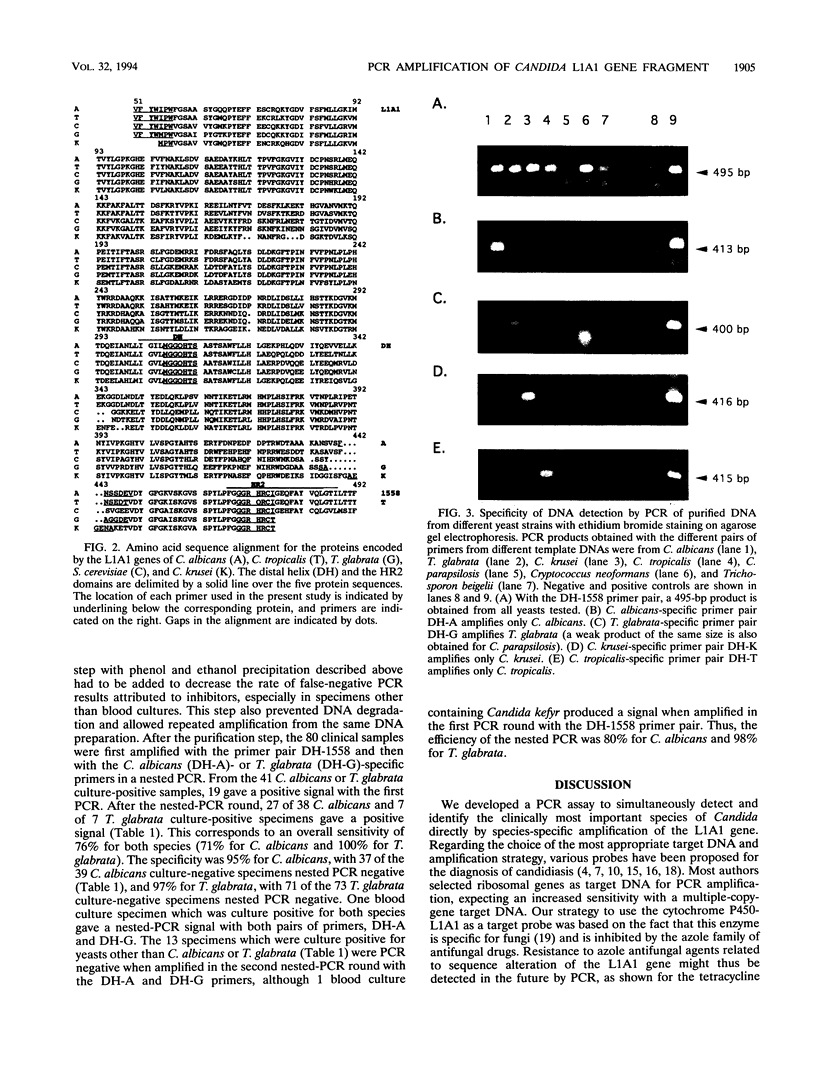
PCR of a Candida albicans cytochrome P-450 lanosterol-alpha-demethylase (P450-L1A1) gene segment is a rapid and sensitive method of detection in clinical specimens. This enzyme is a target for azole antifungal action. In order to directly detect and identify the clinically most important species of Candida, we cloned and sequenced 1.3-kbp fragments of the cytochrome P450-L1A1 genes from Torulopsis (Candida) glabrata and from Candida krusei. These segments were compared with the published sequences from C. albicans and Candida tropicalis. Amplimers for gene sequences highly conserved throughout the fungal kingdom were first used; positive PCR results were obtained for C. albicans, T. glabrata, C. krusei, Candida parapsilosis, C. tropicalis, Cryptococcus neoformans, and Trichosporon beigelii DNA extracts. Primers were then selected for a highly variable region of the gene, allowing the species-specific detection from purified DNA of C. albicans, T. glabrata, C. krusei, and C. tropicalis. The assay sensitivity as tested for C. albicans in seeded clinical specimens such as blood, peritoneal fluid, or urine was 10 to 20 cells per 0.1 ml. Compared with results obtained by culture, the sensitivity, specificity, and efficiency of the species-specific nested PCR tested with 80 clinical specimens were 71, 95, and 83% for C. albicans and 100, 97, and 98% for T. glabrata, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black S. D., Coon M. J. P-450 cytochromes: structure and function. Adv Enzymol Relat Areas Mol Biol. 1987;60:35–87. doi: 10.1002/9780470123065.ch2. [DOI] [PubMed] [Google Scholar]
- Buchman T. G., Rossier M., Merz W. G., Charache P. Detection of surgical pathogens by in vitro DNA amplification. Part I. Rapid identification of Candida albicans by in vitro amplification of a fungus-specific gene. Surgery. 1990 Aug;108(2):338–347. [PubMed] [Google Scholar]
- Chen C., Kalb V. F., Turi T. G., Loper J. C. Primary structure of the cytochrome P450 lanosterol 14 alpha-demethylase gene from Candida tropicalis. DNA. 1988 Nov;7(9):617–626. doi: 10.1089/dna.1988.7.617. [DOI] [PubMed] [Google Scholar]
- Crampin A. C., Matthews R. C. Application of the polymerase chain reaction to the diagnosis of candidosis by amplification of an HSP 90 gene fragment. J Med Microbiol. 1993 Sep;39(3):233–238. doi: 10.1099/00222615-39-3-233. [DOI] [PubMed] [Google Scholar]
- Gotoh O., Tagashira Y., Iizuka T., Fujii-Kuriyama Y. Structural characteristics of cytochrome P-450. Possible location of the heme-binding cysteine in determined amino-acid sequences. J Biochem. 1983 Mar;93(3):807–817. doi: 10.1093/jb/93.3.807. [DOI] [PubMed] [Google Scholar]
- Holm C., Meeks-Wagner D. W., Fangman W. L., Botstein D. A rapid, efficient method for isolating DNA from yeast. Gene. 1986;42(2):169–173. doi: 10.1016/0378-1119(86)90293-3. [DOI] [PubMed] [Google Scholar]
- Hopfer R. L., Walden P., Setterquist S., Highsmith W. E. Detection and differentiation of fungi in clinical specimens using polymerase chain reaction (PCR) amplification and restriction enzyme analysis. J Med Vet Mycol. 1993;31(1):65–75. doi: 10.1080/02681219380000071. [DOI] [PubMed] [Google Scholar]
- Ishida N., Aoyama Y., Hatanaka R., Oyama Y., Imajo S., Ishiguro M., Oshima T., Nakazato H., Noguchi T., Maitra U. S. A single amino acid substitution converts cytochrome P450(14DM) to an inactive form, cytochrome P450SG1: complete primary structures deduced from cloned DNAS. Biochem Biophys Res Commun. 1988 Aug 30;155(1):317–323. doi: 10.1016/s0006-291x(88)81087-8. [DOI] [PubMed] [Google Scholar]
- Kalb V. F., Woods C. W., Turi T. G., Dey C. R., Sutter T. R., Loper J. C. Primary structure of the P450 lanosterol demethylase gene from Saccharomyces cerevisiae. DNA. 1987 Dec;6(6):529–537. doi: 10.1089/dna.1987.6.529. [DOI] [PubMed] [Google Scholar]
- Kan V. L. Polymerase chain reaction for the diagnosis of candidemia. J Infect Dis. 1993 Sep;168(3):779–783. doi: 10.1093/infdis/168.3.779. [DOI] [PubMed] [Google Scholar]
- Lai M. H., Kirsch D. R. Nucleotide sequence of cytochrome P450 L1A1 (lanosterol 14 alpha-demethylase) from Candida albicans. Nucleic Acids Res. 1989 Jan 25;17(2):804–804. doi: 10.1093/nar/17.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. C. Early diagnosis of systemic candidal infection. J Antimicrob Chemother. 1993 Jun;31(6):809–812. doi: 10.1093/jac/31.6.809. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Mabuchi T., Fukazawa Y. New method for detection of Candida albicans in human blood by polymerase chain reaction. J Clin Microbiol. 1993 Dec;31(12):3344–3347. doi: 10.1128/jcm.31.12.3344-3347.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa Y., Mabuchi T., Kagaya K., Fukazawa Y. Isolation and characterization of a species-specific DNA fragment for detection of Candida albicans by polymerase chain reaction. J Clin Microbiol. 1992 Apr;30(4):894–900. doi: 10.1128/jcm.30.4.894-900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musial C. E., Cockerill F. R., 3rd, Roberts G. D. Fungal infections of the immunocompromised host: clinical and laboratory aspects. Clin Microbiol Rev. 1988 Oct;1(4):349–364. doi: 10.1128/cmr.1.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters H. G., Goessens W. H., Meis J. F., Quint W. G. Rapid, polymerase chain reaction-based identification assays for Candida species. J Clin Microbiol. 1993 Apr;31(4):904–910. doi: 10.1128/jcm.31.4.904-910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. W. Metabolism of sterols in yeast. CRC Crit Rev Microbiol. 1978;6(4):301–341. doi: 10.3109/10408417809090625. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Finzel B. C., Gunsalus I. C., Wagner G. C., Kraut J. The 2.6-A crystal structure of Pseudomonas putida cytochrome P-450. J Biol Chem. 1985 Dec 25;260(30):16122–16130. [PubMed] [Google Scholar]
- Reiss E., Morrison C. J. Nonculture methods for diagnosis of disseminated candidiasis. Clin Microbiol Rev. 1993 Oct;6(4):311–323. doi: 10.1128/cmr.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Pang Y., Riley D. E., Hillier S. L., Berger R. C., Krieger J. N. Detection of Tet M and Tet O tetracycline resistance genes by polymerase chain reaction. Mol Cell Probes. 1993 Oct;7(5):387–393. doi: 10.1006/mcpr.1993.1057. [DOI] [PubMed] [Google Scholar]
- Wang J. T., Wang T. H., Sheu J. C., Lin S. M., Lin J. T., Chen D. S. Effects of anticoagulants and storage of blood samples on efficacy of the polymerase chain reaction assay for hepatitis C virus. J Clin Microbiol. 1992 Mar;30(3):750–753. doi: 10.1128/jcm.30.3.750-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]