Abstract
Background. Activation of the thrombospondin-1 (TSP-1)-TGF-β pathway by glucose and the relevance of TSP-1-dependent activation of TGF-β for renal matrix expansion, renal fibrosis and sclerosis have previously been demonstrated by our group in in vivo and in vitro studies.
Design and methods. We investigated renal biopsies (n = 40) and clinical data (n = 30) of patients with diabetic nephropathy. Ten kidneys without evidence of renal disease served as controls. Glomerular and cortical expression of TSP-1, p-smad2/3, fibrosis and glomerular sclerosis (PAS) were assessed by immunhistochemical staining and related with clinical data.
Results. Glomerular (g) and cortical (c) TSP-1 were increased during diabetic nephropathy (g: 2.62 ± 2.65; c: 4.5 ± 4.2) compared to controls (g: 0.67 ± 0.7; c: 1.5 ± 1.2). P-smad2/3 was significantly increased (g: 16.7 ± 12.9; c: 148.7 ± 92.8) compared to controls (g: 7.1 ± 3.6; c: 55 ± 25; P < 0.05). TSP-1 was coexpressed with p-smad2/3 as an indicator of TGF-β activation. TSP-1 correlated with enhanced tubulointerstitial p-smad2/3 positivity (r = 0.39 and r = 0.4, P < 0.05) and glomerular p-smad2/3 correlated with proteinuria (r = 0.35, P < 0.05).
Conclusions. In summary, the present study suggests a functional activity of the TSP-1/TGF-β axis, especially in the tubulointerstitium of patients with diabetic nephropathy. The positive correlation of glomerular p-smad2/3 positivity with proteinuria further supports the importance of the TSP-1/TGF-β system as a relevant mechanism for progression of human type-2 diabetic nephropathy.
Keywords: diabetic nephropathy, proteinuria, TGF-β, TSP-1, tubulointerstitium
Introduction
Diabetic nephropathy is the number one cause of end-stage renal failure in the western world [1]. Despite a steadily increasing number of diabetic patients, there exist few concepts to prevent disease progression and overt renal failure [2]. While predominant changes during early diabetic nephropathy include altered haemodynamics, hyperfiltration and hyperglycaemia, progressive glomerulosclerosis with the presence of Kimmelstiel–Wilson lesions and tubulointerstitial fibrosis can be found during later stages of disease [3,4].
TGF-β is considered the central cytokine for the development of diabetic nephropathy mediating glomerular hypertrophy, matrix expansion and glomerulosclerosis [5]. After its extracellular activation, TGF-β binds to the specific receptors TGF-β-R1 and -R2 mediating phosphorylation of transcription signalling molecules (p-smads) and subsequent transcription of target genes [6,7].
Thrombospondin-1 (TSP-1) is the founding member of a family of matricellular glycoproteins [8]. While predominantly in vitro studies have assigned diverse functions such as phagocytosis, adhesion, chemotaxis, proliferation and migration of cells to TSP-1 [8–10], it has also been identified as an activator of the TGF-β-procytokine complex. In vitro and in vivo studies by others [11,12] and our group [13–15] have shown that TSP-1 binds to the latent TGF-β-procytokine complex causing a conformational change and acting as an endogenous activator of TGF-β in inflammatory disease. Since we recently established TSP-1 as an endogenous activator of TGF-β in experimental diabetic nephropathy in vivo [15], this study aimed to examine the potential relevance of the TSP-1/TGF-β axis for human type-2 diabetic nephropathy.
In the present study, we evaluated the spatial relationship of glomerular as well tubulointerstitial expression of TSP-1 and subsequent TGF-β signalling in biopsies from patients with type-2 diabetic nephropathy and correlated these biopsy data with each other and with clinical data of disease progression.
Material and methods
Source of tissue
Archival tissues from core needle biopsies performed between 1992 and 2003 at the Klinikum Nuremberg (Nuremberg, Germany) were used for this study (n = 40). The morphological diagnosis of diabetic nephropathy was made by the local pathologist unaware of study findings. Each diagnosis was confirmed by the past medical history of diabetes, thereby excluding patients with type 1 diabetes. Control tissues without evidence of renal disease (n = 10) were obtained from distant portions of kidneys surgically excised because of the presence of a localized neoplasm.
Tissue processing and immunohistochemical staining
All biopsies were fixed in 3% paraformaldehyde, embedded in paraffine, and cut into 4 μm sections for indirect immunoperoxidase staining according to standard protocols. Negative controls for immunostaining included either deletion of the primary antibody or substitution of the primary antibody with equivalent concentrations of an irrelevant murine or rabbit mAb. Specific rabbit anti-mouse and (all DakoCytomation, Hamburg, Germany) goat anti-rabbit POD-conjugated (Dianova, Hamburg, Germany) secondary antibodies were applied followed by colour development with AEC (3-amino-9-ethylcarbazole; DakoCytomation), a ready-to-use substrate chromogen.
To perform immunoperoxidase staining, tissue sections were incubated with the following primary and secondary antibodies as indicated at 4°C overnight.
Antibodies used in this study
Antibodies used in this study were Clone A6.1, a murine IgG1 mAb against TSP-1 (Dunn, Asbach, Germany) [15]; a rabbit polyclonal antibody against phosphorylated smad2/3 (p-smad2/3) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) [15] and a rabbit polyclonal antibody against TGF-β1/2 (Santa Cruz Biotechnology Inc.) [15]. PAS stains were used to evaluate glomerulosclerosis and tubulointerstitial fibrosis.
Quantitative analysis of immunostaining
For all stainings, all glomeruli were analysed in a blinded fashion using a 400-fold magnification. Glomerular and cortical TSP-1 were quantified using computer-assisted image analysis software (MetaVue, Visitron Systems, Puchheim, Germany) determining the percentage of area positive for TSP-1. Glomerular and tubulointerstitial expression of TGF-β1/2 were evaluated using a semiquantitative scoring system from 0 to 4, where 0 means <5%, 1 means 6–25%, 2 means 26–50%, 3 means 51–75% and 4 means 76–100% positively stained area per glomerular cross section or tubulointerstitial field of vision, respectively. Assessment of glomerulosclerosis was done via PAS stainings. A semiquantitative scoring system from 0 to 4 was applied where 0 means no sclerosis and 1 means 0–25%, 2 means 26–50%, 3 means 51–75% and 4 means 76–100% of the glomerular tuft area is sclerotic. Fibrosis was assessed on PAS-stained tissues using a semiquantitative scoring system from 0 to 3, where 0 means absence of fibrosis, 1 means 0–25%, 2 means 26–50% and 3 means >50% of fibrotic area. All findings were compared to glomeruli from kidneys without evidence of renal disease. For every biopsy the local nephropathologist evaluated the severity of diabetic nephropathy in a blinded fashion. Thereby, each biopsy was semiquantitatively graded according to the degree of glomerulosclerosis, tubulointerstitial fibrosis and inflammation from 1 to 3 as shown in Table 1 that described the semiquantitative grade of morphology in diabetic nephropathy.
Table 1.
Scoring system for the assessment of diabetic nephropathy
| Parameter | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Glomerulosclerosis | Mildly increased glomerular matrix and sclerosis (up to 30%) | Moderate matrix expansion and sclerosis (<50%) | Extensive matrix expansion, Kimmelstiel–Wilson lesions, global obliteration |
| Tubulointerstitial fibrosis | Mildly increased tubulointerstitial matrix | Increased matrix, initial tubular atrophy widening of the tubulointerstitium | Extensive matrix expansion and tubular atrophy |
| Inflammation | No relevant inflammatory reaction | Inflammation in up to 50% of the section | Severe inflammation in >50% of the tissue section |
Immunohistochemical and fluorescence double staining
Double staining for TSP-1/p-smad2/3 was performed by co-incubation of TSP-1 and p-smad2/3 antibodies followed by different specific Alexa-Fluor antibodies.
Clinical records
At the day of biopsy the following data were collected from the patients corresponding to their clinical record: age, duration of diabetes, proteinuria, albuminuria, presence of retinopathy, presence of diabetic neuropathy, presence duration of hypertension, HbA1c level, serum creatinine and urea.
Statistical analysis
All values were expressed as mean ± SD. Statistical calculations were done using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). In the first step all data were analysed using the Kolmogorov–Smirnov test to test for normal distribution of all parameters. For normally distributed data, statistical significance (defined as a P-value of <0.05) was evaluated using the unpaired t-test followed by an analysis of variance (F-test) and in the case of statistically significant differences regarding variances the Welch-test was used to verify differences of data. For non-parametric testing, the Mann–Whitney U-test was applied. Correlations were calculated using Spearman's algorithm.
Results
Glomerular and cortical TSP-1 as well as TGF-ß expression are increased during diabetic nephropathy
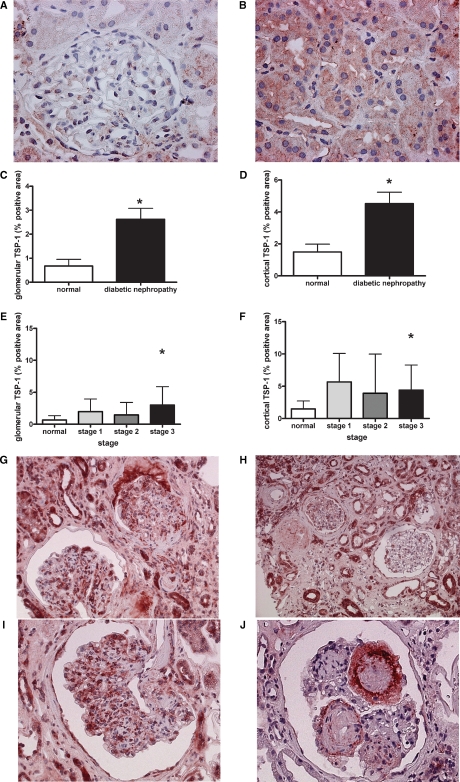
In control kidneys, TSP-1 staining was absent in most glomeruli (0.67 ± 0.68% positive area; Figure 1A) and present only in small amounts predominantly in some tubules within the tubulointerstitium (1.4 ± 1.2% positive area, Figure 1B). Glomerular (2.62 ± 2.65% positive area) as well as cortical (4.5 ± 4.2% positive area; mainly consisting of tubulointerstitial) TSP-1 was increased in diabetic versus control kidneys (both P < 0.05; Figure 1C, D). Differentiation of diabetic nephropathy in three stages of severity demonstrates that in milder diabetic lesions, glomerular and tubulointerstitial, an increase of TSP-1 can be seen (stage 1: 1.98 ± 1.98, stage 2: 1.47 ± 1.94% positive area, Figure 1E–G), but did not reach statistical significance compared to controls due to the low number of biopsies for these groups (stage 1: n = 5, stage 2: n = 5) and a significant variation of diabetic disease and TSP-1 expression in individual glomeruli within the same biopsy. Nevertheless, among all biopsies, glomerular TSP-1 expression correlated with disease stages (r = 0.392, P < 0.05). In kidneys with enhanced diabetic disease, glomeruli demonstrated most prominent TSP-1 expression in various cell types such as mesangial, endothelial and inflammatory cells (stage 3: 3 ± 2.9% positive area; Figure 1E, H–J), which reached statistical significance (stage 3, n = 30, Figure 1E) compared to controls (P < 0.05). In glomeruli with Kimmelstiel–Wilson lesions (Figure 1J), TSP-1 expression could be found at the exterior of these lesions, while the acellular nodules were usually devoid of any TSP-1. In parallel, increased TSP-1 within the cortex, predominantly reflecting tubulointerstitial expression, was most prominent in kidneys with advanced disease (stage 1: 5.7 ± 4.4, stage 2: 3.9 ± 6.1, stage 3: 4.4 ± 3.9% positive area) compared to controls (1.49 ± 1.2 5% positive area, Figure 1F, P < 0.05) and could be localized mainly to inflammatory cells and tubules (Figure 1H).
Fig. 1.
TSP-1 expression is enhanced during type-2 diabetic nephropathy. Immunohistochemistry of TSP-1 in normal kidneys demonstrated the wide absence of TSP-1 in glomeruli (A) but some low level TSP-1 in tubules (B). Glomerular (C) and cortical (D; representing mainly tubulointerstitial) TSP-1 expression were evaluated as described in the Material and methods section and were increased in type-2 diabetic nephropathy. Expression levels of glomerular (E) and cortical (F) TSP-1 varied among biopsies with different severity of lesions. With ongoing disease (G) TSP-1 expression within glomeruli could be detected in various glomerular and inflammatory cells (H, I). In the tubulointerstitium, TSP-1 was mainly confined to tubules and inflammatory interstitial cells (G, H). Kimmelstiel–Wilson lesions demonstrated a typical staining pattern (J) (*P < 0.05 by the Mann–Whitney U-test).
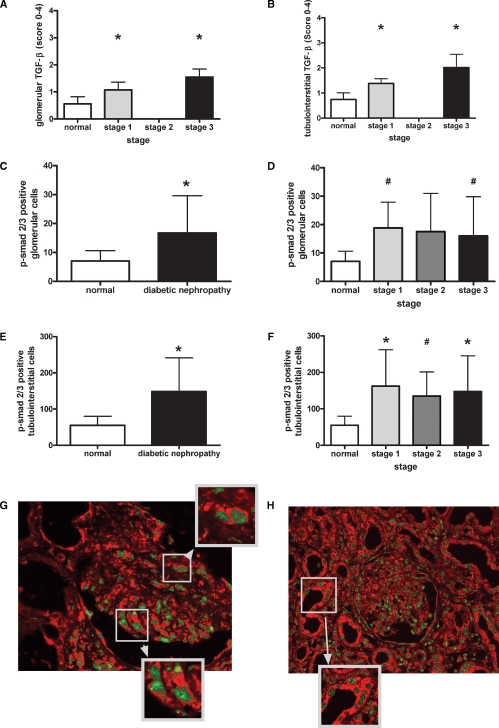
In addition, we also evaluated TGF-β expression in a smaller proportion of renal biopsies (normal: n = 8, stage 1: n = 4, stage 2: n = 0, stage 3: n = 8) to verify the previously published increase of TGF-β during diabetic nephropathy in this study [12,16]. Thereby we found significantly increased glomerular [1.1 ± 0.28 (stage 1) and 1.54 ± 0.29 (stage 3) versus 0.55 ± 0.26; P < 0.05 and P < 0.001; Figure 2A] and tubulointerstitial [1.38 ± 0.18 (stage 1) and 2.0 ± 0.53 (stage 3) versus 0.75 ± 0.27; P < 0.05 and P < 0.001; Figure 2B] expression of TGF-β, as evaluated after staining against TGF-β1/2.
Fig. 2.
Increased TGF-β expression and p-smad2/3 positivity during advanced diabetic nephropathy. Glomerular (A) and tubulointerstitial (B) immunohistochemistry of TGF-β-1/2 were evaluated using a semiquantitative scoring system as described in the Material and methods section. Immunohistochemistry of p-smad2/3 was evaluated by counting positive cells in glomeruli and the tubulointerstitium. Glomerular p-smad2/3 was increased in all diabetic biopsies (C) and biopsies with mild (grade 1) and advanced (grade 3) lesions (D) whereas cortical p-smad2/3 was increased in all subgroups (E, F). Double labelling of TSP-1 and p-smad2/3 was performed as described in the Material and methods section (G, H). Panel G and its zoomed areas depict glomerular co-localization; panel H depicts the clear co-localized TSP-1 and p-smad 2/3 in the tubulointerstitium of diabetic kidneys (*P < 0.05 by the Mann–Whitney U-test; #P < 0.05 by the unpaired t-test and Welch-test).
Increased p-smad2/3 co-localizes and correlates with TSP-1 expression during diabetic nephropathy
After activation of TGF-β, TGF-β receptor signalling led to complexation of smads 2 and 3 and subsequent phosphorylation. Evaluation of p-smad2/3 positive nuclei showed significantly increased positive cell numbers in glomeruli (16. ± 12.9 versus 7.1 ± 3.6; P < 0.05) of biopsies with diabetic nephropathy compared to controls (Figure 2C). Hereby, glomerular numbers of p-smad2/3 positive nuclei were similarly increased in all stages of diabetic glomerulopathy (Figure 2D). In parallel, p-smad2/3 positivity was increased threefold in the tubulointerstitium compared to control biopsies (148 ± 92.8 versus 55 ± 25; P < 0.05, Figure 2E) and this increase was relatively independent of the stage of disease severity (Figure 2F).
To demonstrate that TSP-1 and p-smad2/3 co-localize in biopsies with diabetic nephropathy, fluorescence double labelling of TSP-1 and p-smad2/3 was performed. Thereby, extracellular TSP-1 and nuclear p-smad2/3 clearly localized next to each other in biopsies with type-2 diabetic nephropathy. Examples of co-localization in glomerular and tubulointerstitial diabetic lesions are depicted in Figure 2G and H. Typical areas are shown as a zoomed image.
Activity of the tubulointerstitial TGF-β pathway correlates with the degree of TSP-1 and proteinuria
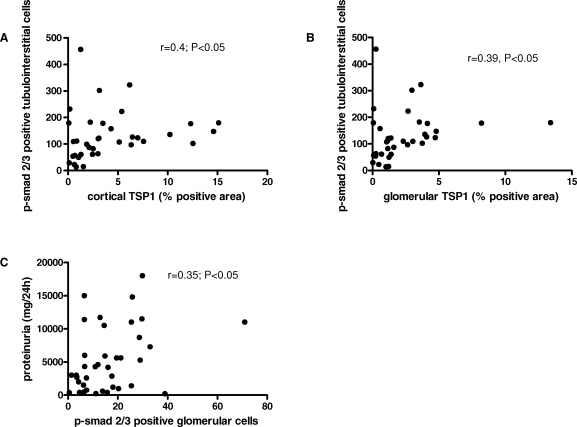
After demonstration of co-localization of TSP-1 and p-smad2/3, we subsequently assessed whether increased TSP-1 expression is directly linked to increased TGF-β activation as indicated by p-smad2/3. Hereby, both cortical and glomerular TSP-1 expression correlated with the amount of p-smad2/3 positive cells in the tubulointerstitium (Figure 3A and B), while no correlation was detected between glomerular TSP-1 and glomerular p-smad2/3.
Fig. 3.
TSP-1 expression and p-smad2/3 positivity correlate in the tubulointerstitium. Correlations were calculated using Spearman's algorithm. Thereby, cortical TSP-1 (A) and glomerular TSP-1 (B) correlated with p-smad2/3 positivity in the tubulointerstitium. The number of glomerular cells positive for p-smad2/3 also correlated with proteinuria in patients with diabetic nephropathy (C).
In this study, clinical data from 30 out of 40 patients were available as shown in Table 2. The mean age of patients was 59.35 ± 13.7 years (versus controls 48.9 ± 19.3 n.s.). The mean duration of diabetes was 12.97 ± 8.8 years, and proteinuria 5504 ± 5372 mg/24 h and albuminuria 2133 ± 2302 mg/24 h were markedly increased. In parallel, serum creatinine values (300.6 ± 114.9 μmol/l) indicated advanced disease in most patients. A summary of diabetes-associated complications is depicted in Table 2. Sixty percent of all patients presented with a prior diagnosis of arterial hypertension and received antihypertensive medication before the renal biopsy. Forty-three percent of patients had been put on ACE inhibitor treatment prior to the renal biopsy that had no influence on expression levels of TSP-1 or p-smad2/3 compared to patients without ACE inhibitors [TSP1 glomerular (%): 1.9 ± 1.1 versus 2.3 ± 1.2, n.s.; TSP-1 cortical (%): 3.1 ± 3.9 versus 3.4 ± 3.2, n.s.; p-smad2/3 glomerular: 13.5 ± 8.8 versus 18.3 ± 10.4 (n), n.s.; p-smad2/3 cortical: 118.6 ± 55.9 versus 128.2 ± 53.9 (n), n.s.]. Differentiation of baseline data from all three subgroups (grade 1–3) compared to controls demonstrates that functional parameters such as proteinuria and serum creatinine are linked to severity of histological lesions (glomerulosclerosis and fibrosis; Table 3).
Table 2.
Baseline parameters of diabetic and non-diabetic patients and presence of diabetic complications
| Parameter | Diabetes group mean ± SD or % (n = 30) | Non-diabetic controls mean ± SD (n = 10) |
|---|---|---|
| Age (years) | 59.35 ± 13.7 | 48.9 ± 19.3 n.s. |
| Duration of diabetes (years) | 12.97 ± 8.8 | 0 |
| Proteinuria (mg/24 h) | 5504 ± 5372* | Not present |
| Albuminuria (mg/24 h) | 2133 ± 2302* | Not present |
| Patients on insulin therapy (n) | 43% (n = 13) | 0 |
| Duration of insulin therapy (years) | 7.6 ± 5.1 | Not present |
| HbA1c level (mg/dl) | 6.87 ± 1.97 | Not present |
| Serum creatinine (mg/dl) | 3.4 ± 1.3* | 0.86 ± 0.28 |
| Serum urea (mg/dl) | 116 ± 37* | 30 ± 10 |
| Presence of hypertension (n) | 63% (n = 19) | 0 |
| Duration of hypertension (years) | 8.1 ± 9.2 | 0 |
| Patients on ACE inhibitor | 43% (n = 13) | 0 |
| therapy (n) | ||
| Prior history of stroke (n) | 20% (n = 6) | 0 |
| Presence of coronary heart | 46% (n = 14) | 0 |
| disease (n) | ||
| Presence of arterial occlusive | 40% (n = 12) | 0 |
| disease (n) | ||
| Presence of retinopathy (n) | 70% (n = 21) | 0 |
| Presence of diabetic | 60% (n = 18) | 0 |
| neuropathy (n) | ||
| History of smoking (n) | 63% (n = 19) | Not present |
n.s., not significant.
*P < 0.05.
Table 3.
Baseline parameters in subgroups with diabetic nephropathy
| Number of patients (n) | Age (years) | Duration of diabetes (years) | HbA1c (%) | Proteinuria (g/24 h) | Serum creatinine (μmol/l) | Fibrosis (scores 0–3) | Glomerulo-sclerosis (scores 0–4) | |
|---|---|---|---|---|---|---|---|---|
| Normal | 10 | 48.9 ± 19.3 | 0 ± 0 | n.a. | n.a. | 76 ± 26.5 | 0.5 ± 0.3 | 0.8 ± 0.3 |
| Grade 1 (mild) | 5 | 50.5 ± 23.2 | 8.6 ± 8.6 | 7.1 ± 2.4 | 0.81 ± 1.1 | 140.5 ± 35.4 | 1.6 ± 0.7 | 2.8 ± 0.8 |
| Grade 2 | 5 | 60.0 ± 9.3 | 15.7 ± 4.6 | 8.15 ± 3.6 | 1.55 ± 1.2 | 92.8 ± 106.1 | 1.85 ± 0.4 | 3.5 ± 0.4 |
| (moderate) | ||||||||
| Grade 3 | 30 | 59.0 ± 13.5 | 13.5 ± 7.4 | 6.79 ± 1.7 | 9.3 ± 19.7 | 358.9 ± 247.5 | 2.4 ± 0.65 | 3.5 ± 0.38 |
| (advanced) |
n.a., data not available.
We then correlated clinical parameters with the expression of TSP-1 and p-smad2/3. Interestingly, beyond the correlation of TSP-1 with the disease stage, the only correlation we detected was present between glomerular p-smad2/3 and the degree of proteinuria (Figure 3C). All other parameters assessed (age, glomerulosclerosis, fibrosis, duration of diabetes, albuminuria, presence of retinopathy, presence of diabetic neuropathy, presence duration of hypertension, HbA1c level, serum creatinine and urea) did not show a direct relationship to TSP-1 or p-smad2/3.
Discussion
Type-2 diabetes is one of the most challenging chronic disorders in the western world [1]. After years of diabetes, diabetic nephropathy is very common and associated with excess morbidity and mortality [2]. Despite some improving grasp regarding the underlying pathophysiology, treatment options after the onset of diabetic nephropathy and chronic kidney disease are still very limited. Whereas elevated blood glucose initially causes haemodynamic alterations, hyperfiltration and activation of glomerular cells [3], as well as excessive matrix deposition, are hallmarks of chronic diabetic nephropathy leading to glomerulosclerosis and tubulointerstitial fibrosis of the kidney [4]. TGF-β is the best-known profibrotic cytokine, clearly being linked to the development of diabetic nephropathy by various experimental studies [17]. To bind to its receptors, latent TGF-β has to be extracellularly activated. Our group recently demonstrated TSP-1 as an important endogenous activator of latent TGF-β in in vivo and in vitro models of glomerulonephritis as well as diabetic nephropathy [13–15]. Therefore, interference with the TGF-β activation process is an interesting therapeutical option to prevent its profibrotic action in the kidney [13].
In the present biopsy study, we wanted to investigate the relevance of the TSP-1/TGF-β axis in human biopsies with type-2 diabetic nephropathy. Dependent on the severity of glomerular and tubulointerstitial lesions, the local nephropathologist divided biopsies into three stages (1–3) of diabetic nephropathy. Glomerular TSP-1 was increased in biopsies with diabetic nephropathy, which was most prominent in advanced disease in areas of glomerulosclerosis and fibrosis (stage 3). In parallel to this finding, we also detected increased expression of TGF-β and of the number of cells positive for the phosphorylated form of the TGF-β signalling complex smad2/3 in glomeruli of patients with diabetic nephropathy compared to controls. TSP-1 and p-smad2/3 were co-localized by fluorescence double staining suggesting the experimentally shown functional relationship [8]. Nevertheless, the degree of glomerular TSP-1 did not directly correlate with the extent of glomerular p-smad2/3 positivity, but did significantly correlate with tubulointerstitial smad2/3 positivity. Although pure speculation, this interesting link of glomerular TSP-1 with tubulointerstitial TGF-β activation may indicate a paracrine action of TSP-1 between different compartments of the kidney. Expression analysis of glomerular TSP-1 as well as TGF-β and connective tissue growth factor (CTGF) has been performed in a recent study by Wahab and colleagues demonstrating in 19 biopsies that glomerular TSP-1, TGF-β and CTGF are increased early on during human diabetic nephropathy [12]. Our findings in 40 renal biopsies are consistent with their data, showing increased glomerular TSP-1 already in incipient diabetic lesions of our patients and a more pronounced increase in more severe diabetic disease. While this excellent study by Wahab and co-workers [12] focused completely on glomerular changes, our study also accounts for changes of the tubulointerstitium.
Hereby, we could demonstrate that cortical (reflecting tubulointerstitial) TSP-1 was increased during diabetic nephropathy and frequently co-localized with p-smad2/3 positivity indicating TGF-β signalling activity. In addition, cortical TSP-1 correlated with the number of tubulointerstitial p-smad2/3 positive cells supporting the concept of a causal relationship between TSP-1 and TGF-β activity in human diabetic nephropathy. The importance of tubulointerstitial changes for the overall time course and progression of human renal disease was established many years ago by Bohle and co-workers [18], while more recent studies confirmed this also for the time course of diabetic nephropathy [19,20]. Our group recently demonstrated in three different experimental nephritis models with interstitial fibrosis that TSP-1 co-localized with active TGF-β within the tubulointerstitium and was an excellent predictor for the later resulting interstitial fibrosis as detected in individual animals [21]. Therefore, the results of this study suggest a similar role for TSP-1 in human diabetic nephropathy.
In addition, correlating a large number of clinical parameters with the degree of glomerular p-smad2/3 positivity or TSP-1, only the extent of glomerular p-smad2/3 positivity positively correlated with the degree of proteinuria but no other clinical parameter as assessed in these diabetic patients. This interesting finding is consistent with the reported direct relationship between urinary excretion of TGF-β and proteinuria as well as TGF-β and glycaemic control, at least early on in disease [22–24]. While we detected a spatial and correlative relation between TSP-1 and the TGF-β activation, we did not find a correlation of either factors with fibrosis/sclerosis in our biopsies from patients with diabetic nephropathy. While this seems to be a surprising result, it has to be considered that detection of the profibrotic TGF-β activity might indicate/reflect active de novo production of matrix molecules in a dynamic process but not necessarily the general status of fibrosis, especially when a given tissue is already quite fibrotic/sclerotic. In our study, frequent acellular Kimmelstiel–Wilson lesions, the degree of fibrosis/sclerosis within these biopsies as well as the clinical data indicate mostly severe disease, where the active component of matrix production may actually already be slowed down.
In summary, the present study demonstrates the spatial and suggests a functional activity of the TSP-1/TGF-β axis in patients with type-2 diabetic nephropathy. Especially in the tubulointerstitium of diabetic nephropathy, TSP-1 is closely linked to TGF-β activity as indicated by p-smad2/3 positivity. The positive correlation of glomerular p-smad2/3 positivity with proteinuria further supports the importance of the TSP-1/TGF-β system as a relevant mechanism for progression of human type-2 diabetic nephropathy.
Acknowledgments
The skilled technical help of S. Weber is gratefully acknowledged. This work was supported by a grant from the Deutsche Forschungsgemeinschaft SFB 423 (TP B6) to C.H.
Conflict of interest statement. None declared.
References
- 1.Ritz E, Rychlik I, Locatelli F, et al. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 2.Ritz E, Stefanski A. Diabetic nephropathy in type II diabetes. Am J Kidney Dis. 1996;27:167–194. doi: 10.1016/s0272-6386(96)90538-7. [DOI] [PubMed] [Google Scholar]
- 3.Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 1999;56:393–405. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:p26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Jim B, Ziyadeh FN. Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol. 2003;23:532–543. doi: 10.1053/s0270-9295(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 6.Bitzer M, Sterzel RB, Bottinger EP. Transforming growth factor-beta in renal disease. Kidney Blood Press Res. 1998;21:1–12. doi: 10.1159/000025837. [DOI] [PubMed] [Google Scholar]
- 7.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 8.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 9.Poczatek MH, Hugo C, Darley-Usmar V, et al. Glucose stimulation of transforming growth factor-beta bioactivity in mesangial cells is mediated by thrombospondin-1. Am J Pathol. 2000;157:1353–1363. doi: 10.1016/s0002-9440(10)64649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro SM, Poczatek M, Schultz-Cherry S, et al. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 12.Wahab NA, Schaefer L, Weston BS, et al. Glomerular expression of thrombospondin-1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia. 2005;48:2650–2660. doi: 10.1007/s00125-005-0006-5. [DOI] [PubMed] [Google Scholar]
- 13.Daniel C, Takabatake Y, Mizui M, et al. Antisense oligonucleotides against thrombospondin-1 inhibit activation of tgf-beta in fibrotic renal disease in the rat in vivo. Am J Pathol. 2003;163:1185–1192. doi: 10.1016/s0002-9440(10)63478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel C, Wiede J, Krutzsch HC, et al. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65:459–468. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 15.Daniel C, Schaub K, Amann K, et al. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56:2982–2989. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 16.Iwano M, Kubo A, Nishino T, et al. Quantification of glomerular TGF-beta 1 mRNA in patients with diabetes mellitus. Kidney Int. 1996;49:1120–1126. doi: 10.1038/ki.1996.162. [DOI] [PubMed] [Google Scholar]
- 17.Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Bohle A, Christ H, Grund KE, et al. The role of the interstitium of the renal cortex in renal disease. Contrib Nephrol. 1979;16:109–114. doi: 10.1159/000402883. [DOI] [PubMed] [Google Scholar]
- 19.Kelly DJ, Chanty A, Gow RM, et al. Protein kinase Cbeta inhibition attenuates osteopontin expression, macrophage recruitment, and tubulointerstitial injury in advanced experimental diabetic nephropathy. J Am Soc Nephrol. 2005;16:1654–1660. doi: 10.1681/ASN.2004070578. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 21.Hugo C, Shankland SJ, Pichler RH, et al. Thrombospondin 1 precedes and predicts the development of tubulointerstitial fibrosis in glomerular disease in the rat. Kidney Int. 1998;53:302–311. doi: 10.1046/j.1523-1755.1998.00774.x. [DOI] [PubMed] [Google Scholar]
- 22.de Muro P, Faedda R, Fresu P, et al. Urinary transforming growth factor-beta 1 in various types of nephropathy. Pharmacol Res. 2004;49:293–298. doi: 10.1016/j.phrs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert RE, Akdeniz A, Allen TJ, et al. Urinary transforming growth factor-beta in patients with diabetic nephropathy: implications for the pathogenesis of tubulointerstitial pathology. Nephrol Dial Transplant. 2001;16:2442–2443. doi: 10.1093/ndt/16.12.2442. [DOI] [PubMed] [Google Scholar]
- 24.Sharma K, Ziyadeh FN, Alzahabi B, et al. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes. 1997;46:854–859. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]