Abstract
Chemopreventive agents generate oxidative stress, which culminates in cell death and may be part of a general mechanism of chemoprevention. The redox-responsive cyclooxygenase (COX)-2, overexpressed during carcinogenesis, has been a target for cancer prevention. To assess the potential link between chemopreventive agents, oxidative stress and COX-2, we studied the chemopreventive sulindac and nitric oxide-donating aspirin (NO-ASA). Both generated oxidative stress and induced COX-2 in various cell lines, more prominently in dying cells. Two antioxidants and an inhibitor of NADPH oxidase abrogated the induction of COX-2 and cell death. Exogenous xanthine/xanthine oxidase, which produce O2−·, had the same effect. Inhibition of caspases and cox-2 knockdown showed that COX-2 did not participate in reactive oxygen species (ROS) generation or cell death induction in response to NO-ASA. Our results support three potentially useful ideas: (i) the concept that ROS are a critical component of the action of chemopreventive agents; (ii) the notion that COX-2 may not be an ideal target for chemoprevention and (iii) the possibility that COX-2 may be overexpressed in cancer cells due to their state of oxidative stress. It is conceivable that, if further substantiated, these findings may inform the rational design of chemotherapeutic strategies, in particular the choice of agents in combination approaches.
Introduction
Chemoprevention emerges as a realistic option for the control of cancer. Several chemopreventive agents generate oxidative stress, which culminates in cell death (1–4). Recently, we proposed that the induction of oxidative stress represents a major proximal event in the mechanism of action of chemopreventive agents (5). On the other hand, cyclooxygenase (COX)-2, progressively overexpressed during the transition from normalcy to malignancy, has been a major molecular target for the prevention of cancer. Several clinical trials aiming to prevent colon and other cancers using COX-2-specific inhibitors have been conducted. Some of them have shown clear-cut efficacy (6,7). This approach has now all but been abandoned due to side effects (8,9). Alternative interpretations of the data have been presented, notably the suggestion that COX-2 inhibitors work through non-COX effects (10). Nevertheless, the generally accepted implication is that COX-2 is central to carcinogenesis and to the action of non-steroidal anti-inflammation drugs (NSAIDs) and COX-2 inhibitors (11,12).
Of particular interest have been reports that (i) COX-2 suppresses apoptosis and thus provides a survival advantage to the neoplastic cell (13,14) and (ii) the expression of COX-2 is induced by oxidative stress in various cells, through mechanisms that may include activation of mitogen-activated protein kinase and/or nuclear factor-κB signaling (15,16).
To assess the potential link between the action of chemopreventive agents, oxidative stress and COX-2, we studied two exemplary compounds, sulindac and nitric oxide-donating aspirin (NO-ASA). Sulindac, a conventional NSAID, has a well-documented chemopreventive effect against colon cancer, both in familial adenomatous polyposis and sporadic colon cancer (17). The novel NO-ASA displays significant anticancer properties, demonstrated in preclinical models of various cancers (18). NO-ASA consists of a conventional aspirin (ASA) molecule linked covalently via a chemical spacer to -ONO2 that releases NO (See Supplementary Figure 8 available Carcinogenesis Online). Compared with conventional ASA, NO-ASA is much more potent in inhibiting the growth of many human cancer cell lines and also in preventing colon and pancreatic cancer in animal tumor models (18).
Here, we report that sulindac sulfide, the active metabolite of sulindac, and NO-ASA induce both oxidative stress and the expression of COX-2, particularly in dying cells. COX-2, however, plays no role in cancer cell growth inhibition by these compounds or in the process of cell death.
Materials and methods
Reagents
Reagents and chemicals were as follows: McCoy’s 5a medium (modified), minimum essential medium (Eagle), RPMI 1640, Ham’s F12K medium and antibiotics were from Fisher-Mediatech (Herndon, VA); fetal calf serum was from Hyclone (Logan, UT); p-NO-ASA [2-(acetyloxy) benzoic acid 4-(nitrooxymethyl)-phenyl ester], its m- and o- positional isomers as well as the denitrated analogues were synthesized by us (19); dihydroethidium (DHE) and 2′,7′-dichlorofluorescein diacetate (DCFDA) were from Calbiochem (San Diego, CA); all other reagents were from Sigma Chemical (St Louis, MO).
Cell culture and cell viability assays
BxPC-3, MIA PaCa-2 human pancreatic cancer cell lines, HT29, Caco-2, LoVo HCT-15 human colon adenocarcinoma cell lines and HUVEC, the human endothelial cell line, were from American Type Culture Collection (Manassas, VA). Cells grown as recommended by American Type Culture Collection were seeded at the indicated density and allowed to attach for 24 h, followed by NO-ASA treatment as indicated. Cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Roche Diagnostics, Indianapolis, IN), following the instructions of the manufacturer.
Western blot
After each treatment, cells were lysed with 1% Triton X-100 lysis buffer and 30 μg of protein cellular lysate was loaded onto sodium dodecyl sulfate–electrophoresis gel and transferred to a polyvinylidene difluoride membrane. COX-1 and COX-2 antibodies were from Cayman Chemical (Ann Harbor, MI). α-Tubulin antibody was from Santa Cruz Biotechnology (Santa Cruz, CA).
Determination of reactive oxygen species
Cells were pretreated with either 5 μM DCFDA or 5 μM DHE in plain RPMI 1640 medium without fetal calf serum or phenol red for 30 min as described (2). Followed by NO-ASA treatment as indicated, the fluorescent intensity was read by SpectraMax from Molecular Devices (Sunnyvale, CA). For flow cytometry assay, cells were treated with different agents and harvested by trypsinizing and resuspended cells with plain RPMI 1640 medium without fetal calf serum or phenol red containing either 5 μM DCFDA or 5 μM DHE and incubated at 37°C for 30 min. Cells were then read by flow cytometry and data analyzed using the CellQuest software.
Measurement of prostaglandin E2
A total of 5 × 105 cells/cm2 seeded overnight were treated with NO-ASA for 6 and 24 h. Prior to adding the NO-ASA, the culture medium was changed to one containing 1% serum. Prostaglandin E2 (PGE2) levels were determined using the Prostaglandin E2 EIA Kit from Cayman Chemical and following their instructions.
Immunofluorescence microscopy
BxPC-3 and HT-29 cells were treated with NO-ASA for 24 h, cytospun onto microscope slides, fixed with 3.7% formaldehyde and immunostained using a monoclonal antibody (mAb) against COX-2 as the primary antibody and fluorescein isothiocyanate-conjugated secondary antibody (Santa Cruz Biotechnology). IgG of the same subtype was used as negative control.
Short-interfering RNA knockdown of cox-2 expression
BxPC-3 cells were treated for 72 h with 20 nM COX-2 short-interfering RNA (siRNA) following the protocol recommended by the manufacturer (Dharmacon, Lafayette, CO). After this, cells were seeded in six-well plates and exposed to NO-ASA as indicated. Lipofectamine 2000 (Invitrogen, Carisbad, CA) was the transfection reagent.
Results
NO-ASA induces the expression of COX-2 in human cancer cell lines
To investigate whether NO-ASA affects the expression of COX, we studied in various human cancer cell lines the levels of COX-1 and COX-2 in response to NO-ASA or ASA.
HT-29 human colon cancer cells, seeded at 5.0 × 105 cells/cm2, were grown overnight and then treated with test compounds for 24 h. The positional isomers of NO-ASA (ortho, meta and para) inhibited cell growth; ASA was much less potent (Table I). The median inhibition concentration (IC50) of cell growth for p-NO-ASA was 21.8 ± 4.4 μM (mean ± SEM for this and all subsequent values); these values vary somewhat from those reported previously (20,21) due to differences in treatment protocol. Consistent with previous findings (22), the o- isomer was more potent than the p-, and the m- was nearly two orders of magnitude less potent than the other two. All NO-ASA isomers concentration-dependently induced COX-2 expression (Figure 1A). The m- was the least potent isomer, requiring concentrations 10- to 20-fold higher than the other two to induce COX-2 expression. None of the NO-ASA isomers induced the expression of COX-1 (Figure 1 and data not shown). Similar to HT-29, NO-ASA induced COX-2 in the CaCo-2 and LoVo colon cancer cell lines (supplementary Figure 1 is available at Carcinogenesis Online).
Table I.
Growth inhibition of human cancer cell lines by NO-ASA and ASA
| Cell line | Cell growth IC50 (μM), mean ± SEM |
|||
| HT-29 | BxPC-3 | Caco-2 | LoVo | |
| NO-ASA | ||||
| o- | 10.9 ± 2.1 | 7.3 ± 1.3 | 2.0 ± 0.6 | 5.2 ± 0.9 |
| m- | 473.5 ± 36.4 | 250.6 ± 27.9 | 300.6 ± 25.5 | 250.4 ± 41.3 |
| p- | 21.8 ± 4.4 | 11.3 ± 3.1 | 9.7 ± 3.1 | 3.9 ± 3.4 |
| ASA | >5000 | >5000 | >3000 | >3000 |
Fig. 1.
NO-ASA induces the expression of COX-2 in colon and pancreatic cancer cell lines. (A) HT-29 colon cancer cells were treated for 24 h with o-, m- or p-NO-ASA or ASA 5 mM and their total cell protein lysates were assessed for COX-2 or COX-1 expression by immunoblotting. α-Tubulin was the loading control. NO-ASA induces the expression of COX-2 concentration-dependently. Bottom panel: HT-29 cells were treated with p-NO-ASA 30 μM or vehicle for 24 h and examined by immunofluorescence microscopy as in Materials and Methods. (a) Control cells; primary antibody = non-specific IgG. (b) Vehicle-treated cells; primary antibody = anti-COX-2 mAb. (c) NO-ASA-treated cells; primary antibody: anti-COX-2 mAb. Note that the last lane in the first, third and fourth immunoblot is from cells treated with conventional ASA. (B) BxPC-3 pancreatic cancer cells were treated for 24 h with o-, m- or p-NO-ASA at the indicated concentrations or ASA 5 mM and their total cell lysates were assessed for COX-2 or COX-1 expression by immunoblotting, showing concentration-dependent induction of COX-2. α-Tubulin was the loading control. Bottom panel: BxPC-3 cells treated with p-NO-ASA 30 μM or vehicle for 24 h were examined by immunofluorescence microscopy as in Materials and Methods. (a) Cells treated with NO-ASA; primary antibody: non-specific IgG. (b) Cells treated with vehicle; primary antibody: anti-COX-2 mAb. (c) Cells treated with NO-ASA; primary antibody: anti-COX-2 mAb. (C) The growth of BxPC-3 cells plated as in Materials and Methods and treated with p-NO-ASA 20 μM for the indicated time periods was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. At each time point, cells treated in parallel with p-NO-ASA 20 μM (+) or vehicle (−) were assayed by immunoblotting for the expression of COX-2 or COX-1. Loading control, α-tubulin. (D) BxPC-3 cells were treated with various concentrations of p-NO-ASA for 6 or 24 h and PGE2 levels were assayed in the culture medium as in Materials and Methods.
NO-ASA also induced COX-2 in the BxPC-3 pancreatic cancer cell line and the HUVEC cell line that is derived from normal human vascular endothelium (23) (Figure 1B). No induction was seen in the cox-null MIA PaCa-2 pancreatic or HCT-15 human colon cancer cells (24,25) (data not shown). The time dependence of the induction of COX-2 by p-NO-ASA 20 μM was studied in BxPC-3 pancreatic cancer cells (Figure 1C). COX-2 was induced by 1 h, increased progressively and reached its maximum by 6 h, remaining stable thereafter at a slightly lower level. The expression of COX-1 remained unchanged. Of interest, the number of cells decreased continuously in response to NO-ASA treatment starting at 1 h, the earliest time of observation.
The induction of COX-2 by NO-ASA was also documented by immunofluorescence (Figure 1). NO-ASA failed to induce the expression of COX-1 at concentrations that induced COX-2 strongly (data not shown). ASA up to 5 mM failed to induce the expression of either COX-1 or COX-2, as determined by immunofluorescence microscopy.
The induced COX-2 is catalytically active
To determine whether the COX-2 induced by NO-ASA was catalytically competent, we measured PGE2 production in BxPC-3 cells (Figure 1D). Cells seeded as previously were washed carefully 24 h later and grown in the presence or absence of NO-ASA or ASA for 6 or 24 h in the medium supplemented with 1% serum (this level of serum does not affect the PG assay). PGE2 levels in the culture medium were increased concentration-dependently in response to NO-ASA, reaching their maximum at 10 μM NO-ASA (P < 0.01) and decreasing by ∼69% at 20 μM NO-ASA compared with the 10 μM NO-ASA levels. As expected, the 24 h values were higher than those obtained at 6 h (P < 0.001), but the pattern of response was similar. Treatment of HT-29 cells with NO-ASA (1–100 μM) following the same protocol generated similar results (data not shown). Thus, the COX-2 induced by NO-ASA is catalytically active.
COX-2 induction is pronounced during cell death
PGE2 enhances the growth of colon cancer cells and inhibits their apoptosis (26), whereas forced COX-2 expression significantly attenuates the induction of apoptosis by NSAIDs (27). Since the induction of COX-2 by NO-ASA is accompanied by suppressed cell growth, we explored the potential role of COX-2 in cell death. Therefore, we evaluated when in the process of cell death COX-2 expression is induced.
COX-2 expression was determined in cells that were either attached (alive) or floating (dying or already dead). After treating three different cell lines with NO-ASA for 24 h, we observed consistently in all that floating cells expressed much more COX-2 than attached cells (Figure 2A–C).
Fig. 2.
Pronounced induction of COX-2 by NO-ASA in dying/dead cells. HUVEC (A), HT-29 (B) and BxPC-3 (C) cells were treated with p-NO-ASA as indicated for 24 h. Cells attached to the culture dish were harvested separately from those floating in the medium; the latter were either dead or dying, as determined by trypan blue staining. Total cell protein lysates were prepared from each group of cells and COX-2 expression was determined by immunoblot. (D) ROS levels were determined in attached (At) and floating (Fl) BxPC-3 cells following treatment with 10 or 15 μM NO-ASA for 4 h and staining with 5 μM DCFDA for 30 min. The fluorescent intensity of DCFDA was determined by flow cytometry. Values for fluorescent intensity (geometric mean) were as follows: control (vehicle treated) cells (all attached) = 10.24; NO-ASA 5 μM: all cells attached = 10.28; NO-ASA 10 μM: attached cells = 15.41, floating cells = 61.53; NO-ASA 15 μM: attached cells = 17.21, floating cells = 62.09.
COX-2 is induced by oxidative stress
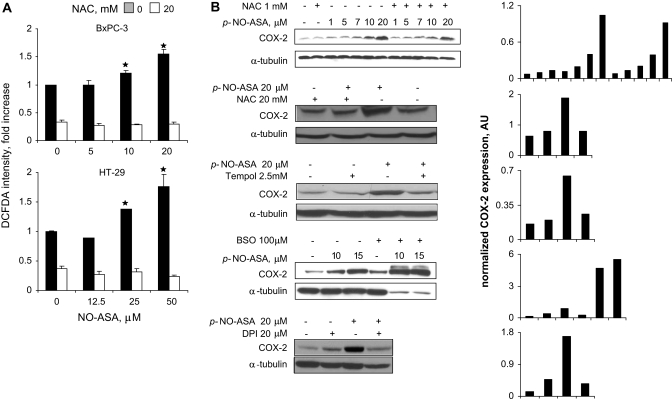
Since COX is known to respond to the redox state of the cell (12), we examined the levels of reactive oxygen species (ROS) in HT-29 and BxPC-3 cells treated with NO-ASA or sulindac sulfide. Both compounds increased concentration dependently the levels of ROS, as determined by the general ROS probe DCFDA (Figures 3A and 4A).
Fig. 3.
NO-ASA induces ROS and COX-2 in pancreatic cancer cells. (A) BxPC-3 and HT-29 cells were treated with one of three concentrations of p-NO-ASA for 3 h. ROS levels were detected by using the general probe DCFDA as in Materials and Methods (30 min staining). Four hours pretreatment with NAC 10 mM abrogated the induction of ROS. (B) BxPC-3 cells were treated with three antioxidants (NAC for 24 h, Tempol for 24 h and diphenylene iodonium for 2 h) or BSO, the inhibitor of glutathione biosynthesis, for 24 h prior to 4 h treatment with p-NO-ASA. COX-2 expression was detected by immunoblotting of total cell protein lysates. While the levels of α-tubulin used as a loading control were markedly reduced in response to the combined treatment with BSO and NO-ASA; Ponceau S staining of the same immunoblot membrane revealed equal protein loading (data not shown). Each lane has been quantified by densitometry and values have been normalized to the corresponding α-tubulin (loading control). The results for each immunoblot are shown in the last column; numbers in the abscissa represent the corresponding immunoblot lanes. *P<0.01 compare to control untreated cells.
Fig. 4.
Sulindac sulfide (SS) induces ROS and COX-2. (A) BxPC-3 cells were treated for 3 h with sulindac sulfide at concentrations half or equal to its 24 h IC50 for growth, harvested and stained with 10 μM DCFDA for 30 min and the fluorescence intensity was analyzed by FACScaliber (upper panel). In the bottom panel, cells were pretreated with 10 mM NAC for 4 h followed by sulindac sulfide treatment for 3 h. Sulindac sulfide increases ROS levels and NAC partially blocks this increase. (B and C) BxPC-3 cells were treated for 24 h (B) or 48 h (C) with sulindac sulfide as indicated with or without a 4 h preincubation with NAC 10 mM. COX-1 and COX-2 levels were assayed by immunoblotting as in Materials and Methods. The 24 h IC50 for growth inhibition is 1.25 mM. Sulindac sulfide induced COX-2 expression that was partially inhibited by NAC.
As shown in Figure 3, ROS levels were increased significantly (P < 0.001) up to 75–80% in these two cell lines when treated with p-NO-ASA at concentrations up to twice its 24 h IC50 for cell growth. This effect was accompanied by a concentration-dependent increase of COX-2 expression, which paralleled the ROS levels.
We also examined the levels of ROS in attached and floating BxPC-3 cells. Using as a probe DCFDA, we observed that at NO-ASA 10μM and 15 μM, the ROS levels of floating cells were ∼4-fold higher (62.09 versus 15.41) than those of attached cells (Figure 2D). The corresponding COX-2 expression levels of attached and floating cells were increased, mirroring the increased levels of ROS.
To further evaluate the role of the cell’s redox state in COX-2 induction, we treated BxPC-3 cells with the antioxidant agents Tempol (28) and N-acetyl-cysteine (NAC) (29) (Figure 3B). Pretreatment of BxPC-3 cells with NAC 1 mM for 4 h blocked modestly the induction of COX-2 by NO-ASA (uppermost immunoblot). Pretreatment of the cells with 20 mM NAC dramatically reduced the expression of COX-2, as did Tempol. NAC inhibited the induction of ROS in both HT-29 and BxPC-3 cells (P < 0.001) (Figure 4A). We also depleted glutathione (GSH) stores in these cells by pretreatment for 24 h with DL-buthionine-(SR)-sulfoximine (BSO) 100 μM, the specific inhibitor of γ-glutamylcysteine synthetase, the rate-limiting enzyme for GSH biosynthesis (30). At the concentration used here, BSO alone induced the expression COX-2 modestly but greatly potentiated the effect of NO-ASA on COX-2 expression, being clearly synergistic with it (Figure 3B). Of note, although the amount of α-tubulin was markedly decreased in cells treated with both p-NO-ASA and BSO (perhaps as a result of cell death), the amount of COX-2 was far greater than that of controls. (Ponceau staining confirmed that the total amount of protein in these lanes was similar to that of the others in this immunoblot.) Finally, the NADPH oxidase inhibitor diphenylene iodonium (31,32) inhibited the induction of COX-2 by NO-ASA (Figure 3B), suggesting an association between O2−· production by this enzyme and COX-2 induction.
Sulindac sulfide inhibited the growth of BxPC-3 cells (24 h IC50 = 1.25 mM), as was expected (33); treatment of BxPC-3 cells with sulindac sulfide increased ROS levels. For example, as seen in Figure 4, after 3 h of treatment with sulindac sulfide at its 24 h IC50 concentration for growth, ROS levels in BxPC-3 cells were increased by up to 185% (P < 0.01). The increase in ROS levels was followed by enhanced COX-2 (but not COX-1) expression. As with NO-ASA, 4 h pretreatment of the cells with NAC 10 mM abrogated the induction of both ROS and COX-2 (Figure 4A and B), an effect paralleled by improved cell survival (data not shown). Longer treatment of BxPC-3 cells with lower concentrations of sulindac sulfide (100 μM for 48 h) also induced COX-2, which was prevented by NAC 10 mM (Figure 4C).
Finally, we assessed whether oxidative stress induced by means other than treatment with NO-ASA and sulindac sulfide induces COX-2. We treated BxPC-3 cells with xanthine (X) and xanthine oxidase (XO), a combination which generates O2−· (34) (Figure 5A–C). The production of O2−· in response to X/XO was documented at 90 min using the DHE probe for the detection of O2−·. The modest fluorescence response of the DCFDA probe indicates the likely presence of small amounts of other ROS. X/XO concentration-dependently induced the expression of COX-2, evaluated after 12 and 24 h of treatment. This effect was accompanied by inhibition of cell growth. Therefore, it is clear that in X/XO-treated cells as well, COX-2 is downstream of ROS production. We also examined the effect of exogenous H2O2 on COX-2 expression. As shown in Figure 5D, treatment of BxPC-3 cells with H2O2 for 3 h increased the expression of COX-2 concentration-dependently. Similar to what was observed in cells treated with BSO (Figure 3B), the β-actin loading control corresponding to the two highest concentrations of H2O2 is decreased, even though the total amount of protein loaded per lane is the same (Ponceau staining). This probably reflects protein degradation in dying/dead cells. The interesting point here, however, is that the levels of COX-2 are greatly increased, in agreement with all our previous findings.
Fig. 5.
The effect of superoxide anion and hydrogen peroxide on COX-2 expression in pancreatic cancer cells. BxPC-3 cells were treated for 12 or 24 h with a combination of X 1 mM and various concentrations of the enzyme XO or p-NO-ASA 20 μM. (A) After BxPC-3 cells were treated for 90 min with either X plus XO or vehicle control, ROS levels were determined using either DHE or DCFDA fluorescent probes, as in Materials and Methods. The corresponding intensity of fluorescence was determined by flow cytometry. (B) The growth of BxPC-3 cells was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay after 24 h of treatment with either a combination of X 1 mM and various concentrations of the enzyme XO or p-NO-ASA 20 μM. The number after XO in the abscissa indicates U/ml. (C) Immunoblots showing the induction of COX-2 by X plus XO, which is dependent upon the amount of XO used and is analogous to that obtained by NO-ASA. α-Tubulin was the loading control. Each lane has been quantified by densitometry and values have been normalized to the corresponding α-tubulin (loading control). The results for each immunoblot are shown in the graphs; letters in the abscissa represent the corresponding immunoblot lanes. (D) BxPC-3 cells were treated for 3 h with various concentrations of H2O2 and the expression of COX-2 was determined by immunoblotting. Loading control, β-actin.
COX-2 is not required for cell growth inhibition and does not cause cell death
COX-2 is the molecular target of the chemopreventive COX-2 inhibitors (11). The implication is that inactivation of COX-2 causes the death of the neoplastic cell. On the other hand, our data show that NO-ASA and sulindac increase the cellular levels of ROS and COX-2 and these events are critical to the cell death induced by these two agents. The implication here is that COX-2 may participate in the induction of cell death by NSAIDs.
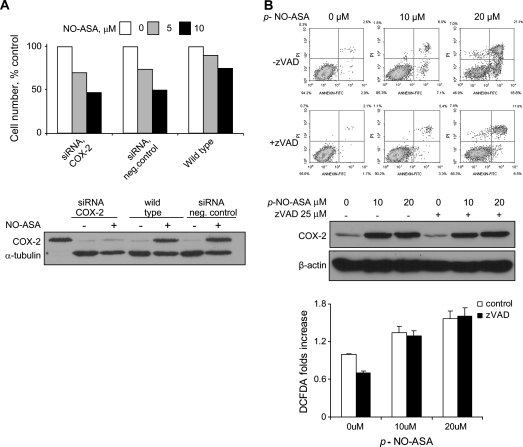
To resolve these issues, we knocked down the expression of cox-2 in BxPC-3 cells using a cox-2-specific siRNA oligonucleotide. In contrast to those transfected with non-specific siRNA or untransfected cells, cells transfected with specific cox-2 siRNA showed essentially no COX-2 expression (Figure 6A). When these cells were treated with 5 or 10 μM NO-ASA for another 24 h, the effect of NO-ASA on cell growth (number of cells alive) was about the same, regardless of the level of COX-2 expression. Thus, inhibition of COX-2 is not required for the induction of cell death by NO-ASA.
Fig. 6.
COX-2- and NO-ASA-induced cell death. (A) BxPC-3 cells transfected with 20 nM COX-2-specific or -non-specific siRNA (negative control) for 72 h, as in Materials and Methods, were treated with p-NO-ASA for 24 h. COX-2 expression was determined by immunoblotting total cell protein lysates COX-2 expression was determined by immunoblotting total cell protein lysates and cell growth using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. Upper panel: the growth of these three types of cells in response to 24 h treatment with NO-ASA. Lower panel: representative immunoblot from cells treated with NO-ASA 10 μM. No COX-2 expression is seen in response to NO-ASA in cells treated with the specific siRNA, in contrast to wild-type (non-transfected) and control BxBC-3 cells. (B) Upper panel: BxPC-3 cells were pretreated for 1 h with or without 25 μM of zVAD, the pan-caspase inhibitor, followed by p-NO-ASA for 18 h. Cells stained with annexin V and propidium iodide were assayed using flow cytometry. The percentage of cells in each of the four quadrants is indicated. Middle panel: the immunoblot shows that COX-2 expression is not affected by treating the cells with the zVAD. Loading control, β-actin. Lower panel: BxPC-3 cells were treated with NO-ASA for 3 h as indicated with or without preincubation of zVAD for 1 h. DCFDA staining shows that zVAD has no effect on ROS production by NO-ASA.
To assess whether COX-2 participates in the induction of cell death, BxPC-3 cells were treated with or without the pan-caspase inhibitor zVAD 25 μM, followed by p-NO-ASA for 18 h (Figure 6B). NO-ASA induced apoptosis concentration-dependently. Apoptotic cells [annexin (+) and propidium iodide (−)] increased from 2.9% in control to 18.8% in cells treated with NO-ASA 20 mM (P < 0.01), whereas those representing secondary necrosis [annexin (+) and propidium iodide (+)] increased from 2.6 to 27.3% (P < 0.01), respectively. Pretreatment with zVAD prevented these changes to a large extent, with the corresponding values being reduced to 6.5 and 17.8% (P < 0.01) (NO-ASA 10 μM gave intermediate responses). ROS levels remained unchanged in response to zVAD evidenced by DCFDA staining (Figure 6B). Despite the reduction in cell death by zVAD, the induction of COX-2 did not change. These findings indicate that cell death is independent of COX-2 induction and appears responsive only to ROS.
Structure–activity relationship for the induction of COX-2 by NO-ASA
As shown above, the three positional isomers of NO-ASA induce COX-2 with different potency. The -ONO2 moiety of NO-ASA releases NO, a property that defines NO-NSAIDs. Denitrated p-NO-ASA (-OH in the place of -ONO2) failed to induce oxidative stress and had no effect on COX-2 expression; denitrated m- and o- isomers gave similar results (supplementary Figure 2 is available at Carcinogenesis Online and data not shown). These data indicate that the -ONO2 moiety is required for these effects. This result is consistent with the notion that the spacer of NO-ASA may be responsible for its effects (35).
Discussion
Our findings document that in cancer cell lines two chemopreventive agents induce oxidative stress, which leads to overexpression of COX-2. The induction of COX-2 by ROS is independent of the agent causing oxidative stress and also of the tissue origin of the cell lines and their phenotype (malignant or not) and is enhanced in dying cells, whose ROS levels are increased further. COX-2 does not participate in the process of cell death or in the cell growth inhibitory effect of these agents.
The induction of COX-2 by NO-ASA was documented both by immunoblotting and by fluorescent microscopy. The induced COX-2 was not an aberrant, metabolically inactive form of COX-2 cross-reacting with the antibody used for its detection; rather, it was catalytically competent as evidenced by its ability to produce PGE2.
The effect of NO-ASA and sulindac sulfide on COX-2 expression is specific since conventional ASA failed to have such an effect and the expression of COX-1 remained unchanged. Of note, cox-null colon and pancreatic cell lines (24) failed to express COX-2 when treated with NO-ASA. The induction of COX-2 occurred in response to all three isomers of NO-ASA, and the levels of COX-2 reflected the cancer cell growth inhibitory potency of each isomer [m-NO-ASA is the least potent (20)]. The induction of COX-2 was both time and concentration dependent and represented a generalized effect in terms of (i) the tissue origin of the cell lines in which it occurs; (ii) their phenotype and (iii) the chemical structure of the compound that induces it. NO-ASA induced COX-2 in four different cell lines; the three of them that are derived from human cancers display a malignant phenotype, whereas the fourth (HU-VE-EC) is a normal cell line. Moreover, two structurally different compounds, NO-ASA and sulindac sulfide, induced COX-2.
The oxidative stress generated by these two chemopreventive agents was responsible for the induction of COX-2. Two antioxidants abrogated this phenomenon, whereas depletion of cellular GSH, the main antioxidant in mammalian cells, induced COX-2 expression, which was enhanced further by NO-ASA. The role of ROS in the induction of COX-2 is also underscored by the induction of COX-2 by O2−·, which was generated by treating cells with X/XO, or by H2O2 directly. Consistent with this notion, diphenylene iodonium, the inhibitor of NADPH oxidase, the enzyme generating O2−·, suppressed COX-2.
Compared with attached cells, floating cells (they are either dying or already dead) had more ROS and more COX-2. The increased levels of COX-2, however, did not contribute to cell death by these agents. First, NO-ASA inhibited the growth of cells whose COX-2 expression was knocked down by siRNA; second, when cell death was blocked by a pan-caspase inhibitor, the induced COX-2 did not change. It is unclear, however, why the dying cell has increased COX-2 levels. One possibility is that COX-2 levels may be increased as a result of slower protein degradation during cell death. Alternatively, the cell, dying in response to an exogenous agent, may try to abort its death by overexpressing a protein like COX-2, which is known to reduce apoptotic susceptibility (27).
Our results support three potentially useful ideas: (i) the concept that ROS is a critical component of the action of chemopreventive agents (5); (ii) the notion that COX-2 may not be an ideal target for chemoprevention (10) and (iii) the possibility that COX-2 may be overexpressed in cancer cells due to their state of oxidative stress (12,36,37). It is conceivable that, if further substantiated, these ideas may inform the rational design of chemotherapeutic strategies, in particular the choice of agents in combination approaches.
In summary, our data establish that two chemopreventive agents, NO-ASA and sulindac sulfide, generate a state of oxidative stress in various cell lines, leading to overexpression of COX-2. The induction of COX-2, however, does not contribute to the cell death induced by these agents. Our data suggest that further studies are needed to assess the role of ROS as part of a general mechanism of action of chemopreventive agents against cancer.
Supplementary material
Supplementary Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (2R01 CA92423 and R01 CA101019).
Supplementary Material
Glossary
Abbreviations
- ASA
aspirin
- BSO
DL-buthionine-(SR)-sulfoximine
- COX
cyclooxygenase
- DCFDA
2′,7′-dichlorofluorescein diacetate
- DHE
dihydroethidium
- IC50
median inhibition concentration
- mAB
monoclonal antibody
- NAC
N-acetyl-cysteine
- NADPH
reduced form of nicotinamide adenine dinucleotide phosphate
- NO-ASA
nitric oxide-donating aspirin
- NSAID
non-steroidal anti-inflammation drug
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- siRNA
short-interfering RNA
- X
xanthine
- XO
xanthine oxidase
References
- 1.Adachi M, et al. Nonsteroidal anti-inflammatory drugs and oxidative stress in cancer cells. Histol. Histopathol. 2007;22:437–442. doi: 10.14670/HH-22.437. [DOI] [PubMed] [Google Scholar]
- 2.Gao J, et al. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc. Natl Acad. Sci. USA. 2005;102:17207–17212. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giardina C, et al. Nonsteroidal anti-inflammatory drugs, short-chain fatty acids, and reactive oxygen metabolism in human colorectal cancer cells. Biochim. Biophys. Acta. 1998;1401:277–288. doi: 10.1016/s0167-4889(97)00140-7. [DOI] [PubMed] [Google Scholar]
- 4.Ng Y, et al. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–1921. doi: 10.1093/carcin/bgi163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigas B, et al. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br. J. Cancer. 2008;98:1157–1160. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruegg C, et al. Non steroidal anti-inflammatory drugs and COX-2 inhibitors as anti-cancer therapeutics: hypes, hopes and reality. Ann. Med. 2003;35:476–487. doi: 10.1080/07853890310017053. [DOI] [PubMed] [Google Scholar]
- 7.Saji S, et al. Novel sensitizing agents: potential contribution of COX-2 inhibitor for endocrine therapy of breast cancer. Breast Cancer. 2004;11:129–133. doi: 10.1007/BF02968291. [DOI] [PubMed] [Google Scholar]
- 8.Lanas A, et al. Prevention of anti-inflammatory drug-induced gastrointestinal damage: benefits and risks of therapeutic strategies. Ann. Med. 2006;38:415–428. doi: 10.1080/07853890600925843. [DOI] [PubMed] [Google Scholar]
- 9.Stewart GS, et al. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell. 1999;99:577–587. doi: 10.1016/s0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- 10.Rigas B, et al. Cancer prevention: a new era beyond cyclooxygenase-2. J. Pharmacol. Exp. Ther. 2005;314:1–8. doi: 10.1124/jpet.104.080564. [DOI] [PubMed] [Google Scholar]
- 11.Cha YI, et al. NSAIDs and cancer prevention: targets downstream of COX-2. Annu. Rev. Med. 2007;58:239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 12.Ulrich CM, et al. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat. Rev. Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 13.Cervello M, et al. Cyclooxygenases in hepatocellular carcinoma. World J. Gastroenterol. 2006;12:5113–5121. doi: 10.3748/wjg.v12.i32.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimura T, et al. Role of cyclooxygenase-2 in the carcinogenesis of gastrointestinal tract cancers: a review and report of personal experience. World J. Gastroenterol. 2006;12:1336–1345. doi: 10.3748/wjg.v12.i9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun KS, et al. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem. Pharmacol. 2004;68:1089–1100. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Fecker LF, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal anti-inflammatory drugs (NSAIDs) Br. J. Dermatol. 2007;156(suppl. 3):25–33. doi: 10.1111/j.1365-2133.2007.07856.x. [DOI] [PubMed] [Google Scholar]
- 17.Giardiello FM. Sulindac and polyp regression. Cancer Metastasis Rev. 1994;13:279–283. doi: 10.1007/BF00666098. [DOI] [PubMed] [Google Scholar]
- 18.Rigas B. The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr. Opin. Gastroenterol. 2007;23:55–59. doi: 10.1097/MOG.0b013e32801145b0. [DOI] [PubMed] [Google Scholar]
- 19.Penning TD, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J. Med. Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 20.Kashfi K, et al. Positional isomerism markedly affects the growth inhibition of colon cancer cells by nitric oxide-donating aspirin in vitro and in vivo. J. Pharmacol. Exp. Ther. 2005;312:978–988. doi: 10.1124/jpet.104.075994. [DOI] [PubMed] [Google Scholar]
- 21.Williams JL, et al. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDs: implications for colon cancer chemoprevention. Cancer Res. 2001;61:3285–3289. [PubMed] [Google Scholar]
- 22.Nath N, et al. NO-donating aspirin inhibits the growth of leukemic Jurkat cells and modulates beta-catenin expression. Biochem. Biophys. Res. Commun. 2004;326:93–99. doi: 10.1016/j.bbrc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Hoshi H, et al. Brain- and liver cell-derived factors are required for growth of human endothelial cells in serum-free culture. Proc. Natl Acad. Sci. USA. 1984;81:6413–6417. doi: 10.1073/pnas.81.20.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanif R, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem. Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 25.Kashfi K, et al. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: evidence of a tissue type-independent effect. J. Pharmacol. Exp. Ther. 2002;303:1273–1282. doi: 10.1124/jpet.102.042754. [DOI] [PubMed] [Google Scholar]
- 26.Qiao L, et al. Selected eicosanoids increase the proliferation rate of human colon carcinoma cell lines and mouse colonocytes in vivo. Biochim. Biophys. Acta. 1995;1258:215–223. doi: 10.1016/0005-2760(95)00100-q. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, et al. Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res. 2002;62:6323–6328. [PubMed] [Google Scholar]
- 28.Schor NF, et al. Exploiting oxidative stress and signaling in chemotherapy of resistant neoplasms. Biochemistry (Mosc.) 2004;69:38–44. doi: 10.1023/b:biry.0000016349.75384.e6. [DOI] [PubMed] [Google Scholar]
- 29.Atmaca G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J. 2004;45:776–788. doi: 10.3349/ymj.2004.45.5.776. [DOI] [PubMed] [Google Scholar]
- 30.Griffith OW, et al. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J. Biol. Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- 31.Li Y, et al. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem. Biophys. Res. Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 32.Matsui T, et al. Diphenyleneiodonium prevents reactive oxygen species generation, tyrosine phosphorylation, and histamine release in RBL-2H3 mast cells. Biochem. Biophys. Res. Commun. 2000;276:742–748. doi: 10.1006/bbrc.2000.3545. [DOI] [PubMed] [Google Scholar]
- 33.Shiff SJ, et al. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J. Clin. Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiraishi H, et al. Oxygen metabolite-induced cytotoxicity to cultured rat gastric mucosal cells. Am. J. Physiol. 1987;253:G40–G8. doi: 10.1152/ajpgi.1987.253.1.G40. [DOI] [PubMed] [Google Scholar]
- 35.Kashfi K, et al. The mechanism of action of nitric oxide-donating aspirin. Biochem. Biophys. Res. Commun. 2007;358:1096–1101. doi: 10.1016/j.bbrc.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 36.Burdon C, et al. Oxidative stress and the induction of cyclooxygenase enzymes and apoptosis in the murine placenta. Placenta. 2007;28:724–733. doi: 10.1016/j.placenta.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halliwell B, et al. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.