Abstract
A test was carried out to see if sucrose could regulate cold-acclimation-associated gene expression in Arabidopsis. In plants and excised leaves, sucrose caused an increase in GUS activity, as a reporter for the activity of the cold-responsive COR78 promoter. This increase was transient at 21 °C but lasted for at least 4 d at 4 °C in continuous darkness. However, at 4 °C with a 16 h photoperiod, GUS activity was similarly high with solutions lacking sucrose or with different concentrations of sucrose. In peeled lower epidermis in the cold dark environment, 40 mM sucrose increased COR78 transcript abundance to substantially above that in the controls, but sorbitol had no effect. Similarly to the cold and dark conditions, sucrose increased COR78 transcript abundance in the epidermis in the warm light and warm dark environments, but not in a cold light environment. Sucrose had much less effect on COR78 transcript abundance in leaves without the lower epidermis. Thus sucrose regulates expression of COR78, possibly mainly in the epidermis, at the level of transcription. Furthermore, 40 mM sucrose at 4 °C for 24 h in constant darkness was sufficient to give the same GUS activity as in fully acclimated plants of the same age in a 16 h photoperiod, although by 48 h, GUS activity had become intermediate between control and fully cold-acclimated plants. Thus sucrose has a regulatory role in the acclimation of whole plants to cold and this may be important during diurnal dark periods.
Keywords: Acclimation, Arabidopsis, cold, COR78, freezing, gene expression, soluble carbohydrates, sucrose
Introduction
Soluble carbohydrates are a central resource in plants and have wide-ranging regulatory effects. Thus they regulate carbohydrate metabolism, assimilate partitioning, development including vascular differentiation, and expression of a large number and variety of genes, and they interact with sensors of the environment (Jeffs and Northcote, 1967; Chiou and Bush, 1998; Arenhas-Huertero et al., 2000; Gibson, 2000; Gonzali et al., 2006; Rolland et al., 2006). Regulation of acclimation to stress is often highly complex and sugar-signalling is involved in this complexity. Its role is solute-specific and thus not osmotic (Kang et al., 2002) although it is connected with ABA signalling (Arenhas-Huertero et al., 2000; Hujjiser et al., 2000; Laby et al., 2000). Although it has been suggested that sugars may regulate cold-acclimation (Guy et al., 1980) the idea has not been tested in whole plants. So far, sugars are implicated in the regulation of cold-acclimation only in cell cultures (Tabaei Aghdaei et al., 2003). Our purpose is to test the regulatory role of sucrose in the cold-acclimation of Arabidopsis plants by testing its effect on cold-responsive gene expression as exemplified by COR78.
Cold induces the rapid accumulation of soluble carbohydrates in plants including Arabidopsis (Levitt, 1980; Pollock, 1984; Wanner and Juntilla, 1999). This reduces cellular dehydration during freezing, can help directly protect macromolecules, can act as a nutritional source during acclimation, and may support recovery (Levitt, 1980; Trunova, 1982; Strauss and Hauser, 1986; Crowe et al., 1992; Eagles et al., 1993; Travert et al., 1997; Klotke et al., 2004). The accumulation of soluble carbohydrates can be induced by cold-inducible gene expression. Thus over-production of the cold-responsive transcription factor CBF3 causes both the acquisition of freezing tolerance and the accumulation of soluble carbohydrates (Gilmour et al., 2000). However, such observations do not rule out a regulatory role for sugars in acclimation: the concepts of regulatory feed-back and feed-forward and of signalling between pathways show how molecules can simultaneously be products of specific metabolic pathways and yet be regulators of their own synthesis and consumption and of other processes.
Light strongly affects acclimation to cold, and Steponkus and Lanphear (1967) suggested that the translocation of soluble carbohydrates from leaves to other organs could explain why this is so. Consistent with this, feeding sugars to either whole plants grown in the dark or to cultured cells induces freezing tolerance (Tumanov and Trunova, 1957; Tumanov et al., 1968; Leborgne et al., 1995; Travert et al., 1997; Tabaei Aghdaei et al., 2003). Like other species, Arabidopsis seedlings increase in freezing tolerance when exogenously supplied with sucrose (Uemura and Steponkus, 2003). Two concentration ranges, 10–35 mM and 30–400 mM, reduced the frequency of different types of membrane lesion (expansion-induced lysis and loss of osmotic responsiveness). Sorbitol had much less effect than sucrose, indicating that the effects were not predominantly osmotic. Uemura and Steponkus (2003) suggested that there were two underlying mechanisms: indirect protection through metabolic use of sucrose, and direct cryoprotection; however, they did not test if a regulatory role in acclimation was involved.
Assimilates, of course, are essential for any process including acclimation. Essential nutrients liable to vary in amount, such as sugars, often signal their availability, and to enter the acclimation process in the absence of adequate resources would be problematic for the plant. Feeding sugars to plants in the cold induces freezing tolerance but is not effective in the warm (Tumanov and Trunova, 1957; Tumanov et al., 1968). By contrast, cell cultures acquire freezing tolerance when supplied with sugars in the cold or in the warm (Dix et al., 1994; Leborgne et al., 1995; Travert et al., 1997; Palonen and Junttila, 1999; Tabaei Aghdaei et al., 2003). Furthermore, in a barley cell culture in either a cold or a warm environment, sucrose regulated expression of a gene that in whole plants was expressed only in response to cold (Tabaei Aghdaei et al., 2003). Tabaei Aghdaei et al. (2003) commented that the regulation of acclimation was unlikely to work identically in all plant tissues. This, they suggested, might explain the different requirements for cold-acclimation in cell cultures compared to those in the more complex whole plant.
The light reaction can regulate cold acclimation (Gray et al., 1997). Any signalling from the photosystem to outside the chloroplast, cell or organ must involve a transportable molecule. Phytochrome is also implicated as a cold sensor (Kim et al., 2002) but, again, another molecule would be needed to pass the signal to different tissues or organs. Speculatively, the effects of light and temperature could also interact to create localized signalling in leaves, for example, by altering the distribution of sugars between intracellular compartments and between cells and the apoplast. Consistent with the latter, Livingston and Henson (1998) found that cold increased apoplastic concentrations of soluble carbohydrates in oat.
Thus, if sucrose has a regulatory role in the acclimation to cold, it is as part of a complex system, and our approach reflected this.
As a molecular marker for acclimation, the expression of COR78 was investigated. COR78 is not involved in nutrition or primary metabolism, and hence could indicate effects that are independent of any nutritional role of sucrose. COR78 codes for a late-embryogensis-abundant (LEA) protein (Fowler et al., 2005); LEA proteins are specialized to a protective role during acclimation (Kosova et al., 2007; Tunnacliffe and Wise, 2007). Activity of β-glucuronidase (GUS) was used as a reporter for the activity of the cold-responsive COR78 (RD29A) promoter (Horvath et al., 1993; Yamaguchi-Shinozaki and Shinozaki, 1994) and qPCR of COR78 was used to test for direct effects on transcript abundance. Survival of freezing was also tested, because finding a molecular effect of supplied sucrose would only be relevant if sucrose also affects the freezing tolerance of plants treated in exactly the same way.
Sucrose supply was not expected to be the only factor affecting acclimation. GUS activity was tested in both the whole plant and excised leaves so that any interactions between sucrose and other parts of the plant or their products could be detected or ruled out, and would show if sucrose could act directly on GUS activity in the leaf. Furthermore, in barley at least, different genes are cold-expressed in the epidermis compared with other tissues (Pearce et al., 1998). Therefore the effects of sucrose were also tested on the lower epidermis itself, after peeling it from the leaf.
Another complication was that recent assimilate could add to our amount of experimentally supplied sucrose. Also, light can directly initiate signals regulating acclimation to cold (Gray et al., 1997; Kim et al., 2002). Therefore, whereas it was thought that sucrose may have a regulatory effect when experimentally supplied to plants or leaves in the dark, it was expected that when plants were in the light any effects would at least be modified and could be suppressed by the light. A further complication was that while any regulatory effects of sucrose on whole plant freezing tolerance might be expected to require cold, this would not necessarily be true of all component parts of the acclimation system (Tabaei Aghdaei, 2003); therefore it could not be assumed that an effect of sucrose on COR78 promoter activity or transcript abundance would necessarily require cold, and it is not required if ABA or drought are applied (Yamaguchi-Shinozaki and Shinozaki, 1994).
For these reasons, plants growing in different environments were tested to compare the effects in the warm and cold and the light and dark. To test the response to exposure to sucrose, Arabidopsis plants were uprooted from the soil and placed with their roots immersed in test solutions, or excised leaves or peeled epidermis were placed on the solutions. The supplied solutions were replaced every 12 h to help maintain the concentrations over several days of treatment.
It is well known that sugars, including sucrose fed to whole plants in the cold and dark, can cause them to acclimate but it is generally assumed that their role is directly protective or is indirectly helpful as a substrate for metabolism. There is no doubt that sugars can have such effects. The question here is whether, in addition, they have a directly regulatory role. The only evidence so far is from a cell culture (Tabaei Aghdaei, 2003). It is investigated here whether sucrose has a regulatory role in the acclimation of whole Arabidopsis plants to cold and to test if the response is different in different environments and tissues.
The specific objectives were to test the hypothesis that sucrose would promote GUS activity and COR78 transcript abundance in the cold and dark; to test whether any effect of sucrose in the dark was suppressed by light, and whether, as in cell cultures (Tabaei Aghdaei, 2003), sucrose had any effect in the warm; to test if there were any differences in the effects of sucrose on COR78 transcript abundance between the epidermis and the leaf; and to test if sucrose had similar effects on freezing tolerance. Thus a broad perspective of the regulatory role of sucrose in the acclimation of whole plants to cold would be obtained.
Materials and methods
Plant growth, treatment, and sampling
Plants of Arabidopsis thaliana (L.) Heynh. ecotype Columbia were grown from seed in autoclaved soil-based potting compost (John Innes No. 2) in a controlled environment of 21 °C, 16 h photoperiod, 300 μmol m−2 s−1 PFD (400–700 nm) for 21 d. For cold exposure, plants were transferred to 4±2 °C day/night, with or without 16 h photoperiod (200 μmol m−2 s−1 PFD, 400–700 nm).
Twenty-one-day-old plants from the 16 h photoperiod warm environment were taken for experiments to test the effects of different solutions in the above warm and cold environments. At this age in our environments the plants were still in the vegetative stage. All solutions (except the sample of water in Fig. 2C) were made up with either half-strength Murashige and Skoog (1962) mineral solution (0.05 Osmol kg−1; Figs 1, 2A, B, 3, 4) or the composition of xylem sap (based on Pate et al., 1975) (Fig. 2C) to which sucrose, mannose, mannitol or sorbitol was added at the molarity to be tested. In some experiments, whole plants were exposed to solutions by gently uprooting them, rinsing their roots in water, and placing them in Petri dishes with their roots in the solution. In these experiments the whole plant was analysed for GUS expression. In other experiments, leaves were tested by excising mature but non-senescent ones from plants and floating them, adaxial surface uppermost, on the solution with their petiole in the solution, and the whole leaves were later taken for analysis. Similar leaves were also taken and the lower epidermis was removed using tweezers; the parts of the lamina where epidermal removal was successful were cut from the rest of the leaf and floated, adaxial surface uppermost with the peeled abaxial surface in contact with the solution.
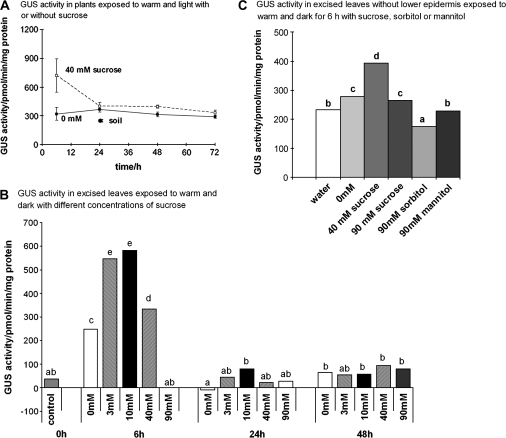
Fig. 2.
GUS activity in Arabidopsis plants (A) and in excised leaves (B) or leaves with lower epidermis removed (C) in the warm on sucrose, mannitol or sorbitol solutions. (A) Plants exposed to solutions in 21 °C (warm) with a 16 h photoperiod (light). (B) Leaves floated on solutions in 21 °C (warm) in continuous dark. (C) The lower epidermis was removed from leaves and the resultant leaf pieces were floated on the solutions for 6 h in the dark. The water treatment contained no added minerals. In (A) bars are ±SE (n=3). In (B) and (C) ANOVA and Tukey's test were used to identify treatment effects; treatments with no letters in common are significantly different (P <0.05; n=3).
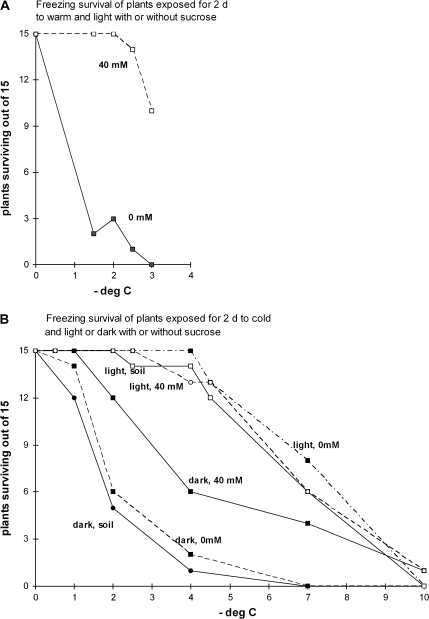
Fig. 1.
Freezing survival of Arabidopsis plants transferred to sucrose solutions for 2 d. (A) Plants from 0 mM or 40 mM sucrose in 21 °C (warm) with a 16 h photoperiod (light). (B) Plants from 0 mM or 40 mM sucrose in 4 °C (cold) either with a 16 h photoperiod (light) or in continuous dark. Plants grown for 2 d in soil in either the warm or cold environment were included as controls. Statistics: (A) More plants survived freezing to temperatures between –1.5 °C and –3.0 °C after 2 d in 40 mM sucrose than in 0 mM sucrose (χ2 tests gave P <0.001 and P <0.01 in replicate experiments). (B) More plants survived in 40 mM sucrose in the dark than in the other two treatments in the dark and fewer survived than in the three treatments in the light (χ2 test gave P <0.001 for all these comparisons).
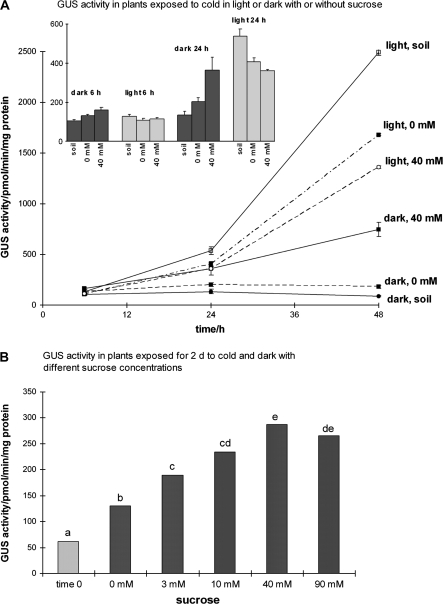
Fig. 3.
GUS activity of Arabidopsis plants in the cold with sucrose. (A) Plants exposed to 0 mM or 40 mM sucrose in 4 °C with a 16 h photoperiod (light) or in continuous dark. The insert shows details for 6 h and 24 h. (B) Plants exposed to 0–90 mM sucrose solutions at 4 °C in continuous dark for 48 h. In (A) bars are ±SE (n=3). In (B) ANOVA and Tukey's test were used to identify treatment effects; treatments with no letters in common are significantly different (P <0.05; n=5).
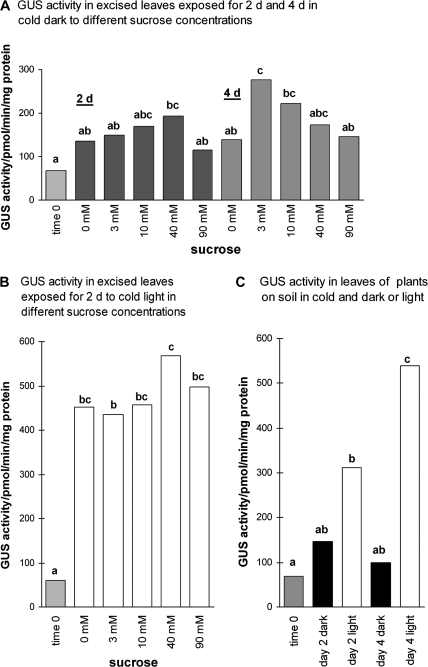
Fig. 4.
GUS activity of excised Arabidopsis leaves in the cold with 0–90 mM sucrose. (A) Leaves floated on 0–90 mM sucrose solutions in an environment of 4 °C in continuous dark for 2 d or 4 d. (B) Same as (A) except that the leaves were maintained in 16 h photoperiod (light) for 2 d. (C) Leaves from control whole plants in soil in 4 °C in 16 h photoperiod (light) or dark, tested at the same time as leaves in (A) and (B). ANOVA and Tukey's test were used to identify treatment effects; treatments with no letters in common are significantly different [P <0.05; n=3 (A, C) or 5 (B)].
Transgenic lines expressing GUS driven by the COR78 promoter
The binary vector pDH78P containing the COR78 promoter controlling expression of the β-glucuronidase gene (GUS) in pBI101.1 was kindly provided by Dr M Thomashow (Horvath et al., 1993). The construct comprised the promoter region of COR78 from +5 bp to –808 bp relative to the transcription start inserted at the BamHI site in pBI101.1 (Rekarte-Cowie, 2002). A. thaliana (L.) Heynh. ecotype Columbia plants were transformed using a dipping method adapted from Bent and Clough (1998) and Clough and Bent (1998). Transformants were selected on agar containing 40 mg ml−1 kanamycin then transferred to soil and further generations similarly selected for kanamycin resistance to obtain F3 seedlings that established lines homozygous for the insert. For details of construction, selection, control lines, and testing, see Rekarte-Cowie (2002).
GUS activity
The method for the GUS assay was adapted from Herrera-Estrella et al. (1994). Protein was extracted into 50 mM sodium phosphate pH 7.0, 10 mM EDTA, 0.1% (w/v) sodium lauryl sarcosine, 0.1% (w/v) Triton X-100, and 10 mM β-mercaptoethanol using sufficient shoot material to give a protein concentration of 1.5–2.5 mg ml−1. Total protein was quantified using the Bradford (1976) method. GUS activity was quantified fluorometrically using 10 mM 4-methylumbelliferyl-β-glucuronide in extraction buffer as substrate. The reaction was stopped using 0.2 M Na2CO3 and emission read at 455 nm with 365 nm as the excitation wavelength using a Shimadzu RF-1501 spectrofluorometer. It was found that samples extracted for protein and assayed for GUS activity after freeze-storage at –80 °C had lost a consistent proportion of their activity compared with samples extracted and assayed immediately (Rekarte Cowie, 2002). Therefore, where possible, samples were extracted and assayed immediately (Figs 2B, 3A), whereas in other experiments samples were stored frozen and analysed after thawing (Figs 2A, 3B, 4). Analysis of variance followed by Tukey's test was used to detect differences between treatments.
Extraction and analysis of RNA
Samples were ground at liquid nitrogen temperature in a pestle and mortar and transferred to 1 ml of TRI-reagent (Sigma) and then treated with 0.2 ml chloroform, shaken, centrifuged, and the aqueous phase recovered; 0.5 ml of isopropanol was added to precipitate RNA which was then recovered by centrifugation. Samples were protected against degradation by the addition of RNase inhibitor (Promega, Madison, USA) and DNA was removed by treatment with RNase-free DNase (Promega, Madison, USA).
To quantify COR78 (At5g52310) transcripts, qPCR was used: real-time PCR of cDNA from reverse-transcribed RNA samples, using the Light Cycler FastStart DNA MasterPlus SYBR Green I kit (Hoffmann-La Roche Ltd, Basel). The standard curve was obtained using a reverse-transcribed sample from plants grown in soil in the cold for 2 h. Primers were 5′-CCGATAACGTTGGAGGAAGA-3′ and 5′-TGATGGAGAATTCGTGTCCA-3′. Identity of the PCR product was confirmed by sequencing. The expression of potential reference genes (coding for an actin and an elongation factor 1 α) varied with treatment, and this has been reported by other researchers (Nicot et al., 2005). However, qPCR data for COR78 per unit total RNA for a time-course during cold-acclimation of Arabidopsis plants grown in soil gave results that parallelled those typically found in the literature for this gene (Hajela et al., 1990; Fowler and Thomashow, 2002). Therefore the qPCR data for relative transcript abundance are expressed per unit total RNA. The values for the lower epidermis and for the leaf minus the lower epidermis were then separately normalized by dividing individual sample values by the mean value for the corresponding samples on 0 mM sucrose in the cold and dark.
Freezing tolerance
Freezing tolerance was tested by plant survival (Pearce, 1980). Plants were carefully uprooted, placed in glass tubes immersed in an oil bath at –1.5 °C and ice was added to nucleate freezing. The bath was cooled between consecutive test temperatures at 10 K h−1, holding at each test temperature for 45 min. At the end of each 45 min period, at each test temperature, some tubes were removed from the bath and the remainder were cooled to the next test temperature. The tubes were thawed at room temperature and the plants replanted and grown in the control environment, covering the plants with cling film for 7 d to reduce moisture loss. Surviving plants were recorded after every 7 d up to 21 d. Numbers of plants surviving were summed across the freezing temperatures tested and, taking into account the numbers tested, differences in survival were tested by χ2.
Results
Freezing tolerance
Sucrose increased plant survival of freezing tolerance in both cold and warm environments (Fig. 1). Plants grown at 21 °C in the light in a mineral solution and then exposed to freezing at –3 °C were killed, whereas plants also given 40 mM sucrose mostly survived (Fig. 1A). Exposure to 20 mM had less effect; it increased survival at –1.5 °C (P <0.05) but all plants were killed at –3 °C (details not shown).
Sucrose also had an effect in the cold with constant darkness. Plants in the cold and dark in either soil or in solution without sucrose had little freezing tolerance, but when 40 mM sucrose was supplied, it enhanced freezing tolerance by about 2 °C (Fig. 1B), to an intermediate level of tolerance between the fully acclimated and the non-acclimated plants.
On the other hand, in the cold with light, 40 mM exogenously supplied sucrose did not enhance freezing tolerance further, which was similarly high whether plants were grown in soil or in solution without sucrose or with 40 mM sucrose (Fig. 1B). The similar tolerance of freezing of plants taken from these treatments in the light, where some plants had been in soil and others had been in solutions, also indicates that uprooting the plants to immerse them in the solutions did not adversely affect the plant's ability to acclimate to freezing.
GUS activity in the warm
Plants that were transferred to 40 mM sucrose in the light in 21 °C for 6 h contained a higher GUS activity than plants without sucrose, but the difference had disappeared by 24 h (Fig. 2A). To understand the effect of sucrose in the warm more fully, the effect was tested of a range of concentrations from 0 mM to 90 mM on GUS activity of detached leaves in constant dark at 21 °C (Fig. 2B). By 6 h, 0 mM, 3 mM, 10 mM, and 40 mM all gave higher GUS activity than before treatment, but 3 mM, 10 mM, and 40 mM sucrose treatments all gave higher GUS activities than 0 mM. The GUS activity with 40 mM sucrose was about half that with 3 mM or 10 mM sucrose, and 90 mM sucrose gave the same GUS activity as before treatment, indicating that a concentration of about 3–10 mM was optimal at 6 h. The effect of sucrose on GUS activity was much less at 24 h and only 10 mM gave significantly higher activity than in 0 mM. There was no effect of supplied sucrose by 48 h.
Leaves from which the lower epidermis had been removed were also used to allow direct contact between the solution supplied and the leaf mesophyll (Fig. 2C). This was to minimize any modification of the solution by its passage through the plant. Mannitol and sorbitol at 90 mM were included to test the effects of osmotica. As with unpeeled leaves, by 6 h 40 mM sucrose increased GUS activity compared with 0 mM (Fig. 2C). Sucrose, mannitol, and sorbitol (90 mM) gave similar or lower GUS activities than in mineral solution or water (Fig. 2C).
GUS activity in the cold
In plants in a 16 h photoperiod at 4 °C, both 0 mM and 40 mM sucrose gave high levels of GUS activity (Fig. 3A). However, at 4 °C and constant darkness, 40 mM sucrose increased GUS activity compared with plants in mineral solution without sucrose and compared with plants in soil, in both of which GUS activity remained low (Fig. 3A).
GUS activity by 48 h in the cold and constant dark with 40 mM sucrose was, like freezing tolerance, intermediate between activity with a 16 h photoperiod and activity in constant dark without sucrose. However, earlier, by 24 h in the cold, the 40 mM sucrose had increased GUS expression in the dark to a similar level to that in plants in the light (insert in Fig. 3A). The solutions were replaced twice daily with fresh solutions and hence the difference between 48 h and 24 h was unlikely to be due to a reduced availability of sucrose in the medium. Furthermore, much lower concentrations were moderately effective: when plants were exposed in the cold and dark to 0–90 mM sucrose for 48 h, all contained a higher GUS activity than at time 0, and all sucrose concentrations from 3 mM to 90 mM gave higher GUS activity than did 0 mM, with a progressive increase in GUS activity up to 40 mM sucrose (Fig. 3B).
The effects of sucrose on excised leaves were also tested in order to detect or rule out dependence on other parts of the plant. Excised leaves were floated on 0 mM to 90 mM sucrose in the cold and dark or with a 16 h photoperiod. When the excised leaves were floated on solutions for 2 d in the dark, only 40 mM sucrose gave a significantly higher GUS activity than at time 0 (Fig. 4A). However, as found when whole plants were fed sucrose, there was a progressive increase in GUS activity from 0 mM to 40 mM. In the warm, the effect of sucrose had been transient, diminishing after 6 h and undetectable by 2 d (Fig. 2B). If there was a similar effect in the cold it might well have progressed more slowly. Therefore, a test was made to see if supplying sucrose for 4 d in the dark gave a lower effect than by 2 d. However, this had similar or, with 3 mM sucrose, more effect than at 2 d but, unlike by 2 d, the highest GUS activities by 4 d were obtained around 3 mM and 10 mM sucrose and GUS activity with 40 mM sucrose did not differ significantly from GUS activity with 0 mM (Fig. 4A). Thus, sucrose had an effect, but the extra time between 2 d and 4 d substantially lowered the optimal exogenous concentration required to induce the response.
On the other hand, in cold and a 16 h photoperiod, GUS activities were similarly high when excised leaves were placed for 2 d in solutions containing either no sucrose or any of the sucrose concentrations 3 mM, 10 mM, 40 mM or 90 mM (Fig. 4B). Comparison amongst the treatments (Fig. 4A, B) and with GUS activity in plants grown on soil (Fig. 4C) indicated that the supply of sucrose to leaves in the cold and dark gave GUS activity that, as with whole plants, was intermediate: it was above the activity in leaves at time 0, in leaves of control plants by 2 d and 4 d on soil in the dark, and in leaves by 4 d on 0 mM sucrose in the dark; but it was below the activity in leaves in a 16 h photoperiod on solutions in the cold and in leaves of plants on soil in a 16 h photoperiod.
COR78 transcript abundance
Lower epidermis peeled from the leaf, and leaf blade with the lower epidermis removed, were floated on sucrose or sorbitol solutions for 2 h. In whole plants acclimated in the normal way, COR78 typically reaches its peak of expression at 6–24 h in the cold, but an increase is detectable earlier (Hajela et al., 1990; Fowler and Thomashow, 2002). An increase in GUS activity was detectable at 6 h (Figs 2, 3A) and earlier (details not shown). Therefore, if sucrose acted at an early stage in the acclimation to cold, an effect on COR78 transcript abundance should be detectable by 2 h.
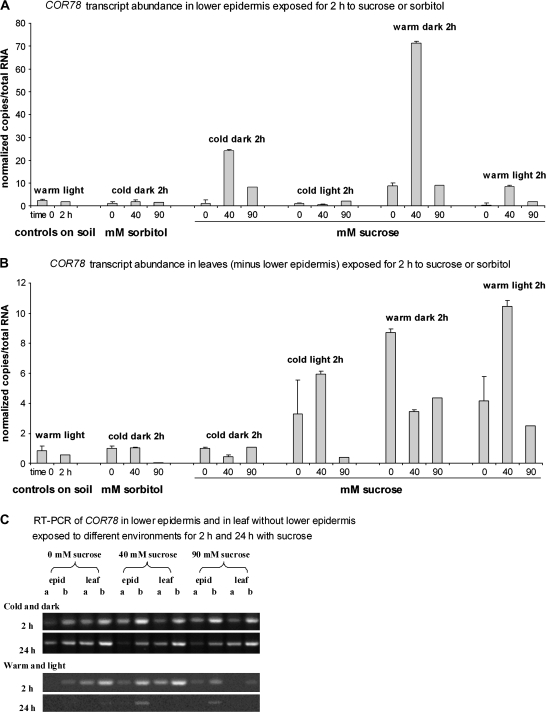
Sucrose at 40 mM considerably increased COR78 transcript abundance in the lower epidermis in the cold and dark, warm and dark, and warm and light environments, to well above transcript abundance in the controls on soil, and 9 times (warm dark) and 25 times (cold dark and warm light) higher than on 0 mM solution (Fig. 5A). Forty mM sorbitol did not increase COR78 transcript abundance in the lower epidermis tested in the cold and dark environment, indicating that the effect with sucrose was not osmotic.
Fig. 5.
COR78 transcripts in Arabidopsis epidermis (A, C) and leaves (B, C) floated on sucrose or sorbitol solutions for 2 h. Cold: 4 °C; warm: 21 °C. (A, B) Normalized transcript amounts in peeled lower epidermis and in leaves without lower epidermis. Data were normalized relative to the mean value from samples on 0 mM sucrose in the cold and dark environment. Bars show SE (n=3). (C) COR78 transcripts from peeled lower epidermis (epid) and leaf without the lower epidermis (leaf) exposed to sucrose solutions for 2 h or 24 h in cold and dark or warm and a 16 h photoperiod (light). Alternate lanes (a and b) show amplifications from reactions with cDNA reverse transcribed from10 ng and 100 ng total RNA.
Sucrose (90 mM) had much less effect than 40 mM sucrose on COR78 transcript abundance in the lower epidermis. Thus, as with GUS activity in the whole leaf, there was an optimal concentration for the effect of exogenous sucrose on the amounts of COR78 transcripts in the lower epidermis in the cold dark and warm dark environments. By contrast, in the cold light, COR78 transcript abundance in the epidermis was low (Fig. 5A) although GUS activity had been high (Fig. 4B).
Sucrose had much less effect on COR78 transcript abundance in the leaves without a lower epidermis (Fig. 5B) than in the lower epidermis (Fig. 5A). In the cold and dark neither sucrose nor sorbitol increased COR78 transcript abundance in leaves without a lower epidermis compared with controls on soil or on 0 mM. In the other environments COR78 transcript abundance was higher on 0 mM than in the controls on soil. In the warm and dark, transcript abundance was also higher with 0 mM than with 40 mM and 90 mM sucrose, but in the warm and light 40 mM gave twice as much as the 0 mM control.
GUS activity in the warm was much lower after 24 h on sucrose solutions than after 6 h (Fig. 2A, B) whereas in the cold it remained at a raised level (Fig. 3A). Therefore it was also tested if COR78 transcript levels were different after 24 h compared with 2 h exposure to the different solutions. By 24 h in the warm and light, transcript levels were lower than at 2 h (in the peeled lower epidermis) or were undetectable (in the leaves without lower epidermis), whereas in the cold and dark they were mostly similar at both time points (Fig. 5C).
Discussion
Exogenously supplied sucrose increased COR78-promoter-driven GUS activity and COR78 transcript abundance in the cold and dark environment. It also promoted expression in the warm, whether light or dark, although only transiently. The effects were similar whether sucrose was fed to whole plants through the roots or directly to the leaves. The response to sucrose even in the cold was rapid, within 2 h. The qPCR results indicate that sucrose regulates COR78 transcript abundance, and the GUS activity, which depends upon effects on the promoter, indicates that it is probably transcription itself that is regulated. Sorbitol did not induce high expression hence the regulatory effect of sucrose was not primarily osmotic.
Unlike in the cold dark environment, when plants grown in the cold light environment were supplied with sucrose it had no detectable effect on GUS activity, which was high with or without exogenously supplied sucrose. Possibly, cold-induced signalling through the photosystem (Gray et al., 1997) overrides any potential effect of the exogenous sucrose. However, this may not explain the lack of response in the epidermis, where chloroplasts are present but not abundant (Pyke and L pez-Juez, 1999), and where COR78 transcript abundance, unlike GUS activity in the whole leaf, was low with or without sucrose in the cold and light.
In the warm and, therefore, in the absence of any cold-induced signal, light did not suppress the effect of sucrose. However, in the warm, both with and without light, the effect of sucrose was transient. The reduction after 6 h of the response could indicate feed-back signalling and negative regulators of acclimation (Pearce et al., 1996; Dong et al., 2006) acting, in the absence of cold, to suppress the initial positive response to sucrose.
The epidermis is of major importance in the adaptation to stresses but its role, structure, and physiology are different from other tissues in the leaf, so differences in the details of how it is adapted to stress are likely. For plant survival of freezing, it is of course as important for the epidermis to survive as for any other part of the leaf. The epidermis is well positioned to receive a cold-induced signal, which could be sugars, from the mesophyll. The effects of sucrose on COR78 transcript abundance in the epidermis in three environments paralleled those for GUS activity in the whole leaf. Could effects in the epidermis explain the GUS activity in the whole leaf? There are only three or four layers of mesophyll cells in Arabidopsis leaves, and the epidermis (lower plus upper) accounts for about one-third of the leaf cell population (Pyke and López-Juez, 1999). The relative increase in COR78 transcripts in the lower epidermis in response to 40 mM sucrose compared with 0 mM sucrose was, depending on the environment, two-to-many fold higher than in the leaf minus the lower epidermis. However, how much the non-epidermal parts of the leaf contributed, is unclear because the leaf samples used in our experiments still carried the upper epidermis (which would be very hard to remove). However, at least a significant proportion of the effect of sucrose on COR78 expression in the leaf may be explained by its expression in the epidermis.
The optimal sucrose concentration for leaf GUS activity in the cold was not constant, changing from 40 mM to 3 mM between the second and fourth days of treatment. However, in the warm, by 6 h the optimal concentration was already around 3 mM and 10 mM. Presumably, if the process by which the optimum changed in the cold also occurred in the warm, it would happen more rapidly, explaining this result. Since the solutions were replenished every 12 h, it would be possible for similar or higher concentrations of sugars to accumulate within the cells than were in the solutions supplied. In cultured cells supplied with 90 mM sucrose, the cells accumulated a similar concentration within the cells, but when supplied with 3 mM sucrose (1 mg g−1) they accumulated sugars (sucrose, glucose, and fructose) to a higher concentration of about 10 mM (∼3.5 mg g−1) (Tabaei Aghdaei et al., 2003). The explanation for the seemingly inconsistent optimum may therefore be that it is, at least in part, intracellular sugars that are sensed rather than the concentration of the exogenous sucrose supplied.
Zero mM sucrose gave a higher GUS activity by 6 h in the warm and dark than at time 0, although lower than the activity with 3 mM, 10 mM and 40 mM sucrose. Presumably this could reflect sugars remaining after the transfer from light to dark at time 0. The lower activity by 24 h with 0 mM would then be due to reduced intracellular concentration resulting from consumption without replacement, reflecting rapid changes in tissue sugar contents within a day (Farrar, 1989).
The concentrations used in our experiments reflected concentrations found in plants during the normal acclimation to cold. The concentrations of sucrose supplied were 3 mM up to 90 mM, corresponding to approximately 1 mg g−1 fresh tissue up to 30 mg g−1 fresh tissue. Most of the sugars present in Arabidopsis during the first few days of exposure to cold are sucrose, glucose, and fructose, although later, by 7 d, raffinose is also present (Gilmour et al., 2000). Our results for content and composition in plants growing in soil during acclimation in our cold and 16 h photoperiod environment show the presence of sucrose, glucose, and fructose, accumulated by 3 d to around 30 mg g−1 fresh tissue (details not shown). Wanner and Juntilla (1999) found that, in Arabidopsis grown in soil and acclimated to cold, there was a range of sugar concentrations depending on the length of the photoperiod and the time in the cold: before exposure to cold there was 4 mg g−1 fresh tissue; by 1 d and 3 d exposure to cold, there were 4 mg g−1 and 3 mg g−1 fresh tissue in plants in constant dark, 7 mg g−1 and 13 mg g−1 fresh tissue in plants in a 6 h photoperiod, and 18 mg g−1 and 40 mg g−1 fresh tissue in plants in an 18 h photoperiod.
But plant sugar contents are largely reflections of vacuolar concentrations whereas any intracellular sensor of sugars may be in the cytosol (Rolland et al., 2006) where it would be difficult to measure concentration. However, since sucrose was supplied exogenously, it is relevant to consider whether our concentrations were similar to apoplastic concentrations. Tetlow and Farrar (1993) found that, in barley leaves (not acclimated to cold), the combined apoplastic concentration of sucrose, glucose, and fructose was 4 mM. Higher concentrations were found by Livingston and Henson (1998) in the apoplast of crowns and leaves of cold-acclimated oat; in guttate from acclimated leaves, the total soluble carbohydrates were approximately 30 mM, of which more than half was sucrose, glucose, and fructose, and the remainder was fructans.
Sucrose supply in the cold and dark had similar effects on freezing tolerance as on GUS activity and COR78 transcript abundance. Could the increased freezing tolerance caused by sucrose supply in the cold and dark be a consequence of the possible regulatory effects of sucrose illustrated by its effect on the activity of the COR78 promoter and on COR78 expression? Cryoprotection probably requires high concentrations of sucrose (Uemura and Steponkus, 2003), whereas effects on freezing tolerance were detected with moderate and low concentrations. Hence, credibly, although not certainly, regulation rather than cryoprotection could explain our effects of sucrose on freezing tolerance.
What significance could regulation by sucrose have in normal acclimation? Overwintering plants that contain a carbohydrate reserve, such as young wheat seedlings, do not require light for acclimation (Andrews et al., 1974). Similarly, as has been shown here, Arabidopsis acclimates if supplied with sucrose in the dark, including activating the COR78 promoter as well as acquiring freezing tolerance. Thus light, although apparently essential for full acclimation of Arabidopsis, is not directly essential for all cold sensing and signalling. During the dark period between the 16 h photoperiod, the direct effects of light-driven signalling would be absent. On the other hand, Arabidopsis plants acclimated in the cold with a 16 h photoperiod accumulate sugars during the day and the amount does not decline significantly at night (Wanner and Juntilla, 1999), so sugar signalling in the dark periods between photoperiods is possible. Also, in the field, during the night plants may often first be exposed to temperatures low enough to trigger acclimation. Our results indicate that if sufficient soluble carbohydrates were present, these might help trigger the first steps in acclimation.
In the field there is typically a lower temperature in the dark period than in the light, and the light period itself can be warm but acclimation will still occur as a result of a cold night (Dexter, 1933; Tysdale, 1933). Cold signalling in the dark period increases freezing tolerance even when plants are exposed to a light period in the cold. Experiments by H Kohn outlined by Levitt (1980) indicated that the transfer of cabbage plants from a constant 5 °C to 5/0 °C day/night caused an increase in freezing tolerance. Similarly, young barley plants exposed to a 10 h photoperiod followed by a 14 h dark period achieved several degrees K more freezing tolerance when grown in 6/2 °C day/night compared to a constant 6 °C (Pearce et al., 1996). Crucially, the lower night temperature also increased the levels of expression of three cold-response gene transcripts with no connection to sugar metabolism, indicating that significant cold-sensing and signalling had occurred in the dark (Pearce et al., 1996). Thus, although constant dark prevents cold-acclimation in plants in the soil (Wanner and Juntilla, 1999), the cold dark period in an environment of alternating day and night periods can substantially enhance cold acclimation. Regulation by sugars accumulated during the photoperiod, but still present in the dark period, would help explain how this occurs.
Acknowledgments
IRC gratefully acknowledges support by the award of the Newcastle University RB Cooke studentship and OSE and KSM gratefully acknowledge support by the government of Libya. The authors gratefully acknowledge the assistance of Colin Muir, Abobakir Elhaj, and of Dr Ethan Hack.
Glossary
Abbreviations
- ABA
abscisic acid
- GUS
β-glucuronidase
- qPCR
quantitative polymerase chain reaction
References
- Andrews CJ, Pomeroy MK, de la Roche IA. The influence of light and diurnal freezing temperature on the cold hardiness of winter wheat seedlings. Canadian Journal of Botany. 1974;52:2539–2546. [Google Scholar]
- Arenhas-Huertero F, Arroyo A, Zhou L, Sheen J, Leon P. Analysis of Arabidopsis glucose-insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes and Development. 2000;14:2085–2096. [PMC free article] [PubMed] [Google Scholar]
- Bent AF, Clough SJ. Agrobacterium germ-line transformation of Arabidopsis without tissue culture. In: Gelvin S, Shilperoort R, editors. Plant molecular biology manual. Netherlands: Kluwer Academic Publishers; 1998. pp. 1–14. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein–dye binding. Annals of Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proceedings of the National Academy of Sciences, USA. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip, a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annual Reviews of Physiology. 1992;54:570–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Dexter ST. Effect of several environmental factors on the hardening of plants. Plant Physiology. 1933;8:123–129. doi: 10.1104/pp.8.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix PJ, Finch I, Burke JI. Genotypic differences in cold tolerance are masked by high sucrose and cytokinin in shoot cultures of sugar-beet. Plant Cell Tissue and Organ Culture. 1994;36:285–290. [Google Scholar]
- Dong CH, Agarwal M, Zhang YY, Xie Q, Zhu JK. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proceedings of the National Academy of Sciences, USA. 2006;103:8281–8286. doi: 10.1073/pnas.0602874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagles CF, Williams J, Louis DV. Recovery after freezing in Avena sativa L., Lolium perenne L. and L. multiflorum Lam. New Phytologist. 1993;123:477–483. doi: 10.1111/j.1469-8137.1993.tb03759.x. [DOI] [PubMed] [Google Scholar]
- Farrar JF. Fluxes and turnover of sucrose and fructans in healthy and diseased plants. Journal of Plant Physiology. 1989;134:137–140. [Google Scholar]
- Fowler SG, Cook D, Thomashow MF. The CBF cold-response pathway. In: Jenks MA, Hasegawa PM, editors. Plant abiotic stress. Oxford: Blackwell; 2005. pp. 71–99. [Google Scholar]
- Fowler SG, Thomashow MF. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathways. The Plant Cell. 2002;14:1675–1690. doi: 10.1105/tpc.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI. Plant sugar-response pathways. Part of a complex regulatory web. Plant Physiology. 2000;124:1532–1539. doi: 10.1104/pp.124.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiology. 2000;124:1854–1865. doi: 10.1104/pp.124.4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P. Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. Journal of Plant Research. 2006;119:115–123. doi: 10.1007/s10265-005-0251-1. [DOI] [PubMed] [Google Scholar]
- Gray GR, Chauvin L-P, Sarhan F, Huner NPA. Cold acclimation and freezing tolerance. Plant Physiology. 1997;114:467–474. doi: 10.1104/pp.114.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CL, Yelenosky G, Sweet HC. Light exposure and soluble sugars in Citrus frost hardiness. Florida Science. 1980;43:265–268. [Google Scholar]
- Hajela RK, Horvath DP, Gilmour SJ, Thomashow MF. Molecular cloning and expression of COR (Cold Regulated) genes in Arabidopsis thaliana. Plant Physiology. 1990;93:1246–1252. doi: 10.1104/pp.93.3.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Estrella L, Leon P, Olsson O, Teeri TH. Reporter genes for plants. In: Gelvin S, Shilperoort R, editors. Plant molecular biology manual. Netherlands: Kluwer Academic Publishers; 1994. pp. 1–32. [Google Scholar]
- Horvath DP, McLarney BK, Thomashow MF. Regulation of Arabidopsis thaliana L. (Heyn) cor78 in response to low temperature. Plant Physiology. 1993;103:1047–1053. doi: 10.1104/pp.103.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hujjiser C, Kortstee A, Pego J, Weisbeek P, Wisman E, Smeekens S. The Arabidopsis SUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: involvement of abscisic acid in sugar responses. The Plant Journal. 2000;23:577–585. doi: 10.1046/j.1365-313x.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- Jeffs LE, Northcote DM. The influence of indol-3yl acetic acid and sugar on the pattern of induced differentiation in plant tissue culture. Journal of Cell Science. 1967;2:77–88. doi: 10.1242/jcs.2.1.77. [DOI] [PubMed] [Google Scholar]
- Kang J-Y, Choi H-I, Im M-Y, Kim SY. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. The Plant Cell. 2002;14:343–357. doi: 10.1105/tpc.010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim YK, Park JY, Kim J. Light signalling mediated by phytochrome plays an important role in cold-induced gene expression through the C-repeat/dehydration responsive element (C/DRE) in Arabidopsis thaliana. The Plant Journal. 2002;29:693–704. doi: 10.1046/j.1365-313x.2002.01249.x. [DOI] [PubMed] [Google Scholar]
- Klotke J, Kopka J, Gatzke N, Heyer AG. Impact of soluble sugar concentration on the acquisition of freezing tolerance in accessions of Arabidopsis thaliana with contrasting cold adaptation: evidence for a role of raffinose in cold acclimation. Plant, Cell and Environment. 2004;27:1395–1404. [Google Scholar]
- Kosova K, Vitamvas P, Prasil IT. The role of dehydrins in plant response to cold. Biologia Plantarum. 2007;51:601–617. [Google Scholar]
- Laby RJ, Kincald MS, Kim D, Gibson SI. The Arabidopsis sugar-sensing mutants sis4 and sis5 are defective in abscisic acid synthesis and response. The Plant Journal. 2000;23:587–596. doi: 10.1046/j.1365-313x.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- Leborgne N, Teulieres C, Travert S, Rols MP, Teissie J, Baudet AM. Introduction of specific carbohydrates into Eucalyptus gunnii cells increases their freezing tolerance. European Journal of Biochemistry. 1995;229:710–717. doi: 10.1111/j.1432-1033.1995.tb20518.x. [DOI] [PubMed] [Google Scholar]
- Levitt J. Responses of plants to environmental stresses. Vol. I. New York: Academic Press; 1980. [Google Scholar]
- Livingston DP, Henson CA. Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiology. 1998;116:403–408. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Nicot N, Hausman JF, Hoffmann L, Evers D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. Journal of Experimental Botany. 2005;56:2907–2914. doi: 10.1093/jxb/eri285. [DOI] [PubMed] [Google Scholar]
- Palonen P, Junttila O. Cold hardening of raspberry plants in vitro is enhanced by increasing sucrose in the culture medium. Physiologia Plantarum. 1999;106:386–392. [Google Scholar]
- Pate JS, Sharkey PJ, Lewis OAM. Xylem to phloem transfer of solutes in fruiting shoots of legumes, studied by a phloem bleeding technique. Planta. 1975;122:11–26. doi: 10.1007/BF00385400. [DOI] [PubMed] [Google Scholar]
- Pearce RS. Relative hardiness to freezing of laminae, roots and tillers of tall fescue. New Phytologist. 1980;84:449–463. [Google Scholar]
- Pearce RS, Dunn MA, Rixon JE, Harrison P, Hughes MA. Expression of cold-induced genes and frost hardiness in the crown meristem of young barley (Hordeum vulgare L. cv. Igri) plants grown in different environments. Plant, Cell and Environment. 1996;19:275–290. [Google Scholar]
- Pearce RS, Houlston CE, Atherton KA, Rixon JE, Harrison P, Hughes MA, Dunn MA. Localization of expression of three cold-induced genes (blt101, blt4. 9, blt14) in different tissues of the crown and developing leaves of cold-acclimated cultivated barley (Hordeum vulgare L. cv. Igri) Plant Physiology. 1998;117:787–795. doi: 10.1104/pp.117.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CJ. Sucrose accumulation and the initiation of fructan biosynthesis in Lolium temulentum L. New Phytologist. 1984;96:527–534. [Google Scholar]
- Pyke K, López-Juez E. Cellular differentiation and leaf morphogenesis in Arabdopsis. Critical Reviews in Plant Sciences. 1999;18:527–546. [Google Scholar]
- Rekarte-Cowie I. Cold acclimation in Arabidopsis. 2002 doi: 10.1093/jxb/ern262. PhD thesis, University of Newcastle upon Tyne, UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez Sheen J. Sugar sensing and signalling in plants: Conserved and novel mechanisms. Annual Review of Plant Biology. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- Steponkus PL, Lanphear FO. Light stimulation of cold acclimation: production of a translocatable promoter. Plant Physiology. 1967;42:1673–1679. doi: 10.1104/pp.42.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G, Hauser H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proceedings of the National Academy of Sciences, USA. 1986;83:2422–2426. doi: 10.1073/pnas.83.8.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaei Aghdaei SR, Pearce RS, Harrison P. Sugars regulate cold-induced gene expression and freezing-tolerance in barley cell cultures. Journal of Experimental Botany. 2003;54:1565–1575. doi: 10.1093/jxb/erg173. [DOI] [PubMed] [Google Scholar]
- Tetlow IJ, Farrar JF. Apoplastic sugar concentrations and pH in barley leaves infected with brown rust. Journal of Experimental Botany. 1993;44:929–936. [Google Scholar]
- Travert S, Valerio L, Fouraste I, Boudet M, Teulieres C. Enrichment in specific soluble sugars of two Eucalyptus cell-suspension cultures by various treatments enhances their frost tolerance via a noncolligative mechanism. Plant Physiology. 1997;114:1433–1442. doi: 10.1104/pp.114.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunova TI. Mechanisms of winter wheat hardening at low temperature. In: Li PH, Sakai A, editors. Plant cold hardiness and freezing stress. Vol. 2. Academic Press; 1982. pp. 41–54. [Google Scholar]
- Tumanov II, Butenko RG, Ogolevets IV. Use of the isolated tissue method for studying hardening of plant cells. Soviet Plant Physiology. 1968;15:625–630. [Google Scholar]
- Tumanov II, Trunova TI. Hardening tissues of winter plants with sugar absorbed from the external solution. Fiziologiya Rastenii. 1957;4:379–388. [Google Scholar]
- Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812. doi: 10.1007/s00114-007-0254-y. [DOI] [PubMed] [Google Scholar]
- Tysdale HM. Influence of light, temperature, and soil moisture on the hardening process in alfalfa. Journal of Agricultural Research. 1933;46:483–515. [Google Scholar]
- Uemura M, Steponkus PL. Modification of the intracellular sugar content alters the incidence of freeze-induced membrane lesions of protoplasts isolated from Arabidopsis thaliana leaves. Plant, Cell and Environment. 2003;26:1083–1096. [Google Scholar]
- Wanner LA, Juntilla O. Cold-induced freezing tolerance in Arabidopsis. Plant Physiology. 1999;120:391–400. doi: 10.1104/pp.120.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. The Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]