Abstract
Background. Serum creatinine concentration is an unreliable and insensitive marker of chronic kidney disease (CKD). To improve CKD detection, the Australasian Creatinine Consensus Working Committee recommended reporting of estimated glomerular filtration rate (eGFR) using the four-variable Modification of Diet in Renal Disease (MDRD) formula with every request for serum creatinine concentration. The aim of this study was to evaluate the impact of automated laboratory reporting of eGFR on the quantity and quality of referrals to nephrology services in Southeast Queensland, Australia.
Methods. Outpatient referrals to a tertiary and regional renal service, and a single private practice were prospectively audited over 3–12 months prior to and 12 months following the introduction of automated eGFR reporting and concomitant clinician education. The appropriateness of referrals to a nephrologist was assessed according to the Kidney Check Australia Taskforce (KCAT) criteria. Significant changes in the quantity and/or quality of referrals over time were analysed by exponentially weighed moving average (EWMA) charts with control limits based on ±3 standard deviations.
Results. A total of 1019 patients were referred to the centres during the study period. Monthly referrals overall increased by 40% following the introduction of eGFR reporting, and this was most marked for the tertiary renal service (52% above baseline). The appropriateness of nephrologist referrals, as adjudicated by the KCAT criteria, fell significantly from 74.3% in the 3 months pre-eGFR reporting to 65.2% in the 12 months thereafter (P < 0.05). Nevertheless, a greater absolute number of CKD patients were appropriately being referred for nephrologist review in the post-eGFR period (24 versus 15 per month). Patients referred following the introduction of eGFR were significantly more likely to be older (median 63.2 versus 59.3 years, P < 0.05), diabetic (25 versus 18%, P = 0.05) and have stage 3 CKD (48% versus 36%, P < 0.01).
Conclusion. The introduction of automated eGFR calculation has led to an overall increase in referrals with a small but significant decrease in referral quality. The increase in referrals was seen predominantly in older and diabetic patients with stage 3 CKD and appeared to result in net benefit.
Keywords: chronic kidney disease, Cockcroft–Gault equation, glomerular filtration rate, Modification of Diet in Renal Disease equation, serum creatinine
Introduction
Chronic kidney disease (CKD) is a major global public health problem. Over the last 25 years, while the world's population has grown by ∼1.5% per annum, the number of individuals being treated with dialysis or kidney transplantation has increased >8% per annum [1]. In 2000, 16% of Australians were estimated to have CKD [2] and by 2005 this figure had risen to 20% or ∼4 million people [3]. The incidence of CKD in the adult population was recently estimated at 0.9% per annum [3] and its increase has largely been driven by population ageing and the epidemics of diabetes, vascular disease and obesity. Not all sectors of the population are affected equally as there is disproportionate representation in the elderly, indigenous peoples and the socially disadvantaged [4,5]. CKD is often not associated with significant symptoms and is unrecognized in 80–90% of cases [6,7]. Its presence is a very strong risk factor for cardiovascular disease, such that individuals with CKD have up to a 10- to 20-fold greater risk of cardiac death than age- and sex-matched controls without CKD [1,8]. Furthermore, patients with CKD are at least 20 times more likely to die from cardiovascular disease than survive to the point of needing dialysis or kidney transplantation [9]. In those who do reach end-stage kidney failure, nearly 30% are referred to a nephrologist late (within 3 months of needing kidney replacement therapy) [10]. Early identification and management of CKD is highly cost effective and can reduce the risk of kidney failure progression and cardiovascular disease by 20–50% [11]. Therefore, increasing the recognition of impaired kidney function, which is often asymptomatic, is a key part of improving health outcomes for patients with CKD.
Historically, the most commonly used measure of overall kidney function in clinical practice has been serum creatinine concentration. Unfortunately, this measurement varies markedly with age, gender and muscle mass and is notoriously insensitive for detecting mild-to-moderate kidney failure, such that patients may lose 50% or more of their kidney function before the serum creatinine value rises above the upper limit of normal [12]. More recently, calculation of estimated GFR (eGFR) using an empirical mathematical formula has been encouraged as a simple, rapid and reliable means of assessing kidney function [13,14]. There are no fewer than 47 different prediction equations currently available, although the 2 most common in use are the Cockcroft–Gault [15] and the four-variable Modification of Diet in Renal Disease (MDRD) formulae [13,16]. The advantages of the MDRD formula are that it only requires knowledge of four simple indices that are readily ascertained by pathology labs (age, gender, race, serum creatinine), does not require knowledge of the patient's weight (making it far more suitable for automated laboratory reporting), does not need correction for body surface area (and therefore does not require knowledge of the patient's height) and has been generally shown to be more precise and accurate than the Cockcroft–Gault equation when the GFR is <60 mL/ min/1.73 m2. In August 2005, the Australasian Creatinine Consensus Working Committee [17] recommended reporting of the four-variable MDRD eGFR simultaneously with creatinine to enhance detection of CKD, thereby facilitating the timely institution of renoprotective therapies and appropriate and timely referral of patients to nephrologists.
The aim of the present study was to prospectively assess the impact of introduction of automated eGFR reporting on the number, patterns and appropriateness of referrals of CKD patients to a tertiary institution, a regional (secondary) hospital and a single private practice in Southeast Queensland, Australia.
Methods
Data collection
Outpatient referrals to a tertiary and regional renal service, and a single private practice were audited over 3 months prior to and 12 months following the introduction of automated eGFR reporting to determine whether this intervention influenced referral patterns overall or according to renal service setting. The tertiary hospital, (a major hospital with a full complement of subspecialty services), served a catchment population of approximately one million and the secondary hospital a population of three hundred thousand. The private practice (where renal services are paid for by the patient and/or their insurance company) served the same population as the secondary hospital but with one other competing practice. The pre-intervention audit period commenced on 23 May 2005 and ended on 22 August 2005, when automated laboratory reporting of eGFR was introduced by the Queensland Health Pathology Service (QHPS, the sole public pathology service in Queensland). The post-intervention audit period extended from 23 August 2005 until 22 August 2006 and took account of the introduction of eGFR reporting in October 2005 by Queensland Medical Laboratory (QML; providing ∼80% of private pathology testing) and in January 2006 by Sullivan & Nicolaides Pathology (S&N; providing ∼20% of local private pathology testing). All three laboratories together accounted for 99% of local pathology services. A more detailed analysis of the impact of eGFR reporting on referral rates was performed at the tertiary centre, which had prospectively collected data on referrals for 15 months prior to the commencement of automated laboratory reporting of eGFR. Throughout the entire study period, an education program was run for general practitioners and hospital staff by local renal services and the Kidney Check Australia Taskforce (KCAT), in the form of lectures, accredited workshops, articles in primary care journals, addenda to pathology reports, online learning, mailed information leaflets, printed office materials and decision support systems embedded in medical software.
Data collected during the audit included age, gender, reason for referral, CKD stage (defined according to K/DOQI criteria) [18], serum creatinine concentration at referral, MDRD eGFR at referral and the presence of a limited range of comorbidities including hypertension (defined as systolic blood pressure >140 mmHg and/or a diastolic blood pressure >90 mmHg), diabetes mellitus and macrovascular disease (defined as a documented history of cerebrovascular, peripheral vascular or ischaemic heart disease). The appropriateness of referrals was determined according to the Kidney Check Australia Taskforce criteria (Table 1).
Table 1.
Kidney Check Australia Taskforce (KCAT) guidelines for indications for referral to a nephrologist
| • | eGFR <30 mL/min/1.73 m2 |
| • | Rapidly declining kidney function (15% decrease in eGFR over 3 months irrespective of baseline level) |
| • | Proteinuria >1g/24 h |
| • | Glomerular haematuria |
| • | Kidney disease and hypertension that proves difficult to control |
| • | Diabetes and eGFR <60 mL/min/1.73 m2 |
Statistics
Results are expressed as mean ± SD for parametric continuous data, median [interquartile range] for non-parametric continuous data, and frequencies and percentages for categorical data. The distributions of categorical variables for the two audit periods were compared using the Chi-square test or Fisher's exact test, as appropriate. Differences in age, eGFR and serum creatinine concentration pre- and post-eGFR reporting were assessed by the Mann–Whitney test. Changes in the quantity and/or appropriateness of referrals over time were analysed by Poisson regression and expressed as incident rate ratios (IRR). For referrals to the tertiary service, where audit data were available over a much longer time period, changes in the quantity and/or appropriateness of referrals over time were analysed by exponentially weighed moving average (EWMA) charts with control limits based on ± 3 standard deviations. Data were analysed using the software packages SPSS for Windows release 12.0 (SPSS Inc., North Sydney, Australia) and Stata/SE 9.2 (College Station, TX, USA). P values <0.05 were considered statistically significant.
Results
Referral patterns
A total of 1019 patients were referred to study centres during the 15 months of surveillance from 23 May 2005 to 22 August 2006. Of these, 175 patients were referred over the 3 months prior to eGFR reporting (58.3 referrals per month) and 844 were referred during the 12 months post-introduction of eGFR reporting (70.3 referrals per month, IRR 1.21, 95% CI 1.02–1.42, P < 0.05). Seventeen patients were <18 years and were therefore excluded from further analysis, leaving 1002 remaining in the study. Sixty-nine percent of the referrals were from primary care, 30% from specialists and 1% from an unrecorded source. There was no significant change in the referral source after eGFR introduction.
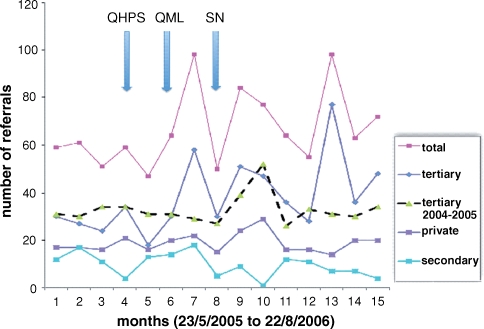
The characteristics of referred patients included in the study are depicted in Table 2. Monthly referral rates increased by 40% following the introduction of automated laboratory reporting of eGFR and concomitant education (Figure 1). The increase in referral rates was most marked for the tertiary renal service (52% above baseline). Patients referred post-eGFR reporting were significantly more likely to be slightly older, have lower eGFR values at referral and have stage 3 CKD compared with those referred prior to the intervention. Moreover, patients referred post-eGFR reporting were significantly more likely to have diabetes mellitus and tended to more frequently have hypertension. No significant differences were observed between the two groups with respect to gender, serum creatinine concentration at time of referral or the presence of macrovascular disease. Interestingly, failure of referred patients to attend their booked renal outpatient clinic increased significantly from 2% to 5% following the introduction of eGFR reporting. The increase in the non-attendance rate was not attributable to increased appointment-waiting times as these did not change throughout the time course of this study. No significant differences in patient characteristics were observed between the tertiary hospital (n = 591), regional hospital (n = 146) and private renal practice (n = 287) (data not shown).
Table 2.
Characteristics of patients referred to renal services during the study period. Results are expressed as number (%) or median (interquartile range)
| Characteristic | Pre-eGFR | Post-eGFR | P |
|---|---|---|---|
| Follow-up time (months) | 3 | 12 | – |
| Number of referrals | 171 | 831 | 0.024 |
| Referral rate per month | 50.3 | 70.3 | 0.024 |
| Failure to attend | 3 (2%) | 44 (5%) | 0.04 |
| Age (years) | 59.3 [47.0–74.1] | 63.2 [51.8–76.1] | 0.01 |
| Male gender | 100 (58%) | 423 (52%) | 0.10 |
| Hypertension | 110/161 (68%) | 559/741 (75%) | 0.06 |
| Diabetes mellitus (%) | 31 (18%) | 209 (25%) | 0.05 |
| Macrovascular disease (%) | 69 (40%) | 294 (36%) | 0.22 |
| eGFR (mL/min/1.73 m2) | 46.4 [24.9–67.9] | 39.6 [28.2–58.6] | 0.04 |
| Serum creatinine (μmol/L) | 130 [90–200] | 140 [105–192] | 0.19 |
| CKD stage | |||
| 1 | 28 (16%) | 80 (10%) | |
| 2 | 34 (20%) | 127 (15%) | |
| 3 | 61 (36%) | 395 (48%) | |
| 4 | 38 (22%) | 193 (23%) | |
| 5 | 10 (6%) | 36 (4%) | 0.01 |
| KCAT adherence (%) | 74.3 | 65.2 | 0.028 |
Fig. 1.
Number of referrals per month during the course of the study. Automated laboratory reporting of eGFR commenced after Month 3.
Appropriateness of nephrologist referrals
The appropriateness of nephrologist referrals, as adjudicated by the KCAT criteria, fell significantly from 74.3% in the 3 months pre-eGFR reporting to 65.2% in the 12 months thereafter (IRR 0.63, 95% CI 0.48–0.82, P < 0.05) (Tables 2 and 3). Nevertheless, a greater absolute number of CKD patients were appropriately being referred for nephrologist review in the post-eGFR period (24 versus 15 per month). The increase in ‘inappropriate’ referrals was largely accounted for by non-diabetic patients with an eGFR in excess of 30 mL/min/1.73 m2. Also of interest was the dichotomy of much higher quality referrals in the public system than in the private sector (75% versus 60%). The reasons for this apparent disparity are uncertain but may relate to differences in socioeconomic background or to differences in perceived ability to access private versus public health care systems (for example, if primary health care providers perceived that patient access to the public health sector was more difficult than for the private sector, the threshold for referral of patients to public services may have been altered accordingly).
Table 3.
Reasons for referral of CKD patients to nephrologists prior to and following the introduction of automated laboratory reporting of eGFR. The differences in reasons for referral between the two time periods were statistically significant (P < 0.001)
| KCAT criteria met | Referral reason | Pre-eGFR (n = 171) | Post-eGFR (n = 831) |
|---|---|---|---|
| Yes | eGFR <30 mL/min/1.73 m2 | 43 (25%) | 203 (24%) |
| Rapidly declining kidney function (15% in eGFR over 3 months | 0 (0%) | 2 (0%) | |
| irrespective of baseline level) | |||
| Proteinuria >1g/24 h | 18 (11%) | 38 (5%) | |
| Glomerular haematuria | 19 (11%) | 48 (6%) | |
| Kidney disease and hypertension that proves difficult to control | 7 (4%) | 41 (5%) | |
| Diabetes and eGFR <60 mL/min/1.73 m2 | 24 (14%) | 107 (13%) | |
| As deemed appropriate by nephrologist (e.g. ADPKD) | 16 (9%) | 95 (11%) | |
| No | CKD but eGFR >30 mL/min/1.73 m2 | 41 (24%) | 289 (35%) |
| Diabetes but eGFR >60 mL/min/1.73 m2 | 3 (1.8%) | 0 (0%) | |
| Not defined | 0 (0%) | 8 (1%) |
Sub-group analysis of tertiary renal unit referrals
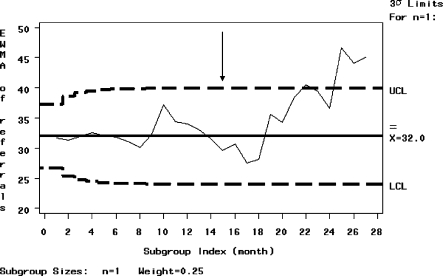
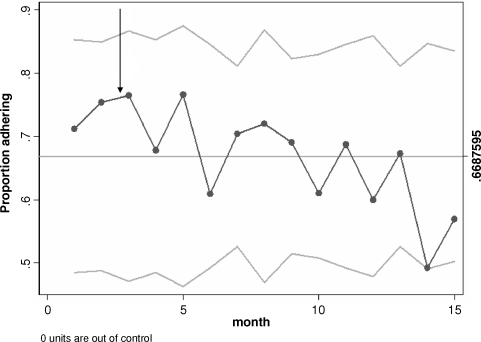
In view of the short period of time over which data were prospectively collected for all study centres prior to eGFR reporting, a sub-group analysis was performed of tertiary renal unit referrals for whom prospective data were available for a much longer period of over 15 months prior to eGFR reporting. The tertiary hospital showed a significant increase in referral rate between the two comparison periods, as demonstrated by the EWMA graph (Figure 2), which shows the referral rate surpassing the upper confidence interval 12 months after the introduction of eGFR reporting. The increase in referral rates was sustained out to 1 year. There was a trend to more inappropriate referrals by the p chart with the graph touching but not surpassing the lower confidence limit at Month 14 of the study (Figure 3).
Fig. 2.
EWMA graph of number of referrals to a tertiary renal centre over time. Automated laboratory reporting of eGFR was introduced at the end of Month 15 (arrow). Upper and lower dashed lines represent the control limits (± 3 standard deviations).
Fig. 3.
EWMA graph of the proportion of referrals to a tertiary renal centre adhering to KCAT referral criteria over time. Automated laboratory reporting of eGFR was introduced at the end of Month 3 (arrow). Upper and lower dashed lines represent the control limits (± 3 standard deviations).
Discussion
The introduction of automated eGFR reporting together with primary healthcare education significantly increased the number of referrals of patients to renal services in southeast Queensland, particularly to the tertiary renal unit. The increase occurred predominantly after the introduction to the private pathology services, reflecting the utilization of these services by general practitioners and private specialists. The majority of patients referred had stage 3 CKD and diabetes and the increased rates of referral reflected an increase in detection and potentially the opportunity for earlier assessment and treatment by specialist services. With any new guideline or tool, there is a learning curve in its usage and there was a trend to more inappropriate referrals, which will need to be addressed with ongoing education.
The relative success of the pre-introduction education undertaken can be seen by comparing the results of this study to that of a similar study in primary care in the UK [19]. Richards et al. [19] showed a seven-fold increase in referrals post-introduction of automated eGFR reporting that was then reduced back to a rate similar to that seen in the current study, following the introduction of a referral assessment service.
The majority of patients with mild to moderate (stages 2 and 3) CKD can and should be managed within the community by general practitioners. A subset of patients will need to be referred to nephrologist services and it is important that the appropriate patients are referred so that effective use of limited outpatient resources occurs. This study showed that although the majority of patients were appropriately referred, there was a trend to poorer quality (Table 2), as defined by the KCAT referral guidelines. Private practice was the exception to this observation, improving from 40% to 48%. Further targeted education to improve the confidence of general practitioners in managing patients with CKD will be of benefit.
There was a significant increase in people who ‘did not attend’ (DNA) following the introduction of automated eGFR. There is an underestimation and underappreciation of the seriousness of CKD and its implications for general health in the community and further education to promote awareness of kidney disease in the community is required.
The increase in diabetics referred post-eGFR is less surprising given that diabetic nephropathy is the largest cause of ESRF in Australia [10]. The introduction of eGFR reporting in this study has led to an increased awareness of CKD in diabetics that will hopefully promote greater attention to modification of renal and cardiovascular risk factors. Given the exponentially increasing number of diabetics it could be suggested that the KCAT indication for referral of all diabetics with an eGFR of <60 mL/min could be further refined, to target those with progressive albuminuria or declining kidney function. Indeed, it is noteworthy that the number of patients referred on the basis of significant proteinuria and/or glomerular haematuria decreased following eGFR introduction, raising the possibility of de-emphasis of the value of urinalysis relative to eGFR in primary care. Given that proteinuria and eGFR contribute equally important and additive information with respect to CKD risk stratification [20], this point should receive greater emphasis in education programs.
The significant increase in the age of the referred patients after intervention could reflect the increasing confidence interval of the MDRD equation with age, and care needs to be taken that patients of extreme age are referred with due consideration of the limitations of the test. Indeed, the recently revised consensus recommendations of the Australasian Creatinine Consensus Working Group [21] state that
eGFR values between 45 and 59 mL/min/1.73 m2 in those of 70 years of age and older should be interpreted with caution. If other signs of kidney damage (e.g. proteinuria, haematuria etc) are not present, a stable eGFR in this range may be consistent with typical GFR for this age and an absence of CKD related complications.
Moreover, recently published reference intervals for eGFR in an apparently healthy Caucasian population demonstrated that MDRD eGFR declines significantly with increasing age [22]. This information has prompted some authors to call for age to be taken into account when using eGFR for CKD diagnosis, staging and management [23,24]. Changes to the KCAT indications for referral of the elderly are currently being considered. Of the patients >75 years, 20% had a normal creatinine concentration (<120 μmol/L). Although a decreased eGFR is still predictive of an increased risk of ESRD and cardiovascular morbidity in the setting of advanced age [25], the requirement for nephrologist review is debatable [7,26].
The principal limitation of the study was the relatively short period of prospective data collection prior to the introduction of eGFR reporting, which reduced the ability to discern changes in referral rates directly attributable to this intervention. The length of follow-up chosen was 12 months post-introduction, which may also have been too short to fully account for the growth in referral rates and may not have reached steady state by the end of this study. The secondary and private centres had smaller referral areas and were therefore less powered to see a significant change. The private practice had one other competing practice that did not take part in this study and may have influenced the results. The study was not designed in a way that could determine the impact of eGFR reporting on awareness of CKD by the referring doctor and other studies examining this very critical point are required. This study also did not examine the economics of eGFR introduction. Finally, the possibility of classification bias could not be excluded since a previous validation study has suggested that 32.4% of subjects were misclassified when MDRD eGFR was used to categorize subjects according to the Kidney Disease Outcomes Quality Initiative CKD classification [27]. Such misclassification has the potential to generate patient anxiety, engender unnecessary and possibly harmful investigation and inappropriately drain precious healthcare resources [28].
In conclusion, automated eGFR reporting by laboratories together with concomitant education significantly increased the referral of patients with stage 3 CKD to nephrologist services and the majority of patients were referred appropriately within the framework of the published guidelines. The intervention therefore resulted in net benefit. As with any new intervention, education of primary care clinicians plays a large role. Since the completion of this study, a number of additional measures have been instigated, including improved feedback to primary services and the sending of a handbook on CKD and its management guidelines to referring practices in an effort to enhance the appropriateness of referrals.
Conflict of interest statement. None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 2.Dunstan D, Zimmet P, Welborn T, et al. AusDiab 2001: The Australian Diabetes, Obesity and Lifestyle Study. Melbourne: International Diabetes Institute; 2001. [Google Scholar]
- 3.Dunstan D, Zimmet P, Welborn T, et al. AusDiab 2005: The Australian, Diabetes, Obesity and Lifestyle Study. Melbourne: International Diabetes Institute; 2005. [Google Scholar]
- 4.El Nahas M. The global challenge of chronic kidney disease. Kidney Int. 2005;68:2918–2929. doi: 10.1111/j.1523-1755.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- 5.Cass A, Cunningham J, Wang Z, et al. Social disadvantage and variation in the incidence of end-stage renal disease in Australian capital cities. Aust NZ J Public Health. 2001;25:322–326. doi: 10.1111/j.1467-842x.2001.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 6.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J Am Soc Nephrol. 2003;14:S131–S138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 7.John R, Webb M, Young A, et al. Unreferred chronic kidney disease: a longitudinal study. Am J Kidney Dis. 2004;43:825–835. doi: 10.1053/j.ajkd.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 10.McDonald S, Excell L. ANZDATA Registry Report 2005. Adelaide, South Australia: Australian and New Zealand Dialysis and Transplant Registry; 2006. [Google Scholar]
- 11.Johnson DW. Evidence-based guide to slowing the progression of early renal insufficiency. Intern Med J. 2004;34:50–57. doi: 10.1111/j.1444-0903.2004.t01-6-.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DW. Use of serum creatinine concentration to assess level of kidney function. Nephrology. 2005;10:S133–S139. [Google Scholar]
- 13.Johnson DW. Use of estimated glomerular filtration rate to assess level of kidney function. Nephrology. 2005;10:S140–S146. [Google Scholar]
- 14.Akbari A, Swedko PJ, Clark HD, et al. Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch.Intern.Med. 2004;164:1788–1792. doi: 10.1001/archinte.164.16.1788. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 17.The Australasian eGFR Working Party Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust. 2005;183:138–141. doi: 10.5694/j.1326-5377.2005.tb06958.x. [DOI] [PubMed] [Google Scholar]
- 18.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 19.Richards N, Harris K, Whitfield M, et al. The impact of population-based identification of chronic kidney disease using estimated glomerular filtration rate (eGFR) reporting. Nephrol Dial Transplant. 2008;23:556–561. doi: 10.1093/ndt/gfm839. [DOI] [PubMed] [Google Scholar]
- 20.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 21.Mathew TH, Johnson DW, Jones GR. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: revised recommendations. Med J Aust. 2007;187:459–463. doi: 10.5694/j.1326-5377.2007.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 22.Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007;72:632–637. doi: 10.1038/sj.ki.5002374. [DOI] [PubMed] [Google Scholar]
- 23.Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: time for a course correction. J Am Soc Nephrol. 2008;19:844–846. doi: 10.1681/ASN.2008010110. [DOI] [PubMed] [Google Scholar]
- 24.Roderick PJ, Atkins RJ, Smeeth L, et al. Detecting chronic kidney disease in older people; what are the implications? Age Ageing. 2008;37:179–186. doi: 10.1093/ageing/afm180. [DOI] [PubMed] [Google Scholar]
- 25.O’Hare AM, Bertenthal D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol. 2006;17:846–853. doi: 10.1681/ASN.2005090986. [DOI] [PubMed] [Google Scholar]
- 26.Glassock RJ, Winearls C. An epidemic of chronic kidney disease: fact or fiction? Nephrol Dial Transplant. 2008;23:1117–1121. doi: 10.1093/ndt/gfn086. [DOI] [PubMed] [Google Scholar]
- 27.Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the modification of diet in renal disease and Cockcroft–Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 28.Grimes DA, Schulz KF. Uses and abuses of screening tests. Lancet. 2002;359:881–884. doi: 10.1016/S0140-6736(02)07948-5. [DOI] [PubMed] [Google Scholar]