Abstract
Background. The benefits of steroid therapy in immunoglobulin A nephropathy (IgAN) have not been established.
Methods. The effect of steroids on kidney survival was retrospectively investigated in 702 patients with IgAN by multivariate analyses.
Results. There were 295 men and 407 women. The median follow-up period was 62 months. One hundred and ninety-four patients were treated with oral steroids (oral steroid group). Thirty-four patients were treated with methylprednisolone (mPSL) pulse therapy (pulse steroid group) followed by oral prednisolone (PSL). In 474 patients, no steroid was used (no steroid group). The urinary protein-creatinine ratio and histological grade were significantly different among treatment groups and were highest in the pulse steroid group followed by the oral steroid group and lowest in the no steroid patients. Serum creatinine was significantly higher in the pulse steroid group than in other two groups. Eighty-five patients developed end-stage renal failure (ESRF) requiring haemodialysis. In multivariate analysis, steroid pulse therapy significantly decreased the risk of ESRF while oral steroid treatment did not improve renal survival in this cohort.
Conclusion. We found that pulse steroid therapy improved kidney survivals in IgAN. Since the clinical findings and histological grade were the most severe in patients treated with mPSL pulse therapy, such therapy may prevent progression of IgAN.
Keywords: histological grade, IgA nephropathy, multivariate analysis, steroid pulse therapy, the Cox proportional hazards model
Introduction
Since Kobayashi et al. first reported the efficacy of steroid treatment in patients with immunoglobulin A nephropathy (IgAN) in 1986 [1], there has been widespread interest in corticosteroid therapy for IgAN [2–17]. However, the efficacy of steroid treatment has not yet been clarified.
In 2003, we reported a randomized control trial (RCT) in patients with IgAN with a glomerular score between 4 and 7, and showed that a low-dose prednisolone (PSL) protocol (20 mg/day as initial dose) had an anti-proteinuric effect but no effect on kidney survival [17,18]. We speculated that an insufficient dose of PSL caused a discrepant effect on proteinuria and on kidney survival. Furthermore, pulse therapy has shown promising results in previous trials [14,17].
The aim of this study was to evaluate the effect of steroid treatment on kidney survival in patients with IgAN. We retrospectively investigated the influence of pulse and oral steroid treatment on kidney survival in 702 patients with IgAN by multivariate analyses using the Cox proportional hazards model.
Materials and methods
Study population
Seven hundred and ninety-four patients with primary IgAN, who had been biopsied between October 1979 and September 2002, were followed up at least 1 year later at Fukuoka Red Cross Hospital. Ninety-two biopsies, which contained <10 glomeruli, were excluded. The remaining 702 patients were included in the study. There were no statistical significant differences in age, sex, urine protein-creatinine ratio (UP-UCR), serum creatinine and incidence of end-stage renal failure (ESRF) between the 702 included patients and 92 excluded patient.
Histological grade based on the glomerular score
As previously reported [18,19], the glomerular score was calculated as the sum of indices of the following three glomerular lesions: (1) hypercellularity; (2) segmental lesions, including crescent, tuft necrosis, tuft adhesion and segmental sclerosis, and (3) global sclerosis.
The glomerular hypercellularity was defined as three or more nuclei in the mesangial area, or endocapillary hypercellularity in any extent. Each glomerulus in a biopsy specimen was given a point according to the semi-quantitatively evaluated extent of involved area by hypercellularity as follows: 1, no hypercellularity; 2, <25% of the glomerular area; 3, 25–50% and 4, ≥50% of the glomerular area. An average of points in each biopsy was calculated and omitted for the figures below the first decimal place. We termed it as the glomerular hypercellularity index.
Crescents, tuft necrosis, tuft adhesions to Bowman's capsule and segmental sclerosis of glomeruli were evaluated together and termed glomerular segmental lesions, because these lesions were observed to be frequently associated with each other.
The index of segmental lesions and the index of global sclerosis were determined according to the percentage of glomeruli showing each lesion out of the total number of glomeruli in a biopsy sample as follows: 0, none; 1, <10% of glomeruli; 2, 10–25%; 3, 25–50% and 4, ≥50% of glomeruli.
The points of indices of three lesions, i.e. hypercellularity, segmental lesions and global sclerosis, were added together and termed as the glomerular score, ranging from 1 to 12.
Histological grade was determined on the basis of the glomerular score as follows: grade I, glomerular score 1 or 2; grade II, glomerular score 3 or 4; grade III, glomerular score 5 or 6; grade IV, glomerular score 7 or 8 and grade V, glomerular score ≥9.
The glomerular score of all kidney biopsies in the present study were evaluated by one investigator (R.K.). As the glomerular score was determined in 1991, kidney biopsies performed before 1991 were re-evaluated by the same person.
Study design and statistics
The influence of clinical parameters, histological grade and treatment on kidney survival was retrospectively examined. The end point of kidney survival was estimated by ESRF requiring haemodialysis therapy. Clinical parameters used for analyses included age, sex, UP-UCR, blood pressure, serum albumin, serum creatinine, total serum cholesterol, serum triglyceride and serum uric acid. As for the treatment, the effect of the method of steroid therapy, the use of the angiotensin converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB), or tonsillectomy was examined. The use of ACE-I or ARB was defined as treatment for at least 6 months. Any antihypertensive agent was permitted to control blood pressure during the follow-up.
The SAS software package was used to perform all statistical analyses. Renal survival in all patients was assessed by the life table method and the log rank statistic. Comparisons among the groups were assessed by the chi-square method, analysis of variance or Kruskal–Wallis method. The statistical significances of the differences in mean of continuous variables, median values or frequencies of categorical variables between the two groups were determined by the multiple t-test, Mann–Whitney U-test or chi-square test with Bonferroni correction.
The crude or multivariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated with the use of the Cox proportional hazards model. In the multivariate model, we selected clinically or biologically plausible risk factors for the determination of kidney survivals as baseline potential confounding factors [19,20], and used the backward procedure with P < 0.05 required to retain each variable in the model including age and sex. Because 21 patients changed treatment groups during the follow-up (12 patients, no steroid to oral steroid; 5 patients, no steroid to pulse therapy; 4 patients, oral steroid to steroid pulse therapy), we also estimated the effect of steroid therapy using the time-dependent Cox proportional hazard regression model including the time-dependent status of treatment and covariates.
P < 0.05 was considered statistically significant.
Results
The baseline characteristics of the study population
The baseline characteristics of the patients are shown in Table 1. The median follow-up period was 62 months, ranging from 6 to 281 months. Eight patients developed ESRF within 1 year of the follow-up. The mean age at biopsy was 33 ± 14 years. There were 295 men and 407 women. The mean systolic or diastolic blood pressure (DBP) was 126 ± 19 mmHg and 75 ± 14 mmHg, respectively. The mean UP-UCR was 1.5 ± 1.9, and the mean degree of haematuria was 2.2 ± 0.9. The mean serum creatinine level was 0.98 ± 0.58 mg/dl (87 ± 51 μmol/l). The mean serum IgA level was 366 ± 126 mg/dl (3.66 ± 1.26 g/l).
Table 1.
Baseline characteristics of study population
| Variables | |
|---|---|
| Number | 702 |
| Duration of follow-up (months) | 62 (6–281) |
| Age (years) | 33 ± 14 |
| Men/women | 295/407 |
| Systolic blood pressure (mmHg) | 126 ± 19 |
| Diastolic blood pressure (mmHg) | 75 ± 14 |
| Urinary protein-creatinine ratio | 1.5 ± 1.9 |
| Urinary haematuria | 2.2 ± 0.9 |
| Serum albumin (g/dl) | 4.2 ± 0.5 |
| Serum creatinine (mg/dl) | 0.98 ± 0.58 |
| Serum uric acid (mg/dl) | 5.6 ± 1.6 |
| Serum total cholesterol (mg/dl) | 198 ± 45 |
| Serum triglycerides (mg/dl) | 123 ± 95 |
| Serum IgA (mg/dl) | 366 ± 126 |
| Histological grade, n (%) | |
| Grade I (glomerular score 1 or 2) | 114 (16.2) |
| Grade II (glomerular score 3 or 4) | 208 (29.6) |
| Grade III (glomerular score 5 or 6) | 203 (28.9) |
| Grade IV (glomerular score 7 or 8) | 145 (20.7) |
| Grade V (glomerular score >9) | 32 (4.6) |
| Steroid therapy, n (%) | 228 (32.3) |
| Use of ACE-I or ARB, n (%) | 241/659 (36.6) |
| Tonsillectomy, n (%) | 28/623 (4.5) |
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin-II receptor blocker.
Duration of follow-up is median (range).
Values are means ± standard deviation or frequencies.
Note: To convert serum creatinine in mg/dl to μmol/l, multiply by 88.4. To convert serum uric acid in mg/dl to μmol/l, multiply by 59.5. To convert triglyceride in mg/dl to mmol/l, multiply by 0.0113. To convert cholesterol in mg/dl to mmol/l, multiply by 0.026. To convert serum IgA in mg/dl to g/l, multiply by 0.01.
One hundred fourteen patients were classified in histological grade I, 208 in grade II, 203 in grade III, 145 in grade IV and 32 in grade V.
Out of 702 patients, 228 patients were treated with steroids. Two hundred forty-one were treated with ACE-I or ARB. Of these, 167 were hypertensive and the remaining 74 patients were normotensive. During the follow-up, 28 patients had tonsillectomy.
The baseline characteristics in each treatment groups
Methylprednisolone (mPSL) pulse therapy, 1 g/day for three consecutive days followed by 30 mg of PSL, was undertaken in 34 patients (pulse steroid group). PSL was tapered and maintained for at least 2 years. Oral steroid therapy was given to 194 patients (oral steroid group). In 46 patients, initial dose of PSL was 60 mg for 4 weeks and PSL was tapered off within 1 year. In 148 patients, the initial dose of PSL was 20 mg or 30 mg for 1 month. PSL was tapered and maintained for at least 2 years. The remaining 474 patients were treated with an anti-platelet agent or with no medication (no steroid group). Table 2 shows the baseline characteristics in each treatment group. UP-UCR was significantly different among groups and was 1.1 ± 1.6, 2.1 ± 1.9 and 3.9 ± 3.2 in no steroid, oral steroid and pulse steroid groups, respectively. UP-UCR was significantly higher in both oral and pulse steroid group than in the no steroid group (P < 0.0001). Furthermore, UP-UCR was significantly higher in the pulse steroid group than in the oral steroid group (P < 0.0001). Serum creatinine was 0.9 ± 0.6, 1.0 ± 0.4 and 1.5 ± 1.0 in the no steroid, oral steroid and pulse groups, respectively, and was significantly higher in the pulse steroid patients compared to the other two groups. The severity of histological grade was significantly different among treatment groups. In the no steroid group, the percentage of patients with histological grade I–V was 23.6, 35.7, 23.6, 15.0 and 2.1, respectively. In the oral steroid group, the percentage of patients with histological grade I–V was 0.5, 19.1, 42.3, 30.9 and 7.2, respectively. The percentage of patients with histological grade I–V was 2.9, 5.9, 26.5, 41.2 and 23.5, respectively, in the pulse steroid group. The histological grade was highest in the pulse steroid group followed by the oral steroid group and lowest in the no steroid patients.
Table 2.
Baseline characteristics of treatment groups
| Variables | No steroid | Oral steroid | Pulse steroid | P | |
|---|---|---|---|---|---|
| Number | 474 | 194 | 34 | ||
| Number of ESRF | 57 | 21 | 7 | ||
| Median duration of follow-up, months (range) | 62 (6–281) | 69 (7–240) | 44 (11–147)b | 0.031 | |
| Age (years) | 33 ± 14 | 33 ± 13 | 33 ± 18 | n.s. | |
| Men/women | 192/282 | 84/110 | 19/15 | n.s. | |
| Systolic blood pressure (mmHg) | 125 ± 19 | 127 ± 19 | 132 ± 24 | n.s. | |
| Diastolic blood pressure (mmHg) | 74 ± 14 | 77 ± 15a | 79 ± 18 | 0.012 | |
| Urinary protein-creatinine ratio | 1.1 ± 1.6 | 2.1 ± 1.9a | 3.9 ± 3.2a,b | <0.0001 | |
| Urinary haematuria | 2.1 ± 1.0 | 2.4 ± 0.9a | 2.8 ± 0.5a | <0.0001 | |
| Serum albumin (g/dl) | 4.3 ± 0.5 | 4.1 ± 0.6a | 3.7 ± 0.6a,b | <0.0001 | |
| Serum creatinine (mg/dl) | 0.9 ± 0.6 | 1.0 ± 0.4 | 1.5 ± 1.0a,b | <0.0001 | |
| Serum uric acid (mg/dl) | 5.5 ± 1.6 | 5.7 ± 1.6 | 6.3 ± 1.5a | 0.016 | |
| Serum total cholesterol (mg/dl) | 193 ± 43 | 205 ± 43a | 238 ± 54a,b | <0.0001 | |
| Serum triglycerides (mg/dl) | 120 ± 100 | 118 ± 68 | 190 ± 118a,b | 0.0001 | |
| Serum IgA (mg/dl) | 362 ± 121 | 385 ± 135 | 313 ± 116b | 0.004 | |
| Histological grade, n (%) | a | a,b | <0.0001 | ||
| Grade I (glomerular score 1 or 2) | n (%) | 112 (23.6) | 1 (0.5) | 1 (2.9) | |
| Grade II (glomerular score 3 or 4) | n (%) | 169 (35.7) | 37 (19.1) | 2 (5.9) | |
| Grade III (glomerular score 5 or 6) | n (%) | 112 (23.6) | 82 (42.3) | 9 (26.5) | |
| Grade IV (glomerular score 7 or 8) | n (%) | 71 (15.0) | 60 (30.9) | 14 (41.2) | |
| Grade V (glomerular score ≥9) | n (%) | 10 (2.1) | 14 (7.2) | 8 (23.5) | |
| Use of ACE-I or ARB, number/total | 133/441 | 90/187a | 18/32a | <0.0001 | |
| Tonsillectomy, number/total | 15/418 | 7/173 | 6/32a,b | 0.003 |
ESRF, end-stage renal failure; n.s, not significant difference; no steroid, no steroid therapy; oral steroid, oral prednisolone therapy; pulse, 1000 mg of methyl-prednisolone pulse therapy followed by oral prednisolone; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin-II receptor blocker.
Duration of follow-up is median (range).
Values are frequencies or means ± standard deviation or frequencies.
aSignificantly different compared to the no steroid group.
bSignificantly different compared to the oral steroid group.
Note: To convert serum creatinine in mg/dl to μmol/l, multiply by 88.4. To convert serum uric acid in mg/dl to μmol/l, multiply by 59.5. To convert triglyceride in mg/dl to mmol/l, multiply by 0.0113. To convert cholesterol in mg/dl to mmol/l, multiply by 0.026. To convert serum IgA in mg/dl to g/l, multiply by 0.01.
Cumulative kidney survival in all patients
During the follow-up period, 85 patients developed ESRF. The cumulative kidney survival rate was 92.1% at 5 years and 81.6% at 10 years from biopsy.
Kidney survival rate according to histological grade analysed by the life table method
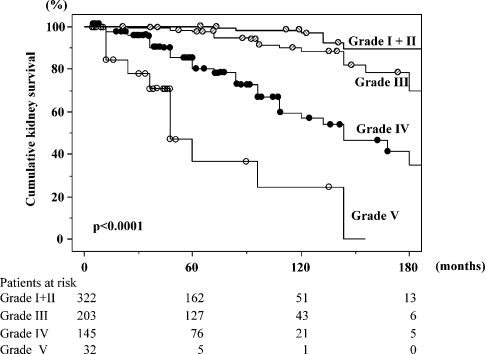
The kidney survival curve analysed by the life table method in each histological grade is shown in Figure 1. The patients with histological grades I and II were analysed together due to the small number of ESRF incidence. Five-year kidney survival in grade I+II, III, IV and V was 99.3, 97.5, 80.5 and 36.7%, respectively. Ten-year kidney survival in grade I+II, III, IV and V was 95.6, 88.3, 57.2 and 24.5%, respectively. Kidney survivals differed significantly among the histological grades (P < 0.0001).
Fig. 1.
Kidney survival curve in each histological grade. The patients with histological grades I and II were analysed together due to the small number of end-stage renal failure incidence. Kidney survivals significantly differed among the histological grades (P < 0.0001). The numbers of patients remaining at 60, 120 and 180 months of follow-up in each histological grade are shown at the bottom.
Risk factors for the development of ESRF
Crude or multivariate-HRs for the development of ESRF are shown in Table 3. In crude analysis, age, UP-UCR, serum creatinine, histological grade, systolic blood pressure (SBP), serum total cholesterol, serum triglyceride and serum uric acid significantly increased the risk of developing ESRF. HR of ESRF for women was significantly lower than men. Higher serum albumin was associated with a significant risk reduction of ESRF.
Table 3.
Crude or multivariate-adjusted hazard ratios for the development of end-stage renal failure
| Univariate analysis | Multivariate analysis (backward method) | ||||
|---|---|---|---|---|---|
| Risk factor | Scale | HR | 95% CI | HR | 95% CI |
| Age (years) | (every 10 years) | 1.43 | (1.23–1.66)** | 1.03 | (0.81–1.31) |
| Women | (versus men) | 0.52 | (0.34–0.80)** | 1.29 | (0.67–2.47) |
| UP-UCR | (every 1) | 1.29 | (1.22–1.35)** | 1.16 | (1.02–1.31)* |
| Serum creatinine | (every 1 mg/dl) | 3.91 | (3.26–4.69)** | 3.95 | (2.50–6.25)** |
| SBP | (every 10 mmHg) | 1.34 | (1.21–1.48)** | 1.03 | (0.87–1.20) |
| Serum albumin | (every 1 g/dl) | 0.27 | (0.19–0.37)** | 0.49 | (0.29–0.84)** |
| Serum total cholesterol | (every 10 mg/dl) | 1.01 | (1.04–1.13)** | – | – |
| Serum triglycerides | (every 10 mg/dl) | 1.00 | (1.03–1.06)** | 1.03 | (1.00–1.06)* |
| Serum uric acid | (every 1 mg/dl) | 1.57 | (1.40–1.75)** | 1.3 | (1.04–1.63)* |
| Histological grade | |||||
| Grade I+II (glomerular score 1–4) | 1 (reference) | 1 (reference) | |||
| Grade III (glomerular score 5 or 6) | 3.58 | (1.40–9.14)** | 3.56 | (1.11–11.39)* | |
| Grade IV (glomerular score 7 or 8) | 16.5 | (7.04–38.79)** | 8.64 | (2.66–28.05)** | |
| Grade V (glomerular score ≥9) | 73.9 | (29.07–187.78)** | 5.74 | (1.31–25.08)* | |
| Steroid therapy | |||||
| No steroid | 1 (reference) | 1 (reference) | |||
| Oral steroid | 0.88 | (0.54–1.46) | 0.61 | (0.30–1.22) | |
| Pulse steroid | 2.6 | (1.18–5.71)* | 0.14 | (0.05–0.44)** | |
| Use of ACE-I or ARB | (versus no use) | 1.09 | (0.66–1.81) | 0.39 | (0.21–0.71)** |
| Tonsillectomy | (versus no tonsillectomy) | 0.86 | (0.27–2.74) | – | – |
UP-UCR, urinary protein-creatinine ratio; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; SBP, systolic blood pressure; HR, hazard ratio.
*P < 0.05, **P < 0.01.
In multivariate analysis, UP-UCR, serum creatinine, serum triglycerides and serum uric acid significantly increased the risk of the development of ESRF (P < 0.05, P < 0.01, P < 0.05, P < 0.05, respectively). Serum albumin significantly decreased the risk of ESRF (P < 0.01). Multivariate adjusted HRs for the development of ESRF in histological grades III, IV and V were 3.56, 8.64 and 5.74, respectively, and significantly increased compared to grade I+II (P < 0.05, P < 0.01, P < 0.05, respectively). As for the steroid treatment, HR of steroid pulse therapy decreased significantly compared to the no steroid group (HR 0.14, 95% CI 0.05–0.44, P < 0.01). On the other hand, HR for the development of ESRF of the oral steroid group did not show any significant difference compared to the no steroid group (HR 0.61, 95% CI 0.30–1.22). These findings were still observed even in the analysis using the time-dependent Cox hazard model with the status of steroid treatment during the follow-up (oral steroid, HR 0.58, 95% CI 0.29–1.17; pulse therapy, HR 0.15, 95% CI 0.05–0.43, P < 0.01). The use of ACE-I or ARB significantly decreased the risk of renal death (P < 0.01). Age, sex, serum total cholesterol, tonsillectomy and SBP did not show any association with ESRF in multivariate analysis. We selected SBP rather than DBP in the multivariate analysis, because the likelihood ratio of the model for SBP showed better fitting than that for DBP.
Adverse effect
In the oral steroid group, two patients developed steroid-induced psychosis and were treated well by the psychiatrist. One patient refused to take PSL after 8 months because of palpitation, increased perspiration and facial blushing. Three patients complained of insomnia and palpitation but were able to tolerate the protocol. One patient had aseptic necrosis of medial epicondyle of left femoral bone after two courses of the low-dose protocol of PSL treatment. The duration between the beginning of PSL and the onset of left knee pain was 6 years. Another patient developed diabetes mellitus, and PSL was tapered immediately. One patient suffered from herpes zoster after 4-month steroid therapy and was treated with aciclovir. In the mPSL pulse group, one patient developed tuberculosis of left cervical lymph node 4 months after the beginning of mPSL and was successfully treated with anti-tubercular agents. The other two patients developed herpes zoster 4 or 5 months after steroid therapy and were treated with acyclovir.
Discussion
A variety of studies have been investigated to confirm the effect of steroid treatment on amelioration of the clinical course of IgAN [1–17]. However, the efficacy of steroid therapy in patients with IgAN has not yet been established.
In our hospital, the treatment of IgAN patients has changed over time. Since the initial 1986 publication of the effects of steroids in IgAN by Kobayashi et al. [1], we started a pilot study of steroid treatment with 60 mg of PSL as initial dose, gradually tapered off within 1 year. We realized the necessity for a RCT to assess the efficacy of steroid treatment in IgAN and performed between 1991 and 1995 a RCT in IgAN with a glomerular score ranging from 4 to 7. A low-dose PSL protocol (initial dose of PSL; 20 mg/day) showed only an anti-proteinuric effect and no effect on kidney survival [18]. Moreover, the protocol failed to demonstrate an anti-proteinuric effect in patients with massive proteinuria, i.e. UP-UCR exceeding 3. Since the amount of proteinuria has been reported to be one of the most important prognosticators in IgAN [21–25] and the effect of steroid therapy on preventing the progression of IgAN is believed to link closely to reduction in urinary protein [14,26], we speculated that a low-dose PSL protocol was insufficient for the treatment of IgAN with moderate histological severity. Steroid pulse therapy has been done in such patients as those with a glomerular score ≥8, UP-UCR exceeding 3.5 g/day and/or elevated serum creatinine level.
Although the present study is retrospective and it is the limitation of this study, we do believe that it is worthy to report our experience of steroid treatment in a large number of Japanese patients with IgAN in a single centre. We used a multivariate analysis using the Cox proportional hazard model.
In the present study, we found that the influence of steroid therapy on the risk of ESRF was differed between the oral and pulse steroid therapy. The HR for the development of ESRF in the patients with steroid pulse therapy decreased significantly compared to the no steroid group. On the other hand, the impact of oral steroid on the risk of ESRF did not reach statistical significance.
Yoshimura et al. first reported the efficacy of mPSL pulse therapy on preventing the progression in eight patients with IgAN showing crescent 10% or more of glomeruli [6]. Two or three courses of 1 g/day mPSL for three consecutive days followed by oral PSL 20 mg/day tapered to 5–10 mg was administrated. Urinary protein excretion significantly decreased, and creatinine clearance significantly increased. After the pulse therapy, they performed second biopsies and found a complete loss or marked decrease in cellular crescent. They suggested that mPSL pulse therapy prevents the progression of IgAN through suppression of new crescent formation as well as transformation of cellular crescent to fibrocellular or fibrous crescent. Hotta et al. [11] also reported that not oral but pulse steroid therapy showed a significant effect on remission of proteinuria and haematuria in their retrospective study using multivariate analysis. They also found that clinical remission was closely related to stable kidney function. Their findings are compatible with our results. In 1999, Pozzi et al. reported a multicentre, randomized and controlled trial designed to compare the effects of a 6-month steroid course with those of supportive therapy in 86 patients with IgAN [14]. Their steroid regimen was three courses of intravenous mPSL, 1 g/day for three consecutive days plus oral PSL, 0.5 mg/kg, on alternate days for 6 months. After 5 years of follow-up, the risk of a doubling in plasma creatinine levels was significantly lower in the treated patients. Recently, they reported a long-term outcome of their previous control trial [17]. Ten-year renal survival was significantly better in the steroid than in the control group (97% versus 53%, P = 0.0003). The end-point of renal survival was a doubling in baseline plasma creatinine levels. The protective effect of mPSL pulse therapy against the progression of IgAN was demonstrated as level-one evidence by their control trial. In the present study multivariate analysis showed that steroid pulse therapy had a significant effect on lowering the risk for the development of ESRF. Considering that the patients treated with steroid pulse therapy were clinically and histologically the most severe cases, steroid pulse therapy seems to be the most effective method among various steroid protocols. However, in our study, the number of patients who received mPSL pulse therapy was only 34. A RCT, which compare the effectiveness of oral steroid and that of pulse steroid therapy, is necessary to reach a conclusion. So far such studies have not yet been reported.
As for the adverse effects of steroid treatment, we got an important message from the patients. Irrespective of the method of steroid therapy, infections such as herpes zoster and lymph node tuberculosis occurred 4–5 months after the initiation of steroid treatment. We should have paid more attention to prevent such infection during steroid therapy. One patient with low-dose oral steroid treatment developed aseptic necrosis of femoral bone after 6 years of steroid therapy. Long-term steroid therapy should be avoided even with low-dose protocol.
We reported previously that the glomerular score related significantly to the outcome of 248 patients with IgAN in univariate life table analysis [19]. In the present study, we reconfirmed the usefulness of the glomerular score to predict the prognosis in 702 patients with IgAN by multivariate analysis. We have applied steroid pulse therapy in IgAN with histological grade over III, that is, the glomerular score ≥5.
In conclusion, we found that pulse steroid therapy improved kidney survival in IgAN. Since the clinical findings and histological grade were the most severe in patients treated with mPSL pulse therapy, such therapy may prevent progression of IgAN.
Acknowledgments
The authors thank Mrs Jean Kawabe for correcting the English.
Conflict of interest statement. None declared.
References
- 1.Kobayashi Y, Fujii K, Hiki Y, et al. Steroid therapy in IgA nephropathy: a prospective pilot study in moderate proteinuric cases. Q J Med. 1986;61:935–943. [PubMed] [Google Scholar]
- 2.Kobayashi Y, Fujii K, Hiki Y, et al. Steroid therapy in IgA nephropathy: a retrospective study in heavy proteinuric cases. Nephron. 1988;48:12–17. doi: 10.1159/000184861. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi Y, Hiki Y, Fujii K, et al. Moderately proteinuric IgA nephropathy: prognostic prediction of individual clinical courses and steroid therapy in progressive cases. Nephron. 1989;53:250–256. doi: 10.1159/000185753. [DOI] [PubMed] [Google Scholar]
- 4.Waldo FB, Alexander R, Wyatt RJ, et al. Alternate-day prednisone therapy in children with IgA-associated nephritis. Am J Kidney Dis. 1989;13:55–60. doi: 10.1016/s0272-6386(89)80117-9. [DOI] [PubMed] [Google Scholar]
- 5.Andreoli SP, Bergstein JM. Treatment of severe IgA nephropathy in children. Pediatr Nephrol. 1989;3:248–253. doi: 10.1007/BF00858524. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura M, Kida H, Saito Y, et al. Effects of methylprednisolone pulse therapy on progressive IgA nephropathy. Jap J Nephrol. 1991;33:761–768. [PubMed] [Google Scholar]
- 7.Kobayashi Y, Hiki Y, Kokubo T, et al. Steroid therapy during the early stage of progressive IgA nephropathy. A 10-year follow-up study. Nephron. 1996;72:237–242. doi: 10.1159/000188848. [DOI] [PubMed] [Google Scholar]
- 8.Waldo FB, Wyatt RJ, Kelly DR, et al. Treatment of IgA nephropathy in children: efficacy of alternate-day oral prednisone. Pediatr Nephrol. 1993;7:529–532. doi: 10.1007/BF00852535. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Waga S, Kakizaki Y, et al. Efficacy of long-term alternate day prednisolone therapy in childhood IgA nephropathy. Clin Exp Nephrol. 1998;2:132–136. [Google Scholar]
- 10.Tsuruya K, Harada A, Hirakata H, et al. Combination therapy using prednisolone and cyclophosphamide slows the progression of moderately advanced IgA nephropathy. Clin Nephrol. 2000;53:1–9. [PubMed] [Google Scholar]
- 11.Hotta O, Miyazaki M, Furuta T, et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis. 2001;38:736–743. doi: 10.1053/ajkd.2001.27690. [DOI] [PubMed] [Google Scholar]
- 12.Welch TR, Fryer C, Shely E, et al. Double-blind, controlled trial of short-term prednisone therapy in immunoglobulin A glomerulonephritis. J Pediatr. 1992;121:474–477. doi: 10.1016/s0022-3476(05)81808-6. [DOI] [PubMed] [Google Scholar]
- 13.Yoshikawa N, Ito H, Sakai T The Japanese Pediatric IgA Nephropathy Treatment Study Group. A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. J Am Soc Nephrol. 1999;10:101–109. doi: 10.1681/ASN.V101101. [DOI] [PubMed] [Google Scholar]
- 14.Pozzi C, Bolasco P, Fogazzi G, et al. Corticosteroids in IgA nephropathy: a randomized controlled trial. Lancet. 1999;353:883–887. doi: 10.1016/s0140-6736(98)03563-6. [DOI] [PubMed] [Google Scholar]
- 15.Shoji T, Nakanishi I, Suzuki A, et al. Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis. 2000;35:194–201. doi: 10.1016/s0272-6386(00)70326-x. [DOI] [PubMed] [Google Scholar]
- 16.Ballardie FW, Roberts ISD. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–148. doi: 10.1681/ASN.V131142. [DOI] [PubMed] [Google Scholar]
- 17.Pozzi C, Andrulli S, Vecchio LD, et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157–163. doi: 10.1097/01.asn.0000103869.08096.4f. [DOI] [PubMed] [Google Scholar]
- 18.Katafuchi R, Ikeda K, Mizumasa T, et al. Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low-dose prednisolone therapy. Am J Kidney Dis. 2003;41:972–983. doi: 10.1016/s0272-6386(03)00194-x. [DOI] [PubMed] [Google Scholar]
- 19.Katafuchi R, Kiyoshi Y, Oh Y, et al. Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. Clin Nephrol. 1998;49:1–8. [PubMed] [Google Scholar]
- 20.Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int. 2006;70:1694–1705. doi: 10.1038/sj.ki.5001794. [DOI] [PubMed] [Google Scholar]
- 21.Katafuchi R, Oh Y, Hori K, et al. An important role of glomerular segmental lesions on progression of IgA nephropathy: a multivariate analysis. Clin Nephrol. 1994;41:191–198. [PubMed] [Google Scholar]
- 22.Alarmartine E, Sabatier JC, Guerin C, et al. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analysis. Am J Kidney Dis. 1991;1:12–19. doi: 10.1016/s0272-6386(12)80284-8. [DOI] [PubMed] [Google Scholar]
- 23.Donadio JV, Bergstralh EJ, Offord KP, et al. Clinical and histopathological associations with impaired renal function in IgA nephropathy. Clin Nephrol. 1994;41:65–71. [PubMed] [Google Scholar]
- 24.D’Amico G, Colasanti G, Barbiano di Belgioioso G, et al. Long-term follow-up of IgA mesangial nephropathy: clinico-histological study in 374 patients. Semin Nephrol. 1987;4:355–358. [PubMed] [Google Scholar]
- 25.Kobayashi Y, Tateno S, Hiki Y, et al. IgA nephropathy: prognostic significance of proteinuria and histological alterations. Nephron. 1983;34:146–153. doi: 10.1159/000183000. [DOI] [PubMed] [Google Scholar]
- 26.Locatelli F, Pozzi C, Vecchio LD, et al. Role of proteinuria reduction in the progression of IgA nephropathy. Ren Fail. 2001;23:495–505. doi: 10.1081/jdi-100104732. [DOI] [PubMed] [Google Scholar]