Abstract
The aryl hydrocarbon receptor (AhR) mediates the carcinogenicity of a family of environmental contaminants, the most potent being 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Increased incidence of lymphoma and leukemia in humans is associated with TCDD exposure. Although AhR activation by TCDD has profound effects on the immune system, precise cellular and molecular mechanisms have yet to be determined. These studies tested the hypothesis that alteration of marrow populations following treatment of mice with TCDD is due to an effect on hematopoietic stem cells (HSCs). Treatment with TCDD resulted in an increased number and proliferation of bone marrow (BM) populations enriched for HSCs. There was a time-dependent decrease in B-lineage cells with a concomitant increase in myeloid populations. The decrease in the B-cell lineage colony-forming unit-preB progenitors along with a transient increase in myeloid progenitors were consistent with a skewing of lineage development from lymphoid to myeloid populations. However, HSCs from TCDD-treated mice exhibited diminished capacity to reconstitute and home to marrow of irradiated recipients. AhR messenger RNA was expressed in progenitor subsets but is downregulated during HSC proliferation. This result was consistent with the lack of response following the exposure of 5-fluorouracil-treated mice to TCDD. The direct exposure of cultured BM cells to TCDD inhibited the growth of immature hematopoietic progenitor cells, but not more mature lineage-restricted progenitors. Overall, these data are consistent with the hypothesis that TCDD, through AhR activation, alters the ability of HSCs to respond appropriately to signals within the marrow microenvironment.
Introduction
The aryl hydrocarbon receptor (AhR) is best known for its ability to mediate the toxicity and carcinogenicity of a family of environmental contaminants, the most potent being 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (1). Increased incidence of leukemia and lymphoma has been associated with exposure of human populations to TCDD and related xenobiotics (2–4), and TCDD is classified as a human carcinogen (4). Xenobiotic AhR ligands are major components of tobacco smoke (5), and the AhR-signaling pathway contributes to tobacco-associated inflammation, acute and chronic lung disease and lung cancer (6–8). Tumors develop in diverse tissues following chronic exposure of rodents to TCDD (9).
Several mechanisms defining possible roles of the AhR in carcinogenesis have been proposed. TCDD has been demonstrated to be a potent tumor promoter (9). However, the AhR may also be involved in cancer initiation since it regulates the expression of enzymes affecting metabolism and disposition of several carcinogens (10). The sustained or inappropriate activity of the AhR as a transcription factor may also stimulate clonal expansion of preneoplastic cells or inhibit apoptosis of these by regulating the expression of genes controlling critical cellular processes (11,12). In particular, the AhR has been shown to have an important role in cell cycle regulation. However, the exact mechanisms have not been detailed, and the effects are probably cell specific (13).
The immune system and immune surveillance mechanisms are important for regulating carcinogenic processes. Given this, it is significant that the dioxins represent some of the most potent immunosuppressive agents known. A single dose of TCDD have been shown to alter both T- and B-cell-dependent immune responses, impair host resistance to a variety of infectious agents and antigens and prevent the rejection of transplanted tumors (14,15). While several cellular and signaling components of the immune system are altered following TCDD exposure, the exact mechanisms by which this chemical disrupts immune functions remain uncertain (15). Nevertheless, these data strongly suggest that the AhR has a normal role in directly regulating important immune cell functions. Limited studies on the immune system in Ahr null-allele (AhR-KO) mice are consistent with this (16–21). However, relationships between the modulation of known AhR-responsive genes and specific functional outcomes in any cells in the immune system have yet to be defined (22).
Given the profound effects of AhR activation on the immune system, the association of hematopoietic diseases with dioxin exposure and that many hematopoietic malignancies are derived from stem and/or progenitor cells, we have been investigating the cellular and molecular events underlying AhR-dependent alterations in bone marrow (BM). The numbers of cells in B-cell compartments decrease following treatment of mice with TCDD (23), but other data suggest that these effects are subsequent to actions of AhR agonists on B-cell precursors (24). Following AhR activation by TCDD, there is also a significant reduction in lymphoid-specific TdT and RAG1 messenger RNA (mRNA) in immature BM cells, and thymic seeding by progenitors is substantially decreased (25,26). The use of radiation–reconstitution chimeras indicated that AhR presence in hematopoietic cells, but not supporting stroma, is necessary for these effects (27,28). However, TCDD treatment actually produces a several fold increase in the number of hematopoietic stem cell (HSC)-enriched Lin−/Sca-1+/cKit+ (LSK) cells (29,30) (see supplementary Figure 1, available at Carcinogenesis Online), which is also dependent on the presence of the AhR in hematopoietic cells (27). TCDD also elicits alterations in LSK cell cycle (31).
These observations suggest that inappropriate AhR activation by TCDD may alter the ability of HSCs to proliferate and differentiate into mature lineage cells. It is also possible that the modulation of HSC functions may be permissive for cancer formation and/or progression. The present studies were designed to dissect the cellular events leading to altered BM subpopulations following AhR activation by TCDD and to begin to define the mechanisms by which these effects occur. Our data are consistent with an interpretation that inappropriate AhR activation by TCDD directly alters the properties of hematopoietic stem/progenitor cells in an environment-dependent manner that impacts their functional capacity.
Materials and methods
Animals and treatment
Four- to five-week-old C57Bl/6J male mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were acclimated for at least 1 week before experimentation. Ahr-KO (Ahr −/−) mice, modified at exon 2 (18), were originally obtained from Jackson Laboratories, and a colony was established at the University of Rochester. A polymerase chain reaction (PCR)-based assay (32) was used to confirm the Ahr genotype of these animals. All animals were maintained and treated in accordance with the guidelines set by the University of Rochester Committee on Animal Resources and the American Association for Laboratory Animal Science. Animals were given food (5001 Rodent Diet; Purina Mills, St. Louis, MO) and water ad libitum and maintained on a 12 h light–dark schedule. TCDD (>99% pure) was obtained from Cambridge Isotopes (Cambridge, MA). From a stock solution of 0.2 mg/ml in dioxane, a standard dosing solution was made in olive oil. Mice were treated with 30 μg TCDD/kg body wt or 0.1 ml olive oil/20 g body wt by intraperitoneal injection or by gavage as indicated. Animals were euthanized by CO2 asphyxiation. Treatment and euthanasia occurred between 9 and 11 a.m. in order to minimize the possible confounding effects of circadian rhythms (31).
Cell isolation for AhR analysis
Total BM was harvested as described previously (24,28). Phenotypic markers outlined in supplementary Figure 1 (available at Carcinogenesis Online) were used to isolate specific cell populations. All antibodies were obtained from BD Biosciences (San Jose, CA) unless otherwise noted. Lineage-positive (Lin+) cells were identified using fluorescent-tagged antibodies against Mac-1 = CD11b (clone M1/70), B220 (RA3-6B2), Gr-1 (RB6-8C5) and Ter-119 (Ter-119). Lin− cells were obtained by blocking the FcγIII/II receptor using antibody clone 2.4G2 and then incubating with biotinylated antibodies against Mac-1, B220, Gr-1, Ter-119, CD3ε (500A2) and CD8α (53-6.7) and streptavidin-linked microbeads. Lin+ cells were removed using magnetic sorting. HSC-enriched (LSK) cells were obtained by incubating Lin− cells with fluorescent-tagged antibodies against Sca-1 (E133-161.7) and cKit = CD117 (2B8) and further sorted using the BD FACSVantage™ SE system. Purity of fractions was >95% for all experiments.
Western blot analysis
Cell fractions were lysed using protein lysis buffer of phosphate-buffered saline (PBS), Tween 20, ethylenediaminetetraacetic acid and protease inhibitors (Roche Complete Mini; Roche Pharmaceuticals, Basel, Switzerland) on ice. Protein in supernatants was quantified using Coomassie Blue reagent (Pierce, Rockford, IL). For BM fractions, 100 μg per lane protein was loaded onto a 7.5% acrylamide gel and for liver controls, 10 μg per lane. After electrophoresis, proteins were transferred to a nitrocellulose membrane and blocked in 5% Blotto [non-fat milk in tris buffered saline-tween (TBST)] (20 mM Tris, 0.5 M NaCl and 0.03% Tween 20, pH 7.5). Membranes were incubated with primary antibodies against AhR (BioMol, Plymouth Meeting, PA) and aryl hydrocarbon receptor nuclear translocation (Arnt) (Novus Biologicals, Littleton, CO) using a 1:5000 dilution in TBST at 4°C. Blots were washed twice in TBST and incubated for 1 h at room temperature in a 1:3000 dilution in TBST of horseradish peroxidase-conjugated secondary antibody. Membranes were washed twice with TBST and developed by chemiluminescence (KPL, Gaithersburg, MD).
RNA isolation, reverse transcription and real-time quantitative PCR
RNA was isolated from Lin-Sca-1+ and LSK cells using the RNeasy Mini/Micro Kit (Qiagen, Valencia, CA) and quantified using a spectrophotometer ND-1000 (NanoDrop Technologies, Wilmington, DE). First-strand complementary DNA synthesis and amplification from 10 ng of RNA were performed using SuperScript™ One-Step RT-PCR with Platinum® Taq (Invitrogen, Carlsbad, CA). The AhR primers’ sequences were forward, 5′-CGGTGCAGAAAACAGTAAAGCC-3′ and reverse, 5′-AAGAAGCTCTTGGCCCTCAGGT-3′. The primers’ sequences for Arnt were forward, 5′-CAGGGACGGTGCCATCTCGAC-3′ and reverse, 5′-CCTGGCAAACCGCTCTTTGTCA-3′. Final reaction mixtures were run in a 2% Ultra Pure Agarose (Invitrogen) gel using Tris-borate-EDTA buffer (0.89 M Tris, 0.89 M boric acid and 20 mM ethylenediaminetetraacetic acid) and visualized using ethidium bromide. RNA for real-time PCR was isolated using the TRIzol reagent (Life Technologies, Gaithersburg, MD) and quantified by spectrophotometry. First-strand complementary DNA was produced following the protocol of SuperScriptII First-Strand cDNA Synthesis system (Invitrogen) using 2.5–5 μg total RNA and oligodT primers. The complementary DNA samples were phenol–chloroform extracted and ethanol precipitated. Real-time quantitative PCR was performed using TaqMan PCR primers and MasterMix (Applied Biosystems, Foster City, CA). Real-time detection and analysis were performed using the Bio-Rad iCycler (Hercules, CA) and data were analyzed using the standard curve method (ABI Prism 7700 Sequence Detection System User Bulletin #2). Samples were run in triplicate and normalized to glyceraldehyde-3-phosphate dehydrogenase RNA.
Phenotyping experiments
Mice were dosed with 30 μg/kg TCDD or olive oil vehicle and euthanized, and BM populations were isolated and prepared for flow cytometry as described above. The most primitive populations were phenotypically defined as long-term HSC, short-term HSC, multipotential progenitors, common lymphoid progenitors and common myeloid progenitors (see supplementary Figures 1 and 2, available at Carcinogenesis Online). For these analyses, the following antibodies were also used: Flt3 (A2F10), IL7R (A7R34), Thy1.1 (HIS51), FcR (93) (eBiosciences, San Diego, CA) and CD34 (RAM34). Analyses of these populations were performed using a BD FACSVantage™ SE flow cytometer and FlowJo software.
Detection of apoptosis
Lin+ cells were isolated from BM as described above. For the detection of apoptotic cells, the Annexin V-fluorescein isothiocyanate Apoptosis Detection Kit I (BD Biosciences) was used according to the manufacturer's instructions. Cells that were in early apoptosis were Annexin V-fluorescein isothiocyanate positive (An+) and propidium iodide negative (PI−). An+ and PI+-stained cells were in end-stage apoptosis and death. An− and PI− cells were considered as viable cells.
Colony-forming unit assays
Mouse colony-forming unit (CFU)-granulocyte macrophage, CFU-granulocyte, CFU-macrophage and CFU-preB assays were performed using MethoCult™ medias (StemCell Technologies, Vancouver, British Columbia, Canada) according to the manufacturer's protocols. For the day 8 [colony-forming unit-spleen (CFU-S)d8] and day 12 (CFU-Sd12) CFU-S assay, TCDD- and olive oil-treated mice were euthanized at day 7 and marrow cells were isolated as described above. Recipient mice (6–8 weeks old) were irradiated with 9.0 Gy from a 137Cs γ-ray source. Four hours after irradiation, these mice were injected intravenously with 50 000 marrow cells from donor TCDD- or olive oil-treated mice. The host mice were euthanized after 8 or 12 days. Spleens were collected, fixed in Telleyesniczky's solution and nodular colonies were counted macroscopically. The high proliferative potential colony-forming cell (HPP-CFC) assay was performed using MethoCult™ M3231 medium (StemCell Technologies) with cytokines and growth factors according to the manufacturer's protocol. Colonies were counted after 14 days in culture. Total HPP-CFC colonies form in the presence of colony stimulating factor-1, stem cell factor, interleukin (IL)-1 and IL-3, whereas colonies generated from the more mature progenitors form in the absence of IL-1 and IL-3. Immature HPP-CFCs are determined as the total HPP-CFC minus mature.
Competitive repopulation experiments
LSK or LSK/CD34− cells from TCDD- or olive oil-treated mice that were CD45.2+ served as donor cells. Recipient CD45.1+ mice were irradiated with two split doses of 5.5 Gy 4 h apart. Three hours after irradiation, these mice were injected intravenously with 5000 LSK or 100 LSK/CD34− donor (CD45.2+) cells together with 200 000 CD45.1+ cells (competitive donor). Six and forty weeks after transplantation, mice were euthanized and BM cells, spleen cells and peripheral blood were collected. Cells were incubated with anti-CD45.2 antibody and subjected to flow cytometry to quantify the multilineage reconstitution of donor versus recipient cells. CD45.2+ cells were phenotyped using the following markers: BM—Sca-1, cKit, Thy1.2, B220, Gr-1 and Mac-1 and spleen and peripheral blood—CD4, CD8, B220, Gr-1 and Mac-1.
Homing experiments
Marrow mononuclear cells from TCDD- or vehicle-treated mice were harvested and resuspended (5 × 106 cells per ml) in Hank's balanced salt solution (CellGro, Lawrence, KS). Cells were labeled with carboxyfluorescein diacetate succinimidyl ester (Invitrogen) at a concentration of 1 μM for 10 min at 37°C. Cells were washed with ice-cold 2% fetal bovine serum Hank's balanced salt solution. Two million cells in 0.2 ml of PBS were injected into the tail vein of previously irradiated mice. Six to twenty h after reconstitution, BM and spleen cells were prepared and suspended in 2% fetal bovine serum in PBS (staining buffer). Red blood cells were lysed, washed once and resuspended in staining buffer and counted, and aliquots (1 × 106 cells per ml) were fixed in 1% paraformaldehyde in PBS. The percentage of carboxyfluorescein diacetate succinimidyl ester+ cells was obtained using channel FL1 of the flow cytometer. Analysis was done using a FACSCalibur™ flow cytometer and FlowJo software. The percentage of homing was calculated as described previously (33).
Analysis of BM after treatment with 5-fluorouracil or cytokines and granulocyte-colony stimulating factor
Forty-eight hours after 5-fluorouracil (5-FU) treatment (150 mg/kg), mice were additionally treated with TCDD (30 μg/kg) or olive oil. Mice were killed at days 3, 5, 9, 11 and 16 days after 5-FU treatment, and BM subsets were quantified as described earlier. In separate experiments, mice were treated subcutaneously once daily for three consecutive days with PBS alone, 15 μg IL-6 per kg + 15 μg granulocyte-colony stimulating factor (G-CSF) per kg or 15 μg IL-11 per kg + G-CSF. Relative levels of Ahr mRNA were determined using real-time PCR as described above.
Bromodeoxyuridine incorporation experiments
For studies examining bromodeoxyuridine (BrdU) incorporation, experiments were conducted as described for the in vivo phenotyping studies with the addition that 2 h before euthanasia mice were injected with 0.1 ml (10 mg/ml solution) of BrdU (Sigma) in PBS. According to the manufacturer's instructions, the Cytofix/Cytoperm Kit and FastImmune anti-BrdU DNAse (BD Biosciences) treatment were used for total BM cells stained against BrdU and fluorescent-tagged B220, Mac-1, Gr-1 or Ter-119 and Lin− cells stained against BrdU, Sca-1 and cKit.
Statistical analysis
Statistical significance was determined by utilization of the two-tailed Student's t-test, with significance level set at P ≤ 0.05.
Results
Exposure to TCDD elicits phenotypic changes in HSC populations
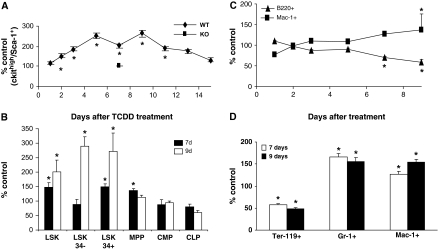
Following the exposure of mice to TCDD, the number of LSK progenitor cells increased, peaking between days 3 and 11 following treatment (Figure 1A). As observed previously (27), an analysis of BM from Ahr−/− mice at day 7 following TCDD treatment indicated no alterations in these cell populations. Analyses of the LSK population at days 7 and 9 assessing the expression of CD34 and Flt3 (supplementary Figures 1 and 2 are available at Carcinogenesis Online) demonstrated that increases in phenotypically defined short-term HSCs and long-term HSCs mainly contributed to the increase in LSK cells (Figure 1B). The increase in multipotential progenitors was marginal and there were no consistent alterations in the phenotypically defined common lymphoid progenitor and common myeloid progenitor populations. As determined previously (19,23,25–27,29), there were no differences in total BM cellularity between TCDD- and vehicle-treated samples (data not shown).
Fig. 1.
TCDD exposure causes changes in marrow cell populations. (A) Time-dependent changes in relative numbers of LSK cells following treatment of wild-type or Ahr-KO mice (four per group per time point) with 30 μg TCDD/kg. Animals were euthanized at the indicated days following treatment, and isolated marrow cells were stained and analyzed by flow cytometry as described in Materials and Methods. (B) Analysis of stem and progenitor cells subsets (see supplementary Figures 1 and 2, available at Carcinogenesis Online) at days 7 and 9 following treatment. (C) Time-dependent changes in the relative numbers of B220+ and Mac-1+ cells following treatment with TCDD. (D) Analysis of Mac-1+, Gr-1+ and Ter-119+ subsets in BM at days 7 and 9 following treatments. *Significantly different from vehicle-treated control (P ≤ 0.05).
AhR activation results in altered numbers of committed lineage cells
The above and previous data indicate that AhR activation by TCDD increases the number of HSCs, yet decreases the number of lymphoid lineage cells (19,23,25–27). It is possible that lineage differentiation is being shifted, either actively or by default in the absence of lymphoid-directed regulatory signals, toward the myeloid lineage. Observed increases in Mac-1+ and Gr-1+ cells in peripheral blood following TCDD treatment (34) are consistent with this. We observed a time-dependent increase in Mac-1+ cells in BM, with a corresponding decrease in B220+ cells (Figure 1C). At 7 and 9 days after TCDD treatment, the numbers of macrophage lineage (cKit−/Sca-1−/Mac-1+) and granulocyte lineage (cKit−/Sca-1−/Gr-1+) cells were significantly increased, whereas Ter-119+ cells were decreased (Figure 1D).
Increased rates of apoptosis could be occurring in cells that make up the immature lymphoid lineage, resulting in decreased prothymocytes (25,26) and immature B cells (19). Conversely, the degree of apoptosis in myeloid-committed cells could be decreased. However, analysis of B220, Ter-119, Gr-1 or Mac-1 lineage cells indicated no changes in apoptosis that could account for increased numbers of myeloid or decreased numbers of B-lineage cells (data not shown).
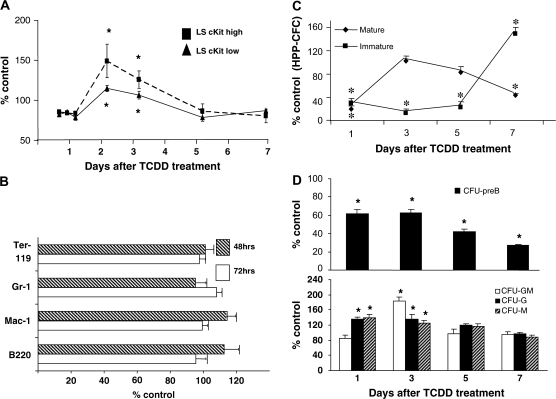
AhR activation elicits increased proliferation of LSK progenitors but not lineage cells
We assessed cellular proliferation by measuring incorporation of BrdU into newly synthesized DNA. Beginning 48 h after TCDD exposure, a substantial increase in BrdU incorporation was seen in LSK progenitor cells (Figure 2A). This suggests that TCDD treatment increases the number of progenitor cells in S phase of the cell cycle. This is consistent with previous data showing a substantially decreased number of LSK cells in quiescence (G0) following TCDD treatment (31). The level of BrdU incorporation into Gr-1+, Mac-1+ or Ter-119+ lineage-committed cell types was not significantly different between TCDD- and vehicle-treated animals (Figure 2B). These data suggest that LSK cell accumulation following TCDD treatment is due, at least in part, to increased cell proliferation.
Fig. 2.
AhR activation alters BrdU incorporation into primitive progenitor cells and alters the number of functional lineage-restricted progenitors. (A) Incorporation of BrdU into LSK cells. Cells were isolated from vehicle- or TCDD-treated mice at various times and a 2 h pulse of BrdU and were stained and analyzed as described in Materials and Methods. Cells were assessed as the number of cells staining positive for BrdU divided by the total number of cells present of that particular phenotype, thus controlling for increased numbers of progenitor cells at later time points. Data are expressed as percentage of vehicle-treated values. Data are an average of three separate experiments, with four mice per group for experiment ± standard error, n = 12. (B) Incorporation of BrdU into lineage cell populations. Cells were harvested from TCDD- or vehicle-treated mice after 48 or 72 h of treatment and a 2 h pulse of BrdU, stained for lineage-specific markers and analyzed as described in Materials and Methods. Data are an average of two separate experiments, with four mice per treatment group ± standard error, n = 8. (C) Marrow cells were isolated at different times after TCDD treatment and HPP-CFC in vivo was determined as described in Materials and Methods. Data presented are as the mean ± standard error of percent control values. *Significantly different from vehicle-treated control (P < 0.05). (D) Marrow cells were prepared from mice at various times after TCDD exposure and short-term colony assays were performed for CFU-preB, CFU-granulocyte macrophage (GM), CFU-granulocyte (G), and CFU-macrophage (M). Data are expressed as percent of vehicle-treated values and are the average of three separate experiments with four mice per experiment ± standard error for n = 12. *Significantly different from vehicle-treated control (P ≤ 0.05).
AhR activation alters the number of functional multipotent and lineage-restricted progenitors
The above observations suggest that following AhR activation by TCDD, lineage differentiation is skewed away from the lymphoid lineage in favor of granulocyte/macrophage lineages. If this is the case, one might expect to see an altered number of lineage-restricted progenitors. Although we did not observe changes in the numbers of phenotypically defined common lymphoid progenitors and common myeloid progenitors, the expression of particular markers may not correspond to functional potential. Consistent with findings of a decrease in B-lineage populations (19,23), the number of functional CFU-preB progenitors decreased over time (Figure 2D). In contrast, there were transient, but significant, increases in CFU-granulocyte, CFU-macrophage and CFU-granulocyte macrophage at days 1 and/or 3 following treatment (Figure 2D) that preceded the increase in Mac-1+ and Gr-1+ cells in marrow.
To further examine the effects of TCDD on functional progenitor populations, we used the in vivo CFU-S assay. Whereas colonies that appear on day 8 (CFU-Sd8) originate from committed myeloerythroid progenitors, multipotent progenitors generate colonies 12 days after transplantation (CFU-Sd12) (35,36). There was a significantly decreased number of CFU-Sd8 colonies arising from cells taken from mice at day 7 following TCDD treatment (supplementary Table 1 is available at Carcinogenesis Online). Since CFU-Sd8 is a function of the combined myeloid and erythroid progenitors, it is possible that this reflects a decreased erythropoiesis since Ter-119 cells were significantly decreased by TCDD treatment (Figure 1D). A previous study found a significant inhibition of erythropoiesis in zebra fish following TCDD exposure (37). Consistent with an increase in the number of LSK progenitor cells and phenotypically defined multipotential progenitors (Figure 1B), there was an increase in CFU-Sd12 at day 7 following TCDD treatment. Overall, these data are consistent with TCDD eliciting an increased presence of multipotent progenitors that have a diminished capacity for differentiation into the lymphoid lineage.
To assess the possible effects of TCDD on immature progenitor cell populations, we performed an HPP-CFC assay. HPP-CFCs are among the most primitive hematopoietic progenitors grown under culture conditions and their assessment has been used as a short-term assay of stem cell potential (38,39). Marrow taken from TCDD-treated mice exhibited a time-dependent decrease in total HPP-CFC, with the most consistent and early effect being on immature populations of these cells (Figure 2C).
HSCs from TCDD-treated mice have a diminished functional capacity
TCDD treatment led to a substantially decreased short-term (6 week) and long-term (40 week) reconstitution activity of either LSK or LSK/CD34− cells (Table IA). The total number of BM cells, the relative percentage of CD45.2+ donor cells and the total CD45.2+ donor cells were all decreased in mice receiving HSCs from TCDD-treated animals. These data are consistent with results from the HPP-CFC experiments (Figure 2C) suggesting effects of TCDD on the functionality of immature multipotent progenitor cells. These data are also consistent with results from a previous investigation that observed an almost total loss in the ability of LSK or LSK/CD34− cells from TCDD-treated animals to reconstitute white cells in peripheral blood of lethally irradiated recipients up to 20 weeks following transplantation (30). However, in that study, the numbers of donor cells in the BM of recipient animals were not assessed. A lineage analysis of the reconstituting BM cells in our study revealed that although the total number of donor CD45.2+ cells of the TCDD-treated group was substantially decreased, the relative distribution of these donor cells within the lineage-committed subsets was not significantly different (Table IB). A similar pattern was observed at 40 weeks following reconstitution of LSK/CD34− cells (data not shown). However, like TCDD-treated animals (Figure 1), the 6-week-recipient mice also demonstrated an increase in the relative percentage of donor CD45.2+ Lin-Sca-1+ and LSK cells in BM (Table IB). Overall, these data suggest that in addition to less donor HSCs from the TCDD-treated group being present in recipient marrow, the HSCs that are there lack full capability to further differentiate.
Table I.
TCDD treatment results in decreased competitive reconstitution and homing ability of HSC
| A. Competitive reconstitution | ||||||
| Treatment of donor animals | Total BM cells per hind limb (× 106) | Percentage of CD45.2+ donors | Total CD45.2+ cells per hind limb (× 106) | |||
| Donor cells = LSK cells (6 weeks) | ||||||
| TCDD (30 μg/kg) | 35 ± 8* | 26 ± 3.7* | 9 ± 2.8* | |||
| Vehicle | 56 ± 20 | 53 ± 4.7 | 30 ± 4.5 | |||
| Donor cells = LSK/CD34− (6 weeks) | ||||||
| TCDD (40 μg/kg) | 22 ± 1.9* | 22 ± 1.0* | 5 ± 0.4* | |||
| Vehicle | 82 ± 7.8 | 27 ± 2.3 | 23 ± 1.0 | |||
| Donor cells = LSK/CD34− (40 weeks) | ||||||
| TCDD (40 μg/kg) | 36 ± 11* | 10 ± 1.3 | 3.5 ± 1.2* | |||
| Vehicle | 106 ± 13 | 19 ± 8.4 | 18 ± 6.2 | |||
| B. Lineage development of LSK donor cells (percentage of CD45.2+ cells in BM subsets) (6 weeks) | ||||||
| Treatment | Lin−/cKit+/Sca-1+ | Lin−/cKit+ | Thy1.2+ | B220+ | Gr-1+ | Mac-1+ |
| TCDD | 22 ± 3.4* | 36 ± 3.3 | 39 ± 5.5 | 35 ± 3.6 | 63 ± 4.0 | 86 ± 4.4 |
| Vehicle | 6 ± 0.5 | 32 ± 0.4 | 34 ± 2.2 | 29 ± 1.6 | 58 ± 5.8 | 83 ± 2.8 |
| C. Homing of hematopoietic cells to BM or spleen (percentage of homing) | ||||||
| Treatment | BM | Spleen | ||||
| TCDD (30 μg/kg) | 14 ± 3.0* | 34 ± 6.7 | ||||
| Vehicle | 23 ± 1.7 | 27 ± 3.4 | ||||
A and B: donor CD45.2+ mice received a single dose of TCDD or olive oil, and at day 7, BM was isolated and LSK and LSK/CD34− subsets were purified. Three hours after irradiation, CD45.1+ recipient mice were injected intravenously with 5000 LSK or 100 LSK/CD34− donor cells together with 2 × 105 CD45.1+ competitor mononuclear BM cells. Recipient mice were killed at 6 or 40 weeks following transplantation and the presence of CD45.2+ donor cells in BM and BM subsets was assessed by flow cytometry. Values are mean ± standard error of n = 3—six mice per group. *Indicates statistically different (P < 0.05) from vehicle-treated group. C: donor mice (n = 6) were treated with TCDD or olive oil. At day 7, mononuclear BM cells were harvested, incubated with carboxyfluorescein diacetate succinimidyl ester and transplanted into recipient mice. After 24 h, BM and spleen cells were harvested and analyzed using flow cytometry. The percentage of homing was calculated using (A × B/C) × 100%, where A = percent carboxyfluorescein diacetate succinimidyl ester cells determined by flow cytometry, B = total organ cellularity and C = 2 × 106 transplanted cells. *P-value < 0.05.
We examined the possibility that the TCDD-elicited defect in reconstitution was due to an effect on homing of cells to BM. Using carboxyfluorescein diacetate succinimidyl ester-labeled cells, we observed a substantially decreased ability of cells from TCDD-treated animals to home to BM following injection into irradiated recipients (Table IC). This suggests that a homing defect is a major contributor to the decreased ability of HSCs from TCDD-treated animals to repopulate irradiated recipients.
TCDD exposure does not affect lineage reconstitution of HSCs in mice treated with 5-FU
To further examine the ability of TCDD to affect HSC/progenitor proliferation and differentiation into lineage cells, we used 5-FU to ablate rapidly dividing progenitor and lineage populations as well as stimulate the proliferation and differentiation of HSCs (40). Following 5-FU treatment, we treated the animals with TCDD at a time (48 h) when there is near maximal ablation of rapidly dividing progenitor and lineage cells and when HSCs are beginning to rapidly proliferate and differentiate. TCDD treatment had no effect on either the time course or the lineage specificity of reconstitution (supplementary Figure 3 is available at Carcinogenesis Online). This suggests that under conditions in which the hematopoietic system is stimulated to replenish its constituents, HSCs are not susceptible to the same effects elicited by TCDD-induced AhR activation observed under resting conditions.
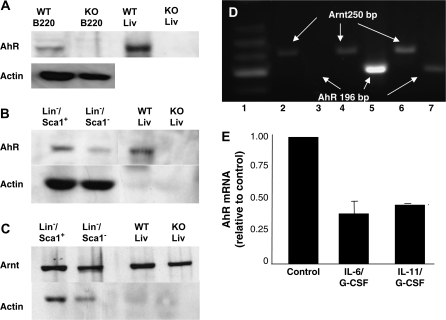
Expression of AhR in BM cell subpopulations
Using chimeric mice, we demonstrated previously that a TCDD-elicited increase in the number of LSK cells was dependent on AhR presence in hematopoietic but not stromal cells (27,28). Although AhR protein is present in crude BM (41), its presence in lineage precursor subpopulations has not been assessed. AhR protein, determined by western blotting, was present in B220+ cells from wild-type but not AhR-KO mice (Figure 3A). Both AhR protein and Arnt protein are expressed in both Lin−/Sca-1+ and Lin−/Sca-1− populations (Figure 3B and C). AhR protein is more highly expressed in the Lin−/Sca-1+ subset, whereas Arnt protein appears to be expressed at approximately equivalent levels in both Lin− subsets. Given the number of animals (40) required to obtain these data, we did not attempt an analysis of more immature subsets that are present in smaller numbers. However, as determined by reverse transcription–PCR, Ahr message was expressed in total BM as well as in Lin−, Lin−/Sca-1+ and LSK subsets (Figure 3D).
Fig. 3.
AhR and Arnt proteins and mRNA are expressed in murine BM subsets, and conditions that stimulate HSC proliferation downregulate Ahr mRNA. (A) Western blot of protein from B220+ cells. Lane 1, wild-type (WT); lane 2, AhR-KO; lane 3, wild-type liver (positive control) and lane 4, AhR-KO liver. (B and C) AhR and Arnt in Lin− cell subsets. Lane 1, Lin−/Sca-1+; lane 2, Lin−/Sca-1−; lane 3, wild-type liver and lane 4, AhR-KO liver. Due to the use of higher protein concentrations in the BM samples (100 μg per lane BM versus 10 μg for liver), the actin levels in the liver samples are much lower than that of the BM samples. (D) Subsets were isolated by magnetic and flow sorting as described in Materials and Methods. RNA from ∼50 000 cells was prepared and reverse transcription–PCR performed using primers spanning regions 466–661 of the Ahr gene (accession # NM_013464) (18) and 88–337 of the Arnt gene (accession # NM_001037737). Lane 1 = low molecular weight ladder. Lanes 2, 4 and 6 are for Arnt. Lanes 3, 5 and 7 are for AhR. Lanes 2 and 3 = LSK from KO mice. Lanes 4 and 5 = Lin−/Sca-1+ from wild-type mice. Lanes 6 and 7 = LSK cells from wild-type mice. (E) Mice were treated subcutaneously once daily for three consecutive days with PBS alone, 15 μg IL-6/kg + 15 μg G-CSF/kg or 15 μg IL-11/kg + G-CSF. Total RNA was isolated from Lin−/Sca-1+ cells. Relative levels of Ahr mRNA were determined using real-time PCR and were normalized to glyceraldehyde-3-phosphate dehydrogenase. These values were then normalized to the PBS control values. Data expressed are mean ± standard error of three separate experiments. Each experiment was performed on RNA isolated and pooled from two mice.
The lack of response to TCDD in 5-FU-treated mice noted above is consistent with data indicating that the Ahr is among those genes downregulated during the proliferation phase of HSCs following treatment of mice with 5-FU, but expressed during periods of quiescence (42). If this is the case, then the Ahr gene should also be downregulated under other conditions that stimulate HSC proliferation. To test this, mice were treated with growth factors and cytokines known to stimulate hematopoiesis. Following daily treatments with IL-6/G-CSF or IL-11/G-CSF, Ahr mRNA was significantly decreased in Lin−/Sca-1+ cells (Figure 3E). These data, together with the previous report (42), suggest that Ahr gene expression is regulated under conditions that affect the proliferation of HSCs.
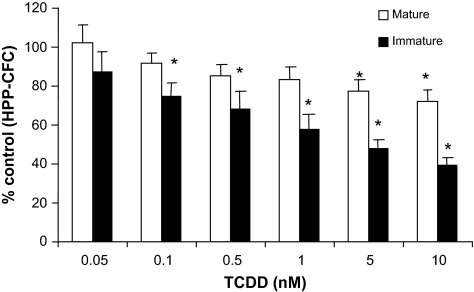
AhR activation directly alters progenitor cell potential in vitro
The above data are consistent with the hypothesis that TCDD, through its ability to activate AhR in HSCs, affects the functional capacity of these cells. The ability of TCDD to directly affect hematopoietic progenitor cell potential was assessed using short-term functional assays. When BM cells were grown under conditions to assess HPP-CFC and exposed to TCDD, there was a concentration-dependent reduction in colonies formed by both mature and immature populations, although the effect, consistent with data obtained following treatment of mice with TCDD (Figure 2C), was greatest on the more immature cells (Figure 4). The addition of TCDD to cultures of cells under conditions to assess CFU-preB, CFU-granulocyte macrophage, CFU-macrophage, CFU-granulocyte and CFU-erythroid had no significant effect on the growth of these cells (data not shown). Together, these data suggest that immature hematopoietic progenitors are directly sensitive to TCDD, with a decrease or loss in sensitivity as maturity increases.
Fig. 4.
TCDD inhibits the colony-forming abilities of HPP-CFC in a concentration-dependent manner in vitro. Marrow cells from mice (n = 20) were separately plated in complete MethoCult media supplemented with colony stimulating factor-1, stem cell factor, IL-1a and IL-3 and exposed to vehicle (dimethyl sulfoxide) or TCDD and described in Materials and Methods. Data are presented as mean values ± standard error. TCDD values were significantly different from dimethyl sulfoxide control (*P < 0.05).
Discussion
These studies demonstrate that TCDD causes an increase in the number of phenotypically defined stem cells in mouse BM. That this persists for as long as 35 days following a single administration (29) is probably due to the long half-life of TCDD (43). The increase in the number of these cells is due, at least in part, to their increased proliferation. This is supported by increased BrdU incorporation (Figure 2) and a decreased percentage of LSK cells in G0 following TCDD exposure (31). In addition, the analyses of BM reconstitution, homing and effects on committed lineage populations strongly suggest that stem cell functions are altered by AhR activation.
Our studies are consistent with those published previously (30), demonstrating that TCDD substantially inhibits HSC function determined by the ability of these cells to repopulate BM in irradiated animals. Our data further suggest that this is primarily due to the inability of these cells to home to BM. It is becoming appreciated that microenvironment-dependent signaling is critical for maintaining the balance between stem cell quiescence, division, differentiation and trafficking within the BM or to other environments for further differentiation and maturation. AhR agonists are known to alter the expression of cell surface proteins and adhesion molecules including those in the cadherin and integrin family (44), as well as β-catenin (45). An attractive hypothesis is that AhR activation in HSCs alters the expression of these or other molecules, resulting in impaired signaling from soluble factors or stromal cells with the marrow niche resulting in decreased homing ability. A recent publication reported that TCDD downregulates the expression of mRNAs for the G-protein-coupled receptor, CXCR4, and its chemokine ligand CXCL12 (SDF-1) in MCF-7 breast cancer cells (46). These molecules are particularly important for HSC homing to and movement within the marrow microenvironment. Early lymphopoiesis is also believed to occur in specific marrow niches controlled by factors within the microenvironment (47). Altered expression of niche-sensing molecules in progenitors may be responsible for the observed TCDD-induced loss of terminal deoxynucleotidyl transferase- and recombinase-activating gene-positive cells in marrow (25,26), loss of developing B cells (23) (Figure 1), decreased CFU-preB (Figure 2), and decreased thymic seeding (25,26). Lacking sufficient signals for differentiation toward the lymphoid lineage, increased numbers of progenitors might then proceed along a default myeloid lineage pathway as compared with what might occur under normal ‘resting’ conditions (48) and as we observed in response to TCDD (Figure 1). Likewise, a TCDD-induced increase number and proliferation of LSK precursor cells may occur due to altered niche binding and loss of quiescence (31,49). Notably, the CXCR4/CXCL12-signaling pathway is also important for the maintenance of the quiescent HSC pool (50). Thus, our data and previous work are consistent with the postulate that AhR dysregulation by TCDD results in the altered ability of HSCs to sense appropriate signals in the BM, leading to altered cycling, trafficking and differentiation potential. Furthermore, this suggests that the AhR may regulate critical genes within HSCs that allow them to differentially respond to signals in their environment. Clearly, however, more work is needed to identify these genes and signaling pathways and demonstrate their relationship with HSC functions.
The data presented here add to previous data consistent with the notion that TCDD alters the function of HSCs through the ability to affect AhR activity within these cells. The AhR is present in phenotypically defined progenitors (Figure 3). As determined by the use of chimeric mice, AhR presence in hematopoietic cells, but not stromal cells, is essential for the increase in HSC number elicited by TCDD (27,30) as well as altered stem cell reconstitution activity (30). In addition, although treatment of mice with TCDD affects developing B cell numbers (Figures 1 and 2), committed B-lineage cells are not affected by direct exposure (24), suggesting a primary effect on more immature precursors. TCDD treatment alters gene expression in hematopoietic precursors (31). TCDD also directly affects the growth of HPP-CFCs (Figure 4), but not more mature lineage-restricted progenitors, under conditions in vitro. Finally, the finding that no effects of TCDD were observed when stem cell populations are stimulated to proliferate following treatment of animals with 5-FU (supplementary Figure 3 is available at Carcinogenesis Online) is also consistent with the argument that, under resting conditions, i.e. without 5-FU treatment, TCDD acts directly on the AhR within HSCs. The Ahr is among those genes turned off during the proliferation phase of HSCs following treatment with 5-FU but is expressed during periods of quiescence (42). Our data using growth factors to stimulate HSC proliferation (Figure 3) are consistent with this.
That Ahr expression in HSCs may be regulated under conditions of quiescence and proliferation has several implications. First, HSC susceptibility to xenobiotic AhR ligands probably depends on the marrow environment. Thus, conditions in which HSCs are stimulated to proliferate may render these cells less susceptible to toxic AhR ligands, similar to what we observed following 5-FU treatment. This may also be a protective mechanism against chemicals that can be metabolized to mutagenic intermediates by the cytochrome P450 isozymes regulated in part by the AhR. If the AhR is downregulated by increased cycling and proliferation of HSCs, then, since under resting conditions some small percentage of HSCs are normally cycling, there may be a subpopulation of HSCs that are less susceptible to TCDD. This might explain our finding that although TCDD substantially inhibits the ability of cells to repopulate the BM in irradiated animals, a percentage of HSCs that did home to BM appeared to differentiate normally (Table I). These data are also consistent with a number of studies indicating that the AhR has a normal functional role in cell cycle (13,51). More specifically for HSCs, the AhR may be important for regulating the balance between quiescence and proliferation by acting as a negative regulator of hematopoiesis with a function of curbing excessive or unnecessary proliferation. That the Ahr gene is downregulated during the proliferation phase of HSCs following 5-FU treatment (42) is consistent with this if buffering AhR activity is necessary for proliferation of normally functioning stem cells to proceed. This concept is supported by additional data we obtained, indicating that LSK cells from AhR-KO mice are hyperproliferative with a significantly greater percentage in G1/S compared with that from wild-type animals (R.W. Garrett and T.A. Gasiewicz, unpublished observations). In agreement with this, a recent publication indicated that the Ahr gene promoter is hypermethylated in human acute lymphoblastic leukemia cells (52); the authors postulated that the Ahr gene is silenced by hypermethylation and that the AhR could be a cell-specific negative regulator of cell proliferation.
The data presented here are consistent with the ability of TCDD via the AhR to affect the functions of immature hematopoietic lineage precursor cells. Although the BM reconstitution studies suggest effects on both short- and long-term HSCs, the data do not allow us to distinguish whether the effects of TCDD are greater on one versus the other or whether the effects are exclusively on these populations versus multipotent progenitor populations as well. This is a reflection of the highly dynamic nature of these populations and the likelihood that our understanding of these, based on the expression of cell surface markers, is very much oversimplified and not representative of their actual functions (53). Furthermore, although, as indicated above, all the available data are consistent with the effect of TCDD on these populations being subsequent to modulation of AhR activity in these cells, we cannot completely rule out the possibility that these actions occur, qualitatively or quantitatively, in conjunction with AhR activation in other cell types, i.e. stromal cells, that are important for regulating functions of HSCs. Nevertheless, it is reasonable to hypothesize that the ability of TCDD to affect HSC functions may be linked to reports of increased incidence of leukemia and lymphoma in populations accidentally exposed to TCDD (2–4). A corollary to the postulate that the AhR has normal role as a negative regulator of HSCs is that dysregulation of AhR expression and/or activity may be associated with the etiology and/or progression of certain hematopoietic diseases including cancers. It has been suggested that modulated AhR activity provides a permissive environment for the selection and expansion of tumor cell clones in human lymphoblastic leukemia (52). Clearly, more work is needed to define these relationships and the mechanisms by which they occur.
In summary, based on the cumulative data presented here and elsewhere (19,23), we favor the hypothesis that TCDD, through AhR activation and modulation of critical genes within HSCs, alters the ability of these cells to respond to signals in their microenvironment. This results in altered HSC numbers and function. Additional investigations are needed to determine the possible link between the actions of TCDD on HSCs and the many functional effects elicited by this chemical on the immune system. It is not without precedent to further propose that other tissue stem cell populations may be similarly sensitive to the actions of xenobiotic AhR ligands. Recently, it was suggested that TCDD-induced activation of skin stem cells and a shift in differentiation commitment of their progeny may represent a primary mechanism for the ability of this chemical to produce chloracne in both humans and animals (54). TCDD has also been shown to disrupt the maturation of granule neuron precursor cells (55). Finally, these and previous data are also consistent with the hypothesis that this bHLH-PAS protein may have a physiological role in regulating quiescence and proliferation of hematopoietic precursors and that dysregulation of this role may contribute to the etiology of certain hematopoietic cancers.
Supplementary material
Supplementary Figures 1–3 and Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (ES04862), Training Grant ES07026 and Center Grant ES01247.
Supplementary Material
Acknowledgments
We thank the members of the Gasiewicz lab, as well as Drs Michael Laiosa and Michael McCabe for their critical reading of this manuscript.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AhR
aryl hydrocarbon receptor
- AhR-KO
AhR null-allele
- Arnt
aryl hydrocarbon receptor nuclear translocation
- BM
bone marrow
- BrdU
bromodeoxyuridine
- CFU
colony-forming unit
- CFU-S
colony-forming unit-spleen
- 5-FU
5-fluorouracil
- HPP-CFC
high proliferative potential colony-forming cell
- HSC
hematopoietic stem cell
- IL
interleukin
- LSK
Lin−/Sca-1+/cKit+
- mRNA
messenger RNA
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
References
- 1.Mimura J, et al. Functional role of AhR in the expression of toxic effects by TCDD. Biochim. Biophys. Acta. 2003;1619:263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 2.Zheng T, et al. Occupation and risk of non-Hodgkin's lymphoma and chronic lymphocytic leukemia. J. Occup. Environ. Med. 2004;44:469–544. doi: 10.1097/00043764-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Consonni D, et al. Mortality in a population exposed to dioxin after the Seveso, Italy, accident in 1976: 25 years of follow-up. Am. J. Epidemiol. 2008;167:847–858. doi: 10.1093/aje/kwm371. [DOI] [PubMed] [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Polychlorinated Dibenzo-Para-Dioxins and Polychlorinated Dibenzofurans. IARC Monogr. Eval. Carcinog. Risks Hum. 1997;69:1–631. [PMC free article] [PubMed] [Google Scholar]
- 5.Dertinger SD, et al. Infuence of aromatic hydrocarbon receptor-mediated events in the genotoxicity of cigarette smoke condensate. Carcinogenesis. 1998;19:2037–2042. doi: 10.1093/carcin/19.11.2037. [DOI] [PubMed] [Google Scholar]
- 6.Dertinger SD, et al. Aryl hydrocarbon receptor signaling plays a significant role in mediating benzo[a]pyrene- and cigarette smoke condensate-induced cytogenetic damage in vivo. Carcinogenesis. 2001;22:171–177. doi: 10.1093/carcin/22.1.171. [DOI] [PubMed] [Google Scholar]
- 7.Thatcher TH, et al. Aryl hydrocarbon receptor (AhR) deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the NF-κB component RelB. Am. J. Pathol. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, et al. Aryl hydrocarbon receptor gene polymorphisms affect lung cancer. Lung Cancer. 2007;56:9–15. doi: 10.1016/j.lungcan.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Knerr S, et al. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol. Nutr. Food Res. 2006;50:897–907. doi: 10.1002/mnfr.200600006. [DOI] [PubMed] [Google Scholar]
- 10.Nebert DW, et al. The role of cytochrome P450 enzymes in endogenous signaling pathways and environmental carcinogenesis. Nat. Rev. Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 11.Vogel CF, et al. Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am. J. Pathol. 2007;171:1538–1548. doi: 10.2353/ajpath.2007.070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bock KW, et al. Ah receptor- and TCDD-mediated liver tumor promotion: clonal selection and expansion of cells evading growth arrest and apoptosis. Biochem. Pharmacol. 2005;69:1403–1408. doi: 10.1016/j.bcp.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Huang G, et al. Multiple mechanisms are involved in Ah receptor-mediated cell cycle arrest. Mol. Pharmacol. 2005;67:88–96. doi: 10.1124/mol.104.002410. [DOI] [PubMed] [Google Scholar]
- 14.Holsapple MP, et al. Tetrachlorodibenzo-p-dioxin-induced changes in immunocompetence: possible mechanisms. Annu. Rev. Pharmacol. Toxicol. 1991;31:73–100. doi: 10.1146/annurev.pa.31.040191.000445. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence BP, et al. Immune modulation by TCDD and related polyhalogenated aromatic hydrocarbons. In: Luebke R, House RH, Kimber I, editors. Immunotoxicology and Immunophamacology. Boca Raton, FL: CRC Press; 2006. pp. 239–258. [Google Scholar]
- 16.Fernandez-Salguero PM, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Salguero PM, et al. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet. Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt JV, et al. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl Acad. Sci. USA. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurmond TS, et al. The aryl hydrocarbon receptor has a role in the in vivo maturation of murine bone marrow B lymphocytes and their response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 2000;165:227–236. doi: 10.1006/taap.2000.8942. [DOI] [PubMed] [Google Scholar]
- 20.Vorderstrasse BA, et al. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol. Appl. Pharmacol. 2001;171:157–164. doi: 10.1006/taap.2000.9122. [DOI] [PubMed] [Google Scholar]
- 21.Shi LZ, et al. The aryl hydrocarbon receptor is required for optimal resistance to Listeria monocytogenes infection in mice. J. Immunol. 2007;179:6952–6962. doi: 10.4049/jimmunol.179.10.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasiewicz TA, et al. Expression and activity of aryl hydrocarbon receptors in development and cancer. Crit. Rev. Eukaryot. Gene Expr. 2008;18:279–321. doi: 10.1615/critreveukargeneexpr.v18.i4.10. [DOI] [PubMed] [Google Scholar]
- 23.Thurmond TS, et al. A single dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin produces a time- and dose-dependent alteration in the murine bone marrow B-lymphocyte maturation profile. Toxicol. Sci. 2000;58:88–95. doi: 10.1093/toxsci/58.1.88. [DOI] [PubMed] [Google Scholar]
- 24.Wyman A, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin does not directly alter the phenotype of maturing B cells in a murine coculture system. Toxicol. Appl. Pharmacol. 2002;180:164–177. doi: 10.1006/taap.2002.9396. [DOI] [PubMed] [Google Scholar]
- 25.Fine JS, et al. Prothymocyte activity is reduced by perinatal 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. J. Pharmacol. Exp. Ther. 1990;255:128–132. [PubMed] [Google Scholar]
- 26.Fine JS, et al. Impairment of prothymocyte activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Immunol. 1990;144:1169–1176. [PubMed] [Google Scholar]
- 27.Staples JE, et al. Thymic alterations induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are strictly dependent on aryl hydrocarbon receptor activation in hemopoietic cells. J. Immunol. 1998;160:3844–3854. [PubMed] [Google Scholar]
- 28.Laiosa MD, et al. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J. Immunol. 2003;171:4582–4591. doi: 10.4049/jimmunol.171.9.4582. [DOI] [PubMed] [Google Scholar]
- 29.Murante FG, et al. Hemopoietic progenitor cells are sensitive targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J mice. Toxicol. Sci. 2000;54:374–383. doi: 10.1093/toxsci/54.2.374. [DOI] [PubMed] [Google Scholar]
- 30.Sakai R, et al. TCDD treatment eliminates the long-term reconstitution activity of hematopoietic stem cells. Toxicol. Sci. 2003;72:84–91. doi: 10.1093/toxsci/kfg002. [DOI] [PubMed] [Google Scholar]
- 31.Garrett RW, et al. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol. Pharmacol. 2006;69:2076–2083. doi: 10.1124/mol.105.021006. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt JV, et al. Molecular characterization of the murine Ahr gene. Organization, promoter analysis, and chromosomal assignment. J. Biol. Chem. 1993;268:22203–22209. [PubMed] [Google Scholar]
- 33.Szilvassy SJ, et al. Homing and engraftment defects in ex vivo expanded murine hematopoietic cells are associated with downregulation in b1 integrin. Exp. Hematol. 2001;29:1494–1502. doi: 10.1016/s0301-472x(01)00751-2. [DOI] [PubMed] [Google Scholar]
- 34.Weissberg JB, et al. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin upon hemostasis and hematologic function in the rat. Environ. Health Perspect. 1973;5:119–123. doi: 10.1289/ehp.7305119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison SJ, et al. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 36.Na Nakorn T, et al. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J. Clin. Invest. 2002;109:1579–1585. doi: 10.1172/JCI15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belair CD, et al. Disruption of erythropoiesis by dioxin in the zebra fish. Dev. Dyn. 2001;222:581–594. doi: 10.1002/dvdy.1213. [DOI] [PubMed] [Google Scholar]
- 38.Kriegler AB, et al. The relationship between different high proliferative potential colony-forming cells in mouse bone marrow. Exp. Hematol. 1994;22:432–440. [PubMed] [Google Scholar]
- 39.Chang H, et al. Standardization of hematopoetic stem cell assays: a summary of a workshop and working group meeting sponsored by the National Heart, Lung, and Blood Institute held at the National Institutes of Health, Bethesda, MD on September 8–9, 1998 and July 30, 1999. Exp. Hematol. 1999;28:743–752. doi: 10.1016/s0301-472x(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 40.Randall TD, et al. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood. 1997;89:3596–3606. [PubMed] [Google Scholar]
- 41.Lavin AL, et al. Expression of functional aromatic hydrocarbon receptor and aromatic hydrocarbon nuclear translocator proteins in murine bone marrow stromal cells. Arch. Biochem. Biophys. 1998;352:9–18. doi: 10.1006/abbi.1998.0587. [DOI] [PubMed] [Google Scholar]
- 42.Venezia TA, et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2:1640–1651. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasiewicz TA, et al. Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab. Dispos. 1983;11:397–403. [PubMed] [Google Scholar]
- 44.Johnson CD, et al. Unraveling gene-gene interactions regulated by ligands of the aryl hydrocarbon receptor. Environ. Health Perspect. 2004;112:403–412. doi: 10.1289/ehp.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez JM, et al. Differential toxicogenomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin in malignant and nonmalignant human airway epithelial cells. Toxicol. Sci. 2002;69:409–423. doi: 10.1093/toxsci/69.2.409. [DOI] [PubMed] [Google Scholar]
- 46.Hsu EL, et al. A proposed mechanism for the protective effect of dioxin against breast cancer. Toxicol. Sci. 2007;98:436–444. doi: 10.1093/toxsci/kfm125. [DOI] [PubMed] [Google Scholar]
- 47.Tokoyoda K, et al. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Kondo M, et al. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 49.Arai F, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Nie Y, et al. CXCR4 is required for quiescence of primitive hematopoietic cells. J. Exp. Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge NL, et al. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J. Biol. Chem. 1998;273:22708–22713. doi: 10.1074/jbc.273.35.22708. [DOI] [PubMed] [Google Scholar]
- 52.Mulero-Navarro S, et al. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis. 2006;27:1099–1104. doi: 10.1093/carcin/bgi344. [DOI] [PubMed] [Google Scholar]
- 53.Orkin SH, et al. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panteleyev AA, et al. Dioxin-induced chloracne—reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp. Dermatol. 2006;15:705–730. doi: 10.1111/j.1600-0625.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 55.Collins LL, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure disrupts granule neuron precursor maturation in the developing mouse cerebellum. Toxicol. Sci. 2008;103:125–136. doi: 10.1093/toxsci/kfn017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.