Abstract
When soil moisture is heterogeneous, sap flow from, and ABA status of, different parts of the root system impact on leaf xylem ABA concentration ([X-ABA]leaf). The robustness of a model for predicting [X-ABA]leaf was assessed. ‘Two root-one shoot’ grafted sunflower (Helianthus annuus L.) plants received either deficit irrigation (DI, each root system received the same irrigation volumes) or partial rootzone drying (PRD, only one root system was watered and the other dried the soil). Irrespective of whether relative sap flow was assessed using sap flow sensors in vivo or by pressurization of de-topped roots, each root system contributed similarly to total sap flow during DI, while sap flow from roots in drying soil declined linearly with soil water potential (Ψsoil) during PRD. Although Ψsoil of the irrigated pot determined the threshold Ψsoil at which sap flow from roots in drying soil decreased, the slope of this decrease was independent of the wet pot Ψsoil. Irrespective of whether sap was collected from the wet or dry root system of PRD plants, or a DI plant, root xylem ABA concentration increased as Ψsoil declined. The model, which weighted ABA contributions of each root system according to the sap flow from each, almost perfectly explained [X-ABA] immediately above the graft union. That the model overestimated measured [X-ABA]leaf may result from changes in [X-ABA] along the transport pathway or an artefact of collecting xylem sap from detached leaves. The implications of declining sap flow through partially dry roots during PRD for the control of stomatal behaviour and irrigation scheduling are discussed.
Keywords: ABA, deficit irrigation, grafting, irrigation scheduling, modelling, partial rootzone drying, sap flow, soil moisture heterogeneity
Introduction
Soil moisture is commonly heterogeneously distributed within the soil profile, with greater root proliferation in the upper surface layers causing localized soil drying while decreased root length density at depth results in comparatively moist subsurface layers (Sharp and Davies, 1985). Although soil moisture sensors distributed throughout the soil profile can monitor water uptake from (and thus estimate sap flow through) roots of different layers (Gu et al., 2004; Leib et al., 2006), the vertical distribution of water uptake will depend on both soil moisture status and root length density. Determining the influence of soil drying per se on water uptake, independent of the influence of root length density, can conveniently be assessed using a split-root plant (Blackman and Davies, 1985), and also provides a good model system to assess the efficacy of different deficit irrigation techniques.
In split-root plants, information on the impact of soil drying on the distribution of water uptake is necessary to model flows of root-to-shoot signals such as abscisic acid (Dodd, 2008; Dodd et al., 2008; Liu et al., 2008), that are produced in response to soil drying and can be transported in the transpiration stream to the shoots to limit plant water use (Davies and Zhang, 1991; Dodd, 2005). Such modelling has been stimulated by the adoption of a particular form of deficit irrigation known as partial rootzone drying (PRD). This technique deliberately imposes soil moisture heterogeneity by independently watering different parts of the rootzone (for example, one side of the row) with the aim of manipulating root-to-shoot signalling to restrict crop water use (Dry et al., 1996). Theoretically, irrigated roots supply sufficient water to the shoots to prevent water deficits (Stoll et al., 2000; Sobeih et al., 2004) while roots in drying soil produce chemical signals that can be transmitted to the shoots, if there is sufficient sap flow through those roots (Dodd et al., 2008). Understanding the relationship between signal transmission and soil water status of different parts of the rootzone might provide a basis to understand the regulation of shoot physiology during deficit irrigation, and to schedule irrigation during PRD.
With this goal in mind, a novel grafting procedure was developed (Dodd, 2007) to determine the contributions of different parts of the root system to total sap flow and leaf xylem ABA concentration ([X-ABA]leaf) of PRD-grown plants. Combining the fractions of sap flow and xylem ABA concentrations from wet and dry parts of the root system in a simple model, better predicted [X-ABA]leaf than the mean (of wet and dry parts of the root system) root xylem ABA concentration ([X-ABA]root). When soil water status of the wet part of the root system remained high, there was an optimal soil water status of the dry part of the root system to maximize ABA export from the entire root system (Dodd et al., 2008). However, in many field experiments with PRD, partial drying of the irrigated roots occurs (Kirda et al., 2004) if irrigation is infrequent. It is therefore important to determine whether the model can still robustly predict [X-ABA]leaf when soil water status of the irrigated root system varies. Possible influences of two different methods of determining the fractions of sap flow from each root system, sap flow sensors in vivo (Dodd et al., 2008) and root pressurization (Salim and Pitman, 1984), on the prediction of [X-ABA]leaf were also assessed.
Materials and methods
Sap flow in vivo and xylem ABA concentration in sap collected from de-topped roots and detached leaves
‘Two root-one shoot’ sunflower (Helianthus annuus L. cv. Tall Single Yellow) plants were created using the grafting procedure previously described (Dodd, 2007; Dodd et al., 2008). Grafted plants resembled an inverted ‘Y’ with the root systems contained in two appressed 0.43 l pots, each designed to fit in a Scholander-type pressure chamber. Plant culture was as previously described (Dodd et al., 2008) except that the plants were grown in a different substrate (John Innes No. 2, J Arthur Bowers, UK). Gravimetric (θ) and volumetric water content of this substrate at field capacity were 0.63 g g−1 and 0.43 cm3 cm−3, respectively, and the bulk density when dry was 0.78 g cm−3. A moisture release curve for this substrate (Dodd et al., 2006) allowed measurements of θ to be converted to soil water potentials (Ψsoil) according to the following relationships. For θ <0.33 g g−1: Ψsoil = –1.105 + 3.065θ. For θ between 0.33 g g−1 and 0.45 g g−1: Ψsoil = –0.419 + 1.67θ–1.70θ2. For θ >0.45 g g−1, Ψsoil was set to –0.01 MPa (which was the lowest pressure that could be measured with the available pressure plates).
Sap flow through each hypocotyl (below the graft union) of the ‘two root-one shoot’ plants was measured using the heat balance technique with commercially available sensors (Model SGA-5, Dynagage®, Dynamax Inc, Houston, TX, USA) as previously described (Dodd et al., 2008). Soil water content of each pot, root water potentials, and (root) xylem ABA concentration, whole plant transpiration rate, leaf water potential, and (leaf) xylem ABA concentration were measured as previously described by Dodd et al. (2008). An overpressure of 0.4 MPa or 0.5 MPa was applied to the leaves or root systems respectively (following measurement of leaf and root water potential, respectively) to collect xylem sap.
Several batches of plants (comprising 10–12 plants per batch) were sequentially produced in the same environmental conditions. Two different irrigation regimes were imposed: deficit irrigation (where equal volumes of water were applied to each pot) and partial rootzone drying where only one pot received water and the other was allowed to dry the soil. Each individual plant received different irrigation volumes in aiming to achieve a range of whole pot soil water contents, estimated during each experiment from measurements of pot weight, and verified later by gravimetric measurement of soil water content. Within the PRD irrigation treatment, the designated ‘wet’ pot was watered at different frequencies (minimum of twice a day) in different plants, in trying to vary soil water content of the wet pot at harvest.
Pressure-induced sap flow and xylem ABA concentration in sap collected above and below the graft union
Since these experiments aimed to measure sap flow (and ABA concentration) above and below the graft union of ‘two root-one shoot’ plants, and the two pots of plants raised as above would not simultaneously fit in the available whole plant pressure chamber, slight cultural modifications (from that described above) were required. Sunflower seeds were placed on two layers of filter paper (Whatman No. 1) moistened with distilled water in a covered Petri dish and allowed to germinate in the dark for 48 h. Two identical plastic bags were placed in a 1.0 l pot (height of 130 mm, diameter of 110 mm, designed to fit in the pressure chamber), and equally filled with the same substrate as above. Before planting, soil water content of each bag was raised to field capacity to aid seedling establishment. A plastic disc of the same diameter as the pot was placed over the surface of the substrate. This disc had two holes (21 mm diameter) in it, spaced at the same dimensions as the split-top lid designed to fit on the pressure chamber (Seel and Jeschke, 1999). One germinated seedling (typical radical length of 20 mm) was placed through the middle of each hole into depressions in the substrate, covered with substrate and watered in. Ten pots were placed into a plastic container (50×33×28 cm), the top of the container covered with aluminium foil (to exclude light and promote hypocotyl extension), and the container placed in a single walk-in controlled environment room (3×4 m) at the Lancaster Environment Centre under environmental conditions previously described by Kudoyarova et al. (2007). Grafting occurred at the same stage of development as above. After graft establishment, plants were watered daily until the beginning of the experiment.
During experiments, half the plants received water to both plastic bags (DI) while the remainder only received water to one plastic bag (PRD). Prior to sap collection, the pot was placed in the pressure chamber, a split-top lid placed on top of the chamber, and each hypocotyl sealed into the chamber lid using a silicone-based dental impression compound (Affinis Fast Regular Body Microsystem, Coltene-Whaledent, Switzerland) which took no more than 5 min to set. To collect sap, the whole shoot was removed 1–3 cm above the graft union, the stump washed with distilled water and then blotted with filter paper three times, to remove any contaminating cell debris. Washing and blotting was performed after sealing the pot into the pressure chamber, thus preventing any increase in soil water content. Preliminary tests showed that applying pressures greater than 0.4 MPa generally resulted in leaks from the pressure seals, and occasionally ejection of the seals. Thus all plants were pressurized (with nitrogen) to 0.4 MPa. To determine the fraction of sap flow from each root system, samples were collected by lightly touching the cut stump with a glass capillary tube, thus avoiding the contamination that results from the radial pressure applied by a collecting sleeve. Samples were collected above the graft union, then the hypocotyls were severed below the graft union, and sap collected as above. If flow rates from both hypocotyls were too high to allow accurate transfer of sap to pre-weighed Eppendorf tubes, sap samples were collected alternately from each hypocotyl. All sap was immediately transferred to pre-weighed Eppendorf tubes then weighed (to determine sap flow rate), frozen in liquid nitrogen, then stored at –20 °C. Sap ABA concentration was measured (as above) using a radioimmunoassay (Quarrie et al., 1988).
Statistical analysis
Within an irrigation treatment (PRD or DI) or combined across irrigation treatments, linear or polynomial regressions determined the significance of relationships between soil and plant variables (Tables 1, 2). Two-way ANOVA determined whether irrigation treatment (Tables 1, 2), root system source (Fig. 5a; wet or dry root systems of PRD plants and DI plants), or data set (Fig. 5b) altered relationships between soil and plant variables. A change in the sensitivity of the y-variable to the x-variable is given by a significant interaction term (x-variable by treatment).
Table 1.
Relationships between sap flow and soil moisture status
| Regression | Data set | θwet (g g−1) | Intercept | Slope |
| Fdry on θdry | Sap flow in vivo I | 0.36±0.02 | –0.92±0.22 | 5.06±0.98 a |
| Sap flow in vivo II | 0.51±0.01 | –0.39±0.13 | 2.04±0.38 b | |
| Pressure-induced sap flow | 0.49±0.02 | –0.75±0.27 | 3.60±0.92 ab | |
| Ψwet (MPa) | ||||
| Fdry on Ψdry | Sap flow in vivo I | –0.09±0.03 | 0.91±0.13 | 1.65±0.32 a |
| Sap flow in vivo II | –0.01±0.00 | 0.41±0.03 | 0.93±0.15 a | |
| Pressure-induced sap flow | –0.01±0.00 | 0.53±0.07 | 1.11±0.29 a |
Regression parameters for relationships between the fraction of total sap flow through (Fdry), and soil water content (θdry) or soil water potential (Ψdry) of the dry part of the root system. Data sets from the sap flow measurements in vivo were discriminated (Fig. 2) using a threshold Ψwet of 0.45 g g−1 (Ψsoil of –0.01 MPa), with data set I including plants where θwet was <0.45 g g−1, and data set II including plants where θwet was >0.45 g g−1. Differences in the slopes of the regressions were determined via two-way ANOVA of data set and θdry or Ψdry. Different slopes (where the interaction term was significant at P < 0.05) are indicated by different letters.
Table 2.
Significance of linear and second order regressions between soil and plant variables
| Relationship | Irrigation | Linear | 2nd order |
| Transpiration rate on whole pot soil water content | Combined | 0.33 | 0.003 |
| Transpiration rate on leaf water potential | Combined | 0.07 | 0.09 |
| Transpiration rate on [X-ABA]leaf | Combined | <0.001 | 0.003 |
| [X-ABA]leaf on leaf water potential | PRD | 0.038 | 0.016 |
| DI | 0.002 | <0.001 | |
| [X-ABA]leaf on whole pot soil water content | Combined | 0.001 | <0.001 |
P values are presented for three distinct data sets: all PRD plants or all DI plants where irrigation treatment by x-variable interaction was significant (P <0.05). Alternatively, a combined data set (including both PRD and DI plants) was analysed where the irrigation treatment by x-variable interaction was not significant.
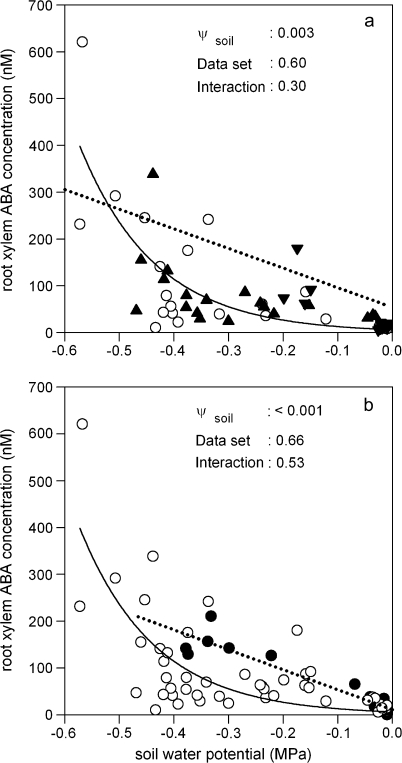
Fig. 5.
The relationship between root xylem ABA concentration and soil water potential of ‘two root-one shoot’ plants grown in two separate pots (a) and two separate pots (open circles) or two plastic bags in a single pot (closed circles) (b). In (a), the wet (closed inverted triangles) and dry (closed triangles) parts of the root system of PRD and DI (open circles) plants are indicated, along with the relationship previously determined (dotted line) for a similar experiment with tomato (Fig. 5 of Dodd, 2007). Each point represents a single root system and linear (dotted lines) or exponential (solid lines) were fitted in SigmaPlot for Windows 2.01. P values determined by two-way ANOVA for irrigation treatment (DI versus wet versus dry) in (a) or data set (two separate pots versus two plastic bags in a single pot) in (b), soil water potential and their interaction are presented.
Results
In a typical ‘two root-one shoot’ plant grown in two separate pots and subjected to partial rootzone drying (Fig. 1a), sap flow through the two hypocotyls was most similar at the beginning of the experiment when soil water content of both pots was similar (data collection began after both pots had been watered to the drip point). As the experiment continued, sap flow through the root system exposed to drying soil decreased such that when the plant was harvested, heat losses by convection by the sap (Qf) from the dry and wet root systems were 4.5 mW and 31 mW, respectively. Thus the fractions of sap flow through dry and wet root systems were 0.13 and 0.87, respectively (Fig. 1a) and the soil water contents of the dry and wet pots were 0.28 and 0.53 g g−1, respectively. By contrast, in a typical deficit-irrigated plant, sap flow through the two hypocotyls was similar throughout two day/night cycles and decreased similarly as the soil dried (Fig. 1b). When the plant was harvested, the fractions of sap flow through the two hypocotyls were 0.43 and 0.57 (Fig. 1b) and the soil water contents of the two pots were 0.17 and 0.18 g g−1, respectively.
Fig. 1.
Sap flow in vivo through hypocotyls of two different ‘two root-one shoot’ grafted plants under partial rootzone drying (a) and deficit irrigation (b). In (a), only the root system designated ‘right’ was watered but in (b), both ‘left’ and ‘right’ root systems were watered. Arrows and the black bars on the x-axis indicate irrigation events and the night periods, respectively. (This figure is available in colour at JXB online.)
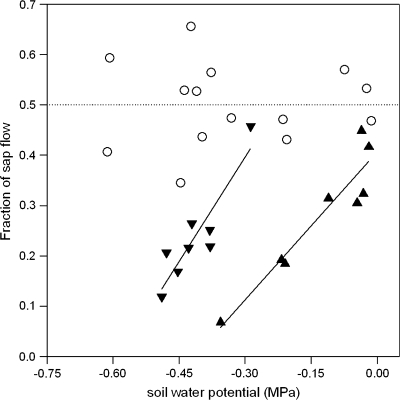
Similar experiments were repeated with a number of plants to examine the effect of different irrigation treatments (PRD versus DI) on the relationship between the fractions of total sap flow through each hypocotyl and soil water potential (Fig. 2). In DI plants, even though absolute sap flow (through the entire plant and each hypocotyl) decreased with Ψsoil (data not shown), the fraction of sap flow through either hypocotyl was similar and varied between 0.34 and 0.66. In PRD plants, the fraction of sap flow through the dry part of the root system (Fdry) significantly decreased with soil water content (θdry– data not shown) or soil water potential of the dry pot (Ψdry). This relationship varied according to the soil water status of the irrigated pot: when soil water potential was less than 0.45 g g−1 (Ψwet < −0.01 MPa), sap flow through the dry part of the root system decreased at a lower threshold soil water potential (Fig. 2). The slope of this decrease was constant, despite varying Ψwet, when Fdry was plotted against Ψdry (Table 1).
Fig. 2.
The relationship between soil water potential and the fraction of total sap flow in vivo from either part of the root system of DI plants (open circles) or the dry part of the root system of PRD plants when soil water potential of the wet pot exceeded (closed triangles) or was less than (closed inverted triangles) –0.01 MPa. Each point represents a single hypocotyl and regression lines were fitted where P < 0.05. (closed triangles) Fdry=0.41 + 0.93Ψdry (r2=0.86) and (closed inverted triangles) Fdry=0.91 + 1.65Ψdry (r2=0.82).
Since different pressures were applied to each root system (a constant overpressure, which exceeded a variable balancing pressure) to collect xylem sap, the effect of soil drying on pressure-induced sap flow (of plants grown in two appressed pots as above) was not examined. Instead, ‘two root-one shoot’ plants were grown in a single pot where the roots were contained in two plastic bags, a constant pressure applied to the root system and sap collected from the stem above the graft union, then each hypocotyl below the graft union. In a plant adequately supplied with water, pressure-induced sap flow through the two hypocotyls was similar (Fig. 3a). Similarly, in a deficit-irrigated plant, pressure-induced sap flow through the two hypocotyls was also similar (Fig. 3c) although total sap flow was much less. In a typical plant subjected to partial rootzone drying, pressure-induced sap flow through the dry part of the root system was 40% less than sap flow through the wet part of the root system (Fig. 3b).
Fig. 3.
Pressure-induced sap flow above the graft union (A) and below the graft union through ‘left’ (L) and ‘right’ (R) hypocotyls of ‘two root-one shoot’ plants under partial rootzone drying (b) and deficit irrigation (a, c). Soil water contents of the left and right pots were 0.44 and 0.49 g g−1 (a), 0.49 and 0.30 g g−1 (b), and 0.29 and 0.27 g g−1 (c), respectively.
Similar experiments were repeated with a number of plants to examine the effect of different irrigation treatments (PRD versus DI) on the relationship between pressure-induced sap flow through each hypocotyl and soil water potential (Fig. 4). In DI plants, the fraction of sap flow through either hypocotyl was similar and varied between 0.4 and 0.6. In PRD plants, the fraction of sap flow through the dry part of the root system significantly (P <0.001) decreased with soil water potential. The relatively few data points where soil water content of the irrigated pot was less than 0.45 g g−1 prevented further discrimination of this relationship (as in Fig. 2). Relationships between the fraction of sap flow through the dry part of the root system (Fdry) and soil water content or potential were compared statistically (Table 1) to determine whether the sensitivity of sap flow to drying soil varied according to the method of sap flow measurement. When the soil water content of the wet part of the root system exceeded 0.45 g g−1, irrespective of whether Fdry was plotted against soil water content (θdry) or soil water potential (Ψdry) of the dry part of the root system, the slope of the relationship in vivo (Fig. 2) was equivalent to that obtained from pressure-induced sap flow measurements (Fig. 4).
Fig. 4.
The relationship between soil water potential and the fraction of total pressure-induced sap flow from the dry part of the root system of PRD plants (closed circles) and either part of the root system of DI plants (open circles). Each point represents a single hypocotyl and a regression line was fitted where P <0.05. Fdry=0.53 + 1.11Ψdry (r2=0.58).
For the ‘two root-one shoot’ plants grown in two separate pots, whole plant transpiration rate was more closely correlated with leaf xylem ABA concentration ([X-ABA]leaf) than either whole pot soil water content or leaf water potential (Table 2). Consequently, the regulation of [X-ABA]leaf was further considered. [X-ABA]leaf significantly increased as leaf water potential or whole pot soil water content declined (data not shown). Irrigation treatment significantly affected the relationship between [X-ABA]leaf and leaf water potential (Table 2), with the slope of this increase greater in DI plants (data not shown).
Root xylem ABA concentration increased as Ψsoil decreased, with samples collected from the dry or wet part of the root system of a PRD plant or from a DI plant giving a similar relationship (Fig. 5a). Tomato (Dodd, 2007) and sunflower had a similar [X-ABA]root when soil water content was < –0.43 MPa or > –0.01 MPa, while at intermediate values it was, on average, 3-fold higher in tomato. However, [X-ABA]root was similarly sensitive to Ψsoil in both species (species×Ψsoil interaction was not significant: P=0.37) despite species differences in absolute [X-ABA]root. The increase in [X-ABA]root with decreasing Ψsoil in ‘two root-one shoot’ sunflowers was similar (data set×Ψsoil interaction was not significant: P=0.53), irrespective of whether plants were grown in two separate pots, or two plastic bags in the one pot (Fig. 5b).
ABA concentration above the graft union was modelled as in Dodd et al. (2008) thus:
| (1) |
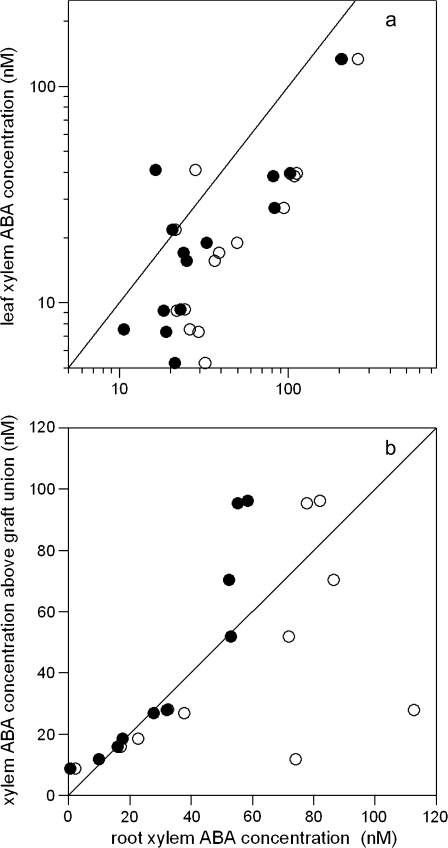
where Fdry, Fwet are the fractions of total sap flow from the dry and wet root systems (Figs 2, 4) and [X-ABA]dry, [X-ABA]wet are the root xylem ABA concentrations from the dry and wet root systems. Shoot xylem ABA concentrations were measured above the graft union in samples collected from leaves detached from plants grown in two separate pots and compared against equation 1 (Fig. 6a). Although equation 1 and the mean root xylem ABA concentration from both pots had a similar predictive ability of [X-ABA]leaf in DI plants (data not shown), equation 1 better predicted [X-ABA]leaf of PRD plants than the mean of [X-ABA]dry and [X-ABA]wet (Table 3). However, both mean and modelled root xylem ABA concentration overestimated [X-ABA]leaf (Fig. 6a).
Fig. 6.
The relationship between detached leaf xylem ABA concentration (a) or xylem ABA concentration above the graft union (b) and mean root xylem ABA concentration (open circles) and a model where [X-ABA]leaf (a) or [X-ABA]above the graft union (b)=Fdry[X-ABA]dry+Fwet[X-ABA]wet (closed circles) for ‘two root-one shoot’ PRD plants grown in two separate pots (a) or two plastic bags in a single pot (b). In both cases the 1:1 relationship is shown as a solid line.
Table 3.
The ability of different models to predict xylem ABA concentration of PRD plants
| Model | Plants in two pots (Fig. 6a) | Plants in one pot (Fig. 6b) |
| Prediction of [X-ABA]leaf | Prediction of [X-ABA]above the graft union | |
| Mean | 32.5±9.0 (16) | 16.3±11.0 (10) |
| Fractional | 19.8±6.7 (16) | –9.8±5.5 (10) |
Shoot xylem ABA concentration ([X-ABA]shoot) was measured in detached leaves or from the stem 1–3 cm above the graft union. For each plant, the difference between model and measurement is calculated as [X-ABA]model minus [X-ABA]shoot. A positive value indicates that the model overestimates [X-ABA]shoot, while a negative value indicates that the model underestimates [X-ABA]shoot. Two different models are indicated: ‘mean’ where [X-ABA]model=mean of [X-ABA]wet and [X-ABA]dry and ‘fractional’ where [X-ABA]model is calculated from equation 1 (see text). Data are means ±SE of the number of values in parentheses.
To determine whether this overestimation was a possible artefact of sap collection from detached leaves, xylem sap was collected both from the stem 1–3 cm above the graft union and below the graft union from plants grown in two plastic bags in the one pot and placed in a specialized ‘split-top’ pressure chamber. Again, equation 1 better predicted [X-ABA]above the graft union of PRD plants than the mean of [X-ABA]dry and [X-ABA]wet (Table 3) and almost perfectly explained [X-ABA]above the graft union for concentrations less than 60 nM (Fig. 6b).
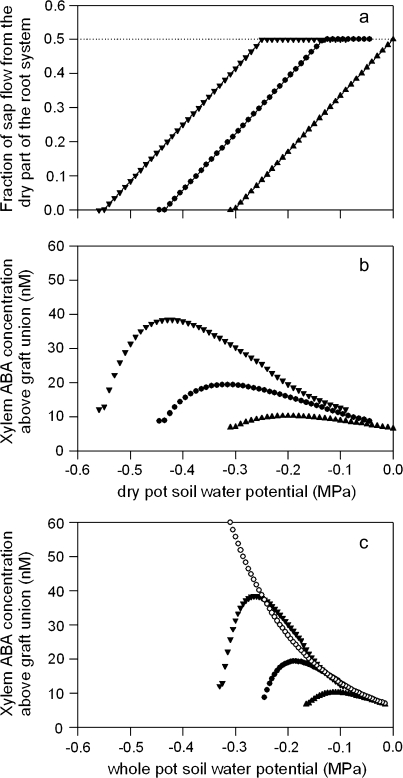
The consequences of equation 1 for [X-ABA]leaf of PRD plants were simulated for a range of irrigated pot soil water status, namely Ψwet of –0.01 MPa, –0.045 MPa, and –0.09 MPa, while varying the dry pot soil water status. At any soil water potential, root xylem ABA concentration was calculated from the exponential relationship fitted to the data from two appressed pots in Fig. 5 ([X-ABA]root=6.309×0.0007Ψsoil; r2=0.55), while the fraction of sap flow from roots in dry soil was calculated from the equation Fdry=mΨdry+c. When Ψwet was –0.09 MPa (analogous to data set II; see Table 1), m and c equalled 1.65 and 0.91, respectively. Maintaining parameter m constant, irrespective of Ψwet (in line with the statistical analysis of Table 1), resulted in parameter c equalling 0.71 and 0.5 for Ψwet of –0.045 MPa and –0.01 MPa, respectively (note that the latter case is realistic since the fraction of sap flow through either of two root systems exposed to an equal, high Ψsoil should equal 0.5). Thus it was possible to calculate the fraction of sap flow from roots in drying soil as Ψdry varied (Fig. 7a). Irrespective of Ψwet, simulated [X-ABA]leaf of PRD plants increased to a maximum, then decreased as the fraction of sap flow from roots in drying soil decreased (Fig. 7b). Thus Ψdry to maximize [X-ABA]leaf of PRD plants varied according to Ψwet. When compared at the same whole pot Ψsoil, there were minimal (<5 nM) differences in simulated [X-ABA]leaf between PRD and DI plants, until the fraction of sap flow through roots of PRD plants in drying soil declined. As Ψdry continued to decline, [X-ABA]leaf of PRD plants decreased relative to DI plants at the same whole pot Ψsoil (Fig. 7c).
Fig. 7.
Simulated relationships between dry pot soil water potential and the fraction of total sap flow from the dry part of the root system of PRD plants (a) and xylem ABA concentration above the graft union calculated from equation 1 (b) when wet pot soil water potential was –0.01 MPa (closed triangles), –0.045 MPa (closed circles) or –0.09 MPa (closed inverted triangles), respectively. Simulated xylem ABA concentration is also plotted against whole pot soil water potential for both PRD and DI (open circles) plants (c).
Discussion
As in previous studies with sunflower (Tardieu et al., 1996; Dodd et al., 2008), plant water use was more closely correlated with leaf xylem ABA concentration than leaf water potential (Table 2). That this occurred in an anisohydric plant like sunflower that does not tightly control its leaf water status (Tardieu et al., 1996) provides further impetus for understanding the factor(s) that regulate [X-ABA]leaf in vivo. Since [X-ABA]leaf was more closely related to soil water status than leaf water potential (Tardieu et al., 1996; Dodd et al., 2008) (Table 2), root xylem ABA concentration was measured in attempting to explain variation in [X-ABA]leaf.
Although plants were grown in two slightly different systems (two appressed pots versus two plastic bags in a single pot) and xylem sap was collected slightly differently in each (a constant overpressure exceeding root balancing pressure versus a constant pressure), the relationship between [X-ABA]root and Ψsoil arising from the two studies was similar (Fig. 5b). While [X-ABA]root increases with decreasing sap flow rate (Else et al., 1995; Dodd et al., 2008), any differences in [X-ABA]root caused by the sap collection technique were much smaller than species differences (Fig. 5a). In another study, grapevine cultivars with a higher [X-ABA]leaf better controlled their leaf water status (Soar et al., 2006b), and it is possible that the lower [X-ABA]root (Fig. 5a) (and [X-ABA]leaf) of sunflower (a known anisohydric species) compared to tomato (which displays more isohydric behaviour in the field; Reid and Renquist, 1997) is of functional significance. Alternatively, species differences in stomatal sensitivity to xylem ABA concentration may be responsible for anisohydric versus isohydric behaviour (Tardieu and Simonneau, 1998).
It has been suggested that measurement of [X-ABA]leaf may provide a marker for comparative water use physiology of different scion–rootstock combinations in grapevine (Soar et al., 2006a). While the relationship between [X-ABA]leaf and soil water status may be robust when soil moisture is more uniformly distributed, as occurs in pots (Dodd, 2007), field-grown plants will often be exposed to heterogeneous soil moisture and differences in rooting patterns between different genotypes (or rootstocks) may be responsible for differences in [X-ABA]leaf. For example, a genotype with a relatively shallow root system restricted to the upper soil profile may be exposed to a relatively uniform soil water status, while a deeper-rooting genotype which deploys some roots in the moist subsoil may source much of its transpirational flux from these roots. Such differences in rooting pattern probably influence root-to-shoot ABA signalling.
Indeed, soil moisture heterogeneity (imposed by partial rootzone drying here) limited the export of ABA from roots to shoots, as sap flow from roots in drying soil decreased with soil drying (Dodd et al., 2008). The effect of diminished sap flow through drying roots of PRD plants (Fig. 2) was evident in soil moisture heterogeneity influencing the relationship between [X-ABA]leaf and Ψleaf such that DI plants had a higher [X-ABA]leaf at a given Ψleaf or Ψsoil (Dodd, 2007; Dodd et al., 2008) (Table 2). Accounting for this diminished sap flow in a simple model (equation 1) improved the prediction of [X-ABA]leaf in PRD plants compared to predictions made by models based on measurements of [X-ABA]root alone (Fig. 6; Table 3; see also Dodd et al., 2008).
However, a related study found that [X-ABA]root of PRD plants was better explained by a predicted mean [X-ABA]root (of wet and dry parts of the root system) based on a predetermined relationship between Ψsoil and [X-ABA]root in plants grown with homogenous soil moisture (Liu et al., 2008), rather than accounting for the fractions of sap flow from different parts of the root system. Measured [X-ABA]root of PRD plants higher than expected (based on relative sap flow from the different parts of the root system) may occur if root pressurization forces water through (drier) parts of the root than those parts employed when water is drawn along water potential gradients in vivo. This hypothesis was explicitly tested, by comparing the effect of Ψsoil on the fractions of in vivo (Fig. 2) and pressure-induced (Fig. 4) sap flow through the dry part of the root system, and proved false, since the slope of the relationship between the fraction of sap flow and Ψsoil was statistically equivalent in both data sets (Table 1).
Another possible explanation for the result that accounting for sap flows did not improve the prediction of [X-ABA]root in PRD plants (Liu et al., 2008), may lie in the particular regression (between Ψsoil and [X-ABA]root in non-irrigated plants) used to predict [X-ABA]root of PRD plants. Although Ψsoil of the dry compartment of PRD plants remained above –0.175 MPa (Fig. 2b of Liu et al., 2008), the regression between Ψsoil and [X-ABA]root (Table 4, Regression A) was extended to a minimum Ψsoil of –0.5 MPa (Fig. 6 of Liu et al., 2008). Accordingly, a new regression (Table 4, Regression B) that included only data where Ψsoil exceeded –0.175 MPa was used to predict [X-ABA]dry and [X-ABA]wet. Measured [X-ABA]root of PRD plants (Fig. 5 of Liu et al., 2008; Table 4, column 2) was compared against mean [X-ABA]root (Table 4, column 5), and [X-ABA]root modelled according to equation 1 (Table 4, column 6), using the fractions of sap flow from wet and dry root systems of PRD plants previously determined (Fig. 3b of Liu et al., 2008).
Table 4.
Modelling root xylem ABA concentration of potato plants exposed to PRD
| Day | Actual | Wet | Dry | Mean | Fractional | Mean-Actual | Fractional-Actual |
| Regression A | |||||||
| 2 | 219 | 146 | 176 | 161 | 158 | −58 | −62 |
| 3 | 224 | 142 | 268 | 205 | 185 | −20 | −40 |
| 4 | 296 | 157 | 436 | 296 | 226 | 0 | −70 |
| 5 | 214 | 176 | 543 | 359 | 218 | 145 | 4 |
| Mean difference | 17±17 (4) | −42±9 (4) | |||||
| Regression B | |||||||
| 2 | 219 | 226 | 245 | 235 | 233 | 16 | 14 |
| 3 | 224 | 223 | 304 | 264 | 251 | 39 | 26 |
| 4 | 296 | 233 | 412 | 322 | 277 | 27 | −19 |
| 5 | 214 | 245 | 481 | 363 | 272 | 149 | 58 |
| Mean difference | 58±7 (4) | 20±13 (4) |
Actual xylem ABA concentration from the entire root system (Fig. 5 of Liu et al., 2008) and that predicted from the wet and dry (designated ‘north’ and ‘south’ in Liu et al., 2008) parts of the root system are compared with a mean (of both wet and dry) root xylem ABA concentration, and a fractional model where [X-ABA]root=Fdry[X-ABA]dry + Fwet[X-ABA]wet. The fraction of sap flow from the dry part of the root system (Fdry) was derived from Fig. 3b of Liu et al. (2008), and for days 2, 3, 4, and 5 was 0.39, 0.34, 0.25, and 0.11, respectively, while Fwet was 1–Fdry. Predicted xylem ABA concentrations from wet and dry parts of the root system were calculated from regressions of xylem ABA concentration (from the entire root system of non-irrigated plants) on soil water potential (as in Fig. 6 of Liu et al., 2008), and water potentials of wet and dry parts of root systems were measured (Fig. 2b of Liu et al., 2008). On days 2, 3, 4, and 5, Ψsoil of the dry part of the root system was –23, –58, –123, and –164 kPa, respectively, and Ψsoil of the wet part of the root system was –12, –10, –16, and –23 kPa, respectively. Two different regressions were fitted: Regression A where [X-ABA] = 2.61Ψsoil+115.2 (as in Fig. 6 of Liu et al., 2008) and Regression B (fitted to all data in Fig. 6 of Liu et al., 2008 where Ψsoil > –175 kPa –the minimum Ψdry achieved by day 5 of PRD) where [X-ABA] = 1.68Ψsoil+206. For each day, the difference between mean or model and measurement is calculated as [X-ABA]model minus [X-ABA]root. A positive value indicates an overestimation of [X-ABA]root, while a negative value indicates an underestimation of [X-ABA]root. The mean difference (±SE) of the number of values in parentheses (analogous to Table 3 above) is provided. Data are xylem ABA concentrations (nM).
Interestingly, prediction of [X-ABA]dry and [X-ABA]wet with Regression B (using the same range of Ψsoil as experienced by PRD plants) altered the conclusion of that study. Using Regression A, as in the original paper (Liu et al., 2008), the mean of [X-ABA]dry and [X-ABA]wet (which over the first drying cycle of PRD overestimated [X-ABA]root by 17 nM) better predicted [X-ABA]root than equation 1 (which underestimated [X-ABA]root by 42 nM). However, using Regression B, equation 1 (which overestimated [X-ABA]root by 20 nM) better predicted [X-ABA]root than the mean of [X-ABA]dry and [X-ABA]wet (which overestimated [X-ABA]root by 58 nM). Some caution should be exercised in interpreting this re-analysis, as mean xylem ABA concentrations, fractions of sap flow, and soil water potentials (re-elaborated from the figures of Liu et al., 2008) rather than data for individual plants, were used. However, in the absence of accurate measurements of [X-ABA]dry and [X-ABA]wet which are only possible in grafted plants, the specific regression fitted between Ψsoil and [X-ABA]root is key in determining the success of equation 1 in forecasting xylem ABA concentrations in the shoot.
However, the predictive ability of equation 1 was influenced by the site (and/or methodology) at which xylem sap samples were collected. That equation 1 consistently overestimated detached leaf xylem ABA concentration of PRD plants (Fig. 6a) was not a deficiency of the model, as the same overestimation was detected when [X-ABA]leaf was plotted against the mean [X-ABA]root of DI sunflower plants (Dodd et al., 2008). Furthermore, this problem was not evident when [X-ABA] was measured above and below the graft union in samples collected by root pressurization (Fig. 6b), suggesting that the graft union itself had little influence on ABA signalling. Although xylem sap collection from detached leaves using a Scholander-type pressure chamber estimated [X-ABA]leaf in sunflower (Tardieu et al., 1996), since samples can be collected in the field (Dodd et al., 1996), direct comparisons against leaf xylem samples collected by root pressurization of intact plants (Schurr et al., 1992) are lacking. Although this comparison was envisaged in this study, failure of the pressure seals at pressures insufficient to collect xylem sap from a leaf in the canopy prevented its realization. However, because [X-ABA]root and [X-ABA]leaf were correlated in DI plants (Dodd, 2007; Dodd et al., 2008), it suggested that measurements of [X-ABA]root and sap flow could usefully predict [X-ABA]leaf of PRD plants.
While a previous study in a different substrate developed a single relationship between the fraction of sap flow through the dry part of the root system and soil water content (Dodd et al., 2008), here two different relationships were distinguished according to the soil water status of the irrigated pot (Fig. 2). However, irrespective of Ψwet, the slope of the decrease in the fraction of sap flow from roots in drying soil was similarly sensitive to Ψdry (Table 1). This indicates that it is still possible to impose PRD (and achieve differential sap flow from wet and dry root systems) even when Ψwet falls below –0.01 MPa, even though many experiments aim to keep part of the root system above this threshold (Leib et al., 2006; Liu et al., 2006). Maintaining some roots above this threshold may explain the limited effect of PRD on stomatal response in some experiments, due to diminished sap flow and signalling from the dry part of the root system (Yao et al., 2001, Dodd et al., 2008). Instead, the effects of PRD on [X-ABA]leaf (and stomatal responses) should be evaluated at a range of (entire rootzone) soil water availabilities by maintaining a Ψsoil difference between different parts of the root system. While equation 1 allows [X-ABA]leaf to be simulated for a wide (theoretical) range of soil water potentials (Dodd, 2008), irrigation frequencies and volumes relative to transpirational losses will determine whether a Ψsoil difference between wet and dry root systems is actually achieved. That soil moisture of plants exposed to PRD in the field is not always heterogeneously distributed (Kirda et al., 2004; Du et al., 2006) may partially explain why irrigation treatment (PRD versus DI) does not always significantly affect shoot physiology.
Continuous soil moisture monitoring (Gu et al., 2004; Leib et al., 2006) can help irrigation managers to impose soil moisture differences between dry and wet root systems during PRD, with the aim of inducing partial stomatal closure. Stomatal responses will depend on signal production by roots in contact with drying soil (Zhang and Davies, 1989), signal transfer to the xylem (Fig. 5), and signal transmission to the shoot depending on the relative sap flow from different parts of the root system (Figs 2, 4). While [X-ABA]leaf cannot be monitored with sufficient temporal resolution to be used in irrigation scheduling, its effects can be determined directly by monitoring plant water use via porometry, infra-red thermography or sap flow (Jones, 2004), or indirectly by quantifying soil moisture depletion (Liu et al., 2008). Modelling [X-ABA]leaf based on differences in Ψsoil between different parts of the root system, and sap flow from those root systems has shown that Ψdry should be maintained within a certain range to maximize ABA signalling (Dodd, 2008; Dodd et al., 2008; Liu et al., 2008; Fig. 7b). However, the fact that this range varies with Ψwet (Fig. 7b) suggests that irrigation scheduling during PRD, based on Ψdry set points, may need to be flexible according to the Ψsoil surrounding the irrigated root system. In practice, in the absence of rainfall, Ψdry decreases continuously during a drying cycle (Gu et al., 2004; Leib et al., 2006) and to avoid ABA concentration decreasing as the soil becomes too dry (Fig. 7b), the wet and dry parts of the rootzone are alternated. While alternation itself transiently increases xylem ABA concentration (Dodd et al., 2006; Topcu et al., 2007; but see Liu et al., 2008), the soil water status at which such alternation events should occur has not been defined physiologically. Instead, soil is allowed to dry during PRD for a pre-determined (usually arbitrary) period of time (Gu et al., 2004; Leib et al., 2006) or until a certain (usually arbitrary) soil water content is reached (Davies et al., 2000; Antolin et al., 2006) before the dry part of the root system is re-irrigated. Whether the ABA modelling approach developed here can inform and complement plant- or soil-based methods of scheduling irrigation of PRD plants remains to be determined.
Despite much interest in, and widespread adoption of, PRD as a management tool (reviewed in Costa et al., 2007; Dodd, 2007; Kirda et al., 2007), concerns remain that its physiological effects are difficult to distinguish from deficit irrigation generally, when the same irrigation volumes are applied. While the physiological bases for differences between DI and PRD plants are only just starting to be understood (Dodd et al., 2008), assessing root-to-shoot signalling of plants exposed to different degrees of soil moisture heterogeneity would seem necessary to determine whether PRD enhances or diminishes (Fig. 7c) signalling according to total soil water availability. Given the range of crops and substrates to which PRD is applied, modelling ABA (and other plant growth regulators) signalling may provide a parsimonious framework of analysis to complement existing multi-factorial field trials.
Acknowledgments
We thank DEFRA (Contract HH3609STX) and the British Council–German Academic Exchange Service (Project 281) for support of this work, and Maureen Harrison for plant care. Gregorio Egea received a travel grant from the FPU programme of the Spanish Ministry of Education, Culture and Sport.
Glossary
Abbreviations
- DI
deficit irrigation
- Fdry, Fwet
fraction of total sap flow from the dry and wet parts of the root system
- Ψleaf, Ψsoil
leaf and soil water potential
- Ψdry, Ψwet
soil water potential of the dry and wet pots
- PRD
partial rootzone drying
- θ
(gravimetric) soil water content
- θdry, θwet
soil water content of the dry and wet pots
- [X-ABA]model
modelled leaf xylem ABA concentration
- [X-ABA]leaf, [X-ABA]root, [X-ABA]shoot
leaf, root, and shoot xylem ABA concentration
- [X-ABA]dry, [X-ABA]wet
root xylem ABA concentration from the dry and wet parts of the root system
References
- Antolin MC, Ayari M, Sanchez-Diaz M. Effects of partial rootzone drying on yield, ripening and berry ABA in potted Tempranillo grapevines with split roots. Australian Journal of Grape and Wine Research. 2006;12:13–20. [Google Scholar]
- Blackman PG, Davies WJ. Root to shoot communication in maize plants of the effects of soil drying. Journal of Experimental Botany. 1985;36:39–48. [Google Scholar]
- Costa JM, Ortuno MF, Chaves MM. Deficit irrigation as a strategy to save water: physiology and potential application to horticulture. Journal of Integrative Plant Biology. 2007;49:1421–1434. [Google Scholar]
- Davies WJ, Bacon MA, Thompson DS, Sobeih W, Gonzalez Rodriguez L. Regulation of leaf and fruit growth on plants in drying soil: exploitation of the plants’ chemical signalling system and hydraulic architecture to increase the efficiency of water use in agriculture. Journal of Experimental Botany. 2000;51:1617–1637. doi: 10.1093/jexbot/51.350.1617. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Annual Review of Plant Physiology and Plant Molecular Biology. 1991;42:55–76. [Google Scholar]
- Dodd IC. Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant and Soil. 2005;274:251–270. [Google Scholar]
- Dodd IC. Soil moisture heterogeneity during deficit irrigation alters root-to-shoot signalling of abscisic acid. Functional Plant Biology. 2007;34:439–448. doi: 10.1071/FP07009. [DOI] [PubMed] [Google Scholar]
- Dodd IC. Measuring and modeling xylem ABA concentration ([X-ABA]) in tomato plants exposed to regulated deficit irrigation (RDI) and partial rootzone drying (PRD) Acta Horticulturae. 2008;792:225–231. [Google Scholar]
- Dodd IC, Egea G, Davies WJ. ABA signalling when soil moisture is heterogeneous: decreased photoperiod sap flow from drying roots limits ABA export to the shoots. Plant, Cell and Environment. 2008;31:1263–1274. doi: 10.1111/j.1365-3040.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Stikic R, Davies WJ. Chemical regulation of gas exchange and growth of plants in drying soil in the field. Journal of Experimental Botany. 1996;47:1475–1490. [Google Scholar]
- Dodd IC, Theobald JC, Bacon MA, Davies WJ. Alternation of wet and dry sides during partial rootzone drying irrigation alters root-to-shoot signalling of abscisic acid. Functional Plant Biology. 2006;33:1081–1089. doi: 10.1071/FP06203. [DOI] [PubMed] [Google Scholar]
- Dry PR, Loveys BR, Botting D, During H. Effects of partial rootzone drying on grapevine vigour, yield, composition of fruit and use of water. In: Stockley CS, Sas AN, Johnstone RS, Lee TH, editors. Proceedings of the 9th Australian wine industry technical conference. Adelaide: Winetitles; 1996. pp. 126–131. [Google Scholar]
- Du TS, Kang SZ, Zhang JH, Li FS, Hu XT. Yield and physiological responses of cotton to partial root-zone irrigation in the oasis field of northwest China. Agricultural Water Management. 2006;84:41–52. [Google Scholar]
- Else MA, Hall KC, Arnold GM, Davies WJ, Jackson MB. Export of abscisic acid, 1-aminocyclopropane-1-carboxylic acid, phosphate, and nitrate from roots to shoots of flooded tomato plants. Accounting for effects of xylem sap flow rate on concentration and delivery. Plant Physiology. 1995;107:377–384. doi: 10.1104/pp.107.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SL, Du GQ, Zoldoske D, Hakim A, Cochran R, Fuselgang K, Jorgensen G. Effects of irrigation amount on water relations, vegetative growth, yield and fruit composition of Sauvignon blanc grapevines under partial rootzone drying and conventional irrigation in the San Joaquin Valley of California, USA. Journal of Horticultural Science and Biotechnology. 2004;79:26–33. [Google Scholar]
- Jones HG. Irrigation scheduling: advantages and pitfalls of plant-based methods. Journal of Experimental Botany. 2004;55:2427–2436. doi: 10.1093/jxb/erh213. [DOI] [PubMed] [Google Scholar]
- Kirda C, Cetin M, Dasgan Y, Topcu S, Kaman H, Ekici B, Derici MR, Ozguven AI. Yield response of greenhouse grown tomato to partial root drying and conventional deficit irrigation. Agricultural Water Management. 2004;69:191–201. [Google Scholar]
- Kirda C, Topcu S, Cetin M, Dasgan HY, Kaman H, Topaloglu F, Derici MR, Ekici B. Prospects of partial root zone irrigation for increasing irrigation water use efficiency of major crops in the Mediterranean region. Annals of Applied Biology. 2007;150:281–291. [Google Scholar]
- Kudoyarova GR, Vysotskaya LB, Cherkozyanova A, Dodd IC. Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. Journal of Experimental Botany. 2007;58:161–168. doi: 10.1093/jxb/erl116. [DOI] [PubMed] [Google Scholar]
- Leib BG, Caspari HW, Redulla CA, Andrews PK, Jabro JJ. Partial rootzone drying and deficit irrigation of ‘Fuji’ apples in a semi-arid climate. Irrigation Science. 2006;24:85–99. [Google Scholar]
- Liu F, Shahnazari A, Andersen MN, Jacobsen SE, Jensen CR. Effects of deficit irrigation (DI) and partial root drying (PRD) on gas exchange, biomass partitioning, and water use efficiency in potato. Scientia Horticulturae. 2006;109:113–117. [Google Scholar]
- Liu F, Song R, Zhang X, Shahnazari A, Andersen MN, Plauborg F, Jacobsen SE, Jensen CR. Measurement and modelling of ABA signalling in potato (Solanum tuberosum L.) during partial root-zone drying. Environmental and Experimental Botany. 2008;63:385–391. [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE, Loveys BR. A monoclonal antibody to (S)-abscisic acid: its characterization and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta. 1988;173:330–339. doi: 10.1007/BF00401020. [DOI] [PubMed] [Google Scholar]
- Reid JB, Renquist AR. Enhanced root production as a feed-forward response to soil water deficit in field-grown tomatoes. Australian Journal of Plant Physiology. 1997;24:685–692. [Google Scholar]
- Salim M, Pitman MG. Pressure-induced water and solute flow through plant roots. Journal of Experimental Botany. 1984;35:869–881. [Google Scholar]
- Schurr U, Gollan T, Schulze ED. Stomatal response to drying soil in relation to changes in the xylem sap composition of Helianthus annuus. II. Stomatal sensitivity to abscisic-acid imported from the xylem sap. Plant, Cell and Environment. 1992;15:561–567. [Google Scholar]
- Seel WE, Jeschke WD. Simultaneous collection of xylem sap from Rhinanthus minor and the hosts Hordeum and Trifolium: hydraulic properties, xylem sap composition and effects of attachment. New Phytologist. 1999;143:281–298. [Google Scholar]
- Sharp RE, Davies WJ. Root growth and water uptake by maize plants in drying soil. Journal of Experimental Botany. 1985;36:1441–1456. [Google Scholar]
- Soar CJ, Dry PR, Loveys BR. Scion photosynthesis and leaf gas exchange in Vitis vinifera L. cv. Shiraz: mediation of rootstock effects via xylem sap ABA. Australian Journal of Grape and Wine Research. 2006a;12:82–96. [Google Scholar]
- Soar CJ, Speirs J, Maffei SM, Penrose AB, McCarthy MG, Loveys BR. Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Australian Journal of Grape and Wine Research. 2006b;12:2–12. [Google Scholar]
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial rootzone drying. Journal of Experimental Botany. 2004;55:2353–2363. doi: 10.1093/jxb/erh204. [DOI] [PubMed] [Google Scholar]
- Stoll M, Loveys BR, Dry PR. Hormonal changes induced by partial rootzone drying of irrigated grapevine. Journal of Experimental Botany. 2000;51:1627–1634. doi: 10.1093/jexbot/51.350.1627. [DOI] [PubMed] [Google Scholar]
- Tardieu F, Lafarge T, Simonneau T. Stomatal control by fed or endogenous xylem ABA in sunflower: interpretation of correlations between leaf water potential and stomatal conductance in anisohydric species. Plant, Cell and Environment. 1996;19:75–84. [Google Scholar]
- Tardieu F, Simonneau T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany. 1998;49:419–432. [Google Scholar]
- Topcu S, Kirda C, Dasgan Y, Kaman H, Cetin M, Yazici A, Bacon MA. Yield response and N-fertilizer recovery of tomato grown under deficit irrigation. European Journal of Agronomy. 2007;26:64–70. [Google Scholar]
- Yao C, Moreshet S, Aloni B. Water relations and hydraulic control of stomatal behaviour in bell pepper plant in partial soil drying. Plant, Cell and Environment. 2001;24:227–235. [Google Scholar]
- Zhang J, Davies WJ. Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant, Cell and Environment. 1989;12:73–81. [Google Scholar]