Abstract
Following exposure to salinity, the root/shoot ratio is increased (an important adaptive response) due to the rapid inhibition of shoot growth (which limits plant productivity) while root growth is maintained. Both processes may be regulated by changes in plant hormone concentrations. Tomato plants (Solanum lycopersicum L. cv Moneymaker) were cultivated hydroponically for 3 weeks under high salinity (100 mM NaCl) and five major plant hormones (abscisic acid, ABA; the cytokinins zeatin, Z, and zeatin-riboside, ZR; the auxin indole-3-acetic acid, IAA; and the ethylene precursor 1-aminocyclopropane-1-carboxylic acid, ACC) were determined weekly in roots, xylem sap, and leaves. Salinity reduced shoot biomass by 50–60% and photosynthetic area by 20–25% both by decreasing leaf expansion and delaying leaf appearance, while root growth was less affected, thus increasing the root/shoot ratio. ABA and ACC concentrations strongly increased in roots, xylem sap, and leaves after 1 d (ABA) and 15 d (ACC) of salinization. By contrast, cytokinins and IAA were differentially affected in roots and shoots. Salinity dramatically decreased the Z+ZR content of the plant, and induced the conversion of ZR into Z, especially in the roots, which accounted for the relative increase of cytokinins in the roots compared to the leaf. IAA concentration was also strongly decreased in the leaves while it accumulated in the roots. Decreased cytokinin content and its transport from the root to the shoot were probably induced by the basipetal transport of auxin from the shoot to the root. The auxin/cytokinin ratio in the leaves and roots may explain both the salinity-induced decrease in shoot vigour (leaf growth and leaf number) and the shift in biomass allocation to the roots, in agreement with changes in the activity of the sink-related enzyme cell wall invertase.
Keywords: Abscisic acid, 1-aminocyclopropane-1-carboxylic acid, indole-3-acetic acid, plant hormones, salt stress, sodium chloride, tomato (Solanum lycopersicum L.), zeatin, zeatin-riboside
Introduction
Salinity is a major factor reducing crop productivity in agriculture as well as a major cause of the abandonment of lands and aquifers for agricultural purposes. Improving the use of such marginal resources requires insight about their limiting effects on plant development. The release of salt-tolerant crops to optimize the use of salt-contaminated water and soil resources has been a much prosecuted scientific goal but with little success to date, as few major determinant genetic traits of salt tolerance have been identified (Flowers, 2004; Munns, 2005). Although maintenance of ionic and water homeostasis is necessary for plant survival, salinity decreases crop productivity both by reducing leaf growth and inducing leaf senescence. This lowers the total photosynthetic capacity of the plant, thus limiting its ability to generate further growth or harvestable biomass and also to maintain defence mechanisms against the stress (Yeo, 2007).
The major physiological processes believed to be involved in the control of plant growth under salinity have been water relations, hormonal balance, and carbon supply, with their respective importance depending on the time scale of the response (Munns, 2002). Salinity affects plant growth in two phases (Munns, 1993). During the initial phase of salinity, the osmotic effect predominates and induces water stress due to the high salt concentration in the root medium. During this phase, shoot growth arrest occurs very quickly (seconds to minutes) but recovers (over several hours) to a new steady-state that is considerably lower than under non-stress conditions. These changes seem to be driven by changes in water relations (Munns, 2002), but during this initial period when osmotic effects predominate (days to weeks), growth seems to be regulated by hormones and/or carbohydrates. During the second phase of the stress (weeks to months), growth is governed by toxic effects due to the high salt accumulation in leaf tissues.
Overall, salinity affects plant productivity by reducing the photosynthetic area by inhibiting cell division and cell expansion rates during leaf growth and by affecting developmental programmes regulating leaf emergence, the production of lateral primordia, and the formation of reproductive organs (Munns, 2002). However, the mechanism(s) that down-regulates leaf growth and shoot development under the osmotic phase of salinity is not known. It has been hypothesized that leaf growth inhibition must be regulated by hormones or their precursors, because the reduced leaf growth rate is independent of carbohydrate supply, water status, nutrient deficiency, and ion toxicity (see Munns and Tester, 2008, for a review). Since plant meristems are actively growing tissues where cell division governs sink strength, environmental signals can modulate plant responses to the growing conditions through changes in phytohormone concentrations, thus controlling assimilate partitioning between different sink tissues (Hartig and Beck, 2006). These hormonal changes not only influence the adaptive response but also affect the normal growth of the harvestable organs and thus influence economic productivity. Hence, plant hormones are considered a primary component of the signalling pathways controlling these processes.
This integrated plasticity in plant development probably involves long-distance communication between different organs with hormones playing an essential role (Sachs, 2005) or differential changes in root and shoot hormone concentrations. Although salinity increased plant ABA concentration in all plant compartments (Wolf et al., 1990; Kefu et al., 1991), its role in growth regulation has been equivocal as different studies have suggested it can inhibit (Dodd and Davies, 1996) or maintain growth by restricting the evolution of ethylene, another potential growth inhibitor (Sharp and LeNoble, 2002). Both cytokinins and auxins act as endogenous mitogens whose concentrations can be environmentally modulated to regulate the formation of roots and shoots and their relative growth (Werner et al., 2001; Sachs, 2005). It has been hypothesized that a decrease in CK supply from the root to the shoot could inhibit leaf growth while a low CK content would promote root growth and thus the root/shoot ratio (van der Werf and Nagel, 1996; Rahayu et al., 2005). It has been reported that salinity decreased the auxin indoleacetic acid (IAA) levels in the roots but not in the leaves of tomato plants (Dunlap and Binzel, 1996), while leaf zeatin concentration declined under osmotic stress in tomato (Walker and Dumbroff, 1981). However, since many of these (relatively scarce) studies were only able to measure one or two of the major hormone groups following a step-change in salinity or any other stress, interpretation of changes in biomass allocation by specific authors has generally favoured the hormones that each author measured, and thus there are several divergent hypotheses of the regulation of biomass allocation (van der Werf and Nagel, 1996; Munns and Cramer, 1996; Sachs, 2005) that co-exist in the literature.
The advent of multi-analyte techniques for hormone quantification allows a far more comprehensive analysis of the changes in plant hormone status following salinity. Accordingly, the endogenous concentrations of five major plant hormones; ABA, ACC, IAA, and two major active cytokinins (Davey and van Staden, 1976) in tomato, Z and ZR, were analysed in leaves of a cultivated tomato genotype submitted to salinity stress (100 mM NaCl for 3 weeks), in order to study the influence of local changes in both plant hormones and ionic status on leaf senescence (Ghanem et al., 2008). In this study, however, root and xylem hormone concentrations were measured to evaluate whether differential hormonal changes in and between roots and shoots regulated growth and biomass partitioning under salinity. Tomato was chosen for this work since it is an economically important crop, whose cropping area (particularly in the Mediterranean) is often limited by the availability of sufficient high quality (non-saline) water for irrigation.
Materials and methods
Plant material and culture conditions
Seeds of tomato (Solanum lycopersicum L.) cv. Moneymaker were obtained from the Tomato Genetics Resource Center (TGRC) (University of California-Davis, CA, USA). Seeds were sown in trays filled with a perlite-vermiculite mix (1:3 v/v proportion) moistened regularly with half-strength Hoagland's nutrient solution. Fourteen days after sowing, the substrate was gently washed from the roots and seedlings placed on polyvinyl chloride plates floating on aerated half-strength Hoagland's nutrient solution in a growth chamber. Solutions were refilled every 2 d and renewed every week.
Plants were grown in a growth chamber under a 16 h daylight period. The air temperature ranged from 25–28 °C during the day and 17–18 °C during the night. Relative humidity was maintained at 70±5% during the night and at 50±5% during the day. Light intensity at the top of the canopy was around 245 μmol m−2 s−1 (PPFD). After 4 d acclimation in control conditions (18 d after sowing), the seedlings were exposed to 0 mM (control) or 100 mM NaCl added to the nutrient solution for three more weeks. Three replications with eight plants per replication and salt treatment were used. An actively growing leaf, present at the moment that salt stress was applied (identified as leaf number 4 by numbering from the base of the plant) was tagged for subsequent growth measurements and harvest for biochemical determinations. Six plants per treatment were harvested for different analyses at 1, 9, 15, and 22 d of salt treatment. Xylem sap was obtained from three plants per treatment immediately after severing the shoot about 2–3 cm above the root system. The root system was placed into a Scholander pressure chamber, and samples obtained by applying a nitrogen pressure similar to the leaf water potential (–0.5 MPa for control plants and about –0.9 MPa for stressed ones) in order to maintain sap flow rates as close as possible to whole plant transpiration rate (Pérez-Alfocea et al., 2000).
Vegetative growth assessment
Six plants per treatment were used for growth analysis. The number of leaves on the main stem with a length >2 cm was recorded every 2 d, and the rate of leaf appearance was estimated from the slope of leaf number versus time. At each harvest, the shoots and roots of each plant were separated and weighed to determine fresh weight (FW) and the root/shoot ratio. The area of the tagged leaf 4 was determined by using a Li-Cor 3100 area meter (Li-Cor Inc., Lincoln, Nebraska, USA). The relative growth rate (RGR) on a FW basis, and the relative expansion rate (RER) of leaf 4, and the RGR of the root system, were evaluated as the increase in FW or leaf area per unit of FW or leaf area present per unit of time and were estimated from the equation [(lnPt2–lnPt1)/(t2–t1)], where Pt2 and Pt1 are the values of each parameter at the end (t2) and at the beginning (t1) of the corresponding growing period.
Hormone extraction and analysis
Cytokinins (zeatin, Z, and zeatin riboside, ZR), indole-3-acetic acid (IAA), and abscisic acid (ABA) were extracted and purified according to the method of Dobrev and Kaminek (2002). One gram of fresh plant material (leaf or root) was homogenized in liquid nitrogen and placed in 5 ml of cold (–20 °C) extraction mixture of methanol/water/formic acid (15/4/1 by vol., pH 2.5). After overnight extraction at –20 °C solids were separated by centrifugation (20 000 g, 15 min) and re-extracted for 30 min in an additional 5 ml of the same extraction solution. Pooled supernatants were passed through a Sep-Pak Plus †C18 cartridge (SepPak Plus, Waters, USA) to remove interfering lipids and plant pigments and evaporated to dryness. The residue was dissolved in 5 ml of 1 M formic acid and loaded on an Oasis MCX mixed mode (cation-exchange and reverse phase) column (150 mg, Waters, USA) preconditioned with 5 ml of methanol followed by 5 ml of 1 M formic acid. To separate different CK forms (nucleotides, bases, ribosides, and glucosides) from IAA and ABA, the column was washed and eluted stepwise with different appropriate solutions indicated in Dobrev and Kaminek (2002). ABA and IAA were analysed in the same fraction. After each solvent was passed through the columns, they were purged briefly with air. Solvents were evaporated at 40 °C under vacuum. Samples then dissolved in a water/acetonitrile/formic acid (94.9:5:0.1 by vol.) mixture for HPLC/MS analysis. Analyses were carried out on a HPLC/MS system consisting of an Agilent 1100 Series HPLC (Agilent Technologies, Santa Clara, CA, USA) equipped with a μ-well plate autosampler and a capillary pump, and connected to an Agilent Ion Trap XCT Plus mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) using an electrospray (ESI) interface. Prior to injection, 100 μl of each fraction extracted from tissues or a similar volume of xylem sap were filtered through 13 mm diameter Millex filters with 0.22 μm pore size nylon membrane (Millipore, Bedford, MA, USA). 8 μl of each sample, dissolved in mobile phase A, was injected onto a Zorbax SB-C18 HPLC column (5 μm, 150×0.5 mm, Agilent Technologies, Santa Clara, CA, USA), maintained at 40 °C, and eluted at a flow rate of 10 μl min−1. Mobile phase A, consisting of water/acetonitrile/formic acid (94.9:5:0.1 by vol.), and mobile phase B, consisting of water/acetonitrile/formic acid (10:89.9:0.1 by vol.), were used for the chromatographic separation. The elution programme maintained 100% A for 5 min, then a linear gradient from 0% to 6% B in 10 min, followed by another linear gradient from 6% to 100% B in 5 min, and finally 100% B maintained for another 5 min. The column was equilibrated with the starting composition of the mobile phase for 30 min before each analytical run. The UV chromatogram was recorded at 280 nm with a DAD module (Agilent Technologies, Santa Clara, CA, USA). The mass spectrometer was operated in the positive mode with a capillary spray voltage of 3500 V, and a scan speed of 22 000 m/z s−1 from 50–500 m/z. The nebulizer gas (He) pressure was set to 30 psi, whereas the drying gas was set to a flow of 6.0 l min−1 at a temperature of 350 °C. Mass spectra were obtained using the DataAnalysis program for LC/MSD Trap Version 3.2 (Bruker Daltonik GmbH, Germany). For quantification of Z, ZR, ABA, and IAA, calibration curves were constructed for each component analysed (0.05, 0.075, 0.1, 0.2, and 0.5 mg l−1) and corrected for 0.1 mg l−1 internal standards: [2H5]trans-zeatin, [2H5]trans-zeatin riboside, [2H6]cis,trans-abscisic acid (Olchemin Ltd, Olomouc, Czech Republic), and [13C6]indole-3-acetic acid (Cambridge Isotope Laboratories Inc., Andover, MA, USA). Recovery percentages ranged between 92% and 95%.
ACC (1-aminocyclopropane-1-carboxylic acid) was determined after conversion into ethylene by gas chromatography using an activated alumina column and a FID detector (Konik, Barcelona, Spain). ACC was extracted with 80% (v/v) ethanol and assayed by degradation with alkaline hypochlorite in the presence of 5 mM HgCl2 (Casas et al., 1989). A preliminary purification step was performed by passing the extract through a Dowex 50W-X8, 50–100 mesh, H+-form resin and later recovered with 0.1 N NH4OH. The conversion efficiency of ACC into ethylene was calculated separately by using a replicate sample containing 2.5 nmol of ACC as an internal standard and used for the correction of data.
Enzyme extraction and assay
For cell wall invertase activity (CWIN, EC 3.2.1.25), the enzyme extracts were prepared essentially as described in Balibrea et al. (1999). Fresh leaf or root tissue samples (100 mg) were frozen with liquid nitrogen and stored at −20 °C until analysis. Samples containing polyvinylpyrrolidone and Fontainebleau sand were homogenized in 1 ml of extraction buffer containing 50 mM HEPES-KOH (pH 7), 10 mM MgCl2, 1 mM Na2EDTA, 2.6 mM DTT, 10% ethylene glycol, and 0.02% Triton X-100. After centrifugation at 20 000 g, the supernatant was discarded and the pellet was washed three times and resuspended in 30 mM acetate buffer (pH 5). The amount of hexose was determined through an enzyme-linked assay monitoring NADH formation at 340 nm, after adding 25 μl 0.6 M sucrose and incubating at 30 °C for 15 min. The proteins were analysed in the pellet after solubilization with 1 M NaCl and the specific enzymatic activities were expressed as nkat mg−1 protein.
Statistical analysis
Data were subjected to an analysis of variance (ANOVA II) using the SAS software (SAS System for Windows, version 8.02). The statistical significance of the results was analysed by the Student–Newman–Keuls test at the 5% level.
Results
Plant development and biomass allocation
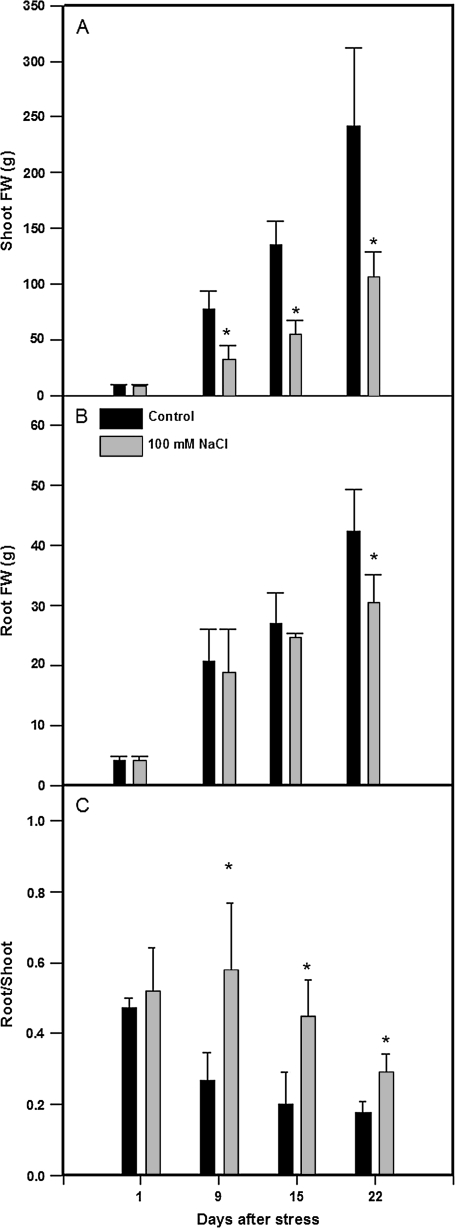
Salinization decreased shoot fresh weight by 50–60% (compared to control plants) from the first week of salinization (Fig. 1A). However, root fresh weight was only significantly affected (30%) after 3 weeks under saline conditions (Fig. 1B). As a consequence, salinization increased the root/shoot ratio by 2-fold compared to control plants, reaching the highest values after the first week of salinization (Fig. 1C).
Fig. 1.
Shoot (A) and root (B) biomass and root/shoot ratio (C) in tomato plants (cv. Moneymaker) grown for 3 weeks on half-strength Hoagland's medium in the absence (black bars) or presence of 100 mM NaCl (grey bars). Data are means of six plants ±SE. Asterisks indicate significant differences between treatments according to Student–Newman–Keuls test at P <0.05.
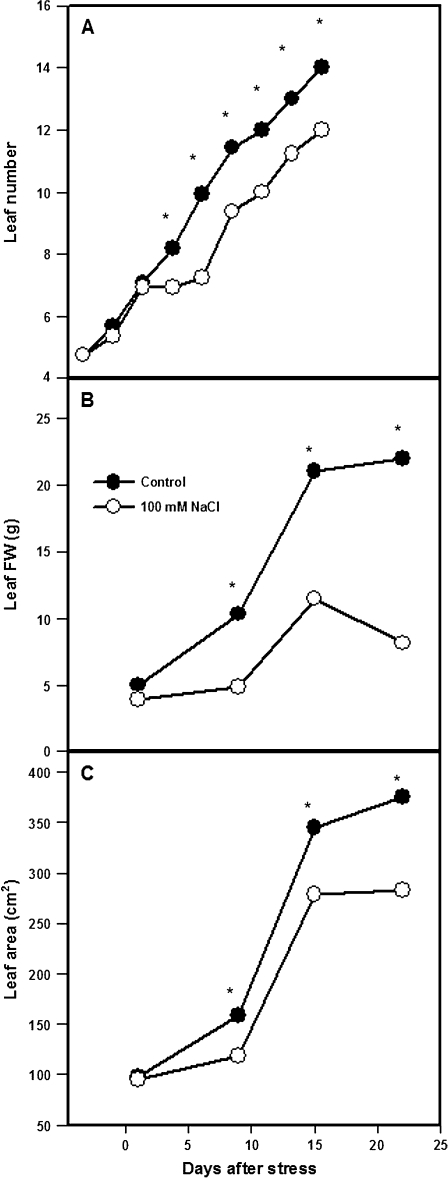
Shoot growth reduction during the first week of salinity was attributable partially to an arrest in the appearance of new leaves, which was detected from day 5 (Fig. 2A). By day 9, the salinized plants had three fewer leaves than the control plants. After this 4 d growth arrest, leaf appearance rate recovered to control values (about 1 new leaf every 2 d).
Fig. 2.
Leaf number (A), leaf 4 biomass (B), and leaf 4 area (C) in tomato plants (cv. Moneymaker) grown for 3 weeks on half-strength Hoagland's medium in the absence (closed circles) or presence of 100 mM NaCl (open circles). Data are means of six plants. Asterisks: see Fig. 1.
Another major factor limiting shoot growth was the impaired development of individual leaves. After 3 weeks of salinity, fresh weight and area of actively expanding leaves (4 g FW and 100 cm2 at the time of imposing salinity) were decreased by 60% and 25%, respectively (Fig. 2B, C).
Hormonal profiling
Cytokinins:
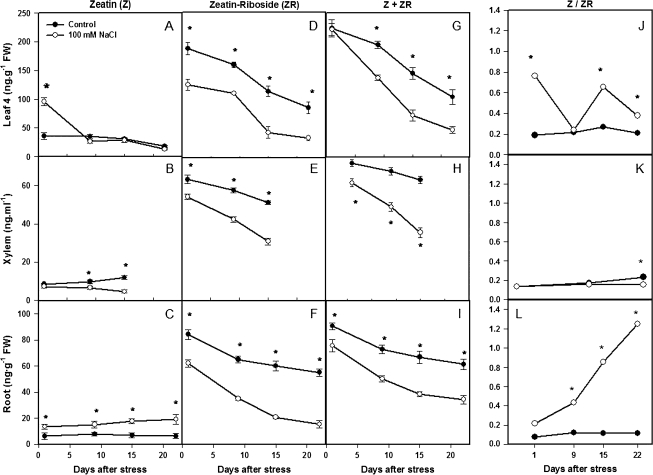
Although ZR concentrations were 2-fold lower in the root than in the leaf (cf. Fig. 3D, F), they showed similar dynamics through the experiment, as they did in xylem sap (Fig. 3E), with an immediate and sustained decrease in ZR concentration following the imposition of salinity. By contrast, the transient (day 1) increase in leaf Z concentration following salinity (Ghanem et al., 2008) was maintained throughout the experiment in the roots (cf. Fig. 3A, C). Xylem sap Z concentrations progressively decreased throughout the experiment (Fig. 3B). Since ZR concentrations were 4.5-, 5-, and 10-fold higher than Z ones in leaves, xylem sap, and roots, respectively, the total CK (Z+ZR) concentration was decreased by salinity throughout the experiment [except on day 1 in the leaves, as noted previously (Ghanem et al., 2008) where the decrease in ZR concentration sustained the increase in Z concentration] (Fig. 3G, H, I).
Fig. 3.
Cytokinin (Z, ZR, and Z+ZR) concentrations and Z/ZR ratio in leaf 4 (A, D, G, J), xylem sap (B, E, H, K), and roots (C, F, I, L) of tomato plants (cv. Moneymaker) grown for 3 weeks on half-strength Hoagland's medium in the absence (closed circles) or presence of 100 mM NaCl (open circles). Data are means of three plants ±SE. Asterisks: see Fig. 1. (Leaf data are replotted from Ghanem et al., 2008.)
Indeed, the ratio between (the putatively more active) zeatin and one of its supposed storage forms (ZR) was maintained almost constant in both leaves and roots under control conditions (Fig. 3J, L). However, salinity provoked a general increase in the Z/ZR ratio following leaf development, but it occurred especially and more progressively in the roots (from 2 to 12 times more than in control roots, following salinization). Only in the xylem sap of control plants did the Z/ZR ratio increase with time, but it was reduced under salinity during the second week (Fig. 3K).
Indoleacetic acid (IAA):
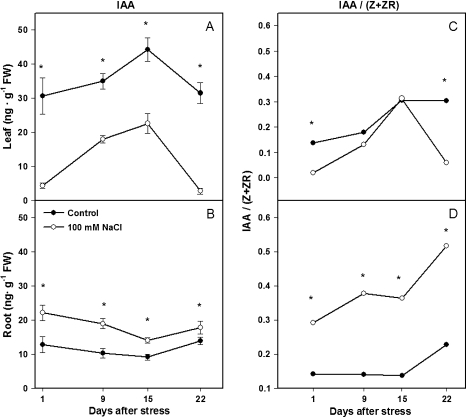
Salinity induced opposite changes in IAA concentrations in leaves and roots. Salinity decreased leaf IAA concentration by 80% (Fig. 4A), but increased root IAA concentration 2-fold (Fig. 4B) during the first day of salt treatment, compared with control plants. These changes remained statistically significant throughout the experiment, even though leaf IAA levels of both control and salinized plants fluctuated with time.
Fig. 4.
IAA concentration and IAA/(Z+ZR) ratio of leaf 4 (A, C) and root (B, D) of tomato plants (cv. Moneymaker) grown for 3 weeks on half-strength Hoagland's medium in the absence (closed circles) or presence of 100 mM NaCl (open circles). Data are means of three plants ±SE. Asterisks: see Fig. 1. (Leaf data are replotted from Ghanem et al., 2008.)
The IAA/(Z+ZR) ratio in the leaves fluctuated in both treatments throughout the experiment, and reached minimum values in salinized plants during the first and third weeks (Fig. 4C), coinciding with the greatest salinity-induced growth reduction (Fig. 2B, C). By contrast, the maximum IAA/(Z+ZR) ratios were in the roots of salinized plants, and were 2–3 times higher than control plants from the first day of salinization (Fig. 4D).
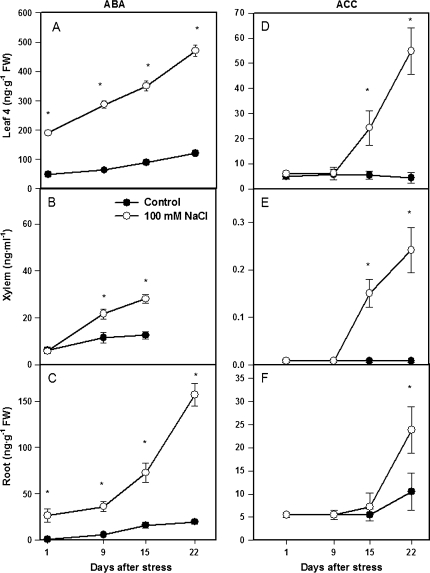
Abscisic acid (ABA) and aminocyclopropane carboxylic acid (ACC):
ABA concentrations of both roots and leaves (although not xylem sap) increased on the first day of salinity and with time in all compartments, reaching up to 8-fold, 4-fold, and 2.3-fold higher concentrations than control plants in roots, leaves, and xylem sap, respectively (Fig. 5A, B, C). Increased ACC concentrations in response to salinization were first detected on day 15 in both leaves and xylem sap, but not in roots (Fig. 5D, E, F). ACC increased with time in all compartments, reaching 2.4-fold, 12-fold, and 30-fold higher than control plants by the end of the experiment in roots, leaves, and xylem sap, respectively.
Fig. 5.
ABA and ACC concentrations of leaf 4 (A, D), xylem sap (B, E), and roots (C, F)) of tomato plants (cv. Moneymaker) grown for 3 weeks on half-strength Hoagland's medium in the absence (closed circles) or presence of 100 mM NaCl (open circles). Data are means of three plants ±SE. Asterisks: see Fig. 1. (Leaf data are replotted from Ghanem et al., 2008.)
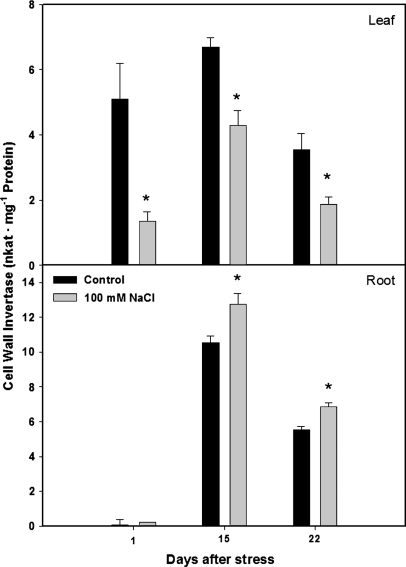
Cell wall invertase activity
Salt-stress significantly decreased CWIN activity in leaf 4 from the first day of salt treatment (Fig. 6). However, root CWIN activity was significantly increased from the second week of salinization.
Fig. 6.
Cell wall invertase activity (CWIN)of leaf 4 and roots of tomato plants (cv. Moneymaker) grown for three weeks on half-strength Hoagland's medium in the absence (black bars) or presence of 100 mM NaCl (grey bars). Data are means of three plants ±SE. Asterisks: see Fig. 1.
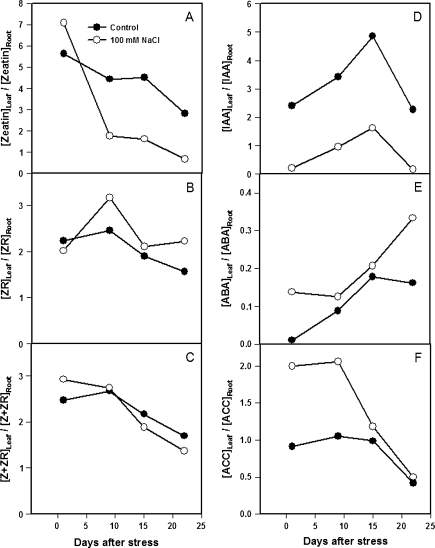
Leaf to root hormonal ratios
Relative changes in hormone concentrations between leaves and roots may explain relative changes in growth ratios and biomass partitioning induced by salt stress. In spite of the small effect on leaf Z concentration, salinity decreased the [Z]leaf/[Z]root ratio by 60–70% compared with the control plants from the first week (Fig. 7A), while it increased the [ZR]leaf/[ZR]root by 40% during the same period (Fig. 7B). Since these changes were somewhat compensatory, the leaf/root ratio for total CK (Z+ZR) was only reduced by 20% during the last two weeks of salinization, and a similar increase occurred on day 1 (Fig. 7C). The most dramatic change induced by salinity was the 70–95% decrease in [IAA]leaf/[IAA]root ratio from the first day of salinity and throughout the experiment (Fig. 7D). As in the case of ZR, the [ABA]leaf/[ABA]root and the [ACC]leaf/[ACC]root ratios were also increased by salinity during the experiment (Fig. 7E, F). Thus, salinity favours the accumulation of CK (especially Z) and IAA in the roots compared to a mature leaf, while it promotes the accumulation of ABA and the ethylene precursor ACC in the leaf compared to the roots.
Fig. 7.
Leaf 4/root hormone concentration ratios for zeatin (A), ZR (B), Z+ZR (C), IAA (D), ABA (E), and ACC (F) in tomato plants (cv. Moneymaker) grown for 3 weeks on half-strength Hoagland's medium in the absence (closed circles) or presence of 100 mM NaCl (open circles). Data are ratios of mean hormonal concentrations of three plants.
Correlation analysis:
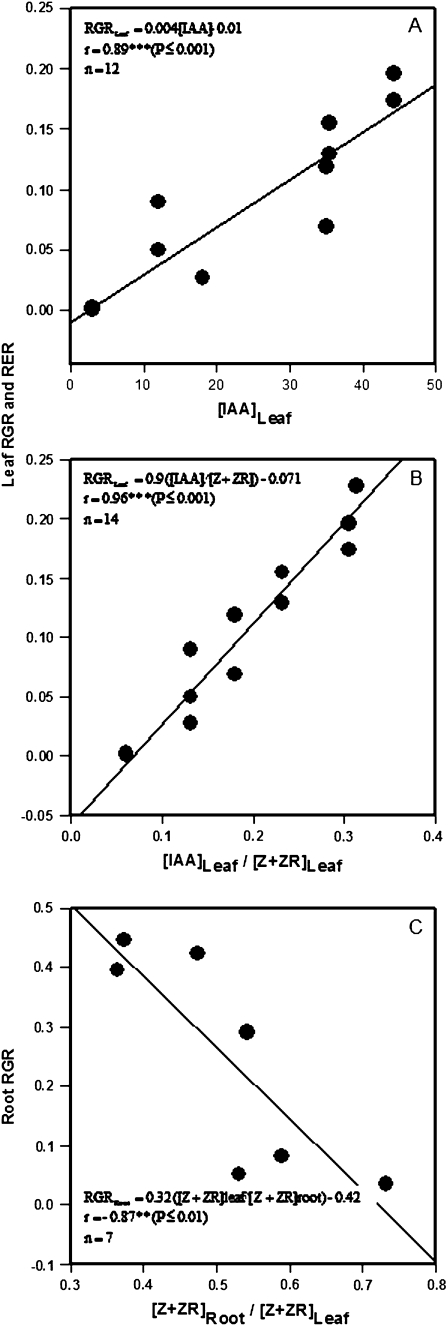
Relative leaf growth (expressed as either fresh weight, RGR, or area, RER) was positively correlated with the leaf IAA concentration (r=0.89, P ≤0.001) and still more closely with the IAA/(Z+ZR) ratio (Fig. 8A, B). No significant correlations were found between the other leaf hormonal concentrations or ratios and leaf growth (Table 1). However, while both shoot and root RGR were positively correlated with leaf Z, ZR, and Z+ZR concentrations (r=0.82–0.93, P ≤0.05; Table 1), the RGR of the root was negatively correlated with the [Z+ZR]root/[Z+ZR]leaf ratio (r = –0.83, P ≤0.05) (Fig. 8C). Interestingly, the accumulation of IAA in the root was negatively correlated with leaf growth parameters, while the ratios of both CK (Z+ZR) and IAA with ABA in the root were positively correlated with shoot growth (Table 1).
Fig. 8.
Linear correlations between leaf RGR or RER and the IAA concentrations (A) or the IAA/(Z+ZR) ratio (B) in leaf 4, and between the RGR of the root and the ratio for CK (Z+ZR) contents in the roots compared to the leaf 4 (C) of tomato plants (cv. Moneymaker) grown for 3 weeks on half-strength Hoagland's medium in the absence or presence of 100 mM NaCl.
Table 1.
Linear correlation coefficients between growth-related parameters (leaf, shoot and root RGR, and leaf RER) and hormonal concentrations and ratios in both leaf 4 and roots on plants grown for 3 weeks on half-strength Hoagland medium in the absence (Control) or presence of 100 mM NaCl
| Leaf hormonal parametersa | ||||||||||||
| Z | ZR | Z+ZR | IAA | ACC | ABA | CK/ACC | CK/ABA | IAA/CK | IAA/ACC | IAA/ABA | ACC/ABA | |
| Leaf RGR | 0.52 | 0.27 | 0.34 | 0.97*** | −0.39 | −0.39 | 0.21 | 0.39 | 0.97*** | 0.29 | 0.46 | −0.21 |
| Leaf RER | 0.48 | 0.01 | 0.10 | 0.82* | −0.20 | −0.18 | −0.02 | 0.14 | 0.96*** | 0.13 | 0.26 | −0.15 |
| Shoot RGR | 0.46 | 0.84* | 0.82* | 0.25 | −0.36 | −0.48 | 0.63 | 0.75* | −0.16 | 0.29 | 0.53 | 0.10 |
| Root RGR | 0.54 | 0.93* | 0.90** | 0.14 | −0.48 | −0.39 | 0.66 | 0.60 | −0.33 | 0.19 | 0.34 | 0.07 |
| Root hormonal parametersa | ||||||||||||
| Leaf RGR | −0.26 | 0.30 | 0.31 | −0.85* | −0.67 | −0.46 | 0.52 | 0.33 | −0.53 | 0.10 | 0.31 | −0.21 |
| Leaf RER | −0.08 | 0.08 | 0.08 | −0.72 | −0.50 | −0.23 | 0.32 | 0.15 | −0.33 | 0.05 | 0.13 | −0.22 |
| Shoot RGR | −0.36 | 0.46 | 0.50 | −0.34 | −0.31 | −0.48 | 0.63 | 0.85** | −0.47 | 0.19 | 0.85** | −0.50 |
| Root RGR | −0.23 | 0.38 | 0.44 | −0.05 | −0.45 | −0.39 | 0.65 | 0.55 | −0.33 | 0.59 | 0.57 | −0.36 |
* P <0.05,** P <0.01, *** P <0.001, n=6–8).
Discussion
Although the economic impact of salinity on tomatoes depends on fruit growth, overall plant growth can first be considered as two local processes (shoot and root development) but also as co-ordinated responses between different organs (biomass partitioning), which must be essentially regulated by hormones (Sachs, 2005). During this time, prior to ion toxicity, when the osmotic stress and nutrient imbalances (e.g. N uptake) mediate changes in hormone concentrations (Pérez-Alfocea et al., 1993; Munns, 2002; Rahayu et al., 2005; Ghanem et al., 2008; Munns and Tester, 2008), growth responses may account for differences in the overall plant productivity by maintaining ion homeostasis and generating new energetic resources (Balibrea et al., 2000; Munns and Tester, 2008).
Shoot growth regulation
Salinity decreased both the total number of leaves (Fig. 2A) and the growth of individual leaves (Fig. 2B, C), thus cell division, elongation, and primordium formation were all decreased. Shoot CK and auxins are probably involved in these processes since they were strongly reduced by salinity in the shoot tissues: about 50% for Z+ZR contents in leaf (Ghanem et al., 2008) and xylem sap, and 50–90% for IAA during leaf development. This idea is supported by the significant correlation found between both the leaf IAA content and the IAA/(Z+ZR) ratio with leaf development under both control and saline conditions (Fig. 8A, B; Table 1). In addition, the leaf CK (ZR and Z+ZR) concentrations were also positively correlated with the overall shoot growth capacity (Table 1), which may be explained not only by promoting cell division but also by delaying leaf senescence under salinity (Ghanem et al., 2008).
The phenotype of salinized tomato plants, where leaf CKX activity was enhanced by salinity (Ghanem et al., 2008), was similar to that of CKX-transgenic tobacco plants (Werner et al., 2001), where total CK contents were also diminished compared to the wild type. In both cases, leaf growth and the leaf appearance rate were strongly reduced, suggesting that modulation of CKs through catabolism is involved in regulating leaf initiation and growth under salinity.
Auxins are also considered to be a positive factor in leaf initiation having antagonistic interactions with CKs (Kepinski, 2006; Shani et al., 2006). Assuming that changes in leaf hormone concentration also occur in other parts of the shoot, delayed leaf appearance (Fig. 2A) could be explained by the decrease in shoot IAA concentration during the first days of salt treatment. The later recovery in leaf appearance rate coincided with the increased IAA concentration and IAA/Z+ZR ratio in the leaf (Fig. 4A, C). Since CK and auxin contents changed in parallel, it is difficult to resolve their independent effects and more functional work is needed, but it is interesting to note that both hormones could exert a major control on the leaf growth rate, as suggested from correlative analysis (Fig. 8A, B; Table 1), probably by promoting cell elongation after cell division.
Although increased leaf ABA accumulation (Fig. 5A) was correlated with impaired shoot development (Figs 1, 2), experiments investigating natural genetic variation in ABA concentration of salinized plants showed a positive (de Costa et al., 2007) or negative (Cramer and Quarrie, 2002; He and Cramer, 1996) correlation between leaf area and ABA concentration, and further studies are needed to determine the physiological significance (if any) of such correlations. That one of the functions of ABA accumulation may be to inhibit ethylene production (Sharp et al., 2000; Sharp and LeNoble, 2002) provided a rationale to measure ACC concentration. That ethylene inhibits shoot growth of salinized plants was suggested by the improved growth of transgenic canola containing ACC deaminase activity (Sergeeva et al., 2006). Although ACC levels were at the limit of detection of our analytical procedures, the fact that leaf and xylem ACC concentrations increased during the third week of salinization (Fig. 5) while growth was inhibited from the first week of salinization (Figs 1, 2), suggests that it was unlikely that ACC was responsible for the initial reduction in leaf growth and leaf appearance rate (Fig. 2).
Root growth regulation
Since CKs promote shoot growth but inhibit root growth (Thomas et al., 1995), low root CK content could stimulate or maintain root growth by prolonging the meristematic phase and thus increasing the number of cells before root elongation took place, as suggested for CKX-overexpressing transgenic plants exhibiting enhanced root development (Werner et al., 2001). Indeed, the relative accumulation of CKs in the roots compared to the leaves (Fig. 7A, C) may be involved in the late root growth reduction under salinity, as these parameters were negatively correlated (Fig. 8C). Interestingly, salinity actually increased root Z concentration from day 1 (Fig. 3C), probably due to conversion from its conjugate ZR (Fig. 3L), which should inhibit root growth rather than promote it. However, variable effects of CKs as a function of concentration and tissue specificity, suggests that a positive role of the increased root Z concentration on root growth cannot be ruled out. Indeed, exogenous CKs, especially the most active form Z, but also its derivatives such as ZR, stimulated tomato root growth (length and frequency) at very low concentrations (10−11 M) but inhibited growth at higher concentrations (Taylor and van Staden, 1998).
The interaction of CKs with other hormones such as auxins can also influence root biomass allocation (Sachs, 2005). Although salinity (300 mM for an unknown period) decreased root IAA concentration in tomato (Dunlap and Binzel, 1996), salt-induced auxin accumulation in the roots increased the auxin/CK ratio (Fig. 4B, D), which may promote cell elongation and root growth. Since auxin is formed in developing and mature leaves and is necessary for the formation of lateral primordia in both root and shoot apical meristems (Kepinski, 2006), a link between acropetal and basipetal auxin transport has been suggested to co-ordinate shoot and root responses to environmental stimuli depending on the organ of detection (Casimiro et al., 2001; Sachs, 2005). Furthermore, IAA may act as a negative feed-back signal temporarily to repress CK synthesis in the roots and their xylem transport to the shoot (Bangerth, 1994; Rahayu et al., 2005), as seems to occur under salinity (this study).
Moreover, increased assimilate partitioning to the roots can be explained by changes in CWIN activity in both roots and leaves, as reported in CK-deficient tobacco plants (Werner et al., 2008). This enzyme regulates sucrose transport by controlling the apoplastic unloading step from phloem (Roitsch et al., 2003). However, although CKs are major hormones establishing sink activity through the co-ordinated induction of extracellular invertases and hexose transporters in order to enhance phloem carbohydrate supply to actively growing tissues (Roitsch et al., 2000), this response seems to depend on the tissue, and Z is unlikely to be responsible for the CWIN induction in the roots. This idea is supported by the induction or maintenance of this enzyme activity reported in the roots of CKX-overexpressing tobacco plants (Werner et al., 2008) with lowered total CK and Z contents (Werner et al., 2001). However, IAA seems to induce both sink (sucrose allocation) and CWIN activities in both young leaves and roots (Roitsch et al., 2003; A. Albacete et al., unpublished results), supporting its role in the relative increase in the root sink strength under salinity.
Similar to its action in the shoot, ABA accumulation may also be necessary to maintain the root growth of salinized plants. Although both WT and ABA-deficient (flacca) tomato plants showed a 50% reduction in root biomass at 200 mM NaCl, reciprocal grafting experiments showed that flacca scions with the same shoot biomass had a much greater root biomass with a WT rootstock than with a flacca rootstock (Chen et al., 2003). As in the shoot, it is possible that root growth of these ABA-deficient rootstocks may have been inhibited by ethylene, since transgenic canola plants containing ACC deaminase (Sergeeva et al., 2006) had greater root growth, although this may be a consequence of improved shoot growth. While root ABA accumulation was apparently sufficient to limit root ACC concentration during the first two weeks of salinization (Fig. 5), a late increase in root ACC concentration coincided with root growth inhibition. Whether this correlation is causative remains to be determined.
Long-distance transport
While local hormone concentrations can influence local growth processes in both roots and shoots, the extent to which a root-applied stress influences long-distance hormone signalling and thus shoot hormone status remains an ongoing research field (Dodd, 2005). Although on some measurement occasions, for some hormones, concentration changes were significant in the roots but not the xylem, salinity only produced opposite changes in root and xylem hormone concentration for zeatin (cf. Fig. 3B, C) and root and shoot hormone concentrations for IAA (cf. Fig. 4A, B). While this correspondence between root and xylem hormone concentrations might suggest that the former regulates the latter, the contributions of roots and shoots to xylem hormone concentrations can only be assessed by detailed flow modelling experiments (Jiang and Hartung, 2008) and/or girdling to block phloem transport to the roots and thus hormone recycling via the xylem (reviewed in Dodd, 2005).
While the roots are the first to perceive the osmotic component of saline stress, rapid loss of shoot turgor within minutes and hours (Munns, 2002) could alter shoot hormone concentrations (Pierce and Raschke, 1980). However, root pressurization during salinity revealed that leaf ABA accumulation was independent of shoot turgor (Kefu et al., 1991), but possibly induced by specific ions such as sodium, disproportionately to their osmotic pressure (Montero et al., 1998). Nutrient relations may also be involved in stress perception by the roots and CKs are considered long-distance signals mediating the shoot response to NO3− availability in the roots (Beck, 1996; Rahayu et al., 2005). Thus NO3−/CI− antagonism at the transport level (Kafkafi et al., 1982) may decrease root nitrate uptake and xylem loading (Cramer et al., 1995), and long-distance CK transport to limit shoot growth under salinity. This idea is supported by observations that only the most tolerant tomato genotypes were able to maintain leaf nitrate concentration (Pérez-Alfocea et al., 1993), while deleterious effects of salinity can be alleviated by increasing NO3− in the substrate (Grattan and Grieve, 1999). However, the contribution of xylem CK delivery to shoot CK status remains controversial (Dodd and Beveridge, 2006).
Conclusions
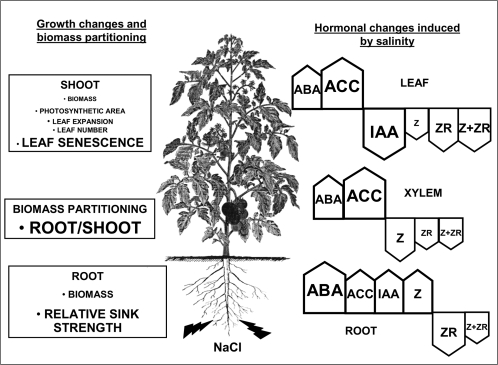
Figure 9 summarizes the different hormonal and growth changes induced by salinity in this study. Although much attention has been directed towards the role of the ‘stress hormones’ ABA and ACC in growth regulation, these do not seem to be the primary factors controlling growth under salinity, at least during the early (osmotic) phases of salinity. Alternatively, cytokinins and auxins seem better candidates in explaining shoot growth impairment and changes in biomass partitioning. More direct evidence on the roles of these changes in hormone concentration are being sought from experiments involving genotypes differing in salt tolerance, tomato mutants, transgenic plants, and grafting procedures.
Fig. 9.
Changes in hormonal concentrations in leaves, xylem sap, and roots in relation to local (shoot, leaf, and root growth) and co-ordinated processes (biomass partitioning) in tomato plants cultivated under salinity (100 mM NaCl) for 3 weeks. The relative increase (arrow up) or decrease (arrow down) in hormone concentration induced by salinity at the end of the experiment is indicated by both arrow and font sizes where the largest, intermediate, and smallest sizes indicate a more than 5-fold, a 2–5 fold, or a less than 2-fold change in hormone concentration.
Acknowledgments
The authors are very grateful to Dr A Torrecillas (CAID, Universidad de Murcia) for his help on hormonal analyses, to the Fonds National de la Recherche Scientifique-FNRS (Belgium) and to the Fundación Séneca (Comunidad Autónoma de Murcia, Spain) for a travel grant to MEG and FPA, respectively, and to the CSIC (Spain) for a research grant to AA (I3P Program). ICD thanks the BBSRC for support of his research on ACC signalling. Research was also supported by the Fundación Séneca de la Región de Murcia, Spain (project 03011/PI/05) and by the CICYT-FEDER project AGL2007-63610/AGR. The authors dedicate this paper to the memory of the late Professors Manuel Caro (CEBAS-CSIC) and Gilles Guerrier (Université d'Orléans, France).
Glossary
Abbreviations
- ABA
abscisic acid
- ACC
1-aminocyclopropane-1-carboxylic acid
- CK
cytokinin
- CWIN
cell wall invertase
- IAA
indole-3-acetic acid
- RGR
relative growth rate
- RER
relative expansion rate
- Z
zeatin
- ZR
zeatin riboside
References
- Balibrea ME, Dell'Amico J, Bolarín MC, Pérez-Alfocea F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiologia Plantarum. 2000;110:503–511. [Google Scholar]
- Balibrea ME, Parra M, Bolarín MC, Pérez-Alfocea F. Cytoplasmic sucrolytic activity controls tomato fruit growth under salinity. Australian Journal of Plant Physiology. 1999;26:561–568. [Google Scholar]
- Bangerth F. Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment and relationship to apical dominance. Planta. 1994;194:439–442. [Google Scholar]
- Beck EH. Regulation of the shoot/root ratio by cytokinins in Urtica dioica: opinion. Plant and Soil. 1996;185:3–12. [Google Scholar]
- Casas JL, Del Río JA, Serrano M, Acosta M. Comparison of 4 methods for the extraction and quantification of 1-aminocyclopropane-1-carboxylic acid (ACC) in tomato fruits. Revista de Agroquimica y Tecnología de Alimentos. 1989;29:191–198. [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, et al. Auxin transport promotes Arabidopsis lateral root initiation. The Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Fu X, Lips H, Sagi M. Control of plant growth resides in the shoot, and not in the root, in reciprocal grafts of flacca and wild-type tomato (Lycopersicon esculentum), in the presence and absence of salinity stress. Plant and Soil. 2003;256:205–215. [Google Scholar]
- Cramer GR, Quarrie SA. Abscisic acid is correlated with the leaf growth inhibition of four genotypes of maize differing in their response to salinity. Functional Plant Biology. 2002;29:111–115. doi: 10.1071/PP01131. [DOI] [PubMed] [Google Scholar]
- Cramer MD, Schierholt A, Wang YZ, Lips SH. The influence of salinity on the utilization of root anaplerotic carbon and nitrogen metabolism in tomato seedlings. Journal of Experimental Botany. 1995;46:1569–1577. [Google Scholar]
- Davey JE, van Staden J. Cytokinin translocation: changes in zeatin and zeatin-riboside levels in the root exudate of tomato plants during their development. Planta. 1976;130:69–72. doi: 10.1007/BF00390846. [DOI] [PubMed] [Google Scholar]
- De Costa W, Zorb C, Hartung W, Schubert S. Salt resistance is determined by osmotic adjustment and abscisic acid in newly developed maize hybrids in the first phase of salt stress. Physiologia Plantarum. 2007;131:311–321. doi: 10.1111/j.1399-3054.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Kaminek M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. Journal of Chromatography. 2002;950:21–29. doi: 10.1016/s0021-9673(02)00024-9. [DOI] [PubMed] [Google Scholar]
- Dodd IC. Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant and Soil. 2005;74:257–275. [Google Scholar]
- Dodd IC, Beveridge CA. Xylem-borne cytokinins: still in search of a role? Journal of Experimental Botany. 2006;57:1–4. doi: 10.1093/jxb/erj021. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Davies WJ. The relationship between leaf growth and ABA accumulation in the grass leaf elongation zone. Plant, Cell and Environment. 1996;19:1047–1056. [Google Scholar]
- Dunlap JR, Binzel ML. NaCl reduces indole-3-acetic acid levels in the roots of tomato plants independent of stress-induced abscisic acid. Plant Physiology. 1996;112:379–384. doi: 10.1104/pp.112.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers TJ. Improving salt tolerance. Journal of Experimental Botany. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- Ghanem ME, Albacete A, Martínez-Andújar C, Acosta M, Romero-Aranda R, Dodd IC, Lutts S, Pérez-Alfocea F. Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.) Journal of Experimental Botany. 2008;59:3039–3050. doi: 10.1093/jxb/ern153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan SR, Grieve CM. Salinity-mineral nutrient relations in horticultural crops. Scientia Horticulturae. 1999;78:127–157. [Google Scholar]
- Hartig K, Beck E. Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biology. 2006;8:389–396. doi: 10.1055/s-2006-923797. [DOI] [PubMed] [Google Scholar]
- He T, Cramer GR. Abscisic acid concentrations are correlated with leaf area reductions in two salt-stressed rapid-cycling Brassica species. Plant and Soil. 1996;179:25–33. [Google Scholar]
- Jiang F, Hartung W. Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. Journal of Experimental Botany. 2008;59:37–43. doi: 10.1093/jxb/erm127. [DOI] [PubMed] [Google Scholar]
- Kafkafi U, Valoras N, Letey J. Chloride interaction with nitrate and phosphate nutrition in tomato (Lycopersicon esculentum L.) Journal of Plant Nutrition. 1982;5:1369–1385. [Google Scholar]
- Kefu Z, Munns R, King RW. Abscisic-acid levels in NaCl-treated barley, cotton and saltbush. Australian Journal of Plant Physiology. 1991;18:17–24. [Google Scholar]
- Kepinski S. Integrating hormone signalling and patterning mechanisms in plant development. Current Opinion in Plant Biology. 2006;9:28–34. doi: 10.1016/j.pbi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Montero E, Cabot C, Poschenrieder C, Barcelo J. Relative importance of osmotic-stress and ion-specific effects on ABA-mediated inhibition of leaf expansion growth in Phaseolus vulgaris. Plant, Cell and Environment. 1998;21:54–62. [Google Scholar]
- Munns R. Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant, Cell and Environment. 1993;16:15–24. [Google Scholar]
- Munns R. Salinity, growth and phytohormones. In: Läuchli A, Lüttge U, editors. Salinity: environment–plants–molecules. Dordrecht: Kluwer Academic Publishers; 2002. pp. 271–290. [Google Scholar]
- Munns R. Genes and salt-tolerance: bringing them together. New Phytologist. 2005;3:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Munns R, Cramer GR. Is coordination of leaf and root growth mediated by abscisic acid? Opinion. Plant and Soil. 1996;185:33–49. [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Pérez-Alfocea F, Balibrea ME, Alarcón JJ, Bolarín MC. Composition of phloem and xylem exudates in relation to the salt-tolerance of domestic and wild tomato species. Journal of Plant Physiology. 2000;156:367–374. [Google Scholar]
- Pérez-Alfocea F, Estañ MT, Santa Cruz A, Bolarín MC. Effects of salinity on nitrate, total nitrogen, soluble protein and free amino acid levels in tomato plants. Journal of Horticultural Science. 1993;68:1021–1027. [Google Scholar]
- Pierce M, Raschke K. Correlation between loss of turgor and accumulation of abscisic-acid in detached leaves. Planta. 1980;148:174–182. doi: 10.1007/BF00386419. [DOI] [PubMed] [Google Scholar]
- Rahayu YS, Walch-Liu P, Neumann G, von Wirén N, Bangerth F. Root-derived cytokinins as long-distance signals for induced stimulation of leaf growth. Journal of Experimental Botany. 2005;56:1143–1152. doi: 10.1093/jxb/eri107. [DOI] [PubMed] [Google Scholar]
- Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK. Extracellular invertase: key metabolic protein and PR protein. Journal of Experimental Botany. 2003;54:513–524. doi: 10.1093/jxb/erg050. [DOI] [PubMed] [Google Scholar]
- Roitsch T, Ehneß R, Goetz M, Hause B, Hofmann M, Sinha AK. Regulation and function of extracellular invertase from higher plants in relation to assimilate partitioning, stress responses and sugar signalling. Australian Journal of Plant Physiology. 2000;27:815–825. [Google Scholar]
- Sachs T. Auxin's role as an example of the mechanisms of shoot/root relations. Plant and Soil. 2005;268:13–19. [Google Scholar]
- Sergeeva E, Shah S, Glick BR. Growth of transgenic canola (Brassica napus cv. Westar) expressing a bacterial 1-aminocyclopropane-1-carboxylate (ACC) deaminase gene on high concentrations of salt. World Journal of Microbiology and Biotechnology. 2006;22:277–282. [Google Scholar]
- Shani E, Yanai O, Ori N. The role of hormones in shoot apical meristem function. Current Opinion in Plant Biology. 2006;9:484–489. doi: 10.1016/j.pbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Sharp R, LeNoble ME. ABA, ethylene and the control of shoot and root growth under water stress. Journal of Experimental Botany. 2002;53:33–37. [PubMed] [Google Scholar]
- Sharp R, LeNoble ME, Else MA, Thorne ET, Gherardi F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. Journal of Experimental Botany. 2000;51:1575–1584. doi: 10.1093/jexbot/51.350.1575. [DOI] [PubMed] [Google Scholar]
- Taylor JLS, Van Staden J. Plant-derived smoke solutions stimulate the growth of Lycopersicon esculentum roots in vitro. Plant Growth Regulation. 1998;26:77–83. [Google Scholar]
- Thomas JC, Smigocki AC, Bohnert HJ. Light-induced expression of ipt from Agrobacterium tumefaciens results in cytokinin accumulation and osmotic-stress symptoms in transgenic tobacco. Plant Molecular Biology. 1995;27:225–235. doi: 10.1007/BF00020179. [DOI] [PubMed] [Google Scholar]
- Van Der Werf A, Nagel OW. Carbon allocation to shoots and roots in relation to nitrogen supply is mediated by cytokinins and sucrose: opinion. Plant and Soil. 1996;185:21–32. [Google Scholar]
- Walker MA, Dumbroff EB. Effects of salt stress on abscisic acid and cytokinin levels in tomato. Zeitschrift fűr Pflanzenphysiologie. 1981;101:461–170. [Google Scholar]
- Werner T, Holst K, Pörs Y, Guivarc'h A, Mustroph A, Chriqui D, Grimm B, Schmülling T. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. Journal of Experimental Botany. 2008;59:2659–2672. doi: 10.1093/jxb/ern134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Srnad M, Schmülling T. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf O, Jeschke WD, Hartung W. Long-distance transport of abscisic-acid in NaCl-treated intact plants of Lupinus albus. Journal of Experimental Botany. 1990;41:593–600. [Google Scholar]
- Yeo AR. Salinity. In: Yeo AR, Flowers TJ, editors. Plant solute transport. Oxford: Blackwell Publishing Ltd; 2007. pp. 340–365. [Google Scholar]